A Framework for Best Practices and Readiness in the Advent of Anti-Amyloid Therapy for Early Alzheimer’s Disease in Asia
Abstract
Advances in biomarker-based diagnostic modalities, recent approval of anti-amyloid monoclonal antibodies for early Alzheimer’s disease (AD; mild cognitive impairment or mild dementia due to AD) and late-stage clinical development of other disease-modifying therapies for AD necessitate a significant paradigm shift in the early detection, diagnosis and management of AD. Anti-amyloid monoclonal antibodies target the underlying pathophysiological mechanisms of AD and have demonstrated a significant reduction in the rate of clinical decline in cognitive and functional outcome measures in patients with early AD. With growing recognition of the benefit of early interventions in AD, an increasing number of people may seek diagnosis for their subjective cognitive problems in an already busy medical system. Various factors such as limited examination time, lack of expertise for cognitive assessment and limited access to specialized tests can impact diagnostic accuracy and timely detection of AD. To overcome these challenges, a new model of care will be required. In this paper, we provide practical guidance for institutional readiness for anti-amyloid therapies for early AD in Asia, in terms of best practices for identifying eligible patients and diagnosing them appropriately, safe administration of anti-amyloid monoclonal antibodies and monitoring of treatment, managing potential adverse events such as infusion reactions and amyloid-related imaging abnormalities, and cross-disciplinary collaboration. Education and training will be the cornerstone for the establishment of new pathways of care for the identification of patients with early AD and delivery of anti-amyloid therapies in a safe and efficient manner to eligible patients.
INTRODUCTION
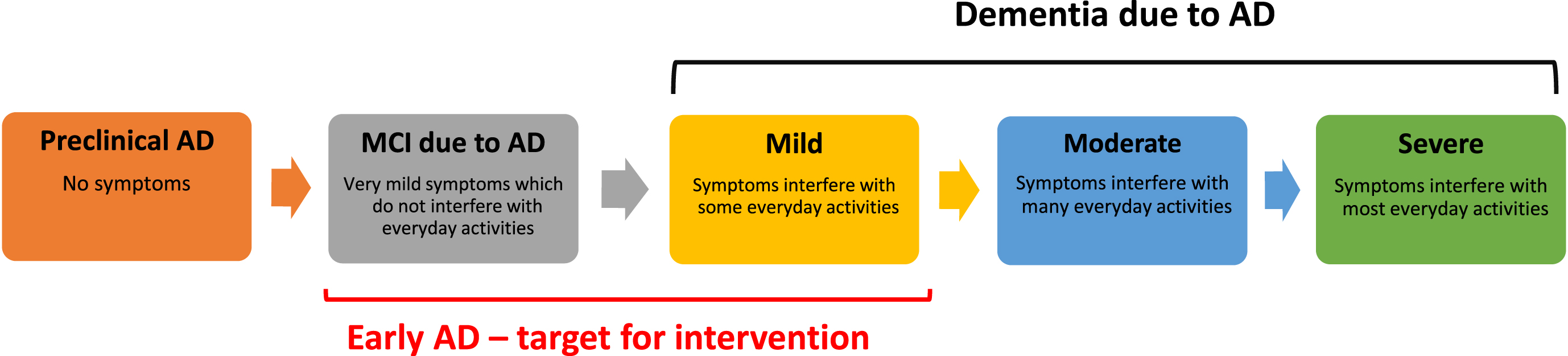
Population ageing and increasing life expectancy have contributed to an increasing prevalence of age-related illnesses worldwide, including dementia related to Alzheimer’s disease (AD) and cerebrovascular disease.1,2 The global prevalence of dementia is projected to reach 135 million by 2050— over 50% of these people are expected to be from the Asia-Pacific region, where the number of people living with dementia will increase from 23 million in 2015 to almost 71 million in 2050.3 AD, the most common cause of dementia accounting for 50–75% of all cases,4–6 has a physical, mental, social, and economic impact on people living with dementia as well as their families and caregivers, in addition to exerting a significant burden on the healthcare systems.6–8 The socioeconomic burden of AD, a chronic progressive neurodegenerative condition, will worsen over the forthcoming years due to population ageing and increasing life expectancy. More recently, there is growing recognition of the benefit of providing early treatment to patients with mild cognitive impairment (MCI; the early symptomatic stage of AD) or mild dementia due to AD (referred to together as early AD hereafter; Fig. 1), allowing function at a higher level for longer.
Fig. 1
Early intervention in the AD continuum. AD, Alzheimer’s disease; MCI, mild cognitive impairment.
Amyloid-β (Aβ) plaques comprising extracellular accumulation of beta amyloid peptide and neurofibrillary tangles formed by intracellular accumulation of hyperphosphorylated tau protein are the hallmarks of AD histopathology.9,10 Consequently, research has focused on therapies that can prevent Aβ formation, lower Aβ levels in the brain, block its aggregation into plaques, take apart existing Aβ plaques, and/or clear the amyloid, thereby potentially slowing the progression of AD.9,10 Indeed, studies of anti-amyloid monoclonal antibodies have demonstrated a significant reduction in the rate of clinical decline in early AD in cognitive and functional outcome measures, while also showing an impact on the pathophysiological process in AD as evidenced by changes in the biomarkers of antibody targets such as amyloid positron emission tomography (PET) and plasma/cerebrospinal fluid (CSF) phosphorylated-tau (p-tau) and total tau.11,12 Historically, therapies for AD have predominantly targeted symptoms, but novel anti-amyloid monoclonal antibodies, such as lecanemab (United States Food and Drug Administration [FDA], Japan Pharmaceuticals and Medical Devices Agency [PMDA], and China National Medical Products Administration [NMPA] approved) and donanemab (in clinical development), offer the potential of slowing down disease progression by targeting the underlying pathophysiological mechanisms of AD.13–16
Given the increasing prevalence of AD and the growing burden on healthcare systems, the advent of anti-amyloid therapies for early AD, advances in biomarker-based detection modalities and late-stage clinical development of other disease-modifying therapies (DMTs) for AD, new models of care and clinical pathways need to be established to identify patients with early AD and deliver anti-amyloid (and other disease-modifying) therapies in a safe and efficient manner to eligible patients. As anti-amyloid agents for early AD receive approval and become available in Asia, healthcare systems need the capacity to detect and diagnose early AD effectively, facilitating timely clinical use of these DMTs. Furthermore, as an increasing number of people seek diagnosis for their subjective cognitive problems, factors such as an already busy medical system, limited examination time for cognitive assessment, limited access to specialized tests, physicians who are not well-informed about dementia disorders and delayed referrals can impact diagnostic accuracy and timely detection of the disease.
