A fixed-spray system for Spotted Wing Drosophila management in high tunnel bramble crops
Abstract
BACKGROUND:
Spotted wing drosophila (SWD) is widely distributed in New York State, with raspberries being especially vulnerable. Its invasion has forced growers to dramatically increase insecticide applications, a significant challenge for high tunnel production.
OBJECTIVE:
A spray system fixed into the tunnel structure was used to apply pesticide sprays to control SWD in research and commercial bramble plantings in 2013 and 2014.
METHODS:
All sprays were applied through a system of microsprinkler nozzles attached to overhead polyethylene tubing supplied by a central pump. Identical applications were made in check tunnels using backpack sprayers. SWD traps were deployed near both treatments to check for adults, and weekly fruit samples were collected and held to rear out larvae infesting the berries.
RESULTS:
2013 results were variable depending on site, ranging from equal low infestations for fixed vs. backpack at the commercial raspberry site, to 4× higher infestations in the fixed-spray blackberry planting vs. the control; in 2014, infestations were 3–4× higher in the fixed-spray than backpack treatments, although infestation levels in both treatments were commercially acceptable. Fixed-sprayer systems may be particularly useful in high tunnels, owing to their greater practicality, efficiency and pesticide safety.
1Introduction
Spotted wing drosophila (SWD) Drosophila suzukii (Matsumura) represents a serious challenge for fruit growers in the Northeastern US and elsewhere. Unlike other fruit flies, SWD has the capacity to lay its eggs in ripening as well as ripe, marketable, soft-skinned fruit. Later maturing berries, such as blueberries, fall raspberries and day-neutral strawberries, appear to be especially vulnerable [1–3]. SWD was first observed in the Northeastern US in 2011, became widespread during the 2012 field season and decimated fall berry crops throughout the region. Over 50% of the blueberry and bramble growers that responded to an end of season survey of small fruit growers in the region conducted by Cornell University reported significant crop loss due to SWD [4].
High tunnels are increasingly being used for berry production in New York and other production regions. Work by Pritts and others [5] has been instrumental in the development and optimization of high tunnels for raspberry and blackberry production, showing that they perform particularly well under high tunnel conditions, with greater yields, an extended harvest season, and greatly improved fruit quality. SWD represents a major economic constraint to the adoption of this profitable production innovation.
Raspberries grown in high tunnels are particularly vulnerable to SWD. The invasion of SWD has forced raspberry growers to dramatically increase insecticide applications to produce marketable fruit, an especially significantlogistical challenge for high tunnel production. Pesticides are the only practical management tools currently available to growers. To achieve a reasonable level of control, they need to be applied frequently (5–7-day spray intervals) and over a long harvest period. These repeated insecticide applications are expensive (fuel and operator expenses plus the pesticides), time-consuming, hazardous, and sometimes not fully effective. Moreover, operating application equipment in the high tunnel environment can be very challenging. Previous work has been conducted in tree fruits using irrigation-type tubing fitted with greenhouse microsprinklers to deliver pesticide sprays directly to the crop canopy from a centralized pump [6]. The supply lines are fixed onto support wires within or above the canopy to optimize spray delivery and coverage.
A fixed system to apply insecticides could help mitigate a number of pest management problems in high tunnel production. Fixed-sprayer systems may be particularly cost-effective in high tunnels, as the framework to support the fixed lines is already present. Once constructed, a fixed-sprayer system should save time in the application of insecticides compared with using conventional application equipment (e.g., a backpack sprayer). Coverage, and therefore effectiveness, could also be improved with a fixed system. Here we report some initial work on the development and evaluation of a fixed-spray system to manage SWD in high tunnel brambles in New York State, USA. The specific objectives for this study were to:
1. Design and construct a fixed-spray system appropriate to different high tunnel bramble production operations, and use it to apply insecticide sprays for seasonal control of SWD.
