Sexual differences and seasonal variations in total phenolics and antioxidant properties in Hippophae rhamnoides leaves
Abstract
BACKGROUND:
Seabuckthorn (SBT) leaves are used for extraction of health promoting compounds and product development.
OBJECTIVE:
The aim of the work was to find out gender differences and seasonal variation in total polyphenol content (TPC) and total antioxidant capacity (TAC).
METHOD:
Leaves of six natural population of SBT, which comprised 200 plants (100 males, 100 females) from the trans-Himalaya were harvested, and their methanolic and acetone extracts were studied for TPC and TAC.
RESULTS:
Males exhibit significantly higher TPC (100.8±23.9 mg GAE/g DW) than females (95.0±23.8 mg GAE/g DW). Similarly, ferric reducing activity was significantly higher in males (6.5±1.1 Fe2+ mmol/g DW) than females (6.1±1.2 Fe2+ mmol/g DW). Significant increase in TPC was observed in male leaves from July to October followed by a significant decrease in November. However, a trend of increase in TPC upto August and a steady decline thereafter was observed in leaves of female SBT. Similarly a trend of an increasing TAC was observed in both the sexes but female leaves were observed to be on an increasing TAC from July to October.
CONCLUSION:
Male SBT leaves exhibit higher TPC and TAC than females; October is the best time for harvesting SBT leaves and; SBT leaves contain significantly higher hydrophilic than lipophilic phenolics and antioxidants.
1Introduction
Seabuckthorn (Hippophae rhamnoides L.) is an ecologically and economically important plant [1]. The species is native to Europe and Asia, but nowadays it is widely grown all over the world. Seabuckthorn (SBT) is dioecious and wind pollinated plant. Traditionally, every part of the plant is being used for a variety of purpose. There are over a hundred popular SBT-based formulations in various pharmacopoeias of Sowa Rigpa (Tibetan medicine) [2]. Although the nutritional and medicinal properties of SBT berries are usually the focus of attention, SBT leaf has been receiving much attention in recent years for its medicinal and therapeutic applications. SBT leaves possess antimicrobial [3–5], anti-viral [6], anti-radiation [7], hepatoprotective [8], cytoprotective [3], adaptogenic [9] and immunoprotective [10] activities. Many of these activities are attributed to high antioxidant capacity including the phenolics. Dried SBT leaves are used for tea and processed for nutraceuticals products.
Antioxidant properties of SBT leaves have been extensively studied [4, 8, 11, 12]. However, SBT is a dioecious plant and hence sexual differences in presence of health promoting compounds in its vegetative parts is expected due to greater reproductive effort in females. Limited studies have been carried on sex-related differences in antioxidant activity and phenolic content in SBT leaves. Górnaś et al. [13] studied lipophilic antioxidants in mixed SBT samples of two females and 10 males. It was found that lipophilic antioxidant is higher in male as compared to female leaves. In contrast, Šnē et al. [14] reported higher total phenolics and antioxidant in female SBT leaves. The antioxidant compounds viz. α-tocopherol, β-tocopherol, γ-tocopherol, plastochromanol-8 and β-carotene were observed to be higher in female than in male SBT leaves [15]. In view of the contrasting results from limited studies, it is felt that more studies involving larger number of samples are needed to better understand the sexual differences in TPC and TAC. Besides the gender differences, the season of harvesting is also known to have a significant effect on the content of antioxidant compounds in leaves [16–19]. SBT leaves can be harvested from June to November with a varying ease of harvesting. But only a small number of studies have been carried on to see the influence of season of harvesting on antioxidant properties of SBT leaves. Morgenstern et al. [20] studied change in antioxidant capacity and phenols during SBT leaf development from April to July. Górnaś et al. [13] studied antioxidants in mixed SBT samples of two female and 10 male harvested in June and October. To the best of our knowledge, studies involving a large number of samples over an extended period of harvesting have not been conducted thus far. In view of emerging importance of SBT leaves for medicinal and therapeutic applications, the present study was undertaken on a larger number of samples with the objective to investigate the role of sexual differences and seasonal variation in phenolic content and antioxidant capacities in SBT leaves.
