Late-season foliar application of mineral compounds effects on postharvest quality of Hayward kiwifruit
Abstract
BACKGROUND:
Late-season foliar application of mineral nutrients is a strategy to achieve higher flower quality, acceptable fruit set and yield in the following spring. However, these treatments may affect current fruit quality and storability.
OBJECTIVES:
This study was conducted to consider the effects of different late-season mineral foliar treatments on postharvest quality of kiwifruit that was presented on vines at the time of foliar application.
METHODS:
Mineral foliar treatments were included urea (0.25%, 0.5% and 1%), zinc sulfate (1000, 1500 and 2000 mg.l–1) and boric acid (500, 1000 and 1500 mg.l–1) alone and combined treatments with urea (0.25%) + H3BO3 (500 mg.l–1) + ZnSO4 (1000 mg.l–1); urea (0. 5%) + H3BO3 (1000 mg.l–1) + ZnSO4 (1500 mg.l–1); urea (1%) + H3BO3 (1500 mg.l–1) + ZnSO4 (2000 mg.l–1) and control group (only water). To determine the best foliar application time in late-season, spraying was done at three different times including September 17, October 7 and October 28 and the fruits postharvest traits were analyzed at harvest time and 90 days after storage.
RESULTS:
In this study, foliar applications of mineral compounds was not shown any negative effect on the fruits quality parameters, but also in some of these treatments positive effect of foliar application were detected on measured parameters. The best performance of foliar application on visible parameters was observed on September 17. Meanwhile, the soluble solids and titratable acidity content was not affected at harvest and after storage time by foliar treatment. Results of this study indicate that dry matter, color indices, and phenol levels were not affected by experimental treatments after storage time. In general, October 7 was indicated as the best spraying time regards into ascorbic acid content and antioxidant activity.
CONCLUSION:
Based on our findings, mineral foliar application can improve appearance characteristics, and nutritional value of kiwifruit that presented on vines at the foliar application time, on September 17 and October 7, respectively.
1Introduction
Initial growth of deciduous fruit trees in spring is supported by remobilization of accumulated nutrients from the previous growing season [13]. Late-season foliar application is considered as an approach to provide sufficient reserve of mineral elements required for plant growth in the following growth season [22]. It has been indicated that most of the nitrogen (N) from fall N fertilization on Hayward kiwifruit vines was partitioned in the perennial organs and used in the next spring. However, to avoid excessive N uptake by the fruit and early softening, caution should be considered by growers and suitable concentrations and time of application must be selected in late-season N applications [57].
The fruit postharvest quality is highly dependent on preharvest factors. So, to achieve a higher fruit quality and yield, it is necessary to optimize preharvest conditions [5]. Among preharvest factors, plant nutrition is a challenging issue, since excessive or unbalanced fertilizer application have been shown to have negative effects on fruit quality [39], and storage properties [58].
Literature to date suggests that N which is a primary constituent of proteins, and is one of the most important elements in the kiwifruit mineral nutrition [39]. Adequate levels of N can increase kiwifruit size [60], however, fruit firmness at harvest and storage time can be negatively affected by excessive amount of N in kiwifruit [26]. Moreover, lower dry matter content, titratable acidity, total phenol, vitamin C and antioxidant activity is found in kiwifruit grown under higher rates of N [29, 32, 60]. N levels also have been associated with increasing soluble solids content at harvest time [7]. However, some other findings suggest that N fertilization rates are not involved in soluble solids content of kiwifruit at harvest and storage time [14].
In addition, micro-nutrients such as zinc (Zn) and boron (B) are considered as a necessery components for different biological functions that may affect tree yield and fruit quality [51]. B and Zn foliar application significantly improved fruit quality in ‘Zard olive [58]. Higher mean fruit weight, size, length, diameter, firmness, soluble solids concentration and titratable acidity was found in pear trees treated by boric acid foliar applications [49].
The main purpose of late-season foliar application of mineral nutrients is improving carbohydrate and mineral storage content in order to achieve a higher quality and quantity of flower and fruit in the next spring season. However, there is a paucity of information related to the effect of late-season foliar application on the postharvest quality of kiwifruit that are presented in kiwifruit vines at the time of foliar application. Therefore, the main objective of the present study was to determine the effect of different late-season foliar treatments of mineral compounds on kiwifruit’s postharvest characteristics that were presented on vines at the foliar application time.
2Material and methods
2.1Plant materials and experimental design
This research was carried out at a commercial orchard in the north of Iran (Vajargah, Guilan with latitude 37°02’N; longitude 50°39’E). The mean annual rainfall and temperature was 1147 mm and 15.7°C, respectively. This study was conducted on sixty-three uniform vines of 8-years old with 4×5 m space, 1:8 male to female ratio, trained on a T-Bar support system with “Tomori” as pollinizer.
Factorial experiment with seven fertilizer types and tree times of spraying are designed in the randomized complete block design. In order to investigate the effect of different late-season foliar application solutions on kiwifruit quality at harvest and at the end of storage time, the following seven foliar application treatments were applied:
F1: Urea (0. 5%); F2: H3BO3 (1000 mg.l–1); F3: ZnSO4 (1500 mg.l–1); F4: Urea (0.25%) + H3BO3 (500 mg.l–1) + ZnSO4 (1000 mg.l–1); F5: Urea (0. 5%) + H3BO3 (1000 mg.l–1) + ZnSO4 (1500 mg.l–1); F6: Urea (1%) + H3BO3 (1500 mg.l–1) + ZnSO4 (2000 mg.l–1); F7: Control. To determine the best foliar application time, spraying was done in early morning at September 17, October 7, and October 28 in 2015 and 2016.
2.2Fruit harvest and postharvest practices
Fruits of treated and untreated (control) vines were harvested when soluble solids content (SSC) reached 6.2– 6.5°Brix. Some harvested fruits were immediately transferred to the laboratory for harvest time analysis and the other fruits were stored at 0.5°C with 90– 95% relative humidity conditions for 90 days to evaluate fruits storage characteristics. 10 individual kiwifruits from each replicates were used to determine fruit quality at harvest and after 90 days storage.
2.3Characterization of fruit quality
2.3.1Weight, firmness and dry matter content
The weight of five fruits from each replicates were measured to the nearest 0. 01 g. Flesh firmness was determined by a hand-held penetrometer (Effegi, model FTO11, Milan, Italy) fitted with an 8 mm tip after skin removal to a vertical depth of 1 mm on both sides of the fruit [18].
Fruits were sliced and portioned from the equator zone of each fruit (including skin, flesh, seeds and core tissues). These portions weight determined before and after oven-dried (70°C for 72 hours) for calculating dry matter.
2.3.2Soluble solids content (SSC) and titratable acidity (TA)
SSC was measured by hand-held refractometer (ATC-1E ATAGO, Japan) using the juice extracted when measuring flesh firmness [19]. To determine titratable acidity, 5 ml of juice from each of the five fruits was mixed with 25 ml of distilled water. Two droplets of phenolphthalein (1%) as a color indicator were added and titrated with 0.1 N NaOH to an endpoint pink (pH 8.2). The results were expressed as percentage of anhydrous citric acid, because of its dominancy in kiwifruit [18].
