Effects of plug plants and bare-root plants on strawberry field performance, fruit quality traits and health-promoting compounds
Abstract
The winter planting system is based on the use of the bare-root plant. It is used extensively in many strawberry cultivation areas characterized by mild winters. Bare-root plants have disadvantages and plug plants represent a valid alternative to the traditional bare-root plant. This study, conducted in Southern Italy, investigated the changes in the fruiting cycle of bare-root and plug plants of strawberry (Fragaria × ananassa), with a focus on fruit quality. Bare root and plug plants for three strawberry genotypes were planted in fields and the differences in yield, quality traits and main bioactive compounds were evaluated. The plants were grown in Scanzano Jonico – Basilicata region (Southern Italy). Yield per plant, fruit size, total soluble solids content, titratable acidity, flesh firmness, skin colour, antioxidant activity (TAC), total phenols (TPH), total anthocyanins (TACY), ascorbic acid content (AA) and phenolic profile were determined. The results confirmed that the start of the harvest for plug plants was significantly earlier than for bare-root plants and plug plants also had higher productivity. The fruit size of plug plants was initially larger than for bare root plants but became significantly smaller when the production flow decreased in May. Plant type did not affect flesh firmness, while the sugar content was lower in plug plants. The fruit colour brightness was higher for plug plants in the first part of the harvest but reduced significantly in the second part. TPH was lower for plug plants, compared to bare-root plants, in the first part of the harvest but higher in the second part, when the fruit size of plug plants decreased significantly. TACY showed a considerable variability and was more influenced by the genotype rather than by the type of plant. Also, TAC, being correlated with TPH, had higher values in plug plants in the second part of the harvest, coinciding with the decrease in size. The content of the most important classes of individual polyphenols (anthocyanins and ellagitannins) was on average higher in plug plants than in bare-root plants. In summary, the plug plant had an early yield start with a production concentrated in March and early April, which is suitable for Mediterranean areas, but the quality traits showed a lot of variability from year to year.
Keywords:
1Introduction
Consumers’ interest in health and nutritional aspects of horticultural products has been increasing in recent years [1–3]. Strawberry (Fragaria×ananassa Duch.) is an economically important crop in the worldwide fruit market due to its attractive fruit, good taste, distinctive flavour and its well-known nutritional value [2, 4]. Strawberry fruits, compared with the other fruits and vegetables, have higher amounts of vitamin C and phenolic compounds, which are known to protect against free radicals [5, 6].
Strawberry is the most important berry crop produced in almost all parts of the world, with different cultivation techniques, harvest seasons and genotypes adapted to different environmental conditions [3, 6, 7].
In temperate zones, the strawberry winter planting system is based on the use of bare-root plants and is used extensively in California [8], Florida [9] and Spain [10]. In Southern Italy, the growers use Spanish or Polish bare-root plants, planted in mid-October [11, 12]. Bare-root plants have weaknesses, mainly in reduced root and vegetative development in nursery fields [12]. This causes variability in the flowering time. However, in the nursery the plant digging date is delayed, to reach an optimal vegetative growth and to satisfy chilling requirements. Furthermore, bare-root plants have high water requirements and a high risk of pathogen contamination in nursery fields [13, 14]. In the same temperate zones, another type of plant, the plug plant, can also be used. This kind of plant is obtained from unrooted runners (tips). The tips are grown in trays containing peat and they are transplanted in the field after 3.5–5 weeks [15]. The advantages of plug plants are: reduced water needs, minimal root damage during transplanting, easy root-taking, rapid growth and more uniform stand, earlier flowering and production, a general increase in yield and lower risk of pathogen contamination [16–22].
Bare-root and plug plant management have been compared in many studies and several authors agree that the use of plug plants increase early yield in strawberry [17, 23, 24]. However, there is still little knowledge of the impact of this type of plant on fruit quality traits [17, 25–27]. The aim of this study was to evaluate the impact of strawberry (Fragaria × ananassa Duch.) plug plants and bare-root plants on yield and fruit quality traits in Southern Italy, where the traditional winter planting system is usually adopted.
2Material and methods
2.1Trial sites and cultivation practices
Field trials were conducted in two consecutive strawberry seasons (2011–12, 2012–13) in Sabato Donato commercial farm, located in Scanzano Jonico, (Italy; 40°25’N/16°42’E). The soil was silty clay loam (sand 18%, silt 42%, clay 40%; pH 8.0), alkaline and with an organic matter content of 2.1%.
The experiment was conducted in a split plot randomized design, with two factors and four replications (30 plants per plant type and genotype). The first factor (main plot) consisted of two plant types: bare-root plants and plug plants. The second factor (sub-plots) consisted of three June-bearing strawberry (Fragaria × ananassa Duch.) varieties ‘Pircinque’, ‘Jonica’ and ‘Sabrosa’.
All plants were supplied by the Italian companies Planitalia srl (‘Sabrosa’) and Coviro Soc. Cons. a r.l. (‘Pircinque’ and ‘Jonica’). The bare-root plants were transplanted on the 10th October of each year. The plug plants were transplanted on the 27th September to allow an early harvest. Plants were grown under tunnel-culture in accordance with the standard management practices. The tunnels were covered in early November and strawberry plants were grown in double rows on raised beds, covered with black polyethylene mulch and spaced 1.2–1.4 m (centre-to-centre); planting density was 7.4 plants per m2.
2.2Yield and average berry weight (fruit size)
Plot harvests were recorded at each picking (1–2 times per week) when the berries were fully red in colour. Total yield per plant was calculated by summing the data of each harvest. Marketable yield was defined as intact fruits having a diameter of > 22 mm. Irregular, rotten and smaller diameter fruits were recorded as discard. Average berry weight per month (January–May) was calculated by the formula (∑ [fp]) P–1 where f is the average berry weight per harvest (ten fruits), p is the marketable yield per plant per harvest, and P is the sum of marketable yields of all pickings (∑p).
2.3Fruit sampling
At three time points (mid-March, mid-April and mid-May) fruit samples were picked in both years. Secondary or tertiary fruits were selected for uniform size, fully red colour and lack of damage. Twenty ripe fruits of the first sample batch were used within ten hours of the picking time to determine flesh firmness, total soluble solids content, skin colour, titratable acidity and ascorbic acid content. A second batch of ripe fruits were collected, freeze dried and ground with a pestle and mortar to a fine homogeneous powder for determining total antioxidant capacity, total polyphenol content, total anthocyanin content; and the determination of the phenolic profile by HPLC-DAD [28].
