A Systematic Review of Rehabilitation for Corticobulbar Symptoms in Adults with Huntington’s Disease
Abstract
Background:
Corticobulbar symptoms have been reported in all stages of Huntington’s disease (HD); aspiration pneumonia associated with swallowing impairment has been identified as the most common cause of death. Whilst recent research has described positive effects of corticobulbar rehabilitation in other neurodegenerative conditions, it is unclear if this is similarly effective in HD. Preliminary evidence in corticospinal rehabilitation has revealed physical therapy and exercise could be beneficial for individuals with HD.
Objective:
This systematic review will explore the literature relative to rehabilitation of corticobulbar symptoms in adults with HD.
Methods:
Two investigators independently searched relevant electronic databases for literature related to corticobulbar rehabilitation in HD, published in English until October 2019. Included studies were critically appraised using the Oxford Centre for Evidence-based Medicine Levels of Evidence, Cochrane Risk of Bias Tool and Scottish Intercollegiate Guidelines Network checklists. Study outcomes included measurements of function, quality of life or neuromuscular physiology.
Results:
Seventy-seven publications were screened with eight studies meeting the inclusion criteria – two randomised control trials and six intervention studies. Validated and objective outcome measures of corticobulbar symptoms were infrequently used. There was a high risk of bias identified in 7/8 studies. The data suggested positive clinical outcomes, no adverse effects and no deterioration observed across longitudinal studies.
Conclusions:
This systematic review documented a lack of high-quality evidence to support the use of rehabilitation to treat corticobulbar symptoms in HD. However, the suggestion of potential positive effects based on available, albeit limited, studies provides justification for further research in this area.
INTRODUCTION
Despite the recent progression in clinical trials and pharmaceutical therapies to slow or alter the progression of Huntington’s disease (HD), there are currently no effective medical treatments [1, 2]. It is therefore important to maximise proactive behavioural interventions to maintain function and improve quality of life. The role of rehabilitation to treat neurodegenerative diseases is a concept with emerging evidence in several conditions, including HD [3–5].
The majority of individuals with HD will develop corticobulbar symptoms [6]. These often present as noticeable changes in speech (dysarthria) and swallowing (dysphagia). Whilst these symptoms may be primarily related to progressive cortical neurodegeneration, evidence also highlights that commonly prescribed anti-choreic medications have side effects which include dysphagia, xerostomia (dry mouth), dysarthria and drowsiness [7–9]. Corticobulbar impairments such as dysphagia and dysarthria are highly correlated with decreased quality of life, independence and increased care giver burden [10–14]. The impact of cognitive impairment and changes in mood, combined with corticobulbar symptoms affect the ability to communicate successfully and participate in meaningful social interactions [15]. Dysphagic and dysarthric symptoms will be the key focus of this review.
Oropharyngeal dysphagia and dysarthria have been widely reported in all stages of HD [15–21]. Whilst these corticobulbar symptoms are highly variable between individuals, there are identified correlations between the severity of dysphagia, dysarthria and disease progression [6, 22]. In HD, all phases of swallowing can be impaired, impacted by choreic movements, cognitive impairment and behavioural changes [6]. Abnormal swallowing physiology in HD compromises swallowing safety with aspiration pneumonia reported as the leading cause of death in this condition [23, 24]. In addition, motor speech changes associated with HD can fall into several dysarthria categories; however, features of hyperkinetic dysarthria are most commonly described in the literature [25, 26]. Speech in HD is typically characterised by rapid involuntary movements of respiration, phonation, and articulation impacting on speech production, prosody and resonance [15, 21, 27]. Many people are unable to communicate effectively at the end stages of the disease.
In recent years, there has been an increase in studies evaluating corticospinal rehabilitation and exercise for individuals with HD [28–31]. Physical therapy and exercise interventions were reportedly well tolerated, resulting in measurable improvement in function during daily activities, cognition and self-reported quality of life [29, 30, 32]. Bilney et al. [5] also reported some functional improvements in gait and balance following multidisciplinary interventions in individuals with HD. European Huntington’s Disease Network Physiotherapy Guidelines have recommended targeted behavioural treatment for people in early to mid-stage HD to improve or maintain functional ability [33]. These guidelines reflect best available evidence and suggest no detrimental effects of intensive behavioural interventions.
This emerging evidence highlights the importance of behavioural interventions and exercise combined with pharmaceutical therapies in the long-term management of HD. An active rehabilitative approach to target corticospinal symptoms has been described as a crucial part of individual HD management [8, 30, 34]; however, the efficacy of rehabilitation targeting corticobulbar symptoms has not yet been reviewed. Compensatory strategies are most commonly applied to manage dysarthria and dysphagia as active rehabilitation has historically been assumed to be detrimental in neurodegenerative populations [3]. Importantly, a careful review of the literature in amyotrophic lateral sclerosis reveals insufficient evidence to suggest that moderate-intensity rehabilitative approaches are contraindicated in this neurodegenerative population [3]. Thus, the present review was conducted to explore the literature relative to rehabilitation of corticobulbar symptoms in adults with HD. This then forms the foundation for future research aimed to maintain or improve corticobulbar function in HD.
MATERIALS AND METHODS
The methodology for this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42017064156) and aligned with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Two investigators independently searched electronic databases for published literature in English until October 2019 using Medline-Ovid, Embase-Ovid, CINAHL, Web of Science (science and social science citation index), Science Direct, Scopus, Pubmed, The Cochrane Library (Cochrane Database of Systematic Reviews), clinical trial registers and best practice guidelines. The search strategy firstly identified the appropriate MeSH terms using the ‘map to MeSH term’ function in the Ovid version on Medline. This process was then repeated for each bibliographic database and relevant papers were screened for additional MeSH terms. These key words were then included in the subsequent literature searches. Key words used were: Huntington, Huntington’s disease, Huntington’s chorea, dysphagia, deglutition, bulbar, swallowing, speech, dysarthria, motor speech, articulation disorders, treatment and rehabilitation. All selected studies were manually cross-referenced to ensure no relevant literature was missed. The search was repeated prior to the final analysis. Abstracts, and then full articles, were reviewed collaboratively to compile a final list for review.
