Susceptibility of strawberry cultivars to root and crown rot caused by Macrophomina phaseolina
Abstract
BACKGROUND: A high incidence of root and crown rot of strawberries (Macrophomina phaseolina) has been observed in major strawberry production regions. In Chile, the pathogen was reported in strawberry plants in 2013. A strategy for disease management is the use of resistant cultivars.
OBJECTIVE: The aim of this study was to evaluate the susceptibility of eleven strawberry cultivars to the disease.
METHODS: Two trials were conducted. A first trial was performed under greenhouse conditions, with plants propagated by runners and inoculated by oat seeds infected with M. phaseolina. The impact of the disease on the growth of the plants and incidence were assessed. The second trial was performed in a growth chamber. Plants were obtained by in-vitro multiplication and were inoculated with a suspension of sclerotia of the same isolate. Plant mortality was assessed.
RESULTS: Significant differences in susceptibility were observed among the eleven strawberry cultivars, with ‘Florida Festival’, ‘Amiga’ and ‘Naiad’ as the least susceptible cultivars, while ‘Florida Fortuna’ was the most susceptible cultivar in both trials.
CONCLUSIONS: Differences were detected in terms of susceptibility of cultivars to root and crown rot of strawberry caused by M. phaseolina. This work contributes to knowledge about the susceptibility of strawberry cultivars to this disease.
1Introduction
Macrophomina phaseolina (Tassi) Goid. is a plant pathogen that attacks a wide range of hosts in more than 500 plant families. It affects crops such as soybean, sorghum, corn, cotton [1], jute [2], sunflower [3], sesame [4], common bean [5], melon [6] and strawberry [7], among others.
The root and crown rot caused by M. phaseolina is an emerging and devastating disease for strawberries. It has been detected in strawberry crops for the last decade, causing serious yield problems in the main producing regions of the world. The disease was reported in strawberry in Illinois in 1958 [8], and after that in France in 1993 [7]. In recent years there have been multiple reports in major growing regions: in 2005 in Florida, United States, and Israel [9, 10]; in 2008 an increase in the incidence of the disease was reported in California, United States [11]; in the same year it was pointed out as an emerging disease in Huelva, Spain [12]; during 2011 the pathogen was identified in Tucumán, Argentina [13], in Iran it was reported in 2012 [14]; and in Australia and Chile it was reported as causing serious damage to the strawberry crop in 2013 [15, 16].
The disease caused by M. phaseolina is favored when the plants have been subjected to water stress and high temperature conditions [17–19]. Symptoms in the strawberry crop include wilting of foliage — older leaves wither and die, young leaves remain alive—, which causes stunted growth. Usually these symptoms first appear after the plants have been established in the field, at the beginning of harvest, or when subjected to stress, resulting in complete collapse or death. The affected plants show necrotic lesions in the inner crown tissue, vascular and cortical, with cracks and orange-brown discoloration [12, 16, 20]
The pathogen forms sclerotia in infected tissues, which allows it to survive in plant debris or in the soil for long time, constituting the initial inoculum of the disease [21]. Pathogens are usually found in the top 20 cm of soil [22]. However, controlling them is difficult because they are resistant structures that tolerate adverse conditions such as high temperatures and drought [23, 24].
In the case of strawberry, disease control is based on the use of pre-transplant fumigants, in order to eradicate or to reduce the initial inoculum. In Chile, it has been a common practice to fumigate with methyl bromide, a fumigant that has been phased-out from 2015 [25]. In countries where methyl bromide has been eliminated, alternative fumigants are applied, such as chloropicrin with 1,3-dichloropropene, metam sodium or metam potassium. In many cases, the emergence of this disease has been attributed to changes in the chemicals used for pre-transplant fumigation[9, 10, 11, 12, 26], a hypothesis that has not yet been demonstrated. In this field several questions remain about the emergence of this disease and the effectiveness of the chemical and non-chemical control available for this and other plant pathogens that inhabit the soil [27, 28]
Given the need to reduce and eventually eliminate fumigation with chemicals and non-chemicals, the search for resistant cultivars certainly rises as an alternative for efficient disease management. However, there is little information on the behavior of commercial strawberry cultivars subjected to infections with Macrophomina phaseolina.
This strategy has been explored in other species, and genotypes with varying degrees of resistance to M. phaseolina have been found in crops like cowpea [29, 30], sorghum [31, 32], soybean [33], common bean [34, 35] and jute[36, 37].
There is very little information on resistance of strawberry to this disease. Some publications refer to a differential response of different genotypes to the infection caused by M. phaseolina [20, 38]. In this context, it is necessary to know the behavior of commercial varieties, in order to move towards obtaining resistant cultivars.
One of the problems in assessing the resistance of cultivars to a disease is finding a methodology capable of discriminating plant response to the pathogens under study. In these procedures, it is necessary to consider the type of plant and the different stages of development, as well as the inoculation methods to be used. The aim of this study was to evaluate the susceptibility of 11 strawberry cultivars to root and crown rot caused by M. phaseolina. To this end, two experiments were performed considering different types of inoculation and phenological stages of plants.