2.3.3Color values and total chlorophyll content
Fruit flesh color was measured using a chromo meter CR-400 (Minolta, Japan). Lightness of color, the intensity of a particular color, and perception of color to the human eye were determined by L*, C*, and h° color parameters [50].
The chlorophyll content of the fruits was extracted based on Hiscox and Israelstam [50] using dimethyl sulphoxide (DMSO) solvent. Briefly, one hundred milligram of leaf tissue was placed in test tube containing 7 ml DMSO and incubated at 65°C for 20 min. The extracted liquid was transferred to another clean tube and the volume made up to a total of 10 ml with DMSO. 200μl of extracted chlorophyll was transferred to a microplate and absorbance was read in an Epoch Microplatle Spectrophotometer (Bio Tek, USA) at 663 and 645 nm against DMSO blank for chlorophyll a and b, respectively. Total chlorophyll (a + b) concentration was determined using the following equation: Total Chlorophyll (g.l-1) = 0.0202 (A645) + 0.00802 (A663) [6]. The total chlorophyll concentration of the extract was calculated from this equation and then was converted to mg.g-1 fresh weight (FW).
2.3.4Total phenolic compound, antioxidant capacity and ascorbic acid
Total phenolic content was determined via the colorimetric method using Folin-Ciocalteau reagent according to Singleton and Rossi [52] with some modification. 200μl of methanolic extract (i.e. 1 mg of fruit tissue in 10 ml of 80% methanol) was mixed with 2.6 ml of deionized water. 200μl of Folin-Ciocalteu’s phenol reagent was added to the mixture. After 6 min, 2.0 ml of 7% (w.v–1) Na2CO3 solution was added to the reaction mixture. Then, the mixture leaved for 60 min at room temperature in a dark place. Absorbance was read at 750 nm using T-60 UV/VIS spectrophotometer (PG Instruments Ltd, UK). Standard curve was made for gallic acid (y = 0.0146x-0.0216, R2 = 0.9953), and the content of total phenolic in the samples were expressed as mg gallic acid equivalents (GAE).100 g-1 fresh weight (FW) of kiwifruit.
Antioxidant activity was measured using DPPH free radical scavenging assay, as described previously by Brand-Williams et al. [10] with some modification. The reaction mixture consisted of 40μl of methanolic extract and 3 ml of 1 mM DPPH radical solution. Finally, the initial absorbance of control (methanol and DPPH) and samples were measured using T-60 UV/VIS spectrophotometer (PG Instruments Ltd, UK) at 515 nm. The percent of inhibition was calculated using the following formula:
Percent of inhibition (%) = [(A515 of control– A515 of sample)/A515 of control]×100.
Concentration of ascorbic acid of kiwifruit was obtained according to the Pearson method [41], using a T-60 UV/VIS spectrophotometer (PG Instruments Ltd, UK) with some modification. The method relies on oxidation of ascorbic acid with 2.6 dichlorphenol-indolphenol. The content of ascorbic acid in samples was calculated using ascorbic acid standard curve (y = 0.001x+0.007, R2 = 0.969).
2.3.5Weight loss
Three individual kiwifruits from each replicate were weighted at the harvest time and 90 days after storage. The weight loss was determined based on following equation [50]:
Percentage weight loss: [(weight at beginning – weight at each sampling time)/weight at beginning]×100
2.3.6Statistical analysis
The collected data were subjected to the analysis of variance using the General Linear Models (GLM) procedure of SAS (version 9.1, SAS Institute, Cary, NC, USA) and means were compared using LSD test at the P≤0.05 level.
3Results and discussion
3.1Fruit weight
The results showed that, foliar application of kiwifruit vines with different fertilizers increased fruit weight compared to control plants. The highest fruit weight was found at the vines treated with urea 1 + B 1500 + Zn 2000 on September 17. However, this amount was not significantly different to other foliar experimental treatments on September 17 and urea 0.5 + B 1000 + Zn 1500 and urea 1 + B 1500 + Zn 2000 treatments on October 7 and 28. Between all experimental times, control vines at all foliar application times and B 1000 on October 28 represented the lowest kiwifruit weights (Fig. 1).