2.4Flesh firmness and skin colour
Twenty fruits were chosen and the flesh firmness of each was measured with an Ametek digital penetrometer with a 6 mm diameter probe (star-shaped plug). Next, the external colour of the fruit, in two opposite areas of the equatorial region, was measured using a Minolta Chromameter reflect II with an 8 mm window providing the three colour coordinates: L*, a* and b*. Colour data were reported as L* (brightness), hue angle (degrees) indicating colour shade (hue = arctan [b* a*–1] where 0°=red-purple; 90°=yellow) and chroma C*=(a*2 + b*2)1/2, which is indicative of colour intensity or saturation. The instruments were calibrated with standard white (Y = 93.96; x = 0.3138; y = 0.3214).
2.5Total soluble solids content and titratable acidity
Juice total soluble solids content (TSS) was measured (°Brix) with an Atago digital refractometer PR-32 Alpha (LaboandCo, Torino, Italy) for twenty fruits. Titratable acidity (TA) was determined using a 702 SM Titrino automatic titrator Metrhom Swiss. A 5.0 g sample of juice was diluted with 25.0 mL distilled water and titrated with 0.1 N sodium hydroxide solution (NaOH) to pH 7.0. The data was reported as meq of NaOH per 100 g of fresh weight (FW).
2.6Ascorbic acid
Ascorbic acid content (AA) was determined by the Merckquant® Ascorbic Acid Test (Reflectoquant®, Merck) using test-strips dipped in strawberry juice [29]. Results were expressed in mg per 100 g FW.
2.7Total phenolic content, total anthocyanin content and total antioxidant capacity
Nutraceutical compounds were determined by slightly modifying the method of Diamanti et al. [30]: 250 mg of freeze-dried fine homogeneous powder were added to 10 mL methanol, stirred, sonicated for 5 min in an ultrasonic bath at 4°C and centrifuged for 30 min at 4,500 rpm. The supernatant was collected in a vial and stored at – 20°C for subsequent analysis. Total phenolic content (TPH) was determined via the Folin-Ciocalteu method as gallic acid equivalents (GAE) after Slinkard and Singleton [31]. Absorbance was measured after two hours using a uv-1601-spectophotometer at 750 nm; the results were expressed as mg GAE per 100 g FW. Total Anthocyanin concentration (TACY) was determined using a pH differential method [32]. The assay was based on the peculiar pH dependent anthocyanin colour change [33]. The samples were diluted (1 : 20, v/v) with solutions of KCl 0.025 mol/L (pH 1.0) and sodium acetate 0.4 mol/L (pH 4.5); the absorbance was measured at 510 and 700 nm in buffers at pH 1.0 and 4.5, respectively, using A = [(A510– A700)pH1.0 – (A510– A700)pH4.5] with a molar extinction coefficient for pelargonidin-3-glucoside of 15,600. The results are reported as mg of pelargonidin-3-glucoside equivalent (Pg-3-gluc) per 100 g FW. Total antioxidant capacity (TAC) was determined by ABTS assay [34]. The radical cation solution was generated overnight with potassium persulfate. The sample reading was carried out after 3 min using a uv-1601-spectophotometer at 734 nm and the results were reported as mmol Trolox equivalent (TE) per 100 g FW.
2.8Phenolic content by HPLC (high-performance liquid chromatography)
The phenolic profile was determined after Andreotti et al. [28]. Samples of phenolic compounds from 0.1 g of freeze-dried fruits were extracted with 1 mL methanol, stirred for 30 s, sonicated for 30 min in an ultrasonic bath at 4 °C and centrifuged for 30 min at 12,500 rpm. The supernatant was filtered through Millipore 0.2μm filters, collected in a vial and stored at – 20°C for analysis using an Agilent 1100 HPLC system with a photodiode array detector (DAD) and an Agilent ZORBAX SB-C18 Rapid Resolution HT column (3.0 × 50 mm, 1.8μm particle size), kept at 35°C in a solution of 0.1 M H3PO4 (solvent A) and methanol (solvent B) as the mobile phase. The solvent gradient was 5% B in A at the beginning of analysis; 50% B in A at 8 min; 100% B at 13 min; and 100% B (isocratic) at 15 min, followed by column preparation for the next analysis, with a total analysis time of 20 min. The flow rate was 0.5 mL/min and chromatograms were recorded at 280, 320, 350 and 510 nm for simultaneous monitoring. The total phenolic compounds were divided into five groups and quantified using external standards: anthocyanins as pelargonidin-3-glucoside (Pg-3-gluc) and cyanidin-3-glucoside (Cya-3-gluc) at 510 nm, flavonols as quercetin-3-gluoside (350 nm), ellagitannins as ellagic acid (280 nm), hydroxycinnamic acids as chlorogenic acid (320 nm) and flavan-3-ols as catechin (280 nm). The phenolic compound count was reported as μg per g of FW.
2.9Statistical analysis
The data were processed by two-way analysis of variance (ANOVA), including the effects of plant type and genotype and their interaction. Significant differences among means were evaluated by the Least Significant Difference (LSD) multiple range test. The coefficient of variation (CV, %) was calculated for each trait. Relationships between parameters were analysed by the Pearson coefficient (r; p < 0.05). Statistics were performed using STATGRAPHICS Centurion (Statpoint Inc., USA).
3Results and discussion
3.1Yield and fruit size
In 2012, the harvest for all varieties was started in late January for plug plants and about 3 weeks later for bare-root plants (Fig. 1). The harvest was finished for both types of plant at the end of May. In 2013, the favourable climatic conditions, with high minimum temperatures in December and January, led to an earlier start than in 2012. The harvest began in mid-January for plug plants while bare-root plants began to bear fruit in early February. Plug plants had higher marketable yield than bare-root plants, for all varieties in both years (Fig. 2).
Fig.1
Starting harvest data per plant type (plug plant and bare-root) of different varieties in 2012 and 2013. *Indicate statistically significant differences between two plant types, variety and year, intra variety via LSD test P < 0.05.