2Materials and methods
2.1Plant material
Two consecutives trials were conducted, using plant material from two propagation systems, and using two different inoculation methods. For the first trial performed in 2013 season (greenhouse trial), the cultivars used were ‘Amiga’, ‘Camarosa’, ‘Florida Elyana’, ‘Florida Festival’, ‘Florida Fortuna’, ‘Fontanilla’, ‘Naiad’ and ‘Siba’, while cultivars ‘Albion’, ‘Fuentepina’ and ‘Santaclara’ were also tested in the second trial (growth chamber trial) (Table 1). For the greenhouse trial season, strawberry plants were obtained from runners in a soilless system. The runners used were 4 to 5 cm long, with 1 expanded leaf and a crown of 2 mm in diameter. They were arranged in seedbeds and, after 5 weeks of growth, 22 plants with a healthy root ball per cultivar were selected. For the growth chamber trial performed in 2014, plants were obtained through in-vitro micropropagation, for which they were multiplied on MS medium [39] and maintained for 6 weeks at 25±1°C, with 16 hours of light and 8 of darkness. For acclimatization, 10 young plants of the same cultivar, with an average of 5 cm height and 3 expanded leaves were placed in plastic containers and kept for 2 months under the same conditions. Two weeks prior to the trial setup, plants were transferred to a growth chamber at a temperature of 28±2°C and under a 12-hour photoperiod. These same conditions were maintained throughout the trial.
2.2Inoculum preparation
For both trials the isolate Mp21.A of M. phaseolina was used. It was selected in a pathogenicity test conducted previously, in which no differences were observed among 22 isolates obtained from strawberry plants grown in San Pedro, Metropolitan region, Chile. To ensure a correct identification, the isolate Mp21.A was molecularly identified using the primers MpkF1 and MpkR1 [40]. For the greenhouse trial, the inoculum consisted in oat seeds infected with the plant pathogen. Oat seeds were placed in 5-kg bags and were twice sterilized for 60 minutes. Bags were inoculated with mycelium from a pure culture and were kept at 30±2°C for two months. For the growth chamber trial, the pathogen was grown on plates containing potato dextrose agar (PDA) medium, and cultured for 4 days at 28°C. Sclerotia and mycelium were removed adding 9 mL of sterile distilled water to the plate and gently raking the surface of the culture with a sterile Pasteur pipette. The suspension was filtered through Miracloth (Calbiochem), and then the sclerotia were dried at 25°C for 3 days. A suspension of sclerotia was prepared in sterile distilled water, at a concentration of 5×105 sclerotia per mL.
2.3Establishment of the trials
For the greenhouse trial, each plant was transplanted to a 1-liter pot with 2/3 peat and 1/3 perlite. Nine grams of inoculated oats with M. phaseolina were added to the substrate. After inoculation, the plants were kept under greenhouse conditions for 10 weeks. During the trial, the plants were cropped as usual, plants were irrigated periodically (two times a week) with 200 mL of water per pot. The trial was performed using a completely randomized design, with an experimental unit of one plant per pot. Treatments included the 8 cultivars, with 18 replicates per treatment. As a control, 4 plants of each cultivar remained uninoculated and were not included in the statistical analysis.
For the second trial, in the growth chamber, the plants were inoculated with 100 mL of the microsclerotia suspension. The trial was performed using a completely randomized design. The experimental unit consisted of a plastic container (1L) with 10 plants. Treatments corresponded to the 11 cultivars described above with four replicates each. Ten uninoculated plants per cultivar were kept as control plants.
2.4Disease assessment
For the greenhouse trial, disease severity was estimated according to symptoms on the aerial part of the plants and damage observed in the crown. The symptomatology of the aerial part was monitored weekly during 10 weeks after inoculation, and was scored based on severity of symptoms according to a scale from 0 to 5 described by Fanget al. [19], where 0 corresponds to a healthy plant and 5 to a dead plant. The severity of tissue damage in the crown was assessed at the end of the trial, by making longitudinal cuts of the crowns. Tissue necrosis was scored based on an arbitrary visual scale, with values from 0 to 5, where 0 corresponds to a completely healthy crown and 5 to a completely affected crown. In order to confirm that the symptoms observed were a consequence of the inoculated pathogen, reisolates on PDA medium, from crown and root tissues, plating pieces of approximately 1.5 mm, from each crown, and from visibly infected roots were performed. The effect of M. phaseolina on plant development was also assessed by measuring the reduction in total dry weight and crown diameter of plants. Plants were dried in an oven at 69°C for 24 hours, and then weighted. The diameter of the crown was determined by performing two perpendicular measurements with a digital Vernier caliper. Reduction in dry weight and diameter were estimated per treatment, based on the difference between each inoculated plant and the average of uninoculated control plants of each cultivar. For the growth chamber trial, plant mortality was assessed during 5 weeks. In order to corroborate the presence of the pathogen, isolations were made from dead plants on PDA medium.
2.5Data analysis
Disease progress curves were constructed from the disease severity data (trial one) and plants mortality data (trial 2),and the areas under the disease progress curve (AUDPC), were calculated. Results obtained were subjected to an analysis of variance (ANOVA), and the means were separated by Tukey’s HSD multiple-range test, at a 5% significance. Kruskal-Wallis test was used for non-parametric variables. Percentage data were Bliss transformed before the analysis. All data collected in the study were analyzed using MINITAB® 16.
3Results
3.1Greenhouse trial
Differences on the disease progress curves among cultivars were observed after one week of the trial onset (Fig. 1), with all cultivars showing symptoms after two weeks. Disease symptoms progressed faster in the cultivars ‘F. Fortuna’ and ‘Siba’, which were the most susceptible cultivars in the trial. On the other hand, ‘F. Festival’ and ‘Amiga’ were the cultivars showing less symptoms of the disease (Fig. 1), while the rest of the cultivars had a similar, intermediate level, of aerial symptoms. None of the cultivars tested were resistant to the disease.
The cultivars separated in two groups according to the AUDPC, based on the severity of the disease on the aerial part of the plants, where ‘Siba’, ‘F. Fortuna’ and ‘Fontanilla’, were the most affected cultivars. The rest of the cultivars had a lower rate of development of aerial symptoms (Fig. 2). Regarding the degree of necrosis in the plants crowns area, ‘F. Fortuna’ showed the highest value, close to 3, which represents plants with necrosis in the pith and vascular system, while no major differences were observed among the other cultivars (Table 2).