2Materials and methods
2.1Sample collection
Six natural population of Hippophae rhamnoides subsp. turkestanica consisting of 100 male and 100 female plants were sampled across the major distribution sites from the Indian trans-Himalaya in October 2014 to study the gender-related differences in TPC and TAC in leaves. Leaves (5 g/plant) were harvested and freeze dried in a Laboratory freeze dryer (ALPHA 2–4 LD plus, Fisher Bioblock Scientific, France) and stored until analysis. The altitude of collection sites ranged from 3203 to 3885 m asl (Table 1). Altitude and location of study sites was established using GARMIN GPS 72, Olathe, Kansas, USA. Ten individual plants (five male and five female each) growing at experimental farm at Defence Institute of High Altitude Research (DIHAR) were selected for studying the seasonal variations in TPC and TAC. Leaves (2 g/plant) were harvested every month in the first week of July to November, freeze dried and stored until analysis.
2.2Chemicals
Solvents and Folin-Ciocalteu reagent were obtained from Merck, Germany. 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), gallic acid and ferrous sulfate hexahydrate, were from Sigma-Aldrich, USA. All the other chemicals used were of analytical grade.
2.3Extraction
Two cycles of extraction, hydrophilic and lipophilic, were performed using the method previously described [21]. Hydrophilic extraction was performed with methanol while lipophilic extraction was done with acetone. Powdered leaf samples (20 to 40 mg) was extracted (n = 3) for 15 min with 1.5 ml methanol in a 2 ml micro centrifuge tube and vortexed at room temperature. The sample was centrifuged at 5600 g for 10 min and the supernatant was recovered. The residue was mixed with 1.5 ml of acetone and the process was repeated as described above. TPC and FRAP were measured directly in the methanolic and acetone extracts and the values were combined mathematically. DPPH was measured in the combined methanolic and acetoneextract.
2.4Determination of total phenolic content
The Folin-Ciocalteu reagent assay was used to determine the TPC [22]. An aliquot of the samples (30 μl) was introduced into 96 well micro-plate followed by 150 μl Folin-Ciocalteu reagent, which was previously diluted with distilled water (1 : 10) and 120 μl sodium carbonate (75 g/l). The micro-plates were vortexed, covered with parafilm and allowed to stand for 30 min. Absorbance at 765 nm was recorded in a micro-plate reader (SpectroMax M2 e, Molecular Devices, Sunnyvale, CA, United States). TPC was expressed in gallic acid equivalents (GAE mg/g DW). The calibration equation for gallic acid was y = 0.014×–0.003 (R2 = 0.995) where y is the absorbance at 765 nm and x is the concentration of gallic acid in mg/l.
2.5Determination of antioxidant capacity
Ferric reducing antioxidant potential (FRAP) assay was conducted using the method previously described [23] with minor modifications [21]. A total of 7.5 μl of extract and 22.5 μl of distilled water were added to 225 μl of freshly prepared FRAP reagent (10 parts of 300 mmol/l sodium acetate buffer at pH 3.6, one part of 10 mmol/l 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ) solution and one part of 20 mmol/l FeCl30.6H2O) and the reaction mixture was incubated for 30 min. The increase in absorbance was measured at 593 nm. The FRAP value was expressed as FeSO40.7H2O mmol/g DW. The calibration equation for FeSO40.7H2O was y = 0.323×–0.103 (R2 = 0.983) where y is the absorbance at 593 nm and x is the concentration of FeSO40.7H2O in mM. Free radical scavenging method by DPPH developed by Brand-Williams et al. [24] was followed with minor modification [21]. A 0.1 mmol/l solution of DPPH in methanol was prepared and 300 μl of the solution was treated with 15 μl of the methanolic and acetone extracted sample. Control was treated with 15 μl of solvent instead of the extract. The mixture was left to stand at room temperature for 30 min before the decrease in absorbance at 517 nm was recorded. Antioxidant value was expressed as IC50, the amount of sample extracted into 1 ml solution necessary to decrease by 50% the initial DPPH concentration. IC50 was derived from the % disappearance vs. concentration plot (concentration means mg of SBT leaf on DW basis extracted into 1 mlsolution).
2.6Statistical analysis
All the experiments were performed in triplicate. The experimental results were expressed as mean±standard deviation (SD) using statistical analysis with SPSS (Statistical Program for Social Sciences, SPSS Corporation, Chicago, Illinois, USA). One way analysis of variance (ANOVA) and post hoc analysis with 2-sided Tukey’s HSD at p≤0.05 level were performed. Student’s t test and Pearson’s correlation analysis were performed to compare and find the correlations among parameters. Regression was performed using MS Excel. Box plots were produced using XLSTAT software.