Studies met the inclusion criteria if they evaluated intervention with adults with manifest Huntington’s Disease, who had reported Shoulson & Fahn Staging 1–5, or presence of ≥ 36 CAG trinucleotide expansion repeats confirmed via genetic testing [35]. Articles with Juvenile HD (JHD) participants were not included as the significantly higher CAG repeats reported in JHD often result in more severe early cognitive and behavioural impairment, as well as faster disease progressions. Articles were included if they aimed to result in long term changes in the underlying neuromuscular substrates that present as corticobulbar symptoms in HD. This included interventions targeting the corticobulbar pathway. Specifically, direct innervation to cranial nerves III, IV, V, VI, VII, IX, X, XII or the muscles of the face, tongue, jaw, pharynx, head and neck. Sensory interventions that aimed to rehabilitate the corticobulbar pathway were also included. Literature excluded from this review were any interventions that focussed solely on compensatory strategies in isolation, such as modified diets, utensil modification, increased supervision.
Data extracted from the studies were grouped, compared and summarised using structured narrative (descriptive) analyses including specific population (e.g. stage of Huntington’s disease), intervention content, intervention effects and outcome measures where possible. Included studies were critically analysed using the Scottish Intercollegiate Guidelines Network (SIGN) Algorithm and Critical Appraisal Checklists [36, 37]. Levels of evidence were rated according to Oxford Centre for Evidence-based Medicine Levels of Evidence [38] and risk of bias was judged using the Cochrane Risk of Bias Tool [39, 40].
RESULTS
In total 888 publications were identified. As detailed in the PRISMA flowchart (Fig. 1), 77 full text articles were reviewed, 19 studies were excluded as they described compensatory management only. Of the remaining 58, only eight studies matched the inclusion criteria: two randomised control trials (RCTs) and six intervention studies evaluated rehabilitative approaches to improve corticobulbar symptoms. It is acknowledged that one of the RCTs evaluated rehabilitation of olfactory (CN I) function, which is not specifically included in the corticobulbar pathways [41]. This study was included given potential impact of olfaction on other corticobulbar symptoms in HD. See Table 1 for a summary of included studies. Using the SIGN Algorithm [36], only two studies were rated as appropriate to assess quality of evidence using the SIGN Critical Appraisal Checklists (see the Supplementary Material) [37]. The remaining studies were critically analysed using the Cochrane Risk of Bias Tool, and descriptively reviewed below. Quantitative data synthesis was not possible as the low yield of articles used varied outcome measures and therefore were not sufficiently homogenous.
Fig.1
PRISMA Flowchart for identifying studies for systematic review.
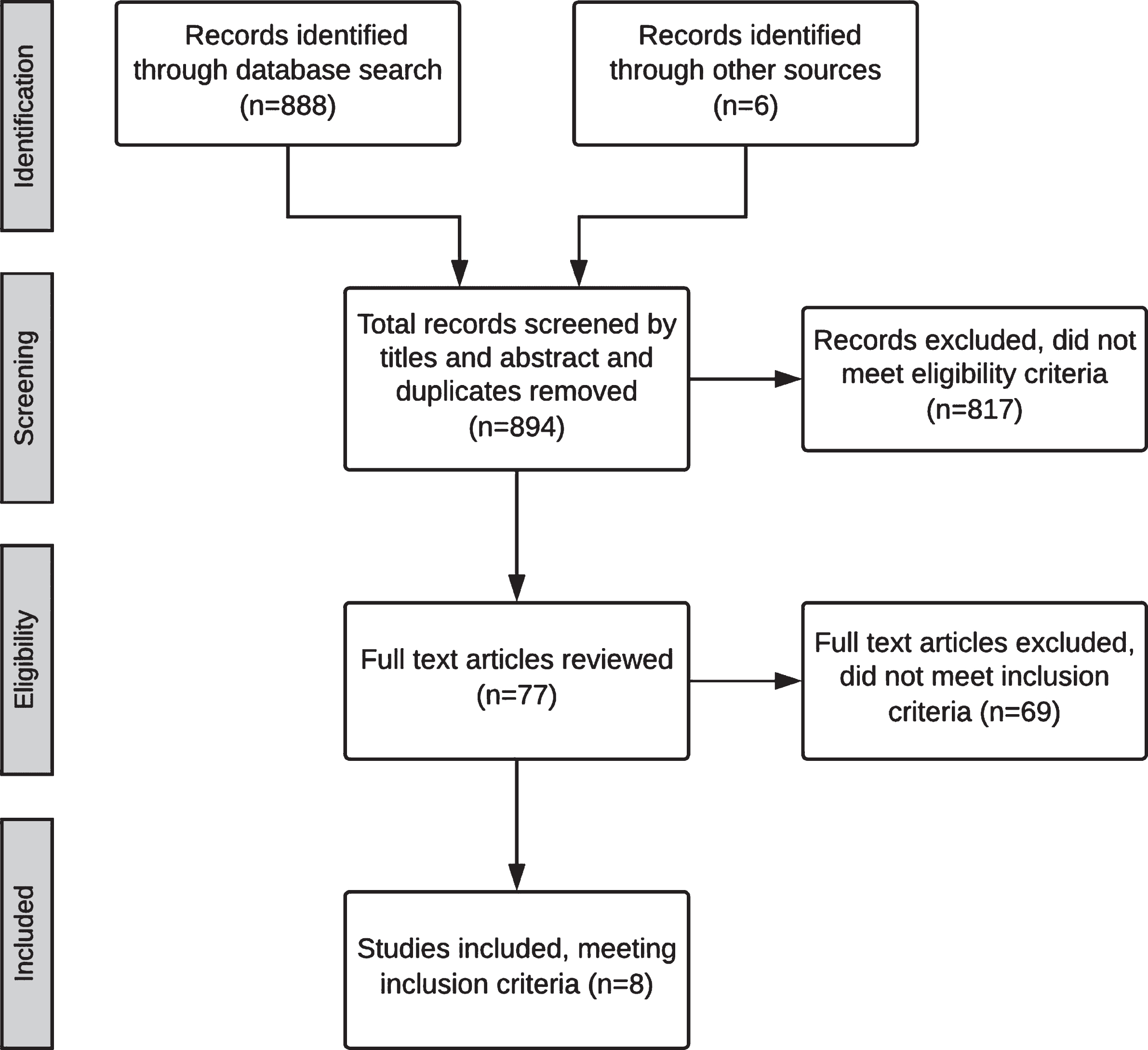
Table 1
Summary of 8 studies included in this systematic review of corticobulbar rehabilitation in adults with Huntington’s disease
| Author | Participants | Study Design & Level of Evidence | Treatment | Outcome Measures | Results/conclusions |
| Leopold & Kagel [47] | N = 12, 11 moderate HD, 1 mild-moderate HD. | Intervention case series (2b) | Modified Valsalva maneuver, modification of diet, utensils, and posture. Individualising swallowing sequence. | Neurological examination, pulmonary function testing, oesophageal manometry, laryngoscopy and videofluoroscopic swallowing study. Diet modification level. | Improved bolus prep and transfer, pre-mature swallow, pharyngeal stasis, nasopharyngeal reflux and aspiration. 8/11 participants returned to a normal diet. 3/11 minimally restricted diet. Maintained for 3 years. |
| Leng et al. [41] | N = 12, mid-late HD in a specialised residential unit. (2 withdrawn with medical complications) | Randomised controlled pilot study. Two group design. (2b) | 1:1 Multisensory stimulation targeting visual, tactile, auditory, olfactory input (MSE, treatment) versus relaxation (control group). 30mins twice weekly for four weeks. | Rehabilitation Evaluation and Behaviour and Mood Disturbance rating scales. Interact observation assessments (pre, during and post-therapy sessions). Physiological measures e.g. respiration and involuntary movements. | Significant difference in mood during MSE sessions, and stimulation level over time. Effects did not generalise between sessions. No other significant differences. |
