A Longitudinal Motor Characterisation of the HdhQ111 Mouse Model of Huntington’s Disease
Abstract
Background: Huntington’s disease (HD) is a rare, incurable neurodegenerative disorder caused by a CAG trinucleotide expansion with the first exon of the huntingtin gene. Numerous knock-in mouse models are currently available for modelling HD. However, before their use in scientific research, these models must be characterised to determine their face and predictive validity as models of the disease and their reliability in recapitulating HD symptoms.
Objective: Manifest HD is currently diagnosed upon the onset of motor symptoms, thus we sought to longitudinally characterise the progression and severity of motor signs in the HdhQ111 knock-in mouse model of HD, in heterozygous mice.
Methods: An extensive battery of motor tests including: rotarod, inverted lid test, balance beam, spontaneous locomotor activity and gait analysis were applied longitudinally to a cohort of HdhQ111 heterozygous mice in order to progressively assess motor function.
Results: A progressive failure to gain body weight was demonstrated from 11 months of age and motor problems in all measures of balance beam performance were shown in HdhQ111 heterozygous animals in comparison to wild type control animals from 9 months of age. A decreased latency to fall from the rotarod was demonstrated in HdhQ111 heterozygous animals in comparison to wild type animals, although this was not progressive with time. No genotype specific differences were demonstrated in any of the other motor tests included in the test battery.
Conclusions: The HdhQ111 heterozygous mouse demonstrates a subtle and progressive motor phenotype that begins at 9 months of age. This mouse model represents an early disease stage and would be ideal for testing therapeutic strategies that require elongated lead-in times, such as viral gene therapies or striatal transplantation.
INTRODUCTION
The presentation of overt motor symptoms, has been shown to occur in the disease progression of Huntington’s disease (HD) in both the human condition [1, 2] and in knock-in mouse models of HD [3–7]. Typically, a clinical diagnosis of manifest HD is based on the total motor score (TMS) of the Unified Huntington’s Disease Rating Scale (UHDRS). Therefore, in order to determine whether the HdhQ111 mouse model accurately reflects the human condition of HD in terms of face, construct and predictable validity, it is important to determine if and when motor signs develop and the severity of these signs. In the present study, heterozygous animals (with one mutant allele, as in most HD patients) were studied in an attempt to appropriately model the human conditionof HD.
A behavioural test battery of motor function was applied longitudinally to determine the time course of motor sign development and progression in the HdhQ111 mouse model of HD. The accelerating rotarod was used to assess motor co-ordination, as it has previously been used extensively to classify motor dysfunction in a range of HD mouse lines [3–12]. A balance beam apparatus was used to determine abnormalities in co-ordinated movement and balance, which have been demonstrated in HD patients [13] and mouse models [5, 11, 14]. In addition, a further test of motor function using an inverted grid was used, as this has been used previously to determine motor function in HD mice [5, 11, 15]. Spontaneous locomotor activity testing was included in the motor test battery to investigate changes in both general locomotor activity levels and disruption to circadian rhythms which manifest as sleep disturbances in HD patients [16–18] and which have also been described in HD mouse lines [2, 15, 19–21]. Gait abnormalities have also been shown in HD patients [22–27] and HD mouse lines [28–30], including the HdhQ111 mouse model [15, 29, 30], thus gait analysis was also included in the longitudinal motor test battery.
In addition to overt motor symptoms, weight loss is common in HD patients [31–33], even in the early stages of the disease [34]. Decreases in body weight as HD progresses have been described in a range of both transgenic and knock-in mouse models [5, 11, 19], including the HdhQ111 mouse line [29, 35]. However, full length human HTT transgenic mice, including BACHD and YAC transgenic mouse lines demonstrate an increase in body weight during HD disease progression [36, 37]. Therefore, it was necessary to determine the body weight progression of the HdhQ111 heterozygous animals to compare and contrast any weight changes seen, to further determine how accurately the HdhQ111 mouse model reflects the human condition. Furthermore, body weight was used to determine the health and wellbeing of animals, 20% weight loss was used to define a humane endpoint in experimental procedures.
Behavioural analyses in heterozygous mice are vital to understand the phenotype observed and the relevance to the human condition of HD. Several behavioural analyses of knock-in mouse models have demonstrated subtle behavioural phenotypes [12, 29, 38] which often lack the severe symptoms demonstrated in late stage human patients. A broad and extensive phenotypic screen has been previously described for HdhQ111 heterozygous mice [29], although this study only characterised animals until 46 weeks of age. Thus, in the present study we sought to extend, develop and verify previous behavioural findings observed in HdhQ111 heterozygous mice by completing a longitudinal motor characterisation over 18 months.
A vast number of mouse models of HD are now available for scientific research means, but before their use, each mouse model must be characterised to determine their suitability in accurately recapitulating the behavioural symptoms of HD. We therefore sought to longitudinally characterise the progression of motor deficits in the HdhQ111 heterozygous knock-in mouse model of HD.
MATERIALS AND METHODS
Animals
HdhQ111 knock-in mice (Jax®, Bar Harbour, Maine, U.S.A.) were bred inhouse on a C57BL/6J background. A total of 56 age matched mice were used (36 HdhQ111/+ animals, with a CAG repeat length range 131–143 and an average of 138 CAG repeats). Of these HdhQ111/+ mice 16 were male and 20 were female. Of the 20 wild type mice 10 were male. Animals were housed in mixed genotype pairs or threes, although some animals had to be separated and singly housed to prevent fighting. Each cage contained modest environmental enrichment of a single cardboard tube and a wooden chew stick. Testing occurred during the light phase from 12.00 hours to 18.00 hours. A female HdhQ111/+ animal was culled at 12 months of age, and 2 male animals (one wild type and one HdhQ111/+) were culled at 16 months of age due to health issues unrelated to HD. Experiments were conducted in accordance with the United Kingdom Animals Scientific Procedures Act (ASPA) 1986. From 1st January 2013, the European Union (E.U.) Directive 2010/63/EU was implemented into UK law by an update of ASPA 1986.
Genotyping
Upon weaning, all HdhQ111 animals were tail tipped for genotyping purposes. Ethyl chloride anaesthetic spray (Vidant Pharma Ltd, Surrey, U.K.) was applied to the tip of the tail before removing a 1 mm section. The tail was then cauterised with a silver nitrate pen and samples were collected in Eppendorf tubes. All samples were then shipped on dry ice to Laragen Inc. (Culver City, California, U.S.A). All genotyping was performed by Laragen Inc. using probe based qPCR to generate the genotype of the animal and corresponding end-point PCR to determine CAG repeat length.
Behavioural methods
Animals were tested at three month intervals in a behavioural test battery that included: locomotor activity, rotarod, inverted grid and balance beam. In addition, at six month intervals a test of gait analysis was added into the test battery, as shown in Fig. 1.
Weight progression
Animals were weighed monthly between 15.00 hours and 16.00 hours. Body weight was also used to determine the health and wellbeing of animals, 20% weight loss was used to define a humane endpoint in experimental procedures.
Rotarod
Animals received 5 days of training on a rotarod apparatus (Ugo Basile, Model Number 47600, Varese, Italy). Day 1 consisted of training with the rod accelerating from 5 revolutions per minute (rpm) to 24 rpm. Training days 2 to 5 consisted of training with the rod accelerating from 5 rpm to 44 rpm. During the training period animals were put back onto the accelerating rod if they fell from the apparatus. Training sessions lasted a maximum of 300 seconds (5 minutes) each and were completed on consecutive days. Animals were then tested on the apparatus which consisted of 2 trials (accelerating from 5 rpm to 44 rpm in a single session). The latency to fall from the rod was recorded for each trial following the activation of a button situated below the rotarod. The values from both trials were averaged to provide the rotarod latency statistic. If the mouse remained on the accelerating beam for whole 5 minute testing period, they were removed at the end of the testing session and given a score of 300 seconds.
Inverted grid test
Animals were placed on a mesh grid (30 cm×30 cm) which had a wooden border. For testing, towels were placed under the apparatus to provide a comfortable landing surface. The grid was then inverted 30 cm above the bench by placing 4 wooden posts under the border of the grid, such that the mouse was suspended upside down on the under surface. Latency to fall from the grid was recorded; the maximum time allowed per trial was 60 seconds.
