Characterization of morphological parameters and biological activity of jujube fruit (Ziziphus jujuba Mill.)
Abstract
BACKGROUND:
A diet rich in fruits and fruit-based products could improve human health. Jujube fruit is getting popularized in the world due to the high content of vitamin C, phenolic, flavonoids, polysaccharides and natural colorant properties.
OBJECTIVES:
The objective of this study was to evaluate the morphological and biological properties (antioxidant activity, total polyphenol, flavonoid and carotenoid content) of 15 genotypes of dry jujube fruit from Nova Kachovka (Ukraine).
MATERIALS AND METHODS:
Morphological characteristics (weight, width, and length), antioxidant activity by two different methods (DPPH, molybdenum reducing antioxidant power) and content of total polyphenols, flavonoids and carotenoids were analyzed in jujube fruit.
RESULTS:
The average weight of jujube fruit ranged from 2.52 to 19.37 g, width from 16.66 to 35.60 mm and length from 18.11 to 40.69 mm. Total polyphenol content ranged from 8.76 to 21.61 mg GAE per g, total flavonoid content from 1.49 to 11.57 μg QE per g and total carotenoid content from 1.53 to 14.31 μg per g. All tested samples exhibited DPPH• radical scavenging activities with values from 11.18 to 16.82 mg TEAC per g. Antioxidant activity by molybdenum reducing antioxidant power method ranged from 209.64 to 481.55 mg TEAC per g of dry matter. Differences between the genotypes were significant in all observed parameters.
CONCLUSION:
The results showed that the jujube fruit is rich in bioactive compounds and can be used in food, medicine and pharmacy industry.
1Introduction
Jujube (Ziziphus jujuba Mill.) is a plant of the family Rhamnaceae traditionally cultivated in the Mediterranean region, Southern and Eastern Asia and South China [1]. Nowadays, jujubes are widely distributed in Asia, Australia and Europe, including Slovakia. The jujubes trees attract the attention of researchers and food producers because of their drought tolerance and perspective of usage of those in food production [2]. Jujube fruits are increasingly eaten fresh or used as the food compounds because of the excellent nutritional properties. Chemically, the jujubes contain 81–83% of moisture, vitamins (A, B complex and especially C vitamin), sugars (22%; galactose, fructose, and glucose), organic acid (citric, malonic, and malic acids), minerals (Ca, K, and Fe), fatty acids (oil content of 0.76–1.8%; oleic acid (71.7%), and linoleic acid (15%), amino acids (0.8%; Asn, Pro, Arg, Ala, Glu, Ser, Asp), fiber (1.3%), and polyphenols [9].
Jujube fruits have been applied in several food products processing, including compotes, alcoholic beverages, chutneys, pickles, cakes and bread, mainly in India and in Africa [4]. However, the jujube fruits possess the health promoting properties and are commonly used in Traditional Chinese Medicine for detoxification, preventing of anemia, analeptic, palliative and immunity improvements. The peel and pulp of jujube fruit were used in folklore medicine for the relief of a cough and cold and as an expectorant. The bark of jujube has been used for antiflatulence, antidiarrhea and anti-vomiting properties while the leaves were consumed as the tea and applied in a cold pack on the head for cold relief and decongestion, including antidiabetes. The seeds of jujube share the sedative and hypnotic effects [10].
The color of jujube peel turns from green to yellow, then to reddish and finally to red during maturation. These peel colors represent maturity stages typically called the green fruit stage, white maturity, half-red maturity and red maturity stage, respectively [3]. Jujube fruits are perishable in the fresh state and deteriorate within ten days under ambient conditions after harvest [5]. Thus, the dried jujubes have been the predominant commercial form due to their long shelf life, convenience and desirable quality [5, 6].
Despite well-known nutritional compounds, the jujube fruit is still underexplored commercially, mainly, because of the content of bioactive compounds, which exhibits the significant health-promoting effect on consumer health [8]. The high antioxidant activity of the extracts from different parts of jujube fruit such as peel, pulp and seeds has been reported. This antioxidant activity has been attributed to the high level of phenolic compounds. Jujube fruit is known to contain a considerable amount of phenolic compounds, including chlorogenic acid, gallic acid, protocatechuic acid and caffeic acid [7]. The quality of available commercially jujube depends on the contents of bioactive compounds such as polyphenols, flavonoids and carotenoids. However, the various factors could influence bioactivity with the geographical environment, cultivar, processing and storage conditions to be among the most important.
Jujubes are an outstanding source of many nutrients and phytochemicals components with still unrevealed potential for commercial use and contribution to a healthy diet. Therefore, the objective of this study was to determine the morphological parameters and biological activity of 15 genotypes of dry jujube fruit (Ziziphus jujuba Mill.) from Ukraine, including detection of antioxidant activity in vitro, phenolic composition and total carotenoid content.
2Materials and methods
2.1Biological material
Jujube fruits (Ziziphus jujuba Mill.) were collected in 2014 from trees growing in the Nova Kachovka, Ukraine. The ripened fruits were picked from trees in red maturity stage. The plants were botanically identified in the Institute of Biodiversity Conservation and Biosafety of the Slovak University of Agriculture in Nitra. Jujube fresh pulp was separated from stone and dried in the oven (Binder 115, Germany) at 38 °C temperature, 48 hours. Samples were identified as ZJ (Ziziphus jujuba) and the numbers from 1 to 15 were used for further identification of genotypes.
2.2Morphological analysis of jujube fruits
The following properties were measured by morphological analysis:
a) Fruit length in mm, n = 15 of each genotype were measured with a digital caliper (Proteco, Czech Republic).
b) Fruit width in mm, n = 15 of each genotype were measured with a digital caliper (Proteco, Czech Republic).
c) Fruit weight in g, n = 15 of each genotype were weighted on an analytical balance (Kern ADB-A01S05, Germany).
d) Fruit jujube stone weight in g, n = 15 of each genotype were weighted on an analytical balance (Kern ADB-A01S05, Germany).
2.3Chemicals
All the chemicals used were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA) and CentralChem (Slovakia).
2.4Sample preparation
The dry jujube pulp was used for detection of total phenolic content and total flavonoid content. An amount of 0.25 g of each sample was extracted with 20 mL of 80% ethanol for 24 h. Then, the sample in 80% ethanol was centrifuged at 4000 rpm (Rotofix 32 A, Hettich, Germany) for 10 min and the supernatant was used for measurement with the DPPH and molybdenum reducing antioxidant power methods.
For detection of total carotenoid content, an amount of 0.5 g of dry jujube pulp was homogenized in the mortar with sea sand and repeatedly extracted with 10 mL of acetone until the sample became colorless. The extract was filtered using a Whatman filter paper.