| Zinzi et al. [43] | N = 40, mild-moderate HD (25 completed study) | Pilot study (2b) | Individual and group intervention (PT, OT & SLT) in an inpatient rehabilitation facility. 3 week block of intensive treatment. Treatment block could be repeated 3 times a year. 8 hours of intervention per day 5 times a week, 4 hours for 1 day, 1 day free. | Zung Depression Scale, Mini-Mental State Examination (MMSE), Barthel Index, Tinetti Scale, Physical Performance Test (PPT). | Each 3 week block of treatment resulted in highly significant (p > 0.001) improvements in motor performance and activities of daily living. No carry-over effect from one admission to the next but no motor decline was detected over two years. Dysarthria and dysphagia specific measurement in Barthel Index and PPT were not stated. |
| Zinzi et al. [13] | N = 40 who had completed at least one block of intensive treatment described above. Average 8.6months follow up. | Retrospective case series, intervention (2b) | A written questionnaire mailed to participants and their carers. | Descriptive and inferential statistics were used. Thematic analyses were also conducted on written texts. | Improvements were reported in speech, swallowing, and several psychosocial aspects: mood, apathy, familiar and social relationships (binomial test, p < 0.05). Improvements in gait, balance, motor control, and fall reduction were also described. Duration of benefits estimated to last 1–3 months by 71% of informants with no carry over to the next admission. |
| Giddens et al. [46] | Case series: N = 14 13 early HD, 1 late HD Pilot study: N = 7, early HD (5 completed study) | Case study & Pilot study (4) | Home-based oral motor, phonatory and respiratory exercises. Twice daily for 30 days | Cranial nerve examination Speech diadochokinetic rates, maximum phonation. | 2/5 patients reported ‘elimination’ of dysphagia. Improved or maintained cranial nerve function and phonation time. |
| Piira et al. [44] | N = 37, early-mid stage HD (31 completed study) | Intervention study (2b) | Daily 1:1 or group therapy (PT, OT & SLT). Exercises focused on improving muscle strength, maintaining function, included unspecified swallowing/speech exercises at least 3 times per week and diet modification at rehabilitation centre. Family/caregiver education training. 3 week block of intensive treatment. Treatment block could be repeated 3 times a year. | MMSE and Unified HD Rating Scale. Motor Function: 6min walking test, TUG, 10m test, BERG balance scale. ADLs: Barthel Index Cognitive function, Hospital Depression & Anxiety Scale, Quality of Life SF 12 questionnaire. | Significant improvements in gait function, balance, quality of life, anxiety and depression, BMI. ADLs and functional ratings remained stable. Significant decline in only one cognitive measure (SDMT). No other decline. Dysarthria and dysphagia specific measurement in Barthel Index were not stated. |
| Piira et al. [45] | N = 6 from, 10 early-mid stage HD who completed previous study above [44]. | Retrospective intervention study (2b) | Daily MDT intervention as described above [44]. 6 admissions of 3 weeks over a 2 year period. | Same outcome measures as above [44]. | No significant decline in gait and balance from 2 year baseline. Some improvement in BMI, QoL, anxiety and depression, did not reach significance. No significant decline in cognition. ADL stable. 4/6 improved motor function. Dysarthria and dysphagia specific measurement in Barthel Index were not stated. |
| Reyes et al. [42] | N = 18, moderate HD. Randomised to control group (n = 9) and training group (n = 9). | Randomised Control Trial (1b) | Home-based inspiratory and expiratory muscle training (5 sets of 5 repetitions) 6 times a week for 4 months. Control group: fixed resistance. Training group: progressively increased resistance. | Spirometry indices, maximum inspiratory and expiratory pressure, 6min walk test, dyspnoea, water swallow test, SWAL-QoL. | Intervention group improved in respiratory outcome measures, time per swallow, SWAL-QoL. Small positive effect on respiratory outcomes for control group. |
Descriptive analysis of key study features
Study design
Table 1 outlines the study designs that were used to evaluate the effectiveness of rehabilitation in individuals with HD. Two studies were RCTs and adopted control groups who received sham treatment [41, 42]. The remaining five studies were within-subject intervention case series/cohort studies [13, 43–46]. Participants were assessed pre- and post-intervention. One of these inpatient study designs then used a non-standardised questionnaire mailed to participants who had completed the first rehabilitative study [13].
