Andean Berry (Vaccinium meridionale Swartz) Juice improves plasma antioxidant capacity and IL-6 levels in healthy people with dietary risk factors for colorectal cancer
Abstract
BACKGROUND:
Vaccinium meridionale is an Andean Berry that contains bioactive compounds with antioxidant and anti-inflammatory properties that can prevent oxidative and pro-inflammatory events associated with colorectal carcinogenesis initiation.
OBJECTIVE:
Determine if Andean Berry Juice (ABJ) can improve plasma antioxidant capacity and reduce pro-inflammatory cytokines of healthy people with dietary factors for colorectal cancer.
METHODS:
Nineteen healthy volunteers received 250 mL of ABJ for 14 days. Plasma was obtained before and after the intervention period to analyze antioxidant status through ABTS and isoprostane methods, IL-1β, TNF-α, IL-6 cytokines and total phenolic content were also analyzed by colorimetric methods.
RESULTS:
Plasma antioxidant capacity increased (0.222 to 0.555 mM Trolox, p≤0.0001) and isoprostane levels were significantly reduced (264 pg/mL to 197 pg/mL) and positively correlated with the total antioxidant capacity (r = 0.037) after juice consumption, whereas total phenol content decreased (7.08 to 7.04 mg gallic acid equivalent/L, p≤0.0249) at the end of intervention. Plasma IL-6 levels were significantly reduced (p = 0.0230), while IL-1β and TNFα did not show changes after juice intake.
CONCLUSIONS:
These results indicate that ABJ daily intake improves plasma antioxidant capacity and reduces levels of the pro-inflammatory cytokine IL-6 associated with colorectal carcinogenesis.
1Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in men and the second in women [1]. The highest prevalence rates have been reported in developed countries, and the lowest rates are found in Africa and South-West Asia [2]. According to the findings of Doll and Peto in 1981 [3] adjusted by Willett in 2005 [4], 35% of deaths caused by CRC are associated with dietary factors, either due to the presence in the diet of carcinogenic agents or due to low intake of foods with preventive properties. According to the study conducted by the World Cancer Research Fund in 2012 [5], there are four dietary factors with convincing and probable evidence that increase the risk of developing CRC: consumption of red meat and processed meat, alcohol consumption, overweight and abdominal fat, and low intake of fiber-containing foods such as fruits, vegetables, cereals and whole grains.
The consumption of red meat is associated with higher levels in the colonic mucosa of nitrates, amines, sulfur, ammonia, branched-chain amino acids and hydrogen sulfide (H2S) associated with a pro-inflammatory state [6, 7]. Alcohol is a prominent carcinogen because excessive consumption leads to the increase of intracellular levels of Reactive Oxygen Species (ROS) [7, 8] that induces chromosomal abnormalities, DNA damage, epigenetic changes, and up-regulation of TNF-α [9]. In addition, alcohol oxidates membrane phospholipids that may be subsequently transformed into isoprostanes, which have been implicated in the progression of carcinogenesis [10]. This type of pro-inflammatory diet leads to oxidative stress activate transcription factors such as the NF-κ pathway associated with the increase in the expression of pro-inflammatory IL-1β, TNFα and IL-6 cytokines [11] involved in the colorectal carcinogenesis initiation [12–15].
Among the chemopreventive mechanisms against CRC present in fruits from the Vaccinium genus, the antioxidant and anti-inflammatory properties have gained special attention. These fruits are a source of poly (phenolic) compounds identified as bioactive, and are able to modulate the expression of molecules involved in the development of CRC as observed in vitro and in vivo studies [16–19].
Vaccinium meridionale Swartz, Andean Berry, is a native Colombian plant that belongs to the Ericaceae family. The fruit is a dark purple globose berry when ripe and has been considered as a potential functional food, due to its high content of phenolic compounds and anthocyanins, giving it an antioxidant capacity similar or greater to the reported values for other Vaccinium species [20, 21]. Maldonado et al 2014 [22] reported the antioxidant capacity of an aqueous extract obtained from this fruit, as well as cytotoxic and anti-proliferative activities against colon adenocarcinoma cells [23]. Recently, Agudelo et al. [24] reported that ABJ inhibits the growth of colon adenocarcinoma cells by apoptotic mechanisms. Therefore, taking into account the importance of health benefits of fruit consumption against colon carcinogenesis, and that juices are a good strategy to increase their intake, we aimed to determine the effect of Andean Berry Juice consumption on the antioxidant capacity of plasma and pro-inflammatory biomarkers in healthy individuals with dietary risk factors for CRC.
2Materials and methods
2.1Plant material
Fresh ripe Andean Berries (Vaccinium meridionale) were harvested from the municipality of El Retiro (Antioquia, Colombia) at an altitude of 2175 meters above sea level and 16°C of temperature in May 2015. Healthy, ripe berries were selected, washed, disinfected (sodium hypochlorite, 100 ppm) and dried. Afterward, the berries were blended for 2 min at 2500 rpm and freeze-dried in a vacuum chamber under pressure (4.27±0.5 mm Hg) at a temperature of –50°C. The resulting freeze-dried powder was stored at room temperature and protected from light in a PET packaging aluminum, to be used as an ingredient in the subsequent preparation of the juice.
2.2Preparation of Andean Berry Juice
The Andean Berry Juice (ABJ) was prepared following the procedure previously described by Franco et al 2016 [23]. Briefly, the freeze-dried powder of Andean Berry was dissolved in 55.4% sterile water and 9% sucrose to obtain a juice of 11.1° Brix, an acidity of 4.33 mg citric acid/mL and pH 3.06. Further, the ABJ was sonicated at 42 kHz, 135 W (Branson B3510 sonicator, Ultrasonic Corporation, USA) for 15 min at RT (25±1°C). The microbiologic analysis of the recount of molds and yeasts was performed according to the Colombian Technical Norms ISO 21521-1 and ISO 21521-2, and taking into account the limits established by the Colombian Resolution 3929/2013 for dehydrated fruits. The microbiological analysis showed no growth of heterotrophic plaques, fecal coliforms, or yeasts or fungi (data not shown). Franco et al 2016 reported the nutritional composition of the ABJ.
