Staining and microscopy of mycorrhizal fungal colonization in preserved ericoid plant roots
Abstract
BACKGROUND:
Visualization of ericoid mycorrhizal colonization using traditional methods relies on either fresh or KOH stored samples. Increasing interest in studying ericoid mycorrhization has highlighted a need for methods which can be used for preserved samples and are simple to implement with commonly available equipment.
OBJECTIVE:
The aim of this study was to improve on traditional techniques for staining ericoid mycorrhizal fungi and microscopically visualizing ericoid mycorrhizal roots which have been preserved.
METHODS:
Ericoid mycorrhizal roots were placed in KOH or frozen at –20 °C for long-term storage. Traditional Trypan Blue staining methods were modified to reduce damage to fine mycorrhizal hyphae and cortical cells. A high light-intensity dark-field microscopy technique was applied to clearly visualize stained mycorrhizae. The novel application was compared to other commonly used practices.
RESULTS:
Trypan Blue staining without KOH storage or clearing allowed for successful staining of ericoid mycorrhizal roots stored at –20 °C. The application of high light-intensity dark-field microscopy provided high contrast visualization of mycorrhizal structures.
CONCLUSIONS:
The modified Trypan Blue staining method was effective on frozen root samples, with dark-field microscopy being particularly effective at visualizing dark colored roots. Advantages to this method are low cost and relatively fast application time. Therefore, this method is a realistic option for large scale analyses with many samples which require long-term preservation.
1Introduction
The under-story vegetation in boreal forests comprises predominantly ericoid plants whose berries provide an important source of nutrition for many organisms. Ericoid plants rely upon mycorrhizal fungi for accessing organic nutrients in harsh, nutrient-poor environments [2]. Ericoid mycorrhizal plants are important to both wild and cultivated berry production in a wide variety of ecosystems. This has led to an increasing interest in studying ericoid mycorrhizal symbiosis for its ecological significance as well as agricultural potential. Ericoid highbush blueberry plants (Vaccinium corymbosum L.) inoculated with mycorrhizal fungi have been shown to gain improved growth and protection against plant pathogens with reduced fertilization requirements [4, 9, 11]. Recent data from fungal genome studies indicate that ericoid fungal species have a significant capacity for decomposition of soil organic matter as their genomes harbor a wide variety of carbohydrate degrading genes [3]. Despite this capability, ecosystems dominated by ericoid and ectomycorrhizal fungi store the most terrestrial C on Earth [1]. As their role in terrestrial C sequestration has not yet been studied intensively, investigations focusing on ericoid plants and their mycorrhizal symbionts will be of great interest in the future. There is also a need to investigate the functions of ericoid mycorrhizae in commercial blueberry fields [10].
Ericoid plant roots are fine structured and ericoid mycorrhizae usually do not form a hyphal mantle, therefore the presence of ericoid mycorrhizal colonization can only be verified using microscopy aided by staining of fungal hyphae. There are several methods available for staining ericoid mycorrhizal roots for microscopy, many of which rely on the original paper by Phillips and Hayman [8]. For instance, Koske & Gemma [5], Nestby et al. [7], Sadowsky et al. [10] and Scagel [12] used modified Phillips & Hayman [8] methods, including clearing of roots in KOH followed by acidification and then staining. As reported by Koske & Gemma [6], the 10% KOH treatment “is harsh and may result in the loss of the cortex from much of the root system”. This is particularly evident in roots which must be preserved long-term and require KOH storage or freezing at –20 °C, with freezing often being necessary for molecular and chemical analyses. Thus, there is a clear need to develop and test methods for staining fragile and thin ericoid plant roots for microscopy that have been stored frozen. Also, as many research projects dealing with ericoid plants are not designed for studying ericoid mycorrhizal colonization but rather other ecological or commercial aspects of ericoid plants, it would be advantageous to be able to re-analyze frozen root samples from such experiments. This capability would provide better understanding of ericoid mycorrhizal ecology and importance for berry cultivation.
The aim of the study was to develop a simple, fast and gentle method for staining and visualizing preserved ericoid plant roots for microscopic detection of mycorrhizal intracellular colonization.