Participants
Treatment groups ranged from five [46] to 40 [43] participants who completed corticobulbar interventions. All studies included individuals at varying stages of the disease which limits comparison of rehabilitation approaches. The Unified Huntington’s Disease Rating Scale (UHDRS) was used in two studies to characterise the stage and severity of the disease [42, 46]. Subcategories of this scale were also used as outcome measures in two other studies [44, 45]. One study included only individuals with mild HD (Stage 1 or 2 according to the UHDRS) [46]. The majority of studies included participants with mild-moderately advanced HD [13, 43–45], whilst Leng et al. [41] included only late-stage (stage 5) HD in their recruitment.
Setting of therapy
Intervention took place in a variety of settings and with different service delivery models. Four studies took place in inpatient facilities and included MDT rehabilitation [13, 43–45], one in a specialist residential home [41], two were home-based programmes of daily exercises [42, 46] and one was in an unspecified outpatient clinic [47]. All studies reported good adherence to therapy programmes and participation in intervention across settings; however, no systematic measurements of adherence were reported.
Intervention
Interventions to maintain or improve speech and swallowing were intensive; six studies evaluated daily intervention [13, 43–46], one completed sessions twice per week [41], and one stated that 11/12 patients were “intensively treated” but did not specify what this consisted of [page 59, 47]. Where specified, the duration of intervention ranged from three weeks [13, 43–45] to four months [42]. Giddens et al. [46] reported that oral motor labial and lingual resistance training, respiratory (glottal adduction) and phonatory exercises were completed twice daily at home, a minimum of four times per week for 30 days. The number of participants that fully adhered to the long-term exercise program was not reported. Similarly, Reyes et al. [42] evaluated inspiratory and expiratory muscle training using a home programme of six training sessions per week for four months. The training group received increased resistance to respiratory pressures over the course of treatment, whilst the control group received a sham protocol with the same device. There were no adverse effects of intensive training and all participants adhered to the protocol, but it is not reported if the adherence to the home programme was specifically measured [42].
Four studies evaluated interventions to maintain or improve speech and swallowing function as part of an intensive inpatient MDT approach [13, 43–45]. Two of these articles specified the intensity of their intervention which focused on physical exercise, social activities, and family/caregiver education teaching sessions [44, 45]. SLT intervention was reported, but specific dysarthria and dysphagia exercises were not described in any of the MDT studies, thus inhibiting replicability. Zinzi et al. [43] included non-specified speech therapy where patients were “taught strategies for swallowing” [page 40, 42] amongst other MDT intervention. This study also completed respiratory exercises using visual and tactile feedback during joint sessions with the physiotherapist and SLT [43].
Leopold et al. [47] provided a description of their dysphagia intervention which included a modified Valsalva manoeuvre (forced exhalation against a pinched nose) alongside compensatory strategies (diet modification, adaptive utensils, optimum positioning). Patients were taught the ‘chew-swallow-cough-swallow sequence’ technique. An unspecified number of the twelve participants with severe dysphagia required non-oral feeding to maintain nutrition and hydration via nasogastric tube until the “compensatory techniques could be instituted and a pureed diet could be safely tolerated” [page 59, 47]. The authors stated that “more severely demented patients required more sessions” [page 60, 47] and greater ongoing supervision post-therapy to target more severe cognitive and motor sequencing deficits; however, this additional therapy was not clearly defined. The duration of the intervention was not stated, and the results were not statistically analysed.
Leng et al. [41] did not focus on dysarthria or dysphagia intervention. Instead, this study measured the effect of multisensory environmental stimulation (MSE) compared to passive relaxation therapy. The standardised MSE intervention was designed for visual, tactile, auditory and olfactory innervation. The authors described the specific intervention sufficiently to allow for replication.
Outcome measures
All intensive MDT intervention studies included broad outcome measures of motor performance (including walking, gait, and balance), ratings of ability to perform activities of daily living (ADL), cognitive measures (typically the Mini-Mental State Examination and UHDRS) and quality of life measures [13, 43–45]. No instrumental or objective measures of speech or swallowing were reported in these studies; however, the Barthel Index (a measure of ADL) includes questions about feeding independence and diet modification. Piira et al. [45] used these baseline measures to report no functional decline in cognition, ADL, gait or balance in participants who continued the rehabilitation programme for another year after initial admission. Zinzi et al. [43] also used the Physical Performance Test (PPT) to measure the functional speed and accuracy of specific tasks. This standardized test included a measure of functional eating. The authors reported significant positive treatment effects on motor and functional performance as measured by the PPT and Tinetti Scale. This improvement was not retained between inpatient admissions. The authors highlighted an overall lack of decline in other cognitive and functional measures such as the Barthel Index over the two year follow up period [43].