2.3Sensory evaluation
Trained judges performed the sensory evaluation in the Sensory Analysis laboratory of the University of Antioquia, according to the Colombian Technical Norms 3501 [25], 3932 [26] and Colombian Technical Guidelines 165 and 226 [27, 28].
2.4Subjects and study design
Women and men between the ages of 18 and 60 years from Universidad de Antioquia (Medellin, Colombia) were recruited via advertisements. After the administration of a questionnaire concerning dietary habits and lifestyle, potential participants were invited to participate in the study and provided written informed consent. We selected 19 individuals who had at least one of the dietary risk factors for colorectal cancer presented in Table 1. Exclusion criteria were as follow: i) family history of colon or rectal cancer; ii) smoking; iii) current use of medication or supplemental vitamins and/or minerals; iv) physical activity for more than 150 min/week; v) any inflammatory bowel disease, cancer, diabetes, gastrointestinal, renal or infectious diseases; vi) pregnancy; vii) vegetarian diet; viii) consumption of more than 2 fruits/day; ix) consumption of two or more servings of vegetables/day. This was a quasi-experimental prospective panel study, without control. The control was the same participant in their initial state before the intervention. The sample size was calculated for the repeated means based on a similar population study [29], in which significant statistical differences were observed in the same tracer variables of our study (the total polyphenol content of the plasma and the antioxidant capacity). Volunteers consumed 250 mL ABJ for 14 days in the morning. Throughout the study, subjects had to avoid the intake of foods rich in anthocyanins, therefore a list of vegetables and fruits that the subjects were not allowed to eat was provided. The subjects kept food diaries during the intervention period to check their compliance with the dietary restriction, through which it was confirmed that all participants had frequent consumption of red meat and processed meat, low consumption of fruits, vegetables and dietary fiber according to Table 1, and a low alcohol intake. No information on quantitative food intake was available as the diaries were dietary food records without any information on quantities. The research protocol was approved by the Ethics Committee of odontology Faculty at Universidad de Antioquia (Concept N° 23-2016, Acta N° 8 of 2016, at Medellin, 30 September of 2016).
Table 1
Dietary risk factors for colorectal cancer according to the World Cancer Research Fund and American Institute for Cancer Research [5]
| Level of evidence | Increased risk | Decreased Risk |
| Convincing | Consumption of 10 grams per day of alcohol (100 mL of wine, 250 mL of beer, 30 mL of rum); | Physical activity for more than 150 min/week |
| Consumption >50 g/day of processed meats | Consumption of foods containing dietary fiber (10 g/day) | |
| Consumption >100 g/day of red meat (pork, beef or lamb); | ||
| Consumption ≤2 servings/day of fruits or vegetables | ||
| Probable | Consumption <200 g/day of cow’s milk |
2.5Blood sampling
At the end of each intervention period, venous blood samples (10 mL) were drawn from each fasting volunteer into evacuated tubes containing sodium heparin. Each whole blood sample was centrifuged for 15 minutes at 3500 rpm. Plasma was carefully separated into Eppendorf tubes and stored at –80°C until use.
2.6Antioxidant activity determined by ABTS•+ (2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) assay
Plasma (10 μL) and stock solution ABTS•+ (990 μL) were mixed and the resulting solution was incubated at room temperature for 30 minutes in the dark. The absorbance was measured at 734 nm against a blank. Trolox was used as a reference standard and the results were expressed as μM Trolox/L [30].
2.7Plasma 8-isoprostanes
Oxygen radicals using a commercial kit (8-Isoprostane ELISA kit, Cayman, USA) measured the 8-isoprostane as biomarkers of oxidation of tissue phospholipids following the manufacturer instructions. This method is based on the competition between 8-isoprostanes present in plasma (50 μL) and an 8-isoprostane-acetylcholinesterase (AchE) conjugate (50 μL) for a limited number of 8-isoprostane-specific rabbit antiserum binding sites. After incubation and wash, the substrate to AChE was added, a yellow product of this enzymatic reaction was measured at 412 nm and is inversely proportional to the amount of 8-isoprostane present in the sample [31]. The results were expressed as pg/mL.
2.8Total phenols in blood plasma
The total phenolic content was determined according to the adapted Folin–Ciocalteu method [32]. Plasma deproteinized by perchloric acid (50 μL) was mixed with 125 μL of Folin–Ciocalteu reagent and 400 μL of sodium carbonate solution (7.1% p/v), and the resulting solution brought to a final volume of 1000 μL. The mixture was stirred and stored at room temperature for 30 minutes in the dark. The absorbance was measured at 760 nm against a control sample. Aqueous solutions of Gallic acid were used to build a calibration curve. The results were expressed as Gallic acid equivalents (GAE)/ml.
2.9Inflammatory biomarkers
At day 1 and at the end of the intervention (day 15) levels of interleukin-1β, interleukin-6, TNF-α, C-Reactive Protein (CRP) and adiponectin were determined in plasma by using the Human ELISA kit (Cayman Chemical and SPI Bio) according to manufacturer’s instructions.
2.10Statistical analysis
The total phenol content, the total antioxidant capacity and the plasma concentration of 8-isoprostanes compared before and after the consumption of ABJ were analyzed by the paired two-tailed test. The difference in the distributions of the levels of TNFα, IL-1β, and IL-6 before and after the intervention was evaluated by the Wilcoxon rank-sum test. The summaries of the distributions include the mean, the standard deviation, the median and the interquartile range (IQR). In order to determine the correlation between the pro-inflammatory biomarkers and the antioxidant capacity in the plasma, the Spearman coefficients (r) were calculated. All data were measured in duplicate and the results were considered significant when p≤0.05. These analyzes were performed with Graph Pad Prism version 7.00 for Windows (GraphPad Software, San Diego California, USA).