When analyzing the effect of the disease on the growth of the plants, the cultivars ‘Amiga’, ‘Camarosa’, ‘F. Festival’, ‘Naiad’ and ‘F. Elyana’ had the lowest reduction of dry weight, while cultivars ‘Siba’, ‘F. Fortuna’ and ‘Fontanilla’ had the highest reduction. Similar results were observed for the reduction of the crown diameter (Table 2).
Through the analysis of the results as a whole, it was determined that the cultivars under study showed different degrees of susceptibility to the pathogen. ‘F. Festival’, ‘Amiga’, ‘Camarosa’ and ‘Naiad’ were the least affected cultivars, considering all parameters. ‘Florida Festival’ was the cultivar with the least disease severity. Meanwhile, ‘Amiga’ presented the lowest effect of the pathogen on plant development. ‘F. Fortuna’ was the most affected cultivar according to severity parameters, whereas ‘Siba’ obtained the worst evaluation due to the reduction in plant growth (Table 2).
3.2Growth chamber trial
Mortality of plants began in the first week after inoculation (Fig. 3). Disease progress rate remained relatively constant in cultivars that had higher mortality by the end of the trial. In the case of the cultivar ‘Santaclara’, plant mortality stabilized after the second week. The same happened with cultivar ‘F. Festival’, although in this case mortality increased again in the fifth week. The area under the disease progress curve shows that the most affected cultivars in terms of mortality of plants were ‘F. Fortuna’ and ‘F. Elyana’, while ‘Santaclara’ and ‘F. Festival’ were the cultivars less affected by M. phaseolina (Fig. 4).
4Discussion
This study allowed the detection of differences in the susceptibility of different genotypes of strawberry to the root and crown rot caused by M. phaseolina, through two experiments with plants of different phenological stages, and using two sources of inoculum.
In the two trials performed, cultivars ‘F. Festival’, ‘Amiga’ and ‘Naiad’ were less susceptible to the attack of M. phaseolina, presenting a low severity of the disease, proper plant growth, and plant mortality lower than 50%. In turn, ‘F. Fortuna’ was the cultivar with the highest susceptibility to the pathogen, showing a high disease severity, both on the aerial part and the crown; moreover, a significant reduction in plants’ dry weight (around 45%) was observed, along with a mortality of plants greater than 70%.
Fang et al. [38], found that the cultivars ‘Albion’ and ‘Aroma’ were less susceptible to M. phaseolina, while cultivars ‘F. Festival’ and ‘Camarosa’ were susceptible to the pathogen, their findings departs from the results of this study where, in both trials, ‘F. Festival’ was less susceptible to the disease, while ‘Albion’ showed a great susceptibility in the growth chamber trial. With respect to ‘Camarosa’, the results are not consistent with each other, which could be attributed to the different inoculation methods. In the study of Fang et al. [36], the inoculation was carried out by inserting a sliver colonized by the pathogen in the plants’ crown area. This is a much more invasive method that departs from natural infection, which occurs through microsclerotia and mycelium of M. phaseolina, found in infected plant debris or in the soil [41]. Another element that could affect the results is the difference in pathogenicity of the isolates used in these studies. A high genetic diversity among strawberry isolates of M. phaseolina from Spanish and Chilean isolates, was determined by SSR markers (unpublished), and these differences in genotypes could be reflected on the phenotypes of these isolates, and therefore in their virulence.
Although in both trials some cultivars behaved similarly regarding their susceptibility to the disease, in the case of ‘Siba’ and ‘Camarosa’, the results were not clear. In the trial under greenhouse conditions, with plants in a soilless system, ‘Camarosa’ showed low susceptibility to the disease, while plants kept under controlled conditions showed high mortality. Moreover, ‘Siba’ was highly susceptible in the trial with greenhouse plants, while it showed medium to low mortality in the second trial.
These results would indicate the importance of the phenological stage and the origin of the plants when evaluating differences between genotypes. Vitro plants are slightly lignified and more susceptible to pathogens, due they lack many of the natural barriers that protect them from attack by harmful microorganisms. This is shown in the results obtained in this study, since higher rates of mortality was observed in the test with plants from in vitro culture (which ranged from 13.9% to 100%).
Although the use of plants obtained through in vitro culture has a number of advantages, such as obtaining a large number of healthy plants within a reasonable period of time, which can also be screened in small spaces due to their smaller size. Some authors agreed that the origin of the plants and their phenologic stage are the most important elements to be considered when evaluating the resistance of strawberry genotypes to different pathogens, since the degree of susceptibility of certain cultivars can be overestimated [42, 43].
Eikemo et al. [43], evaluated different methods for screening strawberry germplasm for resistance to Phytophthora cactorum (Lebert & Cohn) J. Schröt., and found that plants originated in vitro were excessively susceptible to the pathogen, showing good disease symptoms 5 days after inoculation and becoming completely necrotic 10 days after, making extremely important the time in which evaluations should be performed. Meanwhile, Smith et al. [42], assessing the effect of different forms of inoculation and ages of plants on strawberry cultivar resistance to Colletotrichum fragariae A.N. Brooks, found that plants 2 to 4 weeks old from transplant were the most susceptible, and that the best method of inoculation is the one that is closest to the type of inoculation in the field.
Considering that the cultivars used in this work are available in the collections of breeding programs, the results are interesting for designing crossing strategies. Furthermore, the use of plants from vitro (or seed to seedlings) is a tool that can be very effective for evaluating large numbers of plants in early stages of development.
This work contributes to knowledge about the susceptibility of strawberry cultivars to root and crown rot caused by this pathogen, as a basis for the development of breeding programs that enable the development of cultivars that are resistant to this emerging disease.
