Comparison of monomeric anthocyanins and colour stability of fresh, concentrate and freeze-dried encapsulated cherry juice stored at 38°C
Abstract
BACKGROUND:
Anthocyanins are natural colorants and bioactive compounds widely distributed among vegetables and fruits. Unfortunately, in fruit juices stored at room temperature monomeric anthocyanins easily convert to colourless compounds and subsequently to insoluble brown pigments.
OBJECTIVE:
The object of present study is to compare the stability of monomeric anthocyanins and colour changes during storage temperature at 38°C of pasteurized cherry juice, concentrate and freeze-dried juice.
METHODS:
Colour changes of, pasteurized cherry juice (18.7 °Brix), concentrate (61 °Brix) and juice freeze-dried with addition of maltodextrin and arabic gum as encapsulating agents, were evaluated by CIELab parameters, a*, b* and, L*. Anthocyanins content was measured by pH-differential method and total phenolics were determined by Folin-Ciocalteu method. The glass transition temperature (Tg) of the freeze-dried matrix was determined.
RESULTS:
It was found the stability of monomeric anthocyanins and colour retention during storage at 38°C was significantly superior in the freeze-dried encapsulated juice powder than in liquid cherry juices.
CONCLUSIONS:
This is attributed to the protective encapsulation of the anthocyanins in the amorphous matrix of maltodextrin/arabic gum of reduced water activity.
1Introduction
Anthocyanins are bioactive compounds present in many fruits, vegetables and their products. They are natural colorants widely distributed in fruit and vegetables; notably cherries and berries [1]. In addition, anthocyanins have multiple biological roles, e.g. antioxidant activity, anti-inflammatory action, inhibition of blood platelet aggregation and antimicrobial activity, and prevention of cholesterol-induced atherosclerosis [1, 2].
Anthocyanins are the basis for the red, blue and purple colours of fruits, vegetables and their products. Unfortunately, under normal processing and storage at room temperature monomeric anthocyanins transform relatively easy to colourless compounds and subsequently to insoluble brown pigments [3]. Since anthocyanins impart a characteristiccolour to fruits and vegetables they have a considerable impact on consumer sensory acceptance due to perception of inferior product quality [1].
Several studies indicate anthocyanin stability is not merely a function of temperature but is also influenced by intrinsic properties of the product and process conditions. Patras et al. [4] reviewed some important aspects of anthocyanin degradation during thermal processing; conclusions regarding the mechanisms and kinetics of anthocyanin degradation during heat treatment were postulated.
Cherry juice is a good source of anthocyanins, but its stability during heat processing or accelerated storage temperature suffers from the same drawbacks as those from other fruit juices [5]. Arslan [6] studied the effects of degradation preventive agents on storage stability of anthocyanins in sour cherry concentrate. It was concluded the duration and temperature of storage influence anthocyanin stability in cherry juice.
The appearance of a food product is one of the most important quality characteristics for consumer acceptance. As for red fruit–based products such as cherry juice, attractive colour should remain relatively constant throughout shelf life [1]. However, as mentioned above colour stability of cherry juice stored at ambient temperature is a difficult task because of rapid anthocyanin degradation; even at refrigeration temperature of about 4°C [6].
The aim of this research was to study the stability of monomeric anthocyanins and color changes during 38°C storage of fresh (18.7 °Brix), concentrate (61 °Brix) and freeze-dried encapsulated pasteurized cherry juice, to determine the most satisfactory procedure to improve anthocyanin and red colour retention.Temperature of 38°C was selected because it is usually recommended for accelerated shelf life studies of foods to be marketed at ambient temperature [7].
2Materials and methods
2.1Reagents
Maltodextrin Dextrose Equivalent 10 (MD10) from Productos de Maíz S.A., Buenos Aires, Argentina and Arabic Gum from Gelfix S.A., Buenos Aires, Argentina, were used for encapsulation of freeze dried cherry juice.
Ethanol and chlorhydric acid used as solvents for juice extraction were from Biopack, Buenos Aires, Argentina.
Folin–Ciocalteu reagent was purchased from Merck KgaA Darmstadt, Germany. Gallic acid used for phenolic standard curve was obtained from Anedra, Buenos Aires, Argentina.
2.2Samples
Lapins Dark cherry fruit from Tunuyan, Mendoza (Argentina), was provided by Rio Alara S.A. The fruit was frozen at –20°C until processing.
2.2.1Juice
Frozen cherries were thawed in a water bath at 20°C, blanched (87°C, 3 min), destemmed and pitted, and processed in a blender with mesh to obtain the juice. Ascorbic (0.25 g/kg) and citric (4 g/kg) acids were then added; the juice was fractionated and sealed in hermetic plastic bags 50 g each, pasteurized (90°C, 5 min), cooled and stored until used for the study.
2.2.2Concentrate
A fraction of pasteurized juice described above (18.7 °Brix) was evaporated at 40°C in vacuum at 25 mbar in a Laborota Heidolf (Schwabach, Germany) rotary evaporator. The product obtained (61 °Brix) was re-pasteurized, fractionated in sterile and hermetic flasks 10 g each and used for storage studies. This low temperature concentration did not alter anthocyanins content of juice.