Balance beam
Animals were initially trained on the apparatus (1 m in length, 17% angle of ascent, with a 1.5 cm to 0.5 cm taper across the width) to encourage traversing of the beam to the ‘house’ box located at the high end. Training day 1 consisted of placing the mouse on the beam initially close to the ‘house’ box and then increasing the distances away from the ‘house box’ until the animal was at the start area of the beam. The mouse was then placed facing away from the ‘house’ box at the end of the beam (to allow turn time to be measured as a measure of motor coordination) and encouraged to turn around and fully traverse the beam. The second day of training consisted of 2 trials where the animal was placed facing away from the ‘house’ box and needed to turn around and traverse the beam to enter the ‘house’ box at the top. Testing was conducted by 2 consecutive trials which were videotaped to enable analysis. The animal was placed at the far end of the balance beam facing away from the ‘house’ box; the time taken to turn around and the time taken to traverse the beam were recorded. Between each trial mice were given 1 hour to recover before the next trial. While traversing the beam foot slips for the front and hind legs on each side were recorded, one side was live scored and the other video recorded to allow bilateralanalysis.
Spontaneous automated locomotor activity
Animals were placed in a clear Perspex cage (dimensions 40 cm×24 cm×18 cm) on a metal rack through which 3 infrared beams were able to pass, crossing the base of each cage. Locomotor activity was recorded, via non-perseverative infrared beam breaks (beam breaks in duration of less than 3 seconds), for a total of 32 hours on MED-PC® (version 4) software (Vermont, USA) to determine changes circadian cycle mediated activity levels. Animals were allowed ad-libitum access to both food and water which were placed at opposite ends of the clear Perspex cage. Lamps were placed into the testing room and set on a timer to ensure that animals were maintained on their standard 12 hour light/dark cycle, lights on at 06.00 hours and lights off at 18.00 hours.
Gait analysis
Measurement of gait analysis was conducted using a clear Perspex corridor apparatus (65 cm×5 cm×15 cm), as previously described [19] and shown in Fig. 2A, which was lined with a pre-cut piece of white paper. Animals were trained to run to the enclosed darkened box at the end of the corridor by placing the mouse at the far end of the corridor and encouraging them to move towards the goal box at the end. Training was conducted twice for each mouse until the animal readily ran to the end box without encouragement. For testing, the paws of the animal were painted with non-toxic paint (TTS paint, Nottinghamshire, UK); red was used for the front paws and blue for the hind paws. The animal was then placed at the near end of the apparatus and ran to the enclosed goal box at the far end of the apparatus, leaving a print of the associated foot prints on the paper at the base of the apparatus (Fig. 2B). The mouse was then placed into a water bath to wash and groom before being returned to the home cage. The paper print was then allowed to air dry to enable analysis of; stride length, width and overlap for both the front and hind paws of each animal. For each animal, this was calculated using 4 paw prints, this allowed 3 values to be calculated for each measurement (stride length, stride width and overlap) which were then averaged to provide gait measurements (Fig. 2C).
Statistical analysis
Statistical analyses were conducted in IBM SPSS 20 Statistics Software. Typically, three way split plot analyses with ANOVA were used with repeated measures of age and between subject measures of genotype and sex, followed by simple effects analysis. Where significance was found post-hoc tests with Bonferroni corrections were performed. In the cases where missing values were present, due to animals needing to be culled for health reasons, missing data were estimated by an unbiased iterative interpolation procedure within the IBM SPSS statisticalanalysis.
RESULTS
Weight progression results
Male animals were significantly heavier than their female counterparts throughout the 18 months of testing (Fig. 3: Sex; F1,52 = 248.05, p < 0.001). Wild type and HdhQ111/+ animals demonstrated similar body weights at the earlier testing time points, however HdhQ111/+ animals subsequently failed to gain body weight in comparison to their wild type littermates, as demonstrated by the divergence of the wild type and HdhQ111/+ weight curves for both sexes similarly (Fig. 3: Age×Genotype; F16,832 = 6.98, p < 0.001). Post-hoc tests confirmed that the significant difference in body weight began at 11 months of age (p = 0.038) (as shown in Fig. 3).
Spontaneous automated locomotor activity results
Spontaneous locomotor activity was tested longitudinally in HdhQ111/+ animals (Fig. 4). No significant differences were observed in the total non-perseverative beam breaks between HdhQ111/+ and wild type animals, at any age tested. However, animals displayed decreased levels of locomotor activity as they aged (Fig. 4: Age; F5,255 = 24.42, p < 0.001). Furthermore, a significant effect of time of day was observed (Fig. 3: Time; F24,1224 = 116.65, p < 0.001), with animals generally becoming more active in the dark phase (18.00 hours to 06.00 hours) than in the light phase. However no significant differences were observed in locomotor activity between HdhQ111/+ and wild type animals (Fig. 4: Genotype; F1,52 = 0.002, p = n.s.).
Inverted grid test results
HdhQ111/+ animals displayed no impairments in the inverted grid test, in comparison to wild type animals at any of the time points tested (Fig. 5: Genotype; F1,52 = 1.39, p = n.s.). As animals aged their performance on the inverted grid test decreased (Fig. 5: Age; F5,260 = 3.83, p = 0.002). Interestingly, overall, male animals performed significantly worse on the inverted grid test than female animals (Sex; F1,52 = 6.68, p = 0.013).
Rotarod results
Rotarod performance considered over the 18 months of testing demonstrated that HdhQ111/+ animals displayed a decreased latency to fall from the rotarod in comparison to wild type animals (Fig. 6: Genotype; F1,52 = 5.83, p < 0.05). A highly significant effect of age was seen, with animals displaying a decreased latency to fall from the rotarod as they aged (Fig. 6: Age; F5,260 = 15.98, p < 0.001). There was a trend for HdhQ111/+ animals to have a decreased latency to fall from the rotarod as they aged, at 12 months, 15 months and 18 months of age in comparison to wild type animals, but this effect failed to meet significance (Fig. 6: Age×Genotype; F5,260 = 1.35, p = n.s.). Interestingly male animals performed significantly worse than female animals throughout rotarod testing (Sex; F1,52 = 10.46, p < 0.01).
Balance beam results
HdhQ111/+ animals were significantly impaired in all measures of balance beam performance in comparison to wild type animals. Latency to turn on the balance beam was progressively slower as animals aged (Fig. 7A: Age; F5,260 = 13.82, p < 0.001). Although HdhQ111/+ animals did not differ from wild type animals at younger ages, they were progressively slower to turn than wild type controls from 9 months of age (Fig. 7A: Age×Genotype; F5,260 = 3.376, p < 0.01). Interestingly, female animals were shown to turn on the beam significantly faster than male animals (Sex; F1,52 = 5.40, p < 0.05). Male animals were slower to traverse the balance beam than female animals (Sex; F1,52 = 13.78, p < 0.001). Although HdhQ111/+ animals did not differ in traverse time from wild type animals at the earliest ages, they were progressively slower to traverse the balance beam than wild type animals (Fig. 7B: Age×Genotype; F1,52 = 15.29, p < 0.001). Measurement of foot slips made while traversing the balance beam demonstrated that the number of foot slips made by HdhQ111/+ animals did not differ from wild type animals at younger ages. However, HdhQ111/+ animals made progressively more foot slips than wild type animals from 12 months of age (Fig. 7C: Age×Genotype; F5,260 = 40.56, p < 0.001).