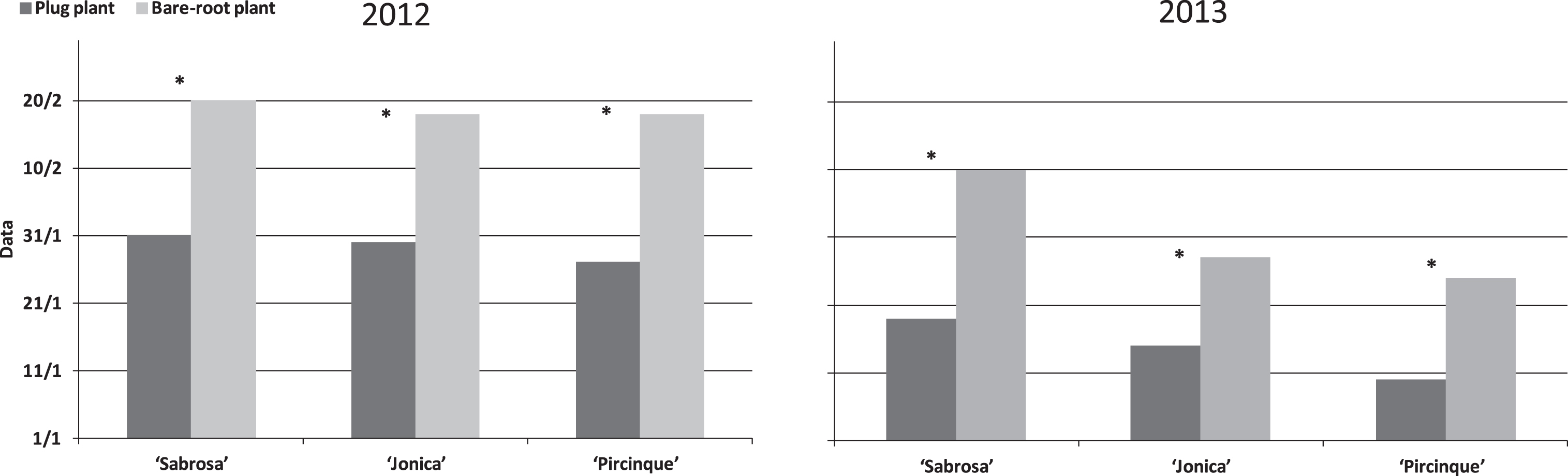
Fig.2
Monthly partitioned and marketable yield (g/plant) of strawberry varieties in 2012 and 2013 years in plug and bare-root plants. *Indicate statistically significant differences between two plant types, variety, month, and year, intra month via LSD test P < 0.05.
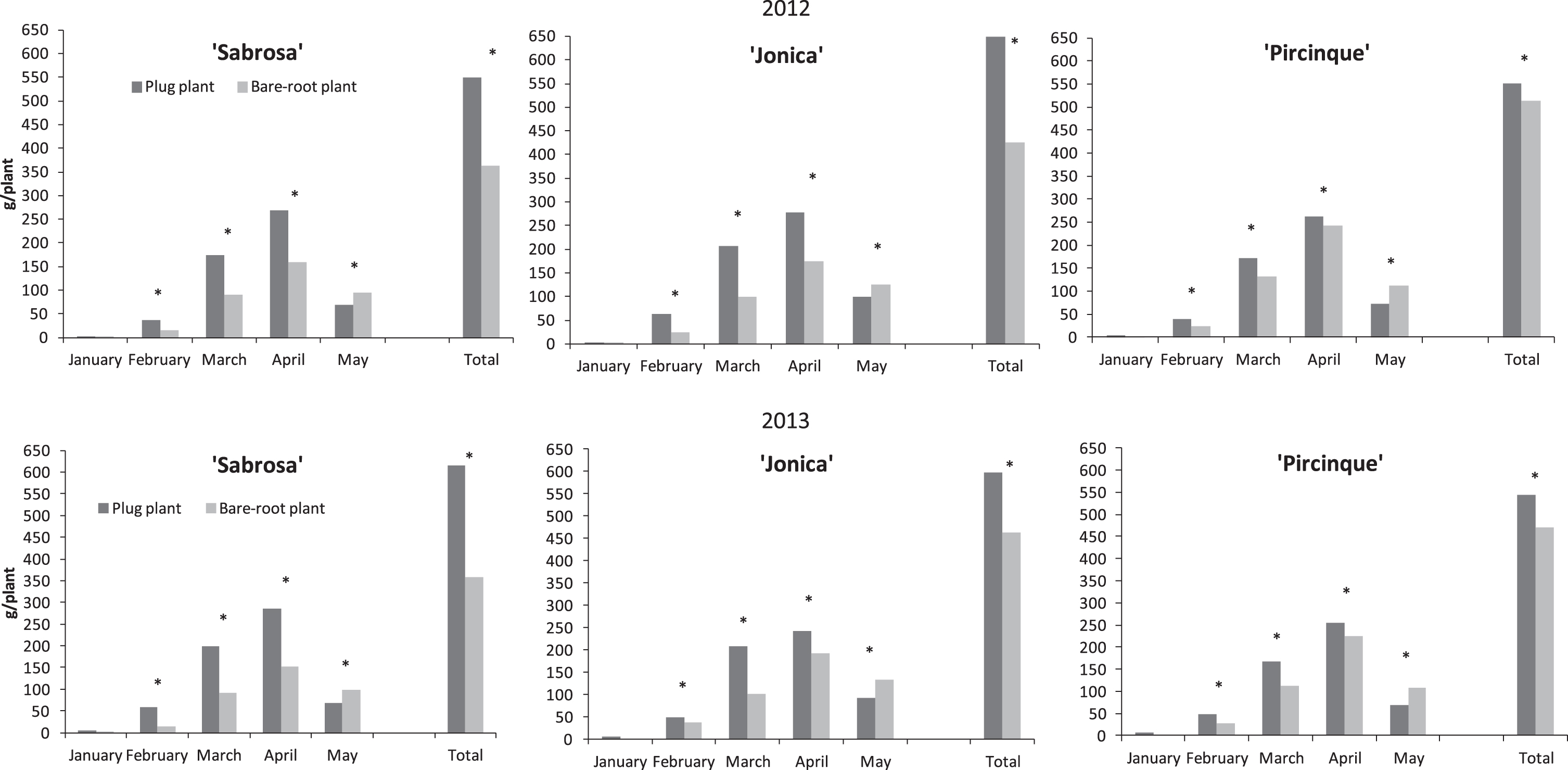
In both years, plug plants for all varieties had a significantly higher yield than bare-root plants, from February to April. In May the yield of bare-root plants was higher than for plug plants.