Fig.1
Effect of foliar application type (F1: Urea (0. 5%); F2: H3BO3 (1000 mg.l–1); F3: ZnSO4 (1500 mg.L-1); F4: Urea (0.25%) + H3BO3 (500 mg.L-1) + ZnSO4 (1000 mg.L-1); F5: Urea (0. 5%) + H3BO3 (1000 mg.l–1) + ZnSO4 (1500 mg.l–1); F6: Urea (1%) + H3BO3 (1500 mg.l–1) + ZnSO4 (2000 mg.l–1); F7: (control) and time (T1: September 17, T2: October 7, T3: October 28) on kiwifruit weight at harvest time. Data were presented as a mean value of the two years.
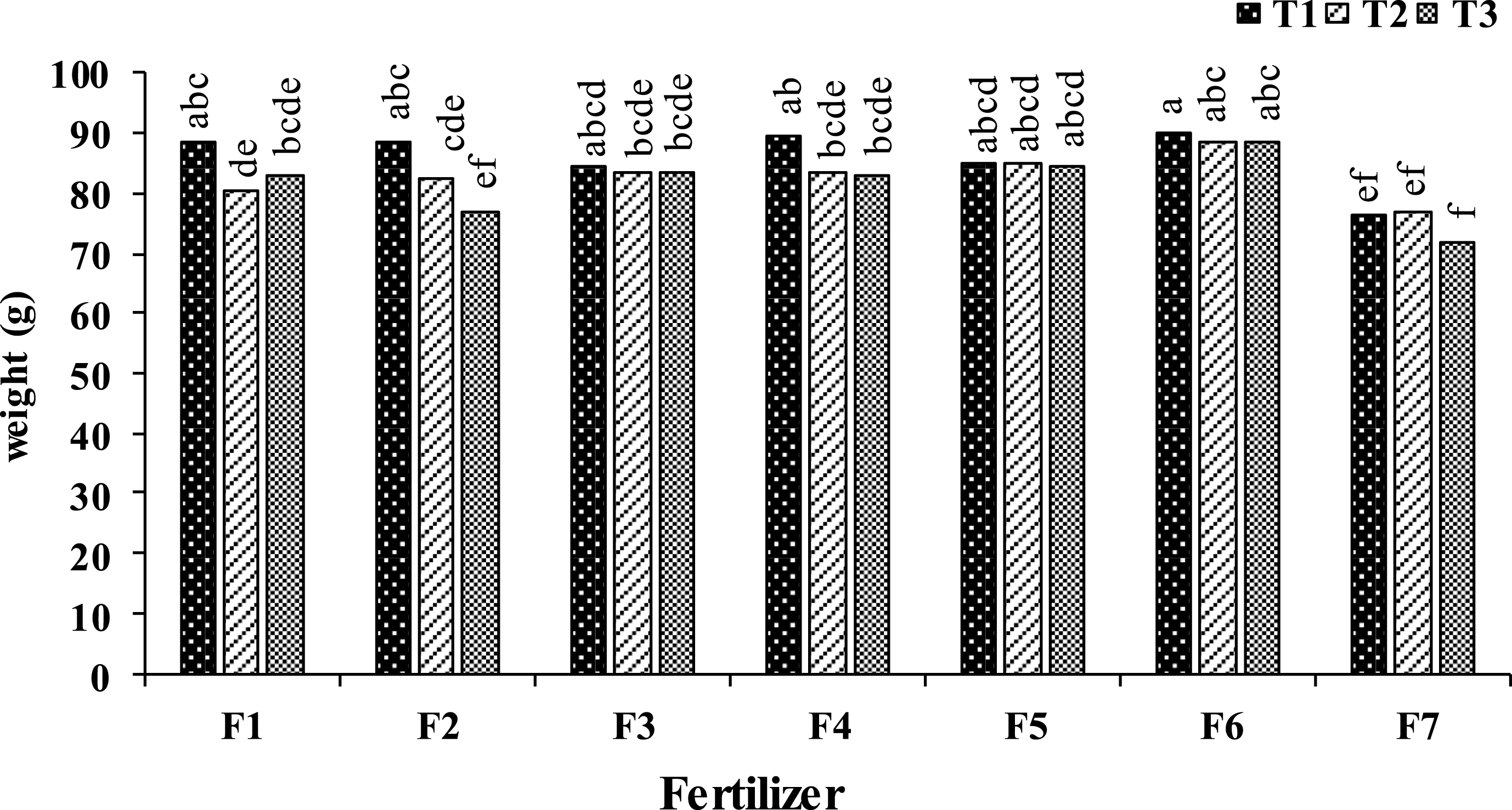
It is clear from fruit weight changes that foliar application on September 17 positively increased fruit weights in all experimental groups. Proper rates of N fertilization can stimulate kiwifruit weight increase [14]. The increase in kiwifruit growth by N balanced levels might be attributed to N role as a major building block of proteins, polyamines, and nucleic acids, and its role in chlorophyll synthesis, improving photosynthesis efficiency of matured leaves and carbohydrate metabolism [34– 43]. On the other hand, the positive effect of B and Zn micronutrients on fruit size as a result of foliar application was reported in peach trees [63]. Fruit size increase is likely associated with auxin level increase which is due to Zn role as a precursor of auxin and B effect on reducing IAA oxidase [1, 42]. The other possible reasons for fruit weight enhancement with B and Zn foliar application can be due to their involvement in cell division, cell elongation, carbohydrates metabolism and proteins synthesis [17, 56]. In general, increase in kiwifruit weight by foliar application on September 17 could be attributed to the longer distance of this time to fruit harvest in comparison to the other application times. It seems foliar application at this time allows the fruit to complete their growth curve at a higher rate, since fruit has sufficient time to use assimilates and nutritious elements compared to the other foliar application times.
3.2Fruit firmness
Foliar application with combined treatments of urea 1 + B 1500 + Zn 2000 and urea 0.5 + B 1000 + Zn 1500 on September 17 led to significant improvement in kiwifruit flesh firmness at harvesting time. However, at the end of the storage time, the highest flesh firmness at urea 1 + B 1500 + Zn 2000 treatment was not significantly different with other foliar treated vine except urea 0.25 + B 500 + Zn 1000 on September 17 (Fig. 2).
Fig.2
Effect of foliar application type (F1: Urea (0. 5%); F2: H3BO3 (1000 mg.l–1); F3: ZnSO4 (1500 mg.L-1); F4: Urea (0.25%) + H3BO3 (500 mg.L-1) + ZnSO4 (1000 mg.L-1); F5: Urea (0. 5%) + H3BO3 (1000 mg.l–1) + ZnSO4 (1500 mg.l–1); F6: Urea (1%) + H3BO3 (1500 mg.l–1) + ZnSO4 (2000 mg.l–1); F7: (control) and time (T1: September 17, T2: October 7, T3: October 28) on kiwifruit flesh firmness at harvest and after three month storage time. Data were presented as a mean value of the two years.
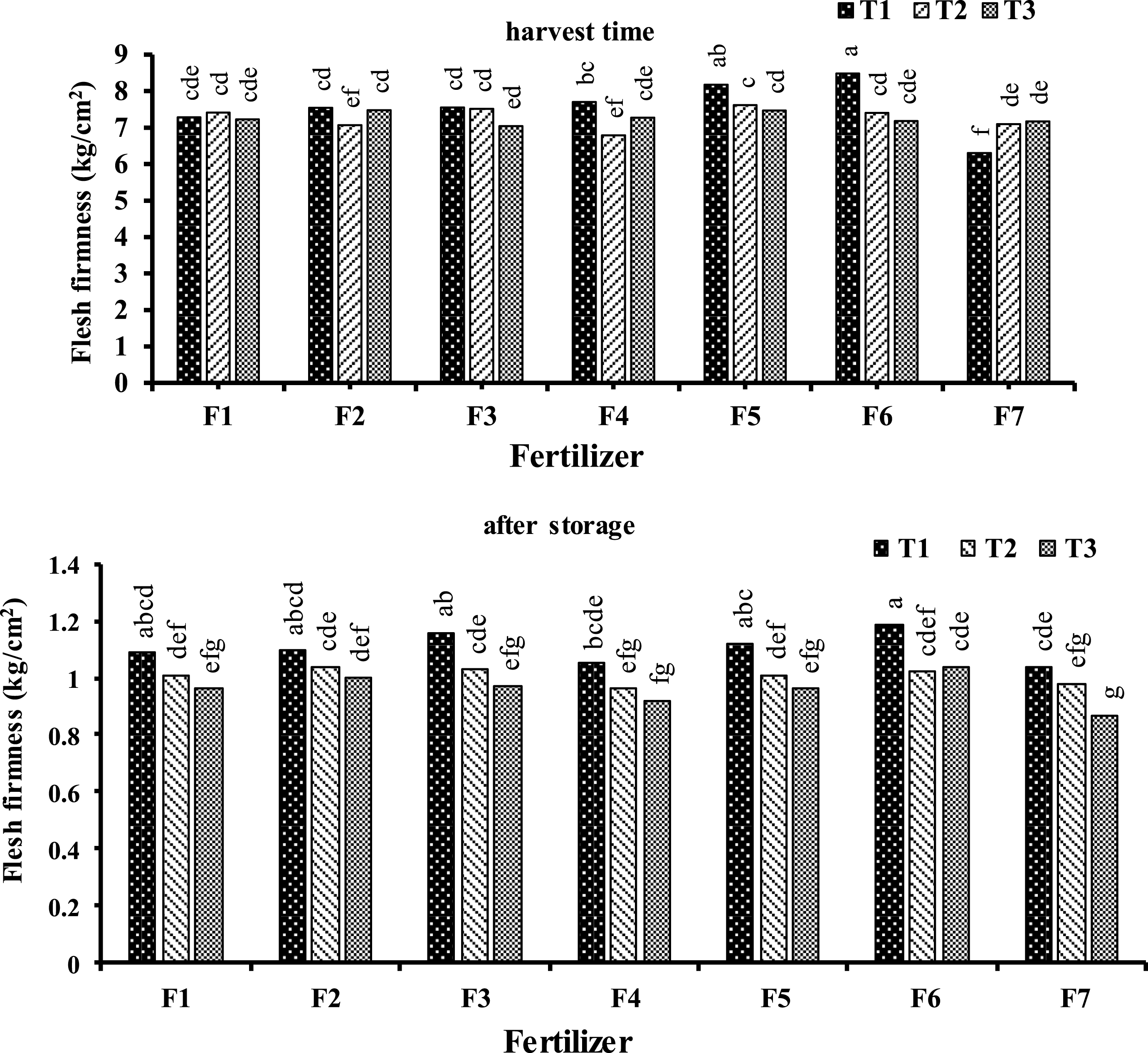
The significant improve in fruit firmness in this study was in agreement with foliar application of ZnSO4 and H3BO3 in peach fruit which application of these fertilizer significantly improve fruit firmness [63]. It might be related to B critical role in cell wall structure as pectic networks stabilizer, cell wall pore size regulator, and cell membrane structures integration [8], and/or Zn induction role in structural maintenance and function of membrane [35]. Although moderate rates of N foliar application and suitable application time had no side effect on fruit firmness [53], excessive amount of nitrogen reduce fruit firmness via vegetative growth induction, and developing shading of plants. Hence, calcium uptake is limited and the N/Ca ratio increased [37]. Since calcium plays a significant role in maintaining fruit quality, decrease in calcium content in kiwifruit can induce fruit softening [47].