2Materials and methods
The ericoid species Vaccinium myrtillus, Vaccinium vitis-idaea, and Calluna vulgaris were grown experimentally for 18 months in homogenized, natural forest humus under simulated boreal forest conditions [13]. The plants were allowed to naturally form ericoid mycorrhizal symbioses within their roots. Root samples were hand washed with cold tap water to remove excess soil.
2.1Staining
Root samples were stored at –20 °C and in 10% KOH solution to test the effects of long-term storage (6 months) on ericoid mycorrhizal roots. Root samples were prepared for microscopic visualization of mycorrhizal colonization using a Trypan Blue staining technique modified from Phillips and Hayman [8]. A second set of samples were stored in 10% KOH for 1 day or 3 days at room temperature and incubated in Trypan Blue at 90 °C for 30 minutes to test if reduced time in both steps prevented damage to fine root cortical cells. Frozen samples were taken directly from –20 °C and incubated in a 1 : 1 solution of lactic acid and glycerol containing 0.05% Trypan Blue at 90 °C for 30 minutes. Frozen roots were not treated with KOH for clearing and therefore did not require neutralization in HCl. As a comparison with the long-term preservation methods being tested, fresh C. vulgaris roots were placed in Trypan Blue for 10 minutes at room temperature.
Immediately after staining with Trypan Blue all root samples were placed in a de-staining solution of 1 : 1 lactic acid and glycerol at room temperature for 15 minutes to remove excess dye. Individual root segments were then arranged on glass microscopy slides. Several root segments from each sample were fitted in parallel under the same 20×50 mm cover slip and embedded with clean de-staining solution, which was then sealed along the sides using transparent nail polish.
2.2Microscopy
A Zeiss Axioskop 2 Plus microscope (Zeiss, Oberkochen, Germany) was used in transmitted light mode with an achromatic-aplanatic universal condenser (0.9 H D Ph DIC) in Dark Field mode and front lens in place. Each prepared slide was observed through an A-Plan 40×/0.65 (Ph2 Var2) objective with maximum illumination and all diaphragms fully opened. The height of the condenser was adjusted to near upper maximum position to produce optimal illumination of the samples. This was achieved by oblique light rays reflecting within the embedding solution surrounding the root fibers, providing increased contrast of roots and stained mycorrhizal fungal cells. Careful adjustment of focus allowed for visualization of colonizing fungal features in several planes. Use of this technique on older microscopes such as an Axioskop 1 (Zeiss, Oberkochen, Germany) is not ideal as their light sources are not sufficient, a 100 watt light source is recommended for clear visualization.
Caution: light intensity should only be increased to maximum after the slide is in place for observation, as the embedding solution reduces the light to a safe viewing level.
2.3Estimation of colonization percentage
By using this method to clearly visualize ericoid mycorrhizal roots it is possible to quantify colonization using the magnified intersections method [6]. This method is performed by recording the mycorrhizal colonization status of each root fiber as it is intersected by the eyepiece crosshair along a vertical transect at 400X magnification. Generally, 50 to 200 vertical transects are done along the length of the slide. This method allows for a simple analysis of colonization at each intersection of the eyepiece crosshair.