3Results and discussion
3.1Effect of plant sex on total polyphenol content and antioxidant activity
High variability in TPC within and among genotypes from different populations was observed. The TPC ranged from 47.2 to 173.1 in male and 29.9 to 165.8 mg GAE/g DW in female between genotypes. Therefore, a variation of 1–3.7 fold in male and 1–5.5 fold in female in TPC was observed. Effect of plant sex on TPC is presented in Table 2. Significantly high variability was observed between the populations. Males showed significantly higher TPC than females (P < 0.001, Student’s t-test) in three out of the six populations. Females showed higher values in two populations and no significant gender differences was observed in the remaining single population. However, the overall mean TPC value of the six population was significantly higher in males (100.8±23.9 mg GAE/g DW) than females (95.0±23.8 mg GAE/g DW) at p≤0.01. In contrast, Šnē et al. [14] reported higher total phenolics in female SBT leaves which could be because of small sample size as observed in PHY and HOR populations in the present study. The overall difference in TPC between male and female SBT leaves in the present study was 5.8%, which is significantly lower than 45% higher TPC reported in male Ginkgo biloba leaves that females [25].
FRAC and DPPH assay are widely used method to test the antioxidant capacity in berries [26, 27]. The ferric reducing activity ranged from 3.9 to 9.5 in male and 2.6 to 9.1 Fe2+ mmol/g DW in female. The difference in FRAP value between the genotypes showing the highest and lowest value was 1–2.4 fold in male and 1–3.5 fold in female. Gender effect on FRAP is presented in Table 3. Significantly high variability was observed between the populations. Males showed significantly higher ferric reducing activity than females (P < 0.001, Student’s t-test) in two populations and no significant gender differences was observed in the remaining four populations. However, the overall mean FRAP value was significantly higher in males (6.5±1.1 Fe2+ mmol/g DW) than females (6.1±1.2 Fe2+ mmol/g DW). The result is in agreement with studies by Górnaś et al. [13] who reported higher antioxidant activities in mixed 10 SBT males than two females. In contrast, Šnē et al. [14] reported higher ferric reducing activity in female than male SBT leaves. The antioxidant compounds viz. α-tocopherol, β-tocopherol, γ-tocopherol, plastochromanol-8 and β-carotene were observed higher in female than in male SBT leaves [15].
Free radical scavenging activity of leaves extract expressed as IC50 is presented in Table 3. A single population showed significantly higher scavenging activities in males than females (P < 0.001, Student’s t-test) and no significant gender differences was observed in the remaining five populations. The overall mean scavenging value was higher in males but the difference was not statistically significant. Higher TPC and TAC with acetone suggests that SBT leaves contains significantly higher hydrophilic than lipophilic antioxidants.
Higher TPC and TAC in male leaves in the present study is in agreement with the fact that in dioecious species the cost of reproduction involves prioritization of resources in fruit development rather than in vegetative growth or protection in females. A major investment in reproduction is generally associated with the disadvantage in terms of oxidative stress and cellular injuries, particularly under adverseconditions [28].
3.2Effect of harvest season on total polyphenol content and antioxidant activity
Effect of the season of harvest on TPC is presented in Table 4. TPC varied significantly during the sampling period. Significant increase in TPC was observed in male leaves from July (66.75±7.16 mg GAE/g DW) to October (93.25±7.14 mg GAE/g DW) followed by a significant decrease in November (7390.4±1096.5 mg GAE/100 g DW). However, increase in TPC was observed upto August in female leaves and then showed a steady declining trend. Decline in TPC from August onward in female as compare to October in male leaves is may be due to higher reproductive efforts by female during the study period (July-November), females developing fruits while males not reproducing. Male contained significantly higher TPC than female leaves from August to November harvesting months (P < 0.001, Student’s t-test). Similar trend was observed in TAC in both the sexes except that female also showed increasing TAC from July to October (Table 4). Progression in harvest season from July to October is related linearly to the increase in TPC (R2 = 0.937) and FRAP (R2 = 0.976) in male leaves (Fig. 1). However, in females the trend of increase was not observed in TPC. In comparison, Morgenstern et al. [20] studied the change in antioxidant capacity and phenols during SBT leaves development from April to July. Antioxidant capacity increased in first week of May and then decreased in third week of the month. A steady increase was observed from June onwards. The phenols decreased initially and then increased steadily during the study period. However, changes in antioxidant capacity and phenols were not studied beyond July. Górnaś et al. studied antioxidants in mixed SBT samples of two female and 10 male harvested in June and October. Higher antioxidant was observed in samples collected in autumn than in summer in both male and female leaves. Results obtained in the present study over an extended harvesting period suggest that October is the best time for harvesting SBT leaves. Ercisli et al. [17] also observed similar trend in antioxidant activity of tea leaves harvested at three commercial harvest seasons (May 15, July 15, September 15). Highest antioxidant activity was observed at 2nd harvest. Increase in TPC and TAC from July to October may be linked to accumulation of health promoting compounds during leaf developmental stages. Decline in TPC and TAC in November may be due to the beginning of leaf senescence in the plant.