Many studies included subjective or non-standardised measures of corticobulbar symptoms. Giddens et al. [46] used an unspecified swallowing screening with no instrumental assessment or functional outcome measures. They reported significant improvements post-therapy on subjective oral motor ratings and diadochokinetic ratings. Two of the six participants self-reported an ‘elimination’ of their swallowing impairment post-therapy, but there were no objective measures of this. Conversely, Leopold et al. [47] described several objective assessments of swallowing including videofluoroscopic swallowing studies, oesophageal manometry, pulmonary function testing, mealtime observation and neurological examination. All participants demonstrated clinical abnormalities of swallowing, speech and voluntary coughing. Each participant was rated on a non-standardised dysphagia severity scale (0 – 5) using instrumental assessment findings. Videofluoroscopic assessment was the only outcome measure repeated post-therapy and was reported descriptively by the authors. No objective timing or displacement measures were used to analyse the videofluoroscopic studies. The study reported that all participants improved and 73% (n = 8) returned to normal diet; the remaining three were advised to have minimal diet restrictions. These improvements were maintained for up to 3 years post-treatment. This was inferred as none of the participants required supplementary or additional non-oral nutrition; however, no objective follow up assessments were reported.∥Reyes et al. [42] used spirometry measures of inspiratory and expiratory pressures pre- and post-treatment. A non-standardised version of the timed water swallowing test was used to measure functional swallowing ability with thin fluids. Participants were instructed to drink 50mls of water three times in each assessment session. The standardised Timed Water Swallow Test is 150mls of water consumed “as fast as is comfortably possible” [48]. Time per swallow, swallowing capacity and volume improved at two months and four months of training. Swallowing related quality of life (SWAL-QoL) improved slightly after four months of training. This study did not include any objective or instrumental measures of swallowing biomechanics. The treatment group had a moderate training effect on maximum inspiratory and expiratory pressures after four months of training. A small effect was also reported for the control group. Improvements were also noted in spirometry measures compared to the control group; however, these differences were not statistically significant. Non-significant improvements were also reported across swallowing measures, including the adapted timed water swallowing test. No studies included secondary information regarding pulmonary status, rate of chest infection or aspiration pneumonia.∥As previously discussed, Leng et al. [41] focused on a multisensory environment to evaluate the effects of visual, tactile, auditory and olfactory stimulation. This study did not include any direct measurements of corticobulbar function, instead the primary measures were of mood and behaviour using the Rehabilitation Evaluation and Behaviour and Mood Disturbance rating scales. Physiological measures including heart rate, respiratory flow and involuntary movements (using the dyskinesia section of the St. Hans Rating Scale) were collected throughout the 12 week observation period. The reliability and validity of these outcome measures were not discussed, particularly in relation to assessment of people with HD. The researchers found an immediate effect of intervention on mood during MSE sessions, and stimulation level over time compared to the control group, but these effects did not generalise between sessions. There were no other significant differences between groups in respect to involuntary movements or other measures. Although speech and swallowing symptoms were not included in this RCT, this study evaluated a rehabilitation which partially targeted olfactory (CN I) function. This study was considered relevant to include to reflect its impact on other corticobulbar symptoms in HD.∥
Risk of bias
A summary of the Cochrane Risk of Bias Tool completed for each study is provided in Table 2. Low risk of attrition bias was identified in 3/8 of studies [13, 41, 42], where complete outcome data were reported. Additionally, there was low risk of bias in selective reporting in 5/8 of studies [41–45]. The method of data collection adopted by Zinzi et al. [13] was judged to have a high risk of bias. Their non-standardised questionnaire was mailed to participants on average 8.6 months post-intervention. This questionnaire asked about their inpatient rehabilitation and care experience; 91.9% (n = 34) of respondents were caregivers and 8.1% (n = 3) respondents were patients who had completed the inpatient intervention. This method of data collection may have introduced a recruitment bias and selective reporting as people generally reported positive improvements on this subjective questionnaire.∥Giddens et al. [46] excluded participants with more severe cognitive or motor impairments creating a risk of selection bias. One participant with late (stage 4) HD was withdrawn from the study as she subjectively perceived increased muscle weakness with the exercise program, however, there were no clinical measures of this. Two participants changed their drug regimens during treatment which created an additional variable. Whilst Leopold et al. [47]’s landmark study published in 1985 is frequently referenced to describe dysphagia in HD, the adopted study design is vulnerable to several identified biases. Blinding of raters was not possible, outcome measures were not validated, or consistently repeated post-therapy. In addition, data analysis of quantitative outcome measures was not conducted.∥Both RCTs included in this review were deemed low risk of bias in several domains [41, 42]. The authors provided a detailed protocol for randomisation and intervention, including baseline and follow up periods to measure any treatment effect. These study designs were replicable and reduced risk of bias, despite the small sample size. Leng et al. [41] used two investigators to facilitate and score the sessions, with good inter-rater reliability reported; however, with this design blinding was not possible. Two participants withdrew from this study due to medical complications which may have increased selection bias, and significant changes in medication were reported for two participants during the study, introducing an additional variable. The other RCT excluded those with moderate-severe corticobulbar symptoms such as lingual chorea and oral weakness which may have introduced a selection bias [42].