References
[1] | Su G , Suh SO , Schneider RW , Russin JS . Host specialization in the charcoal rot fungus, Macrophomina phaseolina . Phytopathology. (2001) ;91: (2):120–126. |
[2] | De BK , Chattopadhya SB , Arjunan G . Effect of potash on stem rot diseases of jute caused by Macrophomina phaseolina . Journal of Mycopathological Research. (1992) ;30: :51–55. |
[3] | Khan SN . Macrophomina phaseolina as causal agent for charcoal rot of sunflower. Mycopathology. (2007) ;5: (2):111–118. |
[4] | Dinakaran D , Mohammed SEN . Identification of resistant sources to root rot of sesame caused by Macrophomina phaseolina (Tassi. ) Goid. Sesame and Sunflower Newsletter. (2001) ;16: :68–71. |
[5] | Mayék-Pérez N , López-Castañeda C , González-Chavira M , Garcia-Espinosa R , Acosta-Gallegos J , de la Vega OM , Simpson J . Variability of Mexican isolates of Macrophomina phaseolina based on pathogenesis and AFLP genotype. Physiological and Molecular Plant Pathology. (2001) ;59: (5):257–264. |
[6] | Jacob CJ , Krarup C , Díaz GA , Latorre BA . A severe outbreak of charcoal rot in cantaloupe melon caused by Macrophomina phaseolina in Chile. Plant Disease. (2013) ;97: (1):141–142. |
[7] | Baudry AA , Morzières JP . First report of charcoal rot of strawberry in France. Acta Horticulturae. (1993) ;348: :485–488. |
[8] | Tweedy B , Powell D . Charcoal rot on strawberry in Illinois. Plant Diseases Reporter. (1958) ;42: :107. |
[9] | Mertely JJ , Seijo TT , Peres NN . First report of Macrophomina phaseolina causing a crown rot of strawberry in Florida. Plant Disease. (2005) ;89: (4):434. |
[10] | Zveibil A , Freeman S . First report of crown and root rot in strawberry caused by Macrophomina phaseolina in Israel. Plant Disease. (2005) ;89: (9):1014. |
[11] | Koike ST . Crown rot of strawberry caused by Macrophomina phaseolina in California. Plant Disease. (2008) ;92: (8):1253. |
[12] | Avilés M , Castillo S , Bascon J , Zea-Bonilla T , Martín-Sánchez PM , Pérez-Jiménez RM . First report of Macrophomina phaseolina causing crown and root rot of strawberry in Spain. Plant Pathology. (2008) ;57: (2):382. |
[13] | Baino OM , Salazar SM , Ramallo AC , Kirschbaum DS . First report of Macrophomina phaseolina causing strawberry crown and root rot in northwestern Argentina. Plant Disease. (2011) ;95: (11):1477. |
[14] | Sharifi K , Mahdavi M . First report of strawberry crown and root rot caused by Macrophominaphaseolina in Irán. Iranian Journal of Plant Pathology. (2012) ;47: (4):161. |
[15] | Hutton DG , Gomez AO , Mattner SW . Macrophomina phaseolina and its association with strawberry crown rot in Australia. International Journal of Fruit Science. (2013) ;13: (1-2):149–155. |
[16] | Sánchez S , Gambardella M , Henríquez JL , Díaz I . First Report of Crown Rot of Strawberry Caused by Macrophomina phaseolina in Chile. Plant Disease. (2013) ;97: (7):996. |
[17] | Dhingra OD , Sinclair JB . Biology and pathogenicity of Macrophomina phaseolina. Viscosa, Brazil. Universidade Federal de Viscosa. (1978) ; pp. 166. |
[18] | Sandhu AMRIT , Singh RD , Sandhu A . Factors influencing susceptibility of cowpea to M phaseolina. Journal of Mycological Plant Pathology. (1999) ;29: :421–424. |
[19] | Fang X , Phillips D , Li H , Sivasithamparam K , Barbetti MJ . Comparisons of virulence of pathogens associated with crown and root diseases of strawberry in Western Australia with special reference to the effect of temperature. Scientia Horticulturae. (2011) ;131: :39–48. |
[20] | Koike ST , Gordon TR , Daugovish O , Ajwa H , Bolda M , Subbarao K . Recent developments on strawberry plant collapse problems in California caused by Fusarium and Macrophomina . International Journal of Fruit Science. (2013) ;(1-2):76–83. |
[21] | Short GE , Wyllie TD . Inoculum potential of Macrophomina phaseolina . Phytopathology. (1978) ;68: :742–746. |
[22] | Campbell CL , Van der Gaag DJ . Temporal and spatial dynamics of microsclerotia of Macrophominaphaseolina in three fields in North Carolina over four to five years. Phytopathology. (1993) ;83: :1434–1440. |
[23] | Papavizas GC . Some factors affecting survival of sclerotia of Macrophomina phaseolina in soil. Soil Biology and Biochemistry. (1977) ;9: (5):337–341. |
[24] | Mihail JD . Macrophomina phaseolina: Spatio-temporal dynamics of inoculum and of disease in a high susceptible crop. Phytopathology. (1989) ;79: :848–855. |
