Anticancer and anti-inflammatory perspectives of Pakistan’s indigenous berry Grewia asiatica Linn (Phalsa)
Abstract
BACKGROUND:
Berries indigenously grown in Asia are known for their diversified nutritional and health promoting properties. Establishing a link between berry consumption and their classical uses in health management however requires detailed research in exploring varied biochemical factors and their therapeutic role in averting risks of chronic disorders.
OBJECTIVE:
The present study was aimed at evaluating anti-inflammatory and anticancer responses of fruit extracts of Grewia asiatica locally known as Phalsa.
METHODS:
Dichloromethane, methanol and 50% hydro-methanolic fractions of fruit were evaluated for polyphenols characterization, quantification and antioxidant assays. Anti-inflammatory and anti-nociceptive responses of fruit extracts were evaluated in rats and mice models, respectively, and cytotoxic activities were measured against MCF-7, HeLa, HEp-2, NCI-H522, HEK-293 cancer cell cultures.
RESULTS:
Phenolics quantification and biological study data suggested 50% hydro-methanolic extracts as maximum carrier of flavonoids (7.92 mgQE/g), anthocyanins (8.1 mg/Kg) and tannins (187.2 mgGAE/g) that significantly (p < 0.05)resulted in higher oxidation inhibition (IC50 41.1 ug/ml), paw edema inhibition (68–74%) and pain mediation in neurogenic phase(31–62%) when administrated at the rate of 400 mg/kg b.w. Maximum cytotoxic activity of G. asiatica (50% hydro-methanolic extracts) was observed against MCF-7 (IC50 34.9 ug/mL), HEp-2 (IC50 80.4 ug/mL) and NCI-H522 (IC50 73 ug/mL) cancer cell lines. LC-ESI-MS/MS characterization of hydro-alcoholic fractions bearing potent biological activities revealed Gallic acid, Ellagic acid, Quinic acid, Calycosin, Vidalenolone, Quercetin, Myricetin, Liquitrigenin and 6-aldehydo-isoophiopogonone. Human equivalent doses of the extracts calculated on the basis of total phenolic contents for anti-inflammatory and nociceptive assays were in range between 6.2–15.8 mg/kg b.w., and 3.1–7.9 mg/kg b.w., respectively.
CONCLUSIONS:
Findings of the study suggest G. asiatica fruit extracts are a potential source of bioactive compounds that might further be explored for anti-inflammatory and anticancer drug discovery and its clinical exploitation. Study concludes supplementation of G. asiatica extracts as possible approach to acquire curative properties in human subjects.
1Introduction
Ever increasing number of deaths associated with cardiovascular diseases, diabetes, cancer, inflammations and many other have gained attention of health experts, researchers and policy makers toward creating strict dietary behavioral changes and promoting healthy eating practices. Plant centric foods, especially fruits and vegetables, have shown a positive effect in averting the risks of chronic health ailments on account of their ability to deliver a wide range of phytochemicals which exhibit anti-oxidant, anti-cancer, and anti-inflammatory activities [1–3].
Excessive generation of free radicals leads toward oxidative stress, protein, and DNA damage. Most fruits are naturally composed of valuable phytochemicals and metabolites possessing the ability for scavenging free radicals in a living system thus showing promising health-promoting properties [2–5]. Berry fruits are rich reservoirs of polyphenols, flavonoids and anthocyanins, and are gaining popularity for the enhanced anti-inflammatory and anticancer effects. Other promising therapeutic features of berry fruits are associated with their potential for being antidiabetic, antipyretic, and anti-aging agents linked to their unique biomolecular composition [6, 7].
Grewia genus (Tiliacae) contains almost 150 species of small to medium shrubs native to the Himalayan region grown in tropical and sub-tropical areas of the world with G. asiatica being the only specie producing edible fruits. This fruit has been widely grown in South Asian countries. Almost 10 species are found in Pakistan, which are primarily grown in Punjab province [8, 9].
G. asiatica, locally known as Phalsa, is traditionally known for its elevated therapeutic and nutritional value. Traditionally consumed during summer months, the juice of G. asiatica is considered to be a therapeutic agent in diabetes, and associated micro and macrovascular comorbidities, i.e., diabetic nephropathy and risk of coronary heart diseases [2, 10]. The phytochemistry of G. asiatica revealed the presence of primary metabolites such as glycosides, alkaloids, essential amino acids, saponins, mucilage, steroids and fixed seed oil [11] while a range of secondary metabolites like naringenin, pelargonidin, cyanidin, quercetin, kaempferol, myricetin, hydroxybenzoic acid and hydroxymethylfurfural were reported from this fruit [2, 12].
The complex phytochemical profile of G. asiatica warrants its exploration to identify health promoting feature of the fruit and to ascertain their potential role for disease prevention. The present research study is an attempt to explore phytochemical profile, antioxidant, anti-inflammatory and anticancer potential of a locally grown cultivar of G. asiatica.
2Material and methods
2.1Plant material
G. asiatica was collected from the district Multan, Punjab, Pakistan in March, 2017 and was verified for varietal identification by qualified pomologists. Berries were processed to yield whole fruit juice and seeds were removed by straining. The juice extracts of the fruit were obtained by successive fractionation with solvents including hexane, dichloromethane, methanol and 50% methanol. Solvents extracts were concentrated under reduced pressure (800 millibar) in a rotary evaporator (Heidolph, Hei-Vap, Germany). The recovery rates of fruit extracts derived by successive extraction process were 0.45% and 1.28% for 100% methanol and 50% methanol, respectively. Extracts recovered were stored at –70 C in ultralow freezer (Sanyo, MDF-U32 V, Japan) for further use.
2.2Phytochemical screening
The qualitative screening of G. asiatica fruit was performed for the presence of secondary metabolites including flavonoids, saponins, tannins, glycosides and alkaloids using standard methods of analysis [13].
2.3Solvents and reagents
Solvents (n-hexane, dichloromethane, methanol, and dimethylsulphoxide), reference/standards (ascorbic acid, gallic acid, tannic acid, quercetin, cyanidin-3-glycosides, indomethacin, and methotrexate), and inflammatory mediators (Carrageenan, PEG2, glutamate, formalin) were purchased from Sigma-Aldrich, USA. MTT3was purchased from BiosesangInc., Korea. Folin-Ciocalteu, Folin-Denis reagent and 2,4,6-Tripyridyl-s-triazine (TPTZ) from Sigma Aldrich, USA. The chemicals, reagents and solvents used in this study were of analytical grade unless otherwise stated.
2.4Experimental animal study
The animal use protocol was approved by the Bioethical Committee at Bahauddin Zakariya University Multan in 2018 under the protocol number 05-18 titled “Exploring in vivo anti-inflammatory properties of indigenous berries of Pakistan”. Albino mice (n – 120) and Wistar rats (n – 80), weighing from 25–30 g and 200– 300 g, respectively were purchased from the Laboratory Animal Rearing Facility at Department of Pharmacy, Bahauddin Zakariya University Multan. The animals were housed two per cage and provided with easy access to water and feed in a controlled environment (25°C ± 4°C, 12/12 light dark cycles) at laboratory animal rearing facility of Institute of Food Science & Nutrition. Hygiene of the cages was rigorously monitored and maintained daily. All experimental procedures were carried out in accordance with the guidelines for the care and use of laboratory animals issued from the Institute for Laboratory Animal Research.
2.5Cancer cell cultures
Cancer cell lines obtained from Husain Ebrahim Jamal Research Institute of Chemistry, (HEJ) Karachi. Dulbecco’s Modified Eagle’s Medium and fetal bovine serum (FBS) was purchased from Bio Whittaker and Gibco BRL Life Technologies, USA.