Table 2
Summary of Cochrane Risk of Bias Tool evaluation. H, high risk of bias, L, low risk of bias, U/C, unclear risk of bias
| Sequence generation | Allocation concealment | Blinding (pts/researchers) | Outcome ax blinding | Incomplete data | Selective reporting | Other bias | |
| Leopold & Kagel [47] | N/A | N/A | N/A | H | H | H | H |
| Leng et al. [41] | L | L | H | H | L | L | U/C |
| Zinzi et al. [43] | N/A | N/A | N/A | U/C | H | L | U/C |
| Zinzi et al. [13] | N/A | N/A | N/A | H | L | H | H |
| Giddens et al. [46] | N/A | N/A | N/A | H | H | H | H |
| Piira et al. [44] | N/A | N/A | N/A | H | H | L | U/C |
| Piira et al. [45] | N/A | N/A | N/A | H | H | L | U/C |
| Reyes et al. [42] | L | L | U/C | U/C | L | L | U/C |
DISCUSSION
Historically, SLT management of HD focused on compensation for speech and swallowing impairment with approaches such as diet modification, increased supervision, postural changes, stabilising the mandible, and visual cues [47, 49]. Indeed, within the screened literature, 17 articles did not meet the inclusion criteria as they only described compensatory management of dysphagia and dysarthria. Whilst these strategies may be important to implement as the disease progresses, it may be worth considering the potential for rehabilitation, particularly for treatment of dysphagia and dysarthria.
This systematic review revealed a lack of high-quality evidence to justify the effectiveness of rehabilitation for corticobulbar symptoms in HD. Despite this, the included studies highlighted preliminary evidence of potential benefits of ongoing research regarding rehabilitation for corticobulbar symptoms, such as dysphagia and dysarthria. Importantly, there was no significant deterioration observed across longitudinal studies, which could be clinically significant in this neurodegenerative disease. This conclusion may be taken cautiously given that the full trajectory of disease progression is not known; however, with a study duration of up to three years, it suggests at the very least, a possible short-term positive impact [13, 43–45, 47]. Further investigation into the effectiveness of specific rehabilitative approaches is warranted as five of the included studies failed to describe which dysarthria or dysphagia interventions were used [13, 43–45, 47]. Of the interventions described in three studies, there was inadequate homogeneity to allow for comparison between rehabilitation methods [41, 42, 46].
In HD, corticobulbar deficits are not isolated to muscle weakness [10, 49]. An alternative approach may be to focus on optimising precision of neuromuscular connections instead of primarily compensatory management. Giddens et al. [46] hypothesised that the significant improvement observed in cranial nerve function following intensive rehabilitation may be attributed to better control and coordination of the muscle groups integral to speech and swallowing. Task specific rehabilitation based on the principles of motor learning may be beneficial in HD management; as these intensive rehabilitative approaches have shown potential to increase neuroplasticity and cortical excitability [50, 51]. Structured rehabilitative programmes which intensively target dysarthria or dysphagia such as Lee Silverman Voice Treatment, expiratory muscle strength training (EMST) and video-assisted swallowing (VAS) therapy have been evaluated in other neurodegenerative diseases. Studies evaluating these rehabilitative approaches have reported benefits following treatment to maintain or improve function in conditions such as amyotrophic lateral sclerosis, multiple sclerosis and Parkinson’s disease, [42, 52–55]. As Reyes et al. [42] evaluated the effects of a structured EMST protocol in HD, this small RCT provided preliminary evidence to suggest this muscle strengthening intervention may be beneficial in HD. This justifies further research into task specific skill-based interventions and muscle strengthening approaches with larger sample sizes and instrumental swallowing outcome measures.
This review also highlighted the lack of objective outcome measures used to evaluate therapy. Only one study included the gold standard videofluoroscopic swallowing studies pre- and post-therapy [47]. However, this study did not include standardised timing or displacement measures of swallowing biomechanics. Whilst one study included a swallowing specific quality of life questionnaire [42], the remaining studies included non-standardised or nonspecific measures of corticobulbar function. The omission of valid and reliable outcome measures is problematic for replicability and evaluation of treatment effects. Furthermore, seven of the eight studies were judged to have a high risk of bias identified in at least one category (Table 2). As often seen in the wider literature of this rare neurodegenerative disease, small sample sizes and underpowered studies were identified as limitations in the majority of the studies included in this review (Table 1). Whilst the disease stage or UHDRS scores were described in most studies, other key participant information such as level of cognition, medication regimes, or presence of depression was not specified consistently.
Although the number of studies is limited and the outcome measures lack objectivity, overall the included studies suggested no adverse effects, and at least the possibility of potential benefits of intensive rehabilitation to improve or maintain function. The therapy programmes were reportedly well adhered to, with minimal withdrawal from intervention and no measurable detrimental effects of an intensive approach across several stages of the disease. This is in line with systematic reviews evaluating corticobulbar rehabilitation in other neurological populations [3]. In addition, this echoes the findings of systematic reviews specifically focused on corticospinal rehabilitation and exercise in HD [5, 29, 32, 33]. These reviews highlight the concept that rehabilitative approaches may be beneficial in HD, counter to traditional belief that active rehabilitation may not be effective or tolerated in neurodegenerative diseases [5]. Conversely, there has been a notable increase in interest and literature since 2003 which has provided evidence to support the feasibility and effectiveness of early MDT intervention to maintain or improve function in corticospinal symptoms of HD. This valuable research may inform further development of rehabilitative approaches to target corticobulbar symptoms such as dysarthria and dysphagia.
Conclusion
There was not sufficient evidence to justify rehabilitation of corticobulbar symptoms on HD. The best available research in early to mid-stage HD suggested short-term improvements in motor function and quality of life following intensive functional rehabilitation. However, there was no evidence of detrimental effects from the available studies. The existing evidence was limited by the risk of bias, with a lack of valid and reliable objective tools used. There was, however, sufficient preliminary evidence to justify further research in this area using well designed intervention studies with standardised, objective outcome measures to evaluate rehabilitative approaches targeting corticobulbar symptoms in individuals with HD.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JHD-190384.