2.2.3Freeze drying and encapsulation procedure
Pasteurized cherry juice (18.7 °Brix) was mixed with Maltodextrin DE10 and Arabic Gum (80:20) at a ratio of 25% of encapsulating agents, poured onto an aluminium tray and frozen at –20°C during 24 h. Then it was freeze dried at room temperature (22 ± 3°C) in a FIC-LI-I-E300-CRT freeze dryer (Buenos Aires, Argentina) operated with a freezing plate and condenser at –40°C and a vacuum of 100 μm Hg during 40 h. Freeze drying did not alter monomeric anthocyanin content of cherry juice. The freeze-dried amorphous carbohydrate microstructure was milled to obtain a powder which was fractionated in sterile, hermetic flasks (5 g) and stored.
2.3Storage conditions
Samples were stored in darkness at 38°C for 33 days (fresh juice) and for 60 days (concentrate and freeze-dried); two samples of juice, concentrate and encapsulated freeze dried juice were removed at selected times to take measurements.
2.4Juice extract
Five grams of juice were extracted twice in 20 ml ethanol: HCl 0.1 N (85:15). The pellets with no detectable residual content of phenolics, were eliminated by centrifugation and the supernatants were mixed and utilized for measurements of total phenolics, monomeric anthocyanins and colour. Samples of concentrate and freeze dried juice were previously reconstituted with water to their original weights.
2.5Methods
2.5.1Physicochemical properties
Total soluble solids content was evaluated with a manual refractometer Atago N2 (Tokyo, Japan). pH was measured at 25°C using a Hanna HI 8424 instrument (Hanna Instruments Inc., Woonsocket, RI, USA).
Water activity was determined using an electronic dew-point water activity meter Aqualab TE (Decagon Devices, Pullman, WA). The equipment was calibrated with saturated salt solutions in the water activity range of interest [8].
Moisture content was performed on 1.5 g of powder in an oven at 90°C up to constant weight.
Glass transition temperature (Tg) of cherry juice freeze dried with maltodextrin + arabic gum was determined by differential scanning calorimetry (DSC) using a DSC822e 104 Mettler Toledo calorimeter (Schwerzenbach, Switzerland). The instrument was calibrated with indium (156.6°C), lead (327.5°C) and zinc (419.6°C). Masurements were performed at a heating rate of 10°C / min. Hermetically sealed 40 μL medium pressure pans were used, (an empty pan served as a reference). Thermograms were then evaluated using Mettler Stare program.
2.5.2Total phenolics
Total phenolics (TP) were determined on the juice extract using the Folin–Ciocalteu method according to Waterhouse [9]. Sample absorbance at 765 nm (PG Instruments T60U UV-Vis spectrophotometer, Leicestershire, United Kingdom) was measured, and phenolic concentrations were expressed as gallic acid equivalent (GAE) in mg/100 g of product, calculated by means of a standard curve of gallic acid.
2.5.3Monomeric anthocyanin content
Monomeric anthocyanin content of juice extracts was determined by pH differential method [10]. Absorbances were read at 510 and 700 nm, and its content was calculated as cyanidin-3-glucoside in mg/100 g of product (MW: 449.2 g mol–1 and ɛ: 26 900 L cm–1mol–1). Monomeric anthocyanin retention (% ) was relative to the initial content considered as 100 % .
2.5.4Colour measurements
Cherry juice color was analyzed using a Minolta Spectrophotometer CM-600d (Konica Minolta Observer), with D65 illuminant and an observer angle of 2°. The colour measurement was obtained by placing 0.4 g of juice or reconstituted juice for concentrate and freeze dried samples, in plastic white containers. CIELab parameters (CIE 1976 L* a* b*) were L* for lightness, a* for redness and b* for yellowness. Calculations of C* ((a* + b*) 1/2) for chroma and h°(arctan b*/a*) for hue angle were made. Total colour difference was calculated as
ΔE* = [(ΔL*)2+(Δa*)2+(Δb*)2]½ and expressed the magnitude of difference between 0 and 33 days of juice storage. The instrument was standardized with a white tile (L* = 91.10, a* = 1.12 and b* = 1.26).
2.6Data analysis
Replicate bottles of each juice product were analyzed at indicated time of storage. All the parameters studied were determined at least by duplicate, and the average was reported. Colour parameters during storing were analyzed applying one-way ANOVA and Student-Newman-Keuls test for multiple means comparisons, using Infostat v.2009 (Universidad Nacional de Córdoba, Argentina). Pearson’s correlations between colour parameter a* and monomeric anthocyanin content were made.
3Results and discussion
Table 1 shows physicochemical characteristics of fresh (18.7 °Brix), concentrate (61 °Brix) and freeze-dried encapsulated cherry juice (aw = 0.10 and 3.8 ± 0.1 % moisture content). Total phenolics and anthocyanin contents in fresh juice are within range reported in literature [11].
Figure 1 compares monomeric anthocyanin stability in the liquid juices (fresh and 61 °Brix concentrate) and in the encapsulated cherry juice powder during storage at 38°C. It can be seen that in fresh and concentrate juices anthocyanin retention decreases rapidly during storage while in the freeze dried powder is much more stable. At 33 days of storage the % anthocyanin retention in the liquid juices (both fresh and concentrate) fell to 11% while it remained at around 90% in the powder.