2.5Detection of antioxidant activity
2.5.1Free radical scavenging activity
Free radical scavenging activity of samples was measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) [11]. An amount of 0.4 mL of sample was mixed with 3.6 mL of DPPH solution (0.025 g DPPH in 100 mL methanol). The absorbance of the reaction mixture was determined with the spectrophotometer Jenway (6405 UV/Vis, England) at 515 nm. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) (10–100 mg/L; R2 = 0.989) was used as the standard and the results were expressed in mg/g Trolox equivalents.
2.5.2Molybdenum reducing antioxidant power
Molybdenum reducing antioxidant power of samples was determined by the method of Prieto et al. [12] with slight modifications. The mixture of the sample (1 mL), monopotassium phosphate (2.8 mL, 0.1 M), sulfuric acid (6 mL, 1 M), ammonium heptamolybdate (0.4 mL, 0.1 M) and distilled water (0.8 mL) was incubated at 90°C for 120 min, then rapidly cooled. The absorbance at 700 nm was detected with the spectrophotometer Jenway (6405 UV/Vis, England). Trolox (10–1000 mg.L-1; R2 = 0.998) was used as the standard and the results were expressed in mg/g Trolox equivalents.
2.6Total polyphenol content
The total polyphenol content was measured by the method of Singleton and Rossi [13] using the Folin-Ciocalteu reagent. A quantity of 0.1 mL of each sample was mixed with 0.1 mL of the Folin-Ciocalteu reagent, 1 mL of 20% (w/v) sodium carbonate and 8.8 mL of distilled water. After 30 min in darkness, the absorbance at 700 nm was measured with the spectrophotometer Jenway (6405 UV/Vis, England). Gallic acid (25–300 mg/L-1; R2 = 0.998) was used as the standard. The results were expressed in mg/g gallic acid equivalents.
2.7Total flavonoid content
The total flavonoid content was determined by the modified method of Willett [14]. A quantity of 0.5 mL of sample was mixed with 0.1 mL of 10% (w/v) ethanolic solution of aluminum chloride, 0.1 mL of 1 M potassium acetate and 4.3 mL of distilled water. After 30 min in darkness, the absorbance at 415 nm was measured using the spectrophotometer Jenway (6405 UV/Vis, England). Quercetin (1–400 mg/L; R2 = 0.9977) was used as the standard. The results were expressed in μg/g quercetin equivalents.
2.8Total carotenoid content
Petroleum ether was pipetted into a separating funnel with the teflon stopcock. The acetone extract of the sample and distilled water were added by flowing along the walls of the funnel. The mixture was allowed to separate into two phases and the aqueous phase was discarded. The petroleum ether phase was washed 2 times with distilled water to remove the residual acetone. The petroleum ether phase was collected in a 50 ml volumetric flask by passing the solution through a small funnel containing 5 g of anhydrous sodium sulfate to remove residual water.
Then, the volumetric flask was made up to volume with petroleum ether and the total carotenoids content were determined from the molar absorption coefficient of β-carotene [15]. The concentration (μg/g) of carotenoids was calculated according to the following formula:
(1)
where: A – absorbance at 445 nm; r – sample dilution; E – molar absorption coefficient E1 %1 cm = 2620; n – sample weight; TCC – total carotenoid content.
2.9Statistical analysis
For statistical analysis, the SAS program was used (the SAS system 9.2.). Correlation coefficients were calculated by CORR analysis.
3Results and discussion
3.1Morphological parameters of jujube fruits
The weight of jujube fruit (Table 1) ranged from 2.52 g (ZJ12) to 19.37 g (ZJ15) with the coefficient of variation of 55.10%, which indicated the very high jujube fruit weight variability. The weight of pulp was from 2.21 g (ZJ12) to 19.29 g (ZJ15) showing the high variability (58.6%) as well. The weight, length and width of jujube fruit stones varied from 0.18 (ZJ07) to 0.88 g (ZJ12), from 18.11 mm (ZJ12) to 40.69 mm (ZJ14) and from 16.66 mm (ZJ07) to 35.60 mm (ZJ15) with high degree of variability of weight but with medium degree of variability for length and width. Karnatovska et al. [16] found the average weight of fruits from 1.00 to 9.5 g in 23 varieties of jujube growing in extreme agroecological condition in Nova Kachovka in Ukraine. Differences in weight of jujube fruits were reported by Ecevit et al. [17], Sivakov et al. [18] and Zhang et al. [19], who found the weight from 4.52 to 12.7 in different plant genotypes. The highest degree of variability of the jujube fruit weight was observed by Jia et al. [20] in variety Shiguang, who determined the average and maximal weight of 34.3 g and 108 g, respectively. Also Brindza et al. [21] found the weight variations from 0.66 to 4.68 g evaluating the collection of jujube seedlings growing in agroecological conditions of Slovakia. Our results are in accordance with the previous studies and support the observations on the high degree of variability between the jujube genotypes.
Table 1
Morphological characteristic of tested jujube fruit
| Parameter | n | min | max |
| V% |
| Fruit weight [g] | 15 | 2.52 | 19.37 | 7.96 | 55.10 |
| Pulp weight [g] | 15 | 2.21 | 19.29 | 7.50 | 58.6 |
| Stone weight [g] | 15 | 0.18 | 0.88 | 0.46 | 55.18 |
| Fruit length [mm] | 15 | 18.11 | 40.69 | 30.93 | 18.05 |
| Fruit width [mm] | 15 | 16.66 | 35.60 | 22.38 | 21.65 |
The correlation coefficients of the linear relationship between the morphological characteristics of the jujube (Table 2) showed the significant linear dependence between the weight and fruit width (r = 0.97). Positive correlation was determined between the weight and length (r = 0.77) and between the length and width of jujube fruits (r = 0.65). There were no correlation between the weight of jujube fruits and jujube stones.
Table 2
The correlation coefficients of linear relationship between the morphological characteristics of tested jujube genotypes
| Parameter | Pulp weight | Fruit length | Fruit width | Stone weight |
| Pulp weight | 1.000 | 0.769*** | 0.976*** | 0.668** |
| Fruit length | 1.000 | 0.653** | 0.517* | |
| Fruit width | 1.000 | 0.703** | ||
| Stone weight | 1.000 |
*P < 0.05; **P < 0.01; ***P < 0.001.
Significant differences in weight of jujube fruit, jujube stones and in the proportion of pulp and stones between the genotypes were observed (Fig. 1). Maximum fruit weight was found in genotype ZJ15 –19.37 g but the proportion of stone to total fruit weight comprised only 4.56%. In contrast, the lowest fruit weight was detected in genotype ZJ12 –2.52 g while the proportion of stone to the total weight of fruit was 12.3%. Genotypes of jujube with big stones are attractive for the producers as those with small stones. Jujube stones contain the valuable seeds, which are rich in biologically active substances, mainly fatty acids. Oil produced from the seeds can be used in pharmaceutical and food industry and hence are intended for practical application [22, 23].
Fig.1
Comparison of weight of fruit and weight of stones in tested jujube genotypes.

