LC-ESI-QTOF-MS profiling, protective effects on oxidative damage, and inhibitory activity of enzymes linked to type 2 diabetes and nitric oxide production of Vaccinium corymbosum L. (Ericaceae) extracts
Abstract
BACKGROUND:
Berries are worldwide recognized as “superfoods” due to the high content of bioactive compounds and the health benefits deriving from their consumption.
OBJECTIVE:
The present study was planned to assess and to compare the chemical profile and the in vitro antioxidant, hypoglycaemic, and anti-inflammatory activities of Vaccinium corymbosum L. berries and leaves extracts obtained by different extraction procedures. Ethanol was chosen as solvent because it is a GRAS (Generally Recognized As Safe) and widely used for the extraction of polar compounds.
METHODS:
Different extraction techniques such asmaceration, ultrasound-assisted extraction, Soxhlet extractor and decoction, have been applied by using food grade ethanol/water as solvent mixture, selected as environmentally friendly solvents. Extracts obtained from fruits and leaves were chemically investigated by liquid chromatography-electrospray ionization quadrupole-time of flight-mass spectrometry (LC-ESI-QTOF-MS) and for their potential in vitro antioxidant, hypoglycaemic, and anti-inflammatory effects.
RESULTS:
Some iridoids were detected for the first time in V. corymbosum. Dried leaves extracted by decoction and ethanol with Soxhlet apparatus showed the highest 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals scavenging activity with IC50 value of 0.77μg/mL, which is 2.2-time lower than that positive control ascorbic acid. A promising inhibition of the production of nitrate/nitrite, critical mediators of inflammation, was found. The decoction of berries showed the highest activity in counteracting nitric oxide (NO.) production. Furthermore, two leaves extracts (decoction of dried leaves and hydroalcoholic maceration of fresh leaves) were particularly active as α-amylase inhibitors with IC50 values of 16.16 and 20.55 μg/mL, respectively.
CONCLUSIONS:
This work could provide valuable basis for future research on V. corymbosum to improve recovery of specific active compounds such as flavonoids and iridoids
Abbreviations
ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
AU | aucubin |
BHT | Butylated hydroxytoluene |
CA | Chlorogenic acid |
DMSO | Dimethyl sulfoxide |
DPPH | 2,2-diphenyl-1-picrylhydrazyl |
FBS | Fetal Bovine Serum |
FRAP | Ferric Reducing Activity Power |
HFF1 | Human Foreskin Fibroblast |
IC50 | Concentration giving 50% inhibition |
IL-2β | Interleukin-2β |
LC-ESI-QTOF-MS | Liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry |
MTT | 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium |
OD550 | Optical density |
QE | Quercetin |
SD | Standard deviation |
1Introduction
Vaccinium L. (Ericaceae) is a genus of about 450 species of shrubs, widely found throughout the Northern Hemisphere and extending south along tropical mountain ranges, especially in Malesia [1]. The shrubs are erect or creeping, with alternate deciduous or evergreen leaves. The small flowers resemble those of the true heaths (Erica), are single, clustered, or in long spikes in the leaf axil. The berries are usually edible. Vaccinium corymbosum L. (highbush blueberry) is native from North America. Now it is locally cultivated in west and central Europe for its edible fruits [2]. It is a deciduous shrub with elliptical leaves, white cylindrical corolla and blue berries 7–10 mm [3].
In traditional medicine, the fruits of V. corymbosum have been mainly used to treat degenerative diseases such as diabetes, Alzheimer’s and Parkinson’s diseases [4, 5]. Fruits are noted for their composition in anthocyanins, flavonoids and phenolic acids. Anthocyanins, which increase in the ripening state to the detriment of other phenolic compounds as flavonoids, are the principal group of constituents. Phenolic acids, followed by flavonoids, represent the dominant classes of compounds in the leaves. Phytochemical studies have revealed the abundance in Vaccinum genus of anthocyanins, but other classes of polar compounds such as phenols, flavonoids, and iridoids are present. Considering the healthy properties of these compounds, the choice of efficient extraction procedures is drawing great attention and solvent extraction is the most used method. The extraction efficiency is affected by several factors including solvent properties, solvent/solid ratio, raw materials particle size, and extraction time and temperature. Crucial is the choice of solvent that must be selective for phytochemical classes with similar polarity, safe, and inexpensive. In several cases, hydroalcoholic solutions demonstrated to be more efficient compared with mono solvent solution [6–8]. The possible explanation could be that majority of phenolic compounds are glycosides and sugar portion is more soluble in water [9]. High temperatures increase both diffusion and solubility, consequently reducing the extraction times. Hence, high temperatures increase the concentration of poorly soluble compounds such as certain flavonoids and iridoids, but also cause the degradation of enzymes and cellular components that would retain the bioactive compounds present [10–12]. Extraction time influences both solubility and transfer of bioactive compounds in the solvent; these processes are correlated with their molecular weight and structure [13, 14]. Increasing time will not affect the extraction after the equilibrium of the solute is reached inside and outside the solid material. Prolonged extraction time could trigger degradative processes of bioactive compounds [14]. In general, a higher extraction yield corresponds to a greater solvent/solid ratio. However, a solvent/solid ratio too high could determine excessive extraction solvent and require a long time for the concentration of the obtained extractive solutions. The conventional extraction methods, including maceration, usually use organic solvents and require a large volume of solvents and long extraction time. Some modern or greener extraction procedures, including ultrasound-assisted extraction, have also been applied in natural products extraction with some advantages such as shorter extraction time and/or less use of organic solvent.
It is well known that phenolic compounds are responsible for a variety of health benefits, in fact present high antioxidant capacity and consequently prevent the risk of disorders linked to oxidative stress, as diabetes and inflammation [15]. The inflammation process comprises several reactions that involve cells, enzymes, transcription factors, and cytokines. During this physiological process, the first step is the migration of immune cells from blood to the inflammation site, here cells release mediators able to recruit other inflammatory cells and release both reactive oxygen species (ROS) and reactive nitrogen species (RNS) as well as pro-inflammatory cytokines. Sometime this process is not regulated and self-limited. This condition known as chronic inflammation is the basis of many diseases, such as cancer, cardiovascular and neurodegenerative diseases, etc [16]. Otherwise, deregulated or chronic inflammatory processes are in the origin of several pathological conditions, such as asthma, diabetes and neurodegenerative diseases, among many others. The search for new alternatives to interfere with key players in inflammation has revealed nature to be a prolific source. Actually, frequently prescribed anti-inflammatory drugs include non-steroidal anti-inflammatory drugs, corticosteroids, and statins. Novel therapies targeting specific cells of the immune system have also been employed. However, there is still a need to develop safer therapeutic anti-inflammatory agents.
Until now, several studies evaluated the chemical profile and the bioactivity of plants from Vaccinium genus and many of them focused on the biological activity of anthocyanins-rich extracts of Vaccinium fruits. Worldwide, the high content of bioactive compounds and the health benefits that derive from the consumption of berry fruits are recognized. However, in recent years the scientific interest regarding the leaves composition and their beneficial effects grows. In fact, several works demonstrated that the leaves chemical profile is similar to that of the fruits or even richer and higher, indicating that they may be used as an interesting alternative source of biomolecules for the development of food supplements, nutraceuticals, or functional foods.
In this context, the aim of the present work is: i) to analyse the role in the bioactivity of V. corymbosum polar extracts of the two main classes of constituents namely flavonoids and iridoids; ii) to find the best extraction procedure to take advantage of the biological properties of these extracts evaluating the role of drying in the composition of the extracts; iii) to investigate the in vitro biological properties of berries and leaves. For this purpose, extracts obtained by using different procedures such as ethanol extraction (maceration, ultrasound and Soxhlet apparatus), hydroalcoholic maceration, and decoction, have been investigated for their chemical profile by using liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry (LC-ESI-QTOF-MS). Their potential nitric oxide inhibitory activity, carbohydrates-hydrolysing enzymes (α-amylase and α-glucosidase) inhibitory activity, and antioxidant properties (by using four in vitro assays such as ABTS, DPPH, FRAP, and β-carotene bleaching tests) were investigated.
2Materials and experimental methods
2.1Chemicals and reagents
Solvents of analytical grade were purchased from VWR International s.r.l. (Milan, Italy). Solvents used for liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry (LC-ESI-QTOF-MS) were purchased from Carlo Erba s.r.l. (Milan, Italy). Ascorbic acid, quercetin, propyl gallate, aucubin, linoleic acid, dimethyl sulfoxide (DMSO), butylated hydroxytoluene (BHT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), interleukin-2 (IL-2), 3(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT), o-dianisidine colour reagent (DIAN), peroxidase-glucose oxidase (PGO), α-amylase from porcine pancreas (EC 3.2.1.1), α-glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salts (ABTS), Tween 20, Folin-Ciocalteu reagent, sodium carbonate, Griess reagent, and β-carotene and L-N-(1-iminoethyl)lysine (L-NIL) were purchased from Sigma-Aldrich S.p.a. (Milan, Italy). Caffeic acid, syringic acid, isoquercitrin, quercetin, and rutin were purchased from Sigma-Aldrich s.r.l. (Orleans, France). Acarbose from Actinoplanes sp. was purchased from Serva (Heidelberg, Germany). Geniposide, myricetin 3-O-glucoside, chlorogenic acid, and hyperoside were purchased from Extrasynthese (Lyon, France).
2.2Plant materials and extraction procedures
Berries (3.8 kg) and leaves (3.1 kg) of Vaccinium corymbosum were collected in Balzata San Giorgio, Rogliano (Cosenza, Southern Italy) (WGS84:39°17′22” N, 16°38′17” E) (CLU26258) in June 2017 and identified by Dr. N.G. Passalacqua, Natural History Museum of Calabria and Botanic Garden (CLU), University of Calabria. Berries and leaves were randomly selected and examined to assess the absence of insect contamination.