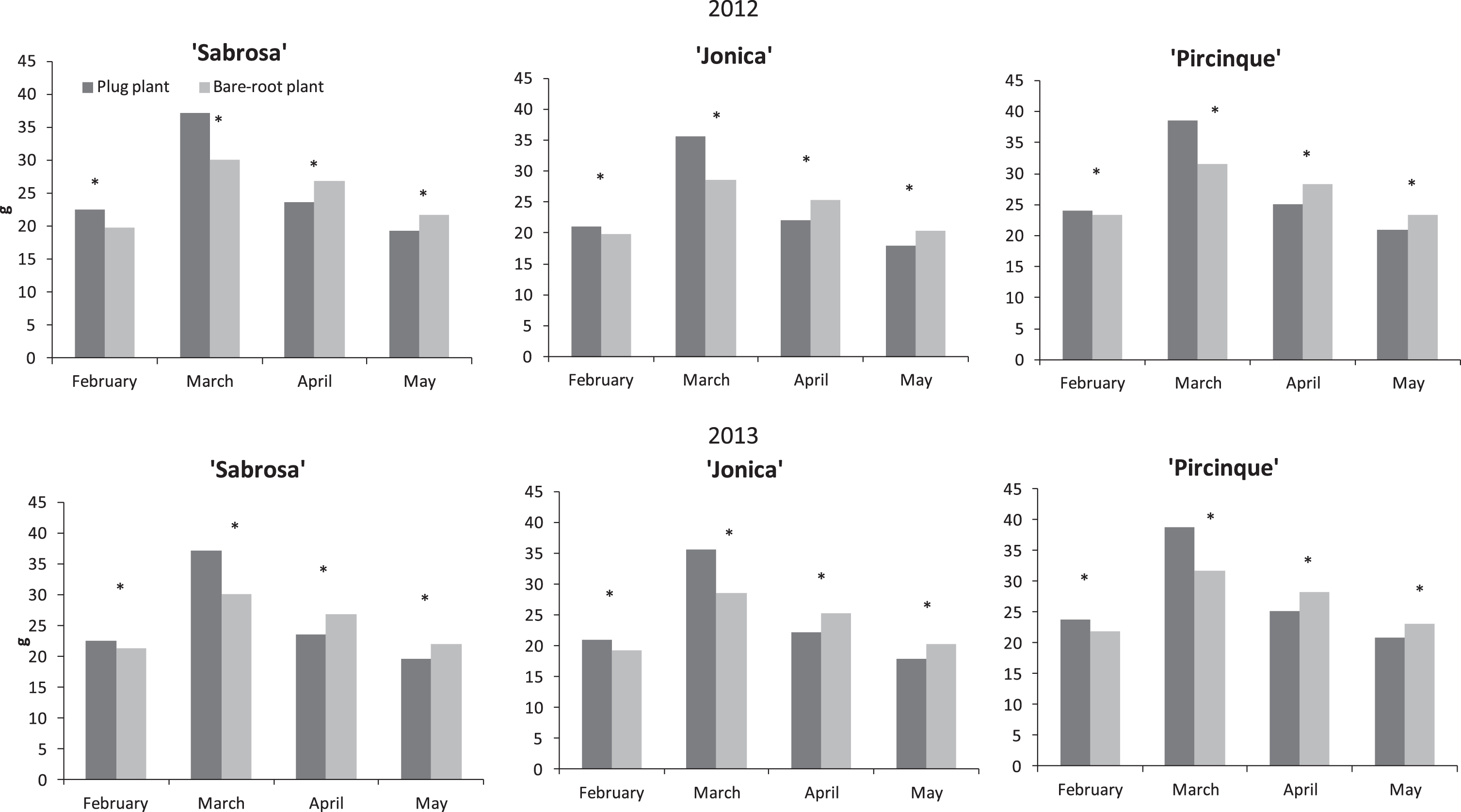
In both years, in the first months of harvest (until March), the plug plants of all varieties provided larger fruit. In contrast, in April and May when most of the production was concentrated, the fruit size provided by plug plants was consistently and significantly smaller than for bare-root plants (Fig. 3).
Fig.3
‘Sabrosa’, ‘Jonica’ and ‘Pircinque’ average fruit weight (g) per month for plug plants and bare-root plants in years 2012 and 2013. *Indicate statistically significant differences between two plant types, variety, month, and year, intra month via LSD test P < 0.05.
3.2Standard quality traits
Flesh firmness was not influenced by plant type growing year or different periods of the harvest season (Fig. 4).
Fig.4
Flesh firmness, total soluble solids content, titratable acidity and colour traits (brightness, chroma and hue angle) of the two plant types (plug plant and bare-root) for the 3 varieties, in 3 months of harvest period, in 2012 and 2013. *Statistically significant differences between two plant types, per variety, month, and year, intra variety via LSD test P < 0.05.
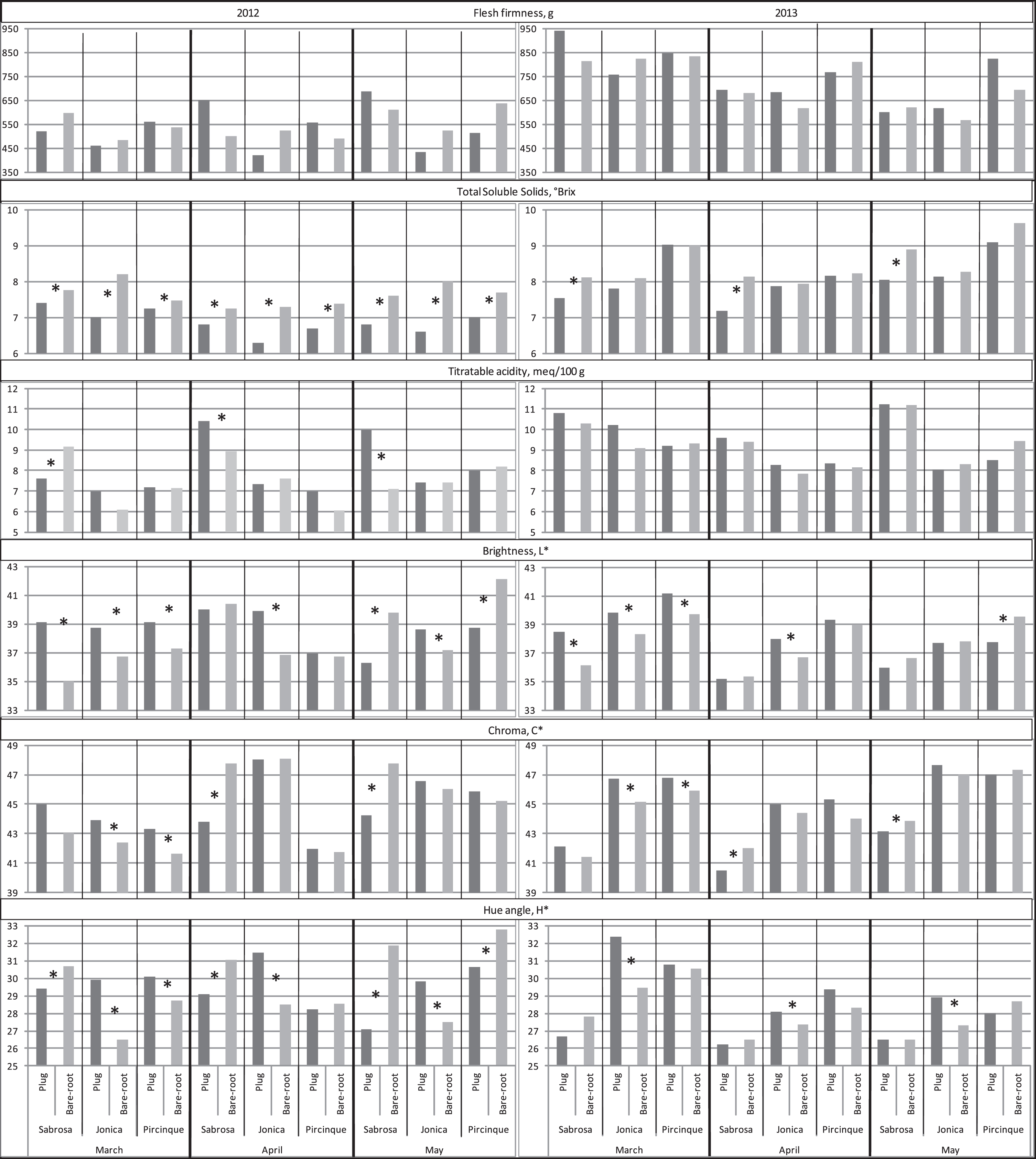
In 2012, lower values for total soluble solids content were found in plug plant fruits for all cultivars and analysis times. In 2013, these results were confirmed only for ‘Sabrosa’ at all time points. The higher plant production of the plug plants was correlated with less fruit sweetness, confirming the negative correlation between these two traits [36]. The higher stress for plug plants, due to their higher yield in comparison to bare-root plants, resulted in a lower TSS. In 2012, in March only, ‘Sabrosa’ plug plants had lower titratable acidity values than bare-root plants, while in April and May bare-root plants had the lower values. In 2013, no differences were found between the types of plant for the three varieties. In 2012, fruit colour brightness (L* value) was higher in March for plug plants of all varieties than for bare-root plants. In April, this was only observed for ‘Jonica’; in May, this was observed for ‘Jonica’ but ‘Sabrosa’ and ‘Pircinque’ had lower values in plug plants. In 2013, similar results were measured in March and April, with fruit colour brightness being higher in plug plants than in bare-root plants. In May, only ‘Pircinque’ plug plant fruits had a lower L* value than those of the corresponding bare-root plants. In 2012, the plug plant chroma values were higher than those of the