3.3Correlation analysis
Table 5 displays the correlation between TPC and antioxidant activity. TPC of male, female and combined samples was significantly correlated with FRAP (0.423, 0.717, 0.581, respectively, p≤0.01) and DPPH (–0.208, –0.551, –0.399, respectively, p≤0.01). Similar result was observed in SBT berry from the trans-Himalaya [29]. Similarly, DPPH scavenging activity (IC50) of male, female and combined samples was significantly correlated with FRAP (–0.48, –0.577, –0.533, respectively p≤0.01).
4Conclusion
Sexual differences and seasonal variation in TPC and TAC in SBT leaves was demonstrated. Males exhibit significantly higher TPC and ferric reducing activity than females. Significant seasonal variation in TPC and TAC was observed in both the sexes. Significant increase in TPC was observed in male leaves from July to October followed by a significant decrease in November. However, increase in TPC in female leaves was observed upto August and then a steady declining trend afterwards due to greater reproductive effort in females. October is the best time for harvesting SBT leaves for higher health promoting compounds content. Leaves contain significantly higher hydrophilic than lipophilic phenolics and antioxidants. Results obtained in this study can be considered for harvesting of SBT leaves for extraction of health promoting compounds and product development.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgments
The study was supported by Defence Research and Development Organisation (DRDO), Ministry of Defence, Government of India. PD, DD and SA are grateful to DRDO for providing Senior Research Fellowship.
REFERENCES
[1] | Stobdan T , Korekar G , Srivastava RB . Nutritional attributes and health application of seabuckthorn (Hippophae rhamnoides L.) – a review. Curr Nutr Food Sci. (2013) ;9: :151–65. |
[2] | Stobdan T , Targais K , Lamo D , Srivastava RB . Judicious use of natural resources: A case study of traditional uses of Seabuckthorn (Hippophae rhamnoides L.) in trans-Himalayan Ladakh, India. Natl Acad Sci Lett. (2013) ;36: :609–13. |
[3] | Upadhyay NK , Kumar Y , Gupta A . Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol. (2010) ;48: :3443–48. |
[4] | Michel T , Destandau E , Floch GL , Lucchesi ME , Elfakir C . Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophae rhamnoides L.) leaf, stem, root and seed. Food Chem. (2012) ;131: :754–60. |
[5] | Arora R , Mundra S , Yadav A , Srivastava RB , Stobdan T . Antimicrobial activity of seed, pomace and leaf extracts of seabuckthorn (Hippophae rhamnoides L.) against foodborne and food spoilage pathogens. Afr J Biotechnol. (2012) ;11: (45), 10424–30. |
[6] | Jain M , Ganju L , Katiyal A , Padwad Y , Mishra KP , Chanda S , Karan D , Yogedra KM , Sawhney RC . Effect of Hippophae rhamnoides leaf extract against Dengue virus infection in human blood-derived macrophages. Phytomedicine. (2008) ;15: :793–99. |
[7] | Khan A , Manna K , Chinchubose , Das DK , Sinha M , Kesh SB , Das U , Dey RS , Banerji A , Dey S . Seabuckthorn (Hippophae rhamnoides L.) leaf extract ameliorates the gamma radiation mediated DNA damage and hepatic alterations. Indian J Exp Biol. (2014) ;52: :952–64. |
[8] | Maheshwari DT , Kumar MSY , Verma SK , Singh VK , Singh SN . Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol. (2011) ;49: :2422–28. |
[9] | Saggu S , Divekar HM , Gupta V , Sawhney RC , Banerjee PK , Kumar R . Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides) leaf extract: A dose dependent study. Food Chem Toxicol. (2007) ;45: :609–17. |
[10] | Mishra KP , Mishra R , Yadav AP , Jayashankar B , Chanda S , Ganju L . A comparative analysis of immunomodulatory potential of Seabuckthorn leaf extract in young and old mice. Biomed Ageing Pathol. (2011) ;1: :61–64. |
[11] | Guan TTY , Cenkowski S , Hydamaka A . Effect of drying on the nutraceuticals quality of sea buckthorn (Hippophae rhamnoides L. ssp sinensis) leaves. J Food Sci. (2005) ;70: :514–18. |