The poor stability of anthocyanins in the liquid juices stored at 38°C is in agreement with literature data, as reviewed in Table 2 which shows the half-life of monomeric anthocyanin degradation (t1/2 = time to reduce concentration to 50 % of its initial value) in various fruit juices (mainly berries) stored at near 38°C. Half-life times ranged between 2.1 days to 31.5 days highlighting the limited stability of monomeric anthocyanins [12–20].
Cherry juice was encapsulated by freeze drying with a mixture of encapsulating agents (maltodextrin and arabic gum) to protect anthocyanins in an amorphous glassy matrix [21, 22]. Cherry juice has a high monosaccharides content; main sugars are fructose and glucose (with small amounts of sucrose). It is well known these sugars (mainly fructose) have very low glass transition temperatures, Tg [23]. Thus, one may expect structural collapse in the amorphous structure if cherry juice is attempted to freeze dried alone. For this reason encapsulating agents (maltodextrin and arabic gum) were used to increase the resulting glass transition temperature; thus improving its physical properties [24].
Figure 2 shows the DSC thermogram for encapsulated cherry juice at a water activity of 0.10; the onset, midpoint and endpoint glass transition temperature values are indicated. Although a glass transition seems to be apparent in the thermogram, the witdth of the glass transition (difference between the end and onset values) is large (over 20°C) suggesting that in this case Tg is actually a transition region, rather than a specific temperature. Since the convention is to report a single temperature, the midpoint glass transition temperature (35.5°C) was taken as most representative of the thermal transition in freeze-dried encapsulated cherry juice [25]. Since the midpoint glass transition temperature (35.5°C) is close to the storage temperature (38°C) one may explain the stability of anthocyanins (Fig. 1) due to the existence of a cuasi glassy state. It is known that physical changes in an amorphous matrix are time dependent being a function of (T-Tg), where T is the storage temperature. In present conditions this difference (38 –35.5°C) is very small and the system would behave as in the glassy state, at least for the time-scale of present work (60 days) [24]. As observed in Fig. 3 visual examination of the dry cherry powder after 60 days storage at 38°C revealed absence of collapse/caking therefore confirming a glassy-like behavior.
Laine et al. [26] reported that a polyphenol-rich raspberry extract was stabilized by freeze-drying with maltodextrins as material coating, and found the freeze-dried particles were stable over long periods providing to polyphenols an effective protection against the oxidation phenomenon during their storage. Estupiñan et al. [27] found that addition of maltodextrin DE20 improved the color and stability of antioxidants in freeze-dried powders from Andes berry during storage. Osorio et al. [28] also reported that the stability of anthocyanins was enhanced by spray drying encapsulation in maltodextrin and/or arabic gum matrixes.
The attractive red colour of cherry juice plays such a vital role in consumer sensory acceptance; therefore, it is highly desirable it should remain relatively constant throughout shelf life. Figure 4 shows colour parameter a* (redness) as a function of storage time for liquid juices (fresh and concentrate) and encapsulated juice of low water activity. It can be seen that parameter a* decreases rapidly from the beginning of storage for both liquid cherry juices but remained more or less constant for the encapsulated cherry juice. These results resemble those showed in previous Fig. 2, which compared the monomeric anthocyanin stability of liquid juices (regular and 61 °Brix concentrate) and encapsulated juice, suggesting that the loss of anthocyanins is associated to loss of characteristic red colour. For fresh and concentrate juices a high correlation between parameter a* and anthocyanin content during storage was found, being R = 0.94 and 0.95 respectively (P < 0.05).
Figure 5 shows the changes in all colour parameters: a* (redness), b* (yellowness), L* (lightness), as well as calculated purity (C*) and hue angle (h°) values, for liquid juices and encapsulated juice before and after 33 days storage at 38°C. Overall, it can be observed that colour parameters experimented significant modifications in the liquid juices (18.7 and 61 °Brix) after 33 days at 38°C, with decreased a* and increased b* resulting in a higher tone (h°) value and in a brownish juice. For the encapsulated juice the changes were much less important. Total colour difference (ΔE*) values were 11.2 ± 0.1, 18.6 ± 0.5 and 2.2 ± 0.3 for fresh, concentrate and freeze dried juice respectively, indicating more noticeable visual changes in colour during storage in liquid systems; these changes being more relevant for concentrate juice. Particularly in this case significant increase of lightness (L*) and decline of saturation (C*) were observed; and this may be attributed to non-enzymatic browning reactions which are known to proceed faster at intermediate water activity [29]. Changes in L* and C* values produce fading of colour that could reflect decolourization of anthocyanins and a shift from vivid to a duller colour respectively. Similar results were obtained in strawberry puree storing 8 weeks at 25°C as a consequence of processing and storage [30].
4Conclusions
In addition to contributing to bioactive properties, attractive colour is one of the most important sensory characteristics of cherry juice. However, in pasteurized liquid juices (fresh and concentrate) both anthocyanin content and red colour are unstable and susceptible to degradation during storage at 38°C. On the contrary, the encapsulated cherry juice powder of low water activity, exhibited a good stability of both anthocyanins and colour parameters.