3Results
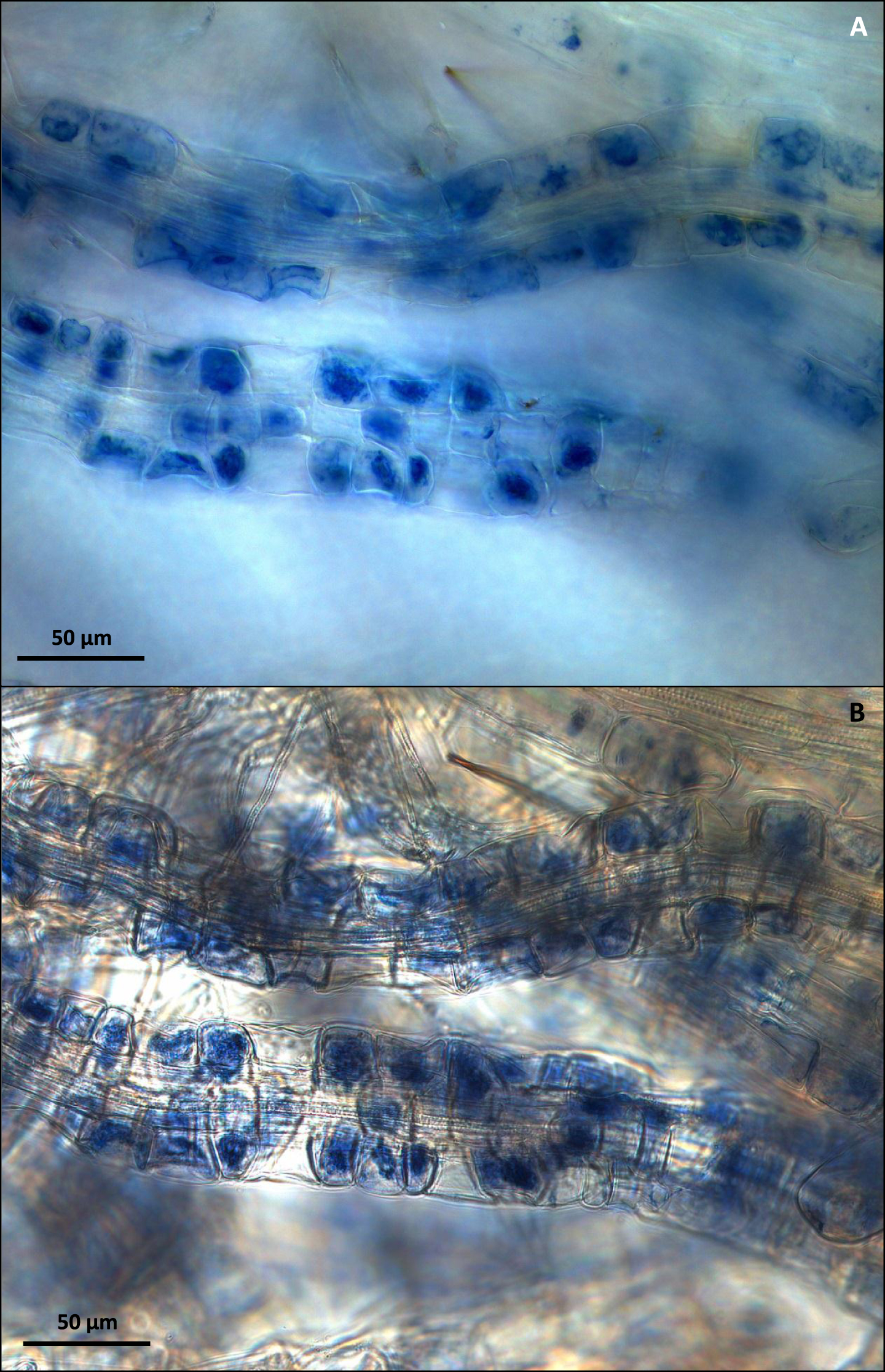
Storage of ericoid roots in 10% KOH for both 1 and 3 days was highly damaging to cortical cells of all tested species, with nearly all roots becoming completely disrupted (Fig. 1A). Directly stained fresh C. vulgaris roots (Fig. 1B) allowed for high quality dark-field microscopic visualization of fungal structures, but the freeze-dye method gave even better results, even after long storage of roots at –20 °C (Fig. 1C). The visualization of mycorrhizal colonization with the freeze-dye method produced the best results also with mycorrhizal roots of V. myrtillus and V. vitis-idaea (Fig. 2A and 2B). The high light-intensity dark field microscopy (Fig. 3A) provided superior visualization of root cells when compared with traditional bright field microscopy (Fig. 3B).
Fig.1
(A) C. vulgaris roots after KOH treatment and Trypan Blue staining resulting in total loss of root cortical cells. (B) Fresh C. vulgaris roots after staining in Trypan Blue for 10 minutes at room temperature and displaying intracellular hyphal coiling (HC). (C) Mycorrhizal C. vulgaris roots after freezing at –20 °C and staining with Trypan Blue and displaying intracellular hyphal coiling (HC). Bar = 50μm.