2.6Quantification of total phenolics, flavonoids, anthocyanins and tannins contents of juice extracts
Total phenolic contents were determined by Folin-Ciocalteu (FC) colorimetric method [14] using gallic acid as standard. Absorbance was recorded at 765 nm and values were recorded in triplicate using ethanol as a blank. Total flavonoid contents were determined using AlCl3 assay [15]. Samples absorbance was read at 510 nm using spectrophotometer (UV-Vis 3000, ORI, Germany). Quercetin standard curve was plotted and samples results were expressed as mg quercetin equivalents per gram (mg QE/g) of the dried weight. Total anthocyanins contents were calculated by pH differential method [16] with little modifications. Total tannin contents were determined using tannic acid as standard [17]. Absorbance of test samples was measured at 725 nm and a standard curve was plotted to calculate concentration of tannins and the results were expressed as mg tannic acid equivalent/g of the dried extracts
2.7Determination of antioxidant activity
2.7.1DPPH free radical scavenging assay
The DPPH free radical scavenging activity of G.asiatica fruit was carried out as previously described [18]. Briefly, 1 mL of the test sample was added to 3 mL of DPPH solution (0.004%). Sample reagent mixtures were incubated in a dark chamber at 25°C for a period of 30 min and absorbance was spectrophotometrically recorded at 517 nm (UV-Vis 3000, ORI, Germany). Ascorbic acid was used as positive control. DPPH radical scavenging activity of the juice extracts was calculated using following equation and the results were expressed as IC50.
2.7.2Ferric reducing antioxidant power (FRAP)
Ferric reducing antioxidant power of G. asiatica was determined as previously described [19] using ferrous sulphate as standard. The test sample (100 μL) was added to FRAP working solution and the reaction mixture incubated for 10 min at 37°C. The absorbance was read at 593 nm (UV-Vis 3000, ORI, Germany) and results reported as mmol/g.
2.7.3Hydrogen peroxide (H2O2) scavenging activity
H2O2 scavenging activity of G.asiatica fruit was determined by the method of Ruch et al. [20]. Sample extracts were added to 0.6 mL H2O2 solution (40 mM) and incubated for 10 minutes. Samples absorbance was read (230 nm) spectrophotometrically (UV-Vis 3000, ORI, Germany) against a phosphate buffer blank. Hydrogen peroxide scavenging activities of G. asiatica extracts were calculated as follow.
Where Ao was the absorbance of the control and As was the sample absorbance.
2.8Anti-inflammatory activity
2.8.1Carrageenan, formaldehyde and PGE2-induced paw edema assessment
Carrageenan-induced anti-inflammatory activity of G. asiatica fruits was determined as described by Morris [21]. Wister rats with normal paw were divided into 8 groups, namely normal control (received water), indomethacin (100 mg/kg) and dichloromethane, methanol, 50% methanol extracts-treated groups. Each extract wasadministered to the Wistar rats at the levels of 200 and 400 mg/kg b.w., respectively. Thirty minutes post-treatment, 0.1 mL carrageenan, prepared in 0.9% normal saline, was intraperitoneally adminstrated in the right hind paw of each animal. One hundred microlitre of formalin solution (4%) was injected into the right hind paw of each rat in formaldehyde induced edema assessment [22]. PGE2 induced anti-inflammatory response of G. asiatica extracts was determined by PGE2 solution (0.001 mg/mL as phlogistic agent) administration to the right hind paw of rats [23]. Linear paw circumference was noted by using plethysmometer at 0, 1, 2 and 3 hours of carrageenan administration. However linear paw circumference measurement was performed at 0, 3, 6, 12, 24 hours post formalin administration and at 0, 30, 60, 120 mins of post PEG2 administration in formaldehyde and PGE2– induced edema assessment, respectively. The increase in paw circumference was used as measurement of inflammation.
2.8.2Formalin and glutamate-induced pain behavior
Anti-nociceptive response of G. asiatica fruits extracts was determined by formalin induced paw licking response among Wistar rats [24]. Two hundred and fifty microlitre formalin (2.5%) was injected into the right hind paw of each mice treated with normal saline (control), Indomethacin (positive control), dichloromethane extracts (200 and 400 mg/kg b.w.), methanol extracts (200 and 400 mg/kg b.w.), 50% methanol extracts (200 and 400 mg/kg b.w.). Twenty microlitre glutamate (10 μmol/paw) solution was injected into plantar aponeurosis surface of the right hind paw of each mice in glutamate-induced nociceptive response assessment [25]. Licking responses of treated animals was observed at early neurogenic pain phase for 0–5 minutes while anti-inflammatory pain stage was determined at 20–25 post formalin administration period.
Body surface area (BSA) normalization method was used to translate tested doses of extracts from animal to human [26]. Values for conversion of animal doses to human equivalent doses based on BSA were derived from Food and Drug Administration draft guidelines data [27].
2.9Anticancer activity
2.9.1Methyl thiazolyl tetrazolium (MTT) assay
Cytotoxic response of G. asiatica extracts was performed as suggested by Roy et al. [28]. Test samples was prepared in 1% dimethylsulphoxide (DMSO) and diluted to final concentration between 0.5–200 μg/mL in microtiter plate wells. Incubation of microtiter plate was performed at 37°C for 48 hrs and 50 μL of the MTT solution (5 mg/mL) was added to each well. Second incubation was performed for under dark at 37°C for 4 hrs. Absorbance was measured at 570 nm in a microplate reader (Tecan Austria GmbH) to elucidate reduction in MTT. The effect of the test compound on cell viability was calculated using untreated cells as control. Inhibition activity of the novel compounds (%) against the tested cell cultures was calculated using formula given here under:-
Where At, Ab and Ac represents absorbance of test, blank and control
2.10LC-ESI-MS/MS analysis
The successive fractions, exhibiting significantly higher antioxidant, anti-inflammatory and anticancer activities were further explored for bioactive compounds identification using LC MS/MS (Thermo Electron Corporation, USA) [29]. The direct injection mode with Electron Spray Ionization was adopted for detection purpose, at positive mode. The capillary temperature, sample flow rate, and mass range were maintained at 280°C, 8 μL/min and 50 to 1000 m/z, respectively. The collision induced energy during MS/MS mainly depending upon nature of parent molecular ion was kept in between 10 to 45. Every compound was optimized for MS parameters in order to obtain better ionization, ion transfer and to ensure optimum signal of parent and daughter fragments by analytes infusion and manually operating the parameters. The source parameters were identical for all of the analytes. Analysis of ESI-MS/MS acquired data was performed using manual, Xcalibur (Xcalibur 3.0). Structural elucidation was done using ChemDraw (ChemDraw Ultra 8.0) and then compared with previously published data.
2.11Statistical analysis
Data derived from this study are expressed as mean±Standard Error of Means (SEM) for three measurements. Statistical differences between the control and treatments were analyzed by analysis of variance (ANOVA), Dunnett’s test using graph pad prism and p < 0.05 was considered significant (*p < 0.05, **p < 0.01, ***p < 0.001).
3Results and discussions
3.1Phytochemical constituents of G. asiatica extracts
Maximum total phenolic contents were recorded in dichloromethane extracts of G. asiatica i.e., 243.33 mgGAE /g followed by methanol and 50% hydromethanolic extracts i.e., 189.9 and 177.2 mgGAE/g, respectively (Table 1). Identical concentration of phenolic contents was reported in an earlier study by Asghar et al. [30] wherein aqueous and 60% methanolic extracts of G. asiatica pulp exhibited 205 mg GAE/g and 126 mg GAE/g total phenolics contents, respectively. Variable concentrations of total flavonoids, ranging between 1.61–7.92 mg QE/g, was found in different extracts of G. asiatica. Hydro-alcoholic extracts were observed with highest flavonoids contents followed by methanolic (4.81 mgQE/g). Contrary to their high phenolics content, comparatively low levels of tannins among different extracts were observed in dichloromethane extracts i.e., 1.61 mgQE/g (Table 1). A similar trend was recorded for anthocyanin concentrations among different extracts wherein 50% hydro-methanolic extracts were found to carry 8.1 mg/kg anthocyanin content whereas dichloromethane extracts recovered 0.98 mg/kg total anthocyanins from G. asiatica fruit. Flavonoids constitute around 60% of the polyphenols and their high degree of hydroxylation promotes rapid degradation [31]. Extraction and stability of biomolecules like flavonoids from berries increase with organic solvent-water mixtures and at lower extraction temperature, pH [32]. Hydro-methanolic extracts (50%) offered high affinity to flavonoids including anthocyanins thus yielding better recovery of bioactive compounds than reported earlier. Higher affinity of total tannins contents was also observed for solvent polarity and maximum levels of tannins were observed in 50% hydro-methanolic extracts (187.22 mgGAE/g) while the least concentration was recorded in dichloromethane extracts (42.33 mgGAE/g) (Table 1). G. asiatica has been cited as a good source of phenolic acids, flavonoids and a small amount of tannins. However, more promising results have been delivered from the present study compared with earlier studies where G. asiatica methanolic extracts were found to deliver 144 mg GAE/g total phenolics, 4.61 mg QE/g total flavonoids and 4.9 mg/kg anthocyanins [33, 34].