The jujube fruits start to be very attractive for food producing not only in Asia but also in other countries, including Ukraine and Slovakia. Our findings on the morphologyical characteristics of fruits are important for plant breeding and practically significant. Results of the present study indicate that jujube genotypes could be sorted according to the fruit and stones size.
3.2Antioxidant activity of jujube measured with the DPPH and molybdenum reducing antioxidant power methods
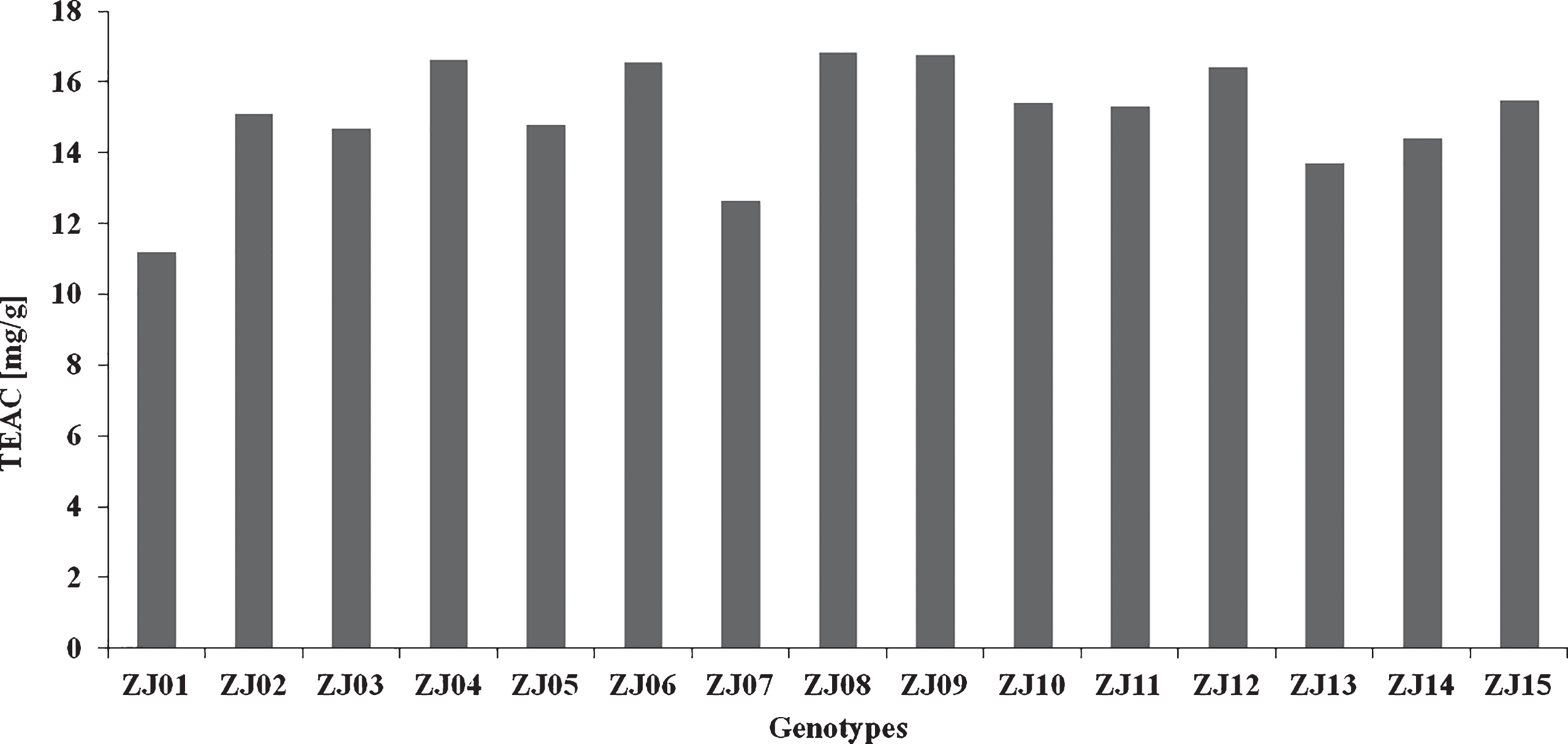
The antioxidant activity of jujube genotypes evaluated by the DPPH method (Fig. 2) ranged from 11.18 (ZJ01) to 16.82 (ZJ08) mg TEAC/g. The degree of mean variability of parameters was confirmed by the variation coefficient (V = 10.68%) in all the genotypes tested.
Fig.2
Antioxidant activity of the jujube genotypes evaluated by the DPPH method.
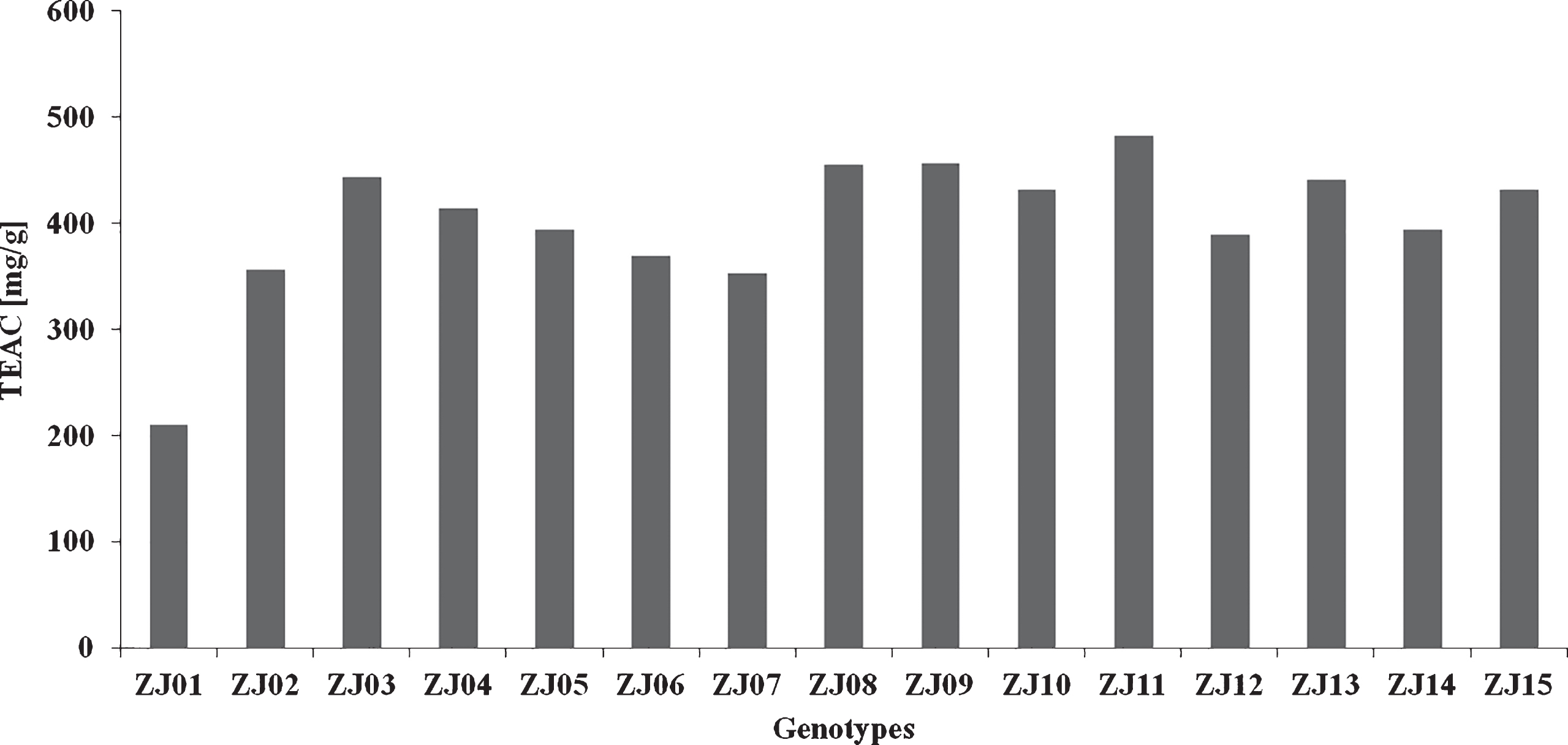
The antioxidant activity evaluated by the molybdenum reducing antioxidant power (Fig. 3) varied from 209.64 (ZJ01) to 481.54 (ZJ11) mg TEAC/g. The degree of mean variability of parameters was confirmed by the variation coefficient (V = 16.36%) in tested genotypes.