Berries were harvested at maturity stage, defined by visual colour and size measurement. Berries and leaves were divided in two parts in order to obtain fresh and dried products. Leaves (1.5 kg) were dried at room temperature for 7 days in the dark. Fruits (2 kg) were dried for 7 days at 50 °C using a gravity convection oven (Thermo Scientific Heraeus, Germany). During the process, the temperature was stable (its distribution is based on warm air moving upwards). Daily determination of fruits weight was measured with weight electronic scale until the weight was stable.
Plant materials (1.8 kg and 760 g of fresh and dried berries, respectively, and 1.6 kg and 570 g of fresh and dried leaves, respectively) were subjected to extraction by: maceration using ethanol (1:2 w/v for fresh fruits, 1:5 w/v for dried fruits, 1:1.5 w/v for fresh leaves, 1:4 for dried leaves; 1 L, 3×72 h) and a 60% v/v hydroalcoholic solution of ethanol (1:2 w/v for fresh fruits, 1:5 w/v for dried fruits, 1:1.5 w/v for fresh leaves, 1:4 for dried leaves; 1 L, 3×72 h); ultrasound-assisted extraction by using Branson 3800 ultrasonic system, series CPXH (130 W, 40 kHz frequency (Milan, Italy) and ethanol as solvent (1:2 w/v for fresh fruits, 1:1.5 w/v for dried fruits, 1:0.75 w/v for fresh leaves, 1:3 for dried leaves; 150 mL, 3×1 h); Soxhlet apparatus (conventional glass with an extraction chamber with a diameter of 8 cm and a height of 30 cm, accompanied by a flask of capacity of 1 L) using ethanol as solvent (1:4 w/v for fresh fruits, 1:10 w/v for dried fruits, 1:15 w/v for fresh leaves, 1:20 for dried leaves; 600 mL, 7 cycles); and decoction (1:1 w/v, 30 min for fruits, 1:10 w/v, 10 min for fresh leaves, and 1:20 w/v, 10 min for dried leaves). Extraction yields are reported in Table 1.
Table 1
Extraction yield (%) and total phenols, flavonoids, and iridoids content of V. corymbosum extracts
| V. corymbosum | Yield ( %)a | TPC (mg CA/g)b | TFC (mg QE/g)c | TIC (mg AU/g)d |
| Fruits | ||||
| F1A | 10.11±1.14 | 32.81±0.32 | 30.87±0.25 | 104.04±0.71 |
| F1B | 9.90±0.95 | 34.93±0.21 | 29.33±0.22 | 71.33±0.63 |
| F1C | 7.84±0.73 | 35.47±1.23 | 28.53±0.44 | 104.71±0.77 |
| F1D | 13.05±1.3 | 34.87±0.85 | 31.87±0.37 | 92.22±0.55 |
| F1E | 7.23±0.70 | 32.22±0.53 | 30.53±0.12 | 106.70±0.86 |
| F2A | 26.21±2.74 | 31.61±0.32 | 28.53±0.25 | 91.33±0.33 |
| F2B | 25.13±2.50 | 31.87±0.58 | 28.65±0.35 | 98.61±1.32 |
| F2C | 6.71±0.65 | 32.82±1.06 | 27.87±0.44 | 80.58±0.21 |
| F2D | 32.15±3.24 | 29.40±0.67 | 27.27±0.52 | 110.42±1.74 |
| F2E | 14.04±1.40 | 29.27±0.45 | 26.47±0.27 | 100.73±2.57 |
| Leaves | ||||
| L1A | 16.21±1.62 | 470.27±3.32 | 250.70±2.07 | 126.11±1.62 |
| L1B | 18.70±1.92 | 469.33±2.41 | 230.13±1.79 | 104.03±1.21 |
| L1C | 9.63±0.94 | 394.60±2.37 | 274.41±2.66 | 89.33±1.18 |
| L1D | 24.15±2.40 | 442.44±2.66 | 189.87±1.83 | 86.24±1.02 |
| L1E | 8.71±0.95 | 464.45±3.63 | 232.23±1.72 | 124.69±1.44 |
| L2A | 17.75±1.83 | 337.33±2.27 | 199.07±2.54 | 137.32±1.87 |
| L2B | 24.01±2.42 | 375.47±2.72 | 261.32±3.01 | 107.11±2.69 |
| L2C | 20.52±2.13 | 444.13±3.90 | 273.31±2.74 | 83.34±1.22 |
| L2D | 31.71±3.20 | 325.30±2.54 | 265.26±2.78 | 104.02±1.54 |
| L2E | 17.03±1.70 | 382.66±3.16 | 138.13±1.86 | 100.64±1.96 |
F1: fresh fruits extracts, F2: dried fruits extracts; L1: fresh leaves extracts; L2: dried leaves extracts. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol extraction Soxhlet apparatus; E. Ethanol ultrasound-assisted extraction. Data are reported to mean±Standard Deviation (SD) (n = 3). TPC: Total Phenols Content; TFC: Total Flavonoids Content; TIC: Total Iridoids Content. aExpressed as (g dried extract/ g plant materials)×100. bmg of chlorogenic acid (CA) equivalents/g of extract. cmg of quercetin (QE) equivalents/g of extract. dmg of aucubin (AU) equivalents/g of extract.
2.3Total phytochemicals (phenols, flavonoids, iridoids) content
The total phenols content (TPC) was determined as previously described [17]. In brief, 0.2 mL of Folin-Ciocalteu reagent, 2 mL of water, and 1 mL of Na2CO3 15% (w/v) and 100μL of extract (1.5 mg/mL) were mixed. The absorbance was read at 765 nm using a UV-Vis Jenway 6003 spectrophotometer (Milan, Italy), after 2 h of incubation at 25 °C. The TPC was expressed as milligrams of chlorogenic acid equivalents (CA)/g of extract. For the determination of total flavonoids content (TFC) a method previously described was employed [18]. Distilled water (4 mL) and sodium nitrite 5% (w/v) (0.3 mL) were mixed to the extract (1.5 mg/mL). After 5 min, 0.6 mL of 10% (w/v) AlCl3 was added, and after 6 min 2 mL of 1 M NaOH and 2.1 mL of distilled water were additional to final mixture. Absorbance was measured at 510 nm. The TFC was calculated as milligrams of quercetin equivalents (QE)/gram of extract. The total iridoids content (TIC) was determined spectrophotometrically at 609 nm, following the colorimetric method based on the Trim and Hill reaction. The reaction containing 400μL of extract (1.5 mg/mL) and 4.0 mL of Trim-Hill reagent (acetic acid/0.2%) was heated at 100 °C for 5 min. The presence of iridoids was confirmed by blue coloration of solution. The total iridoids content was expressed as milligrams of aucubin equivalents (AU)/g of extract. All samples were analysed in triplicate.
2.4LC-ESI-QTOF-MS analyses
V. corymbosum extracts were analysed by liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry (LC-ESI-QTOF-MS) by using an HPLC (U-3000, Thermo, Courtaboeuf, France) coupled to an ESI-QTOF-MS (Maxis II, Bruker, Champs sur Marne, France). The chromatographic separation was performed at 35 °C using a column C18 (Acclaim RSLC polar advantage II, 100×2.1 mm, 2.2μm) with a speed of flow of 0.3 mL/min. The injection volume was 2μL. Elution solvents consists of a mixture of 0.1% formic acid, 10% methanol and water (phase A), and 0.1% formic acid in acetonitrile (phase B). The elution gradient was as follows: 0 to 2 min 95% A; 2 to 7 min, 95 to 85% A; 7 to 15 min, from 85 to 50% A; 15 to 18 min, 50 to 20% A; 18 to 19 min, 20% and 19 to 21 min, 20 to 95% A. Chromatograms were obtained at four wavelengths such as 240, 270, 340, and 510 nm. Mass spectra were acquired in positive mode using the following parameters: ESI 3500 V, m/z 50–1200, MS 2 Hertz. Chromatograms of selected leaves and fruits extracts are reported in Supplementary Materials (Fig. S1–S9).
2.5Cell viability assay
2.5.1Cell cultures
Dulbecco’s modified Eagle’s medium (Gibco BRL, Life Technologies) integrated with 100 U/mL penicillin, 4.5 g/L glucose, 100μg/mL streptomycin and 15 % fetal bovine serum were used for cultured of Human Foreskin Fibroblast (HFF1), obtained from the American Type Culture Collection (Rockville, MD, USA). To obtain the same experimental conditions and precision in the measurements the cells were plated at a constant density.
2.5.2MTT test
Cell viability was assess using 3(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT) test on a 96-well plate (8×103 cells/well) [19]. Previously, cells were incubated at 37 °C in humidified atmosphere of 5% CO2 to allow cell attachment; after 24 h the cells were treated with various concentration of extracts (12.5–250μg/mL) for 24 h. The number of living cells was determined based on formazan production that is proportional to cell vitality. The presence of metabolic activity allows the conversion of tetrazolium salt to coloured formazan. The optical density was measured at 570 nm. Results were calculated as percentage cell viability respect to untreated cells (control).
2.6Assessment of nitrite/nitrate concentrations
The inhibitory activity of V. corymbosum extracts on nitrite production was measured by the Griess method [20]. This method is based on the diazocopulation reaction between Griess reagent and nitrites. HFF1 cells were pre-treated for 90 min with V. corymbosum extracts (at the concentration of 12.5μg/mL), and stimulated for 30 min with 10μg/mL of interleukin-2β. In the cells, total nitrite concentration was calculated by adding 250 μL of Griess reagent to 250μL of medium. The optical density was measured at 550 nm (OD550). Results were obtained by comparison with OD550 of standard aqueous solutions of sodium nitrite and expressed as IC50 values respect to untreated and interleukin stimulated cells. L-N6-(1-iminoethyl)lysine (L-NIL) (1μg/mL) was used as a positive NO production inhibitor able to reduce about of 50% iNOS activity.