REFERENCES
[1] | Wild EJ , Tabrizi SJ . Therapies targeting DNA and RNA in huntington’s disease. Lancet Neurol. (2017) ;16: (10):837–47. |
[2] | Bonelli RM , Hofmann P . A systematic review of the treatment studies in Huntington’s disease since 1990. Expert Opin Pharmacother. (2007) ;8: (2):141–53. |
[3] | Plowman EK . Is there a role for exercise in the management of bulbar dysfunction in amyotrophic lateral sclerosis? J Speech Lang Hear R. (2015) ;58: (4):1151–66. |
[4] | Van Hooren M , Baijens L , Voskuilen S , Oosterloo M , Kremer B . Treatment effects for dysphagia in Parkinson’s disease: A systematic review. Parkinsonism Relat Disord. (2014) ;20: (8):800–7. |
[5] | Bilney B , Morris ME , Perry A . Effectiveness of physiotherapy, occupational therapy, and speech pathology for people with Huntington’s disease: A systematic review. Neurorehabilitation Neural Repair. (2003) ;17: (1):12–24. |
[6] | Heemskerk AW , Roos RA . Dysphagia in Huntington’s disease: A review. Dysphagia. (2011) ;26: (1):62–6. |
[7] | Walker FO . Huntington’s disease. Lancet. (2007) ;369: (9557):218–28. |
[8] | Mason SL , Barker RA . Advancing pharmacotherapy for treating Huntingtons disease: A review of the existing literature. Expert Opin Pharmacother. (2016) ;17: (1):41–52. |
[9] | Higgins DS . Huntington’s disease. Curr Treat Options Neurol. (2006) ;8: (3):236–44. |
[10] | Manor Y , Oestreicher-Kedem Y , Gad A , Zitser J , Faust-Socher A , Shpunt D , et al. Dysphagia characteristics in Huntington’s disease patients: Insights from the Fiberoptic Endoscopic Evaluation of Swallowing and the Swallowing Disturbances Questionnaire. CNS Spectr. (2018) :1–6. |
[11] | Cubo E , Rivadeneyra J , Armesto D , Mariscal N , Martinez A , Camara RJ . Relationship between nutritional status and the severity of Huntington’s disease. A Spanish multicenter dietary intake study. J Huntingtons Dis. (2015) ;4: (1):75–85. |
[12] | Clarke G , Fistein E , Holland A , Tobin J , Barclay S , Barclay S . Planning for an uncertain future in progressive neurological disease: A qualitative study of patient and family decision-making with a focus on eating and drinking. BMC Neurol. (2018) ;18: (1):115. |
[13] | Zinzi P , Salmaso D , Frontali M , Jacopini G . Patients’ and caregivers’ perspectives: Assessing an intensive rehabilitation programme and outcomes in Huntington’s disease. J Public Health. (2009) ;17: (5):331–8. |
[14] | Ekberg O , Hamdy S , Woisard V , Wuttge–Hannig A , Ortega PJD . Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia. (2002) ;17: (2):139–46. |
[15] | Hartelius L , Carlstedt A , Ytterberg M , Lillvik M , Laakso K . Speech disorders in mild and moderate Huntington disease: Results of dysarthria assessments of 19 individuals. J Med Speech Lang Pathol. (2003) ;11: (1):1–14. |
[16] | Michou E , Trender-Gerhard I , Gerhard A , Craufurd D , Herholz K , Hamdy S . Pilot observations from a multimodal imaging study in mild dysphagic patients in early stage huntington’s disease (HD). Dysphagia. (2017) ;32: (1):172–3. |
[17] | Alves TC , Cola PC , Santos RR , Motonaga SM , da Silva RG . Swallowing endoscopy findings in Huntington’s disease: A case report. CoDAS (São Paulo). (2016) ;28: (4):486–8. |
[18] | Lee TH , Lee JS , Kim WJ . High resolution impedance manometric findings in dysphagia of Huntington’s disease. World J Gastroenterol. (2012) ;18: (14):1695–9. |
[19] | de Tommaso M , Nuzzi A , Dellomonaco AR , Sciruicchio V , Serpino C , Cormio C , et al. Dysphagia in Huntington’s disease: Correlation with clinical features. Eur Neurol. (2015) ;74: (1-2):49–53. |
[20] | Cruickshank TM , Thompson JA , Domínguez D , Juan F , Reyes AP , Bynevelt M , et al. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: An exploratory study. Brain Behav. (2015) ;5: (2). |
[21] | Chan JC , Stout JC , Vogel AP . Speech in prodromal and symptomatic Huntington’s disease as a model of measuring onset and progression in dominantly inherited neurodegenerative diseases. Neurosci Biobehav Rev. (2019) ;107: :450–60. |
[22] | Heemskerk AW , Verbist BM , Marinus J , Heijnen B , Sjogren EV , Roos RA . The Huntington’s Disease Dysphagia Scale. Mov Disord. (2014) ;29: (10):1312–6. |
[23] | Heemskerk A-W , Roos RA . Aspiration pneumonia and death in Huntington’s disease. PLoS currents. (2012) ;4. |
[24] | Rodrigues FB , Abreu D , Damásio J , Goncalves N , Correia-Guedes L , Coelho M , et al. Survival, mortality, causes and places of death in a European Huntington’s disease prospective cohort. Mov Disord Clin Pract. (2017) ;4: (5):737–42. |
[25] | Lansford KL , Liss JM . Vowel acoustics in dysarthria: Speech disorder diagnosis and classification. J Speech Lang Hear Res. (2014) ;57: (1):57–67. |
[26] | Diehl SK , Mefferd A , Ya-Chen L , de Riesthal M , Claassen DO . Motor speech phenotypes in Huntington disease. Neurotherapeutics. (2018) ;15: (4):1175–6. |
[27] | Rusz J , Klempíř J , Tykalová T , Baborová E , Čmejla R , Růžička E , et al. Characteristics and occurrence of speech impairment in Huntington’s disease: Possible influence of antipsychotic medication. J Neural Transm. (2014) ;121: (12):1529–39. |
[28] | Quinn L , Busse M , Carrier J , Fritz N , Harden J , Hartel L , et al. Physical therapy and exercise interventions in Huntington’s disease: A mixed methods systematic review protocol. JBI Database System Rev Implement Rep. (2017) ;15: (7):1783–99. |