Fig.3
Antioxidant activity of jujube genotypes evaluated by the molybdenum reducing antioxidant power.
The antioxidant activity of jujube fruits in the present study was comparable with Koley et al. [27], who reported the results from 14.18 to 39.64% obtained with DPPH method in 12 Indian jujube cultivars. Parkash et al. [24] antioxidant activity testing results were 69.7±4.3% in dry jujube fruit, but Ali-Shad et al. [26] found the antioxidant activity to be between 34.9% and 47.3% in Pakistan. In Europe, the antioxidant activity of Spanish jujube by the ABTS method was from 23.85 to 47.37 mmol TEAC/100 g [3]. Antioxidant activity of jujube fruits could be influenced by the degree of maturity [1, 25]. The best antioxidant activity was present in half-red, respectively in last (red) degree of maturity stage. Since there are differences in genotypes of jujube cultivars grown under different geographic and agroecological conditions, our results are difficult to compare with the previous reported. Our study confirms the free radical scavenging ability in jujube grown in Ukraine indicating that the antioxidant properties possess the fruits of the cultivar in the Western Europe agroecological conditions. Wang et al. [28] reported that the polysaccharides of jujube can exhibit strong antioxidant and anticancer activity and can be used as safe compounds for functional foods showing that the current knowledge of jujube antioxidant activity is underestimated.
3.3Total carotenoid content in jujube fruits
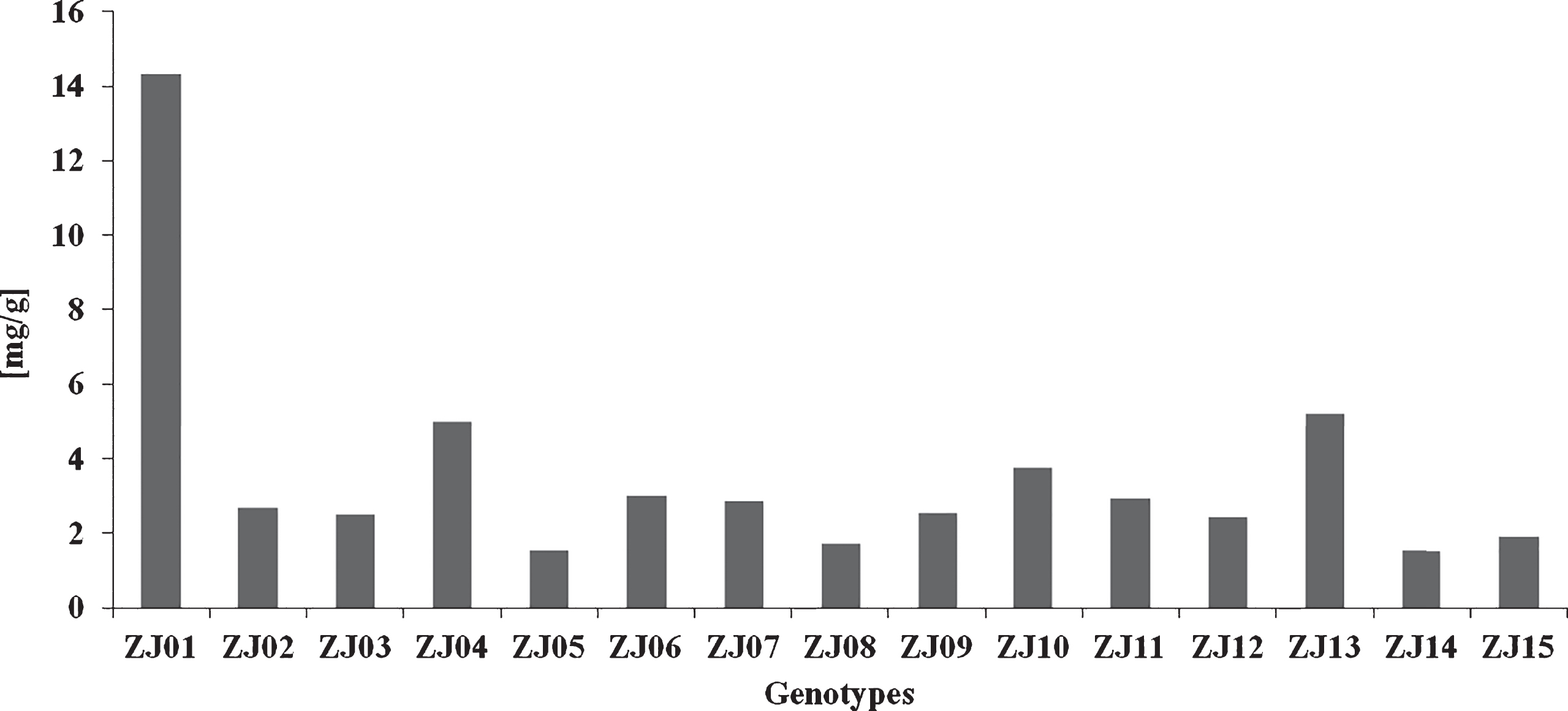
The total carotenoid content (Fig. 4) was the highest in ZJ1 (14.31 μg/g) but the lowest in ZJ05 (1.52 μg/g), ZJ14 (1.52 μg/g) and ZJ08 (1.72 μg/g) with the high degree of variation (V = 88.16%) in jujube fruits confirmed. Variations in the content of the total carotenoids were reported previously. Guil-Guerrero et al. [29] found the total carotenoid content from 4.12 to 5.98 mg/100 g while San and Yildirim [30] from 7 to 35 μg/100 g in the fresh mass of jujube fruits. Pareek and Dhaka [32] showed the mean total carotenoid content of 21 μg/100 g for fresh jujube fruits. The content of total carotenoids increases during ripening and Ezhilarasi and Tamilmani [31] reported that the colour changes in jujube fruits were attributable to the loss of chlorophyll and the synthesis of carotenoids. The colour turn from green into red is a consequence of chlorophyll degradation and accumulation of a large amount of carotenoids within the plastids as the chloroplasts present in the mature green fruit are transformed to chromoplasts. Dietary carotenoids from jujube fruits can provide health benefits in decreasing the risk of disease, particularly certain cancers and eye disease [33]. β-carotene of jujube carotenoids may have the beneficiary effect due to its ability to be converted to vitamin A.