Table 1
Phytochemical composition, antioxidant, and free radical scavenging activities of DCM, MeOH and 50% MeOH extracts of G. asiatica
| Parameter | DCM extracts | MeOH extracts | 50% MeOH extracts |
| Total phenolic contents (mg GAE/g) | 177±0.2 | 190±0.4 | 243±0.2 |
| Total flavonoid contents (mg QE/g) | 1.61±0.05 | 4.81±0.4 | 7.9±0.7 |
| Total anthocyanins contents (mg/kg) | 0.98±0.04 | 4.12±0.1 | 8.1±0.9 |
| Tannin contents (mg GAE/g) | 42±0.6 | 64±0.3 | 187±0.9 |
| FRAP (mmol/g) | 43±0.6 | 27±0.7 | 14±0.2 |
| DPPH (IC50 μg/mL) | 153±2.3 | 77±1.1 | 41±1.0 |
| H2O2 (%) | 33±1.44 | 43±0.4 | 73±0.6 |
Values are Means±S. D. DCM extracts = 100% dichloromethane extracts. MeOH = 100% methanol extracts. 50% MeOH = Methanol:water (50 : 50v/v).
3.2Antioxidant activity of G. asiatica extracts
Berries are potential source of bioactive compounds including phenolics, flavonoids, anthocyanins, hydrolysable tannins and B vitamins that strongly influence free radical scavenging activities [35]. Phytochemical screening of G. asiatica fruit confirmed the presence of phytoconstitutents including tannins and flavanoids that significantly (p < 0.005) attributed antioxidant, anti-inflammatory and anticancer responses to alcoholic and hydro-alcoholic extracts. Free radical scavenging assay (DPPH), Scavenging of hydrogen peroxide (H2O2) and ferric reducing antioxidant power (FRAP) are considered as reliable techniques to evaluate total antioxidant activity [36]. Antioxidant activity assessment of G. asiatica fruit extracts revealed hydro-methanolic extracts to be more promising in delivering free radical scavenging activity (Table 1). Minimum IC50 value was recorded for hydro-methanolic extracts in DPPH assay while 73% radical scavenging activity was observed in H2O2 assay. Dichloromethane extracts exhibited maximum ferric reducing antioxidant power (FRAP) i.e., 43 mmol/g contrary to methanol and hydro-methanolic extracts where FRAP value was observed as 27 mmol/g and 14.2 mmol/g, respectively. Earlier, 50% methanolic extracts of G. asiatica leaves were reported with relatively higher IC50 value i.e., 56.4 μg/mL [37]. Increased antioxidant activities of G. asiatica hydro-methanolic extracts seen in this study are in agreement to the previous findings wherein combinations of solvents were reported to increase recovery of a range of phenolic compound that attribute synergistic effect in scavenging free radicals or inhibiting their production [38].
3.3Anti-oedematous activity of G. asiatica extracts
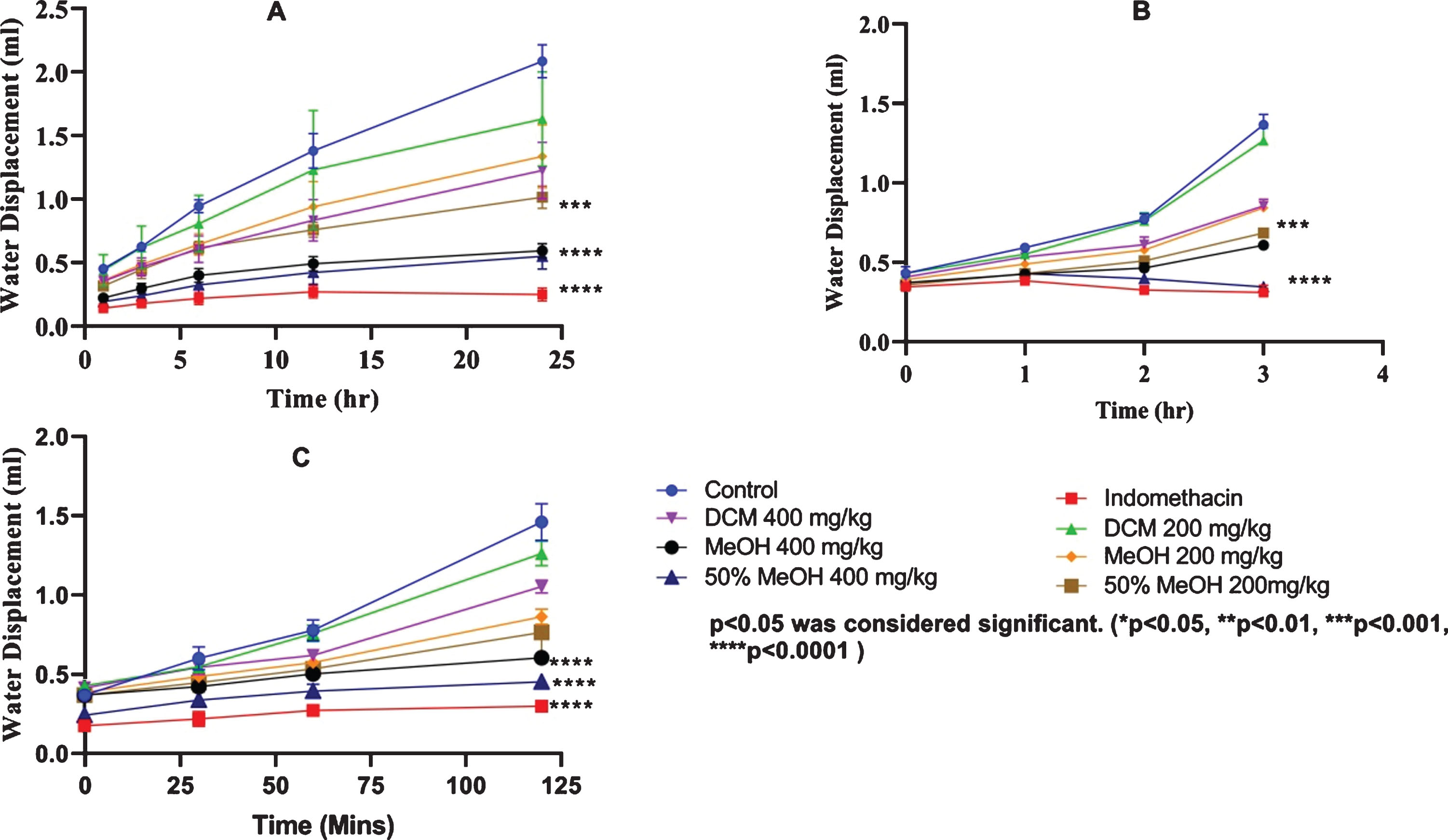
Methanolic and 50% hydro-methanolic fractions dispensed at the rate of 400 mg/kg b.w., generated significant inhibition of formaldehyde-induced paw edema with maximum inhibitory effect of G. asiatica fruit extracts recorded for hydro-methanolic extracts i.e., (73.6%, p < 0.0001) and methanolic extracts (71.5%, p < 0.0001). Comparatively higher paw edema inhibitory effect was noticed in rats administrated with indomethacin i.e., (87.9%, p < 0.0001) (Fig. 1). Ameliorative effect of hydro-methanolic extracts administration (400 mg/kg b.w.,) was also observed in carrageenan induced paw edema wherein fruit extracts presented comparable inhibitory response to indomethacin i.e., 68% (p < 0.0001) and 71% (p < 0.0001), respectively within 3 hrs of extract/drug administration (Fig. 1). Hydro-methanolic extracts (50%) also offered 68.7% (p < 0.0001) inhibition in PEG2 induced paw edema in comparison with control in 120 min of extracts administration while indomethacin presented relatively higher rate of inhibition to PGE2 induced paw edema i.e., 79% (p < 0.0001) (Fig. 1). Anti-inflammatory and analgesic activity of G. asiatica root bark extracts were rightly attributed to their phytochemicals including phenolics and flavonoids by [39]. The study further argued methanolic extracts of G. asiatica root bark to induce 59% inhibition in paw edema at equal concentration as has been reported in this study. However, as shown in Fig. (1), G. asiatica hydro-methanolic fruit extracts were found more effective in reducing inflammation.