With a view of providing practical guidance on anti-amyloid therapies for early AD, this article presents a framework for best practices and readiness to prepare for the administration of anti-amyloid therapies for early AD in Asia, identifies challenges to introducing these new therapies, and highlights future directions to support optimal use of these new therapies. Given the diverse healthcare infrastructures and resource availabilities in the different countries in Asia, the new models and pathways to enable timely and effective identification, diagnosis and treatment of early AD may differ by countries, hence the framework should be adapted to suit localsettings.
METHODS
The framework and recommendations were developed by experts in the related field (neurologists [dementia specialists] and neuroradiologist) across Asia (China, Hong Kong, Taiwan, Thailand, Singapore, and Korea) who have much experience in managing patients with MCI and dementia in their practice. Following review of the literature and available prescribing information (PI) for approved anti-amyloid agents for early AD, the experts met virtually in August 2023 and discussed approaches to early AD patient identification, treatment delivery and monitoring of patients, and potential challenges associated with use of anti-amyloid agents for early AD in Asia. Based on their clinical experience in Asia and the available literature, the experts subsequently drafted the framework and recommendations for best practices and readiness for anti-amyloid therapies for early AD in the Asian context, which were refined through multiple discussions.
RESULTS
Our framework for best practices and readiness for anti-amyloid therapies for early AD in Asia is based on 5 pillars: clinical evaluation and diagnostic modalities; infusion protocols and infrastructure; monitoring, identification and management of amyloid-related imaging abnormalities (ARIA) and other adverse events (AEs) associated with anti-amyloid therapies; cross disciplinary collaboration; and education (Figs. 2 and 3). In the context of the Asian region, we outline the best practices, in terms of what is needed to deliver anti-amyloid therapies efficiently and safely to eligible patients with early AD, potential challenges to implementing best practices, and recommendations to help achieve readiness with best practices.
Fig. 2
Pillars of the framework for best practices and readiness for anti-amyloid therapies for early AD in Asia. AD, Alzheimer’s disease; AE, adverse event; ARIA, amyloid-related imaging abnormalities.