3Results and discussion
The intake of fruit as juice has gained great acceptance in recent years; it has been estimated that the intake of juices has increased significantly compared to the consumption of whole fruit [33]. Since berry juices contain polyphenolic antioxidant compounds that may be involved in the inhibition of chronic inflammatory processes, they might be consumed regularly as part of a normal diet to reduce the incidence of chronic non-communicable diseases.
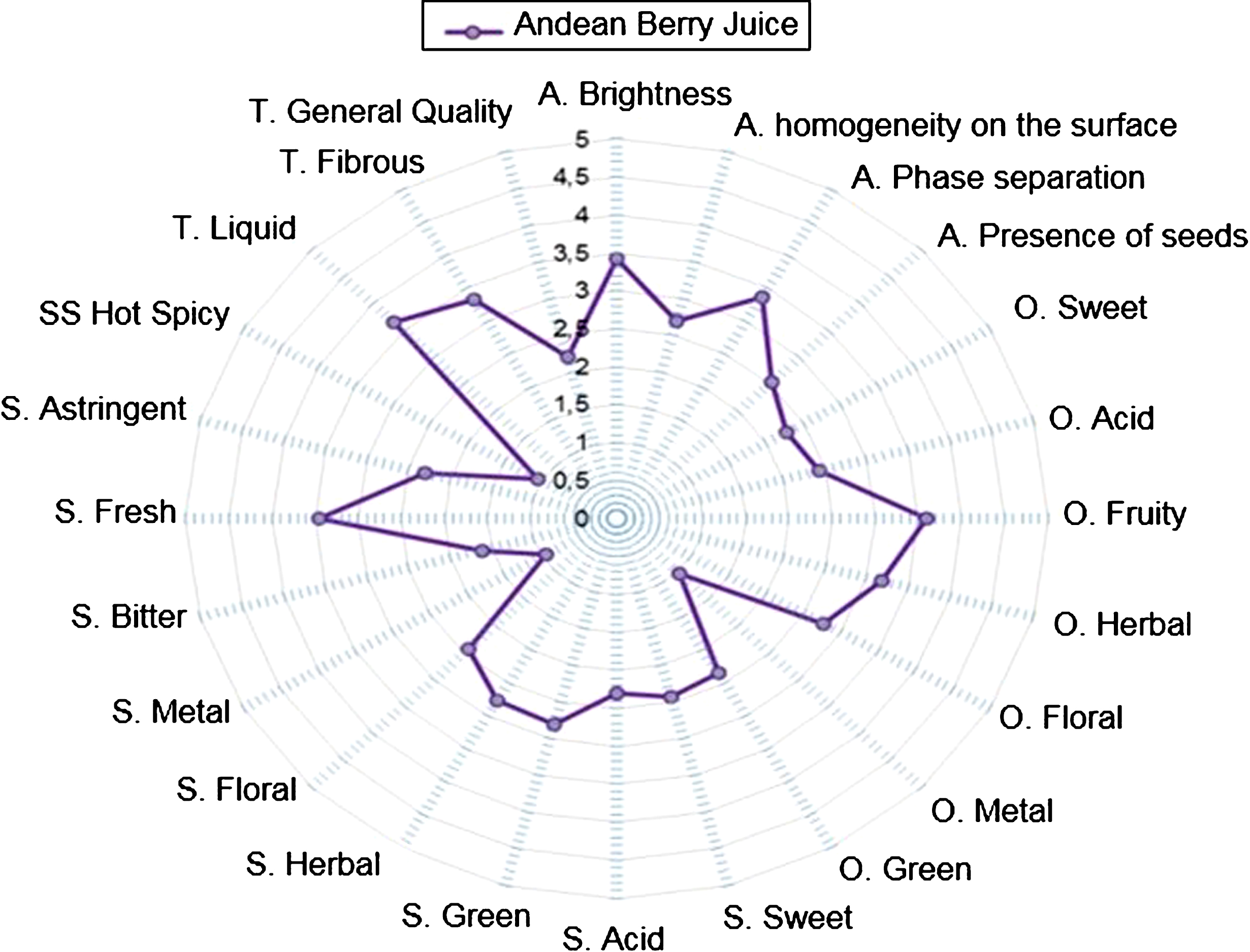
In this study, we began describing the sensory profile of this product by a multidimensional approach to identify descriptors that can be used to determine the quality of a product, giving the maximum information to establish a sensory profile. We used this method to evaluate the quality of ABJ. The quality of this product was measured with a score of 3 to 1, where 3 is of high quality and 1 is of low quality. All other descriptors are evaluated with a score of 0 to 5, where 0 is the low intensity of the sensory attribute and 5 is the high intensity of the sensory attribute. Figure 1 shows the sensory profile description of ABJ performed by a trained jury. In the appearance descriptors, the juice had an intensity greater than 3.0 for brightness and phase separation; an intensity lower than 3.0 for the attributes of homogeneity due to the presence of seeds. In the descriptions of the odors, the juice presented intensities higher than 3.0 corresponding to the fruity and herbaceous odor; intensities lower than 3.0 for sweet, acid, floral, metallic and green odors. In relation to the attributes of flavor, sweet taste, acid, green, herbal, floral, metallic, bitter, astringent and spicy was 3.5; and the fresh taste was 3.5. In general, the panel of 10 experts judged the quality of the product as 2.2, which is considered a product of good sensory quality. Therefore, we consider that this juice can easily be incorporated into the human diet as a strategy to increase or promote not only the consumption of fruits as agents rich in antioxidant compounds, but to prevent the inflammatory state by modulating biomarkers present in chronic non-communicable diseases associated with oxidative stress and chronic inflammation [11, 12].