3.3Soluble solids content (SSC), Dry matter content (DMC), and titratable acidity (TA)
Different type and time of fertilization had no significant effect on fruit SSC and TA content at harvest and the end of storage time (data not shown). Foliar application of urea 1 + B 1500 + Zn 2000 and urea 0.5 + B 1000 + Zn 1500 combined fertilizers on September 17 increased significantly kiwifruit DMC at harvest time (Table 1), whereas there were no significant effect found in DMC between all treatments (fertilizers and times) at the end of storage time (data not shown).
Table 1
Effect of foliar application type (F) and time (T) on kiwifruit dry matter concentration, total phenolic content and color parameters (L*, C* and h°) at harvest time. Data were presented as a mean value of the two years
| Fertilizers | Times | Dry matter (%) | Phenol (mg GAE/ 100 g FW) | L* | C* | h° |
| F1 | T1 | 15.56b - d | 72.89ab | 53.90g - i | 42.65a - e | 110.39a |
| T2 | 14.78d | 66.97e - g | 54.30f - h | 43.12a - c | 110.33a | |
| T3 | 13.08e - h | 66.27d - g | 53.02i | 43.21a - c | 110.32a | |
| F2 | T1 | 15.89a - c | 71.42a - f | 55.68a - e | 41.79e - g | 109.65b - d |
| T2 | 14.78d | 72.79a - c | 56.36ab | 43.09a - c | 110.06a - c | |
| T3 | 13.69e - g | 70.69a - f | 54.53e - h | 42.83a - d | 110.36a | |
| F3 | T1 | 15.37cd | 73.55a | 53.81g - i | 42.76a - d | 110.38a |
| T2 | 12.93gh | 67.88b - g | 56.20a - c | 42.49b - f | 109.53c - e | |
| T3 | 13.85ef | 67.69c - g | 55.99a - d | 41.60fg | 109.72bc | |
| F4 | T1 | 15.60bc | 72.30a - d | 55.30a - f | 41.64fg | 108.97ef |
| T2 | 13.59e - g | 70.10a - g | 55.59a - e | 42.29c - f | 110.07a - c | |
| T3 | 13.04gh | 66.48fg | 55.20b - f | 43.11a - c | 109.73bc | |
| F5 | T1 | 16.21ab | 73.22a | 53.69hi | 42.81a - d | 110.19ab |
| T2 | 15.49b - d | 67.92b - g | 55.51a - f | 42.40b - f | 110.41a | |
| T3 | 13.61e - g | 66.51fg | 54.76e - h | 41.72e - g | 109.67b - d | |
| F6 | T1 | 16.58a | 74.78a | 56.55a | 43.53a | 110.32a |
| T2 | 15.58b - d | 72.27a - d | 53.60hi | 43.27ab | 110.00a - c | |
| T3 | 13.96e | 71.81a - e | 53.00i | 41.33g | 108.78f | |
| F7 | T1 | 12.91gh | 65.08g | 55.50a - f | 42.38b - f | 109.60cd |
| T2 | 13.59e - g | 66.36gf | 54.99c - g | 41.55fg | 109.63b - d | |
| T3 | 13.90e | 66.33fg | 55.60e - h | 41.92d - g | 109.14d - f | |
| Significance | ||||||
| F | ** | ** | ** | ** | ** | |
| T | ** | ** | ** | ** | ** | |
| F×T | ** | ** | ** | ** | ** |
F1: Urea (0. 5%); F2: H3BO3 (1000 mg.l–1); F3: ZnSO4 (1500 mg.l-1); F4: Urea (0.25%) + H3BO3 (500 mg.l-1) + ZnSO4 (1000 mg.l–1); F5: Urea (0. 5%) + H3BO3 (1000 mg.l–1) + ZnSO4 (1500 mg.l–1); F6: Urea (1%) + H3BO3 (1500 mg.l–1) + ZnSO4 (2000 mg.l–1); F7: control. T1: September 17, T2: October 7, T3: October 28. Different letters within a column indicate significant differences by LSD test. *, ** and NS are significant at p≤0.05, at p≤0.01 and not significant, respectively.
Although the values of SSC and TA were not affected by experimental foliar treatments in this study, Zn and B application can increase SCC in fruits [16– 48]. The effect of Zn on SSC can be due to its role in carbohydrate and nucleic acid metabolism, and also to the activities of various enzyme involved in these biochemical reaction [3], whereas the effect of B can be associated to its roles in the transportation of sugar and carbohydrate metabolism [35] that might be the reason to increase of SSC. Decreasing in carbohydrate synthesis was reported in association with higher N uptake [36– 38]. However, application of suitable N compound can increase fruit SSC [7]. In addition to SSC, fruit TA also was positively or negatively affected by application of ZnSO4 and H3BO3 in strawberry and blackcurrant, mango, guava, and pomegranate [1, 15, 27, 40, 48]. There are few reports of N fertilization effects on kiwifruit acidity. Similar to our results, no significant effect on fruit TA was detected when kiwifruit vines fertilized with various levels of N [39, 59, 60].
It has already been determined that high levels of N fertilization resulted in higher absorption of water and lower DMC [54] but optimum concentration of N compounds increased carbohydrate production and enhanced fruit DMC [62]. On the other hand, B can play a positive role in facilitating sugars transport through the formation of B-sugar complexes, and also increase leaf photosynthesis rate via its role on physiological processes [61]. Moreover, Zn effect on increasing fruit fresh and dry weight might be back to the role of enzymes participating in carbohydrate metabolism [9]. The lack of significant increase in SSC, TA and DMC levels is likely due to spraying mineral nutrients in our study was not great enough to increase these parameters.