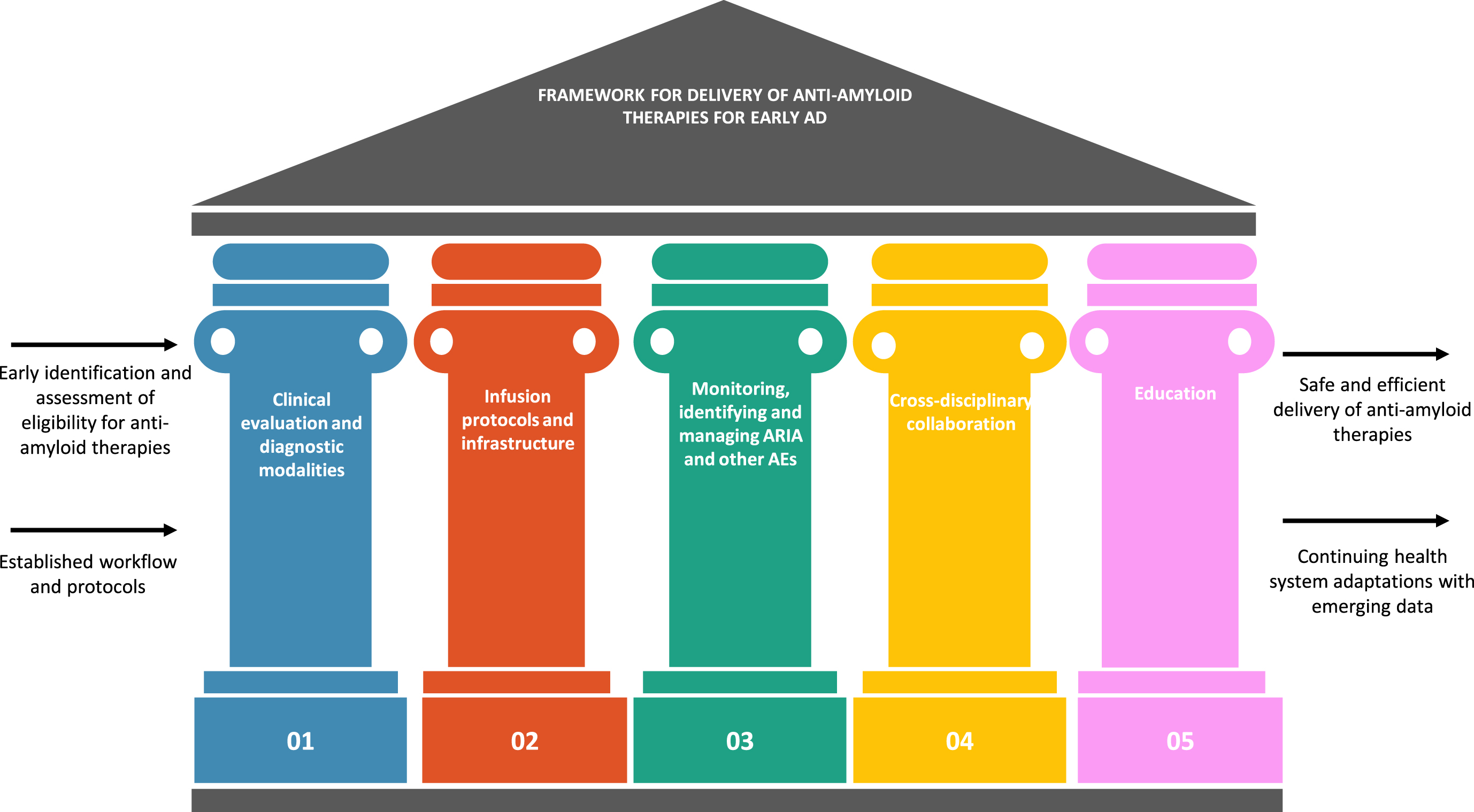
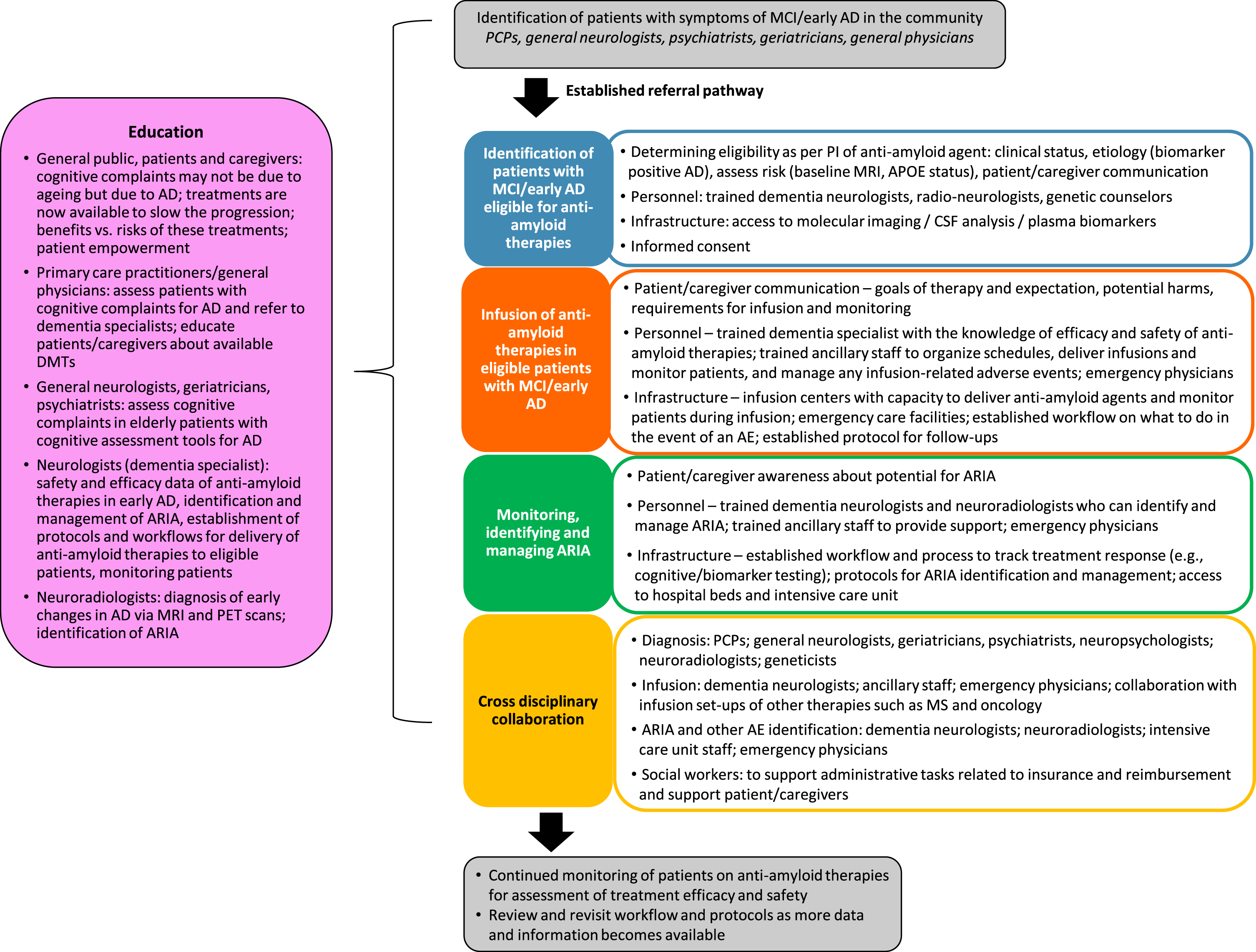
Fig. 3
Best practices workflow for the delivery of anti-amyloid therapies for patients with early AD. AD, Alzheimer’s disease; AE, adverse event; ARIA, amyloid-related imaging abnormalities; CSF, cerebrospinal fluid; DMT, disease modifying therapy; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; MS, multiple sclerosis; PCP, primary care physician; PET, positron emission tomography; PI, prescribing information
Early identification of patients with early AD
Anti-amyloid therapies have been shown to slow functional and cognitive decline in patients with early AD and can hence be beneficial in this population.11,12 However, the diagnosis of early AD can be challenging due to a pervasive belief among healthcare providers and patients/caregivers that cognitive changes experienced by the elderly are related to the normal ageing process, and very little can be done to alleviate these changes.17–23 There is also an element of stigma associated with the term ‘dementia’ which precludes prompt consultation with healthcare providers.18,20,24,25
Furthermore, primary care physicians are usually the first point of contact for the elderly with symptoms suggestive of AD, such as memory complaints, in chronic care settings such as hypertension or diabetes clinics; they are often not aware of early symptoms of AD and/or are not equipped to assess the patients for their cognitive impairment.21–23 In addition to time constraints, primary care physicians may also lack the knowledge and awareness of screening tools and the interpretation of results, and the potential next steps.21–23 Nevertheless, primary care can play a vital role in the early detection and management of AD, particularly given the rapidly ageing populations and a shortage of dementia specialists.26,27 Education and training of primary care physicians on how to assess cognitive concerns in their elderly patients, how to communicate with patients and manage expectations, and when and who to refer patients to will go a long way in empowering them to take a more active role in the process. Other stakeholders such as geriatricians, general neurologists and psychiatrists can also be enlisted and trained to contribute to the early identification of patients with AD. Additionally, standardized workflows and checklists will aid these healthcare providers in patient identification and referral by promoting efficiency in the referral process.