[12] | Korekar G , Stobdan T , Chaurasia OP , Singh SB . Phenolic content and antioxidant capacity of various solvent extracts from seabuckthorn (Hippophae rhamnoides L.) fruit pulp, seeds, leaves and stem bark. Acta Aliment Hung. (2011) ;40: :449–58. |
[13] | Górnaś P , Šnē E , Siger A , Segliņa D . Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: The effect of harvest time, sex, drying and extraction methods. Ind Crops Prod. (2014) ;60: :1–7. |
[14] | Šnē E , Segliņa D , Galoburda R , Krasnova I . Content of phenolic compounds in various sea buckthorn parts. Proc Latvian Acad Sci B. (2013) ;67: :411–15. |
[15] | Górnaś P , Šnē E , Siger A , Segliņa D . Sea buckthorn (Hippophae rhamnoides L.) vegetative parts as an unconventional source of lipophilic antioxidants. Saudi J Biol Sci. (2016) ;23: :512–16. |
[16] | Anderson JV , Chevone BI , Hess JL . Seasonal variation in the antioxidant system of eastern white pine needles. Plant Physiol. (1992) ;98: :501–08. |
[17] | Ercisli S , Orhan E , Ozdemir O , Sengul M , Gungor N . Seasonal variation of total phenolic, antioxidant activity, plant nutritional elements, and fatty acids in tea leaves (Camellia sinensis var. sinensis clone Derepazari 7) grown in Turkey. Pharm Biol. (2008) ;46: :683–87. |
[18] | Brahmi F , Mechri B , Dabbou S , Dhabi M , Hammami M . The efficacy of phenolic compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind Crops Prod. (2012) ;38: :146–52. |
[19] | Giampieri F , Tulipani S , Alvarez-Suarez JM , Quiles JL , Mezzetti B , Battino M . The strawberry: Composition, nutritional quality, and impact on human health. Nutr. (2012) ;28: :9–19. |
[20] | Morgenstern A , Ekholm A , Scheewe P , Rumpunen K . Changes in content of major phenolic compounds during leaf development of sea buckthorn (Hippophae rhamnoides L.). . Agr Food Sci. (2014) ;23: :207–19. |
[21] | Korekar G , Stobdan T , Arora R , Yadav A , Singh SB . Antioxidant capacity and phenolic content of apricot (Prunus armeniaca L.) kernel as a function of genotype. Plant Food Hum Nutr. (2011) ;66: :376–83. |
[22] | Singleton VL , Rossi JA . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. (1965) ;16: :144–58. |
[23] | Ikram EHK , Eng KH , Jalil AMM , Ismail A , Idris S , Azlan A , et al . Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J Food Compos Anal. (2009) ;22: :388–93. |
[24] | Brand-Williams W , Cuvelier ME , Berset C . Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft Und-Technologie. (1995) ;28: :25–30. |
[25] | Koczka N , Móczár Z , Stefanovits-Bányau Ė . Ombódi A . Differences in antioxidant properties of ginkgo leaves collected from male and female trees. Acta Pharm. (2015) ;65: :99–104. |
[26] | Amatori S , Mazzoni L , Alvarez-Suarez JM , Giampieri F , Gasparrini M , Forbes-Hernandez TY , Afrin S , Provenzano AE , Persico G , Mezzetti B , Amici A , Fanelli M , Battino M . Polyphenol-rich strawberry extract (PRSE) slows in vitro and in vivo biological activity against invasive breast cancer. Sci Rep. (2016) . doi: 10.1038/srep30917 |
[27] | Jiménez-Aspee F , Theoduloz C , Ávila F , Thomas-Valdés S , Mardones C , Baer DV , Schmeda-Hirschmann G . The Chilean wild raspberry (Rubus geoides Sm.) increases intracellular GSH and protect against H2O2 and methylglyoxal-induced damage in AGS cells. Food Chem. (2016) ;194: :908–19. |
[28] | Juvany M , Müller M , Pintó-Marijuan M , Munné-Bosch S . Sex-related differences in lipid peroxidation and photoprotection in Pistacia lentiscus. J Exp Bot. (2014) ;65: :1039–49. |
[29] | Korekar G , Dolkar P , Singh H , Srivastava RB , Stobdan T . Variability and the genotypic effect on antioxidant activity, total phenolics, carotenoids and ascorbic acid content in seventeen natural population of seabuckthorn (Hippophae rhamnoides L.) from trans-Himalaya. LWT-Food Sci Technol. (2014) ;55: :157–62. |
Figures and Tables
Fig.1
Relation between total phenolic content (A-B) and antioxidant capacity (C-D) in male and female Seabuckthorn leaves with harvest season (July-October).