3.4Color indices and chlorophyll pigment content
The highest amount of all L*, C* and h° indices at harvest time was found in the vines treated with urea 1 + B 1500 + Zn 2000 on September 17 (Table 1). However, these indices after 90 days storage were not significantly affected in regards to type and time of foliar application (data not shown). In this study chlorophyll concentration was statistically significant among vines treated with different foliar fertilizers (Table 2). The highest total chlorophyll content at harvest time was found in urea 1 + B 1500 + Zn 2000 treatment on September 17 followed by Zn 1500 and B 1000 on September 17 and urea 1 + B 1500 + Zn 2000 on October 7. At the end of storage period, the total chlorophyll concentration in urea 1 + B 1500 + Zn 2000 foliar treatment at all three times application, urea 0.5 + B 1000 + Zn 1500, Zn 1500 and B 1000 on September 17 was significantly higher than other interactions. However control vines had the lowest total chlorophyll concentration at both harvest and after storage time (Table 2).
Table 2
Effect of foliar application type (F) and time (T) on kiwifruit total chlorophyll content, antioxidant activity and ascorbic acid content at harvest time and after three month storage time. Data were presented as a mean value of the two years
| Fertilizers | Times | Chlorophyll (mg.100g–1FW) | Antioxidant activity (% DPPH) | Ascorbic acid (mg.100 g–1FW) | |||
| Harvest | After storage | Harvest | After storage | Harvest | After storage | ||
| F1 | T1 | 1.64b - d | 0.81fg | 49.23f | 29.82fg | 71.67d - g | 53.25g |
| T2 | 1.43fg | 0.86d - f | 63.10a | 43.50b | 86.91a | 66.93bc | |
| T3 | 1.44fg | 0.89c - e | 53.33c - f | 36.79c - e | 72.09d - f | 43.92i | |
| F2 | T1 | 1. 7ab | 0.95a - c | 51.52ef | 23.76hi | 75.09de | 45.54hi |
| T2 | 1.25j | 0.73h - c | 63.81a | 34.50c - f | 82.04a - c | 63.37b - d | |
| T3 | 1.31hi | 0.69h - i | 54.01b - f | 37.05cd | 66.46f - i | 41.43i | |
| F3 | T1 | 1.77a | 0.97ab | 52.65de | 24.06h | 58.14kl | 56.40e - g |
| T2 | 1.34g - i | 0.76hg | 64.08a | 51.96a | 86.92a | 62.27bc | |
| T3 | 1.63c - d | 0.93a - d | 51.30ef | 26.09gh | 64.96h - k | 63.63b - d | |
| F4 | T1 | 1.09j | 0.74h - i | 59.85b - e | 36.15c - e | 64.80h - k | 58.21d - g |
| T2 | 1.30hi | 0.84ef | 58.44a - d | 36.15c - e | 84.50ab | 63.37b - d | |
| T3 | 1.35g - i | 0.94b - d | 53.54c - f | 37.82c | 64.96h - k | 61.16c - f | |
| F5 | T1 | 1.66bc | 0.94a - c | 49.57f | 32.67c - f | 59.49j - l | 44.20i |
| T2 | 1.47ef | 0.70h - i | 59.89ab | 54.47a | 83.77ab | 68.65ab | |
| T3 | 1.36gh | 0.81fg | 54.50b - f | 33.84c - f | 76.00c - e | 61.92b - e | |
| F6 | T1 | 1.78a | 1.00a | 59.66ab | 37.48c | 76.80c - e | 41.94i |
| T2 | 1.68a - c | 1.00a | 64.12a | 56.79a | 87.00a | 73.93a | |
| T3 | 1.52ef | 0.96a - c | 55.85b - e | 34.56c - f | 77.83b - e | 56.47e - g | |
| F7 | T1 | 1.55de | 0.65j | 49.48f | 18.37i | 78.34b - d | 38.88i |
| T2 | 1.59c - e | 0.67ji | 41.83g | 29.75fg | 71.27e - h | 55.75e - g | |
| T3 | 1.07j | 0.63j | 58.98b - f | 31.75d - f | 53.51l | 54.50fg | |
| Significance | |||||||
| F | ** | ** | ** | ** | ** | ** | |
| T | ** | ** | ** | ** | ** | ** | |
| F×T | ** | ** | ** | ** | ** | ** | |
F1: Urea (0. 5%); F2: H3BO3 (1000 mg.l–1); F3: ZnSO4 (1500 mg.l–1); F4: Urea (0.25%) + H3BO3 (500 mg.l–1) + ZnSO4 (1000 mg.l–1); F5: Urea (0. 5%) + H3BO3 (1000 mg.l–1) + ZnSO4 (1500 mg.l–1); F6: Urea (1%) + H3BO3 (1500 mg.l–1) + ZnSO4 (2000 mg.l–1); F7: control. T1: September 17, T2: October 7, T3: October 28. Different letters within a column indicate significant differences by LSD test. *, ** and NS are significant at p≤0.05, at p≤0.01 and not significant, respectively.
It is well established that fruit appearance and color are distinctive features in acceptance and rejection of fruit in consumer point of view and market price [50]. In kiwifruit the higher color indices is equal to the greater postharvest quality [4, 24]. In addition, micro fertilizers application severely postpone fruit ripening, and consequently fruit color indices after prolonged storage remain higher than control fruits [40]. Considering our findings, foliar application of different macro and micro nutrients at late-season time didn’t show any changes on fruit color indices after storage time. It seems changes in fruit color components could be related to nonenzymatic reactions, and the decomposition of chlorophyll and other pigments [50]. However, the kiwifruit green color which is originated from chlorophyll pigments considered as an important visible quality parameter related to marketing success [31]. It seems the mechanism behind chlorophyll increase in this study is due to Zn role in porphobilonogen formation, a precursor for the formation of chlorophyll [45]. Furthermore, degradation of chlorophyll was apparently postponed with H3BO3 application. It is likely to be associated with the inhibition of ethylene synthesis and reduction of abscisic acid content [23]. Moreover, the increase of chlorophyll in association with raising N amount was reported before, since N play a key role in chlorophyll structure [34]. Cheng and Fuchigami [13] also noticed that total Rubisco activity increased linearly with N content. Therefore, it could be concluded that mineral nutrients application can increase the chlorophyll concentration in kiwifruit.
3.5Total phenolic compound, antioxidant activity and ascorbic acid levels
At harvest time, fruits of vines that treated with urea 1 + B 1500 + Zn 2000 on September 17 had the highest values of total phenol, but the difference was not significant with many other foliar treatments (Table 1). At the end of storage time fruit total phenol did not show any significant differences among experimental groups (data not shown). However, kiwifruit antioxidant activity and ascorbic acid levels at harvest and after 90 days storage were significantly affected by interaction of foliar application type and time. In all experimental foliar treatments the highest levels of kiwifruit antioxidant activity at harvest time were observed on October 7 in comparison to control fruits. Nevertheless, at the end of storage time, urea 1 + B 1500 + Zn 2000, Zn 1500, and urea 0.5 + B 1000 + Zn 1500 on October 7 represent higher content of kiwifruit antioxidant activity (Table 2). In similar to antioxidant activity, all foliar applications on October 7 led to higher ascorbic acid concentration at harvest time. After three months storage period, the highest ascorbic concentration accumulated in fruits of vines treated by urea 1 + B 1500 + Zn 2000 on October 7, however, this amount was not significantly different to urea 0.5 + B 1000 + Zn 1500 treatment (Table 2).