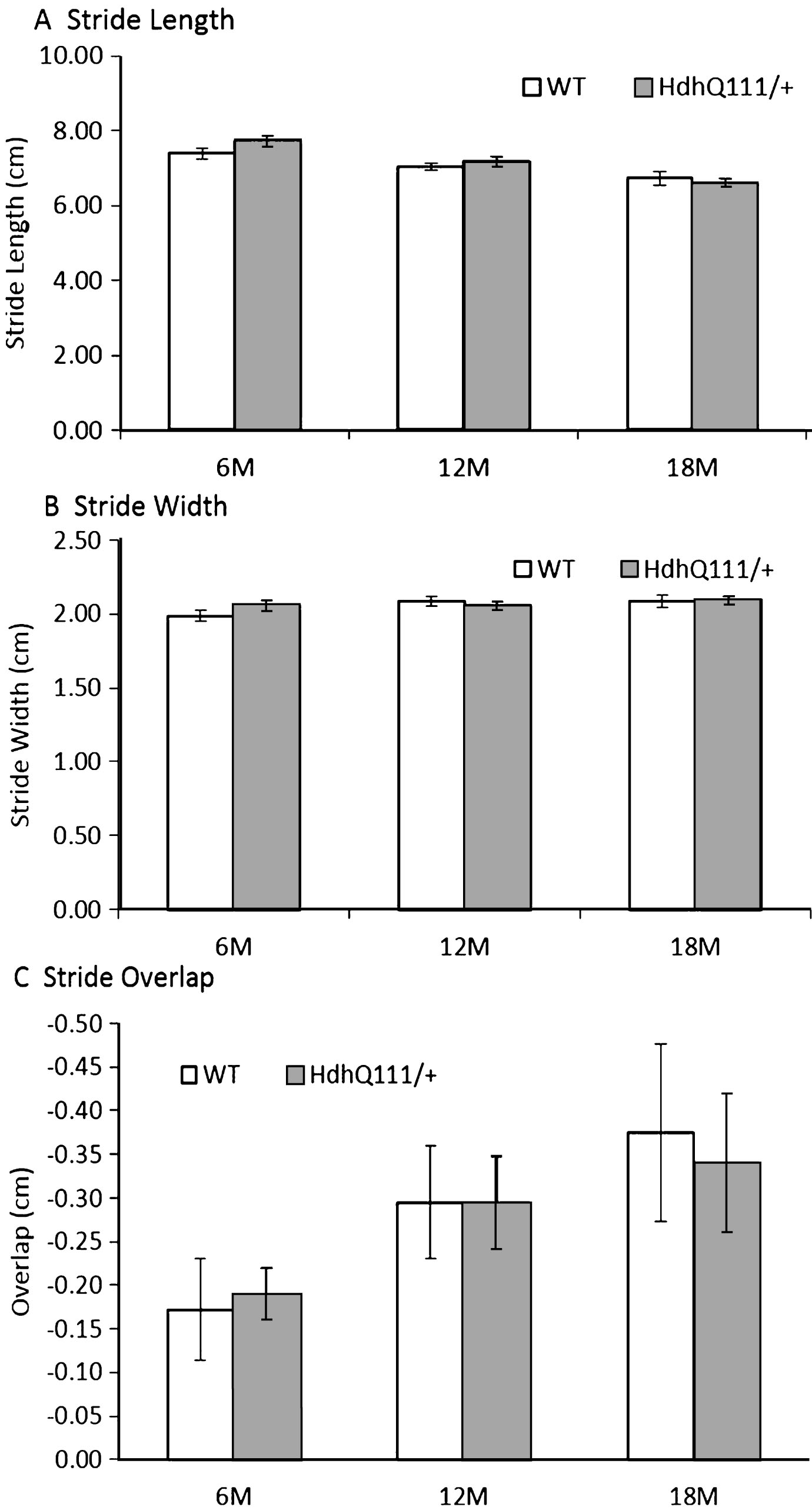
Gait analysis results
HdhQ111/+ animals did not show any impairments in gait in comparison to wild type animals in stride length (Fig. 8A: Genotype; F1,52 = 0.04, p = n.s.), stride width (Fig. 8B: Genotype; F1,52 = 0.02, p = n.s.) or stride overlap (Fig. 8C: Genotype; F1,52 = 0.02, p = n.s.). However, as animals aged, their stride length decreased (Fig. 8A: Age; F2,104 = 15.37, p < 0.001), as did their stride overlap (Fig. 8C: Age; F2,104 = 4.36, p < 0.05). However, no significant effect of age was seen in stride width (Fig. 8B: Age; F1,52 = 3.61, p = n.s.).
DISCUSSION
The results of the longitudinal motor characterisation of HdhQ111/+ animals demonstrated that significant motor deficits occurred in balance beam performance from 9 months of age in HdhQ111/+ heterozygous animals. Significant deficits in rotarod performance were observed in HdhQ111/+ heterozygous animals in comparison to wild type animals, although these were not progressive over time. HdhQ111/+ heterozygous animals had a significant inability to gain weight in comparison to wild type animals from 11 months of age.
The observed weight differences replicate previous findings in both transgenic and knock-in mouse models of HD [5, 11, 19], although the progression and extent of the failure to gain weight or weight loss observed, varies with each particular mouse model. The progressive failure to gain weight demonstrated in HdhQ111/+ animals from 11 months of age may be reflective of the weight abnormalities shown in the human condition [31, 32, 34, 39]. However, the human condition often represents a weight loss rather than a failure to gain weight. Although the underlying reasons for the inability to gain weight, observed in this case, are still unknown. The lack of observable chorea in HdhQ111/+ mice, despite an inability to gain weight, may lead to the conclusion that an underlying metabolic problem in the cause. This suggestion has been explored in HD patients [40–42] and in other mouse strains [43–46], although, it is yet to be explored in the HdhQ111 mouse model of HD.
The results obtained on several of the motor tests can be sensitive to the confounding effects of body weight. The significant difference between the weight of male and female animals may explain some of the sex differences observed in the motor tests, as some of the motor tests such as the rotarod and inverted grid test are sensitive to the effects of gravity. Furthermore, significant sex differences and interactions between sex and genotype were inconsistently produced throughout the longitudinal motor characterisation. The effect, if any, which the weight changes had on the motor read outs for the genotypes presented here, is uncertain. Although, if weight were to influence the motor results obtained for genotype, it may be that heavier animals, in this case the wild type animals, are likely to be disadvantaged in tasks such as the rotarod in comparison to their HdhQ111/+ littermates, which is not demonstrated in the results. It could also be argued that a decreased body weight may be advantageous in other motor tests such as the balance beam, although this is not the case in the results observed.
Significant motor deficits were seen in HdhQ111/+ animals in turn time and traverse time on the balance beam apparatus from 9 months of age, thus suggesting an inability to initiate movement and subsequently move along the beam. Furthermore, the increased number of foot slips that HdhQ111/+ animals made while crossing the balance beam compared to wild type animals at 12, 15 and 18 months of age, are indicative of progressive impairments in motor co-ordination and balance. Sex differences were demonstrated, with female animals generally displaying a more rapid time to turn and traverse the beam, although these differences between sexes were not consistently produced over time and their level of significance was not as high as that of the genotype differences. Problems with balance are particularly important to consider in people who are affected by HD, as this can lead to an increased risk of falls [47], social isolation and thus a reduced quality of life [48–50].
Further motor deficits were demonstrated in HdhQ111/+ animals in rotarod performance, as HdhQ111/+ animals displayed a decreased latency to fall from the rotarod in comparison to wild type animals, although this difference was not progressive over time. These deficits in rotarod performance are indicative of problems in co-ordinated movement in HdhQ111/+ animals. Significant gender differences were observed in rotarod performance, with female animals performing significantly better on the rotarod than male animals. These gender differences may be attributable to differences in weight between male and female animals. This suggestion has previously been described in other outbred mouse strains [51]. The results observed in rotarod and balance beam performance reflect previous findings of impaired motor learning and co-ordination in HdhQ111/+ mice at 46 weeks of age [29].
Although HdhQ111/+ animals displayed significant deficits in balance beam traverse time from 9 months of age, no significant deficits were seen in spontaneous locomotor activity in HdhQ111/+ animals in comparison to wild type animals. These results are in contrast to a previous study which found hypo-activity in the dark phase in HdhQ111/+ animals in comparison to wild type animals. Our results may be explained by the suggestion that when HdhQ111/+ animals are free to move in the locomotor activity cages no significant differences in activity are observed. Whereas, when HdhQ111/+ animals are challenged to traverse a beam they find the task significantly harder than wild type animals. Furthermore, due to the study design, habituation effects of repeatedly testing the same animals in a battery of motor tests should also be considered as a possible explanation as to the differences between the present study and a previous study which found hypo-activity in HdhQ111/+ mice [29] The lack of significant deficits in spontaneous locomotor activity, suggest that the observed deficits in both rotarod and balance beam may specifically involve motor co-ordination and balance.
Locomotor activity was studied in the HdhQ111/+ mouse model of HD because significant deficits in circadian rhythm had been previously demonstrated in HD patients [16–18, 21]; however, such differences were not observed in this case in the HdhQ111/+ mouse model. Overall, animals displayed decreased activity as they aged, although this may be the result of the natural aging process it may also be attributable to habituation effects. As animals were tested in the motor test battery every 3 months, they were required to be tested a total of 6 times within the test battery. Therefore, it could be the case that animals became increasingly habituated to the locomotor activity boxes and thus moved less during subsequent testing. This is suggested in particular by the large decline in locomotor activity between the first and second tests, at 3 months of age and 6 months of age respectively, before other motor deficits were apparent. Furthermore, this suggestion may also be valid for the other motor tasks, as the same animals were used repeatedly in the longitudinal testing paradigm, this meant that animals received repeated testing on the motor tests at a young age; therefore this exposure and testing at a young age may well have affected future motor test read outs. To prevent these types of habituation or practice effects occurring in future longitudinal motor characterisations, a new group of animals could be tested at each time point, although this would have considerable ethical and financial implications and could no longer be considered true longitudinal testing.