Maintaining anthocyanin content as well as a stable red colour in cherry juice during storage at room temperature is problematic; thus juice encapsulation in a dried matrix of low water activity could be used as a strategy for product stabilization.
Acknowledgments
Authors acknowledge to Rio Alara S.A (Buenos Aires, Argentina) for donating the cherry fruits.
References
1 | Busso Casati C, Sanchez V, Baeza R, Magnani N, Evelson P, Zamora MC (2012) Relationships between colour parameters, phenolic content and sensory changes of processed blueberry, elderberry and blackcurrant commercial juices International Journal of Food Science and Technology 47: 8 1728 |
2 | Mazza G, Miniati E (1993) Anthocyanins in fruits, vegetables, and grains Mazza G, Miniati E Boca Raton CRC Press |
3 | Markakis P (1982) Stability of Anthocyanins in Foods Markakis P Anthocyanins As Food Colors New York Academic Press 163 |
4 | Patras A, Brunton NP, O’Donnell C, Tiwari BK (2010) Effect of thermal processing on anthocyanin stability in foods; mechanisms and kineticsof degradation. Review Trends in Food Science & Technology 21: 1 3 |
5 | Kelebek H, Selli S (2011) Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars International Journal of Food Science & Technology 46: 12 2530 |
6 | Arslan D (2015) Effects of degradation preventive agents on storage stability of anthocyanins in sour cherry concentrate Agronomy Research 13: 4 892 |
7 | Labuza TP, Schmidl MK (1985) Accelerated shelf life testing of foods Food Technology 39: 9 57 |
8 | Favetto GJ, Resnik SL, Chirife J, Ferro Fontá C (1983) Statistical evaluation of water activity measurements obtained with the Vaisala Humicap humidity meter Journal of Food Science 487: 2 534 |
9 | Waterhouse AL (2001) Determination of total phenolics Wrolstad RE Current Protocols in Food Analytical Chemistry I1.1.1. New York Wiley & Sons |
10 | Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy Wrolstad R, Acree T, An H, Decker E, Penner M, Reis D, Schawrtz S, Shoemaker C, Spoms P Current Protocols in Food Analytical Chemistry 1st.edn F1.2.1. New York John Wiley and Sons, Inc |
11 | Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE (2006) Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women J Nutr 136: 4 981 |
12 | Busso Casati C, Baeza R, Sanchez V, Catalano A, López P, Zamora MC (2015) Thermal degradation kinetics of monomeric anthocyanins, colour changes and storage, effect in elderberry juices Journal of Berry Research 5: 1 29 |
13 | Alighourchi H, Barzegar M (2009) Some physicochemical characteristics and degradation kinetic of anthocyanin of reconstituted pomegranate juice during storage Journal of Food Engineering 90: 2 179 |
14 | Kirca A, Cemeroğlu B (2003) Degradation kinetics of anthocyanins in blood orange juice and concentrate Food Chemistry 81: 4 583 |
15 | Kirca A, őzkan M, Cemeroğlu B (2006) Stability of black carrot anthocyanins in various fruit juices and nectars Food Chemistry 97: 4 598 |
16 | Kirca A, őzkan M, Cemeroğlu B (2007) Effects of temperature solid content and pH on stability of black carrot anthocyanins Food Chemistry 101: 1 212 |
17 | Wang WD, Xu SY (2007) Degradation kinetics of anthocyanins in blackberry juice and concentrate Journal of Food Engineering 82: 3 271 |
18 | Cemeroğlu B, Velioğlu S (1994) Degradation kinetics of anthocyanins in sour cherry juice and concentrate Journal of Food Science 59: 6 1216 |
19 | Martínez Zambrano J, Rojas Sarmiento H, Borda Guerra G, Hastamorir Caro A, Medina Rianño M (2011) Estabilidad de antocianinas en jugo y concentrado de agraz (Vaccinium Meridionalle Sw.) Revista Facultad Nacional de Agronomía - Medellín 64: 1 6015 |
20 | Harbourne M, Jacquier JC, Morgan D, Lyng J (2008) Determination of the degradation kinetics of anthocyanins in a model juice system using isothermal and non-isothermal methods Food Chemistry 111: 1 204 |
21 | Galmarini M, Maury C, Mehinagic E, Sanchez V, Baeza R, Mignot S, Zamora MC, Chirife J (2013) Stability of individual phenolic compounds and antioxidant activity during storage of a red wine powder Food and Bioprocess Technology 6: 12 3585 |
22 | Sanchez V, Baeza R, Galmarini M, Zamora MC, Chirife J (2013) Freeze-drying encapsulation of red wine polyphenols in an amorphous matrix of maltodextrin Food and Bioprocess Technology 6: 5 1350 |
23 | Roos Y (1995) Phase Transitions in Foods NY, USA Academic Press |
24 | Roos Y, Karel M (1991) Phase transition of mixtures of amorphous polysaccharides and sugars Biotechnology Progress 7: 1 49 |
25 | Cherian G, Chinachoti P (1996) 2H and 170 Nuclear magnetic resonance study of water in gluten in the glassy and rubbery state Cereal Chem 73: 5 618 |
26 | Laine P, Kylli P, Heinonen M, Jouppila K (2008) Storage stability of microencapsulated cloudberry (Rubus chamaemorus) phenolics Journal of Agriculture and Food Chemistry 56: 23 11251 |
27 | Estupiñan DC, Schwartz SJ, Garzón GA (2011) Antioxidant activity, total phenolics content, anthocyanin, and color stability of isotonic model beverages colored with Andes berry (Rubus glaucus Benth) anthocyanin powder Journal of Food Science 76: 1 S26 |
28 | Osorio C, Acevedo B, Hillebrand S, Carriazo J, Winterhalter P, Morales AL (2010) Microencapsulation by spray-drying of anthocyanin pigments from Corozo (Bactris guineensis) fruit Journal of Agricultural and Food Chemistry 58: 11 6977 |
29 | Labuza TP, Warren RM, Warmbier HC (1977) The physical aspects with respect to water and non-enzymatic browning Adv Exp Med Biol 86: B 379 |
30 | Howard LR, Brownmiller C, Prior RL (2014) Improved color and anthocyanin retention in strawberry puree by oxygen exclusion Journal of Berry Research 4: 2 107 |
Figures and Tables
Fig.1
Comparison of monomeric anthocyanins retention in: ▴ freeze dried encapsulated (aw = 0.10), ♦ concentrate (61 °Brix) and ∘ fresh (18.7 °Brix) cherry juices stored at 38°C.