bare-root plants in March for all varieties. In April and May only, ‘Sabrosa’ plug plants had a lower value than the bare-root plants. Similar results were also obtained in the second year for all varieties. In 2012, plug plants’ hue values were lower than those of bare-root plants in all months for the ‘Sabrosa’ variety; the opposite result was found for ‘Jonica’. Variable results were recorded for ‘Pircinque’: higher hue values were found in plug plants in March, no significant differences in April, but lower values in May. In 2013, plug plants’ hue values were higher than those of the bare-root plants only for ‘Jonica’, confirming the results previously obtained.
3.3Antioxidant capacity
AA is one of the main bioactive compounds of strawberry fruits and a main determinant of their antioxidant capacity [37, 38]. In both years, in all the three months and for all varieties, plug plants and bare-root plants did not show any significant differences (Fig. 5). This result was partly in disagreement with the study of Crespo et al. [37] who detected a negative correlation between daily production (g per day) and AA content.
Fig.5
Ascorbic acid (AA), total phenolics (TPH), total anthocyanins (TACY) and total antioxidant capacity (TAC) of the two plant types (plug plant and bare-root), for the 3 varieties, in 3 months of harvest period, in 2012 and 2013. *Statistically significant differences between two plant types, per variety, month, and year, intra variety via LSD test P < 0.05.
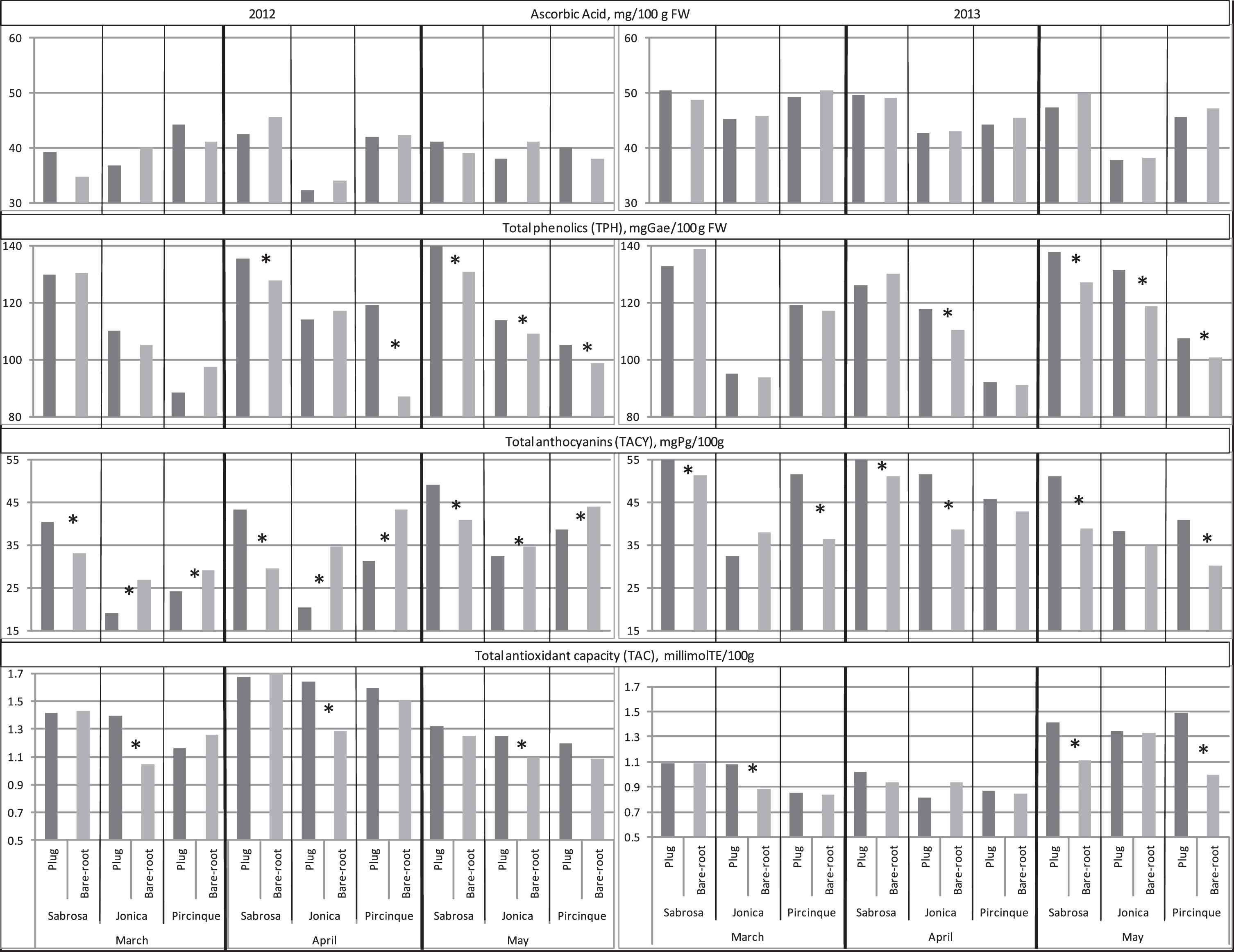
Strawberry fruits contained considerable amounts of TPH with putative health-promoting effects. In 2012, in March no differences were detected between the TPH values of the two plant types; in April, for ‘Sabrosa’ and ‘Pircinque’ varieties, and in May for all varieties. Fruit plug plant values were higher than bare-root plants. In March 2013, TPH results confirmed the data obtained for the previous year. In April, the TPH of ‘Jonica’ (and for all varieties in May) was higher for plug plants. A significant negative correlation between TPH and fruit weight was found (r = –0.45*), in agreement with previous studies [36, 39]. Phenolics are mainly concentrated in fruits skin [4], larger fruits have a lower surface-to-pulp ratio and, hence, lower TPH than the smaller ones. In this study, the smaller size of the fruit from the plug plants in April and May explained (even if not for all varieties and years) the higher content of TPH in fruit produced from plug plants.