[25] | Marcotte M , Banks J , Porter I , Pizano M , Besri M . Evaluations of critical use nominations for methyl bromide and related matters. UNEP Technology and Economic Assessment Panel. UNON Nairobi, Kenya. (2007) ; 102. |
[26] | Zveibil A , Mor N , Gnayem N , Freeman S . Survival, host-pathogen interaction, and management of Macrophomina phaseolina on strawberry in Israel. Plant Disease. (2012) ;96: (2):265–272. |
[27] | Chamorro M , Domínguez P , Medina JJ , Miranda L , Soria C , Romero F , De los Santos B . Assessment of chemical and biosolarization treatments for the control of Macrophomina phaseolina in strawberries. Scientia Horticulturae. (2015) ;192: :361–368. |
[28] | Chamorro M , Miranda L , Domínguez P , Medina JJ , Soria C , Romero F , De los Santos B . Evaluation of biosolarization for the control of charcoal rot disease (Macrophomina phaseolina) in strawberry. Crop Protection. (2015) ;67: :279–286. |
[29] | Muchero W , Ehlers JD , Close TJ , Roberts PA . Genic SNP markers and legume synteny reveal candidate genes underlying QTL for Macrophomina phaseolina resistance and maturity in cowpea [Vigna unguiculata (L) Wal]. BMC Genomics. (2011) ;12: (1):8. |
[30] | Singh S , Lodha S . Varietal resistance of cowpea to Macrophomina phaseolina (Tassi) Goid causing dry root-rot and its control. Indian Journal of Agriculture Science. (1986) ;56: :552–555. |
[31] | Diourte M , Starr JL , Jeger MJ , Stack JP , Rosenow DT . Charcoal rot (Macrophomina phaseolina) resistance and the effects of water stress on disease development in sorghum. Plant Pathology. (1995) ;44: :196–202. |
[32] | Tesso TT , Claflin LE , Tuinstra MR . Analysis of stalk rot resistance and genetic diversity among drought tolerant sorghum genotypes. Crop Science. (2005) ;45: :645–652. |
[33] | Smith GS , Carvil ON . Field screening of commercial and experimental soybean cultivars for their reaction to Macrophomina phaseolina . Plant Disesase. (1997) ;81: :363–368. |
[34] | Olaya G , Abawi GS , Weeden NF . Inheritance of resistance to Macrophomina phaseolina and identification of RAPD markers linked to the resistance genes in beans. Phytopathology. (1996) ;86: :674–679. |
[35] | Songa W , Hillocks RJ , Mwangombe AW , Buruchara R , Konno WK . Screening common bean accessions for resistance to charcoal rot (Macrophomina phaseolina) in Eastern Kenya. Exp Agric. (1997) ;33: :459–468. |
[36] | Mandal RK , Sarkar S , Saha MN . Field evaluation of white jute (Corchoruscapsularis L. ) germplasm against Macrophominaphaseolina (Tassi) Goid under Sorbhog conditions. Environment and Ecology. (2000) ;18: (4):814–818. |
[37] | Biswas C , Dey P , Karmakar PG , Satpathy S . Next-generation sequencing and micro RNAs analysis reveal SA/MeJA1/ABA pathway genes mediated Systematic Acquired Resistance (SAR) and its master regulation via production of phased, trans-acting siRNAs against stem rot pathogen Macrophomina phaseolina in a RIL population of jute (Corchorus capsularis). Physiological and Molecular Plant Pathology. (2014) ;7: :76–85. |
[38] | Fang X , Phillips D , Verheyen G , Li H , Sivasithamparam K , Barbetti MJ . Yields and resistance of strawberry cultivars to crown and root diseases in the field, and cultivar responses to pathogens under controlled environment conditions. Phytopathologia Mediterranea. (2012) ;51: :69–84. |
[39] | Murashige T , Skoog F . A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum. (1962) ;15: (3):473–497. |
[40] | Babu B , Saxena A , Srivastava A , Arora D . Identification and detection of Macrophomina phaseolina by using species- specific oligonucleotide primers and probe. Mycologia. (2007) ;99: :797–803. |
[41] | Kaur S , Dhillon GS , Brar SK , Vallad GE , Chand R , Chauhan VB . Emerging phytopathogen Macrophomina phaseolina: Biology, economic importance and current diagnostic trends. Critical Reviews in Microbiology. (2012) ;38: (2):136–151. |
[42] | Smith BJ , Black LL , Galletta GJ . Resistance to Colletotrichum fragariae in strawberry affected by seedling age and inoculation method. Plant Disease. (1990) ;74: (12):1016–1021. |
[43] | Eikemo H , Stensvand A , Tronsmo AM . Evaluation of methods of screening strawberry cultivars for resistance to crown rot caused by Phytophthora cactorum . Annals of Applied Biology. (2000) ;137: (3):237–244. |
Figures and Tables
Fig.1
Disease progress curves according to severity of aerial symptoms of plants inoculated with Macrophomina phaseolina, maintained under greenhouse conditions. Disease severity was measured according to an arbitrary 0 –5 scale.