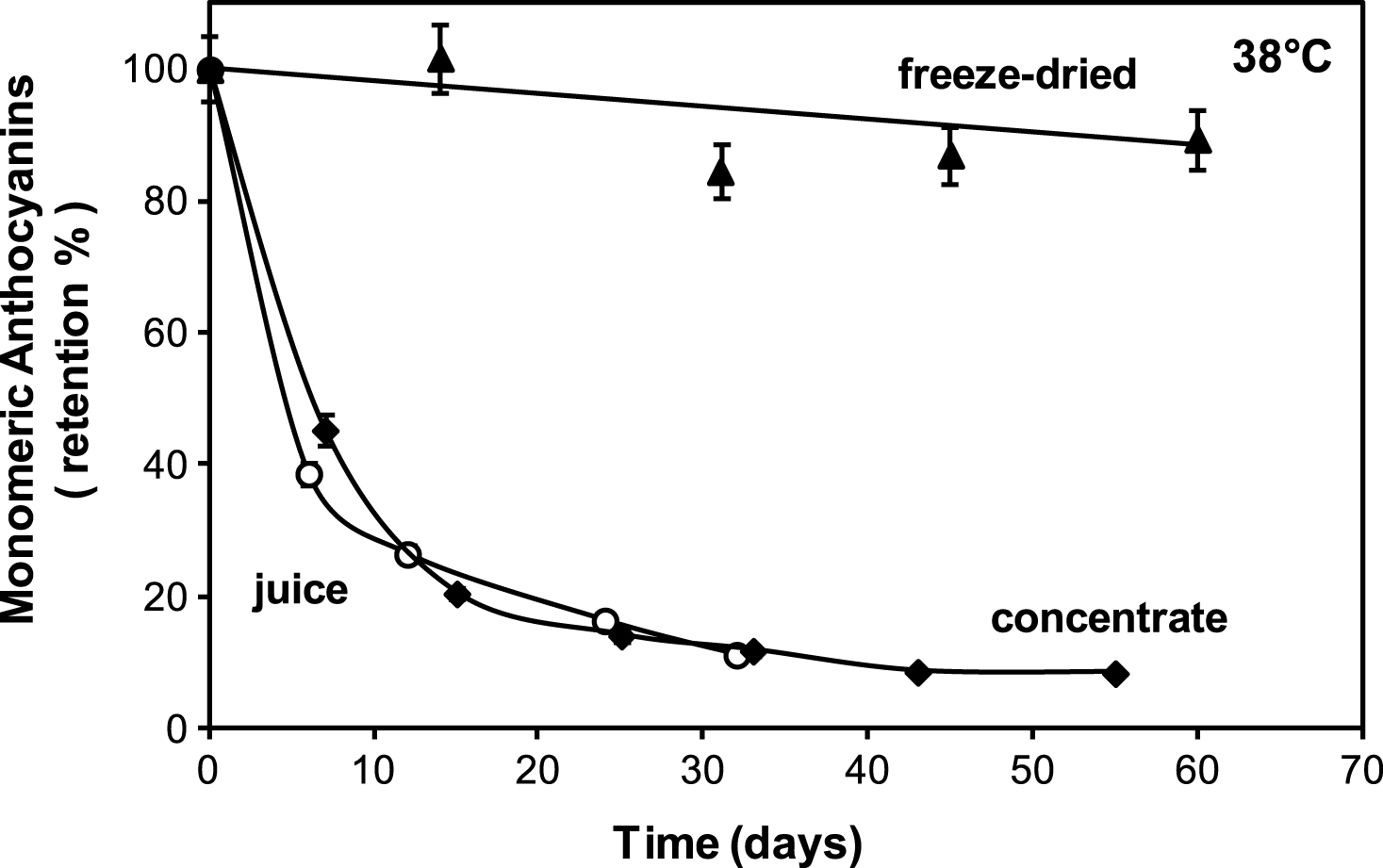
Fig.2
DSC thermogram for cherry juice freeze-dried encapsulated in a maltodextrin/arabic gum matrix of aw = 0.10.