Fig.1
Dose-dependent anti-inflammatory effect of G.asiatica Linn in different models of inflammation and noceiption. Label:A. Formaldehyde induced rat’s hind paw edema B. Carrageenan induced rat’s hind paw edema, C. PEG2 induced rat’s hind paw edema.
3.4Anti-nociceptive and anti-inflammatory activity of G. asiatica extracts
G. asiatica fruit extracts derived from dichloromethane, methanolic and hydro-methanolic extracts were exposed to formalin-induced paw licking in mice. Administration of methanolic and 50% hydro-methanolic extracts (400 mg/kg) exhibited significant inhibition of pain in both neurogenic phase (p < 0.0001) and anti-inflammatory phase (p < 0.0001) in comparison with the control (Fig. 2). Methanolic and 50% hydro-methanolic extracts of G. asiatica protected study animals from painful stimulation of formalin in dose dependent manner with a maximum effect being 62.9% and 62.6% for methanolic and hydromethanolic extracts, respectively at 400 mg/kg b.w.(Fig. 2). Similar responses of G. asiatica fruit methanolic and hydro-methanolic extracts were noticed in glutamate-induced nociceptive response in a mice model and significant (p < 0.0001) anti-nociceptive effect of G. asiatica was observed in comparison with control and standard drug (Fig. 2). Methanolic extracts of G. asiatica have been reported to show antipyretic and anti-inflammatory activity in dose dependent manner (125–500 mg/kg b.w.) citing 51–62% inhibition of writhing and 28 –36% inhibition of paw edema post 3 hrs acetic acid and carrageenan administration, respectively [40]. Carrageenan-induced paw edema model is considerd as a standard method to explore anti-inflammatory activity of test samples because it is characterized by the release of prostaglandins (PGs), bradykinins, serotonin, histamine and substance P [21, 41]. Significant response of G. asiatica fruit extracts administration to rats in reducing paw edema can be attributed to a marked decline in production of inflammatory markers thus validating G. asiatica fruit extracts’s ability to modulate stress-induced inflammatory responses. The G. asiatica hydro-methanolic extracts significantly suppressed vasodilation, vascular permeability and other inflammatory phases by inhibiting the release of inflammatory mediators and possibly COX-2 enzyme expression in the paw tissues. Human equivalent doses (HED) calculated on the basis of phenolic contents of G. asiatica extracts are presented in Table 4. The results suggest anti-inflammatory and anti-nociceptive responses of G. asiatica fruit extracts in human subjects may be achieved by delivering berries phenolics in range 6.2 – 15.8 mg/kg b.w., and 3.1–7.9 mg/kg b.w., respectively. Considering varying the phenolics extraction efficiency of solvents deployed in this study as mentioned in section 2.1 and Table 1, 3–6 servings of seedless fraction of G. asiatica fruit (serving size 100–150 g) may deliver 433–945 mg total phenolics to satisfy HED for anti-inflammatory and anti-nociceptive responses in a 60 kg individual. Absorption rates of G. asiatica phenolics are not completely known. Theoretically, the referred amount of fruit can anticipate suggested results by considering the highest rate of polyphenols intestinal absorption. Else otherwise, the desired concentration of phenolics could effectively be delivered by oral supplementation at the rate of 2–4 g/day.