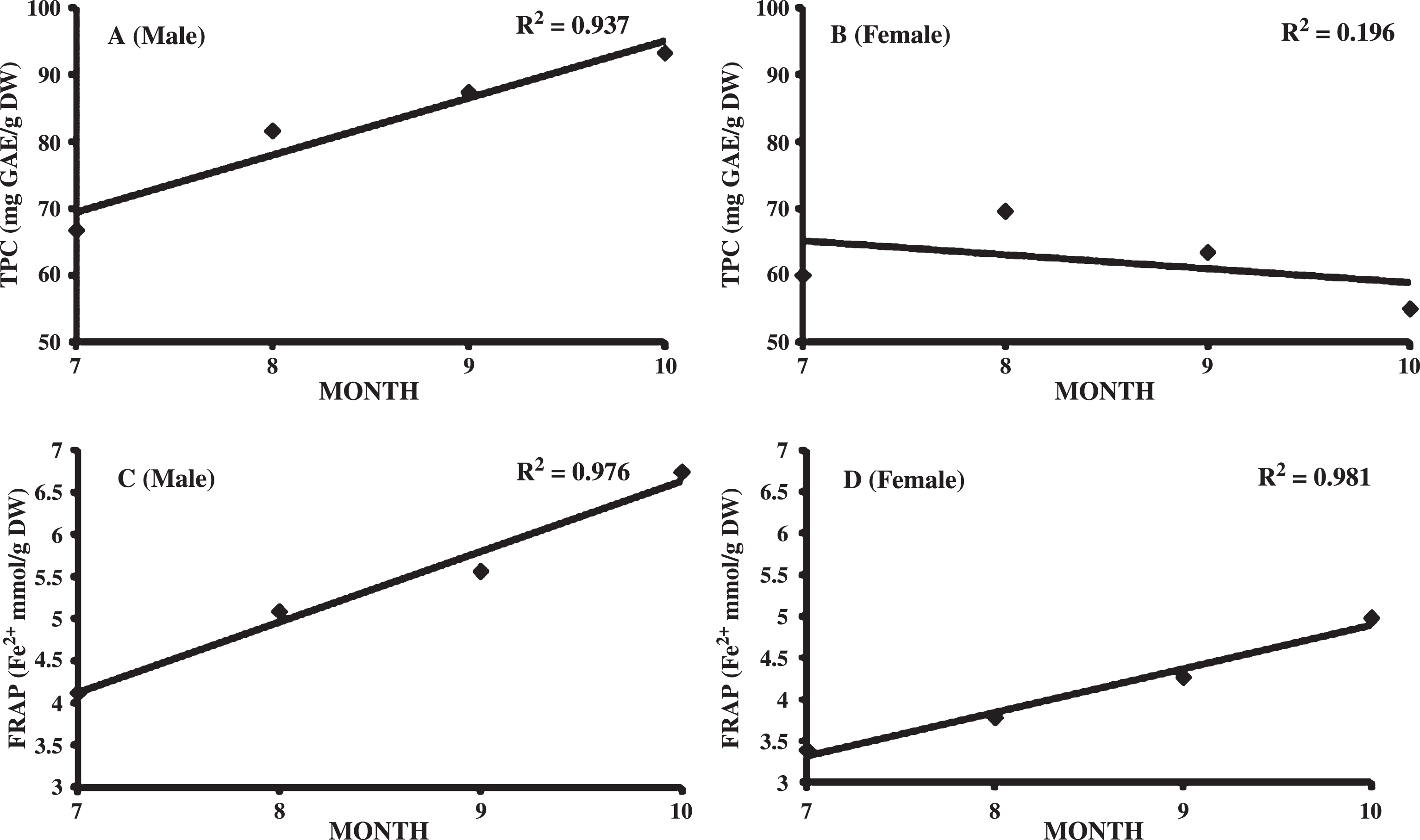
Table 1
Locations of six natural populations of H. rhamnoides L. from Indian trans-Himalaya
| Sampling Locations | Population ID | Latitude (N) | Longitude (E) | Altitude (m) ASL | Sample size (numbers) | |
| Male | Female | |||||
| Spituk | SPT | 34°07′7″ | 77°30′4″ | 3203±5.6 | 20 | 20 |
| Chuchot | CHU | 34°05′4″ | 77°35′9″ | 3239±5.0 | 17 | 17 |
| Shey | SHY | 34°04′1″ | 77°37′7″ | 3260±4.6 | 17 | 17 |
| Phyang | PHY | 34°11′5″ | 77°30′1″ | 3636±49.6 | 16 | 16 |
| Horzey | HOR | 34°12′1″ | 77°35′3″ | 3812±24.8 | 15 | 15 |
| Sakti | SKT | 34°02′1″ | 77°48′6″ | 3885±37.3 | 15 | 15 |
Table 2
Sexual difference in total phenolic content (mg GAE/g DW) of H. rhamnoides (100 males, 100 females) leaves
| Population ID | Male leaves | Female leaves | ||||||||
| Hydrophilic | Lipophilic | Combined1 | Hydrophilic | Lipophilic | Combined1 | |||||
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | |||||
| SPT | 101.60±20.47b | 2.27±0.26bc | 103.86±20.58b | 61.53 | 142.45 | 107.76±19.95c | 2.27±0.40bc | 110.03±20.05c | 76.72 | 165.83 |