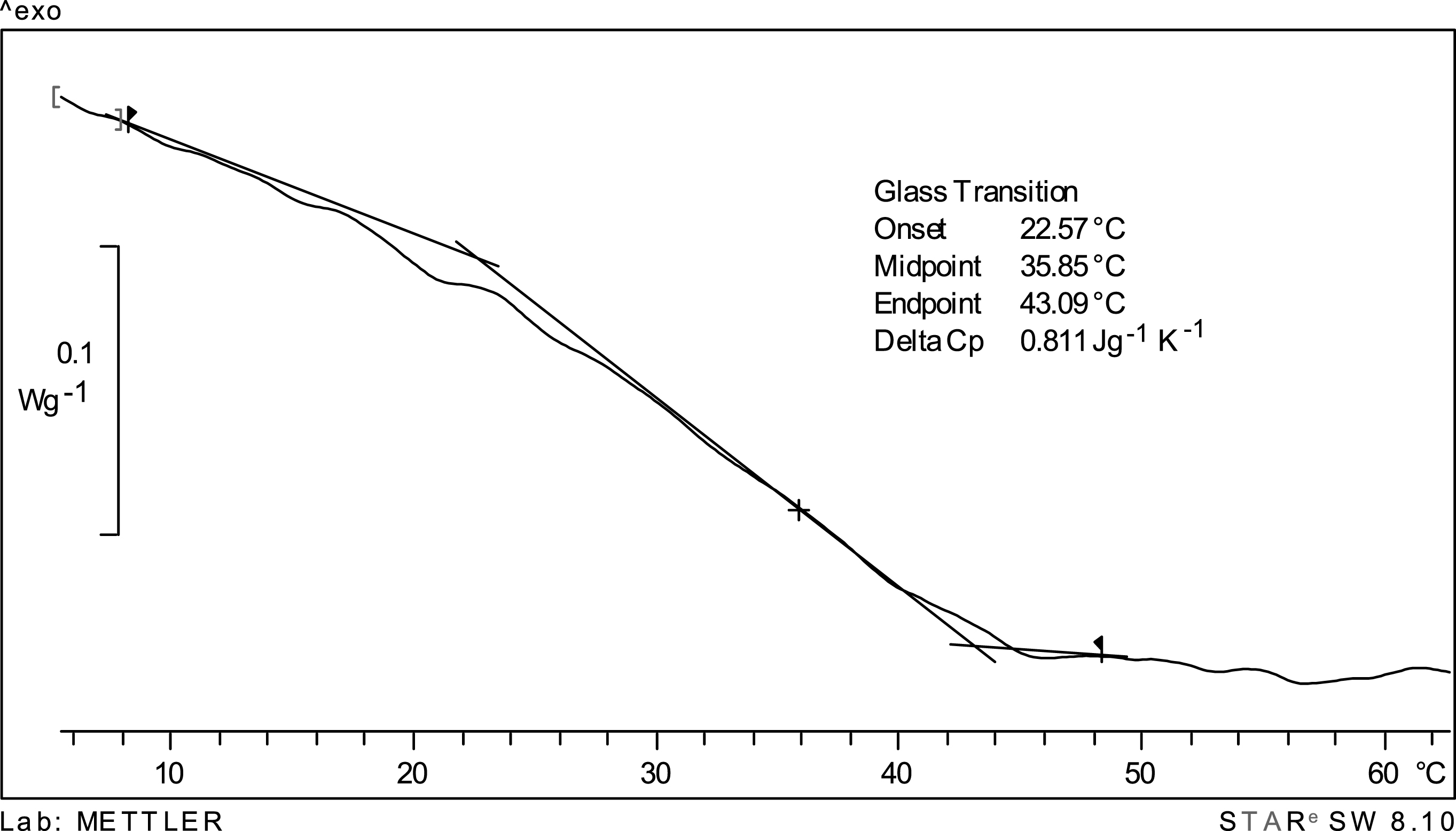
Fig.3
Encapsulated cherry juice powder (aw = 0.10) before and after 60 days storage at 38°C –left side: before storage; right side: after storage.

Fig.4
Comparison of a* (redness) colour parameter of: ▴ freeze dried encapsulated (aw = 0.10), ♦ concentrate (61 °Brix) and ∘ fresh(18.7 °Brix) cherry juices stored at 38°C.