Fig.4
Total carotenoid content in jujube genotypes.
3.4Total polyphenol and flavonoid content in jujube fruits
The total polyphenol content (Fig. 5) in jujube genotypes ranged from 8.76 (ZJ12) to 21.61 mg GAE/g (ZJ06). The correlation coefficient (V = 23.60%) confirms the high variability of the parameter (Table 3). The total polyphenol content varied widely from 1.09 to 106.8 mg GAE/g in studies in Africa and Asia, respectively [24, 34, 35]. Differences between the cultivars were described and the total polyphenol content was from 4.29 to 6.00 mg GAE/g in five cultivars of jujube in Gao et al. [36] study. Polyphenol content was from 7.45 to 13.21 mg GAE/g in ten genotypes of jujube from different geographical localities in Sun et al. [37] study. Ali-Shad et al. [26] compared the total polyphenol content in hexane and methanol extracts of jujube and found that the mean values were higher in methanol extracts (21.74 mg GAE/g). In our mind, the differences between the present and previously conducted studies may be attributable to the plant geographical origin as well as the different methods of extraction. There are few data on bioactive nutrients, especially on the content and composition of polyphenols of jujube from Ukraine. Gallic, protocatechuic, p-coumaric, ferulic and sinapic acids were found to be the predominant compounds of polyphenols found in jujube extracts [10], while protocatechuic, gallic, chlorogenic and caffeic acids were identified in the dried peel, pulp and seed of Chinese jujubes [38]. Pawlowska et al. [39] reported that the betulinic acid was present in all parts of jujube plant and exhibited anti-inflammatory and antibacterial activity [40].
Fig.5
Total polyphenol content in jujube genotypes.
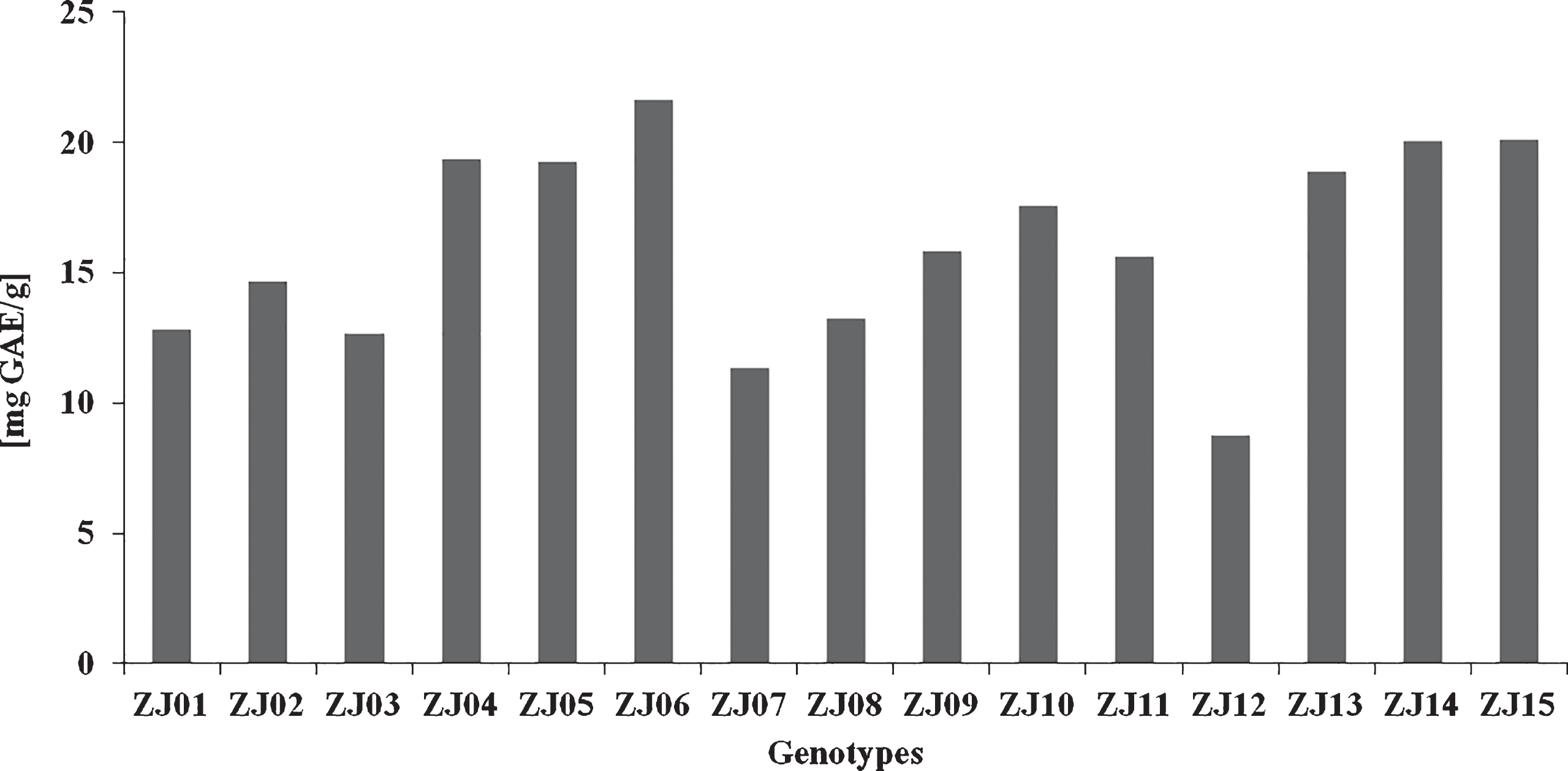
Table 3
Variability of biological activity in jujube genotypes
| Parameter | n | min | max |
| V% |
| Carotenoids [μg/g] | 15 | 1.53 | 14.31 | 3.59 | 88.16 |
| Polyphenols [mg GAE/g] | 15 | 8.76 | 21.61 | 16.11 | 23.60 |
| Flavonoids [μg QE/g] | 15 | 1.49 | 11.57 | 5.64 | 53.19 |
| DPPH method [mg TEAC/g] | 15 | 11.18 | 16.82 | 15.06 | 10.68 |
| Molybdenum reducing power method [mg TEAC/g] | 15 | 209.64 | 481.55 | 401.15 | 16.36 |
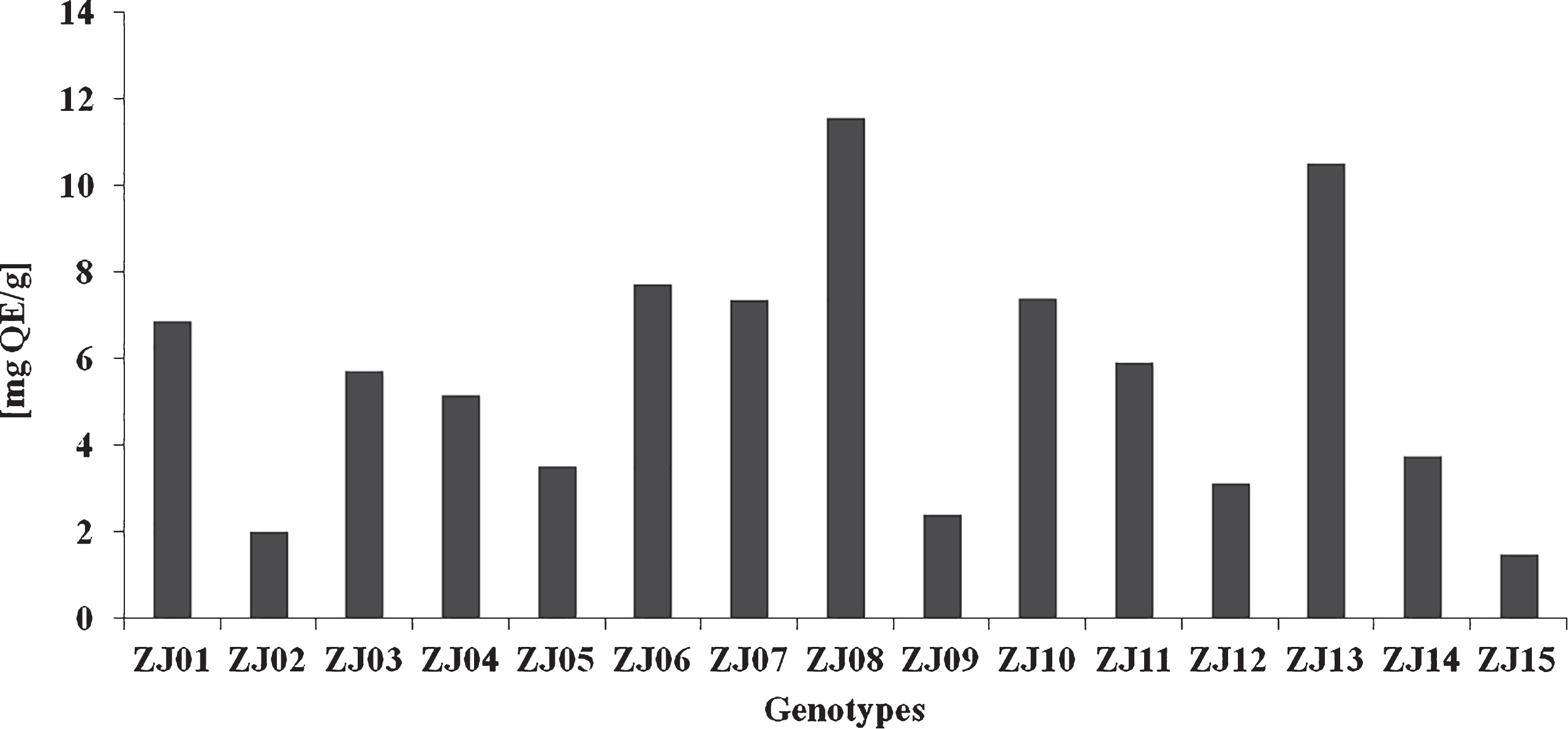