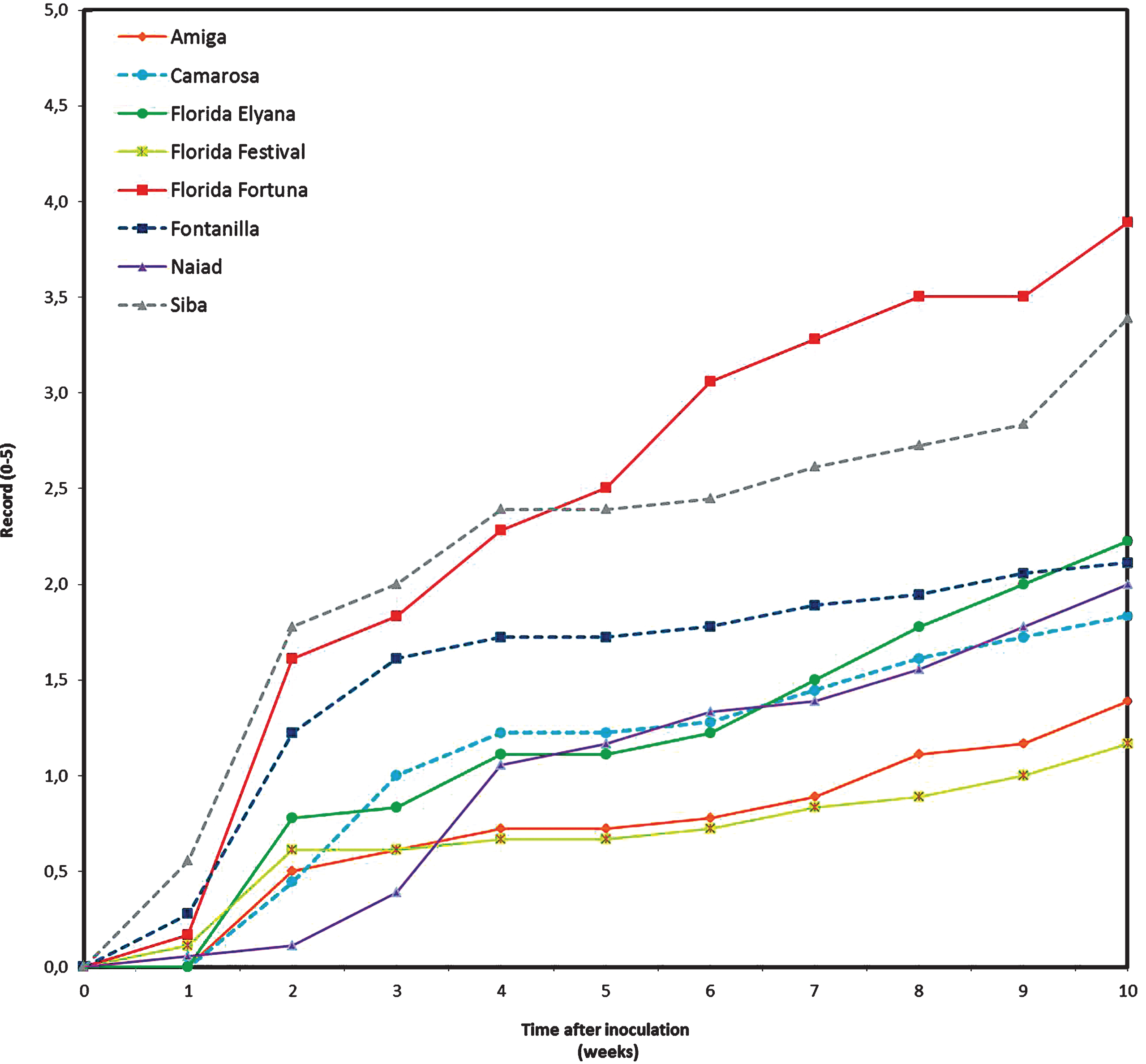
Fig.2
Area under the disease progress curve (AUDPC) according to severity of aerial symptoms, of strawberry plants inoculated with Macrophomina phaseolina, maintained under greenhouse conditions. Bars indicate standard deviation. Columns with the same letter are not different according to Tukey’s HSD (p≤0.05).
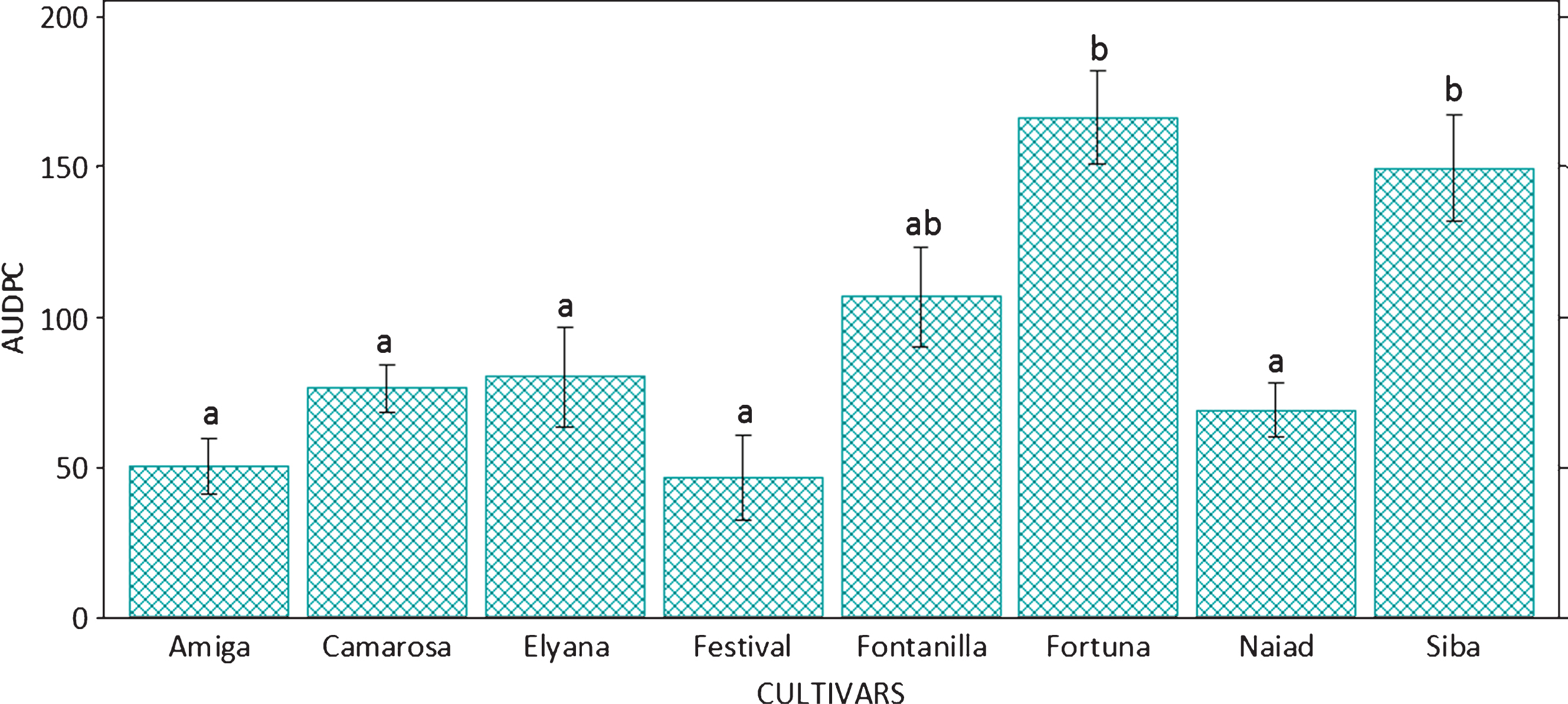
Fig.3
Disease progress curves according to plant mortality of 11 strawberry cultivars inoculated with Macrophomina phaseolina, and maintained under controlled conditions.