Family members are usually the first to notice symptoms of early AD and their participation in the diagnosis and management journey is crucial but is often overlooked. The general public should be educated on the importance and benefit of bringing cognitive complaints in their elderly family members to the attention of their primary care physician at the earliest opportunity. Increasing awareness of the availability of potential therapies to slow cognitive decline when initiated early in the disease continuum will improve attitudes towards an AD diagnosis and will foster confidence in seeking treatment. Early detection of AD could empower patients and their caregivers to proactively make decisions about future treatment and prepare for the cognitive and behavioral changes associated with disease.
Clinical evaluation and diagnostic modalities
Diagnostic requirements for anti-amyloid therapies depend on the identification of patients who may be eligible to receive these therapies. Patients should be selected for newly introduced anti-amyloid therapies strictly based on the approved label of the therapies.28 The US FDA, Japan PMDA, and China NMPA have approved lecanemab for patients with MCI or mild dementia due to AD,13–15 but the prescribing information may differ from country to country, and these should be used to determine patient eligibility.
Patient selection should begin with a diagnosis of MCI or mild dementia due to AD, with AD etiology confirmed based on biomarker positivity and cognitive testing to determine cognitive impairment severity. The diagnosis of MCI and mild dementia requires extensive neuropsychological and functional assessment which may not be possible in resource restricted Asian countries. While the Mini-Mental Status Examination is widely used in the clinical trials of anti-amyloid therapies to establish the severity of cognitive impairment,12,16 this may not be routinely used in primary clinical practice in Asia. Cognitive assessment tools that are familiar and culturally appropriate in respective Asian countries, with population-appropriate cutoff points, may need to be used instead. One possibility is the Quick Dementia Rating Scale which is a brief questionnaire which has been validated in Asian countries.29–31 Other tools that are commonly used in Asia include the Montreal Cognitive Assessment,32,33 the Korean Dementia Screening Questionnaire,34,35 and the Visual Cognitive Assessment Test.36
Evidence of brain amyloidosis, determined by elevated amyloid uptake on PET or increased Aβ42, Aβ42/Aβ40, and p-tau/Aβ42 in the CSF, is required for the initiation of anti-amyloid therapies.12,16 The measurement of CSF biomarkers may be more accessible compared with amyloid PET but needs facilities and expertise for a lumbar puncture and sample interpretation.37 Furthermore, a baseline magnetic resonance imaging (MRI) scan to exclude dementia disorders other than AD and to evaluate the extent of cerebrovascular involvement and identify other abnormalities which may be associated with a higher risk of ARIA, such as lobar microbleeds, is also needed. Consequently, MRI and PET/CSF biomarker facilities are an absolute requirement along with trained dementia neurologists, neuroradiologists and nuclear medicine specialists who are experienced in assessing MRI and PET scans.
Although apolipoprotein E ɛ4 (APOE4) is the strongest risk factor for non-familial AD, APOE testing has not been recommended in the past for the management of patients with AD because of a limited clinical utility. However, phase III studies of anti-amyloid monoclonal antibodies demonstrated an increased incidence of ARIA among APOE4 carriers, with much larger effects observed among ɛ4 homozygotes compared to heterozygotes.11,12 Since APOE4 genotypes are associated with an increased risk of ARIA,38,39 APOE4 genotyping is highly recommended to inform patient decisions regarding anti-amyloid therapy. The practice for APOE4 testing and communication of results to patients and their family varies across Asia, but genetic counselling regarding the implications of APOE testing is important and may require the involvement of genetic counselors. As such, we suggest that APOE4 genetic testing be offered to patients in whom biomarker tests support anti-amyloid treatment and when patients are interested in pursuing these treatments. Patient consent prior to the test and education and counseling will be essential, as is the involvement of caregivers and family members. The results of the APOE4 genetic test will have implications for the family of the patient undergoing the test— the biological children of ɛ4 homozygotes will definitely be ɛ4 carriers and this information can impact their mental well-being as well as future life choice and planning.39
Open communication with patients and caregivers about the implications of the AD diagnosis and the APOE4 status, and the expectation from therapy is essential. Dementia specialists need to involve patients and their caregivers at every step of the care/treatment journey for the alignment of expectations, benefits versus risks and goals of therapy. It is particularly important that patients and caregivers understand that anti-amyloid therapies will not cure AD but will potentially slow disease progression, and they may be associated with side effects.
Countries in Asia have diverse healthcare infrastructures and it should be acknowledged that cost constraints could preclude PET/MRI scans, CSF biomarker analysis, and APOE4 testing. Blood-based biomarkers are being researched and may become available as screening as well as diagnostic tools in the future.40 However, for eligible patients to receive anti-amyloid therapies in a safe and efficacious manner, it is important that dementia specialists undergo comprehensive education about the available data for these therapies so that they can perform all mandatory clinical and diagnostic evaluations to identify appropriate patients.