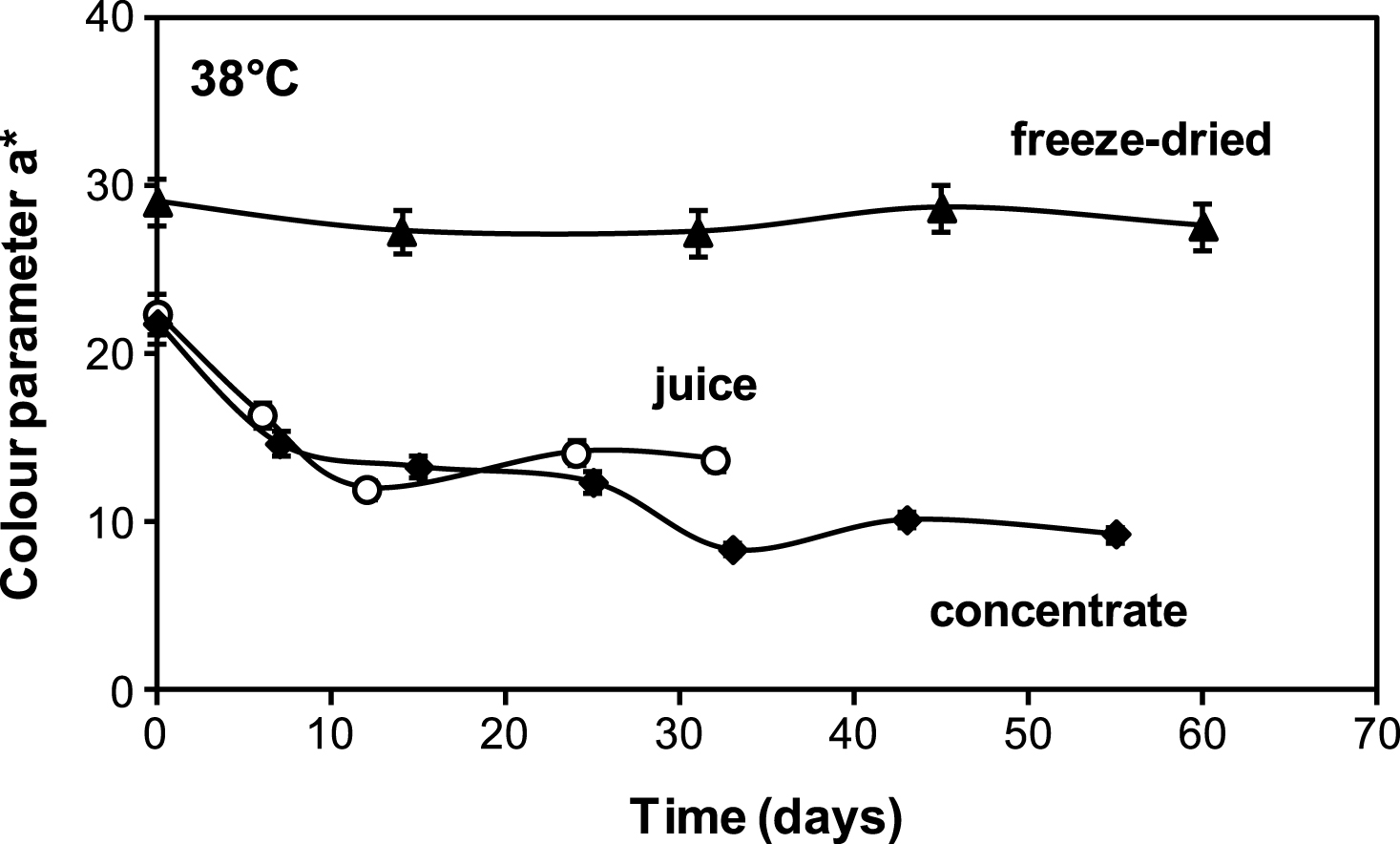
Fig.5
Comparison of all colour parameters (L*, a*, b*, C*, h°) in freeze dried encapsulated, concentrate (61 °Bx) and fresh (18.7 °Bx) cherry juice at 0 and 33 days stored at 38°C. Different letters above the data bars indicate that colour parameter differed between storage time, P < 0.05, Student-Newman-Keuls test.
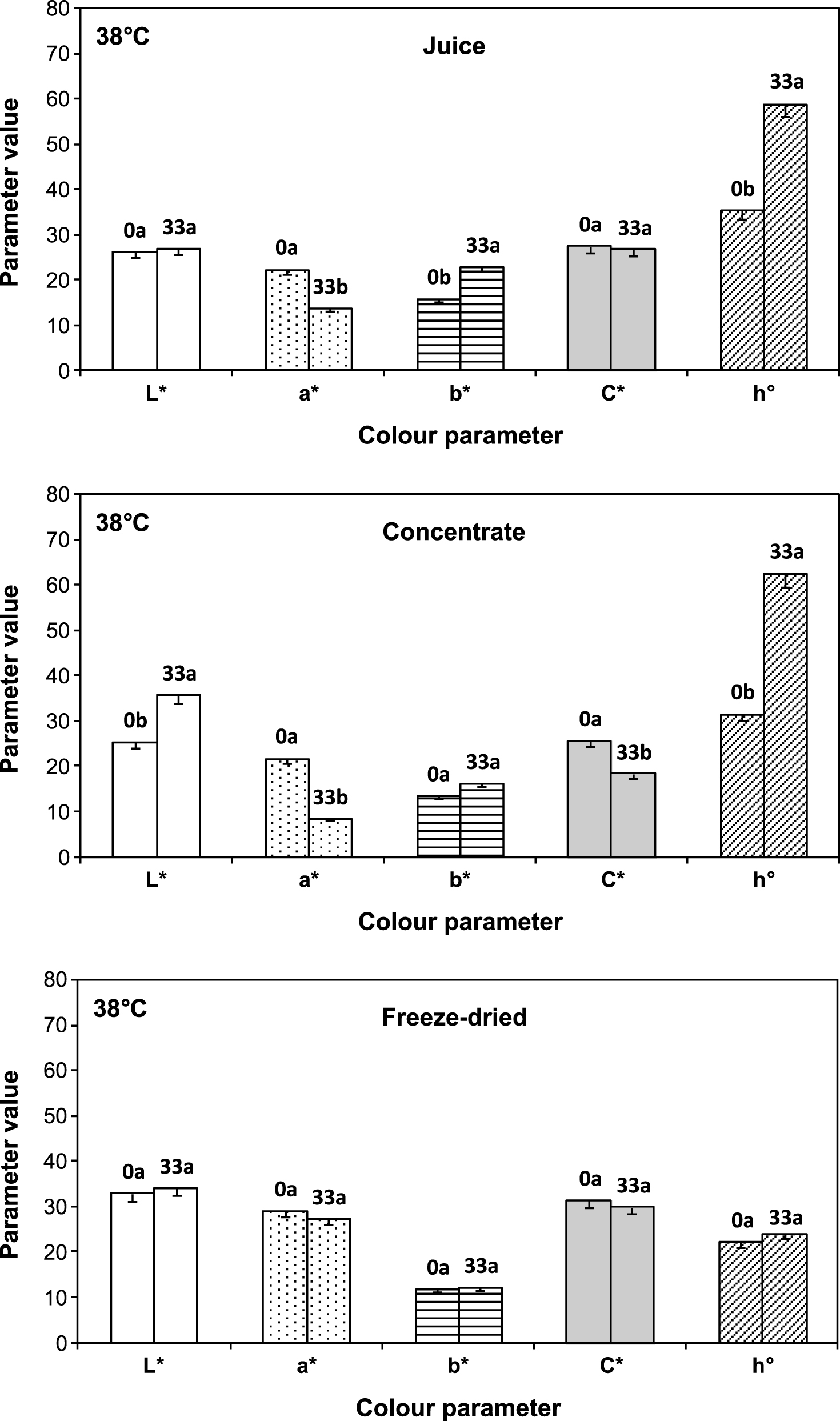
Table 1
Physico-chemical characteristics of cherry juices used in this study
| Total soluble solids (°Brix) | pH | aw | Total phenolics content (mg/100 g) | Monomeric anthocyanins (mg/100 g) | |
| Fresh juice | 18.7 ± 0.1 | 3.71 ± 0.01 | 0.973 ± 0.001 | 159 ± 11 | 23.5 ± 0.2 |
| Juice Concentrate | 61.0 ± 0.1 | 3.90 ± 0.01 | 0.824 ± 0.001 | 459 ± 20 | 86.2 ± 2.9 |
| Freeze-dried encapsulated juice | 0.100 ± 0.001 | 392 ± 30 | 67.5 ± 4.0 |
Values are means ± standard errors.
Table 2
Half-life of monomeric anthocyanin degradation (t1/2) in various (fresh and concentrate) fruit juices (mostly berries) stored at near 38°C
| Fruit juice | °Brix | pH | Storage Temperature (°C) | Previous thermal treatment | t ½ (days) | Reference |
| Elderberry | 36.8 | 3.6 | 40 | Pasteurized at 80°C | 27.7 | Busso Casati et al., 2015 |