The total flavonoid content (Fig. 6) varied from 1.49 (ZJ15) to 11.58 μg QE/g (ZJ08). The variation coefficient (V = 53.19%) supported the observations on high variability (Table 3) of this parameter. Our results correspond to Ali-Shad et al. [26] study, who found the amount of flavonoid from 3.25 mg QE/g (hexane extract) to 15.66 mg QE/g (methanol extract) in jujube fruits. Novel flavonoids –swertish, spinosine and zivulgarin from jujube fruit and semen were described in study Gong et al. [41] there the swertish and spinosine were exerted the sedative effect to humans. Differences between the content of flavonoids were observed between studies and rutin, myricetin, quercetin, apigenin and kaempferol were identified in jujube fruit in the study of Siriamornpun et al. [10], while Gao et al. [36] found three major flavonoid compounds – epicatechin, catechin and rutin. The differences in individual flavonoids between the studies may be due to the genetic variability of the plants leading to dissimilarities in the biosynthesis of flavonoids in these varieties [10].
Fig.6
Total flavonoid content in jujube genotypes.
Strong negative correlation (Table 4) was detected between the total carotenoid content and antioxidant activity identified with the DPPH method (r = –0,643) and between the total carotenoid content and antioxidant activity determined by the molybdenum reducing power method (r = –0.732). Significant correlation was not identified between the polyphenol content and antioxidant activity. These results clearly show that the antioxidant activity and the content of carotenoids, polyphenols, flavonoids are not directly proportional hence in every method applied for examination the different combination of biochemical components should beconsidered.
Table 4
The correlation coefficients of linear relationship between the biological activity of tested jujube genotypes
| Parameter | Carotenoids | Polyphenols | Flavonoids | DPPH method | MRP method |
| Carotenoids | 1.000 | –0.161 | 0.239 | –0.643** | –0.732*** |
| Polyphenols | 1.000 | –0.060 | 0.213 | 0.216 | |
| Flavonoids | 1.000 | –0.163 | 0.043 | ||
| DPPH method | 1.000 | 0.667** |
MRP –molybdenic reducing power; **P < 0.01; ***P < 0.001.