In contrast to our finding, B and Zn foliar application increased total phenolic content in olive and grape berries [46, 55]. It is may be due to expression of the genes that mediated in phenolic compounds biosynthesis up regulated by B and Zn application. In addition, B role has been reported before in the metabolism of phenolic compounds [33]. The consistent phenol level in our kiwifruits from different foliar applications may be related to times, and levels of application.
The influences of mineral nutrient such as Zn and B on antioxidant activity were reported before through increasing phenol level [15, 55]. On the other hand, mineral foliar application (N, Zn and B) can increase carbohydrate concentration in plants, and it seems the excess amount of carbohydrates are used for antioxidants biosynthesis [10, 43, 44].
Our results are in agreement with Latocha [30], which expressed that the ascorbic acid level was not constant and could be affected by growing conditions, such as fertilization. Ascorbic acid in plants is synthesized from sugars which are supplied through photosynthesis [32]. So, the possible role of mineral nutrients (N, Zn and B) on photosyntate accumulation could participate in increasing ascorbic acid levels. On the other hand, increase in ascorbic acid content might be due to catalytic activity of Zn and B on its bio-synthesis from its precursor (glucose-6-phosphate) or inhibition of its conversation into dehydro ascorbic acid by enzyme ascorbic acid oxidation or both [48].
3.6Weight loss
Compared to the control group, a significant reduction in kiwifruit weight loss at the end of storage time was found in all foliar treatments on September 17 and October 7 with the exception of urea 0.25 + B 500 + Zn 1000 (Fig. 3).
Fig.3
Effect of foliar application type F1: Urea (0. 5%); F2: H3BO3 (1000 mg.l–1); F3: ZnSO4 (1500 mg.L-1); F4: Urea (0.25%) + H3BO3 (500 mg.L-1) + ZnSO4 (1000 mg.L-1); F5: Urea (0. 5%) + H3BO3 (1000 mg.l–1) + ZnSO4 (1500 mg.l–1); F6: Urea (1%) + H3BO3 (1500 mg.l–1) + ZnSO4 (2000 mg.l–1); F7: (control) and time (T1: September 17, T2: October 7, T3: October 28) on kiwifruit weight loss after three month storage. Data were presented as a mean value of the two years.
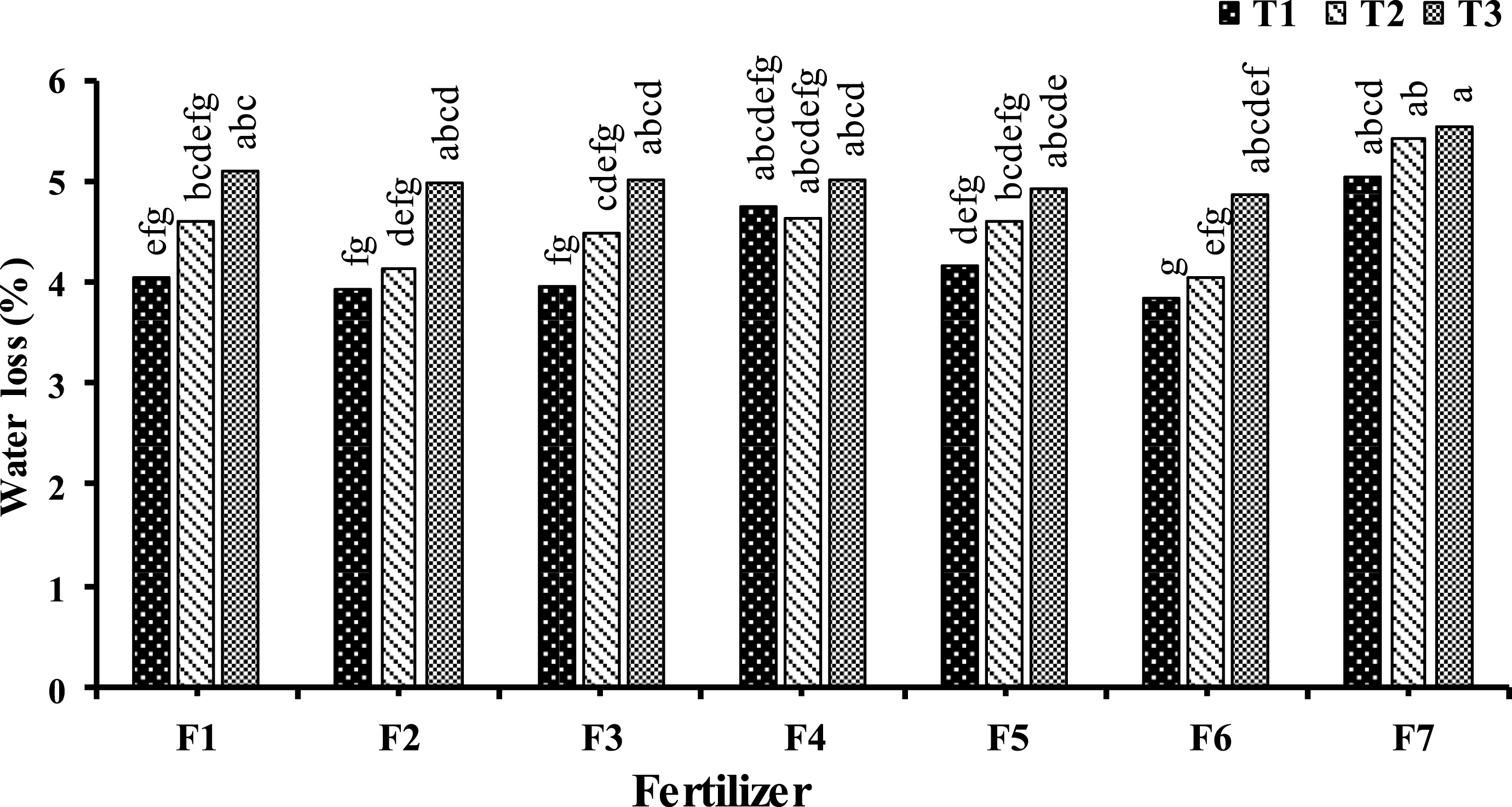
The role of Zn and B in reducing weight loss has been reported in guava and mango previously [21, 28]. The lower weight loss of fruits can be related to the role of B and Zn in the structure of the cell wall and maintaining membrane integrity [8, 35]. B in association with membrane constituents plays an important role in maintenance of structural integrity of plasma membrane [11]. Also, the presence of B in the cell wall as a borate diol diester, which cross-links two chains of rhamnogalacturonan II (RG-II), suggesting that B plays an important role in cross-linked pectic network to regulate the mechanical and biochemical properties of the cell wall [20]. In addition, the contribution of Zn in membrane integrity maintenance is due to its interaction with phospholipids and sulphydryl groups of membrane proteins [12].