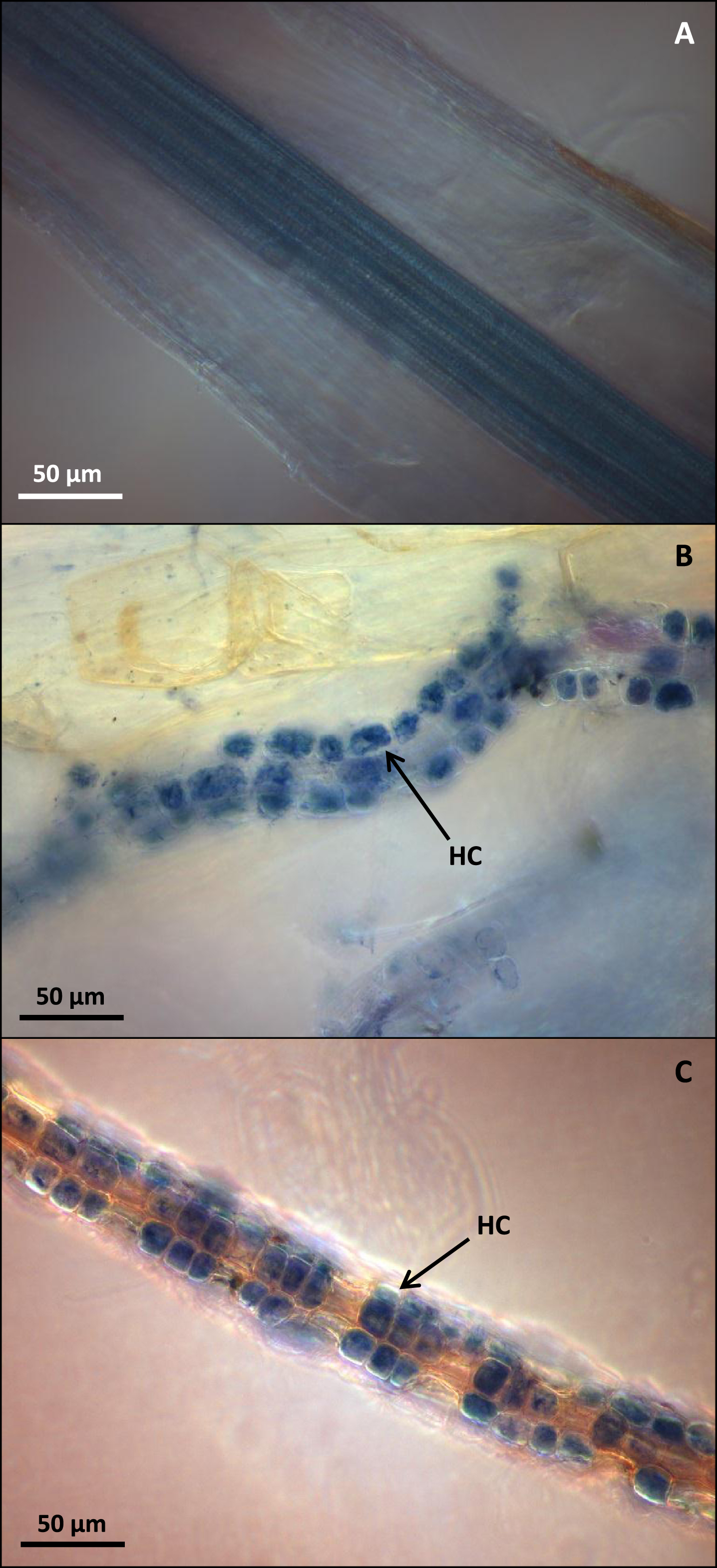
Fig.2
(A) Mycorrhizal V. myrtillus roots after freezing at –20 °C and staining with Trypan Blue and displaying intracellular hyphal coiling (HC). (B) Mycorrhizal V. vitis-idaea roots after freezing at –20 °C and staining with Trypan Blue and displaying intracellular hyphal coiling (HC). Bar = 50μm.