2. Assess efficacy of a fixed-spray system in preventing fruit infestation by SWD.
3. Evaluate spray deposition and distribution in the canopy of bramble plantings using a fixed-spray application system.
2Methods
2.1Objective 1. Construction and operation of a fixed-spray system
In mid-July 2013, an arrangement of fixed tubing and nozzles for pesticide application was installed in each of three high tunnel systems currently under bramble production in New York State: a high tunnel raspberry research planting at the NYS Agricultural Experiment Station in Geneva, a blackberry research planting at the Cornell Horticulture Department high tunnels in Ithaca, and a commercial high tunnel raspberry operation at Stonewall Hill Farm in Stephentown, NY. In July 2014, the trial was repeated at just the commercial raspberry site in Stephentown, following a modification to the nozzle system, described below.
For the raspberry systems (Geneva and Stephentown), the main supply lines consisted of 2-cm polyethylene irrigation tubing strung above the planted rows, and affixed to the cross-struts of the high tunnel structure using cable ties, with 6-mm micro-tubing drop lines suspended down to the plant canopy every 1.5 m along each side of each row. Each drop line was fitted with a DAN 7000 series microsprinkler (Jain Irrigation USA, Fresno, CA) having an 8-mm orifice and a flat circular pattern spreader; each unit contained a 138-kPA check valve. The nozzles were oriented laterally facing toward the row center, producing a spray profile in the vertical plane and directed slightly into the canopy. In the blackberry high tunnel system (Ithaca), the structure was similar, but because of the higher canopy density of this crop, the drop lines were suspended every 0.75 m along the sides of the rows, and an additional center overhead supply line was used to contact the row middles from drop lines spaced every 1.5 m; nozzles on this line were oriented with the spray profile being horizontal over the canopy. In July 2014, a similar row-middle line of nozzles was added to the commercial raspberry planting in Stephentown, to improve spray coverage to the inside areas of the plant canopy. All supply lines were connected to a PVC manifold (mounted on a board near the tunnel entrance) fitted with an individual pressure gauge and ball valve for each line; the manifold in turn was connected to a portable wheeled greenhouse sprayer (Nifty Nursery-Cart model, Rears Manufacturing, Eugene, OR) with a 95-L tank and a 3 HP gasoline motor powering a diaphragm pump. Each tunnel consisted of three planted rows, ranging from 27–44 m in length and 10 m in width with 3 rows of plants per tunnel; only a single line was operated at a time in order to optimize spray pressure along the extent of the line.
To make an application, all lines were first filled by sequentially opening each valve to receive spray solution from the pump until the line pressure reached 138 kPA, just before the check valves opened. Then, one valve at a time was opened to increase the pressure to 207 kPa and spray the pesticide solution from one line, for a total application time of 30 seconds, to wet the canopy foliage adjacent to the line of nozzles. The next line’s valve was then opened as the first one was closed, and the process was continued similarly until all six lines were allowed to spray; the total application required an average of approximately 57 L for the area sprayed within the tunnel (ca. 0.03 ha). To recover pesticide solution remaining in the tubing after spraying was completed, a length of hose attached to a valve on the PVC manifold drained off most of the contents of the supply lines into a container; this was used to fill a backpack sprayer for treating check rows in an adjacent high tunnel planting at each planting site, which was not fitted with the fixed-spray system. In 2014, an air compressor was incorporated into the system at the Stephentown site, and was used to expel the residual spray solution from the lines and onto the plant canopies after the pump was switched off at the end of a spray application. This greatly simplified the handling process of the spray material, and made the application process much quicker, besides ensuring that the entire system was purged of all spray solution.
2.2Objective 2 – Assessment of insecticide treatment efficacy
The two principal products used for control of SWD were Delegate [spinetoram] (245–420 g/ha; labeled rate is 210–420 g/ha) and Assail [thiamethoxam] (350 g/ha; labeled rate is 315–371 g/ha), to each of which was added 240 g sugar/100 L as a feeding stimulant [7]. Sprays were applied weekly, and rotated on the following schedule in 2013: Delegate - 29 Jul, 19 and 26 Aug, 16 and 23 Sept; Assail - 5 and 12 Aug, 2 and 9 Sept. At Stephentown, additional sprays of Mustang Max [zeta-cypermethrin] were applied at 0.3 L/ha (labeled rate) on 25 September and 2 October. All applications were made at dusk to minimize exposure to foraging bees. In 2014, insecticide applications were made at the Stephenson commercial planting on the following schedule (at the same respective rates): Delegate, 26 July; 3 and 25 August; 1 and 23 September; 1 October; Assail, 11 and 17 August; 7 and 17 September. On each insecticide application date, the plantings in the adjacent check high tunnels received the same corresponding spray, applied using a backpack sprayer (Solo USA, Newport News, VA).