4Conclusion
It is well established that late-season foliar application of mineral elements can improve mineral and carbohydrate reserves and improving flowering, fruit set and yield in the next growing season. Foliar application on October 28 increased kiwifruit vines carbohydrate and mineral reserves (Authors unpublished data). In addition, foliar application at this time did not show any negative effect on kiwifruit postharvest quality. Therefore, it could be concluded that October 28 is an excellent time for foliar application to increase vine reserves without negative effect on kiwifruits quality. On the other hand, the majority of postharvest traits was positively affected by late-season foliar application, particularly on September 17 and October 7. Fruit weight, and firmness increase and reduction in weight loss are considered as important visible quality parameters which effects on kiwifruit market success. The current study findings suggest that September 17 is the effective time for foliar application to improve these visible quality parameters. While, October 7 was determined as an appropriate application time to increase fruits nutritional value. Hence, if the goal of late season foliar application is to only improve the postharvest characteristics of fruits (appearance or nutritional value), one of these two times is highly recommended.
References
[1] | Abdollahi M , Eshghi S , Tafazoli E . Interaction of paclobutrazol, boron and zinc on vegetative growth, yield and fruit quality of strawberry (Fragaria×Ananassa Duch. Cv. Selva). J Biol Environ Sci. (2010) ;4: (11), 67–75. |
[2] | Abdollahi1 M , Eshghi S , Tafazzoli E , Moosavi N . Effects of paclobutrazol, boric acid and zinc sulfate on vegetative and reproductive growth of strawberry cv. Selva. J Agr Sci Tech. (2012) ;14: :357–63. |
[3] | Alloway BJ . Zinc in soils and crop nutrition, second ed. published by IZA and IFA, Brussels, Belgium and Paris, France;. (2008) . p. 35. |
[4] | Amodio ML , Colleli G , HaseyJK , Kader AA . A comparative study of composition and postharvest performance of organically and conventionally grown kiwifruits. J Sci Food Agr. (2007) ;87: :1228–36. |
[5] | Antunes MDC , Neves N , Curado S , Rudrigoes F , Franco J , Panagopoulos T . The effects of calcium applications on kiwifruit quality preservation during storage. Acta Hortic. (2007) ;753: :727–32. |
[6] | Arnon DI. Copper enzymes in isolated chloroplasts, polyphenoxidase in beta vulgaris. Plant Physiol. (1949) ;24: :1–15. |
[7] | Benge JR , De Silva HN , Banks NH , Jeffery PB . Empirical modeling of postharvest changes in the firmness of kiwifruit. Postharvest Biol Technol. (2000) ;19: :211–20. |
[8] | Blevins DG , Lukaszewski KM . Boron in plant structure and function. Annu Rev Plant Physiol Plant Mol Biol. (1998) ;49: :481–500. |
[9] | Brand IA , Heinickel A . Key enzymes of carbohydrate metabolism as targets of the 11.5-kDaZn2+-binding protein (Parathymosin). J Biol Chem. (1991) ;266: :20984–9. |
[10] | Brand-Williams W , Cuvelier ME , Berset C . Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. (1995) ;28: :25–30. |
[11] | Cakmak I , RoÉmheld V . Boron deficiency-induced impairments of cellular functions in plants. Plant Soil. (1997) ;193: :71–83. |
[12] | Cakmak I . Role of zinc in protecting plant cells from reactive oxygen species. New Phytol. (2000) ;146: :185–205. |
[13] | Cheng L , Fuchigami LH . CO2 assimilation in relation to nitrogen in apple leaves. J Hortic Sci Biotechnol. (2000) ;75: :383–7. |
[14] | Costa G , Lain O , Vizzotto G , Johnson S . Effect of nitrogen fertilization on fruiting and vegetative performance, fruit quality and postharvest life of kiwifruit cv. Hayward. Acta Hortic. (1997) ;444: :279–84. |
[15] | Davarpanah S , Tehranifar A , Davarynejad GH , Abadía J , Khorasani R . Effects of foliar applications of zinc and boron nano-fertilizers onpomegranate (Punica granatum cv. Ardestani) fruit yield and qualitySci Hortic. (2016) ;210: :1–8. |
[16] | Dobroluybskii OK , Strakhov VG , Tanurkov GR . Effect of micro fertilizers on yield and quality of grape in Ukrainian South. Agrokhimiya. (1981) ;10: :135–7. |
[17] | Emami A . Methods of plant analysis. Soil and Water Research Institute1, Technical Issue, No. 982. |
[18] | Fattahi J , Fifaii R , Babri B . Postharvest quality of kiwifruit (Actinidia deliciosa cv. Hayward) affected by pre-storage application of salicylic acid. South-west J Hortic Biol Environ. (2010) ;1: (2), 175–86. |
[19] | Feng J , MacKay BR , Maguire KM . Variation in firmness of packed in Hayward kiwifruit. Acta Hortic. (2003) ;610: :211–8. |
[20] | Fleischer A , O’Neill MA , Ehwald R . The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. (1999) ;121: :829–38. |
[21] | Goswami AK , Shukla HS , Kumar P , Mishra DS . Effect of pre-harvest application micro-nutrients on quality of guava cv. Sardar Hort Flora Res Spectrum. (2012) ;1: (1):60–3. |
[22] | Harris RW , Clark JR , Matheny NP . Arboriculture: Integrated Management of Landscape Trees, Shrubs, and Vines (3rd ed.). Prentice Hall, Upper Saddle River, NJ; .(1999) . p. 687. |
[23] | Hashemabadi D , Hosheinzade M , Kaviani B , Musavi M , keyghobadi S , Zahiri S . Effect of nano-silver and boric acid on extending the vase life of cut rose (Rosa hybrid L). J Env Biol. (2014) ;35: :833–88. |
[24] | Hassani F , Garousi F , javanmard M . Edible coating based on whey protein concentration-rice bran oil to maintain the physical and chemical properties of the kiwifruit (Actinidia deliciousa). TJS. (2012) ;10: :26–34. |
[25] | Hiscox JT , Israelstam G . A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. (1979) ;57: :1332–4. |
[26] | Johnson RS , Mitchell FG , Costa G . Nitrogen influences kiwifruit storage life. Acta Hortic. (1997) ;444: :285–9. |
[27] | Kazemi M . Influence of foliar application of iron, calcium and zinc sulfate on vegetative growth and reproductive characteristics of strawberry cv. Pajaro. TJS. (2014) ;1: :21–6. |
[28] | Kumar OV , Kumar G . Effect of pre-harvest foliar sprays of zinc on post-harvest changes in the quality of mango cv. Dashehari. Acta Hortic. (1989) ;231: :763–70. |
[29] | Lancaster JE , Macrae EA . Definition of preferred taste and aroma characteristics Kiwifruit New Zealand Report for Project 283/99; (2000) . |