The lack of genotype differences in the inverted grid test is perhaps unsurprising due to the relatively insensitive nature of the behavioural test, with the majority of animals performing close to the 60 second ceiling in the protocol. The inverted grid test is highly likely to be influenced by differences in body weight, with heavier animals falling from the grid more rapidly than lighter animals. However, if this were to be the case it is likely to disadvantage wild type animals rather than HdhQ111/+ animals, which is not the case in the observed results. Furthermore, the time that each trial was conducted for (60 seconds) is in accordance with the relevant licencing requirements to minimise pain, suffering, distress and lasting harm to animals; however this may not be long enough to draw out any genotypic differences. It is plausible that sensitivity to detect genotype and aging effects may be increased by extending the test duration to 2 or 3 minutes, but this is yet to be investigated. Therefore, the inverted grid test used in this case, may not be an appropriately sensitive test measure. Attempts to use a mechanical grip strength metre [52] have been used successfully in observing deficits in grip strength in other mouse models [53, 54] and in the R6/2 mouse model of HD [55]. Therefore, in future longitudinal motor characterisations it may provide more meaningful data if a mechanical grip strength metre were used in behavioural testing.
No significant gait abnormalities were observed in HdhQ111/+ animals in the present study, although the characterisation was only conducted until 18 months of age, therefore it may be the case that such abnormalities would have developed at a later time point. However, motor abnormalities were demonstrated at earlier ages and thus for practical, financial and welfare reasons it was pre-planned to end the motor characterisation at 18 months of age. Our results are in contrast to others who have found gait abnormalities in the HdhQ111/+ mice at 46 weeks [29], although in this case the methods used to determine gait performance were automated and thus comparatively more sophisticated than the methods utilised here. In another study in both HdhQ111/+ and HdhQ111/Q111 mice, gait abnormalities were found at 24 months of age [30], utilising a similar method to that employed here, although these animals were bred on a CD1 background strain, which is different to the background strain of the animals used in this study. Thus, the background strain and equipment used for the gait testing are both likely to impact upon the results observed.
In some tests (inverted grid, rotarod, balance beam) motor impairments in HdhQ111/+ animals in comparison to their wild type littermates, were more marked in male animals than in female animals. The gender differences between male and female animals do not appear to be stable within the behavioural results of the motor tests and often there was no interaction between sex and genotype. Therefore, overall genotypic differences were of primary concern in determining whether the HdhQ111/+ mice accurately reflects the motor symptoms of HD. The sex differences could be attributable weight differences among the sexes, although it is plausible to use a single sex of animals in behavioural testing, this has significant practical, ethical and financial implications and as HD is a disease which affects both sexes equally, both sexes were used in behavioural testing to reflect this.
Sex differences have previously been observed in the HdhQ111 mouse line [29] and male animals have been shown to demonstrate increased anxiety [35]. Anxiety measurements were not conducted in this particular study, and it is noteworthy that the literature regarding anxiety problems in the HdhQ111 mouse model has shown conflicting results. Decreased anxiety has been demonstrated in HdhQ111/+ mice on a C57BL/6J background strain at 11 weeks of age [29]. Whereas, others have shown increased anxiety in male HdhQ111/+ mice on a CD1 background strain [35]. Therefore, further studies are required to determine if the HdhQ111 mouse model of HD reflects any of the symptoms of anxiety that are shown in the human condition.
In summary, the longitudinal motor characterisation presented here, demonstrated a gradual decrease in the ability of HdhQ111/+ animals to gain weight in comparison to their wild type littermates. The emergence of motor deficits in rotarod performance in HdhQ111/+ animals from 12 months of age and in balance beam performance from 9 months of age demonstrates subtle and progressive motor deficits which resemble early stage HD, as the deficits are specific in nature and lack the gross dyskinesia observed in late stage HD patients. These findings are crucial in determining how accurately the HdhQ111 mouse model of HD reflects motor signs observed in the human condition.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
The authors would like to thank the Medical Research Council for funding this work via a PhD studentship for Dr. Emma Yhnell. The authors would also like to thank Mr. Yateen S. Patel and Mr. David J. Harrison for his technical assistance.
REFERENCES
[1] | Kremer H , Group HS . Unified Huntington’s disease rating scale: Reliability and consistency. Mov Disord. (1996) ;11: (2):136–42. |
[2] | Hefter H , Homberg V , Lange HW , Freud H-J . Impairment of rapid movement in Huntington’s disease. Brain (1987) ;110: (3):585–612. |
[3] | Trueman RC , Brooks SP , Jones L , Dunnett SB . Rule learning, visuospatial function and motor performance in the Hdh Q92 knock-in mouse model of Huntington’s disease. Behav Brain Res (2009) ;203: (2):215–222. |
[4] | Hickey MA , Kosmalska A , Enayati J , Cohen R , Zeitlin S , Levine MS , et al., Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington’s disease mice. Neuroscience (2008) ;157: (1):280–295. |
[5] | Brooks S , Higgs G , Jones L , Dunnett SB . Longitudinal analysis of the behavioural phenotype in Hdh(CAG) 150 Huntington’s disease knock-in mice. Brain Res Bull (2012) ;88: (2):182–188. |
[6] | Smith GA , Rocha EM , McLean JR , Hayes MA , Izen SC , Isacson O , et al., Progressive axonal transport and synaptic protein changes correlate with behavioral and neuropathological abnormalities in the heterozygous Q175 KI mouse model of Huntington’s disease. Hum Mol Genet (2014) ;23: (17):4510–4527. |
[7] | Heng MY , Duong DK , Albin RL , Tallaksen-Greene SJ , Hunter JM , Lesort MJ , et al., Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet (2010) ;19: (19):3702–3720. |
[8] | Laforet GA , Sapp E , Chase K , McIntyre C , Boyce FM , Campbell M , et al.,Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington’s disease. J Neurosci (2001) ;21: (23):9112–9123. |
[9] | Southwell AL , Warby SC , Carroll JB , Doty CN , Skotte NH , Zhang W , et al., A fully humanized transgenic mouse model of Huntington disease. Hum Mol Genet (2013) ;22: (1):18–34. |
[10] | Cheng P-H , Li C-L , Her L-S , Chang Y-F , Chan AW , Chen C-M , et al., Significantly differential diffusion of neuropathological aggregates in the brain of transgenic mice carrying N-terminal mutant huntingtin fused with green fluorescent protein. Brain Struct Funct (2013) ;218: (1):283–294. |
[11] | Brooks SP , Janghra N , Workman VL , Bayram-Weston Z , Jones L , Dunnett SB . Longitudinal analysis of the behavioural phenotype in R6/1 (C57BL/6J) Huntington’s disease transgenic mice. Brain Res Bull (2012) ;88: (2):94–103. |
[12] | Rising AC , Xu J , Carlson A , Napoli VV , Denovan-Wright EM , Mandel RJ . Longitudinal behavioral, cross-sectional transcriptional and histopathological characterization of a knock-in mouse model of Huntington’s disease with 140 CAG repeats. Exp Neurol (2011) ;228: (2):173–182. |
[13] | Johnson KA , Bennett JE , Georgiou N , Bradshaw JL , Chiu E , Cunnington R , et al., Bimanual co-ordination in Huntington’s disease. Exp Brain Res (2000) ;134: (4):483–489. |
[14] | Heng MY , Tallaksen-Greene SJ , Detloff PJ , Albin RL . Longitudinal evaluation of the Hdh (CAG) 150 knock-in murine model of Huntington’s disease. J Neurosci (2007) ;27: (34):8989–8998. |