In 2013, to assess efficacy of the insecticide treatments in preventing SWD fruit infestation, samples of maturing fruit were taken at each site weekly, beginning the first week of August and continuing until 23 September (Ithaca blackberries), 7 October (Geneva raspberries), or 21 October (Stephentown raspberries). Samples were held at room temperature (22°C) in the lab to rear out any larvae in the fruit to the adult stage. Numbers of samples taken ranged from 8–13 per site, each consisting of 10–20 berries (∼50–100 g total), taken from both the fixed-spray planting and the check planting at each site. In 2014, fruit samples were taken weekly at the Stephentown site from 25 July to 8 October.
During the last week of July 2013, SWD adult traps were deployed adjacent to the high tunnel systems at each site to obtain an indication of local population pressure near each planting. Traps consisted of plastic 1-L deli cups containing a fermented yeast+flour mixture, with apple cider vinegar as a drowning medium [8, 9]. During 2014, traps were again set out at the Stephentown site, and were in place from mid-July until early October.
2.3Objective 3 - Evaluation of spray deposition
To measure spray deposition from the fixed-spray system in the fully developed canopy, on 25 September 2013 at the Geneva site, water-sensitive dye indicator cards (Syngenta, Basel, Switzerland) were stapled onto the leaves on the outside portion of the row as well as in the inside center of the canopy, both on the leaf tops and undersides, and on the left and right sides of candidate rows. Three replications of the evaluation were run, in which a total of 16 cards were placed in the inside center of the canopy, and 10 cards were placed on the outside of the row, one each on the top and underside of the leaf, on each side of the row. The system was run for 30 seconds with water only, and video imaging software was used to assess average card coverage [10]. On 24 July 2014, a similar spray deposition trial was conducted at the Stephentown site, with 10 cards placed in the center of each replicate, including some affixed to fruits, with 2 or 3 replicates per row. However, in this trial, the cards were sprayed either with the nozzles of the newly installed row-middle lines turned off, or with the center nozzles turned on, to assess the level of improved coverage provided by the center spray lines.
3Results
3.1Monitoring SWD adult activity
Numbers of SWD adults captured in the adult traps during 2013 were very low initially, and began to increase starting in mid-August, particularly at the Ithaca and Stephentown sites (Fig. 1A–C). In 2014, a similar peak in flight activity at Stephentown was seen at the end of September. At this farm, captures in traps placed inside the high tunnel both years showed that the tunnel structure was not able to effectively prevent SWD adults from entering(Fig. 1A, D), and although numbers were considerably lower than those caught outside of the tunnel, their presence inside was sufficient to pose a threat of fruit infestation. To protect the fruit from attack by undetected SWD females, preventive insecticide treatments were started in late July each year.
3.2Insecticide treatment efficacy
2013. At Stephentown, a commercial site where ripe fruit was picked nearly daily, there were generally low total numbers of flies reared from the fruit, with no major difference between the fixed-spray and backpack sprayer treatments (Table 1). A time course comparison of SWD adults obtained from the individual berry samples shows the highest number of flies obtained from the 2 and 10 Sep samples (Fig. 2A), with few or no flies found on the remaining sample dates. Although these dates did not correspond with any peak in adult captures in the traps placed inside the Stephentown tunnel, there was an increase in the number of SWD adults captured outside the tunnel during this period (Fig. 1A). The peak trap capture of flies at this site occurred on 25 Sep, which was not reflected in berry sample infestations.