Infusion protocols and infrastructure
Currently available anti-amyloid therapies are administered by intravenous infusion every 2-4 weeks, with each infusion requiring at least 1 h.28 Following the determination of eligibility, patients will need to undergo recurrent infusions of anti-amyloid therapy. Logistically, this will require infusion centers with the capacity to provide these infusions and administrative staff to schedule the infusions and follow-up with the patients. With sustained efforts to identify patients with AD early in the continuum, an increase in the number of patients who might be eligible for anti-amyloid therapies could require the establishment of new infusion centers specifically for anti-amyloid therapies and/or increasing the capacity of existing infusion centers for other therapies, such as those for multiple sclerosis, rheumatology, and oncology. Opportunities for cross disciplinary collaboration and other innovative private-public models of collaboration should be explored to build capacity.
Infusion-related reactions and ARIAs are the most common side effects of anti-amyloid therapies that infusion centers must be prepared to deal with appropriately and expeditiously. In clinical trials, infusion reactions including fever, chills, headache, rash, nausea, vomiting, abdominal discomfort and elevated blood pressure, were usually mild-to-moderate in severity, and typically resolved within 24 h following the infusion.12,28 As such, patients should be carefully monitored during each infusion and followed-up periodically thereafter. Care pathways and protocols with clearly assigned responsibilities for the management of infusion reactions must be established and communicated to all infusion center staff who should be trained to identify and address infusion reactions. Infusion centers should also be prepared for unforeseen severe reactions, such as anaphylaxis— immediate access to emergency physicians and critical care facilities is essential.
Patients and caregivers must be educated about the possibility of infusion reactions so that they can alert the infusion center staff in a timely manner. They should be provided with a clear explanation of the administration of infusions and the frequency, and the contact information of nurses and treating/infusing physicians with instructions to report in case of an unexplained symptom.
Monitoring, identifying, and managing ARIA
Imaging abnormalities related to amyloid, referred to as ARIA, have been observed in clinical trials of anti-amyloid therapies in AD.11,12,28 ARIA can be associated with edema (ARIA-E) or with hemorrhagic changes (ARIA-H) and can co-occur.41 The incidence of ARIA observed in clinical trials is variable with the different anti-amyloid monoclonal antibodies, with the incidence of ARIA-E ranging from 12.6% – 36.8% (symptomatic ARIA-E: 2.8% – 6.1%) and the incidence of ARIA-H ranging from 17.3% – 31.4%.11,12 Although ARIA is not limited to people who received anti-amyloid therapies, its occurrence in this population highlights the need for risk assessment, monitoring, and response strategies. Increased awareness of ARIA is needed to ensure that appropriate monitoring and management protocols are in place for the current and future use of amyloid-targeting treatments in clinical practice.
Common symptoms of ARIA observed in studies of anti-amyloid therapies in early AD included dizziness, headache, visual disturbances and increased mental confusion; however, it must be noted that most cases of ARIAs in these studies were asymptomatic.12,28,42 As such, in addition to the baseline MRI, safety MRI scans before specific infusions as outlined in the prescribing information of approved agents are recommended to minimize the likelihood of worsening or recurrence of ARIA. In patients at higher risk of ARIA, such as APOE4 homozygotes and those who had ARIA on previous MRI scans, additional scans are recommended.28,43
Care pathways for the management of symptomatic and asymptomatic ARIA should be developed, clearly outlining when the anti-amyloid therapies should be discontinued and any additional treatment strategies that need to be employed. These pathways can be based on the protocols included in the prescribing information of the therapies, but it is important for these pathways to be flexible to evolve as more information becomes available. These care pathways should clearly assign roles and responsibilities to follow when ARIA is detected and all members of the care team must be trained adequately to monitor patients, identify ARIAs and manage these events appropriately. Access to other hospital resources, such as emergency rooms, intensive care units, beds in hospital wards for admission, laboratory tests, medications (such as anti-seizure drugs) and inpatient MRI and electroencephalography, must be ensured. Cross-disciplinary training and collaboration can ensure optimal utilization of hospital resources that could be required for monitoring and managing ARIA.
Since the findings of ARIA may be subtle and the treatment pathways will be based on the grade of ARIA, neuro-radiology expertise will be critical. Neuroradiologists should be trained to detect ARIA on MRI scans; radiologists who are not specifically trained to detect ARIA may miss subtle lesions. Above all, dementia specialists should be primarily responsible for administering anti-amyloid therapies and monitoring treatment response and safety, and they should be well-versed with the efficacy and safety profiles of anti-amyloid therapies. The number of dementia specialists, particularly those who are aware of anti-amyloid therapies and willing to administer them, may be low compared to the number of patients who might be eligible for anti-amyloid therapies. Concerted efforts are needed to increase the knowledge and training of dementia specialists for administering anti-amyloid therapies safely and effectively. At the same time, general neurologists, geriatricians, and psychiatrists can be trained to perform these clinical and diagnostic assessments to reduce the burden on dementia specialists.