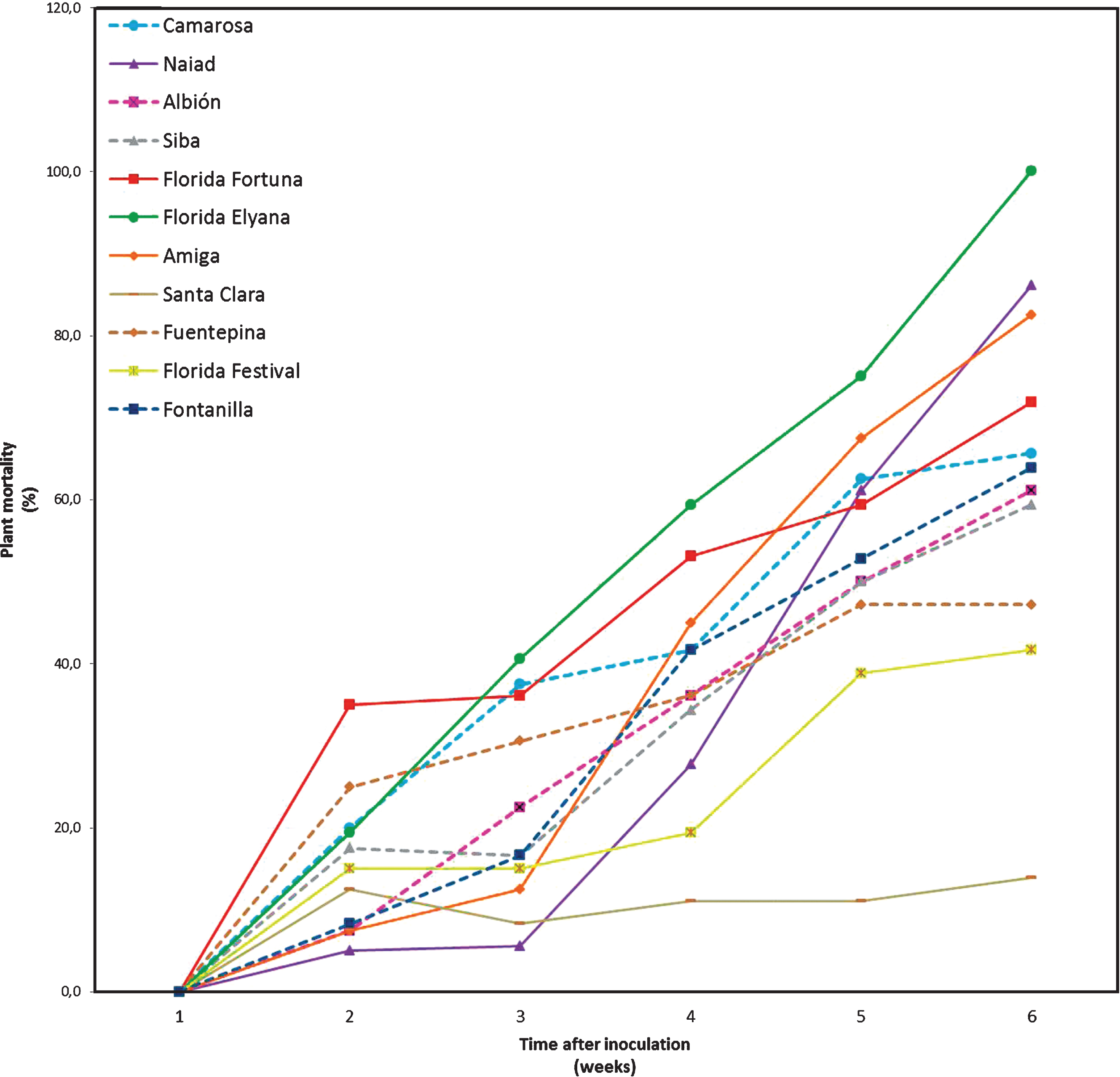
Fig.4
Area under the disease progress curve (AUDPC) according to mortality of plants of 11 strawberry cultivars inoculated with Macrophomina phaseolina, maintained under controlled conditions. Bars indicate standard deviation. Columns with the same letter are not different according to Tukey’s HSD (p≤0.05).