| Pomegranate | 13.7 | 3.2 | 37 | Pasteurized at 93°C | 25.4 | Alighourchi et al, 2009 |
| Bloor orange | 45 | 3.4 | 37 | Concentrated at 80°C in Rotavapor | 2.1 | Kirca et al., 2003 |
| Bloor orange | 69 | 3.4 | 37 | Concentrated at 80°C in Rotavapor | 3.1 | Kirca et al., 2003 |
| Black carrot in various juices | 9.9–26.2 | 3.0–3.9 | 37 | Pasteurized at 85 °C | 12–16 | Kirca et al., 2006 |
| Black carrot | 30 | 4.3 | 37 | Pasteurized at 85°C | 28.7 | Kirca et al., 2007 |
| Black carrot | 45 | 4.3 | 37 | Pasteurized at 85°C | 31.5 | Kirca et al., 2007 |
| Black carrot | 64 | 4.3 | 37 | Pasteurized at 85°C | 28 | Kirca et al., 2007 |
| Blackberry | 8.9 | 2.9 | 37 | Pasteurized at 85°C | 11.7 | Wang and Xu, 2007 |
| Blackberry | 65 | 2.9 | 37 | Pasteurized at 85°C | 9.4 | Wang and Xu, 2007 |
| Sour cherry | 45 | 3.2 | 37 | Pasteurized | 14 | Cemeroglu et al., 1994 |
| Sour cherry | 71 | 3.1 | 37 | Pasteurized | 11 | Cemeroglu et al., 1994 |
| Agraz (Vaccinium meridionale sw) | 2.5 | 3 | 37 | Pasteurized at 85°C | 4.9 | Martínez Zambrano et al., 2011 |
| Agraz (Vaccinium meridionale sw.) | 19.5 | 2.6 | 37 | Pasteurized at 85°C | 21 | Martínez Zambrano et al., 2011 |
| Model blackcurrant juice | 11 | 3.4 | 40 | Fruits blanched in boiling water 2 min | 9.4 | Harbourne et al., 2008 |