2.7Antioxidant activity
The antioxidant activity of V. corymbosum berries and leaves extracts was investigated by applying four in vitro tests described below. The radicals scavenging activity was assessed by using two different spectrophotometric methods such as 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diamonium salts (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) tests. The ABTS test was performed following the procedure of Loizzo et al. [21]. Briefly, a solution of ABTS+ and potassium persulfate was prepared and left overnight. The solution was diluted with ethanol until an absorbance of 0.70 measured at 734 nm (Perkin Elmer 40 UV-VIS spectrophotometer, Milan, Italy). Extracts and diluted ABTS+ solution were mixed and after 5 min and the absorbance was read. The ABTS· + radicals scavenging activity was calculated as follow: [(A0–A)/A0]×100, where A0 is the absorbance of the control reaction and A is the absorbance in the presence of the extract. The DPPH test was performed using procedure previously described [21]. The DPPH radicals scavenging activity was calculated as follow: [(A0−A1)/A0]×100, where A0 is the absorbance of the blank, and A1 is the absorbance in the presence of the sample. In both tests, ascorbic acid was the positive control.
The β-carotene bleaching test was done following the procedure previously described [22]. The β-carotene solution was added to linoleic acid and 100% Tween 20. The emulsion was mixed with 200μL of extracts (1–100μg/mL). The absorbance was read at 470 nm against a blank at t = 0 and after 30 and 60 min of incubation. The positive control was propyl gallate.
In the Ferric Reducing Activity Power (FRAP) assay, FRAP reagent was prepared as previously reported, and added to the methanol solution of the extracts tested at the concentration of 2.5 mg/mL [23]. The absorption was measured at 595 nm. The positive control was butylated hydroxytoluene (BHT).
2.8α-Amylase inhibitory activity test
In the α-amylase inhibitory activity test, 25.3 mg of enzyme were make soluble in 100 mL of cold distilled water [24]. The starch solution was prepared by adding 125 mg of potato starch in 25 mL of sodium phosphate buffer 20 mM and sodium chloride 6.7 mM (at 65 °C for 15 min). The colorimetric reagent was made by mixing a sodium potassium tartrate solution (24 g of sodium potassium tartrate in 16 mL of sodium hydroxide 2 M) and 96 mM 3,5-dinitrosalicylic acid solution (0.88 g of acid in 46 mL of water). V. corymbosum extracts (40μL) at concentration ranged from 12.50 to 1000μg/mL, and control were added to the starch solution and left to react at 25 °C with enzyme. The absorbance was read at 540 nm. Acarbose was selected as positive control.
2.9α-Glucosidase inhibitory activity test
In this assay, the enzyme (1 mg) was dissolved in 10 mL of ice-cold distilled water to make the enzyme solution and a maltose solution was prepared by adding 12 g of maltose in 300 mL of 50 mM sodium acetate buffer [24]. The o-dianisidine colour reagent (DIAN) solution was prepared by dissolving 1 tablet in 25 mL of distilled water and peroxidase-glucose oxidase (PGO) system-colour reagent solution was made by dissolving 1 capsule in 100 mL of ice cold distilled water. V. corymbosum extracts (5μL) at concentration ranged from 12.50 to 1000μg/mL and control were stirred to maltose solution at 37 °C. The reaction was started with addition of the enzyme solution and was stopped after 30 min of incubation at 37 °C by adding a solution of perchloric acid. The supernatant of tube of the first step was mixed with DIAN and PGO and was left to incubate for 30 min at 37 °C. The absorbance was read at 540 nm. Acarbose was the positive control.
2.10Statistical analysis
Statistical analysis was performed using one-way Analysis of Variance (ANOVA), followed by Dunnett’s multiple comparison test. The concentration giving 50% inhibition (IC50) was determined by nonlinear regression with the use of Prism GraphPad Prism, version 4.0 for Windows (GraphPad Software, San Diego, CA). The concentration-response curve was obtained by plotting the percentage inhibition versus concentration. Differences within and between groups were evaluated by one-way analysis of variance test (ANOVA) followed by a multiple comparison Dunnett’s test (α= 0.05) compared with the positive control. Pearson’s correlation coefficient (r) was used for investigating the relationship between total phytochemicals content and biological activities. Linear regression, correlation, assessment of repeatability, calculation of average and relative standard deviations (SD) were performed using Microsoft Excel 2010 software.
3Results and discussion
3.1Extraction yields and total phytochemicals content
Table 1 shows the extraction yields and the total phytochemicals content of V. corymbosum. Except for decoction, extraction yields from dried berries were higher than fresh berries. This difference was not observed with the leaves. The highest extraction yields were obtained by using ethanol with Soxhlet apparatus for the extraction of dried fruits and leaves (32.1 and 31.7%, respectively), followed by hydroalcoholic and ethanolic maceration of dried berries (26.2 and 25.1%, respectively). Except for dried fruits, the lowest extraction yields were obtained by using the ultrasound-assisted extraction procedure. The spectrophotometric determinations of the total phenols, flavonoids, and iridoids content evidenced a similar content of these phytochemicals in the extracts with some variations according to the extraction technique and plants organs used.
The analysis of TPC allows to find the following trend decoction > hydroalcoholic maceration > Soxhlet apparatus (EtOH)>ethanolic maceration > ultrasound-assisted extraction (EtOH). The entire data collection allows founding that dried fruits extracts showed a slightly lower total phytochemicals content than fresh fruits extracts. This could be attributed to the fact that, during drying, the peel, which is the part richer in polyphenols, is the first component to be affected by degrading effects [25]. Broadly, leaves extracts are richer in phenols and flavonoids than fruits extracts. Interesting data were obtained with the ethanol maceration of fresh leaves that showed a total phenols and iridoids content of 470.27 mg of chlorogenic equivalents/g of extract and 126.11 mg of aucubin equivalents/g of extract, respectively. This extract L1A, also has one of the best flavonoid levels (250.70 mg of quercetin equivalents/g of extract).
Among dried leaves extracts, the best phenols (444.13 mg of chlorogenic acid equivalents/g of extract) and flavonoids (273.31 mg of quercetin equivalents/g of extract) contents were obtained from the decoction, whereas the best iridoids content (137.32 mg of aucubin equivalents/g of extract) was observed after ethanolic maceration. There are few studies in the literature on the total phytochemicals content of V. corymbosum leaves and none has studied or revealed the presence of iridoids. According with our data, Ehlenfeldt et al. [26] compared different varieties of V. corymbosum and found a higher total phenols content in the leaves (23.58 mg/g ∼ 66.29 mg/g) compared to the fruits (0.20 mg/g ∼ 1.75 mg/g).
3.2LC-ESI-Q-TOF-MS profile
The main phytochemical compounds described in literature in V. corymbosum berries are anthocyanins. Their extraction need of an acidified alcoholic solution and for this reason, herein they were not detected. Compounds were identified based on UV spectra, molecular weight (m/z ion [M + H]+), and chemotaxonomic significance [27–38]. The presence of hyperoside (quercetin-3-O-galactoside), isoquercitrin (quercetin-3-O-glucoside), myricetin 3-O-glucoside, quercetin, rutin (quercetin-3-O-rutoside), caffeic acid, geniposide, and chlorogenic acid were verified with authentic standards. The principal classes detected in V. corymbosum berries (Table 2) and leaves (Table 3) extracts are flavonoids, phenolic acids, and iridoids. In particular, ten phenolic acids, thirteen flavonoids and four iridoids were detected. Leaves showed a greater chemical diversity than fruits and only some of identified compounds are biosynthesized by both fruits and leaves.
Table 2
Identification of chemical compounds in V. corymbosum fruits extracts using the LC-ESI-QTOF-MS technique
| Compounds | Rt (min) | Molecular Formule | MH+ | Error (ppm) | Score (%) | MS fragment | UV λ (nm) | Fresh Fruits | Dried Fruits | Ref. | ||||||||
| F1A | F1B | F1C | F1D | F1E | F2A | F2B | F2C | F2D | F2E | |||||||||
| Phenolic acids | ||||||||||||||||||
| Caffeic acid | 5.5 | C9H8O4 | 181.0500 | 0.4 | 100 | 295, 322 | √ | √ | 33 | |||||||||
| 3,5-di-O-Caffeoylquinic acid | 10.3 | C25H24O12 | 517.1346 | 0.3 | 100 | 244, 330 | √ | √ | √ | √ | √ | 35 | ||||||
| Chlorogenic acid | 9.8 | C16H18O9 | 355.1029 | 0.3 | 100 | 242, 325 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 33 | |
| Syringic acid | 10.6 | C9H10O5 | 199.0606 | 0.6 | 100 | 218, 274 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 42 | |
| 2,3,4 Trihydroxybenzoic acid | 2.1 | C7H6O5 | 171.0293 | 0.1 | 100 | 255, 292 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 36 | |
| Flavonoids | ||||||||||||||||||
| Hyperoside (*) | 12.8 | C21H20O12 | 465.1031 | 0.2 | 100 | 303.0499 | 217, 278, 350 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Isoquercitrin (*) | 12.8 | C21H20O12 | 465.1031 | 0.2 | 100 | 303.0499 | 215, 253, 353 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Myricetin 3-O-glucoside | 12.0 | C21H20O13 | 481.0982 | 0.8 | 100 | 319.0389 | 212, 266, 364 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Quercetin | 16.8 | C15H10O7 | 303.0504 | 0.1 | 100 | 213, 255, 353 | √ | √ | 31 | |||||||||
| Quercetin 3-O-arabinoside | 13.4 | C20H18O11 | 435.7749 | 0.5 | 100 | 303.0499 | 213, 253, 353 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Rutin | 12.4 | C27H30O16 | 611.1612 | 0.6 | 100 | 303.0499 | 213, 253, 352 | √ | √ | 31 | ||||||||
| Syringetin 3-O-glucoside | 13.1 | C23H24O13 | 509.1295 | 0.7 | 100 | 347.2967 | 219, 346 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Iridoids | ||||||||||||||||||
| Dihydromonotropein | 12.1 | C16H24O11 | 393.1396 | 0.2 | 95 | 237 | √ | 49 | ||||||||||
| Geniposide | 10.6 | C17H24O10 | 389.1447 | 0.1 | 100 | 239 | √ | √ | 32 | |||||||||
| Scandoside | 10.4 | C17H24O10 | 391.1240 | 0.2 | 100 | 241 | √ | 49 | ||||||||||
F1: fresh fruits extracts, F2: dried fruits extracts. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol extraction Soxhlet apparatus; E. Ethanol ultrasound-assisted extraction. (*) interchangeable.