[15] | Menalled L , El-Khodor BF , Patry M , Suárez-Fariñas M , Orenstein SJ , Zahasky B , et al., Systematic behavioral evaluation of Huntington’s disease transgenic and knock-in mouse models. Neurobiol Dis (2009) ;35: (3):319–336. |
[16] | Wiegand M , Möller A , Lauer C , Stolz S , Schreiber W , Dose M , et al., Nocturnal sleep in Huntington’s disease. J Neurol (1991) ;238: (4):203–8. |
[17] | Videnovic A , Leurgans S , Fan W , Jaglin J , Shannon KM . Daytime somnolence and nocturnal sleep disturbances in Huntington disease. Parkinsonism Relat Disord (2009) ;15: (6):471–474. |
[18] | Aziz NA , Anguelova GV , Marinus J , Lammers GJ , Roos RA . Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington’s disease. Parkinsonism Relat Disord (2010) ;16: (5):345–350. |
[19] | Carter RJ , Lione LA , Humby T , Mangiarini L , Mahal A , Bates GP , et al., Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci (1999) ;19: (8):3248–3257. |
[20] | Morton AJ , Wood NI , Hastings MH , Hurelbrink C , Barker RA , Maywood ES . Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci (2005) ;25: (1):157–163. |
[21] | Wulff K , Gatti S , Wettstein JG , Foster RG . Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci (2010) ;11: (8):589–599. |
[22] | Delval A , Krystkowiak P , Blatt JL , Labyt E , Dujardin K , Destée A , et al., Role of hypokinesia and bradykinesia in gait disturbances in Huntington’s disease. J Neurol (2006) ;253: (1):73–80. |
[23] | Grimbergen YA , Knol MJ , Bloem BR , Kremer BP , Roos RA , Munneke M . Falls and gait disturbances in Huntington’s disease. Mov Disord (2008) ;23: (7):970–976. |
[24] | Hausdorff JM , Cudkowicz ME , Firtion R , Wei JY , Goldberger AL . Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in parkinson’s disease and Huntington’s disease. Mov Disord (1998) ;13: (3):428–437. |
[25] | Koller WC , Trimble J . The gait abnormality of Huntington’s disease. Neurology (1985) ;35: (10):1450. |
[26] | Rao AK , Muratori L , Louis ED , Moskowitz CB , Marder KS . Spectrum of gait impairments in presymptomatic and symptomatic Huntington’s disease. Mov Disord (2008) ;23: (8):1100–7. |
[27] | Reynolds NC Jr , Myklebust JB , Prieto TE , Myklebust BM . Analysis of gait abnormalities in Huntington disease. Arch Phys Med Rehabil (1999) ;80: (1):59–65. |
[28] | Lin C-H , Tallaksen-Greene S , Chien W-M , Cearley JA , Jackson WS , Crouse AB , et al., Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Hum Mol Genet (2001) ;10: (2):137–144. |
[29] | Hölter SM , Stromberg M , Kovalenko M , Garrett L , Glasl L , Lopez E , et al., A broad phenotypic screen identifies novel phenotypes driven by a single mutant allele in Huntington’s disease CAG knock-in mice. PloS One (2013) ;8: (11):e80923. |
[30] | Wheeler VC , Gutekunst C-A , Vrbanac V , Lebel L-A , Schilling G , Hersch S , et al., Early phenotypes that presage late-onset neurodegenerative disease allow testing of modifiers in Hdh CAG knock-in mice. Hum Mol Genet (2002) ;11: (6):633–640. |
[31] | Kremer H , Roos R . Weight loss in Huntington’s disease. Arch Neurol (1992) ;49: (4):349. |
[32] | Stoy N , McKay E . Weight loss in Huntington’s disease. Ann Neurol (2000) ;48: (1):130. |
[33] | Van Raamsdonk JM , Gibson WT , Pearson J , Murphy Z , Lu G , Leavitt BR , et al., Body weight is modulated by levels of full-length huntingtin. Hum Mol Genet (2006) ;15: (9):1513–1523. |
[34] | Djousse L , Knowlton B , Cupples L , Marder K , Shoulson I , Myers R . Weight loss in early stage of Huntington’s disease. Neurology (2002) ;59: (9):1325–1330. |
[35] | Orvoen S , Pla P , Gardier AM , Saudou F , David DJ . Huntington’s disease knock-in male mice show specific anxiety-like behaviour and altered neuronal maturation. Neurosci Lett (2012) ;507: (2):127–132. |
[36] | Gray M , Shirasaki DI , Cepeda C , Andre VM , Wilburn B , Lu X-H , et al., Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci (2008) ;28: (24):6182–6195. |
[37] | Slow EJ , Van Raamsdonk J , Rogers D , Coleman SH , Graham RK , Deng Y , et al., Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet (2003) ;12: (13):1555–1567. |
[38] | Menalled LB , Kudwa AE , Miller S , Fitzpatrick J , Watson-Johnson J , Keating N , et al., Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington’s disease: zQ175. PloS One (2012) ;7: (12):e49838. |
[39] | Aziz N , van der Burg J , Landwehrmeyer G , Brundin P , Stijnen T , Roos R . Weight loss in Huntington disease increases with higher CAG repeat number. Neurology (2008) ;71: (19):1506–1513. |
[40] | Browne SE , Bowling AC , Macgarvey U , Baik MJ , Berger SC , Muquit MM , et al., Oxidative damage and metabolic dysfunction in Huntington’s disease: Selective vulnerability of the basal ganglia. Ann Neurol (1997) ;41: (5):646–653. |
[41] | Koroshetz WJ , Jenkins BG , Rosen BR , Beal MF . Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann Neurol (1997) ;41: (2):160–165. |
[42] | Mazziotta JC , Phelps ME , Pahl JJ , Huang S-C , Baxter LR , Riege WH , et al., Reduced cerebral glucose metabolism in asymptomatic subjects at risk for Huntington’s disease. New Engl J Med (1987) ;316: (7):357–362. |
[43] | Weydt P , Pineda VV , Torrence AE , Libby RT , Satterfield TF , Lazarowski ER , et al., Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1α in Huntington’s disease neurodegeneration. Cell Metab (2006) ;4: (5):349–362. |
[44] | van der Burg JM , Bacos K , Wood NI , Lindqvist A , Wierup N , Woodman B , et al., Increased metabolism in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis (2008) ;29: (1):41–51. |
[45] | Duan W , Guo Z , Jiang H , Ware M , Li X-J , Mattson MP . Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A (2003) ;100: (5):2911–2916. |
[46] | Tsang TM , Woodman B , Mcloughlin GA , Griffin JL , Tabrizi SJ , Bates GP , et al., Metabolic characterization of the R6/2 transgenic mouse model of Huntington’s disease by high-resolution MAS 1H NMR spectroscopy. J Proteome Res (2006) ;5: (3):483–492. |
[47] | Busse M , Wiles CM , Rosser AE . Mobility and falls in people with Huntington’s disease. J Neurol Neurosurg Psychiatry (2009) ;80: (1):88–90. |
[48] | Helder D , Kaptein A , Van Kempen G , Van Houwelingen J , Roos R . Impact of Huntington’s disease on quality of life. Mov Disord (2001) ;16: (2):325–330. |
[49] | Ho AK , Gilbert AS , Mason SL , Goodman AO , Barker RA . Health-related quality of life in Huntington’s disease: Which factors matter most? Mov Disord (2009) ;24: (4):574–578. |
[50] | Ready RE , Mathews M , Leserman A , Paulsen JS . Patient and caregiver quality of life in Huntington’s disease. Mov Disord (2008) ;23: (5):721–726. |
[51] | McFadyen M , Kusek G , Bolivar V , Flaherty L . Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes, Brain Behav (2003) ;2: (4):214–219. |
[52] | Van Riezen H , Boersma L . A new method for quantitative grip strength evaluation. Eur J Pharmacol (1969) ;6: (3):353–356. |
[53] | Barnéoud P , Lolivier J , Sanger DJ , Scatton B , Moser P . Quantitative motor assessment in FALS mice: A longitudinal study. Neuroreport (1997) ;8: (13):2861–2865. |
[54] | Glynn D , Drew CJ , Reim K , Brose N , Morton AJ . Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Hum Mol Genet (2005) ;14: (16):2369–2385. |
[55] | Hickey M , Gallant K , Gross G , Levine M , Chesselet M-F . Early behavioral deficits in R6/2 mice suitable for use in preclinical drug testing. Neurobiol Dis (2005) ;20: (1):1–11. |
Figures and Tables
Fig.1
Longitudinal hand testing time scale. Animals were tested in the longitudinal motor test battery every 3 months. Rotarod, balance beam, inverted grid and locomotor activity testing occurred every 3 months, from 3 months of age until 18 months of age. Additional tests of gait analysis were completed at the 6 month, 12 month and 18 month time points.
Fig.2
Gait analysis apparatus and representative images of the results obtained. A. A photograph of the gait corridor apparatus used in gait analysis. B. Representative image of the results obtained from gait analysis. C. Schematic illustration of gait analysis measurements of stride length, stride width and stride overlap.