| CHU | 123.88±21.83c*** | 2.19±0.22ab | 126.07±21.84c*** | 93.03 | 173.06 | 91.67±25.43b | 2.95±0.40e*** | 94.62±25.34b | 51.02 | 152.44 |
| SHY | 86.87±18.28a*** | 2.48±0.40c | 89.66±18.90a*** | 47.19 | 131.67 | 74.98±10.95a | 2.53±0.49d | 77.51±11.15a | 51.99 | 97.03 |
| PHY | 93.43±15.18ab | 2.03±0.28a | 95.46±15.37ab | 63.69 | 124.28 | 109.59±16.45c*** | 2.11±0.30ab | 111.70±16.70c*** | 81.86 | 144.57 |
| HOR | 84.71±12.82a | 2.49±0.39c | 87.21±12.88a | 67.04 | 120.58 | 94.36±13.65b*** | 2.38±0.35cd | 96.89±14.10b*** | 73.41 | 125.42 |
| SKT | 99.91±29.63b*** | 3.12±0.66d*** | 103.02±29.70b*** | 58.07 | 170.36 | 76.76±26.49a | 2.01±0.38a | 78.77±26.76a | 29.88 | 132.01 |
| Average | 98.24±23.82** | 2.41±0.60 | 100.83±23.92** | 47.19 | 173.06 | 92.64±23.66 | 2.37±0.49 | 95.02±23.82 | 29.88 | 165.83 |
Values represented as mean±SD. For each column, different lowercase letters indicate significantly different at p < 0.05, as measured by 2-sided Tukey’s HSD between populations. 1Combined: Values of hydrophilic and lipophilic extract combined mathematically. **Value significantly higher than that of opposite sex at p≤0.01; ***Value significantly higher than that of opposite sex at p≤0.001.