Table 3
Identification of chemical compounds in V. corymbosum leaves extracts using the LC-ESI-QTOF-MS technique
| Compounds | Rt (min) | Molecular Formule | MH+ | Error (ppm) | Score (%) | MS fragment | UV λ (nm) | Fresh leaves | Dried leaves | Ref. | ||||||||
| L1A | L1B | L1C | L1D | L1E | L2A | L2B | L2C | L2D | L2E | |||||||||
| Phenolic acids | ||||||||||||||||||
| Caffeic acid | 5.5 | C16H22O11 | 181.0500 | 0.4 | 100 | 295, 322 | √ | 33 | ||||||||||
| 4-O-Caffeoylquinic acid methyl ester (*) | 8.9 | C17H20O9 | 369.1185 | 0.3 | 99 | 294, 328 | √ | √ | √ | √ | √ | √ | √ | √ | 38 | |||
| 5-O-Caffeoylquinic acid methyl ester (*) | 10.2 | C17H20O9 | 369.1185 | 0.9 | 89 | 294, 328 | √ | √ | √ | √ | √ | √ | √ | √ | 38 | |||
| 3-O-Caffeoylquinic acid methyl ester (*) | 11.1 | C17H20O9 | 369.1185 | 0.7 | 91 | 294, 328 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 38 | ||
| 3,5-di-O-Caffeoylquinic acid methyl ester | 14.3 | C26H26O12 | 531.1502 | 0.3 | 100 | 244, 330 | √ | √ | 35 | |||||||||
| Caffeoyl coumaroylquinic acid | 14.3 | C25H24O11 | 501.1396 | 0.2 | 98 | √ | √ | 35 | ||||||||||
| Chlorogenic acid | 9.8 | C16H18O9 | 355.1029 | 0.3 | 100 | 242, 325 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 33 | ||
| Flavonoids | ||||||||||||||||||
| Hyperoside(*) | 12.8 | C21H20O12 | 465.1031 | 0.2 | 100 | 303.0499 | 217, 278, 350 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Isoquercitrin(*) | 12.8 | C21H20O12 | 465.1031 | 0.2 | 100 | 303.0499 | 215, 253, 353 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Isorhamnetin malonylglycoside | 14.0 | C25H24O15 | 565.1193 | 0.1 | 100 | 316.267 | 212, 254, 355 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 37 |
| Isorhamnetin 3-β-D-galactoside | 13.3 | C22H22O12 | 479.1189 | 0.1 | 99 | 316.267 | 212, 255, 368 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 45 |
| Kaempferol 3-O-glucoside | 13.2 | C21H20O11 | 449.1077 | 0.2 | 100 | 287.2287 | 210, 265, 346 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Myricetin 3-O-a-L-arabinooside (*) | 12.6 | C20H18O12 | 451.0876 | 0.2 | 100 | 319.0389 | 219, 253, 365 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 45 |
| Myricetin 3-O-glucoside | 12.0 | C21H20O13 | 481.0982 | 0.8 | 100 | 319.0389 | 212, 266, 364 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Quercetin | 16.8 | C15H10O7 | 303.0504 | 0.1 | 100 | 213, 255, 353 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 | |
| Quercetin acetylglycoside | 13.9 | C23H22O13 | 507.1138 | 0.2 | 98 | 303.0499 | 213, 252, 354 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 39 |
| Quercetin 3-O-arabinoside (*) | 13.4 | C20H18O11 | 435.7749 | 0.5 | 100 | 303.0499 | 213, 253, 353 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Quercitrin | 13.6 | C21H20O11 | 449.1079 | 0.3 | 100 | 303.0499 | 213, 254, 356 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Rutin | 12.4 | C27H30O16 | 611.1612 | 0.6 | 100 | 303.0499 | 213, 253, 352 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Syringetin 3-O-glucoside | 13.1 | C23H24O13 | 509.1295 | 0.7 | 100 | 347.2967 | 219, 346 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 31 |
| Iridoids | ||||||||||||||||||
| Dihydromonotropein | 12.1 | C16H24O11 | 393.1396 | 0.2 | 95 | 237 | √ | √ | √ | √ | √ | 49 | ||||||
| Geniposide | 10.6 | C17H24O10 | 389.1447 | 0.1 | 100 | 239 | √ | √ | √ | √ | √ | 32 | ||||||
| Scandoside | 10.4 | C16H22O11 | 391.1240 | 0.2 | 100 | 241 | √ | √ | √ | √ | √ | 49 | ||||||
| Vaccinoside | 15.2 | C25H28O13 | 537.1608 | 0.3 | 97 | 246 | √ | √ | √ | √ | √ | 40 | ||||||
L1: fresh leaves extracts; L2: dried leaves extracts. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol extraction Soxhlet apparatus; E. Ethanol ultrasound-assisted extraction. (*) or isomer.
Five phenolic acids, seven flavonoids, and three iridoids characterized berry extracts, while seven phenolic acids, thirteen flavonoids and four iridoids were reported in leaves extracts. Berries and leaves biosynthesized chlorogenic acid, and caffeic acid. The other metabolites such as caffeoylquinic acid derivatives (3-O-, 4-O-, 5-O-caffeoylquinic acid methyl ester, 3,5-di-O-caffeoylquinic acid methyl ester and caffeoyl-coumaroylquinic acid) were found only in leaves while 2,3,4-trihydroxybenzoic acid, syringic acid, 3,5-di-O-caffeoylquinic acid were produced by fruits. Moreover, differences related to the extraction procedure must be taken into consideration. In all fruits and leaves extracts was found chlorogenic acid. Only ethanol maceration both fresh fruits (F1A) and leaves (L1A) and decoction of fresh fruits (F1C) has allow to extract caffeic acid and 3,5-di-O-caffeoylquinic acid was found in all the fresh fruits extracts (F1). Other caffeoylquinic derivatives, 3,5-di-O-caffeoylquinic acid methyl ester and caffeoyl-coumaroyl-quinic acid, have been highlighted, respectively, after extraction of fresh leaves using ethanol by maceration (L1A), and Soxhlet apparatus (L1D). Furthermore, only in the decoction of dried leaves were not reported the presence of caffeoylquinic acid methyl ester isomers but found in all other leaves extracts.
The flavonoids, namely myricetin 3-O-glucoside, syringetin 3-O-glucoside, and quercetin derivatives such as arabinoside, galactoside (hyperoside), and glucoside (isoquercitrin) were found in all fruits and leaves extracts. Other flavonoids, namely isorhamnetin 3-O-β-D-galactoside, isorhamnetin malonylglycopyranoside, kaempferol 3-O-glucoside, quercetin acetylglucopyranoside, myricetin 3-O-α-L-arabinoside, quercetin, quercitrin, and rutin were characterized in all leaves extracts. Rutin and quercetin were extracted also with ethanol maceration of fresh fruits (F1A) and hydroalcoholic maceration of dried fruits (F2B).
The four iridoids identified in V. corymbosum extracts were scandoside, geniposide, vaccinoside, and dihydromonotropein. With the exception of vaccinoside only detected in leaves extracts, iridoids are present in both leaves and berries. Their extraction is affected by the used procedure.
Geniposide, scandoside, and dihydromonotropein were found in all fresh leaves extracts (L1), with the exception of hydroalcoholic maceration extract (L1B). The extraction of these three iridoids from the dried leaves is more difficult; geniposide, scandoside, and vaccinoside were only detected after ethanolic maceration (L2A) while dihydromonotropein was detected after decoction (L2C). The presence of vaccinoside is observed in all extracts of fresh leaves (L1) with the exception of that resulting from decoction (L1C). Our results showed that it was better to work with fresh fruits to extract iridoids. Indeed, among iridoids that we have been able to identify, geniposide, scandoside, and dihydromonotropin were obtained exclusively after ethanolic maceration (F1A). Our results showed that it would be easier to selectively obtain geniposide from fresh fruits by using decoction (F1C), dihydromonotropein from dried leaves by using decoction (L2C), and vaccinoside from fresh leaves by using hydroalcoholic maceration (L1B). The identification of phytochemicals is in agreement with previously published data. In the Vaccinium genus, hydroxycinnamic acids derivatives are abundant and they are described both in leaves and fruits [30, 35, 39–42]. The majority of flavonoids identified characterizes the genus and is classically found in Ericaceae family [28, 29, 32, 36, 37].
This study revealed the presence of isorhamnetin malonylglycoside and quercetin acetylglucopyranoside. These compounds are less frequently described in the plant kingdom. The iridoids herein detected are previously described in the Vaccinium genus or Ericaceae family [41, 43–45].