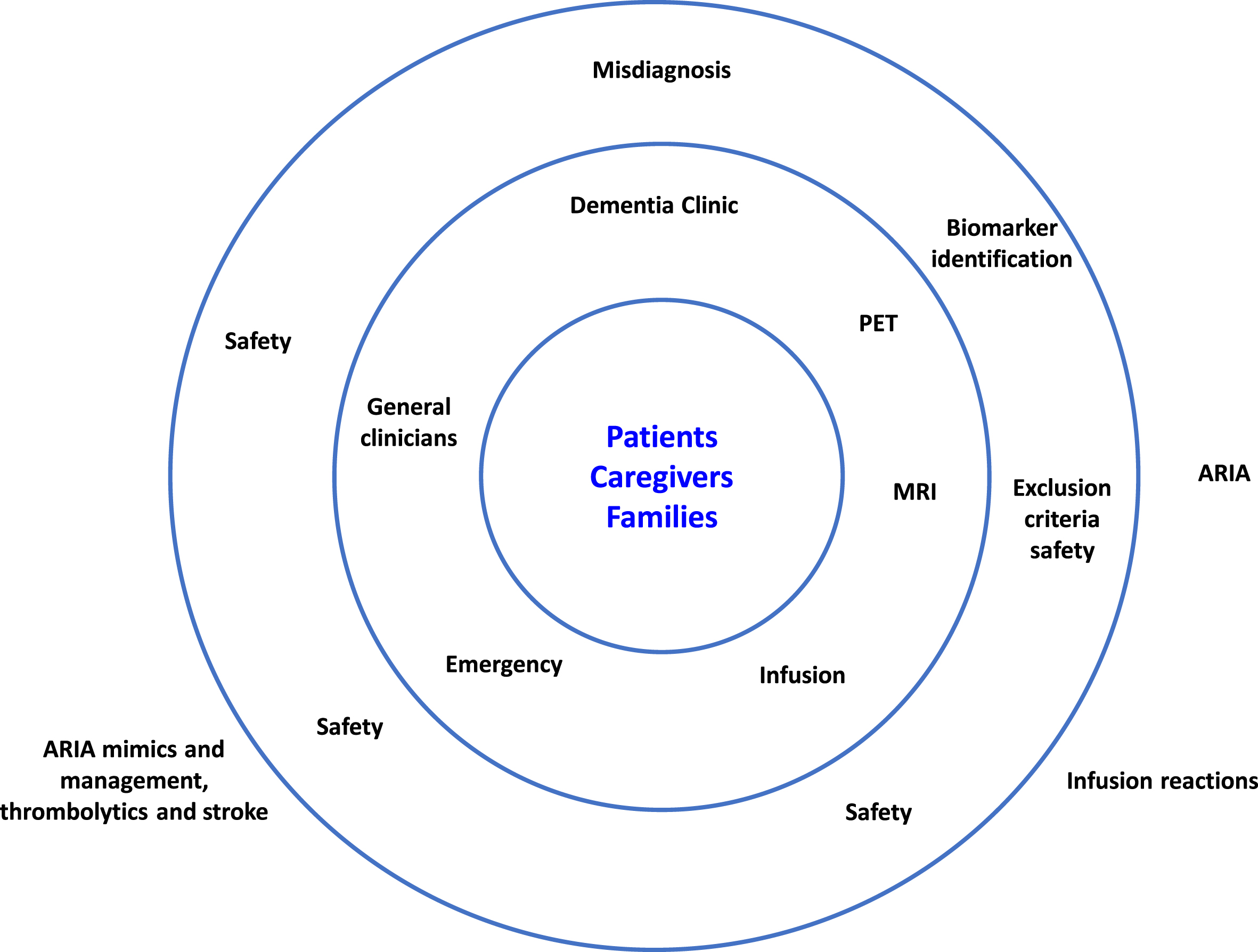
Patients with early AD who are deemed eligible for anti-amyloid therapies and their caregivers should be made aware of the benefits versus risk of these therapies so they can make an informed decision. Patients and caregivers must understand the risk of occurrence of ARIA and the potential consequences, and the need for safety MRI scans. To empower patients and caregivers, the care team should offer clear explanations of the safety issues and the measures in place to mitigate these issues (Fig. 4).
Fig. 4
Safeguarding patients from adverse events of anti-amyloid therapies. ARIA, amyloid-related imaging abnormalities; MRI, magnetic resonance imaging; PET, positron emission tomography.
Cross-disciplinary collaboration
Cross-disciplinary collaboration is important at all stages from early identification of patients with early AD to treatment and management with anti-amyloid therapies. One of the primary challenges is the disparity in the number of neurologists versus patients. Although Asia constitutes 60% of the world’s population, it contains only 20% of the world’s neurologists and there is significant variability in neurologic care across different countries depending on the level of economic development and healthcare infrastructure and the system of healthcare financing.26 With the rapidly ageing population and the efforts to identify AD early in the disease process in light of the availability of anti-amyloid therapies, a significant increase in the number of patients requiring assessment and management is anticipated. To ease the burden on dementia specialists, other healthcare practitioners, such as primary care physicians, geriatricians, general neurologist or psychiatrists, neuropsychologists, can offer valuable support in the identification and assessment of patients with early AD. While dementia specialists should be the primary healthcare professional responsible for the management of early AD, collaboration with radiologists/nuclear medicine specialists for diagnosis and monitoring, emergency staff to provide urgent care in case of infusion reactions/ARIA/other AEs, and geneticists for APOE4 testing and educating patients and caregivers on implications will be required. Dementia specialists who are educated and trained in the use of anti-amyloid therapies can lead the education of physicians in these disciplines to ensure complete alignment in terms of the protocols and pathways. When the therapies become available in Asia, social workers can support administrative tasks related to insurance and reimbursement as well as provide patient and caregiver support.
Ideally, fully resourced centers/clinics should be established for AD dementia diagnosis and care, where anti-amyloid therapies can be provided to eligible patients with early AD and these patients can then be monitored and managed appropriately. However, resource constraints could preclude such clinics. Cross-disciplinary collaboration can address this challenge; care pathways to facilitate the delivery of anti-amyloid therapies can be introduced to existing pathways for other conditions, such as multiple sclerosis and cancer.
Education
Education and training are the foundation for the identification of patients with AD at the very early stage and the efficient and safe delivery of anti-amyloid therapies to eligible patients with early AD. Educational efforts should be consistent and targeted to specific challenges and barriers throughout the patient journey.