In the Geneva and Ithaca high tunnel systems, approximately 2.5 times as many flies were obtained from berries in the fixed-spray treatments as from the check plantings. The greatest numbers of adults were obtained from samples collected during the period from mid-August to mid-September (Fig. 2B, C), which did correspond to the period of increasing SWD flies in the traps at these sites. In Ithaca and Geneva, which were both research sites, the ripening fruit was not harvested as frequently, and the Ithaca blackberry planting was furthermore much more vigorous than the raspberry plantings, which likely resulted in spray coverage not being as thorough. The number of berry samples from which no SWD adults emerged was uniformly low in both treatments at the Geneva and Ithaca sites (ranging from 0–2), and in contrast, high (8–10) for the two treatments at the Stephentown site (Table 1).
2014. Total numbers of SWD adults obtained from berry samples in the fixed-spray treatment at Stephentown were generally 3–4 times higher than those found in the backpack-treated berries (Table 1), despite the addition of row center nozzles to the fixed-spray installation in that tunnel. Nearly all of these adults were obtained from samples taken between late August and late September (Fig. 2D), which once again corresponded with the period of SWD adult captures in traps, particularly outside the high tunnel (Fig. 1D). Additionally, approximately 67% of the infested fruits were taken from a region in one of the rows under 2-3 nozzles (a 4–5 m-long section) that experienced clogging problems associated with the addition of sugar to the sprays. This will be addressed in future operations by installing valves at the ends of the supply lines that will allow them to be flushed with water between spray applications. The number of berry samples yielding no SWD adults from the fixed-spray treatment was roughly half that seen from the backpack treatment (Table 1). Despite these numerical differences, overall levels of infestation using both the fixed-spray system and the backpack application method were relatively low and commercially acceptable, according to the grower cooperator.
3.3Assessment of spray deposition
Measurements of the spray deposition in 2013 showed that spray coverage was highly variable, but predictably best on the outside of the canopy, and markedly better on the tops of the leaves (40–100% coverage, above the average seen in orchard field trials) than on the undersides (1–26%) (Table 2). Cards in the inside center of the canopy were less well covered (16–67% on leaf tops, 1–8% on undersides). Results in the 2014 evaluation showed that the center line nozzles provided a great improvement in coverage to the row middles (Table 2), and good coverage on cards attached to individual fruits.
4Conclusions
These initial trials demonstrated the potential utility and effectiveness of a fixed-spray system in efforts to control SWD in high tunnel bramble plantings. Although efficacy and coverage of the pesticide sprays using this type of system was not always equivalent to that achieved from a backpack sprayer, the overall results of these trials show that this approach to applying sprays has promise for benefitting berry growers in need of a solution to the SWD challenge. As seen at the commercial Stephenson trial site, where ripe fruit was picked every day, the successful management of this pest also depends on frequent picking of mature fruits to eliminate potential infestation sites for incoming flies to attack. In contrast, at the research sites, where ripe fruit was infrequently removed, overall levels of infestation were much higher than at the commercial site, regardless of application method, but particularly so for the fixed-spray system. In addition, the very dense canopy present in the blackberry high tunnel most likely exacerbated the challenge of getting adequate coverage using the fixed system. It was here where we found the highest levels of infestation. Trap captures of SWD adults verified the presence of significant population pressure at all of the trial sites, although as frequently seen in other crop locations, trap numbers and timings did not always correspond closely with fruit infestation incidence and patterns [9].
We feel that the basic design of the fixed-spray systems as currently configured could benefit from further modifications, and the efficacy of their use could be more thoroughly documented by more detailed evaluation procedures. Some potential new areas of investigation going forward include shortening the spray duration time, as the system may be running too long and in effect washing off the active ingredient; changes in rates of water and insecticide may affect coverage and efficacy. It would also be helpful to assess spray coverage on the fruit by using a fluorescent tracer dye. Quantifying pesticide residue levels on the fruit, or conducting bioassays using lab-reared flies to see how efficacy changes over time, would give a clearer indication of direct spray efficacy. Pesticide handling and conservation could be improved through the use of a dosing pump for direct pesticide injection, rather than mixing pesticide solutions in the tank. Finally, there may be cultural practices that increase coverage on the plant surfaces, such as positioning of canes, or cane pruning.