Fig.1
Sensory profile of the ABJ. Conventions; A: appearance, O: smell, F: taste, T: texture.
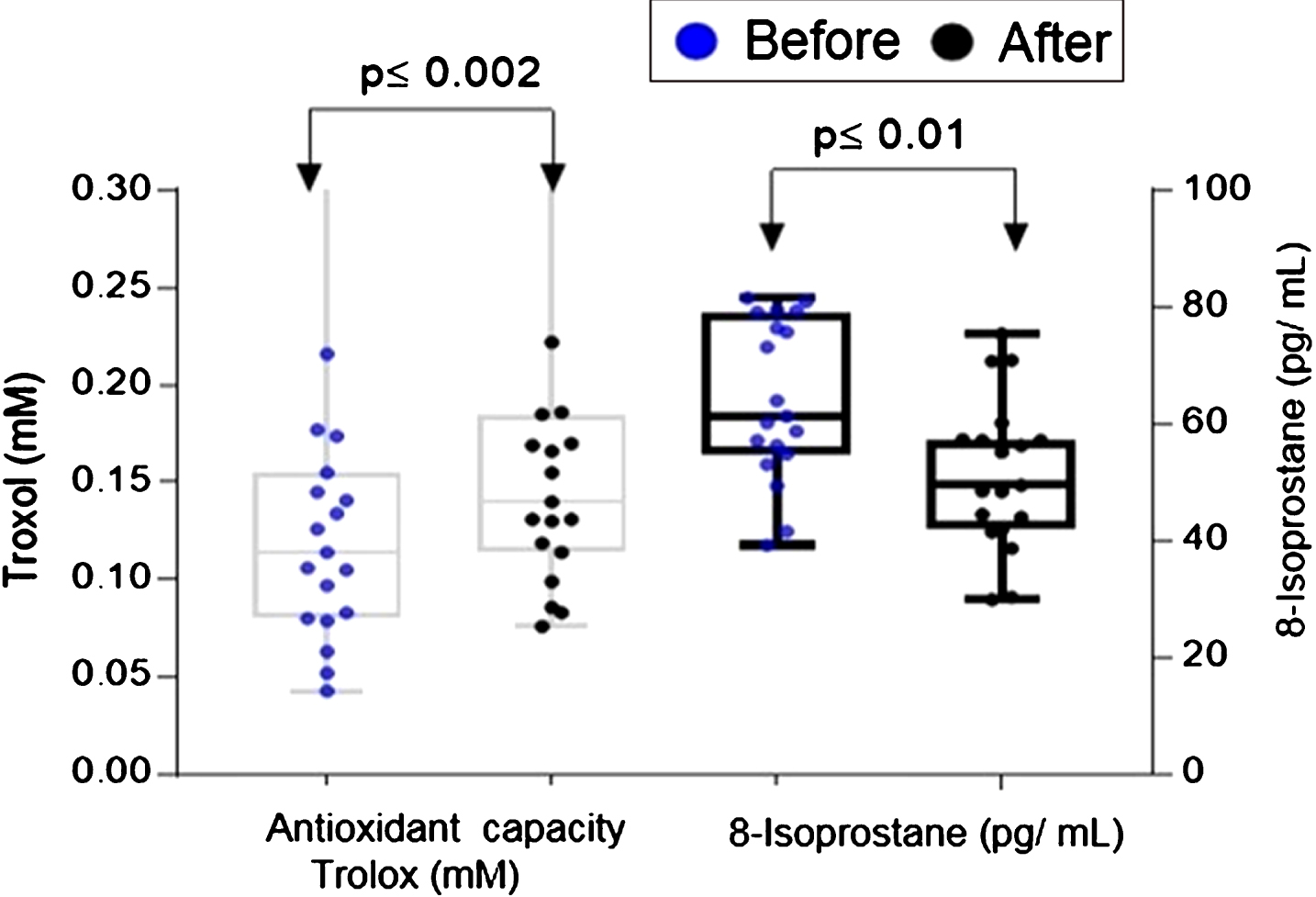
When comparing the basal plasma antioxidant status (0.222 Trolox mM) with the levels at the end of the intervention (and 0.052 Trolox mM), the plasma antioxidant capacity increased up to four times (Fig. 2) with a significance of 0.002. In relation to the 8-isoprostanes levels, the paired two-tailed test showed a significant reduction (p≤0.0001) in the plasma of participants at the end of the intervention (Fig. 2). The mean of the 8-isoprostanes levels in plasma at the end of the study was 197±64 pg/mL; on the contrary, the median at baseline was 264±44 pg/mL. The total concentration of phenols in plasma showed a significant decrease (p≤0.0392), these oscillated between 4.2 and 10.2 (mg of EAG / L) with a median of 7.04 at the end of the study, while the baseline presented a median of 7.084 mg of EAG / L.
Fig.2
Plasma total antioxidant capacity and 8-isoprostanes levels before and after 14 day intake of ABJ in healthy individuals with dietary risk factors associated with CRC. The comparison was performed by paired two-tailed t-test. The difference in distributions for total antioxidant capacity was analyzed by the Wilcoxon rank sum test.
Consumption of fruits rich in polyphenols, such as berries of the genus Vaccinium, may reduce the risk of developing CRC (RR = 0.85, 95% CI = 0.75–0.96 for 3 servings/day) [34]. To become bioactive compounds, these polyphenols must undergo various intestinal transformations; however, the bioavailability of the polyphenols in the berries depends on their specific structural subfamilies. In this study, we report a reduction in total plasma phenols after 14 days of ABJ intake compared to the baseline (p≤0.0392). Although anthocyanins are the most abundant polyphenol compounds present in ripe Andean Berry [20, 21], the high molecular weight affects its absorption in the small intestine. For example, after intake for seven days of 45g of lyophilized black raspberries, the total anthocyanins content in plasma and urine was 1% because they cannot be absorbed in the native form [35]. However, anthocyanins pass from the small intestine into the large intestine where they are catabolized by the colonic microbiota, which allows a diversity of phenolic acids including the acid 4-O-methyl-gallic, which is absorbed and passed into the circulatory system [36].