Fig.2
Dose dependent anti-nociceptive response of G. asiatica fruit extracts. Label:A. Formalin induce mice’s hind paw licking B. Glutamate induce mice’s hind paw licking.
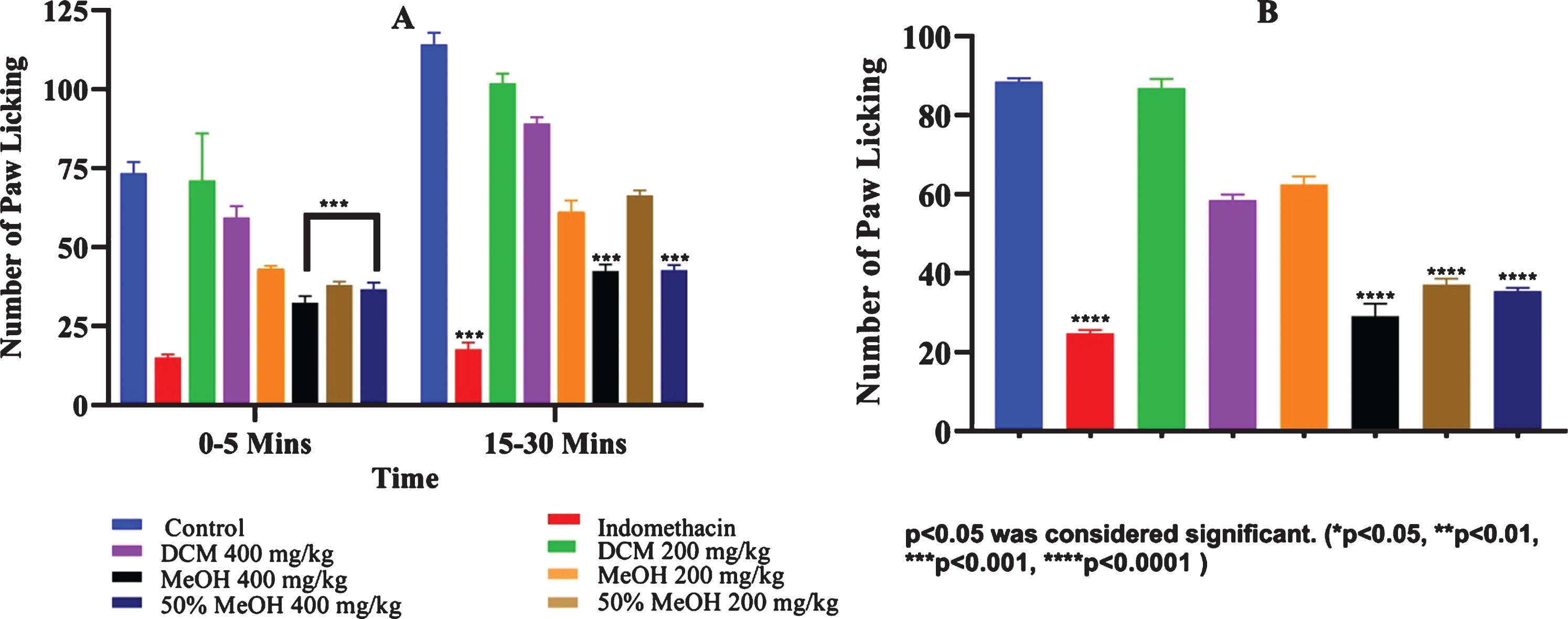
Table 2
Profiling of bioactive components identified by LC-MS/MS analysis among 50% hydro-methanolic fraction of G.asiatica fruit
| Rt (min) | Molecular weight | ESI-MSn (Ions) | Chemical formula | Identification | References |
| 2.64 | 273 | 255, 241, 225, 213, 205. 195, 183 | C15H1005 | Genisteun-d4 | [60] |
| 6.42 | 431 | 430.2, 371, 341, 311, 283.33, 268.92 | C21H20O10 | Vitexin | [59] |
| 9.81 | 169 | 169, 127, 125 | C7H608 | Gallic acid | [61] |
| 10.16 | 291 | 273.17, 165.08, 141.25 | C15H1406 | Epicatechin | [55] |
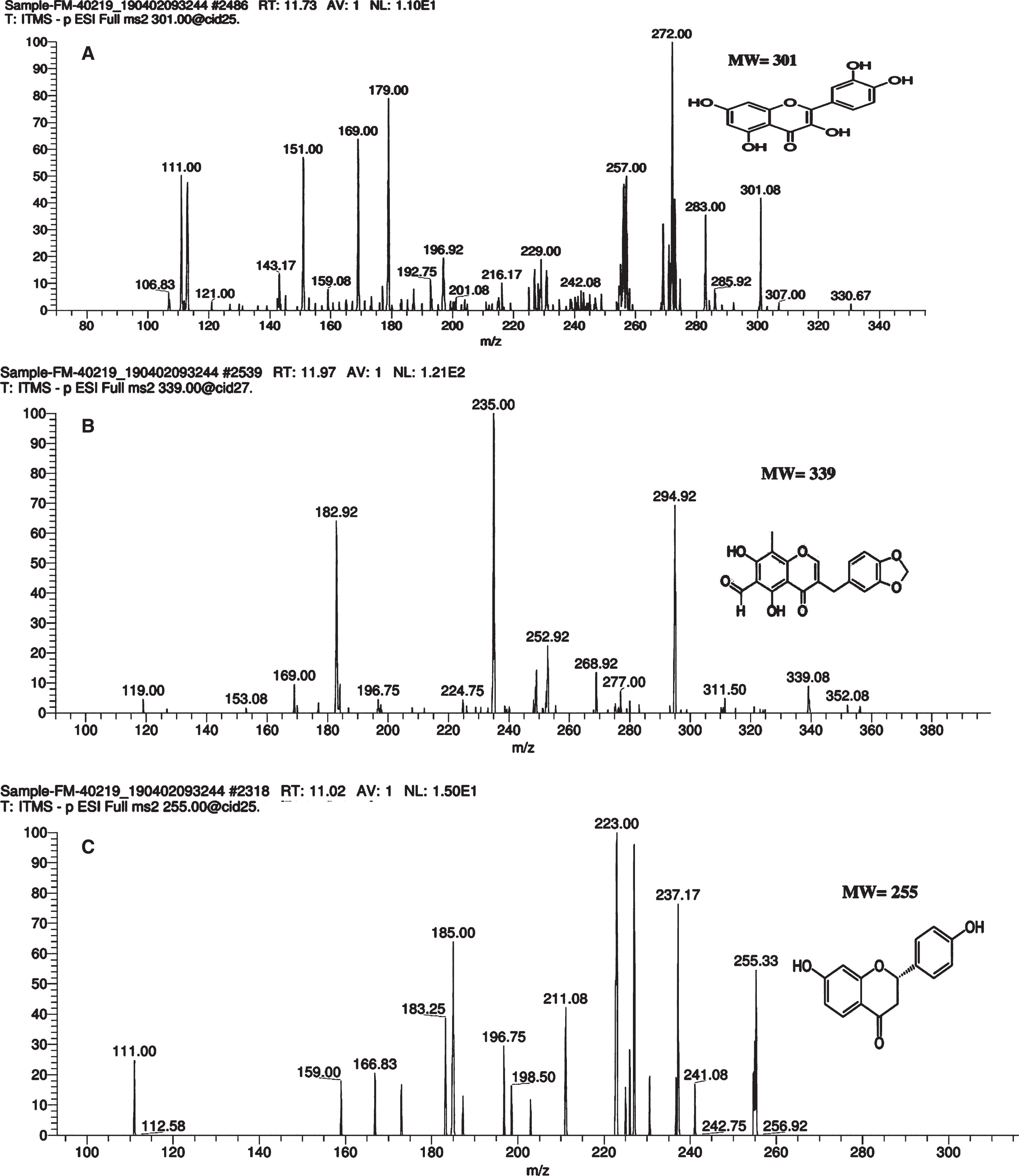
| 11.02 | 256 | 255, 241, 237.17, 223, 211, 185, 183, 159 | C15H12O4 | Liquiritigenin | [62] |
| 11.73 | 301 | 301.08, 272, 179, 151, 121, 106 | C15H1007 | Quercetin | [54] |
| 11.85 | 317 | 317.25, 299, 272.08179, 151, 101 | C15H1008 | Myricetin | [54] |
| 11.97 | 339 | 339, 311, 294.92, 268.92, 182.92, 169 | C19H14O7 | 6-aldehydo-isoophiopogonone | [62] |
Table 3
Profiling of bioactive components identified by LC-MS/MS analysis among 50% hydro-methanolic fraction of G.asiatica fruit
| Rt (min) | Major MS/MS m/z (intensity) | ESI-MSn (Ions) | Chemical formula | Identification | References |
| 2.22 | 179 | 179.17, 161.17, 134. 92 | C9H804 | Caffeic acid | [61] |
| 3.74 | 234.51 | 235.08, 203.08 | C13H1404 | Vidalenolone | [58] |
| 15.51 | 169 | 169, 127, 125.08 | C7H608 | Gallic acid | [61] |
| 16 | 191 | 191, 173, 129 | C7H1206 | Quinic acid | [55] |
| 17.27 | 283 | 265, 255, 241, 239, 223, 211, 183 | C16H12O5 | Calycosin | [56] |
| 17.68 | 301 | 301.08, 285.92, 283, 257, 229, 179 | C14H608 | Ellagic acid | [57] |
| 18.63 | 354.31 | 353.25, 191, 173 Or 354.33, 191, 177, 174 | C16H1809 | Cholorogenic acid | [61] |
| 19.63 | 423 | 422.6, 404.8, 387, 369, 301 | C19H18011 | Mangiferin | [48] |
Table 4
Human equal doses of Grewia asiatica extracts on the basis of phenolic contents
| Assay and species | Type of extract | Animal dose (mg/kg) | Weight (kg) Animal/Human | BSA (m2) Animal/Human | Km factor Animal/Human | HED mg/Kg |
| Carrageenan, formaldehyde and PEG2 induced edema in rat’s hind paw | Methanol (100%) | 38 | 0.15/60 | 0.025/1.6 | 6/37 | 6.16 |
| 76 | 12.32 | |||||
| Methanol (50%) | 48.6 | 7.88 | ||||
| 97.2 | 15.76 | |||||
| Formalin and glutamate-induced licking of the paw in mice | Methanol (100%) | 38 | 0.02/60 | 0.007/1.6 | 3/37 | 3.08 |
| 76 | 6.16 | |||||
| Methanol (50%) | 48.6 | 3.94 | ||||
| 97.2 | 7.88 |
BSA = Body surface area; Human equivalent dose (HED) = Animal dose (mg/Kg)×Animal Km/Human Km.
3.5Anticancer activity of G. asiatica fruit extracts
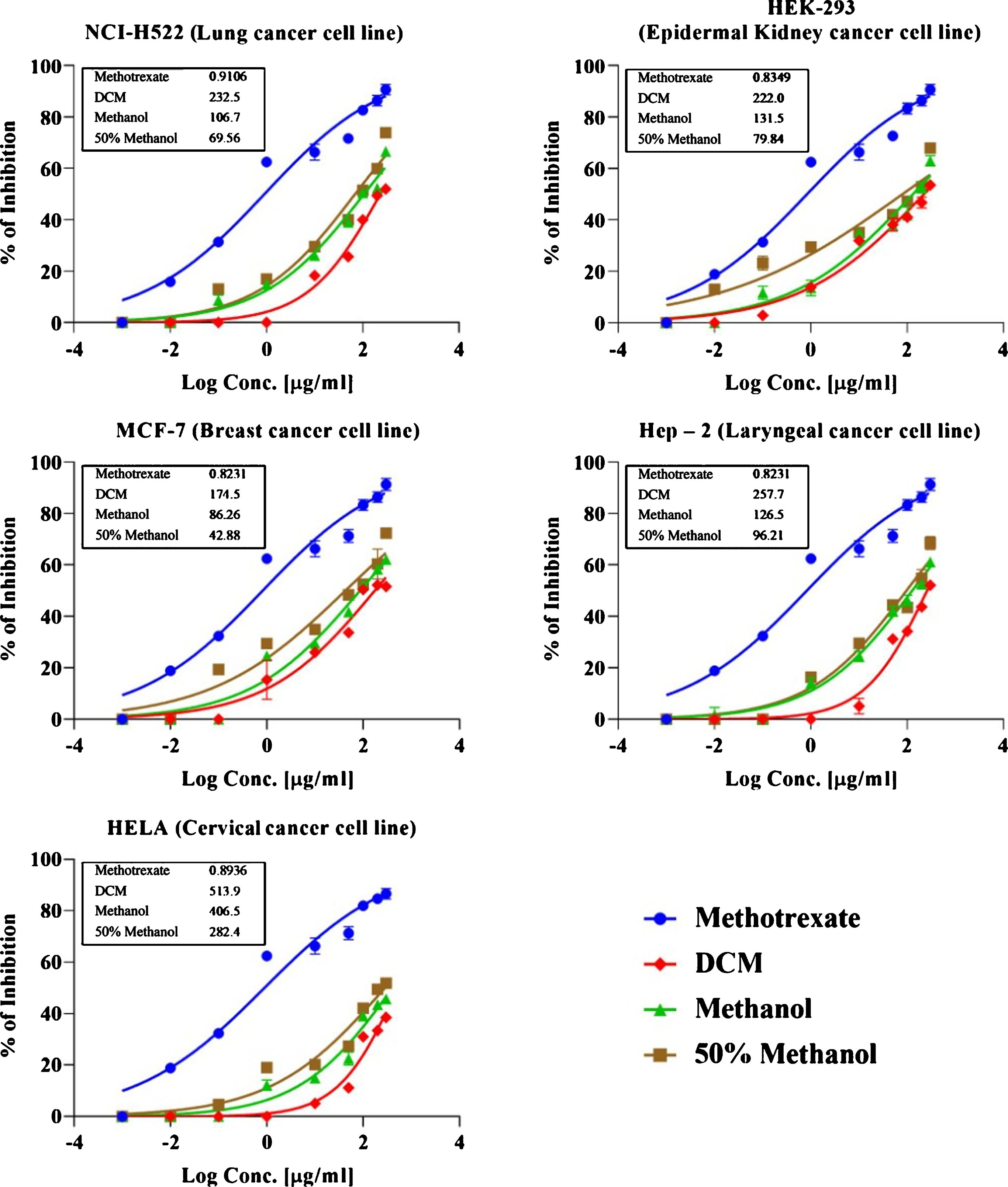