As elucidated previously, there is a widespread misconception in the general public as well as general physicians and primary care physicians that cognitive complaints in the elderly are a normal part of ageing and a diagnosis of AD is pointless because there is no available cure.17–24 Furthermore, most general neurologists who do not manage patients with AD dementia routinely in their practice are not likely to be aware of the research and progress that led to the development of anti-amyloid therapies for early AD. For that matter, dementia specialists may not be aware of the emerging clinical research data for anti-amyloid therapies unless they were involved in the clinical trials. Education for general physicians and primary care physicians should be directed towards increasing their awareness of the importance of further assessment of elderly patients presenting with cognitive symptoms and improving their capability for administering screening tests. They should be empowered to refer appropriate patients upstream to specialists. Specialists, such as general neurologists, psychiatrists, and geriatricians can be trained to further assess patients referred from the community and administer diagnostic tests. These specialists are uniquely placed to support dementia specialists in the initial assessment of patients and in providing information to patients and caregivers about the potential of anti-amyloid therapies. Dementia specialists can benefit from education and training on anti-amyloid therapies, their efficacy and safety data, identification and management of ARIA, and strategies for communicating with patients and their caregivers, while neuroradiologists should be trained for reading MRI and PET scans to detect early changes in AD, MRI sequencing protocols and criteria, and to identify ARIA.
The general public and patients with dementia and their families/caregivers should be educated so that they are encouraged to undergo further assessment for cognitive complaints. They should also be made aware of the availability of DMTs for early AD and the difference between DMTs and symptomatic treatments of AD. Although anti-amyloid therapies have the potential to change the treatment landscape of AD, the general public should understand that these therapies will not cure AD. It is important to manage treatment expectations and goals of care from the outset. Before initiating treatment, patients and their caregivers should have access to all available information regarding the administration, frequency, potential side effects, benefits versus risks of the therapies, so that they are empowered to advocate for themselves.
FUTURE DIRECTIONS
Current clinical practice for the management of AD revolves around symptomatic treatment and infrequent use of diagnostic testing modalities leads to late-stage diagnosis. Further understanding of the pathophysiological processes underlying AD and the development of therapies targeting these processes signals an upcoming paradigm shift in the approach to managing AD towards biomarker-based early detection, diagnosis, and therapeutic interventions. To adapt to this evolving scenario, healthcare systems need to rethink the existing pathways for the diagnosis and management of AD and develop collaborative and multidisciplinary pathways and framework with a patient-centered focus.40 Data for anti-amyloid therapies will continue to become available, both from clinical trials and real-world studies, and it will be important to revisit and rework the pathways considering emerging evidence.
Anti-amyloid agents for early AD are novel therapies supported by rigorous data; however, how this data translates into clinical settings is yet to be seen. Results from post-marketing studies and registries will provide further evidence for the efficacy and safety of these agents and identify other measures for evaluation such as clinically meaningful responses and patient-related outcomes.
To account for regional differences based on ethnicities and differences in healthcare systems and infrastructure, appropriate use recommendations for specific anti-amyloid therapies can be developed by each country in Asia based on the local PIs and accounting for practice differences. These recommendations should ideally be developed under the auspices of the local dementia societies by dementia specialists who have been involved in clinical studies of the specific agents. While the global registration trials of the available anti-amyloid therapies have enrolled patients from Asia, further studies specifically in Asian populations with MCI or mild dementia due to AD could improve the confidence of local healthcare providers.
Further research and development of blood-based biomarkers for AD could potentially transform the way screening for AD and treatment monitoring is performed. Blood-based biomarkers in the future could alleviate cost and access-related issues associated with PET or CSF biomarkers. Furthermore, primary care physicians could potentially use blood-based biomarkers to identify patients who would benefit from a referral to specialists for further diagnostic tests.
Cost and accessibility issues will arise when anti-amyloid agents for early AD become available in Asia and will have to be dealt with according to the local healthcare and reimbursement systems.
CONCLUSION
Anti-amyloid monoclonal antibodies are promising disease-modifying therapies that have the potential to offer benefits to patients with early AD who would otherwise have been treated symptomatically. These agents will be particularly important in Asia where the number of people living with AD is expected to more than double by the year 2050. It is imperative for healthcare systems across the different countries in Asia to prepare for the availability of anti-amyloid therapies by establishing new clinical pathways and models of care. Early identification of AD will be crucial, and biomarkers will guide the diagnosis, treatment initiation and patient monitoring. Incorporation of anti-amyloid monoclonal antibodies into the treatment paradigm for eligible patients will require strong multidisciplinary collaboration, thorough clinical evaluation, development of protocols and infrastructure for infusion and treatment monitoring, and comprehensive training of all involved medical personnel. Clear and transparent communications about anti-amyloid therapies and the risks and benefits associated with them to patients and their families/caregivers will ensure their engagement and acceptance. Finally, consistent and continuous educational efforts underscoring the importance of early diagnosis of AD in this era of innovative therapies, aimed at the general public, primary care physicians and specialists, will be essential for optimizing patientoutcomes.
AUTHOR CONTRIBUTIONS
Jae-Hong Lee (Conceptualization; Methodology; Project administration; Supervision; Visualization; Writing – original draft; Writing – review & editing); Jianping Jia (Visualization; Writing – original draft; Writing – review & editing); Yong Ji (Visualization; Writing – original draft; Writing – review & editing); Nagaendran Kandiah (Visualization; Writing – original draft; Writing – review & editing); SangYun Kim (Visualization; Writing – original draft; Writing – review & editing); Vincent Mok (Visualization; Writing – original draft; Writing – review & editing); Ming-Chyi Pai (Visualization; Writing – original draft; Writing – review & editing); Vorapun Senanarong (Visualization; Writing – original draft; Writing – review & editing); Chong Hyun Suh (Visualization; Writing – original draft; Writing – review & editing); Christopher L. H. Chen (Conceptualization; Methodology; Project administration; Supervision; Visualization; Writing – original draft; Writing – review & editing).