The costs of constructing and operating a fixed-spray system, while not exorbitant, do represent an investment that should be assessed on the basis of time and labor saved, as well as materials needing to be purchased. For a high tunnel such as at the Stephenson site (44 m in length), the system’s tubing, microsprinklers, gauges, valves and fittings totaled approximately US $1200. Added to this would be the cost of the motorized pump sprayer, currently $3500. This would bring total equipment and materials cost to approximately $4700, which would need to be amortized over the lifetime of the system. Although we do not have actual data on the durability of such a system in a high tunnel, previous work [6] using a comparable structure installed in an apple planting for 3 years, during which time it was exposed to the elements year-round, showed no need for major repair or replacement, making this a reasonable conservative estimate of its longevity. In comparison, a hand-pumped backpack sprayer, such as was used in these trials, can be purchased for approximately $150 (or about $600 for a more efficient motorized mist blower backpack unit).
Time and convenience factors also need to be considered in contrasting the two application methods. For the fixed-spray system, each application including mixing and clean-up required 30 min, compared with 90 min for the multiple spray loads required to adequately cover all the rows in the tunnel using a backpack sprayer. Applicator and pollinator safety is an integral component of any comparison of the two treatment technologies. To minimize applicator exposure to the treated plants, the backpack sprays were applied while walking backwards along the rows, which added to the inconvenience of the operation. In contrast, the fixed-spray system could be operated without the need for the applicator to travel through the planting while the sprays were being applied. Also, because less light is required for the system to be operated, the grower was able to wait until dusk to spray, giving honey bees and bumble bees inside the tunnel during the day adequate time to return to their hives for the evening. Backpack spraying needed to be started earlier in the afternoon to ensure adequate visibility, which meant greater potential pesticide exposure for the pollinators.
In summary, we believe that the availability of a fixed-spray system could make growing high tunnel raspberries more feasible in the current production environment where SWD is present. Fixed-spray systems may also prove practical for smaller field plantings of high-value blueberries and raspberries. Importantly, the adoption of fixed-spray systems for berry crops would reduce the time investment needed for control sprays and grower exposure to the insecticides.
Acknowledgments
We gratefully acknowledge the efforts of our collaborating trial site personnel, Dale Ila Riggs, Laura McDermott, Marvin Pritts, Rich Raba, and Courtney Weber; and our technical and engineering assistance: Bill Larzelere, Steve Hesler, Jordi Llorens, Changyuan Zhai, Johanna Elsensohn, Tessa Lessord, Chrissy Dodge, Gabrielle Brind-Amour, McKenzie Schessl, and Allison Wentworth. This work was funded by the support of the New York Farm Viability Institute.