Cytoprotective effects of polyphenols in normal cells and their simultaneous cytotoxic response toward cancerous cells make plant polyphenols as safer anticarcinogenic biomolecules [42]. Nexus to significant anti-inflammatory activities of G. asiatica fruit extracts that are linked with inhibition in the production of potential carcinogens such as hydrogen peroxide, hydroxyl radicals and superoxide, methanolic and 50% methanolic fractions were tested for anticarcinogenic activity. The extracts were not merely recorded to anticipate reduction in oxidative stress induced by DPPH, FRAP and H2O2 but also yielded significant inhibition in cancer progression among different cancer cell lines. The effect of G. asiatica extracts against breast cancer cells (MCF-7), epidermal kidney cancer cells (HEK-293), cervical cancer cells (HeLa), laryngeal cancer cells (HEp–2) and lung cancer cells (NCI-H522) is presented in Fig. (3). In line with their biological responses, dichloromethane and methanol extracts of G. asiatica were observed to be less effective against various cancer lines as compared to hydro-methanolic extracts that exhibited anti-cancer activity at concentration between 0.1 – 300 μg/mL. Concentrations of G. asiatica hydro-methanolic extracts responsible for inhibiting 50% cancer cells of breast cancer, lung cancer, laryngeal cancer, epidermal kidney cancer, and cervical cancer were 34.87 μg/mL, 73.01 μg/mL, 80.41 μg/mL, 98.35 μg/mL and 239.9 μg/mL, respectively. Cancer cells inhibitory concentration of standard drug(methotrexate) when tested at concentration in range between 0.01–100 μM/mL were observed as 18.5 μM/mL for NCI H522, 50.68 μM/mL for HEP-2, 18.52 μM/mL for HELA, 19.16 μM/mL for MCF-7 and 34.87 μM/mL for HEK 293 cells (Fig. 3). The results of present study found in agreement with an earlier study by Marya et al. [43] where IC50 calculated in MTT assay against HEp-2, NCl-H 522, HEK 293, HeLa, MCF 7 and NCl-H 522 were reported as 50.31, 53.88, >100, 58.65 and 59.03 (μg/mL), respectively. In another study [44] leaves of G. asiatica were explored for their possible anticancer activity, extracts were proven effective against all cancer cell lines at IC-50 of 53.70 μg/mL, 54.90 μg/mL, 199.5 μg/mL and 177.8 μg/mL against HL – 60, K-562, MCF-7 and HeLa cells, respectively.
Fig.3
Dose response curve for cytotoxicity activity of G.asiatica extracts against cancer cell lines
3.6Identification of compounds by LC-MS/MS
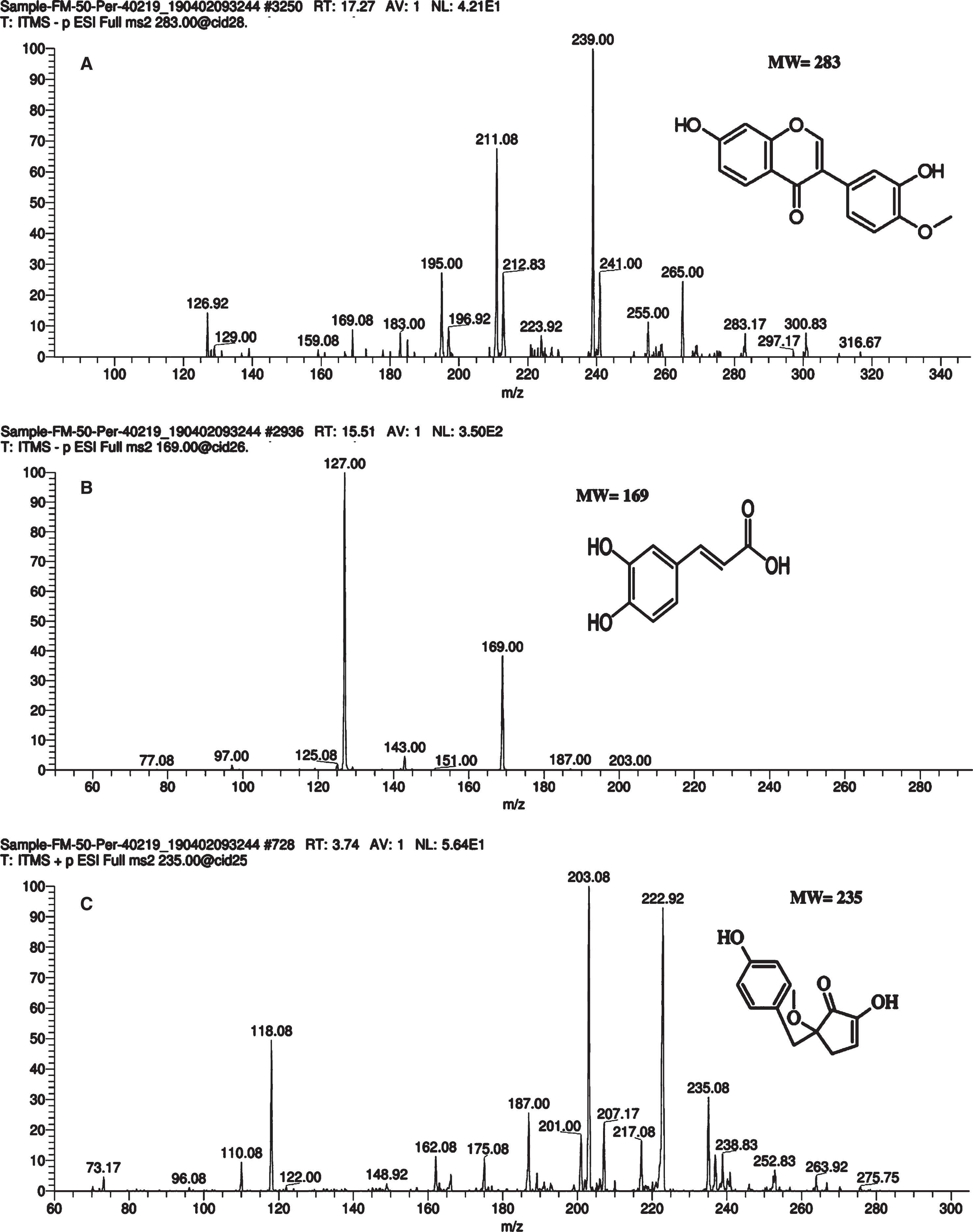
The successive fractions exhibited potential anti-inflammatory and anticancer activities were further subjected to LC MS/MS analysis for the identification of bioactive compounds by comparing their masses with the available literature. The 50% methanolic fraction contained calycosin, ellagic acid, caffeic acid, gallic acid, quinic acid, cholorogenic acid, vidalenolone and mangiferin (Table 3). Previously, there has been no data available on Calycosin, Vidalenolone and mangiferin levels from G. asiatica (Fig. 4). Calycosin holds potential to cure oxidative stress, inflammation, and cancer as has been reported in an earlier study on Chinese herbal plant Radix astragalus by Gao et al. [45]. Reported compound has also been documented to anticipate reduction in migration and invasion capacity of human breast cancer cells [46]. Similarly, Vidalenolone, a novel phenolic metabolite reported in this study was previously isolated as a natural antioxidant from the tropical red alga Vidalia sp. Moreover, Mangiferin–a potential pharmacological compound has been extensively reported in mango fruit [48] however, methanolic and hydro-methanolic extracts of G. asiatica were also identified as carrier of Mangiferin.