3.3Nitrate/nitrite as critical mediators in inflammation
A large body of literature has demonstrated that chronic inflammation is one of the key factors in the development of many disorders and diseases strictly related to oxidative stress [46]. It is well known that vegetables and fruits exert anti-inflammatory and antioxidant activities due to their valuable phytochemicals content [47]. In our study, all tested extracts showed NO inhibitory activity. Among these extracts, fruits elicited an anti-inflammatory effect higher respect to leaves (Table 5). In particular, F1C (IC50 value of 19.51 μg/mL) and F2C (IC50 value of 18.93 μg/mL), obtained by decoction, presented a more significant activity in inhibiting NO production responsible for the early inflammatory response.
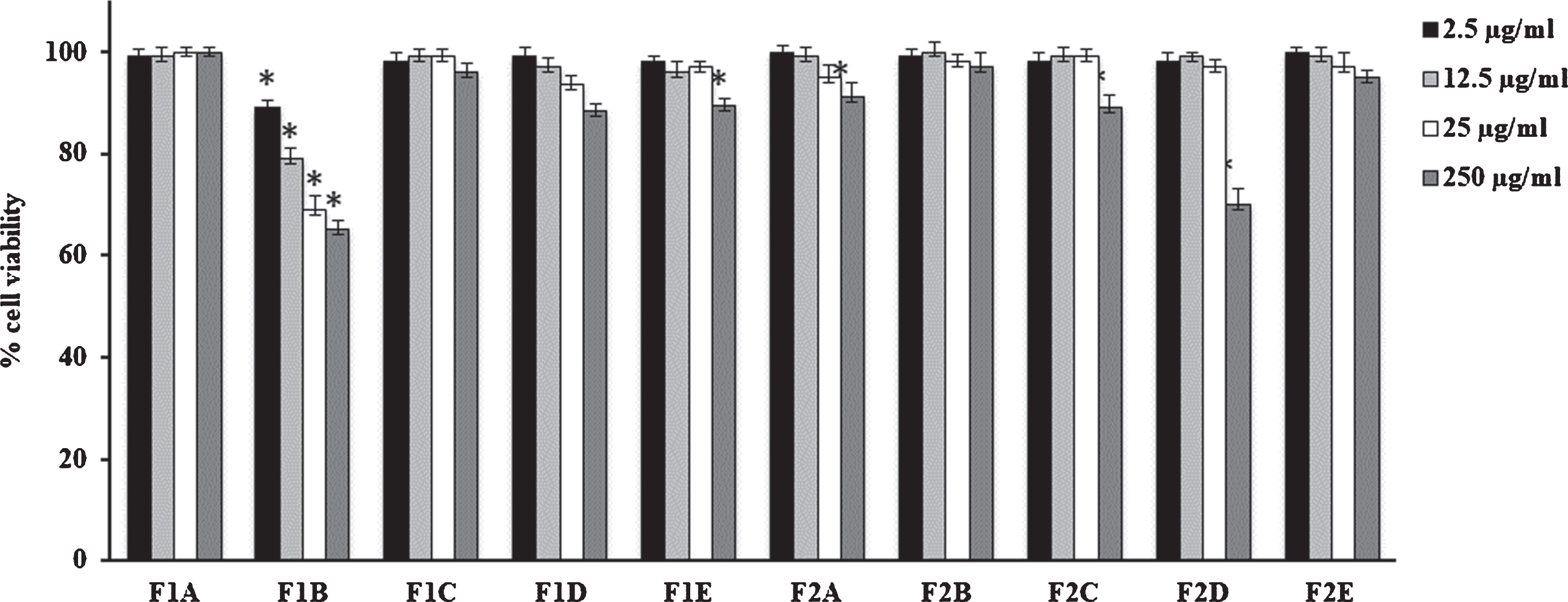
V. corymbosum berries and leaves extracts did not modify cell viability performed on HFF1 cells independently of the extraction technique used (Figs. 1 and 2). Only F1B extract showed a slight toxicity respect to dried fruit and fresh/dried leave extracts. In particular, at 12.5μg/mL it is able to reduce cell viability nearly to 80% compared to control.
Fig. 1
Cell viability in HFF1 cells treated for 24 h with leaves extracts at different concentrations (2.5–250μg/mL). Values are the mean±SD of four experiments in triplicate. *Significant vs untreated control cells: p < 0.001. L1: fresh leaves, L2: dried leaves. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol Soxhlet extraction; E. Ethanol ultrasound-assisted extraction.
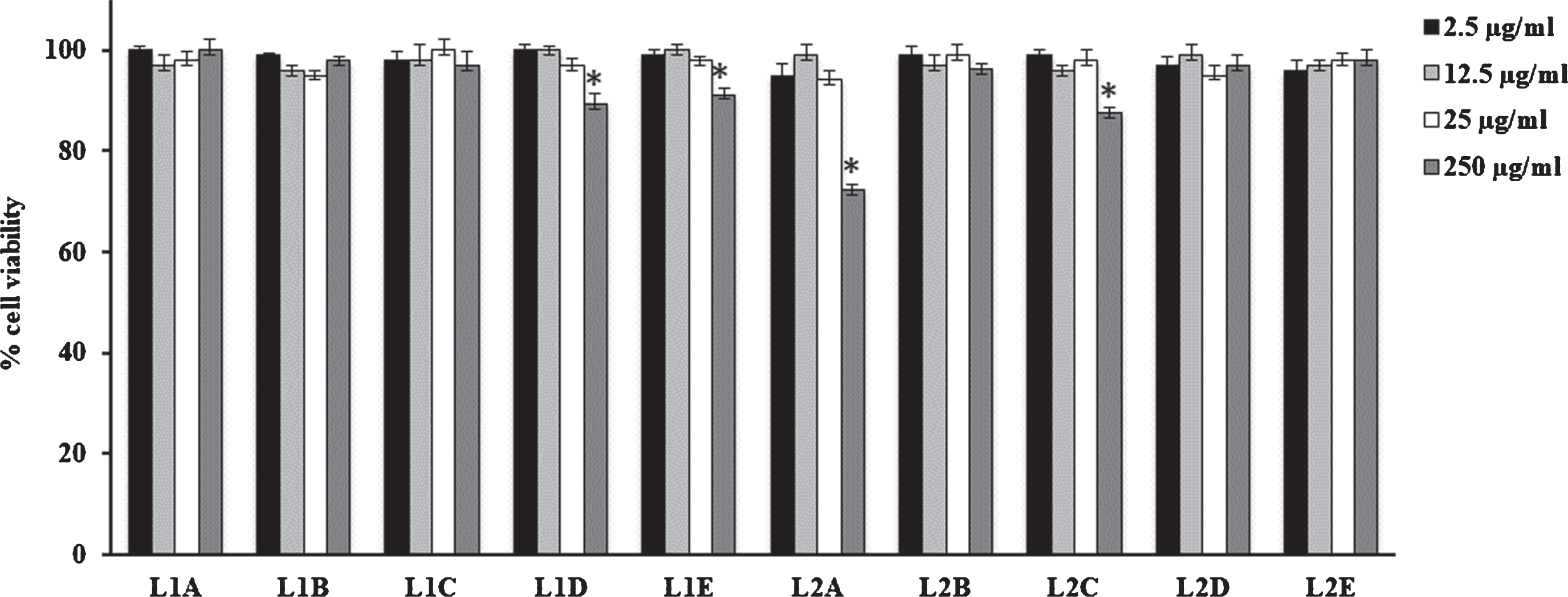
Fig. 2
Cell viability in HFF1 cells treated for 24 h with fruits extracts at different concentrations (2.5–250μg/mL). Values are the mean±SD of four experiments in triplicate. *Significant vs untreated control cells: p < 0.001. F1: fresh fruits, F2: dried fruits. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol Soxhlet extraction; E. Ethanol ultrasound-assisted extraction.
3.4Antioxidant activity
Several in vitro assays were developed and employed to measure the antioxidant activity of foods and their derived products [48, 49]. In this work, ABTS, DPPH, β-carotene bleaching and FRAP tests were done. ABTS, DPPH and FRAP tests are based on electron transfer mechanism. β-Carotene bleaching test was used to investigate the counteract effect of a sample on radicals induced by oxidation of fatty acid [50, 51].
In ABTS test, leaves extracts showed a greater antioxidant power than fruits extracts and their IC50 values are lower than that reported for the positive control ascorbic acid (IC50 value of 1.70μg/mL) (Table 4).