REFERENCES
1 | Lee JC, Bruck DJ, Curry H, Edwards D, Haviland DR, Van Steenwyk RA, Yorgey BM(2011) The susceptibility of small fruits and cherries to the spotted-wing drosophilaDrosophila suzukii. Pest Manag Sci67: 13581367 |
2 | Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton O’Neal SD, Zalom FD(2011) Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its range and damage potentialJ Integ Pest Manag2: G1G7 |
3 | Burrack HJ, Fernandez GE, Spivey T, Kraus DA(2013) Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivorePest Manag Sci69: 11731180 |
4 | http://www.northeastipm.org/neipm/assets/File/SWDWG-2012-Damage-Survey.pdf |
5 | Heidenreich C, Pritts M, Demchak K, Hanson E, Weber C, Kelly M. High tunnel raspberries and blackberries. Dept. Horticulture, Cornell Univ., Ithaca; 2012. Available from: http://www.hort.vt.edu/ghvegetables/documents/High%20Tunnel%20Construction%20and%20Crop%20Production/Cornell%20High%20Tunnel%20Raspberries%20and%20Blackberries%202012.pdf |
6 | Agnello A, Landers A(2006) Current progress in development of a fixed-spray pesticide application system for high-density apple plantingsNY Fruit Quarterly14: 42226 |
7 | Cowles RS, Rodriguez-Saona C, Holdcraft R, Loeb GM, Elsensohn JE, Hesler SP(2015) Sucrose improves insecticide activity against Drosophila suzukii (Diptera: Drosophilidae)J Econ Entomol108: 2640653 |