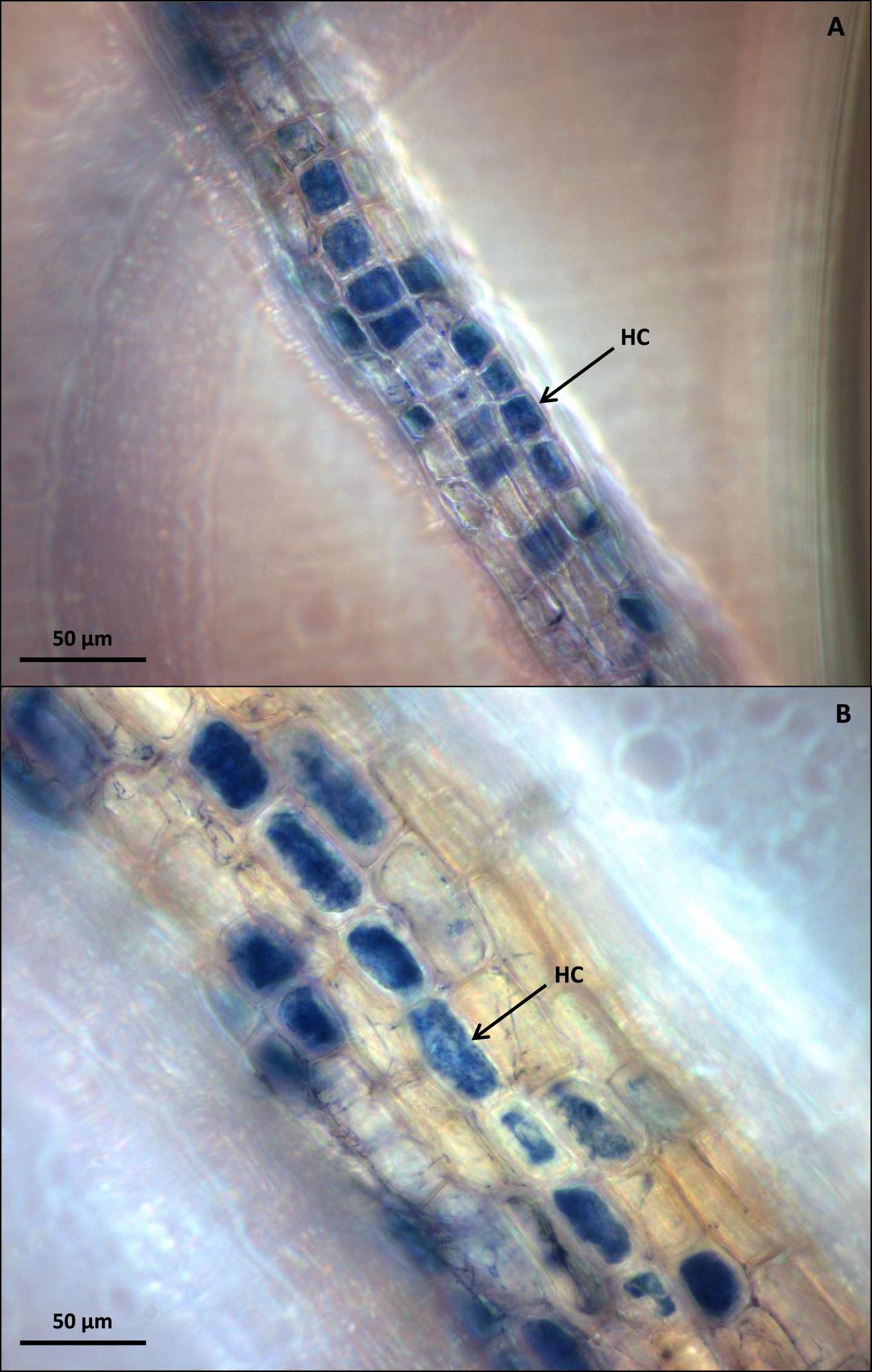
Fig.3
(A) C. vulgaris mycorrhizal roots visualized by high light-intensity dark field microscopy. (B) Comparative visualization of the same C. vulgaris mycorrhizal roots using bright field microscopy. Bar = 50μm.
4Discussion
The combined staining and microscopy methods described here provide good visual clarity and detection of ericoid mycorrhizal colonization in roots frozen for later analyses with the additional benefits of simplicity, rapid application and low cost. With practice, a slide preparation of stained ericoid mycorrhizal roots takes under one hour to complete and time of microscopy is reduced as the high light-intensity dark-field method makes it easier to observe mycorrhizal structures in several focus planes. Firstly, staining of mycorrhizal hyphae was successful after freezing of ericoid mycorrhizal roots at –20 °C, while preserving fine hyphae on the surface of roots and internal hyphal structures. Traditional methods describe clearing of roots in KOH followed by HCl neutralization and staining, which in our case was shown to be damaging to fine ericoid plant roots and any hyphae on or within them. As the microscopy utilized in this method provided enhanced visual clarity and illumination, it was possible to observe mycorrhization even in darkly colored root cortical cells. Therefore, a clearing step using KOH was entirely unnecessary and fragile hyphal structures were preserved.