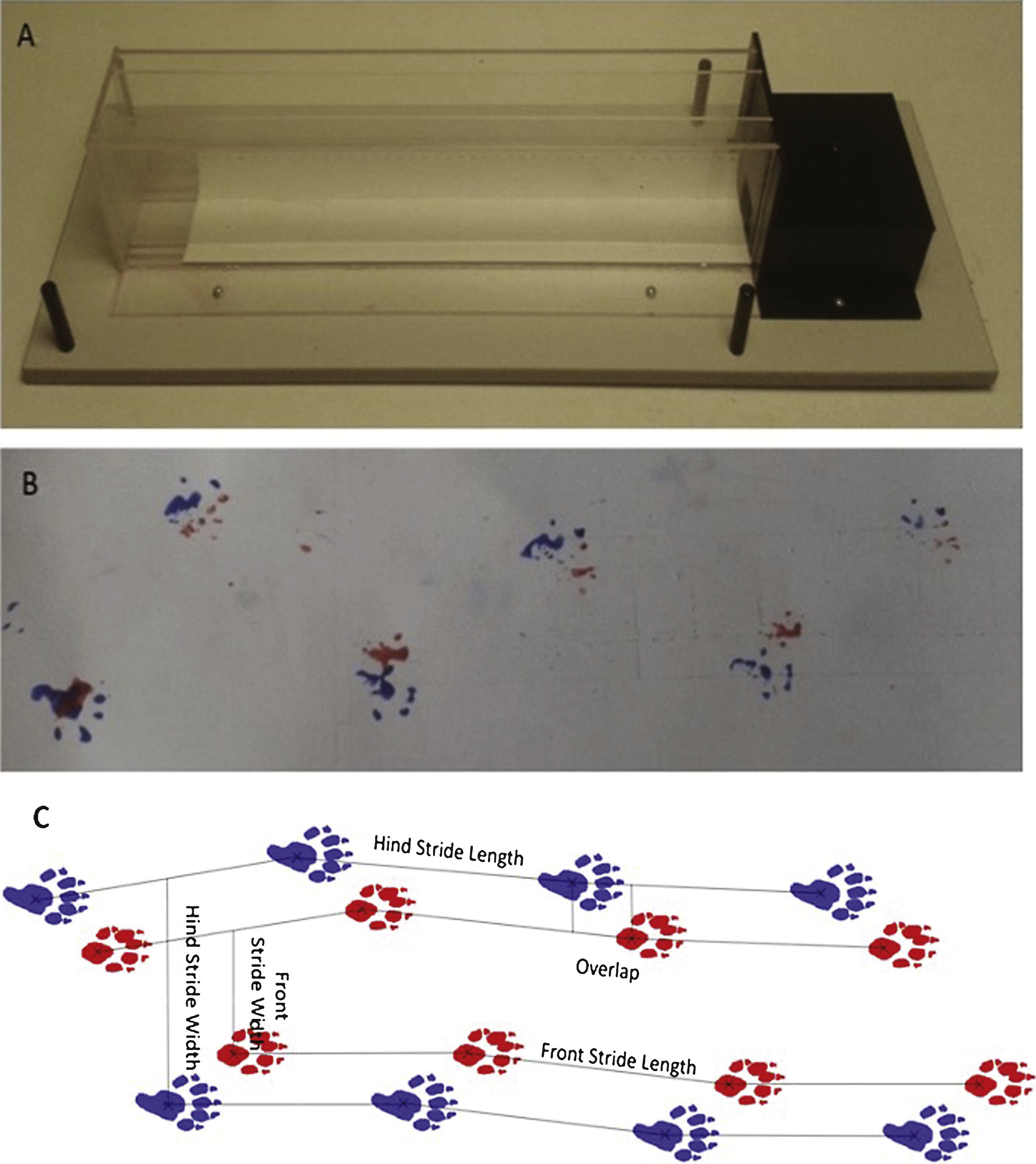
Fig.3
Weight progression of HdhQ111/+ animals over 18 months. Mice were weighed at 2 months of age and subsequently every month. The data represents the mean weight of HdhQ111/+ and wild type animals averaged to provide the data point. Data was analysed using a repeated measures ANOVA. The data represents a total of 36 HdhQ111/+ animals, of which 16 were male and 20 wild type animals, of which 10 were male. Error bars represent standard error of the mean. Significant differences are stated for males and females combined. Asterisks represent the significance level for a genotype comparison at a particular age after multiple testing correction *p < 0.05, **p < 0.01, ***p < 0.001.
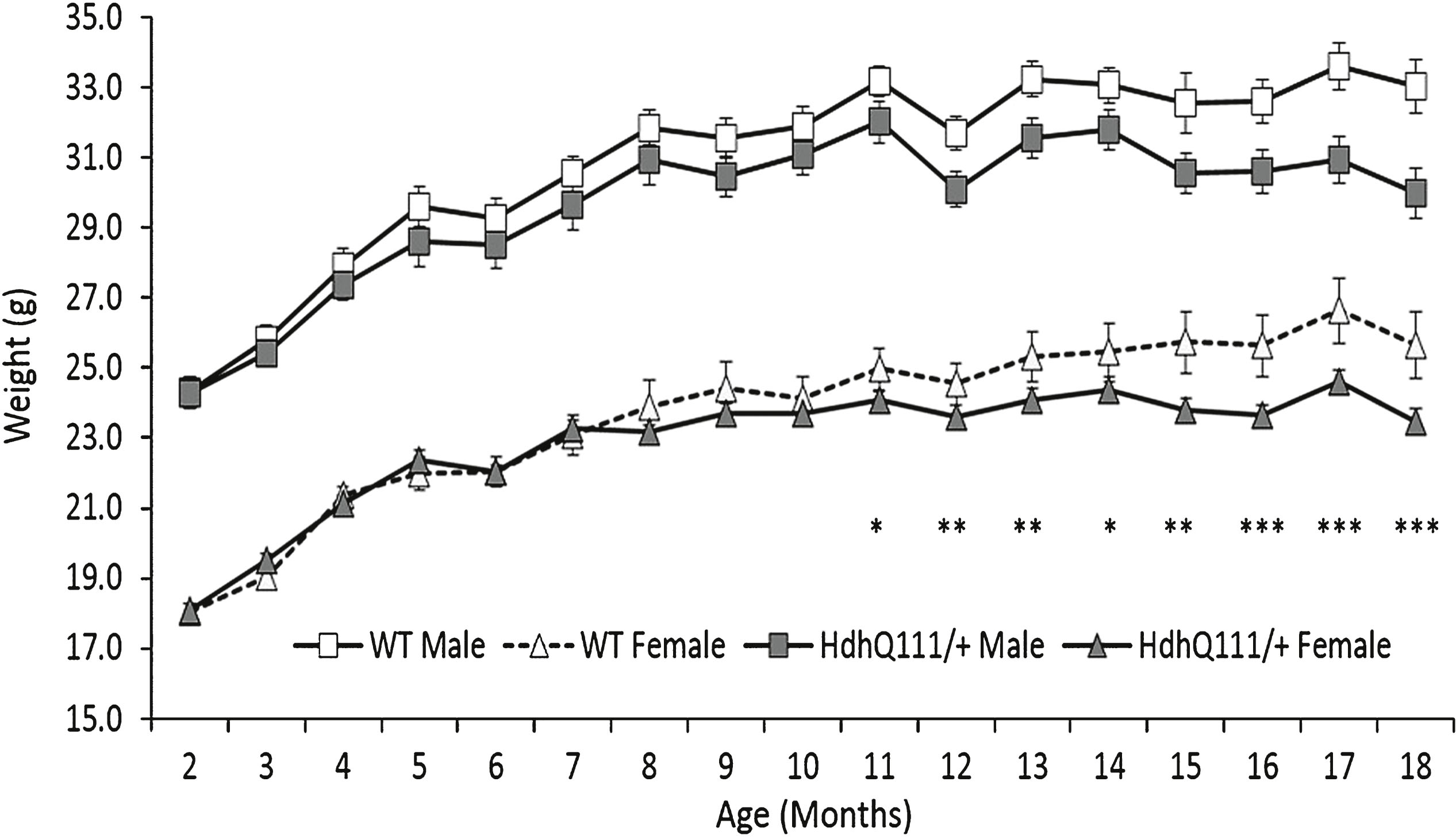
Fig.4
Spontaneous locomotor activity longitudinal results for HdhQ111/+ animals over 18 months. Results are shown over 24 hours (from 15.00 hours to 15.00 hours) at each time point. The data represents a total of 36 HdhQ111/+ animals, of which 16 were male and 20 wild type animals, of which 10 were male. A main effect of time was demonstrated at each age, with animals generally more active in the dark phase (18.00 hours to 06.00 hours). No significant differences were demonstrated between genotypes at any age. No significant sex differences were demonstrated thus data for males and females was combined. Error bars represent standard error of the mean.