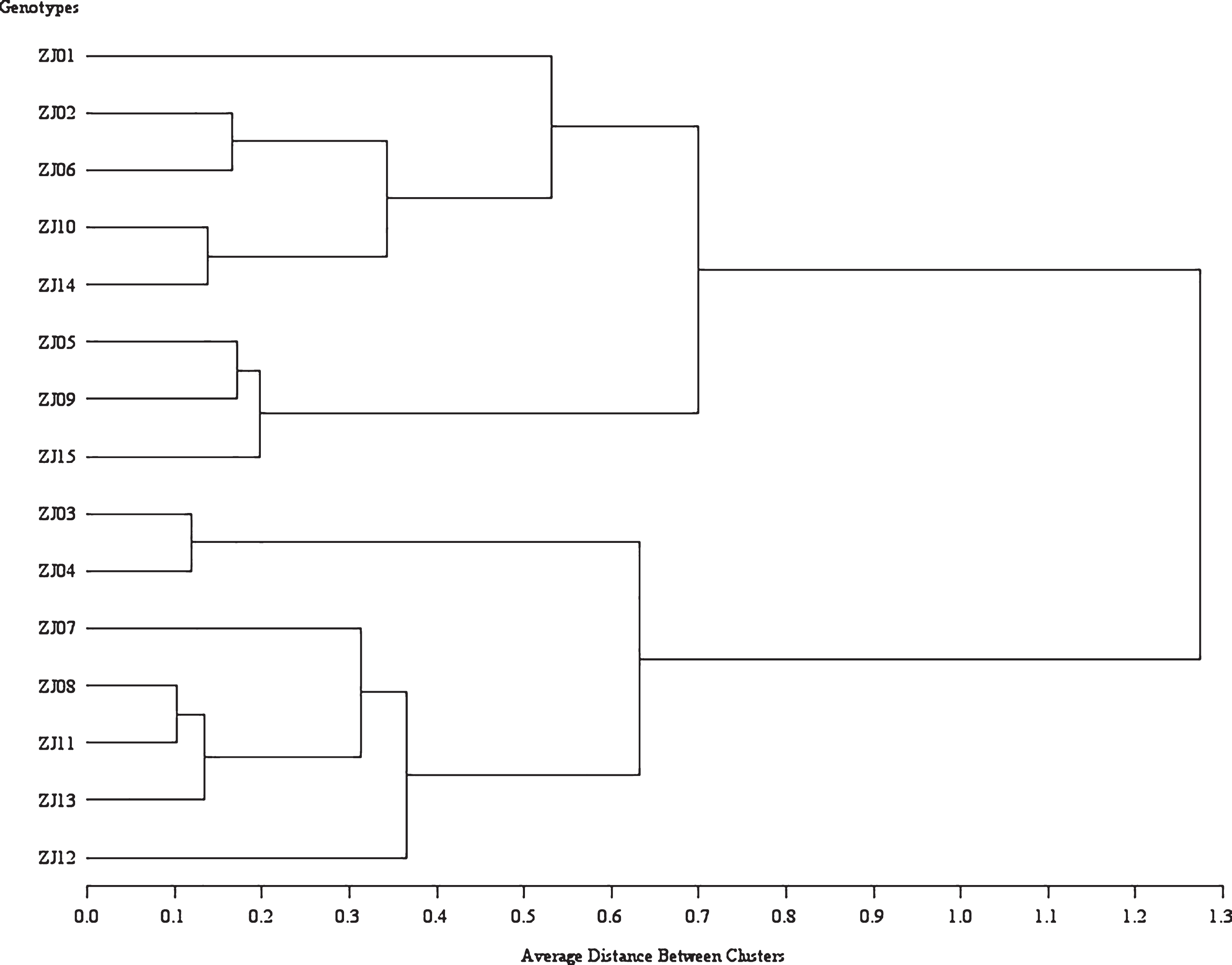
Based on the data obtained in our study we provided the determination of phenotypic relatedness by the method of discriminant analysis (Fig. 7). A comparison clearly shows the different genotypes and grouping and the significant differences between them.
Fig.7
Comparison of phenotypic relatedness of the tested jujube genotypes according to the morphological and biological properties.
4Conclusion
The present study revealed the significance differences between the 15 jujube genotypes regarding the morphological parameters, antioxidant activity, total polyphenol, flavonoid and carotenoid content. The report confirmed that the correlation between the antioxidant activity and total polyphenol, flavonoid and carotenoid content was not found. The practical use of jujube genotypes is highlighted for genetic breeding that allow setting specific targets for further research study. Practically, the study confirmed the use of the fruits of jujube of the examined genotypes in the food industry as raw material for the preparation of various food products with significant phytopharmaceutical properties. Ziziphus jujuba Mill. can be a potential fruit species for expanding the use of resources in Slovakia.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgments
The publication was prepared with the active participation of researchers in international network AGROBIONET, as a part of international program “Agricultural biodiversity to improve nutrition, health and quality of life” (TRIVE ITMS26110230085) within the project ITEBIO-ITMS 26220220115 “Support of technologies innovation for special bio-food products for human healthy nutrition”.
References
[1] | Gündüz K , Saracoglo O . Changes in chemical composition, total phenolic content and antioxidant activities of jujube (Ziziphus jujuba Mill.) fruits at different maturation stages. Acta Scientiarum Polonorum Hortorum Cultus. (2014) ;13: :187–95. |
[2] | Wu ChS , Gao QH , Kjelger RK , Guo XD , Wang M . Yields, phenolic profiles and antioxidant activities of Ziziphus jujube Mill. in response to different fertilization treatments. Molecules. (2013) ;18: :12029–40. |
[3] | Wang B , Huang Q , Venkitasamy Ch , Chai H , Gao H , Cao W , Lu X , Pan Z . Changes in phenolic compounds and their antioxidant activity in jujube (Ziziphus jujuba Mill.) during three edible maturity stages. LWT –Food Science and Technology.. (2016) ;66: :56–62. |
[4] | Zozio S , Serent A , Cazal G , Mbéguité-A-Mbéguité D , Ravion S , Pallet D , Abel H . Changes in antioxidant activity during the ripening of jujube (Ziziphus mauritiana L.). Food Chemistry. (2016) ;150: :448–56. |
[5] | Kov X , Chen Q , Li X , Li M , Kan C , Chen B , Zhang Y , Xue Z . Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chemistry. (2015) ;173: :1037–44. |
[6] | Gao QH , Wu CS , Yu JC , Wang M , Ma YJ , Li CM . Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. Journal of Food Sciences. (2012) ;12: :1218–25. |
[7] | Zhao H , Zhang H , Yang S . Phenolic compounds and its antioxidant activities in ethanolic extracts from seven cultivars of Chinese jujube. Food Science and Human Wellness. (2014) ;3: :183–90. |
[8] | Bastos VJ , NBeves LC , Silva PMC , Shahab M , Colombo RC , Roberto SR . Harvest point determination of Indian fruit (Ziziphus mauritiana L.) based on physicochemical and functional parameters. Scientia Horticulture. (2016) ;213: :392–402. |
[9] | Wojdylo A , Barrachina AAC , Legua O , Hernandez F . Phenolic composition, ascorbic acid content, and antioxidant activity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chemistry. (2016) ;201: :307–14. |
[10] | Siriamornpun S , Weerapreeyaku N , Barusrux S . Bioactive compounds and health implications are better for green jujube fruit that for ripe fruit. Journal of Functional Foods. (2015) ;12: :246–55. |
[11] | Sánchés-Moreno C , Larrauri A , Saura-Calixto F . A procedure to measure the antioxidant efficiency of polyphenols. Journal of Sciences and Food Agricultural. (1998) ;76: :270–6. |
[12] | Prieto P , Pinera M , Aguilar M . Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry. (1999) ;269: :334–7. |
[13] | Singleton VL , Rossi JA . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Agricultural. (1965) ;6: :144–58. |
[14] | Willett WC . Balancing life-style and genomics research for disease prevention. Science. (2002) ;292: :695–8. |
[15] | STN 56 0053:1986. Determination of vitamin A and its provitamins. Praha: ÚNN, (1986) . |
[16] | Karnatovska M , Brindza J , Grygorieva O , Derevjanko V , Kochanova Z , Birova D . Jujube fruit (Zizyphus jujuba Mill.) variability determination. 1st International Scientific Conference on Medicinal, Aromatic and Spice Plants. Book of Scientific Papers and Abstracts. Nitra. (2007) ;219. SBN 987-80-8069-973-4. |