8 | Iglesias LE, Nyoike TW, Liburd OE(2014) Effect of trap design, bait type, and captures on Drosophila suzukii (Diptera: Drosophilidae) in berry cropsJ Econ Entomol107: 15081518 |
9 | Burrack HJ, Asplen M, Bahder L, Collins J, Drummond FA, Guedot C, Isaacs R, Johnson D, Banton A, Lee JC, Loeb G, Rodriguez-Saona C, Van Timmeren S, Walsh D, McPhie DR(2015) Multi-state comparison of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in blueberries and caneberriesEnviron Entomol10.1093/ee/nvv022 |
10 | Novartis. Water-sensitive paper for monitoring spray distribution. 5th ed. Basel, Switzerland: Novartis; 2000 |
Figures and Tables
Fig.1
Spotted Wing Drosophila adults caught in traps in commercial and research high tunnel sites, NY 2013-2014.
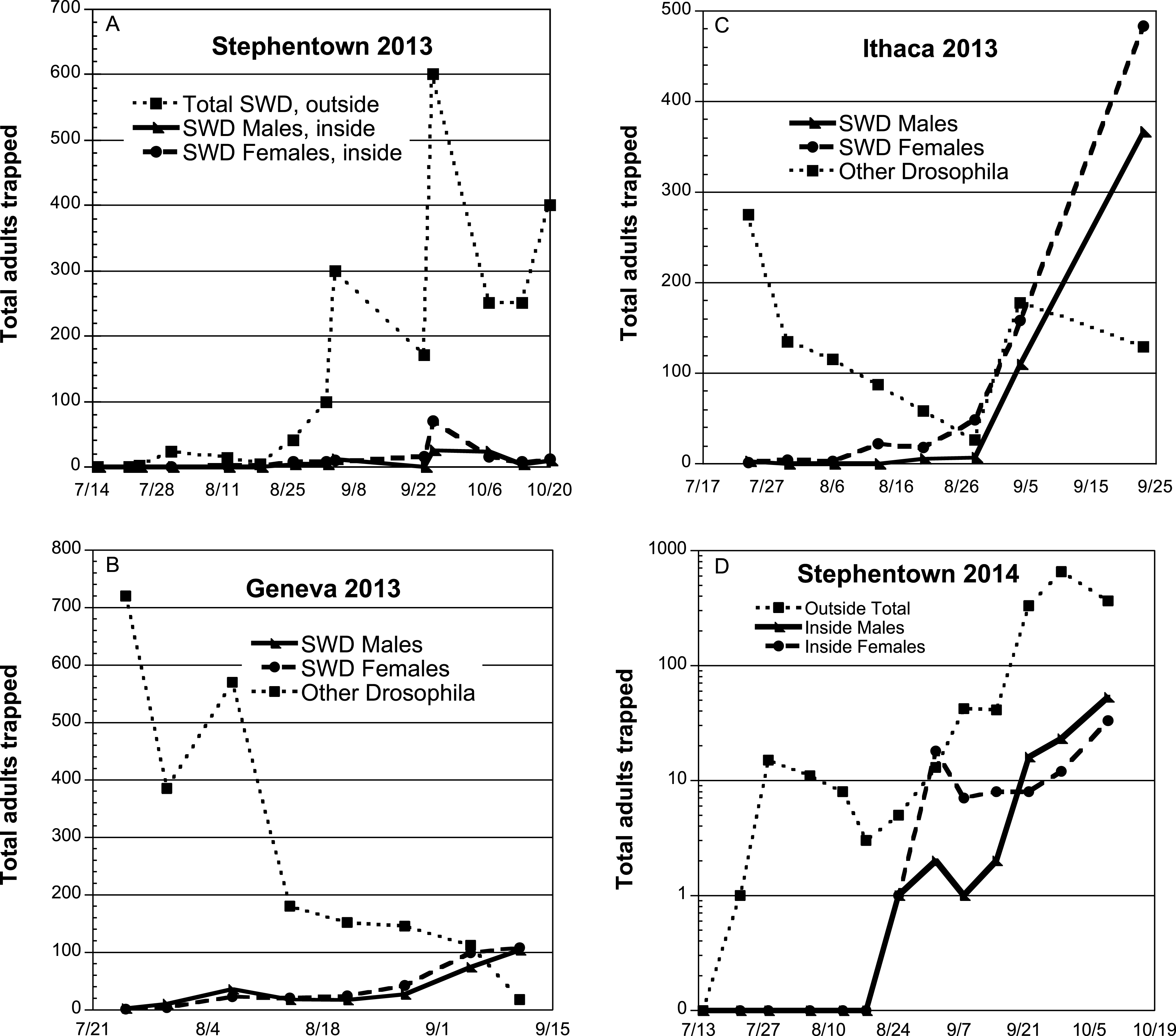
Fig.2
Time course of mean numbers (±SEM) of Spotted Wing Drosophila adults emerging from individual samples of berries held under laboratory conditions to assess infestation levels after treatment using either fixed-spray or backpack pesticide applications in commercial and research high tunnel sites, NY 2013-2014.
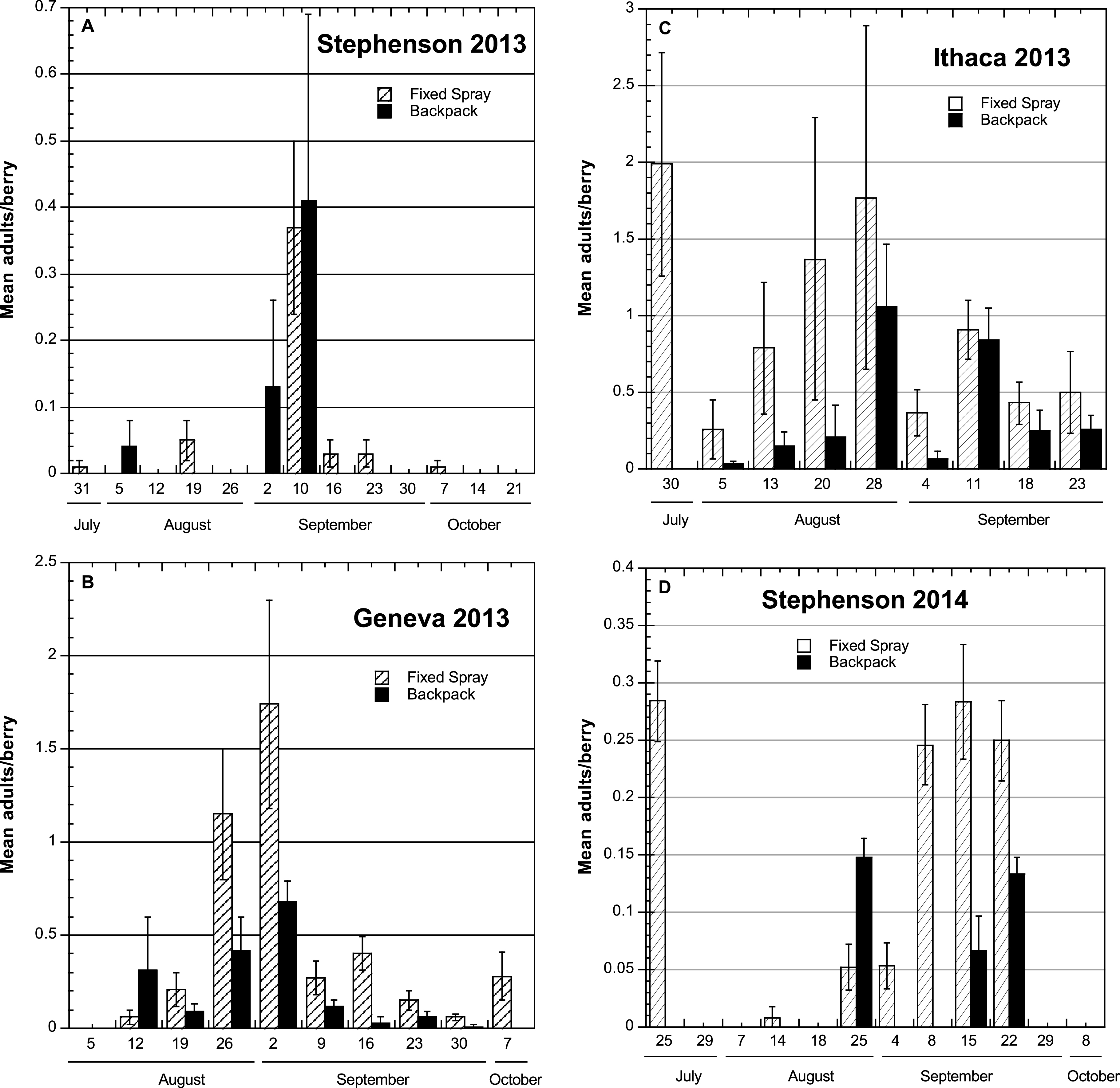
Table 1
Mean (SEM) emerged spotted wing Drosophila adults from maturing berries sprayed Using either fixed-spray system or backpack sprayer. New York, 2013-14
| Site | Crop | Application method | n Samples | Total SWD/g fruit | Total SWD/ berry | # samples with no SWD |
| 2013 | ||||||
| Geneva | raspberries | fixed-spray | 10 | 1.63 (0.05) | 4.31 (0.14) | 1 |