Table 4
In vitro antioxidant activity of V. corymbosum extracts
| V. corymbosum | ABTS test | DPPH test | FRAP test | β-Carotene bleaching test | |
| IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μM Fe (II)/g) | IC50 (μg/mL) | ||
| 30 min | 60 min | ||||
| Fruits | |||||
| F1A | 3.80±0.44∧ | 89.71±2.32** | 5.54±0.34** | 14.13±1.20** | 54.29±2.40** |
| F1B | 1.14±0.12∧ | 53.55±1.29** | 2.19±2.28** | 40.40±4.83** | 53.07±2.30** |
| F1C | 5.31±0.56∧ | 50.64±1.21** | 11.42±2.32** | 4.65% a ∧ | 13.74% a ∧ |
| F1D | 5.32±0.51∧ | 51.61±1.23** | 5.42±0.97** | 39.60±3.01** | 62.65±1.63** |
| F1E | 3.41±0.35∧ | 52.58±1.25** | 1.64±0.35** | 14.97±1.63** | 88.17±2.82** |
| F2A | 34.31±1.48** | 71.88±2.56** | 16.47±2.46** | 40.59±4.22** | 57.67±5.21** |
| F2B | 18.22±1.22** | 195.75±1.92** | 5.88±0.39** | 52.51±5.32** | 45.64% a ∧ |
| F2C | 8.83±0.57** | 54.52±1.55** | 0.80±0.09** | 4.65% a ∧ | 20.27% a ∧ |
| F2D | 3.40±0.33∧ | 74.69±1.81** | 7.36±0.21** | 25.48±2.30** | 87.89±8.90** |
| F2E | 3.81±0.21∧ | 144.14±3.56** | 12.62±2.15** | 41.25% a ∧ | 27.85% a ∧ |
| Leaves | |||||
| L1A | 4.62±0.51∧ | 36.09±0.45** | 23.83±1.25** | 8.97±0.26∧ | 13.19±0.16** |
| L1B | 3.43±0.03∧ | 26.46±0.28** | 24.56±1.67** | 19.01±0.25** | 26.42±0.21** |
| L1C | 1.09±0.01∧ | 15.75±0.12** | 20.28±1.89** | 34.0% a∧ | 34.91% a ∧ |
| L1D | 1.10±0.02∧ | 28.43±0.32** | 25.63±1.56** | 47.35±0.48** | 40.78±1.35** |
| L1E | 1.06±0.03∧ | 26.46±0.25** | 23.94±1.67** | 53.73±0.59** | 45.20±0.43** |
| L2A | 11.05±0.11** | 36.31±1.33** | 26.34±1.71** | 9.72±0.09∧ | 33.27±1.36** |
| L2B | 1.90±0.20∧ | 29.42±1.29** | 22.92±1.98** | 64.80±0.85** | 89.20±2.82** |
| L2C | 0.77±0.06∧ | 12.77±0.82∧ | 22.12±1.45** | 25.58% a ∧ | 44.82% a∧ |
| L2D | 0.77±0.07∧ | 34.34±1.36** | 24.35±1.64** | 49.23±0.52** | 43.41±0.86** |
| L2E | 1.50±0.01∧ | 28.43±1.25** | 23.37±1.56** | 48.43% a ∧ | 84.42±0.92** |
| Positive control | |||||
| Ascorbic acid | 1.70±0.21 | 5.03±0.80 | |||
| BHT | 63.20±4.31 | ||||
| Propyl gallate | 1.01±0.01 | 1.02±0.01 | |||
F1: fresh fruits extracts, F2: dried fruits extracts; L1: fresh leaves extracts; L2: dried leaves extracts. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol extraction Soxhlet apparatus; E. Ethanol ultrasound-assisted extraction. aat a concentration of 100μg/mL. Data are expressed as means±S.D. (n = 3). ABTS test: One-way ANOVA ***p < 0.0001 followed by a multicomparison Dunnett’s test **p < 0.05, ∧p > 0.05 compared with ascorbic acid. DPPH assay: One-way ANOVA ***p < 0.0001 followed by a multicomparison Dunnett’s test: **p < 0.05, ∧p > 0.05 compared with ascorbic acid. FRAP test: One-way ANOVA ***p < 0.0001 followed by a multicomparison Dunnett’s test **p < 0.05 compared with BHT. β-Carotene bleaching test 30 min: One-way ANOVA ***p < 0.0001 followed by a multicomparison Dunnett’s test: **p < 0.05, ∧p > 0.05 compared with prolyl gallate. β-Carotene bleaching test 60 min: One-way ANOVA ***p < 0.0001 followed by a multicomparison Dunnett’s test: **p < 0.05, ∧p > 0.05 compared with prolyl gallate.
Dried leaves extract obtained by decoction resulted the most active as DPPH radicals scavenging agent with an IC50 value of 12.77μg/mL. This aqueous extract (L2C) exhibited also a highest ABTS radicals scavenging activity with IC50 value of 0.77μg/mL. The same value of ABTS radical scavenging activity was found when dried leaves were extracted by ethanol (Soxhlet apparatus, L2D). Promising results were obtained with L1E, L1C and L1D. The extract from decoction of fresh leaves showed a similar activity as DPPH radicals scavenging agent (L1C, IC50 of 15.75μg/mL).
In ABTS assay, extract obtained by hydroalcoholic maceration resulted the most active with an IC50 value of 1.14μg/mL. This result is not confirmed in DPPH test where fruits showed IC50 values in the range 50.64–195.75μg/mL for F1C and F2B, respectively.
V. corymbosum leaves evidenced greater FRAP activity than fruit extracts. The ethanolic maceration of the dried leaves showed the highest activity with FRAP value of 26.34μM Fe(II)/g. However, this value is 2.4-time lower than the positive control BHT (63.20μM Fe(II)/g). A great variability in protection of lipid peroxidation was observed. Among fruits, F1A and F1E showed the best performance after 30 min incubation with IC50 values of 14.13 and 14.97μg/mL, respectively whereas fresh leaves extracted by hydroalcoholic maceration (IC50 value of 8.97μg/mL) showed the highest activity followed by L2A sample (IC50 value of 9.72μg/mL). The same trend was observed also after 60 min of sample incubation however with higher IC50 values. It is interesting to note that both fruits and leaves extracted by decoction did not inhibit lipid peroxidation.
Some studies that investigated the antioxidant activity of V. corymbosum leaves are present in literature. Namiesnik et al. [52] analysed V. corymbosum fruits from Poland that were lyophilized and extracted by using a hydroalcoholic solution of methanol/water (1:1). After removal of methanol the aqueous solution was extracted with diethyl ether and ethyl acetate. The obtained extracts (diethyl ether, ethyl acetate, and water) were tested for their composition and antioxidant activity. A DPPH radicals scavenging activity of 108.09μM TE/g DW and a percentage of inhibition of lipid peroxidation of 80.1% at 100 ppm for the aqueous extract were found.
In the same year, Podsedek et al. [53] investigated the antioxidant activity of the aqueous phase of the acetone (70% v/v) extract of V. corymbosum cv Toro fruits from Poland. This extract showed an ABTS radical scavenging activity of 27.09μM TE/g fruit and a FRAP value of 16.86μM Fe(II)/g. These data are in line with our results. Previously, Rodrigues et al. [54] investigated the antioxidant potential of V. corymbosum cv Bluecrop. The ultrasonic methanol extract showed radical scavenging potential with values of 1253.90 and 1244.13μM/100 g FW for ABTS and DPPH, respectively. Value of 699.78μM/100 g FW was found in FRAP assay.
3.5In vitro α-amylase and α-glucosidase inhibitory activity
The hypoglycaemic capacity of V. corymbosum extracts was evaluated through the inhibition of α-amylase and α-glucosidase. The results are reported in Table 6. As evident, some V. corymbosum extracts showed an appreciable activity against α-glucosidase. Among fruits extracts, significant results were obtained by hydroalcoholic maceration of fresh leaves (L1B) and Soxhlet (EtOH) apparatus dried leaves (L2D) extracts against α-amylase with IC50 values of 20.55 and 16.16μg/mL, respectively. The activity of these extracts was 2.4 and 3 times higher than positive control for L1B and L2D, respectively. In addition, selectivity values of 10.47 and 16.03 were found for α-amylase.
Table 5
Inhibitory effects of V. corymbosum extracts on NO production in HFF1 cells
| V. corymbosum | IC50 (μg/mL) |
| Fruits | |
| F1A | 25.72±1.65 |
| F1B | 25.30±0.86 |
| F1C | 19.51±1.11 |
| F1D | 25.12±1.12 |
| F1E | 25.30±0.99 |
| F2A | 21.55±0.99 |
| F2B | 21.26±1.32 |
| F2C | 18.93±1.61 |
| F2D | 26.04±1.24 |
| F2E | 20.16±0.79 |
| Leaves | |
| L1A | 27.17±1.21 |
| L1B | 28.41±1.11 |
| L1C | 27.17±0.65 |
| L1D | 28.41±1.08 |
| L1E | 24.03±0.81 |
| L2A | 26.48±1.23 |
| L2B | 24.41±1.54 |
| L2C | 28.52±1.36 |
| L2D | 34.71±1.34 |
| L2E | 29.76±0.87 |
| Positive control | |
| L-N6-(1-Iminoethyl)lysine | 1.00±0.01 |
F1: fresh fruits extracts, F2: dried fruits extracts; L1: fresh leaves extracts; L2: dried leaves extracts. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol extraction Soxhlet apparatus; E. Ethanol ultrasound extraction. Data are expressed as means±S.D. (n = 4).
Table 6
Carbohydrates-hydrolysing enzymes inhibitory activity of V. corymbosum extracts
| V. corymbosum | IC50, μg/mL | Selectivity Index (SI) | ||
| α-Amylase | α-Glucosidase | α-Amylase | α-Glucosidase | |
| Fruits | ||||
| F1A | 33.79% a | 189.81±4.15**** | – | – |
| F1B | 4.37% a | 298.42±3.56**** | – | – |
| F1C | 36.73% a | 666.29±7.64**** | – | – |
| F1D | 46.08% a | 195.84±2.65**** | – | – |
| F1E | 24.13% a | 139.88±2.31**** | – | – |
| F2A | 4% a | 188.06±4.56**** | – | – |
| F2B | 195.94±4.58**** | 373.74±5.62**** | 1.91 | 0.52 |
| F2C | 16.82% a | 146.89±1.24**** | – | – |
| F2D | NA | 139.88±1.47**** | – | – |
| F2E | 680.30±8.32**** | 76.57±0.85**** | 0.11 | 8.88 |
| Leaves | ||||
| L1A | 564.68±3.25**** | 230.10±4.56**** | 0.41 | 2.45 |
| L1B | 20.55±0.74**** | 215.21±3.68**** | 10.47 | 0.09 |
| L1C | NA | 195.94±1.47**** | – | – |
| L1D | 36.92% a | 134.63±1.57**** | – | – |
| L1E | NA | 341.34±3.62**** | – | – |
| L2A | 178.42±1.85**** | 247.62±2.51**** | 1.39 | 0.72 |
| L2B | 417.54±2.69**** | 262.51±2.78**** | 0.63 | 1.59 |
| L2C | NA | 8.80±0.06**** | – | – |
| L2D | 16.16±0.01**** | 259.00±5.69**** | 16.03 | 0.06 |
| L2E | 325.57±2.35**** | 176.67±8.45**** | 0.54 | 1.84 |
| Positive control | ||||
| Acarbose | 50.01±1.43 | 35.50±1.10 | 0.71 | 1.41 |
F1: Fresh fruits; F2: Dried fruits; L1: Fresh leaves; L2: Dried leaves. A. Ethanolic maceration; B. Hydroalcoholic maceration; C. Decoction; D. Ethanol Soxhlet extraction; E. Ethanol ultrasound-assisted extraction. a at a concentration of 1 mg/mL. Data are expressed as means±S.D. (n = 3). NA: not active. Differences within and between groups were evaluated by one-way ANOVA followed by a multicomparison Dunnett’s test (α= 0.05): ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.1 compared with the positive control. a Selectivity for α-amylase is defined as IC50 (α-glucosidase)/IC50 (α-amylase). b Selectivity for α-glucosidase is defined as IC50 (α-amylase)/IC50 (α-glucosidase).