Fig.4
Mass spectrum of bioactive compounds in G. asiatica 50% hydro-methanolic extracts analyzed by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). Label:A: Calycosin B: Gallic acid C: Vidalenolone.
Likewise, 6-aldehydo-isoophiopogonone, Liquiritigenin, Quercetin, Myricetin, Genisteun-d4, Epicatechin, gallic acid, ellagic acid and Vitexin were identified in methanolic and hydro-methanolic fractions of G. asiatica (Table 2). The compounds for the first time reported in G. asiatica are 6-aldehydo-isoophiopogonone, Liquiritigenin, Genisteun-d4 and Vitexin (Fig. 5). Liquiritigenin and 6-aldehydo-isoophiopogonone had been extracted earlier from Glycyrrhiza radixand Ophiopogon japonicas plants. Studies on these fractions reported Liquiritigenin and 6-aldehydo-isoophiopogononeto hold potent antioxidant, anti-allergic, anti-inflammatory, and anti-hyperlipidemic properties [49, 50]. Likewise, Genistien-d4- an isoflavone and Vitexin – an epigenin flavone isolated from G. asiatica extracts had been reported to possess anti-inflammatory and anticancer properties against colon and breast cancer cells [51, 52].Biological properties of G. asiatica fruit extracts reported in this study correlate with isolated bioactive compounds that had already been reported as potential antioxidant, anti-inflammatory and anti-cancerous agents.
Fig.5
Mass spectrum of bioactive compounds in G. asiatica 50% hydro-methanolic extracts analyzed by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). Label:A: Quercetin B: 6-aldehydo-isoophiopogonone C: Liquiritigenin
4Conclusion
Findings of this study confirmed that successive fractions of G. asiatica fruit (DCM, methanol and 50% hydro-methanol) as good source of bioactive compounds like other berries with high medicinal and functional food properties. The study validates therapeutic properties of G. asiatica fruit extracts in inflammation, pain, and cancer mediation thus suggesting their use in drug discovery and development of functional ingredients for therapeutic foods development. This study further defined hydro-methanolic extraction of G. asiatica as better carrier of gallic acid, Vitexin, Liquiritigenin, Genisteun-d4 and 6-aldehydo-isoophiopogonone, thefirst time identified in G. asiatica, while pure methanolic extraction was found as a promising technique to isolate ellagic acid, caffeic acid, quinic acid, vidalenolone, calycosin and mangeferin from G. asiatica fruit. The study suggests that G. asiatica extracts should be further explored in evaluating biological activities of fruit bioactive isolates in order to better understand their metabolic responses in clinical cell models.
Funding
The authors report no funding.
Conflict of interest
The authors declare no conflict of interest to this study.
Acknowledgments
Authors are thankful to Higher Education Commission of Pakistan for supporting Research of Mr. Muhammad Qamar, Indigenous Ph.D Scholarship holder at Institute of Food Science & Technology, Bahauddin Zakariya University, Multan, Pakistan. Authors would like to thank Prof. Dr. Ronald G. Labbe, Department of Food Science, University of Massachusetts Amherst for language editing and providing technical assistance in creating manuscript revision.
References
[1] | Goswami S , Jain R , Masih H . Antioxidant and DNA protection potential of Grewia asiatica L. leaves acetone extract. Journal of Pharmacognosy and Phytochemistry. (2018) ;SP1 212–17. |
[2] | Choudhary IM , Siddiqui J , Abbaskhan A , Naheed S , Adhikari A , Awalia JJ , et al. Bio-active antioxidants from plant foods for neutraceutical product development. (2018) ; United States patent application US 13/759: :820. |
[3] | Halpin HA , Morales-Suárez-Varela MM , Martin-Moreno JM . Chronic disease prevention and the new public health. Public Health Review. (2010) ;32: (1):120–54. |
[4] | Sharma N , Sharma V , Abrol V , Panghal A , Jaglan S , et al. An Update on Bioactive Natural Products from Endophytic Fungi of Medicinal Plants. In Pharmaceuticals from Microbes. (2019) ; Springer, Cham 121–53. |
[5] | Sharma KV , Sisodia R . Hepatoprotective efficacy of Grewia asiatica fruit against oxidative stress in swiss albino mice. Iranian Journal of Radiation Research. (2010) ;8: (2):75–85. |
[6] | Veberic R , Slatnar A , Bizjak J , Stampar F , Mikulic-Petkovsek . Anthocyanin composition of different wild and cultivated berry species. Food Science and Technology. (2015) ;60: (1):509–17. |
[7] | Sarma AD , Mallick AR , Ghosh A . Free radicals and their role in different clinical conditions: an overview. International Journal of Pharmaceutical Sciences and Research. (2010) ;1: (3):185–92. |
[8] | Khan RS , Asghar W , Khalid N , Nazir W , Farooq M , Ahmed I , et al. Phalsa (Grewia asiatica L) fruit berry a promising functional food ingredient. Journal of Berry Research. (2019) ;9: (2):1–15. |
[9] | Ullah W , Uddin G , Siddiqui BS . Ethnic uses, pharmacological and phytochemical profile of genus Grewia. Journal of Asian Natural Products Research. (2012) ;14: (2):186–95. |
[10] | Shukla R , Sharma DC , Pathak N , Bajpai P . Genomic DNA Isolation from High Polyphenolic Content Grewia asiatica L. Leaf Without Using Liquid Nitrogen. Iranian Journal of Science and Technology. (2018) ;42: (2):347–51. |
[11] | Gupta MK , Sharma PK , Ansari SH , Lagarkha R . Pharmacognostical evaluation of Grewia asiatica fruits. International Journal of Plant Sciences. (2006) ;1: :249–51. |
[12] | Chattopadhyay S , Pakrashi S . Triterpenes from Grewia-asiatica. Journal of the Indian Chemical Society. (1975) ;52: :553–553. |
[13] | Harborne J . Methods of plant analysis. Phytochemical methods. (1984) ; Springer, Ed. pp. 1–36. |
[14] | Hossain MA , Shah MD . A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arabian Journal of Chemistry. (2015) ;8: (2):66–71. |
[15] | Oriakhi K , Oikeh EI , Ezeugwu N , Anoliefo O , Aguebor O , Omoregie ES , et al. Comparative antioxidant activities of extracts of Vernonia amygdalina and Ocimum gratissimum leaves. The Journal of Agricultural Science. (2013) ;6: (1):13. |
[16] | Galik S . Determination of The Anthocyanin Concentration in Table Wines and Fruit Juices Using Visible Light Spectrophotometry. Cell Biology. (2012) ;2: :1–12. |
[17] | Polshettiwar S , Ganjiwale R , Wadher S , Yeole P . Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian Journal of Pharmaceutical Sciences. (2007) ;69: (4):574–6. |
[18] | Alara O , Abdurahman N , Mudalip SA , Olalere O . Effect of drying methods on the free radicals scavenging activity of Vernonia amygdalina growing in Malaysia. Journal of King Saud University Science. (2017) ; In Press. |
[19] | Zahin M , Aqil F Ahmad I . Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. (2010) ;703: (2):99–107. |
[20] | Ruch RJ , Cheng SJ , Klainig JE . Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. (1989) ;10: (6):1003–8. |
[21] | Morris CJ . Carrageenan-induced paw edema in the rat and mouse. Inflammation protocols. (2003) ; Springer, Ed. pp. 115–21. |
[22] | Brownlee G . Effect of deoxycortone and ascorbic acid on formaldehyde-induced arthritis in normal and adrenalectomised rats. Lancet. (1950) ;268: :157–9. |
[23] | Muruganandan S , Pant S , Srinivasan K , Chandra S , Tandan S , Lal J , et al. Inhibitory role of Syzygium cumini on autacoid-induced inflammation in rats. Pharmacology. (2002) ;46: (4):482–6. |
[24] | Hunskaar S , Hole K . The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. (1987) ;30: :103–14. |
[25] | Beirith A , Santos AR , Calixto JB . Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain research. (2002) ;924: (2):219–28. |