| backpack | 10 | 0.62 (0.03) | 1.72 (0.07) | 2 | ||
| Ithaca | blackberries | fixed-spray | 9 | 1.45 (0.08) | 8.39 (0.46) | 0 |
| backpack | 8 | 0.62 (0.04) | 2.88 (0.19) | 0 | ||
| Stephentown | raspberries | fixed-spray | 13 | 0.24 (0.01) | 0.50 (0.02) | 8 |
| backpack | 13 | 0.26 (0.02) | 0.58 (0.04) | 10 | ||
| 2014 | ||||||
| Stephentown | raspberries | fixed-spray | 12 | 0.51 (0.05) | 1.18 (0.12) | 5 |
| backpack | 12 | 0.13 (0.02) | 0.35 (0.05) | 9 |
Table 2
Spray deposition on water-sensitive cards in high tunnel fixed-spray systems, New York 2013-14
| Location | Avg. % card coverage | ||
| 2013, Geneva | Rep 1 | Rep 2 | Rep 3 |
| Left - canopy outside, top of leaf | 37.5 | 93.1 | 86.3 |
| underside of leaf | 0.9 | 9.2 | 5.2 |
| Left - canopy inside, top of leaf | 40.0 | 33.9 | 47.0 |
| underside of leaf | 0.9 | 0.5 | 0.8 |
| Right - canopy outside, top of leaf | 69.1 | 100.0 | 65.0 |
| underside of leaf | 9.7 | 26.4 | 9.1 |
| Right - canopy inside, top of leaf | 15.8 | 26.0 | 67.0 |
| underside of leaf | 7.7 | 1.7 | 2.0 |
| 2014, Stephentown | Rep 1 | Rep 2 | Rep 3 |
| Middle nozzles off | |||
| Upper canopy outside | 4.7 | 77.7 | |
| Lower canopy outside | 11.7 | 77.5 | |
| Center of canopy, top | 5.1 | 4.4 | |
| Middle nozzles on | |||
| Upper canopy outside | 100.0 | 22.9 | 5.4 |
| Lower canopy outside | – | 15.5 | 11.4 |
| Center of canopy, top | 50.2 | 95.6 | 10.0 |
| Fruit | 84.2 | 80.6 | 45.0 |