Decoction of dried leaves (L2C) exhibited the highest α-glucosidase inhibitory activity with an IC50 value of 8.80μg/mL, 4.4 times higher than the positive control.
Interesting results were obtained also by ethanol ultrasound-assisted extraction of dried fruits (F2E) with an IC50 value of 76.57μg/mL. Interesting data were obtained by L2C, L1D and L1C. These extracts showed a high inhibition against α-glucosidase, but no activity against α-amylase.
Johnson et al. [55] have compared inhibition capacity against α-amylase and α-glucosidase of various cultivar of V. corymbosum fruits grown at the same location under the same environmental conditions cv commercial samples. Different cultivars showed high percentage of inhibition, for both α-amylase (91.79∼103.32%) and α-glucosidase (103.22∼193.61%), respect to commercial V. corymbosum sample (86.80 and 75.54% for α-amylase and α-glucosidase, respectively).
Cheplick et al. [56] investigated phenolic-linked bioactive functionality of V. corymbosum in type 2 diabetes management during fruit maturation. The mature fruit showed higher α-amylase, α-glucosidase inhibitory activity, and significant potential to improve glucose metabolism, compared to immature fruits. Previously, McDougall et al. [57] tested the inhibitory activity against α-amylase and α-glucosidase of polyphenols-rich extracts from different berries. Blackcurrant and blueberry extracts more readily inhibited α-glucosidase while raspberry and strawberry extracts were more effective α-amylase inhibitors. The inhibitory effects of various phenolic acids on α-amylase and α-glucosidase activities were studied [58]. Chlorogenic acid showed high inhibition against these enzymes with IC50 values of 9.10 and 9.24μg/mL, respectively for α-amylase and α-glucosidase.
In a clinical study, 45 g of V. corymbosum powder as smoothie were administered to 32 volunteers (adults, obese, and insulin resistant) for breakfast and dinner, for six weeks. At the end of this period, the participants showed improved insulin sensitivity. In conclusion, studies confirmed that V. corymbosum berries exhibited anti-diabetic properties and protection of pancreatic β-cells from glucose-induced oxidative stress [59]. Discordant results were reported by Ştefănescu et al. [34] that studied the effects of V. corymbosum dried leaves against diabetes. None positive effects were observed after administration of hydroalcoholic V. corymbosum leaves extracts in diabetic rats, compared with the control (non-diabetic rats). A great majority of studies have focused on the effect of V. myrtillus leaves and fruits extracts in diabetes [34, 60].
3.6Correlation between phytochemical content and bioactivity
Pearson’s correlation analyses were done to predict the relationship between the phytochemicals compounds found in extracts and bioactivities. Analysing extracts from fresh fruits, TPC showed a strong significant positive correlation (r value of 0.89) with FRAP test, while a moderate correlation was found between TFC and β-carotene bleaching test after 60 min of incubation (r = 0.69). Same moderate correlation (r = 0.68) was reported for the TIC and ABTS test.
Moreover, in dried fruits the radical scavenging activity against ABTS was positive correlated with TFC (r value of 0.79). A positive correlation was found between TFC of fresh fruits and TIC of dried fruits and anti-inflammatory activity with values of r = 0.71 and 0.81, respectively.
Analysing fresh leaves, positive correlations were found between TPC and DPPH and FRAP test with r values of 0.83 and 0.76, respectively. This implies the ability of TPC to act as reducing agents and hydrogen donors in neutralising free radical. On the other hand, antioxidant activity of dried leaves may be related to the TIC. In fact, very strong correlations between this phytochemicals class and ABTS, DPPH, and FRAP tests with r values of 0.91, 0.82 and 0.93, respectively, were found. The high correlation found in this study between flavonoids and iridoids and the investigated bioactivities allow us to suppose that the healthy properties of V. corymbosum may be related not only to the anthocyanins, the main phytochemical compounds present in Vaccinium genus, but also to other compounds, such as flavonoids and iridoids.
4Conclusion
In summary, a phytochemical screening by LC-ESI-Q-TOF-MS, four different in vitro antioxidant assays, α-amylase and α-glucosidase inhibitory activity tests, and the anti-inflammatory activity assay by means of the effects on the NO production were executed in order to compare and to correlate the chemical composition of berries and leaves of V. corymbosum with their biological activities. V. corymbosum extracts are characterised by the presence of flavonoids, phenolic acids, and iridoids as dominant classes of constituents. These phytochemical classes are associated with the bioactivities reported for the different extracts. To the best of our knowledge, this is the first study that revealed the presence of iridoids in V. corymbosum. A promising inhibition of the mediator of inflammation, NO, was found. Fruits extracted by decoction showed the highest activity in counteracting NO production.
Considering that V. corymbosum berries are edible, their consumption may be helpful for the treatment of inflammatory disorders. Extracts obtained by decoction of dried leaves and by hydroalcoholic fresh leaves showed a promising α-amylase inhibitory activity. These extraction procedures applied to V. corymbosum leaves have the potential to improve recovery of hypoglycaemic compounds. Overall, our results indicated that extracts of V. corymbosum devoid of anthocyanins and rich in flavonoids and iridoids might be considered a source of natural active agents.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgments and Funding
The authors would like to thank dr. N.G. Passalacqua of the Botany Department at the University of Calabria (Italy) for the botanical identification of the plant species. The Authors would like to thank Arul Marie of the Plateau Technique de Spectrométrie de Masse Bio-Organique for the access to the instrument (Laboratoire Molécules de Communication et Adaptation des Microorganismes (MCAM, UMR 7245)-Sorbonne Universités, Muséum National d’Histoire Naturelle, CNRS - Paris, France).
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JBR-200536.
References
[1] | Kloet VE . Manual of the flowering plants of Hawaii, Bishop Museum Special Publication. (1990) ;83: :591–5. |
[2] | Može Š , Polak T , Gašperlin L , Koron D , Vanzo A , Poklar Ulrih N , Abram V . Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.), J. Agric. Food Chem. (2011) ;59: :6998–7004. |
[3] | Tutin TG , Heywood VH , Burges NA , Valentine DH , Walters SM , Webb DA . Flora Europaea. (1972) ;3: :12–3. |
[4] | Hong SM , Soe KH , Lee TH , Kim IS , Lee YM , Lim BO . Cognitive improving effects by highbush blueberry (Vaccinium corymbosum L.) vinegar on scopolamine-induced amnesia mice model, J Agric Food Chem. (2018) ;66: :99–107. |
[5] | Pervin M , Hasnat MA , Lim JH , Lee YM , Kim EO , Um BH , Lim BO . Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators, J Nutr Biochem. (2016) ;28: :103–13. |
[6] | Thoo YY , Ho SK , Liang JY , Ho CW , Tan CP . Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia), Food Chem. (2010) ;120: :290–5. |
[7] | Jayaprakasha GK , Singh RP , Sakariah KK . Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro , Food Chem. (2001) ;73: :285–90. |
[8] | Yilmaz Y , Toledo RT . Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols, J Food Compos Anal. (2006) ;19: :41–8. |
[9] | Ignat I , Volf I , Popa VI . A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables, Food Chem. (2011) ;126: :1821–35. |
[10] | Lim YY , Murtijaya J . Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods, LWT Food Sci Technol. (2007) ;40: :1664–9. |
[11] | Náthia-Neves G , Tarone AG , Tosi MM , Maróstica Júnior MR , Meireles MAA . Extraction of bioactive compounds from genipap (Genipa americana L.) by pressurized ethanol: Iridoids, phenolic content and antioxidant activity. Food Res Int. (2017) ;102: :595–604. |
[12] | Mokrani A , Madani K . Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.), Fruit Sep Purif Technol. (2016) ;162: :68–76. |
[13] | Belwal T , Dhyani P , Bhatt ID , Rawal RS , Pande V . Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM), Food Chem. (2016) ;207: :115–24. |
[14] | Vuong QV , Golding JB , Stathopoulos CE , Nguyen MH , Roach PD . Optimizing conditions for the extraction of catechins from green tea using hot water, J Sep Sci. (2011) ;34: :3099–106. |
[15] | Rodriguez-Mateos A , Feliciano RP , Cifuentes-Gomez T , Jpe S . Bioavailability of wild blueberry (poly)phenols at different levels of intake, J Berry Res. (2016) ;6: :137–48. |