The ABJ was prepared using the whole fruit, including the epidermis where the highest concentration of anthocyanins and fiber is found, due to which some participants reported in the first three days of intervention diarrheal episodes, which may have contributed to decreasing the total phenol content in plasma. However, when determining the plasma antioxidant capacity at the end of the study, the samples were able to inhibit up to 4 times more the oxidation of the ABTS radical in ABTS+. These findings suggest that the antioxidant capacity of ABJ in plasma may be attributed to components other than anthocyanins, such as Gallic acid.
In a previous study, our group used a model of gastrointestinal digestion in vitro to study the free phenolic bioavailability of ABJ. The Gallic acid was the phenolic acid with the highest bioavailability in most stages of gastrointestinal digestion: mouth (721.51%), stomach (529.17%), small intestine and colon (up to 158.89 and 33.91%, respectively) (unpublished data). Gallic acid has been associated in the prevention of lipids peroxidation. This phenolic acid has a 3-OH group and ortho-dihydroxyl substitution, which gives it the antioxidant capacity based on electron transfer (ET) [37], such as that used in the protonation of the ABTS radical •. The correlation analysis (Table 2) indicates that the decrease in the levels of 8-isoprostanes can be explained up to 37% by the increased improvement of plasma antioxidant status in healthy individuals that ingest ABJ regularly for 14 days.
Table 2
Spearman correlation coefficients (r) for antioxidant capacity, IL-6, and 8-isoprostanes levels in plasma at the end of the intervention
| IL-6 | Antioxidant capacity | 8-isoprostanes | |
| IL-6 | 1 | – | – |
| Antioxidant capacity | 0.30375163 | 1 | – |
| 8-isoprostanes | 0.32459533 | 0.377295286 | 1 |
The 8-isoprostanes has been accepted as a biomarker to monitor oxidative stress in vivo, since it is a product of lipid peroxidation from arachidonic acid present in phospholipids of the cell membrane, and consequently increases in blood and urine [38]. In agreement with our results, Davinelli et al (2015) [39] designed a double-blind, placebo-controlled study and reported a decrease in urinary levels of 8-isoprostanes after ingestion of anthocyanin extract of maqui berry. A reduction of 8-isoprostanes levels by 60% was also observed in the urine of patients with premalignant lesions in the esophagus who received 6 g (women) or 45 g (men) of freeze-dried black raspberries for 6 months [40].
There is a close link between chronic inflammation and CRC, one of the conditions of greatest risk for this type of cancer is represented by inflammatory bowel disease (IBD) [41]. The use of non-steroidal anti-inflammatory drugs (NSAIDs) reduces mortality from sporadic colon cancer and may reduce the number of adenomas in patients with familial adenomatous polyposis [42]. Several studies suggest that plasma levels of TNFα, IL-1β, and IL-6 are increased in patients with CRC. These cytokines can have value as biomarkers for early diagnosis [43]. Given the above, we set out to determine the plasma levels of TNFα, IL-1β, and IL-6. No significant differences were found after ingestion of ABJ in the plasma of the volunteers who completed the study (Fig. 3). On the other hand, the concentration of IL-6 decreased significantly after the intervention compared to baseline levels (p≤0.03), with a median of 35.4 pg / mL at the beginning of the intervention and a median of 23.2 pg / mL at the end of the 14 days of ABJ intake (Fig. 3).
Fig.3
Plasma IL-1β, IL-6 and TNFα levels before and after intake of ABJ for 14 days in healthy participants with dietary factors associated with risk for CRC. The difference in the distribution of inflammatory cytokines was analyzed using the Wilcoxon signed rank test.
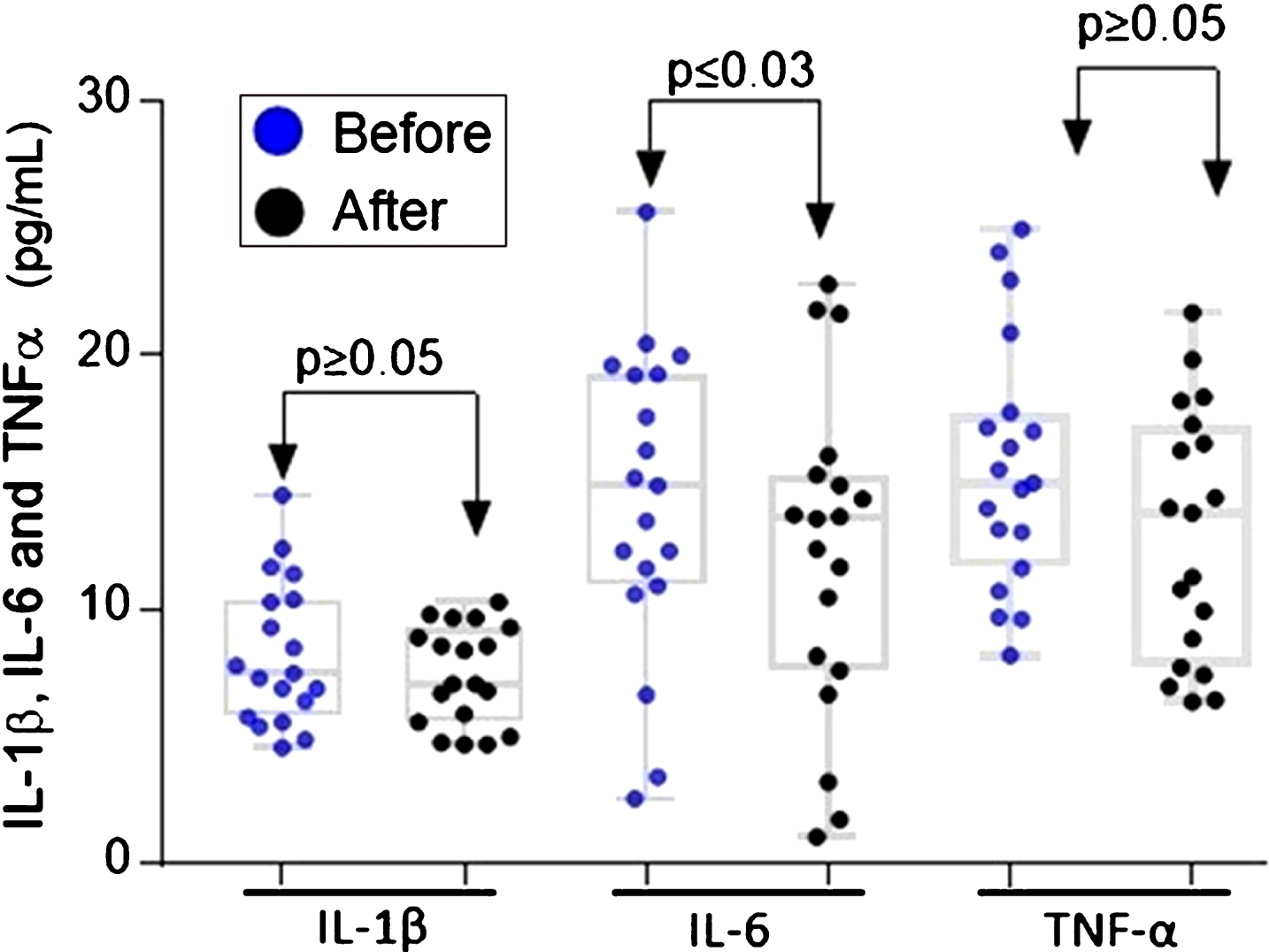
Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine that also has an apoptotic effect, induces cell proliferation, and induces differentiation. Al Obeed in 2014 [44] collected colon tissue samples from 30 patients with CRC and found that the TNF-α gene up-regulated and associated with stage III and IV neoplasms compared to premalignant tumor stages (p = 0.004), indicating that the high expression of this cytokine is associated with advanced stages of the tumor. In the present study, no significant differences were found in the medians of the TNF-α plasma levels after ABJ intake, because participants of this study were healthy, and the expression of TNF-α is evident in advanced stages of carcinogenesis and not in the initial stages.
Interleukin 1β (IL-1β) is a pro-inflammatory cytokine, which induces the expression of TNFα, IL-6, among other pro-inflammatory mediators and growth promoters of tumor cells [45]. In an interesting randomized study of four years, Bobe et al (2010) [19] determined that consumption of fiber, fruit, and vegetables rich in polyphenols inhibits the occurrence of colorectal adenoma. The number of flavonoids consumed by 1905 participants with a confirmed diagnosis of adenoma was estimated through a food frequency questionnaire. The researchers concluded that patients with flavonoid intake above the median 29.7 mg/day and decreased IL-1β concentrations had the lowest risk of advanced adenoma recurrence (OR = 0.37, 95% CI = 0.15–0.94) [19]. In our research, although the participants included in this study present dietary habits that may increase the risk for developing CRC long-term, they did not present significant differences in the plasma IL-1β concentration after intake of ABJ for 14 days. This supports the theory that IL-1β is a potential biomarker for advanced stages of CRC [46, 47].
Several experimental and clinical studies have linked the cytokine IL-6 with the pathogenesis of CRC. The increase in the expression of IL-6 has been associated with advanced stages of CRC and a decrease in patient survival. Unlike other cytokines, IL-6 is expressed in the earliest stages of carcinogenesis since this is key in the regulation of inflammation through the activation of the pathway STAT-3 [48]. In a randomized controlled dose-response trial with 60 volunteers, it was demonstrated that consumption of lyophilized strawberries in adults with abdominal adiposity (BMI: 36±5 kg/m2) and elevated serum levels of lipids during 12 weeks, decreased significantly the serum levels of IL-6, whereas no differences were found in the levels of IL-1β [49]. The intake of dietary polyphenols, especially from berries, has been associated with anti-inflammatory effects in experimental models, although there are few supportive clinical studies [50]. In our study, we reported for the first time that consumption of ABJ decreases by 19.4% (p = 0.0230) the plasma levels of IL-6 (Fig. 3), one of the most important cytokines in the initiation and promotion of colon carcinogenesis.
In conclusion, the ABJ evaluated in this study has adequate sensory properties, which increased by 4-fold the antioxidant capacity of the plasma of participants who ingested 250 mL of the juice for 14 days. In addition, this juice reduced the oxidative stress biomarker 8-isoprostanes and the pro-inflammatory cytokine IL-6. These data highlight the chemopreventive potentials of Andean Berry in colorectal cancer prevention.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgments
Author Carlos Daniel Agudelo was supported by a scholarship from the Francisco José de Caldas Institute for the Development of Science and Technology (COLCIENCIAS). Author Ivan Luzardo-Ocampo was supported by a scholarship from the Consejo Nacional de Ciencia y Tecnología (CONACYT) [grant number: 384201]. Authors would like to thank M.Sc. Kenia Vázquez Sánchez for her support in the preparation of samples. This work was supported by a grant from the Comité para el Desarrollo de la Investigación (CODI) from Universidad de Antioquia [grant number 2015-7505].