Table 3
Sexual difference in total antioxidant capacity of H. rhamnoides (100 males, 100 females) leaves
| Population | Male leaves | Female leaves | ||||||
| ID | 1FRAP (FeSO4.7H2O mmol/g DW) | 2IC50 (mg/ml) | 1FRAP (FeSO4.7H2O mmol/g DW) | 2IC50 (mg/ml) | ||||
| Hydrophilic | Lipophilic | 3Combined | 4Combined | Hydrophilic | Lipophilic | 3Combined | 4Combined | |
| SPT | 7.07±1.17c | 0.13±0.01a | 7.21±1.18c | 0.34±0.10a | 7.21±1.04c | 0.14±0.02ab | 7.35±1.05c | 0.32±0.18a |
| CHU | 7.01±0.61c*** | 0.14±0.02ab | 7.15±0.60c*** | 0.33±0.03a*** | 6.05±1.20b | 0.18±0.03d*** | 6.23±1.21b | 0.37±0.03a |
| SHY | 5.75±0.71ab | 0.15±0.02bc | 5.90±0.73b | 0.40±0.27ab | 5.63±0.62ab | 0.17±0.02c*** | 5.80±0.62b | 0.42±0.29a |
| PHY | 6.86±0.63c*** | 0.16±0.02c** | 7.02±0.64c*** | 0.38±0.05ab | 5.54±0.70ab | 0.15±0.02b | 5.69±0.72ab | 0.37±0.03a |
| HOR | 6.09±0.91b | 0.15±0.02c*** | 6.24±0.91b | 0.46±0.17bc | 6.08±0.82b | 0.13±0.02a | 6.21±0.83b | 0.44±0.13a |
| SKT | 5.28±1.26a | 0.15±0.03c*** | 5.08±1.80a | 0.54±0.20c | 5.07±1.32a | 0.13±0.02a | 5.19±1.34a | 0.65±0.35b |
| Average | 6.37±1.14*** | 0.15±0.2 | 6.51±1.14*** | 0.41±0.18 | 5.96±1.19 | 0.15±0.03 | 6.11±1.20 | 0.43±0.23 |
Values represented as mean±SD. For each column, different lowercase letters indicate significantly different at p < 0.05, as measured by 2-sided Tukey’s HSD between populations. 1FRAP: Ferric reducing antioxidant potential. 2IC50: Inhibitory concentration, the amount of sample extracted into 1 ml solution necessary to decrease by 50% the initial DPPH concentration. 3Combined: Values of hydrophilic and lipophilic extract combined mathematically. 4Combined: Values of combined hydrophilic and lipophilic extract measured. **Value significantly higher than that of opposite sex at p≤0.01, ***Value significantly higher than that of opposite sex at p≤0.001.
Table 4
Seasonal variation in total phenolic content and total antioxidant capacity of H. rhamnoides (5 males, 5 females) leaves
| Month | Male | Female | ||||
| 1TPC | 2FRAP | 3IC50 | 1TPC | 2FRAP | 3IC50 | |
| July | 66.75±7.15a | 4.12±0.20a*** | 0.52±0.20c | 59.97±16.63abc | 3.39±0.50a | 0.69±0.57a |
| August | 81.59±10.71bc** | 5.09±0.49b*** | 0.26±0.09b*** | 69.57±13.04c | 3.78±0.37ab | 0.52±0.16a |
| September | 87.38±6.13cd*** | 5.62±0.51bc*** | 0.19±0.08ab*** | 63.45±8.02bc | 4.27±0.32c | 0.73±0.16a |
| October | 93.25±7.14d*** | 6.74±0.57d*** | 0.12±0.04a*** | 55.02±8.04ab | 4.98±0.33d | 0.85±0.63a |
| November | 73.90±10.97ab*** | 5.68±0.85c*** | 0.31±0.18b*** | 51.47±6.60a | 4.09±0.47bc | 0.94±0.43a |
| Average | 80.57±12.68*** | 5.45±1.02*** | 0.28±0.19*** | 59.89±12.56 | 4.10±0.66 | 0.75±0.45 |
Values represented as mean±SD. For each column, different lowercase letters indicate significantly different at p < 0.05, as measured by 2-sided Tukey’s HSD between months. 1TPC: Total phenolic content (mg GAE/g DW). 2FRAP: Ferric reducing antioxidant potential (FeSO4.7H2O mmol/g DW). 3IC50: Inhibitory concentration, the amount of sample extracted into 1 ml solution necessary to decrease by 50% the initial DPPH concentration. **Value significantly higher than that of opposite sex at p≤0.01; ***Value significantly higher than that of opposite sex at p≤0.001.
Table 5
Pearson correlation to estimate the interrelationship between TPC, IC50, and FRAP
| Male | Female | Combined | |||||||
| 1TPC | 2FRAP | 3IC50 | 1TPC | 2FRAP | 3IC50 | 1TPC | 2FRAP | 3IC50 | |
| 1TPC | 1 | 0.423** | –0.208** | 1 | 0.717** | –0.551** | 1 | 0.581** | –0.399** |
| 2FRAP | 1 | –0.481** | 1 | –0.577** | 1 | –0.533** | |||
| 3IC50 | 1 | 1 | 1 | ||||||
**Correlation is significant at p≤0.01. 1TPC: Total phenolic content (mg GAE/g DW). 2FRAP: Ferric reducing antioxidant potential (FeSO40.7H2O mmol/g DW). 3IC50: Inhibitory concentration, the amount of sample extracted into 1 ml solution necessary to decrease by 50% the initial DPPH concentration.