[26] | Reagan-Shaw S , Nihal M , Ahmad N . Dose translation from animal to human studies revisited. FASEB Journal. (2008) ;22: (3):659–61. |
[27] | Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers, U.S. Food and Drug Administration, Rockville, Maryland, USA. (2002) . |
[28] | Roy M , Chakraborty S , Siddiqi M , Bhattacharya RK . Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pacific Journal of Cancer Prevention. (2002) ;3: :61–7. |
[29] | Steinmann D , Ganzera M . Recent advances on HPLC/MS in medicinal plant Analysis. Journal of Pharmaceutical and Biomedical Analysis. (2011) ;55: (4):744–57. |
[30] | Asghar MN , Khan IU , Sherin L , Ashfaq M . Evaulation of Antioxidant Activity of Grewia asiatica Berry Using 2, 2’-Azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) and N, N-Dimethyl-p-phenylenediamine Radical Cations Decolourization Assays. Asian Journal of Chemistry. (2008) ;20: (7):5123–132. |
[31] | Vallinas MG , Castejón MG , Casado AR , Molina AR . Dietary phytochemicals in cancer prevention and therapy: a complementary approach with promising perspectives. Nutrition Reviews. (2013) ;71: (2):585–99. |
[32] | Boeing JS , Barizao EO , e Silva BC , Montanher PF , de Cinque Almeida V , Visentainer JV , et al. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries. Application of principal component analysis. Chemistry Central Journal. (2014) ;8: (1):48. |
[33] | Srivastava J , Kumar S , Vankar PS . Correlation of antioxidant activity and phytochemical profile in native plants. Nutrition and Food Science. (2012) ;42: (2):71–9. |
[34] | Khattab HA , El-Shitany NA , Abdallah IZ , Yousef FM , Alkreathy HM , et al. Antihyperglycemic potential of Grewia asiatica fruit extract against streptozotocin-induced hyperglycemia in rats. Oxidative Medicine and Cellular Longevity. (2015) ; Article ID 549743. |
[35] | Skrovankova S , Sumczynski D , Mlcek J , Jurikova T , Sochor J . Bioactive compounds and antioxidant activity in different types of berries. International journal of molecular science. (2015) ;16: (10):24673–706. |
[36] | Lim CSH , Lim SL . Ferric reducing capacity versus ferric reducing antioxidant power for measuring total antioxidant capacity. Laboratory Medicine. (2013) ;44: (1):51–55. |
[37] | Gupta MK , Lagarkha R , Sharma DK , Sharma PK , Singh R , Ansari HS , et al. Antioxidant activity of the successive extracts of Grewia asiatica leaves. Asian Journal of Chemistry. (2007) ;19: (5):3417. |
[38] | BrglezMojzer E , Hrncic MK , Skerget M , Knez Z , Bren U . Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. (2016) ;21: (7):901. |
[39] | Paviaya US , Kumar P , Wanjari MM , Thenmozhi S , Balakrishnan BR . Analgesic and anti-inflammatory activity of root bark of Grewia asiatica Linn. in rodents. Ancient Science of Life. (2013) ;32: (3):150. |
[40] | Akhtar B , Ashraf M , Javeed AL , Sharif A , Akhtar MF . Analgesic, antipyretic and antinflammatory activities of Grewia asiatica fruit extracts in albino mice. Acta Poloniae Pharmaceutica. (2016) ;73: (4):983–9. |
[41] | Murray CW , Porreca F , Cowan A . Methodological refinement in the mouse paw formalin test an animal model of tonic pain. Journal of Pharmacological and Toxicological Methods. (1988) ;20: (2):175–86. |
[42] | Ramos S . Cancer chemoprevention and chemotherapy: Dietary polyphenols and signaling pathways. Molecular Nutrition & Food Research. (2008) ;52: (5):507–26. |
[43] | Marya B , Dattani KH , Patel DD . In vitro cytotoxicity evaluation of aqueous fruit and leaf extracts of Grewia asiatica using MTT assay. Pharmaceutical Chemistry Journal. (2011) ;3: (3):282–7. |
[44] | Kakoti BB , Selvan VT , Manikandan L , Gupta M , Mazumder UK , Das B , et al. Antitumor and in-viro activity of Grewia asiatica Linn. against Ehlrich’s ascites carcinoma cell lines. Pharmacology online. (2011) ;3: :956–60. |
[45] | Gao J , Liu ZJ , Chen T , Zhao D . Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharmaceutical biology. (2014) ;52: (9):1217–22. |
[46] | Li S , Wang Y , Feng C , Wu G , Ye Y , Tian J . Calycosin inhibits the migration and invasion of human breast cancer cells by down-regulation of Foxp3 expression. Cellular Physiology and Biochemistry. (2017) ;44: (5):1775–84. |
[47] | Zhong W , Zhang Z , Zhao P . The impact of initial systemic inflammatory response after aneurysmal subarachnoid hemorrhage. Turkish Neurosurgery. (2016) ;27: (3). |
[48] | Kole L , Giri B , Manna SK , Pal B , Ghosh S . Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. European Journal of Pharmacology. (2011) ;653: (1–3):8–15. |
[49] | Suryawanshi S , Asthana RK , Gupta RC . Simultaneous estimation of mangiferin and four secoiridoid glycosides in rat plasma using liquid chromatography tandem mass spectrometry and its application to pharmacokinetic study of herbal preparation. Journal of Chromatography. (2007) ; 858: (1-2):211–19. |
[50] | Yoo HD , Ketchum SO , France D , Bair K , Gerwick WH . Vidalenolone, a novel phenolic metabolite from the tropical red alga Vidalia sp. Journal of natural products. (2002) ;65: (1):51–53. |
[51] | Wang D , Lu J , Liu Y , Meng Q , Xie J , Wang Z , Teng L . Liquiritigenin induces tumor cell death through mitogen-activated protein kinase-(MPAKs-) mediated pathway in hepatocellular carcinoma cells. BioMed research international; (2014) . |
[52] | Yancui W , Feng L , Zongsuo L , Liang P , Bangqing W , Jing Y , Yingying S , Cunde M . Homoisoflavonoids and the antioxidant activity of Ophiopogon japonicus root. Iranian Journal of Pharmaceutical Research. (2017) ;16: (1):357–65. |
[53] | Zhou P , Wang C , Hu Z , Chen W , Qi W , Li A . Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC cancer. (2017) ;17: (1):813. |
[54] | Gates PJ , Lopes NP . Characterisation of flavonoid aglycones by negative ion chip-based nanospray tandem mass spectrometry. International journal of analytical chemistry. (2012) . |
[55] | Saldanha L , Vilegas W , Dokkedal A . Characterization of flavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules. (2013) ;18: (7):8402–16. |
[56] | Mehmood A , Hussain A , Irshad M , Hamayun M , Iqbal A , Rahman H , Ayaz S , et al. Cinnamic acid as an inhibitor of growth, flavonoids exudation and endophytic fungus colonization in maize root. Plant physiology and biochemistry. (2019) ;135: :61–68. |
[57] | Yan L , Yin P , Ma C , Liu Y . Method development and validation for pharmacokinetic and tissue distributions of ellagic acid using ultrahigh performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Molecules. (2014) ;19: (11):18923–35. |
[58] | Laghari AQ , Memon S , Nelofar A , Laghari AH . Extraction, identification and antioxidative properties of the flavonoid-rich fractions from leaves and flowers of Cassia angustifolia. American Journal of Analytical Chemistry. (2011) ;2: (08):871. |
[59] | Krasteva I , Nikolov S . Flavonoids in Astragalus corniculatus. Química Nova. (2008) ;31: (1):59–60. |
[60] | Kang J , Hick LA , Price WE . A fragmentation study of isoflavones in negative electrospray ionization by MSn ion trap mass spectrometry and triple quadrupole mass spectrometry. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up-to-the-Minute Research in Mass Spectrometry. (2007) ;21: (6):857–868. |
[61] | Riaz M , Rasool N , Iqbal M . Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) analysis of Russeliaequisetiformis extract. Bulgarian chemical communications. (2017) ;49: (2):354–59. |
[62] | Rahman H , Khan I , Hussain A , Shahat AA , Tawab A , Qasim M , et al. Glycyrrhizaglabra HPLC fractions: Identification of AldehydoIsoophiopogonone and Liquirtigenin having activity against multidrug resistant bacteria. BMC complementary and alternative medicine. (2018) ;18: (1):140. |