[17] | Ecevit FM , Şan B , Dilmaç UT , Hallaç Türk F , Yildirim AN , Polat M , Yildirim F . Selection of superior ber (Ziziphus jujuba L.) genotypes in Çivril region. Tarim Bilimleri Dergisi. (2008) ;1: :51–6. |
[18] | Sivakov L , Georgiev D , Ristevski B , Mitreski Z . Pomological and technological characteristics of Chinese jujube (Zyziphus jujuba) in Macedonia. Jugoslovensko Vocarstvo. (1988) ;224: :387–92. |
[19] | Zhang H , Jiang L , Ye S , Ye YB , Ren FZ . Rapid SNP discovery and a RAD-based high-density linkage map in jujube (Ziziphus Mill.). Food and Chemical Toxicology. (2010) ;48: :1461. |
[20] | Jia YF , Lu R , Duan Y , Ma W . A New Zizyphus jujuba cultivar ‘Shiguang’. Shijiazhuang. Pomology Institute, Hebei Academy of Agriculture and Forestry Sciences, Shijiazhuang 050061, (2010) . China. |
[21] | Brindza J , Karnatovská M , Grygorieva O , Vietoris V , Kucelová L , Erdélyová G . Morphological and organoleptic nature of Ziziphus jujuba Mill. Potravinarstvo. (2011) ;5: :1–11. |
[22] | Yoon J . Hair growth promoting effect of Zizyphus jujuba essential oil. Food and Chemical Toxicology. (2010) ;48: :1350–4. |
[23] | Al-Reza SM , Bajpai VK , Kang SC . Antioxidant and antilisterial effect of essential oil and organic extracts from Zizyphus jujuba. Food and Chemical Toxicology. (2009) ;47: :2374–80. |
[24] | Prakash D , Upadhyay G , Gupta C . Total phenol and antioxidant activity of some fruits and their under-utilized parts. International Food Research Journal. (2013) ;20: :1717–24. |
[25] | Wang C , Cheng D , Cao J , Jiang W . Antioxidant capacity and chemical constituents of Chinese jujube (Ziziphus jujuba Mill.) at different ripening stages. Food Sciences and Biotechnology. (2013) ;22: :639–44. |
[26] | Shad A , Ahmad S , Ullah R , AbdEl-Salam Naser M , Fouad H , Ur R , Najeeb H , Hidayat SW . Phytochemical and biological activities of four wild medicinal plants. The Scientific World Journal. (2014) ;5: :1–7. |
[27] | Koley TK , Kaur Ch , Nagal S , Walia S , Jaggi S , Sarika S . Antioxidant activity and phenolic content in genotypes of Indian jujube (Ziziphus mauritiana L.). Arabian Journal of Chemistry. (2016) ;9: :1044–52. |
[28] | Wang D , Zhao Y , Jiao Y , Yu L , Yang S , Yang X . Antioxidative and hepatoprotetive effects of the polysaccharides from Ziziphus jujube cv. Shaanbeitanzo. Carbohydrate Polymers. (2012) ;88: :1453–9. |
[29] | Guil-Guerrero JL , Diaz Delgado A , Matallana Gonzalez MC , Torija Isasa M . Fatty acids and carotenes in some ber (Ziziphus jujuba Mill) varieties. Plant Foods for Human Nutrition. (2004) ;59: :23–7. |
[30] | San B , Yildirim AN . Phenolic, alpha-tocopherol, beta-carotene and fatty acid composition of four promising jujube (Ziziphus jujuba Miller) selections. Journal of Food Composition and Analysis. (2010) ;23: :706–10. |
[31] | Ezhilarasi C , Tamilmani A . Influence of paddy husk on the ripening fruit of Ziziphus mauritiana L. ARPN Journal of Agricultural and Biological Science. (2009) ;6: :29–42. |
[32] | Pareek S , Dhaka RS . Association analysis for quality attributes in ber. Indian Journal of Arid Horticulture. (2008) ;3: :77–80. |
[33] | Johnson EJ . The role of carotenoids in human health. Nutrition in Clinical Care. (2002) ;5: :56–65. |
[34] | Elaloui M , Laamouri A , Fabre J , Mathieu C , Vilarem G , Hasnaoui B . Distribution of free amino acids, polyphenols and sugars of Ziziphus jujuba pulps harvested from plants grown in Tunisia. Natural Product Research. (2015) ;29: :94–7. |
[35] | Li JW , Ding SD , Ding XI . Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochemistry. (2005) ;11: :3607–13. |
[36] | Gao QH , Wu PT , Liu JR , Wu CS , Parry JW , Wang M . Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Scientia Horticulturae. (2011) ;130: :67–72. |
[37] | Sun YF , Liang ZS , Shan CJ , Viernstein H , Unger F . Comprehensive evaluation of natural antioxidants and antioxidant potentials in Ziziphus jujuba Mill. var. spinosa (Bunge) Huex HF. Chou fruits based on geographical origin by TOPSIS method. Food Chemistry. (2011) ;124: :1612–9. |
[38] | Zhang H , Jiang L , Yubin Y , Fazheng R . Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujube Mill) from China. Food and Chemical Toxicology. (2010) ;48: :1461–5. |
[39] | Pawlowska AM , Camangi F , Bader A , Braca A . Flavonoids of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (Rhamnaceae) fruits. Food Chemistry. (2000) ;112: :858–62. |
[40] | Preeti Tripathi S . Ziziphus jujube: A phytopharmacological review. International Journal of Research and Development in Pharmacy and Life Sciences. (2014) ;3: :959–66. |
[41] | Gong C , Yanjing B , Yuying Z , Jing T , Yi L , Guangzhong T , Libin M , Ning L , Xiaojie X . Flavonoids from Ziziphus jujuba Mill var. spinasa. Tetrahedron. (2000) ;56: :8915–20. |