[30] | LatochaP . The comparison of some biological features of Actinidia arguta cultivars fruit. Hort Land Arch. (2007) ;28: :105–9. |
[31] | LawesGS . The effect of shading on the chlorophyll content of Hayward kiwifruit. NZJ Crop and Hort Sci. (1989) ;17: :245–9. |
[32] | Lee SK , Kader AA . Preharvest and postharvest factors influencing vitamin C content in horticultural crops. Postharvest Biol Technol. (2000) ;20: :207–20. |
[33] | Liakopoulos G , Karabourniotis G . Boron deficiency and concentrations and composition of phenolic compounds in Olea europaea leaves: A combined growth chamber and field study. Tree Physiol. (2005) ;25: :307–15. |
[34] | Malakouti MJ , Majidi A , Sacheshmepour M , Dehghani F , Shahabi AA , Keshavarz P , Basirat M , Rastegar H , Taheri M , Gandomkar A , Tadayon M.S , Asadi A , Kiani SH , Bybordi A , Mahmoudi M , Saleh J , Mostasharim M , Manouchehri S , Afkhami M , Rasouli MH , Mozaffari V . Nutritional disorders, determination of quality indices and optimum levels of nutrients in fruits grown on the calcareous soils of Iran. Soil and Water Research Institute. Sana Publication;. (2005) . pp. 319–25. |
[35] | Marschner H . Mineral Nutrition of Higher Plants. London: Academic Press; (1995) . pp. 301–6 . |
[36] | Marschner H . Mineral Nutrition of Higher Plants. London: Academic Press; (1995) . p. 889. |
[37] | Mills T , Boldingh H , Blattman P , Green S , Meekings J . Nitrogen application rate and the concentration of other macronutrients in the fruit and leaves of Gold kiwifruit. J Plant Nutr. (2008) ;31: :1656–75. |
[38] | Morton AR . Kiwifruit (Actinidia spp.) vine and fruit responses to nitrogen fertilizer applied to the soil or leaves. PhD Thesis, Massey University, Palmerston North, New Zealand; (2013) . |
[39] | Pacheco C , Calouro F , Vieira S . Influence of nitrogen and potassium on yield, fruit quality and mineral composition of kiwifruit. IJEE. (2008) ;2: :517–21. |
[40] | Patel V . Effect of Pre-harvest Sprays of Calcium, Zinc and Boron for Delayed Ripening and Prolonged Stroreability of Guava (Psidium guajava L.) cv. L-49 of fruits. Master of Science Thesis in Mandsaur University, India; (2013) . |
[41] | Pearson D . The Chemical Analysis of Foods (6th edition). Churchill, London; (1970) . p. 233. |
[42] | Puzina TI . Effect of zinc sulfate and boric acid on the hormonal status of potato plants in relation to tuberization. Russ J Plant Physiol. (2004) ;51: (2), 209–15. |
[43] | Ramezanian A , Rahemi M , Vazifehshenas MR . Effect of foliar application of calcium chloride and urea on quantitative and qualitative characteristics of pomegranate fruits. Sci Hortic. (2009) ;121: :171–5. |
[44] | Roussos PA , Tassis A . Effects of girdling, nitrogen, zinc and auxin foliar spray applications on mandarin fruit “Nova” quality characteristics. Emir J Food Agric. (2011) ;23: :431–9. |
[45] | Ryugo K . Fruit culture: It is Science and Art. John Wiley and Sons; (1988) . pp. 259–61. |
[46] | Saadati S , Moallemi N , Mortazavi SMH , Seyyednejad SM . Effects of zinc and boron foliar application on soluble carbohydrate and oil contents of three olive cultivars during fruit ripening. Sci Hortic. (2013) ;164: :30–4. |
[47] | Sale P , Clark C . On the nutrition of Hayward kiwifruit - putting it all together- deciding on nutritional programme. Orchardist New Zealand; (2002) . p. 44–7. |
[48] | Sandipkumar P . Effect of micronutrients spray on yield, quality and retention of mango fruit (Mangifera indica L.) cv. Amrapali under middle Gujarat condition. Anand Agricultural University, India; (2015) . |
[49] | Shalan AMN . Impact of boric acid spraying date with different concentrations on yield and fruit quality of Pyrus communis cv. ‘Le-conte pear trees.. J. Plant Prod. (2013) ;4: (10), 1479–91. |
[50] | Shiri MA , Ghasemnezhad M , Fatahi Moghadam J , Ebrahimi R . Effect of CaCl2 sprays at different fruit development stages on postharvest keeping quality of “Hayward” kiwifruit. J Food Process Preserv. (2015) ;40: 624-–35. |
[51] | Shoeib MM , El Sayed A . Response of Thompson seedless grape vines to the spray of some nutrients and citric acid. Minia J Agric Res Dev. (2003) ;23: (4), 681–98. |
[52] | Singleton VL , Rossi JA . Colorimetry of total phenolics with phosphomolybdic– phosphotungstic acid reagents. Am J Enol Vitic. (1965) ;16: :144–58. |
[53] | Smith GS , Miller SA . Osmotic effects on performance and fruit quality of kiwifruit vines. Acta Hortic. (1992) ;297: :331–6. |
[54] | Snelgar WP , Minchin PEH , Blatmann P , Hall AJ . Sink priority on ‘Hayward’ kiwifruit vines. NZJ Crop Hortic Sci. (2012) ;40: :253–63. |
[55] | Song CZ , Liu MY , Meng JF , Chi M , Xi ZM , Zhang ZW . Promoting effectof foliage sprayed zinc sulfate on accumulation of sugar and phenolics inberries of Vitis vinifera cv. merlot growing on zinc deficient soil. Molecules. (2015) ;20: :2536–54. |
[56] | Sourour MM . Effect of foliar application of some micronutrient forms on growth, yield, fruit quality and leaf mineral composition of Valencia orange trees grown in North Sinai. Alexandria J Agri Res. (2000) ;45: (1), 269–85. |
[57] | Tagliavini M , Inglese P , Rombola A . Root uptake, storage and remobilisation of autumn applied nitrogen to kiwifruit (Actinidia deliciosa) vines. Agron J. (2000) ;20: :23–30. |
[58] | Talaie A , Taheri M . The effect of foliar spray with N, Zn and B on the fruit set and cropping of Iranian local olive trees. Acta Hortic. (2001) ;564: :337–41. |
[59] | Testoni A , Granelli G , Pagano A . Mineral nutrition influence on the yield and the quality of kiwifruit. Acta Hortic. (1990) ;282: :203–8. |
[60] | Vizzotto G , Lain O , Costa G . Relationship between nitrogen and fruit quality in kiwifruit. Acta Hortic. (1999) ;498: :165–72. |
[61] | Wimmer MA , Eichert T . Review: Mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci. (2013) ;203: :25–32. |
[62] | Xia G , Cheng L . Foliar urea application in the fall affects both nitrogen and carbon storage in young ‘Concord’ grapevines grown under a wide range of nitrogen supply. J Am Soc Hortic Sci. (2004) ;129: :653–9. |
[63] | Yadav V , Singh PN , Yadav P . Effect of foliar fertilization of boron, zinc and iron on fruit grwoth and yield of low-chill peach cv. Sharbati. Inter J Scientific Res. (2013) ;3: :1–6. |