TACY was negatively correlated to colour traits L*, chroma and hue (r=– 0.55*, – 0.54* and – 0.51* respectively), indicating that high TACY corresponded to darker fruit with a more saturated colour, supporting the findings of Cocco et al. [36]. In 2012, plug plants showed higher content of TACY for ‘Sabrosa’ in all the three months; in the other two varieties the opposite was found. In 2013, results were variable: only ‘Sabrosa’ confirmed the results of the previous year, while plug plants of ‘Jonica’ (in April) and ‘Pircinque’ (in March and May) had higher TACY content than the corresponding bare-root plants; the opposite of the results of the previous year.
TAC is taken as the cumulative action and synergic interaction of all the antioxidant compounds and, hence, represents overall free radical scavenging capacity. In 2012, ‘Jonica’ plug plants showed higher TAC values than bare-root plants in all the three harvest months. No significant differences were found for the other two varieties in these three months. In 2013, ‘Jonica’ plug plants showed higher TAC values than bare-root plants only in March. No significant differences were found in April. In May, the TAC of ‘Sabrosa’ and ‘Pircinque’ plug plants were higher than those of the bare-root plants.
It is well known that phenolic compounds are closely associated with TAC. We found a significant positive correlation between TAC and TPH (r = 0.51**), in agreement with others [4, 36, 38, 40]. A significant negative correlation between TAC and fruit size was also observed (r=– 0.56*), showing a higher antioxidant capacity in smaller fruits.
3.4HPLC analysis of phenolics
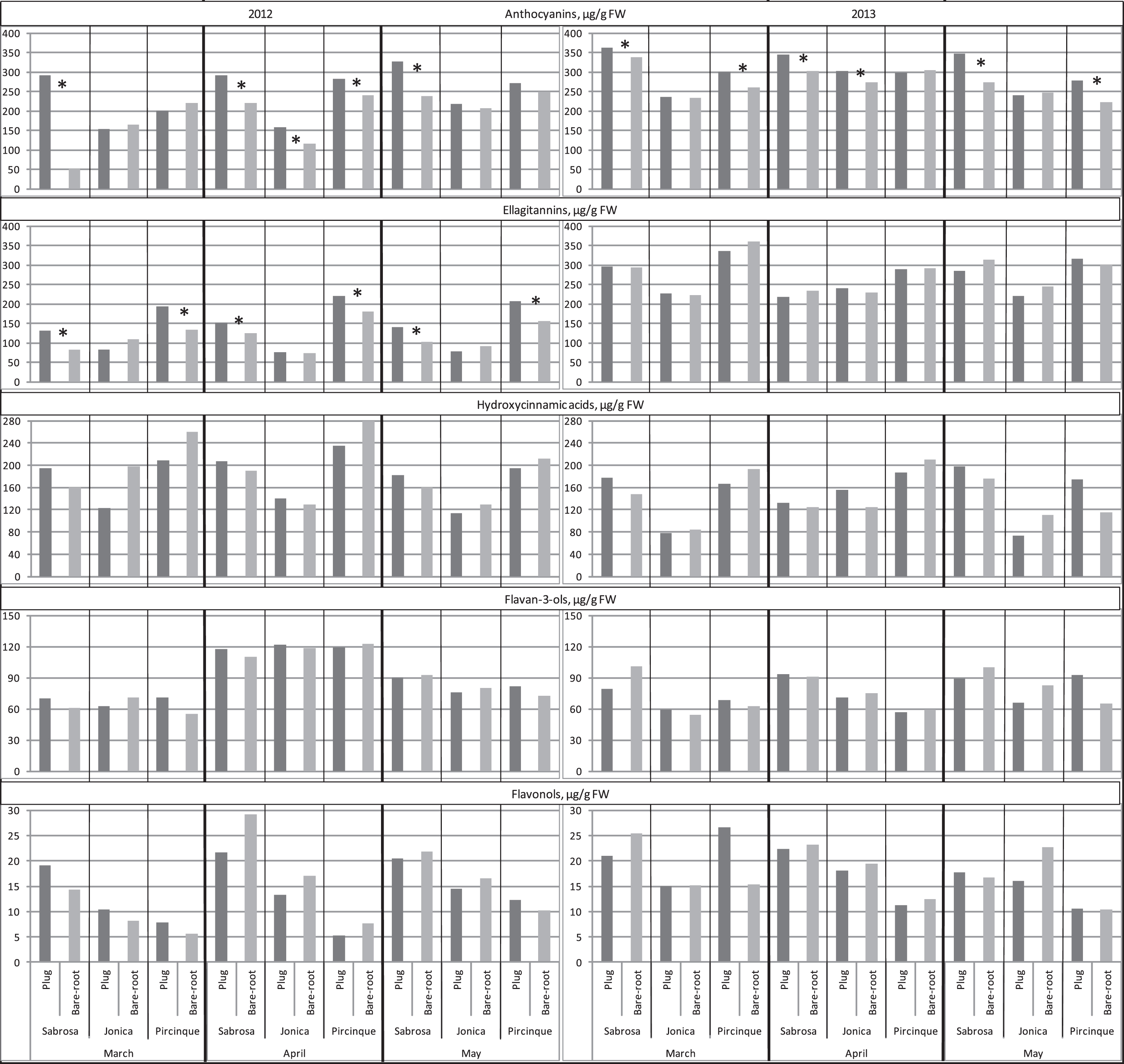
The five classes of phenolic compounds identified were anthocyanins, ellagitannins, hydroxycinnamic acids, flavan-3-ols and flavonols, confirming previous findings [36]. The main anthocyanin pelargonidin– 3– glucoside (Pg-3-gluc) was more than 95% of the total anthocyanin content (data not shown). Plug plants had higher average values for Pg-3-gluc in fruits, with some differences among the cultivars (Fig. 6), indicating that strawberry anthocyanin content was strongly affected by genotype, as previously reported [36, 41, 42]. The values for ‘Sabrosa’ plug plants were higher than for bare-root plants in all three months for both years; for ‘Jonica’, this was the case in April in both years; and for ‘Pircinque’, in April 2012 and March and May 2013. Predictably, Pg-3-gluc was correlated with TACY (r = 0.77*), in agreement with other findings [36, 38, 43]. In agreement with Crespo et al. [37] no significant correlation between Pg-3-gluc and TAC was found, indicating that anthocyanins are not the only important TAC determinants. Moreover, a significant negative correlation between fruit colour parameters and Pg-3-gluc content was found (r=– 0.31* and – 0.41* for chroma and hue, respectively), indicating that a high Pg-3-gluc count corresponded to darker colour, confirming previous results [44, 45].