References
[1] | Global Burden of Disease Cancer Collaboration, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study, JAMA Oncol (2017) ;3: (4):524–48. doi: 10.1001/jamaoncol.2016.5688 |
[2] | Pointet AL , Taieb J . Cáncer de colon, EMC - Tratado de Medicina (2017) ;21: , 1–7. |
[3] | Doll R , Peto R . The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today, J Nat Cancer Inst (1981) ;66: (6):1191–308. |
[4] | Willett WC . Diet and cancer: An evolving picture, JAMA. (2005) ;293: (2):233–4. |
[5] | World Cancer Research Fund / American Institute for Cancer Research Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC: AICR, (2012) . |
[6] | Nyangale E , Mottram D , Gibson G . Gut microbial activity, implications for health and disease: The potential role of metabolite analysis. J Proteome Res. (2012) ;11: (12):5573–85. doi: 10.1021/pr300637d |
[7] | Cho E , Lee JE , Rimm EB , Fuchs CS , Giovannucci EL . Alcohol consumption and the risk of colon cancer by family history of colorectal cancer. Am J Clin Nutr. (2012) ;95: (2):413–9. doi: 10.3945/ajcn.111.022145 |
[8] | Sabitha K , Venugopal B , Rafi , Ramana K . Role of antioxidant enzymes in glucose and lipid metabolism in association with obesity and type 2 diabetes. Am J Med Sci Med. (2014) ;2: (1):21–4. doi: 10.12691/ajmsm-2-1-5 |
[9] | Praud D , Rota M , Rehm J , Shield K , Zatoński W , Hashibe M , La Vecchia C , Boffetta P . Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer. (2016) ;138: (6):1380–7. doi: 10.1002/ijc.29890 |
[10] | Dorjgochoo T , Gao YT , Chow WH , Shu XO , Yang G , Cai Q , Rothman N , Cai H , Li H , Deng X , Franke A , Roberts LJ , Milne G , Zheng W , Dai Q . Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr. (2012) ;96: (2):405–14. doi: 10.3945/ajcn.112.034918 |
[11] | Gloire G , Legrand-Poels S , Piette J . NF-kappa B activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol. (2006) ;72: (11) :1493–505. |
[12] | Saha S , Lee S , Won J , Choi H , Kim K , Yang G , Dayem A , Sang-Goo C . Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci. (2017) ;18: (7):1544. doi: 10.3390/ijms18071544 |
[13] | Wong M , Ziring D , Korin Y , Desai S , Kim S , Lin J , Gjertson D , Braun J , Reed E , Singh RR . TNFalpha blockade in human diseases: Mechanisms and future directions. Clin Immunol. (2008) ;126: (2):121–36. doi: 10.1016/j.clim.2007.08.013 |
[14] | Waldner MJ , Foersch S , Neurath MF . Interleukin-6 - A key regulator of colorectal cancer development. Int J Biol Sci. (2012) ;8: (9):1248–53. doi: 10.7150/ijbs.4614 |
[15] | Jedinak A , Dudhgaonkar S , Sliva D . Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiol. (2010) ;215: (3):242–49. doi: 10.1016/j.imbio.2009.03.004 |
[16] | Seeram N , Adams L , Hardy M , Heber D . Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J Agric Food Chem. (2004) ;52: (9):2512–17. |
[17] | Brown EM , Latimer C , Allsopp P , Ternan NG , McMullan G , McDougall GJ , Stewart D , Crozier A , Rowland I , Gill CI . In vitro and in vivo models of colorectal cancer: Antigenotoxic activity of berries. J Agric Food Chem. (2014) ;62: (18):3852–66. doi: 10.1021/jf4050759 |
[18] | Lala G , Malik M , Zhao C , He J , Kwon Y , Giusti MM , Magnuson BA . Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr Cancer. (2006) ;54: (1):84–93. |
[19] | Bobe G , Murphy G , Albert PS , Sansbury LB , Lanza E , Schatzkin A , Colburn NH , Cross AJ . Serum cytokine concentrations, flavonol intake and colorectal adenoma recurrence in the PolypPrevention Trial. Br J Cancer. (2010) ;103: (9):1453–61. doi: 10.1038/sj.bjc.6605915 |
[20] | Gaviria CA , Ochoa CI , Sánchez NY , Medina CI , Lobo M , Galeano PL , Mosquera AH , Tamayo A , Lopera YE , Rojano BA . Propiedades antioxidantes de los frutos de agraz o mortiño (Vaccinium meridionale Swartz), In: Ligarreto GA, editor. Perspectivas del cultivo de agraz o mortiño (Vaccinum meridionale Swartz) en la zona altoandina de Colombia. Bogotá: Universidad Nacional de Colombia. (2009) , pp. 93–112. |
[21] | Gaviria CA , Ochoa CI , Sánchez N , Medina C , Lobo M , Mosquera AJ , Tenorio-Tamayo A , Lopera-Pérez Y , Rojano B . Actividad antioxidante e inhibición de la peroxidación lipídica de extractos de frutos de mortiño (Vaccinium meridionale SW). Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. (2009) 8: (6):519–18. |
[22] | Maldonado-Celis ME , Arango-Varela SS , Rojano BA . Free radical scavenging capacity and cytotoxic and antiproliferative effects of Vaccinium meridionale Sw. against colon cancer cell lines. Rev Cubana Plantas Med. (2014) ;19: (2):172–84. |
[23] | Franco-Tobón YN , Rojano BA , Alzate-Arbeláez AF , Morales-Saavedra DM , Maldonado-Celis ME . Efecto del tiempo de almacenamiento sobre las características fisicoquímicas, antioxidantes y antiproliferativa de néctar de agraz (Vaccinium meridionale Swartz). Arch Latinoam Nutr. (2016) ;66: (4):261–71. |