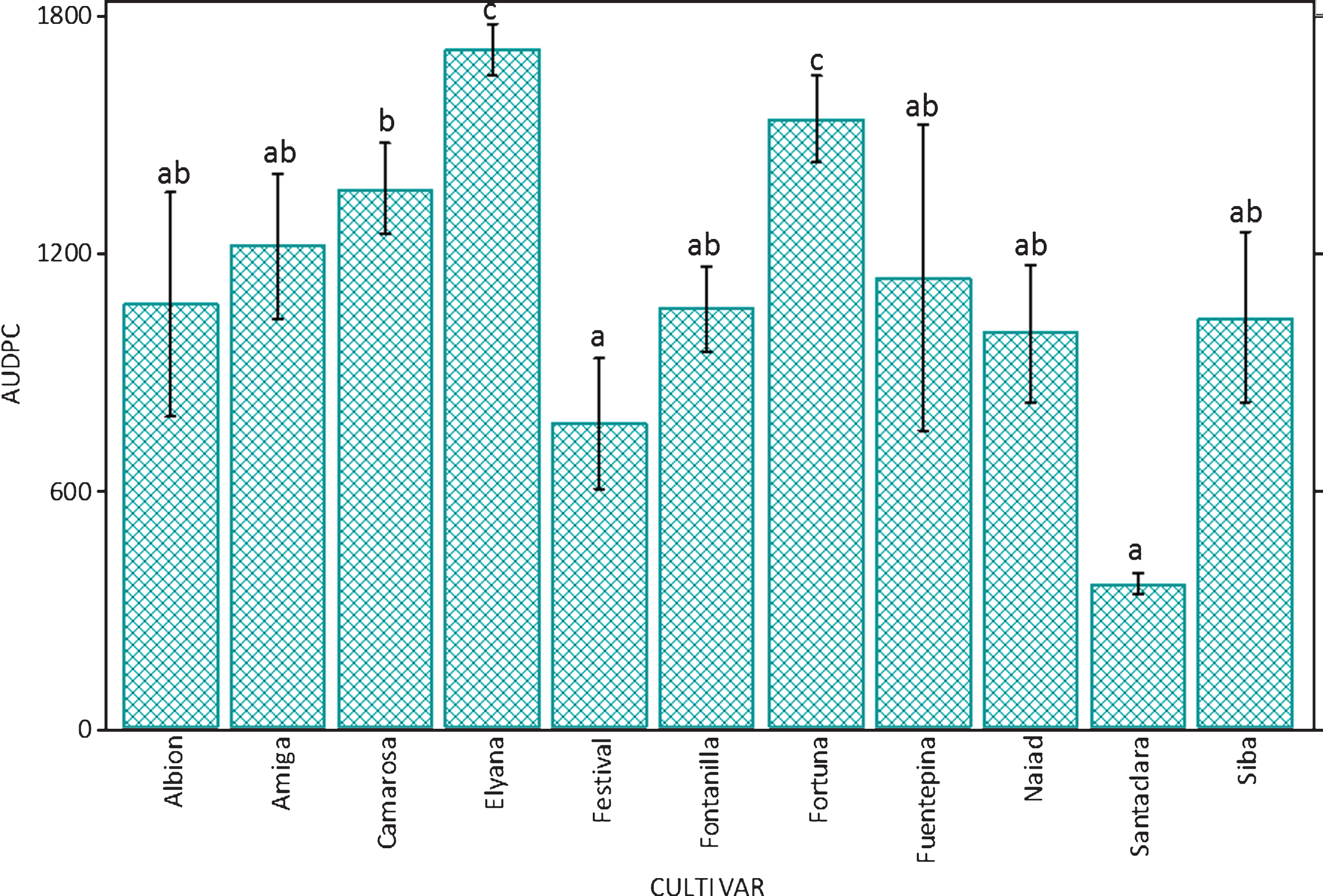
Table 1
Cultivars used in the study
| Cultivar | Source |
| Albión | University of California –United States of America |
| Amiga | Research and Training Institute for Agriculture and Fisheries (IFAPA) –Spain |
| Camarosa | University of California –United States of America |
| Florida Elyana | University of Florida –United States of America |
| Florida Festival | University of Florida –United States of America |
| Florida Fortuna | University of Florida –United States of America |
| Fontanilla | Research and Training Institute for Agriculture and Fisheries (IFAPA) –Spain |
| Fuente Pina | Research and Training Institute for Agriculture and Fisheries (IFAPA) –Spain |
| Naiad | Italian Consortium of Nurseries (CIV) –Italy |
| Santa Clara | Research and Training Institute for Agriculture and Fisheries (IFAPA) –Spain |
| Siba | Italian Consortium of Nurseries (CIV) –Italy |
Table 2
Susceptibility of strawberry cultivars against Macrophomina phaseolina infection, measured as severity of symptoms and growth reduction
| Cultivar | Severity (0–5)1 | Growth Reduction (%)1,2 | ||||||
| Aerial | Crown | Dry Weight | Crown Diameter | |||||
| Florida Festival | 1.2 | a | 0.3 | a | 21.5 | a | 3.9 | ab |
| Amiga | 1.4 | ab | 0.8 | ab | 6.7 | a | 0.3 | a |
| Camarosa | 1.8 | ab | 1.0 | ab | 13.0 | a | 3.0 | ab |
| Naiad | 2.0 | ab | 0.9 | ab | 22.9 | ab | 1.4 | ab |
| Fontanilla | 2.1 | ab | 0.8 | ab | 43.5 | bc | 9.1 | bc |
| Florida Elyana | 2.2 | b | 1.1 | ab | 29.2 | ab | 6.6 | abc |
| Siba | 3.4 | c | 1.6 | b | 53.6 | c | 12.9 | c |
| Florida Fortuna | 3.9 | c | 3.0 | c | 44.9 | bc | 8.5 | bc |
| Average | 2.3 | 1.2 | 29.5 | 5.7 | ||||
Means followed by the same letter in each column are not significantly different, according to Tukey’sHSD test (p≤0.05). 1Parameters assessed 10 weeks after inoculation. 2Reduction calculated based on uninoculated control plants.