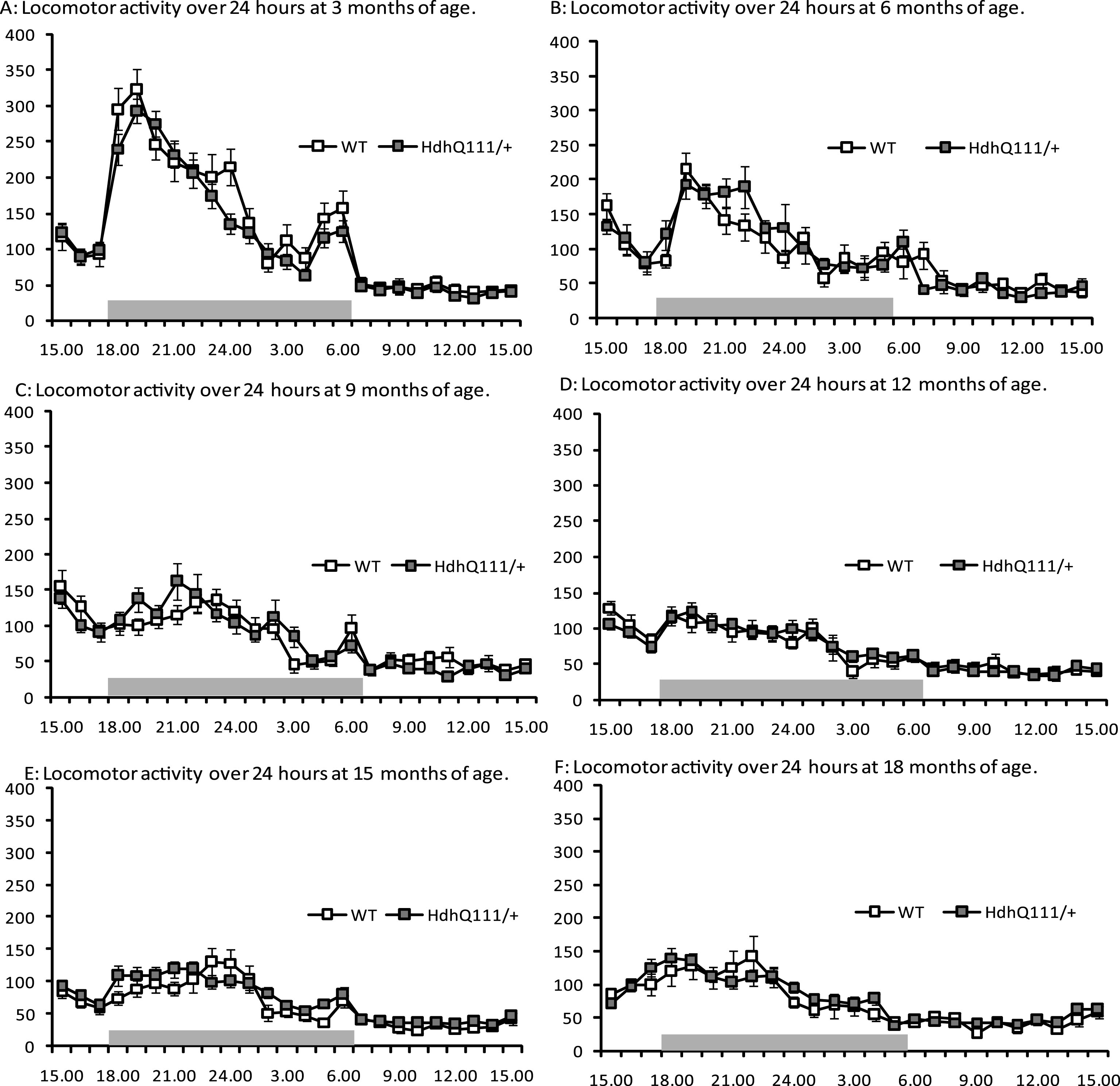
Fig.5
Inverted grid test results for HdhQ111/+ animals over 18 months. Mice were tested at 3 months of age and subsequently every month. The average latency to fall represents an average of 2 trials for HdhQ111/+ and wild type animals at each time point. The data represents a total of 36 HdhQ111/+ animals, of which 16 were male and 20 wild type animals, of which 10 were male. Error bars represent standard error of the mean.
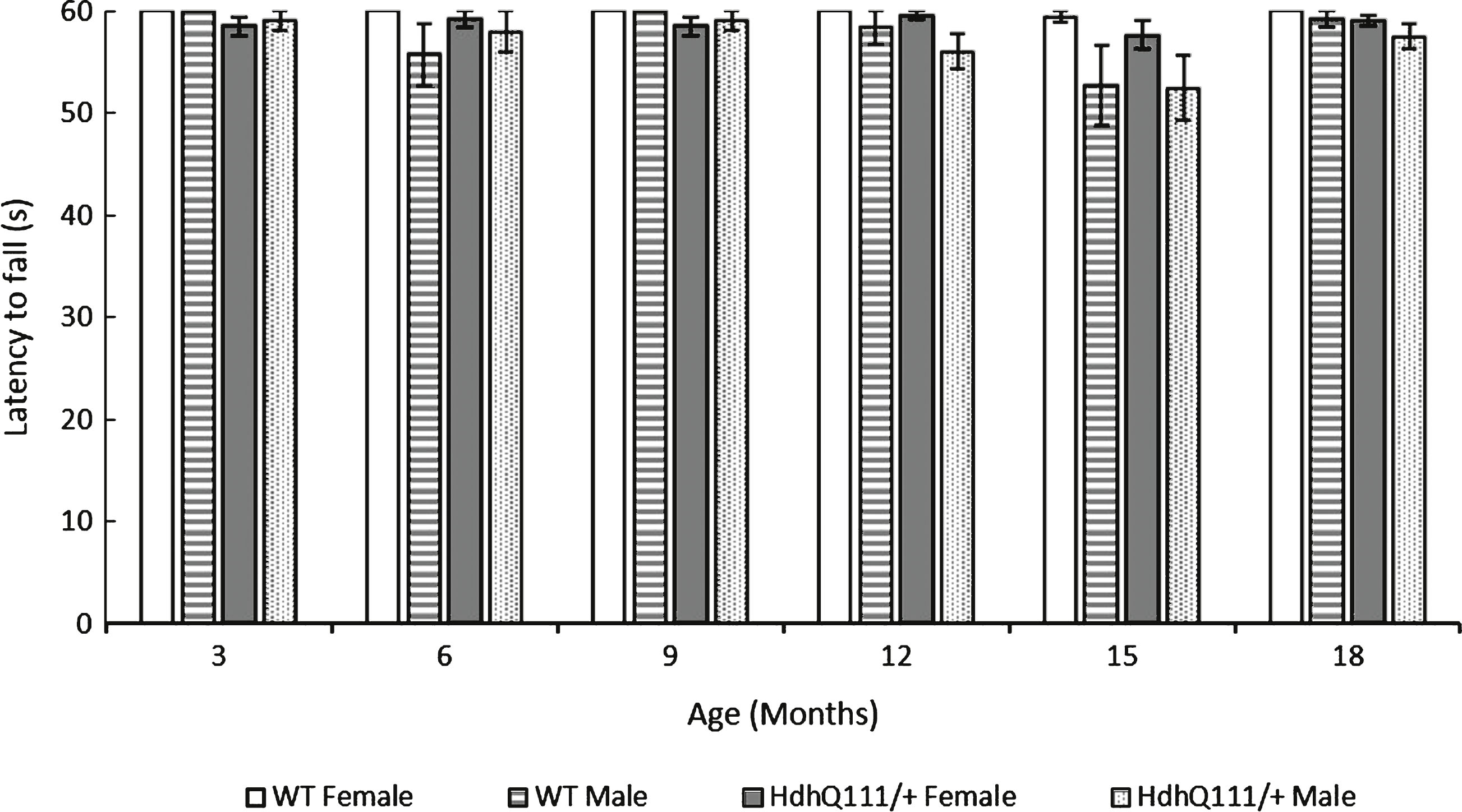
Fig.6
Rotarod performance in HdhQ111/+ animals over 18 months. The data represents a total of 36 HdhQ111/+ animals, of which 16 were male and 20 wild type animals, of which 10 were male. Data is shown as an average of 2 trials. Error bars represent standard error of the mean.