Ellagitannins were the second phenolic compound studied. In 2012, ‘Sabrosa’ and ‘Pircinque’ plug plants had fruits with higher values than bare-root plants in all the three months. No significant differences were observed in 2013. The content of ellagitannins and TACY showed a significant correlation (0.53**), similar to Pg-3-gluc. The content of other phenolic compounds (hydroxycinnamic acid, flavan-3-ol and flavonol) in strawberry plants was not affected by plant type or genotype in either year.
Fig.6
Concentrations of phenolic compounds (anthocyanins, ellagitannins, hydroxycinnamic acids, flavan-3-ols and flavonols μg/g FW) determined by HPLC of the two plant types (plug plant and bare-root), for the 3 varieties, in 3 months of harvest period, in 2012 and 2013. *Statistically significant differences between two plant types, per variety, month, and year, intra variety via LSD test P < 0.05.
4Conclusions
This study confirmed that the start of the harvest for plug plants was significantly earlier than for bare-root plants, for all varieties in both years. The plug plants had a higher early productivity in February, March and April for all varieties. In May, the production slowed down with respect to the bare-roots plants. The fruit size of plug plants was larger than that of bare-root plants until March, but it decreased in April and May, when the yield was highest, and was significantly smaller than the berry weight of bare-root plants. This behaviour negatively affects the costs of production as the time needed to pick fruits is related to fruit size, so it is necessary to spend more time to collect small fruits.
With regards to the qualitative parameters of the fruit, the type of plant did not affect flesh firmness, TA, hue, AA, TAC and TACY, but it markedly influenced the other parameters.
The fruit of the plug plants had higher brightness and chroma in the first part of the harvest, compared with the bare-root plants, and higher TPH content in the second part. During harvest time, anthocyanins and ellagitannins were higher in the fruits of the plug plants than in those of the bare-root plants, while the sugar content was lower for the plug plant fruits.
Growers could successfully cultivate plug plants, if the purpose was to anticipate the harvest. In fact, the technique is well established in Sicily in order to take advantage of the favourable environmental conditions. This allows a considerable advance in ripening. Suitable nursery scheduling could allow plug plants to be supplied at a specific time such that it was possible to guarantee to the growers good results in terms of early harvesting time, taking into account the decrease in fruit size and the consequent increase in picking costs.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Acknowledgments
The authors wish to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) in Brazil, for the scholarship in Italy, process BEX 9734/11-2, issued to the student Carine Cocco.
References
[1] | Scalzo J , Politi A , Pellegrini N , Mezzetti B , Battino M . Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. (2005) ;21: :207–13. |
[2] | Crespo P , Ançay A , Carlen C , Stamp P . Strawberry cultivar response to tunnel cultivation. Acta Hortic. (2009) ;838: :77–81. |
[3] | Correia PJ , Pestana M , Martinez F , Ribeiro E , Gama F , Saavedra T , Palencia P . Relationships between strawberry fruit quality attributes and crop load. Scientia Hortic. (2011) ;130: :398–403. |
[4] | Capocasa F , Scalzo J , Mezzetti B , Battino M . Combining quality and antioxidant attributes in the strawberry: The role of genotype. Food Chemistry. (2008) ;111: (4):872–8. |
[5] | Roussos PA , Denaxa NK , Damvakaris T . Strawberry fruit quality attributes after application of plant growth stimulation compounds. Sci. Hortic. (2009) ;119: :138–46. |
[6] | Tulipani S , Marzban G , Herndl A , Laimer M , Mezzetti B , Battino M . Influence of environmental and genetic factors on health-related compounds in strawberry. Food Chemistry. (2011) ;124: :906–13. |
[7] | Kosar M , Kafkas E , Paydas S , Baser KHC . Phenolic composition of strawberry genotypes at different maturation stages. Journal of Agricultural and Food Chemistry. (2004) ;52: (6):1586–9. |
[8] | Voth V , Bringhurst RS . Culture and physiological manipulation of California strawberries. Hort Science. (1990) ;25: :889–92. |
[9] | Reekie JY , Hicklenton PR , Duval J , Chandler C , Struik PC . Manipulating transplant morphology to advance and enhance fruit yield in strawberry. Acta Hortic. (2003) ;626: :235–40. |
[10] | Lopez Aranda JM . The cultivation of the strawberry in Huelva. In “The strawberry crop at Huelva”. Junta de Andalusia. (2008) :103–70. |
[11] | Recupero S , D’Anna F , Arcuti P . “The very early maturing productions of strawberry in Southern environments”. Rivista di Frutticoltura e di Ortofloricoltura. 1995. |
[12] | Faedi W , Gufo S , Bernardini D . Piantagioni autunnali di piante fresche di fragola in Sicilia. Atti Incontro Frutticolo S.O.I. su: “La coltura della fragola”. Ferrara, 14 febbraio. (1986) :42–44. |
[13] | Maas JL . Opportunities to Reduce the potential for disease infection and spread with strawberry plug plants. Acta Hortic. (1998) ;513: :409–16. DOI: 10.17. |
[14] | Poling EB , Maas JL . “Strawberry plug transplant technology”. Acta Hort. (1998) ;513: :393–401. |
[15] | Pritts M , Handley D . “Strawberry production guide for the Northeast, Midwest, and Eastern Canada”. Natural Resource, Agriculture, and Engineering Service (NRAES), Ithaca, New York. (1998) :14853–5701. |
[16] | Poling EB , Parker K . “Plug production of strawberry transplants”. Advances in strawberry production (USA). (1990) ;9: :37–9. |