[29] | Fritz NE , Rao AK , Kegelmeyer D , Kloos A , Busse M , Hartel L , et al. Physical therapy and exercise interventions in Huntington’s disease: A mixed methods systematic review. J Huntingtons Dis. (2017) ;6: :217–35. |
[30] | Quinn L , Busse M . The role of rehabilitation therapy in Huntington disease. Handb Clin Neurol. (2017) ;144: :151–65. |
[31] | Petzinger GM , Fisher BE , McEwen S , Beeler JA , Walsh JP , Jakowec MW . Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in parkinson’s disease. Lancet Neurol. (2013) ;12: (7):716–26. |
[32] | Dolbow JD , Ly H , Elwert N , Gassler J . Effects of exercise environment and protocol intensity on the efficacy of rehabilitation care for patients with Huntington’s disease: A comprehensive review. Int J Exerc Sci. (2019) ;12: :456–70. |
[33] | EHDN Standards of Care Working Group; Rae D , Hamilton A , Miedzybrodzka ZA . Standard of Care in Huntington’s Disease. European Huntington’s Disease Network; (2013) [Available from: http://www.euro-hd.net/html/network/groups/physio. |
[34] | Bilney B , Morris ME , Denisenko S . Physiotherapy for people with movement disorders arising from basal ganglia dysfunction. N Z J Physiother. (2003) ;31: (2):94–100. |
[35] | Shoulson I , Fahn S . Huntington disease: Clinical care and evaluation. Neurology. (1979) ;29. |
[36] | Scottish Intercollegiate Guidelines Network S. Algorithm for classifying study design for questions of effectiveness http://www.sign.ac.uk/assets/study_design.pdf2011 [Available from: http://www.sign.ac.uk/assets/study_design.pdf |
[37] | Scottish Intercollegiate Guidelines Network S. SIGN 50: A guideline developer’s handbook https://www.sign.ac.uk/checklists-and-notes.html: NHS Quality Improvement Scotland Equality and Diversity Office; 2011. |
[38] | Phillips B , Ball C , Sackett D , Badenoch D , Straus S , Haynes B , et al. Oxford Centre for Evidence-based Medicine – Levels of Evidence (2009) [Available from: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. |
[39] | Higgins JP , Altman DG , Gøtzsche PC , Jüni P , Moher D , Oxman AD , et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) ;343: :d5928. |
[40] | Higgins JPT , Green S . Cochrane Handbook for Systematic Reviews of Interventions The Cochrane Collaboration2011 [Version 5.1.0 [Available from: http://www.handbook.cochrane.org. |
[41] | Leng TR , Woodward MJ , Stokes MJ , Swan AV , Wareing L-A , Baker R . Effects of multisensory stimulation in people with Huntington’s disease: A randomized controlled pilot study. Clin Rehabil. (2003) ;17: (1):30–41. |
[42] | Reyes A , Cruickshank T , Nosaka K , Ziman M . Respiratory muscle training on pulmonary and swallowing function in patients with Huntington’s disease: A pilot randomised controlled trial. Clin Rehabil. (2015) ;29: (10):961–73. |
[43] | Zinzi P , Salmaso D , De Grandis R , Graziani G , Maceroni S , Bentivoglio A , et al. Effects of an intensive rehabilitation programme on patients with Huntington’s disease: A pilot study. Clin Rehabil. (2007) ;21: (7):603–13. |
[44] | Piira A , Van Walsem MR , Mikalsen G , Nilsen KH , Knutsen S , Frich JC . Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: A prospective intervention study. PLoS Curr. (2013) ;5. |
[45] | Piira A , Van Walsem MR , Mikalsen G , Øie L , Frich JC , Knutsen S . Effects of a two-year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: A prospective intervention study. PLoS Curr. (2014) ;6. |
[46] | Giddens CL , Coleman AE , Adams CM . A home program of speech therapy in Huntington’s disease. J Med Speech Lang Pathol. (2010) ;18: (2):1–11. |
[47] | Leopold NA , Kagel MC . Dysphagia in Huntington’s disease. Arch Neurol. (1985) ;42: (1):57–60. |
[48] | Hughes TA , Wiles CM . Clinical measurement of swallowing in health and in neurogenic dysphagia. Q J Med. (1996) ;89: (2):109–16. |
[49] | Kagel MC , Leopold NA . Dysphagia in Huntington’s disease: A 16-year retrospective. Dysphagia. (1992) ;7: (2):106–14. |
[50] | Athukorala RP , Jones RD , Sella O , Huckabee M-L . Skill training for swallowing rehabilitation in patients with parkinson’s disease. Arch Phys Med Rehabil. (2014) ;95: (7):1374–82. |
[51] | Herman T , Giladi N , Hausdorff J . Treadmill training for the treatment of gait disturbances in people with Parkinson’s disease: A mini-review. J Neural Transm. (2009) ;116: (3):307–18. |
[52] | Plowman EK . Impact of expiratory strength training in amyotrophic lateral sclerosis expiratory training in ALS. Muscle Nerve. (2016) ;54: (1):48–53. |
[53] | Troche M , Okun M , Rosenbek J , Musson N , Fernandez H , Rodriguez R , et al. Aspiration and swallowing in parkinson disease and rehabilitation with EMST A randomized trial. Neurology. (2010) ;75: (21):1912–9. |
[54] | Chiara T , Martin AD , Davenport PW , Bolser DC . Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: Effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Arch Phys Med Rehabil. (2006) ;87: (4):468–73. |
[55] | Manor Y , Mootanah R , Freud D , Giladi N , Cohen JT . Video-assisted swallowing therapy for patients with Parkinson’s disease. Parkinsonism Relat Disord. (2013) ;19: (2):207–11. |