Compared to more advanced and potentially cost prohibitive visualization techniques such as Differential Interference Contrast (DIC) or electron microscopy, high-quality microscopy of mycorrhizae using dark-field microscopy is advantageous. This method requires minimal training and is achievable using the majority of modern light microscopes without expensive additional equipment. The ability to clearly observe mycorrhizal structures reduces ambiguity when identifying mycorrhizal colonization and provides more accurate estimation of colonization frequency.
The primary advantage of this method is the short application time, with quick staining and simple microscopy providing the capability to analyze large numbers of samples which can also be preserved by freezing at –20 °C, allowing for further molecular and chemical analyses. Reducing time of analyses in commercial berry production or field scale ecological studies is critical to producing results. The capability of this method may become increasingly relevant with growing interest in utilizing ericoid mycorrhizae for commercial purposes, as well as current research which indicates the potential importance of ericoid mycorrhizae in global carbon sequestration. The presented method produces successful staining and microscopy of frozen ericoid mycorrhizal root samples. Thus, freezer-stored ericoid root samples from old, completed experiments could be re-analyzed for quantification of ericoid mycorrhizal colonization.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgments
The authors gratefully acknowledge the Academy of Finland for supporting this work (grant numbers 292699, 293365).
References
[1] | Averill C. , Turner B.L. , Finzi A.C. . Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature. (2014) ;505: (7484):543–5. |
[2] | Cairney J.W. , Meharg A.A. . Ericoid mycorrhiza: A partnership that exploits harsh edaphic conditions. European Journal of Soil Science. (2003) ;54: (4):735–40. |
[3] | Kohler A. , Kuo A. , Nagy L.G. , Morin E. , Barry K.W. , Buscot F. , Canbäck B. , Choi C. , Cichocki N. , Clum A. , Colpaert J. . Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics. (2015) ;47: (4):410–5. |
[4] | Koron D. , Gogala N. . The use of mycorrhizal fungi in the growing of blueberry plants (Vaccinium corymbosum L.). Acta Horticulturae. (2000) ;525: :101–6. |
[5] | Koske R.E. , Gemma J.N. . A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research. (1989) ;92: (4):486–8. |
[6] | McGonigle T.P. , Miller M.H. , Evans D.G. , Fairchild G.L. , Swan J.A. . A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytologist. (1990) ;115: (3):495–501. |
[7] | Nestby R. , Krogstad T. , Joner E. , Vohnik M. . The effect of NP fertilization on European blueberry (Vaccinium myrtillus L.) development on cultivated land in mid-Norway. Journal of Berry Research. (2014) ;4: (3):147–57. |
[8] | Phillips J.M. , Hayman D.S. . Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. (1970) ;55: (1):158-IN18. |
[9] | Prodorutti D. , Pertot I. , Giongo L. , Gessler C. . Highbush blueberry: Cultivation, protection, breeding and biotechnology. The European Journal of Plant Science and Biotechnology. (2007) ;1: (1):44–56. |
[10] | Sadowsky J.J. , Hanson E.J. , Schilder A.M. . Root colonization by ericoid mycorrhizae and dark septate endophytes in organic and conventional blueberry fields in Michigan. International Journal of Fruit Science. (2012) ;12: (1-3):169–87. |
[11] | Scagel CF . Inoculation with ericoid mycorrhizal fungi alters fertilizer use of highbush blueberry cultivars. Hort Science. (2005) ;40: (3):786–94. |
[12] | Scagel CF . Isolate-specific rooting responses of Leucothoe fontanesiana cuttings to inoculation with ericoid mycorrhizal fungi. The Journal of Horticultural Science and Biotechnology. (2005) ;80: (2):254–62. |
[13] | Timonen S. , Sinkko H. , Sun H. , Sietiö O.M. , Rinta-Kanto J.M. , Kiheri H. , Heinonsalo J. . Ericoid roots and mycospheres govern plant-specific bacterial communities in boreal forest humus. Journal of Microbial Ecology. (2016) ). doi: 10.1007/s00248-016-0922-6 |