[24] | Agudelo CD , Arango S , Cortés-Mancera F , Rojano B , Maldonado ME . Antiproliferative and pro-apoptotic effects of Andean berry juice (Vaccinium meridionale Swartz) on human colon adenocarcinoma SW480 cells. J Med Plants Res. (2017) ;11: (24):393–402. doi: 10.5897/JMPR2017.401 |
[25] | Icontec. Norma Técnica Colombiana (NTC) 3501. Análisis Sensorial. Vocabulario. Bogotá: Icontec, (2012) . |
[26] | Icontec. Norma Técnica Colombiana (NTC) 3932. Análisis Sensorial. Metodología Identificación y selección de descriptores para establecer un perfil sensorial por aproximación multidimensional. Bogotá: Icontec, (1996) . |
[27] | Icontec. GTC 165. Análisis Sensorial. Metodología. Guía general. Bogotá: Icontec, (2012) . |
[28] | Icontec. GTC 226. Análisis Sensorial. Guía general para el diseño de cuartos de prueba. |
[29] | Sadowska-Krępa E , Kłapcińska B , Podgórski T , Szade B , Tyl K , Hadzik A . Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol Sport. (2015) ;32: (2):161–8. doi: 10.5604/20831862.1144419 |
[30] | Re R , Pellegrini N , Proteggente A , Pannala A , Yang M , Rice-Evans C . Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. (1999) ;26: (9-10):1231–7. |
[31] | Pradelles P , Grassi J , Maclouf J . Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: An alternative to radioimmunoassay. Anal Chem. (1985) ;57: (7):1170–3. |
[32] | Singleton V . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. (1999) ;299C: (1):152–78. doi: 10.1016/S0076-6879(99)99017-1 |
[33] | IAlimentos. Lo Natural se toma la Industria de Jugos [online]. (2017) . Disponible: https://revistaialimentos.com/noticias/lo-natural-se-toma-la-industria-jugos [citado 15 de Marzo de 2017]. |
[34] | Johnson C , Wei C , Ensor J , Smolenski D , Amos C , Levin B , Berry D . Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. (2013) ;24: (6):1207–22. doi: 10.1007/s10552-013-0201-5 |
[35] | Fang J . Bioavailability of anthocyanins. Drug Metab Rev. (2014) ;46: (4):508–20. doi: 10.3109/03602532.2014.978080 |
[36] | Espín J , García-Conesa M , Tomás-Barberán FA . Nutraceuticals: Facts and fiction. Phytochem. (2007) ;68: (22-24):2986–3008. |
[37] | Griffiths HR . Antioxidants and protein oxidation. Free Radic Res. (2000) ;33: (Suppl):S47–58. |
[38] | van‘t Erve TJ , Kadiiska MB , Masson RP . Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox Biol: (2017) ;12: :582–99. doi: 10.1016/j.redox.2017.03.024 |
[39] | Davinelli S , Bertoglio JC , Zarrelli A , Pina R , Scapagnini G . A Randomized clinical trial evaluating the efficacy of an anthocyanin-maqui berry extract (Delphinol®) on oxidative stress biomarkers. J Am Coll Nutr. (2015) ;34: (Suppl 1):28–33. doi: 10.1080/07315724.2015.1080108 |
[40] | Kresty LA , Frankel WL , Hammond CD , Baird ME , Mele JM , Stoner GD , Fromkes JJ . Transitioning from preclinical to clinical chemopreventive assesments of lyophilized blackraspberries: Interim results show berries modulate markers of oxidative stress in Barrett’sesophagus patients. Nutr Cancer. (2006) ;54: (1):148–56. |
[41] | Itzkowitz SH , Yio X . Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am J Physiol Gastrointest Liver Physiol. (2004) ;287: (1):G7–17. |
[42] | Ruder EH , Laiyemo AO , Graubard BI , Hollenbeck AR , Schatzkin A , Cross AJ . Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. (2011) ;106: (7):1340–50. doi: 10.1038/ajg.2011.38 |
[43] | Knüpfer H , Preiss R . Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J Colorectal Dis. (2010) ;25: (2):135–40. doi: 10.1007/s00384-009-0818-8 |
[44] | Al Obeed , Alkhayal K , Sheikh , Zubaidi , Vaali-Mohammed M , Boushey R , Mckerrow J , Abdulla M . Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J Gastroenterol. (2014) ;20: (48):18390–6. doi: 10.3748/wjg.v20.i48.18390 |
[45] | Krelin Y , Voronov E , Dotan S , Elkabets M , Reich E , Fogel M , Huszar M , Iwakura Y , Segal S , Dinarello CA , Apte RN . Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. (2007) ;67: (3):1062–71. |
[46] | Apte RN , Dotan S , Elkabets M , White MR , Reich E , Carmi Y , Song X , Dvozkin T , Krelin Y , Voronov E . The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. (2006) ;25: (3):387–408. |
[47] | Schetter AJ , Nguyen GH , Bowman ED , Mathe EA , Yuen ST , Hawkes JE , Croce CM , Leung SY , Harris CC . Association of inflammation-related and microRNA gene expression with cancer-specific mortality of colon adenocarcinoma. Clin Cancer Res. (2009) ;15: (18):5878–87. doi: 10.1158/1078-0432.CCR-09-0627 |
[48] | Wang SW , Sun YM . The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review). Int J Oncol. (2014) : 44: (4):1032–40. doi: 10.3892/ijo.2014.2259 |
[49] | Schell J , Scofield RH , Barrett JR , Kurien BT , Betts N , Lyons TJ , Zhao YD , Basu A . Strawberries improve pain and inflammation in obese adults with radiographic evidence of knee osteoarthritis. Nutrients. (2017) ;9: (9):949. doi: 10.3390/nu9090949 |
[50] | Kristo AS , Klimis-Zacas D , Sikalidis AK . Protective role of dietary berries in cancer. Antioxidants. (2016) ;5: (4):37. doi: 10.3390/antiox5040037 |