[16] | Ginwala R , Bhavsar R , Chigbu DGI , Jain P , Khan ZK . Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin, Antioxidants. (2019) ;8: :35–63. |
[17] | Gao X , Ohlander M , Jeppsson N , Björk L , Trajkovski V . Changes in antioxidant effects and their relationship to (Hippophae rhamnoides L.) during maturation, J Agric Food Chem. (2000) ;48: :1485–90. |
[18] | Yoo KM , Lee CH , Lee H , Moon BK , Lee CY . Relative antioxidant and cytoprotective activities of common herbs, Food Chem. (2008) ;106: :929–36. |
[19] | Malfa GA , Tomasello B , Sinatra F , Villaggio G , Amenta F , Avola R , Renis M . “Reactive” response evaluation of primary human astrocytes after methylmercury exposure, J Neurosci Res. (2014) ;92: :95–103. |
[20] | Mariotto S , Esposito E , Di Paola R , Ciampa A , Mazzon E , de Prati AC , Darra E , Vincenzi S , Cucinotta G , Caminiti R , Suzuki H , Cuzzocrea S . Protective effect of Arbutus unedo aqueous extract in carrageenan-induced lung inflammation in mice, Pharmacol Res. (2014) ;57: :110–24. |
[21] | Loizzo MR , Abouali M , Salehi P , Sonboli A , Kanani M , Menichini F , Tundis R . In vitro antioxidant and antiproliferative activities of nine Salvia species, Nat Prod Res. (2014) ;28: :2278–85. |
[22] | Loizzo MR , Sicari V , Pellicanò T , Xiao J , Poiana M , Tundis R . Comparative analysis of chemical composition, antioxidant and anti-proliferative activities of Italian Vitis vinifera by-products for a sustainable agro-industry, Food Chem Toxicol. (2019) ;127: :127–34. |
[23] | Loizzo MR , Tundis R , Chandrika UG , Abeysekera AM , Menichini F , Frega NG . Antioxidant and antibacterial activities on foodborne pathogens of Artocarpus heterophyllus Lam. (Moraceae) leaves extracts, J Food Sci. (2010) ;75: :291–5. |
[24] | Tundis R , Bonesi M , Sicari V , Pellicanò TM , Tenuta MC , Leporini M , Menichini F , Loizzo MR . Poncirus trifoliata (L.) Raf.: Chemical composition, antioxidant properties and hypoglycaemic activity via the inhibition of α-amylase and α-glucosidase enzymes, J Funct Foods. (2016) ;25: :477–85. |
[25] | Ehlenfeldt MK , Camp MJ , Wang SY . α-Glucosidase inhibitory activity and antioxidant capacity in the peel and pulp of mixed species blueberry hybrids, Plant Gen Res. (2015) ;13: :190–4. |
[26] | Ehlenfeldt MK , Prior RL . Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry, J Agric Food Chem. (2001) ;49: :2222–7. |
[27] | Bhattachartya S , Christensen KB , Olsen LC , Christensen LP , Grevsen K , Faergeneman NJ , Kristiansen K , Young JF , Oksbejerq N . Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans, J Agric Food Chem. (2013) ;61: :11033–40. |
[28] | Chan EWC , Lim YY , Ling SK , Tan SP , Lim KK , Khoo MGH . Caffeoylquinic acids from leaves of Etlingera species (Zingiberaceae), LWT- Food Sci Technol. (2009) ;42: :1026–30. |
[29] | Clifford MN , Wu W , Kirkpatrick J , Rakesh Jaiswal R , Kuhnert N . Profiling and characterisation by liquid chromatography/multi-stage mass spectrometry of the chlorogenic acids in Gardeniae Fructus, Rapid Commun. Mass Spectrom. (2010) ;24: :3109–20. |
[30] | Friedrich H , Schönert J . Phytochemical investigation of leaves and fruits of Vaccinium myrtillus, Planta Med. (1973) ;24: :90–100. |
[31] | Karikas GA , Euerby MR , Waigh RD . Constituents of the stems of Arbutus unedo , Planta Med. (1987) ;53: :223–4. |
[32] | Reynertson KA , Wallace AM , Adachi S , Gil RR , Yang H , Basile MJ , D’Armiento J , Weinstein IB , Kennelly EJ . Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora), J Nat Prod. (2006) ;69: :1228–1230. |
[33] | Sakakibara J , Koto T , Yasue M . Studies on the constituents of Vaccinium bracteatum Thunb. II. On the constituents of the flowers, particularly on the structure of vaccinoside, a new iridoids glycoside. Yakugaku Zasshi. (1973) ;93: :164–70. |
[34] | Ştefănescu BE , Szabo K , Mocan A , Crişan G . Phenolic compounds from five Ericaceae species leaves and their related bioavailability and health benefits, Molecules. (2019) ;24: :2046. |
[35] | Su Z . Anthocyanins and flavonoids of Vaccinium L, Pharm. Crops. (2012) ;3: :7–37. |
[36] | Wald B , Wray V , Galensa R , Herrmann K . Malonated flavonol glycosides and 3,5-dicaffeoylquinic acid from pears, Phytochemistry. (1989) ;28: :663–4. |
[37] | Zadernowski R , Naczk M , Nesterowicz J . Phenolic acid profiles in some small Berries, J Agric Food Chem. (2005) ;53: :2118–24. |
[38] | Zhao P , Tanaka T , Hirabayashi K , Zhang YJ , Yang CR , Kouno I . Caffeoyl arbutin and related compounds from the buds of Vaccinium dunalianum , Phytochemistry. (2008) ;69: :3087–94. |
[39] | He XJ , Liu RH . Cranberry phytochemicals: isolation, structure elucidation, and their antiproliferative and antioxidative activities, J Agric Food Chem. (2006) ;54: :7069–74. |
[40] | Olennikov DN , Tankhaeva LM . Phenolic compounds from Rhododendron dauricum from the Baikal region, Chem Nat Compd. (2010) ;46: :471–3. |
[41] | Tenuta MC , Tundis R , Xiao J , Loizzo MR , Dugay A , Deguin B . Arbutus species (Ericaceae) as source of valuable bioactive products, Crit Rev Food Sci Nutr. (2019) ;59: :864–81. |
[42] | Yan X , Murphy BT , Hammond GB , Vinson JA , Neto CC . Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon), J Agric Food Chem. (2002) ;50: :5844–9. |
[43] | Blumberg JB , Camesano TA , Cassidy A , Kris-Etherton P , Howell A , Manach C , Ostertag LM , Sies H , Skulas-Ray A , Vita JA . Cranberries and their bioactive constituents in human health, Adv Nutr. (2013) ;4: :618–32. |
[44] | Brighenti F , Valtuena S , Pellegrini N , Ardigo D , Del Rio D , Salvatore S , Piatti P , Serafini M , Zavaroni I . Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects, Br J Nutr. (2005) ;93: :619–25. |
[45] | Heffels P , Müller L , Schieber A , Weber F . Profiling of iridoid glycosides in Vaccinium species by UHPLC-MS. Food Res Int. (2017) ;100: :462–8. |
[46] | Norris GH , Blesso CN . Dietary and endogenous sphingolipid metabolism in chronic inflammation, Nutrients. (2017) ;2017: :E1180. |
[47] | Bonesi M , Loizzo MR , Acquaviva R , Malfa GA , Aiello F , Tundis R . Anti-inflammatory and antioxidant gents from Salvia genus (Lamiaceae): an assessment of the current state of knowledge, Antiinflamm. Antiallergy Agents Med Chem. (2017) ;16: :70–86. |
[48] | Floegel A , Kim DO , Chung SJ , Koo SI , Chun OK . Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods, J Food Compos Anal. (2011) ;24: :1043–8. |
[49] | Puchau B , Zulet MA , Gonzalez de Echavarri A , Hermsdorff HH , Martinez JA . Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults, Nutrition. (2009) ;26: :534–41. |
[50] | Huang D , Ou B , Prior RL . The chemistry behind antioxidant capacity assays, J Agric Food Chem. (2005) ;53: :1841–56. |
[51] | Pervin M , Hasnat MA , Lim BO . Antibacterial and antioxidant activities of Vaccinium corymbosum L. leaf extract, As Pac J Trop Dis. (2013) ;3: :444–53. |
[52] | Namiesnik J , Vearasilp K , Nemirovski A , Leontowicz H , Leontowicz M , Pasko P , Martinez-Ayala AL , Gonzalez Aguilar GA , Suhaj M , Gorinstein S . In vitro studies on the relationship between the antioxidant activities of some berry extracts and their binding properties to serum albumin, Appl Biochem Biotechnol. (2014) ;172: :2849–65. |
[53] | Podsedek A , Majewska I , Malgorzata R , Sosnowska D , Koziolkiewicz M . In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits, J Agric Food Chem. (2014) ;62: :4610–7. |
[54] | Rodrigues E , Poerner N , Rockenbach II , Gonzaga LV , Mendes CR , Fett R . Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil, Ciencia e Tecnologia de Alim. (2011) ;31: :911–7. |
[55] | Johnson MH , Lucius A , Meyer T , Gonzalez de Mejia E . Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-amylase and α-glucosidase by highbush blueberry (Vaccinium corymbosum), J Agric Food Chem. (2011) ;59: :8923–30. |
[56] | Cheplick S , Sarkar D , Bhowmik P , Shetty K . Phenolic bioactives from developmental stages of highbush blueberry (Vaccinium corymbosum) for hyperglycemia management using in vitro models, Can J Plant Sci. (2015) ;95: :653–62. |
[57] | McDougall GJ , Shpiro F , Dobson P , Blake A , Stewart D . Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase, J Agric Food Chem. (2005) ;53: :2760–6. |
[58] | Oboh G , Agunloye OM , Adefegha SA , Akinyemi AJ , Ademiluyi AO . Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): a comparative study, J Basic Clin Physiol Pharmacol. (2015) ;26: :165–70. |
[59] | Karcheva-Bahchevanska DP , Lukova PK , Nikolova MM , Mladenov RD , Iliev IN . Effect of extracts of bilberries (Vaccinium myrtillus L.) on amyloglucosidase and α-glucosidase activity, Folia Medica. (2017) ;59: :197–202. |
[60] | Bljajić K , Petlevski R , Vujić L , Čačić A , Šoštarić N , Jablan J , Saraiva de Carvalho I , Zovko Končić M . Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves, Molecules. (2017) ;22: :1–14. |