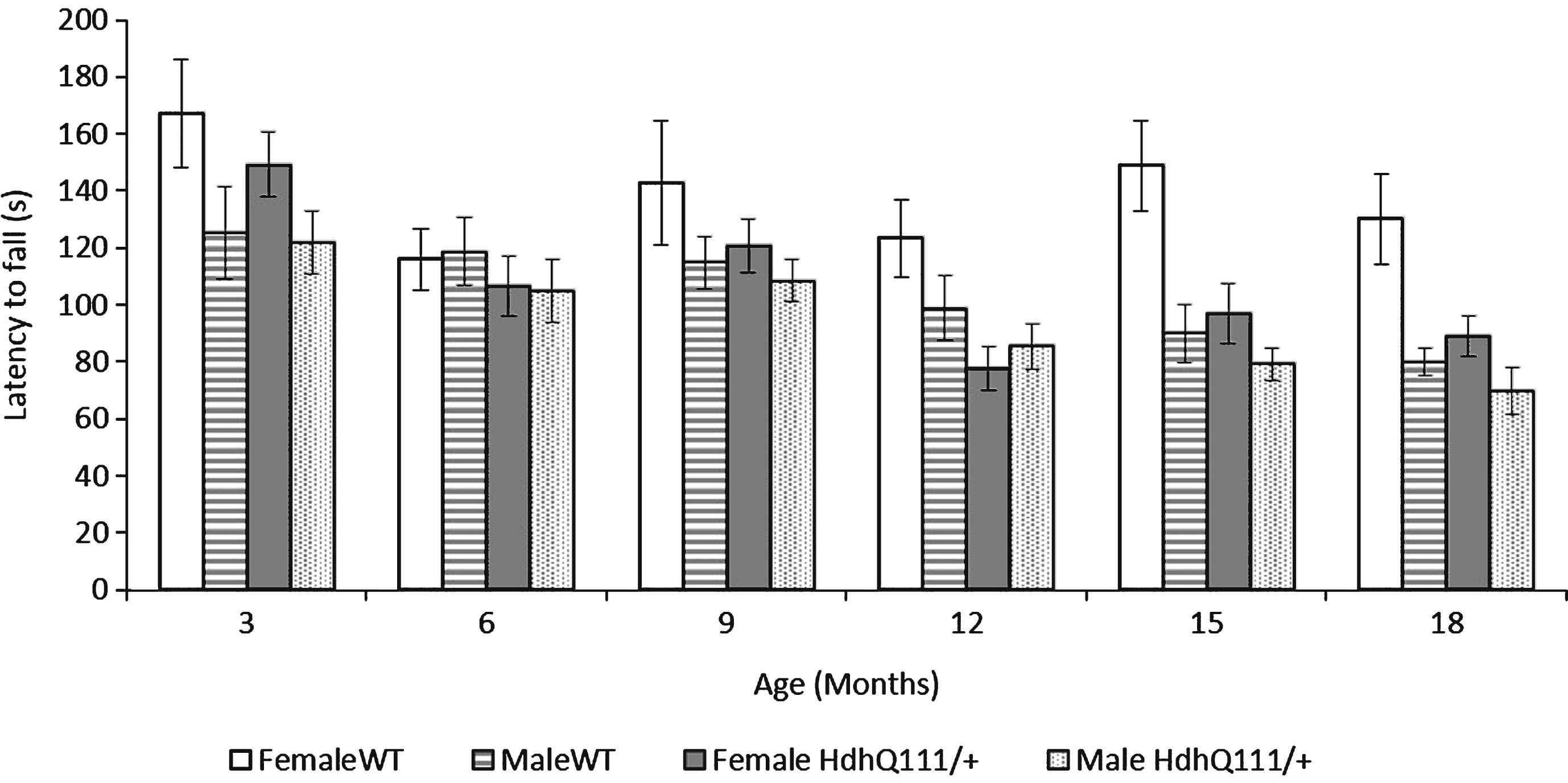
Fig.7
Balance beam performance in HdhQ111/+ animals over 18 months. A. Time to turn on the balance beam demonstrated HdhQ111/+ animals were significantly slower to turn on the beam than wild type animals, from 9 months of age. B. Traverse time on the balance beam showed HdhQ111/+ animals were significantly slower to traverse the beam than wild type animals from 9 months of age. C. Total number of foot slips made while crossing the beam demonstrated HdhQ111/+ animals made significantly more foot slips from 12 months of age. The data represents a total of 36 HdhQ111/+ animals, of which 16 were male and 20 wild type animals, of which 10 were male, sexes shown separately. Error bars represent standard error of the mean. Asterisks represent the significance level for a genotype comparison at a particular age after multiple testing corrections *p < 0.05, **p < 0.01, ***p < 0.001.
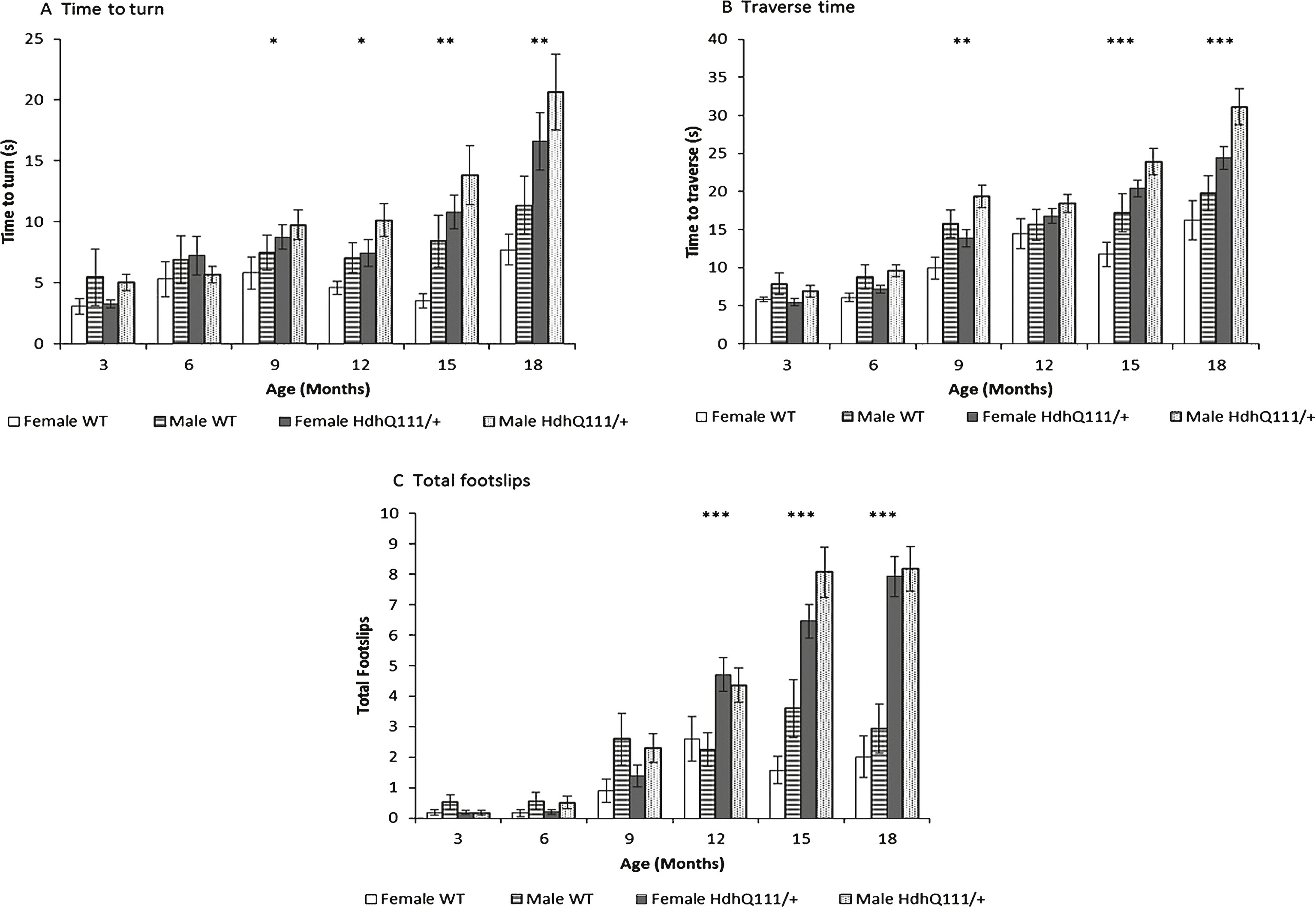
Fig.8
Gait analysis results for HdhQ111/+ animals over 18 months. No significant differences were observed between HdhQ111/+ and wild type animals in any measure of gait analysis. The data represents a total of 36 HdhQ111/+ animals, of which 16 were male and 20 wild type animals, of which 10 were male. Data was combined for males and females as no significant differences between the sexes were demonstrated. Error bars represent standard error of the mean.