[17] | Hochmuth G , Cantliffe D , Chandler C , Stanley C , Bish E , Waldo E , Legard D , Duval J . “Fruiting responses and economics of containerized and bare-root strawberry transplants established with different irrigation methods”. HortTechnology. (2006) ;16: (2):205–10. |
[18] | Bish EB , Cantliffe DJ , Chandler CK . “Strawberry daughter plant size alters transplant growth and development”. VIII International Symposium on Timing Field Production in Vegetable Crops. (1997) ;533: :121–6. |
[19] | Sances FV . Conventional and organic alternatives to methyl bromide on California strawberries. Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions. (2003) :24.1–24.3. |
[20] | Morey L . Strawberry plugs: Pros and cons. Florida Grower. (2001) ;94: :6–7. |
[21] | Kokalis-Burelle N . “Effects of transplant type, plant growth-promoting rhizobacteria, and soil treatment on growth and yield of strawberry in Florida”. Plant and soil. (2003) ;256: (2):273–80. |
[22] | Durner EF , Poling EB , Maas JL . “Recent advances in strawberry plug transplant technology”. HortTechnology. (2002) ;12: (4):545–50. |
[23] | D’Anna F , Caracciolo G , Alessandro R , Faedi W . “Effects of plant type on two strawberry cultivars in Sicily”. VII International Strawberry Symposium. (1049) :553–6. |
[24] | Bish EB , Cantliffe DJ , Chandler CK . Temperature conditioning and container size affect early season fruit yield of strawberry plug plants in a winter, annual hill production system. Hort Science. (2002) ;37: :762–4. |
[25] | Gilreath JP , Santos BM , Noling JW , Locascio SJ , Dickson DW , Rosskopf EN , Olson SM . Performance of containerized and bare-root transplants with soil fumigants for Florida strawberry production. Hort Technology. (2006) ;16: :461–5. |
[26] | Hennion B , Schupp J , Longuesserre J . ‘Fraisimotte’: A strawberry plug plant developed by CIREF in France. Proc. Third Intl. Strawberry Symp. Acta Hort. (1997) ;439: :469–74. |
[27] | Menzel CM , Toldi A . “An evaluation of containerized plants for strawberries growing in a subtropical environment”. Hort Technology. (2010) ;20: (4):786–93. |
[28] | Andreotti C , Ravaglia D , Ragaini A , Costa G . Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Annals of Applied Biology. (2008) ;153: :11–23. |
[29] | Camin F , Perini M , Bontempo L , Fabroni S , Faedi W , Magnani S , Baruzzi G , Bonoli M , Tabilio MR , Musmeci S , Rossmann A , Kelly SD , Rapisarda P . Potential isotopic and chimica markers for characterising organic fruits. Food Chemestry. (2011) ;125: :1072–82. |
[30] | Diamanti J , Capocasa F , Denoyes B , Petit A , Chartier P , Faedi W , Mezzetti B . Standardized method for evaluation of strawberry (Fragaria×ananassa Duch.) germoplasm collections as a genetic resource for fruit nutritional compounds. Journal of Food Composition and Analysis. (2012) ;28: (2):170–8. |
[31] | Slinkard K , Singleton VL . Total phenol analysis: Automation and comparison with manual methods. American Journal of Enology and Viticulture. (1977) ;28: (1):49–55. |
[33] | Giusti MM , Wrolstad RE . Characterization and measurement of anthocyanins by UV visible spectrescopy. Current protocols in food analytical chemistry. (2001) :19–31. |
[34] | Lo Scalzo RL , Genna A , Branca F , Chedin M , Chassaigne H . Anthocyanin composition of cauliflower (Brassica oleracea L. var. botrytis) and cabbage (B. oleracea L. var. capitata) and its stability in relation to thermal treatments. Food Chemistry. (2008) ;107: (1):136–44. |
[35] | Re R , Pellegrini N , Proteggente A , Pannala A , Yang M , Rice-Evans C . Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. (1999) ;26: (9):1231–7. |
[36] | Cocco C , Magnani S , Maltoni ML , Quacquarelli I , Cacchi M , Antunes LEC , D’Antuono LF , Faedi W , Baruzzi G . “Effects of site and genotype on strawberry fruits quality traits and bioactive compounds”. Journal of Berry Research. (2015) ;5: (3):145–55. |
[37] | Crespo P , Bordonaba JG , Terry LA , Carlen C . Characterisation of major taste and health-related compounds of four strawberry genotypes grown at different Swiss production sites. Food Chemistry. (2010) ;122: (1):16–24. |
[38] | Tulipani S , Mezzetti B , Capocasa F , Bompadre S , Beekwilder J , De Vos CHR , et al. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. Journal of Agricultural and Food Chemistry. (2008) ;56: (3):696–704. |
[39] | Anttonen MJ , Hoppula KI , Nestby R , Verheul MJ , Karjalainen RO . Influence of fertilization, mulch color, early forcing, fruit order, planting date, shading, growing Environment, and genotype on the contents of selected phenolics in strawberry (Fragaria×ananassa Duch.) fruits. Journal of agricultural and food chemistry. (2006) ;54: (7):2614–20. |
[40] | Meyers KJ , Watkins CB , Pritts MP , Liu RH . Antioxidant and antiproliferative activities of strawberries. Journal of agricultural and food chemistry. (2003) ;51: :6887–92. |
[41] | Carbone F , Preuss A , De Vos RCH , D’Amico E , Perrotta G , Bovy AG , et al. Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant, Cell & Environment. (2009) ;32: (8):1117–31. |
[42] | Josuttis M , Carlen C , Crespo P , Nestby R , Dietrich H , Kruger E . A comparison of bioactive compounds fruit from Europe affected by genotype and latitude. Journal of Berry Research. (2012) ;2: :73–95. |
[43] | Crecente-Campo J , M Nunes-Damaceno M , Romero-Rodriguez MA , Vazquez-Odériz ML . Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria×ananassa Duch, cv Selva). Journal of Food Composition and Analysis. (2012) ;28: :23–30. |
[44] | Prior RL , Cao G , Martin A , Sofic E , McEwen J , O’Brien C , et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. Journal of Agricultural and Food Chemistry. (1998) ;46: (7):2686–93. |
[45] | Pineli LDLDO , Moretti CL , dos Santos MS , Campos AB , Brasileiro AV , Córdova AC , et al. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. Journal of Food Composition and Analysis. (2011) ;24: (1):11–6. |