ACKNOWLEDGMENTS
The authors would like to thank Dr Shilpa Mudgal from In Vivo Communications Asia Pte. Ltd. for medical writing contribution which was funded by Eisai Co. Ltd.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Jianping Jia is an Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review. Nagaendran Kandiah is a scientific advisor for Nurophet, Neuroglee and Neurophet; has received honorarium for lectures and presentation from Eisai, Schwabe, Danone and Menarini; has received grant support from Schwabe (2023–2025) and MiRXES (2022–2024); has a provisional patent for 2 digital cognitive diagnostic app; and is an Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review. SangYun Kim has received lecture fees and advisory fees from Eisai. Ming-Chyi Pai has received consulting fees from Eisai and Eli Lilly, and honorarium for lectures and presentations from Eisai, Novartis and Eli Lilly. Vorapun Senanarong has received consulting fees from Eisai. Christopher Chen has received consulting fees from Eisai, Actinogen, Cerecin and Cerboriva. Jae-Hong Lee, Yong Ji, Vincent Mok, and Chong Hyun Suh have no conflict of interest to report.
REFERENCES
1. | Nichols E , Steinmetz JD , Vollset SE , et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health (2022) ; 7: : e105–e125. |
2. | Li X , Feng X , Sun X , et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2019. Front Aging Neurosci (2022) ; 14: : 937486. |
3. | Dementia warning for the Asia-Pacific region. Lancet Neurol (2015) ; 14: : 1. |
4. | Karantzoulis S and Galvin JE . Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev Neurother (2011) ; 11: : 1579–1591. |
5. | Alzheimer’s disease facts and figures. Alzheimers Dement (2023) ; 19: : 1598–1695. |
6. | World Health Organization. Dementia, https://www.who.int/news-room/fact-sheets/detail/dementia accessed 16 April 2024 . |
7. | Wimo A , Guerchet M , Ali GC , et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement (2017) ; 13: : 1–7. |
8. | Kandiah N , Choi SH , Hu CJ , et al. Current and future trends in biomarkers for the early detection of Alzheimer’s disease in asia: expert opinion. J Alzheimers Dis Rep (2022) ; 6: : 699–710. |
9. | Leisher S , Bohorquez A , Gay M , et al. Amyloid-lowering monoclonal antibodies for the treatment of early Alzheimer’s disease. CNS Drugs (2023) ; 37: : 671–677. |
10. | Hampel H , Hardy J , Blennow K , et al. The amyloid-β pathway in Alzheimer’s disease. Mol Psychiatry (2021) ; 26: : 5481–5503. |
11. | Sims JR , Zimmer JA , Evans CD , et al Donanemab in early symptomatic Alzheimer disease. JAMA (2023) ; 330: : 512. |
12. | van Dyck CH , Swanson CJ , Aisen P , et al. Lecanemab in early Alzheimer’s disease. N Engl J Med (2023) ; 388: : 9–21. |
13. | Harris E . Alzheimer drug lecanemab gains traditional FDA approval. JAMA (2023) ; 330: : 495. |
14. | Biogen “LEQEMBI®” (Lecanemab) Approved for the Treatment of Alzheimer’s Disease in China, https://investors.biogen.com/news-releases/news-release-details/leqembir-lecanemab-approved-treatment-alzheimers-disease-china (2024, accessed 16 April 2024). |
15. | Eisai Co. Ltd. ‘LEQEMBI® Intravenous Infusion’ approved for the treatment of Alzheimer’s disease in Japan, https://www.eisai.com/news/2023/news202359.html (2023, accessed 16 April 2024). |
16. | Mintun MA , Lo AC , Duggan Evans C , et al. Donanemab in early Alzheimer’s disease. N Engl J Med (2021) ; 384: : 1691–1704. |
17. | Song D , Yu D and Sun Q . Perception and knowledge of dementia prevention and its associated socio-demographic factors in China: A community-based cross-sectional study. Front Neurosci (2022) ; 16: : 1093169. |
18. | Alzheimer’s Disease International. Dementia in the Asia Pacific region. https://www.alzint.org/resource/dementia-in-the-asia-pacific-region/ (2014). |
19. | Hossain M , Crossland J , Stores R , et al. Awareness and understanding of dementia in South Asians: A synthesis of qualitative evidence. Dementia (2020) ; 19: : 1441–1473. |
20. | Li Y-T , Bai J-X , He J-M , et al. The mediating role of attitudes towards dementia on the relationship between dementia knowledge and behaviors towards persons with dementia: a cross-sectional study. J Multidiscip Healthc (2023) ; 16: : 4213–4225. |
21. | Leung CW , Lam TP , Wong KW , et al. Early detection of dementia: The knowledge and attitudes of primary care physicians in Hong Kong. Dementia (2020) ; 19: : 830–846. |
22. | Wang Y , Xiao LD , Luo Y , et al. Community health professionals’ dementia knowledge, attitudes and care approach: a cross-sectional survey in Changsha, China. BMC Geriatr (2018) ; 18: : 122. |
23. | Gong N , Yang D , Zou J , et al. Exploring barriers to dementia screening and management services by general practitioners in China: a qualitative study using the COM-B model. BMC Geriatr (2023) ; 23: : 55. |
24. | Ali MF , Ja’afar NIS , Krishnan TG , et al. Dementia awareness among elderly at risk for developing mild cognitive impairment: a cross sectional study at a university-based primary care clinic. BMC Geriatr (2023) ; 23: : 496. |
25. | Aihara Y , Kato H , Sugiyama T , et al. Public attitudes towards people living with dementia: A cross-sectional study in urban Japan (innovative practice). Dementia (2020) ; 19: : 438–446. |
26. | Tan C-T . Neurology in Asia. Neurology (2015) ; 84: : 623–625. |
27. | Gauthier S , Rosa-Neto P , Morais JA , et al. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London, https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf (2021, accessed 16 February 2024). |
28. | United States Food and Drug Administration. Highlights of Prescribing Information. LEQEMBI®, https://www.accessdata.fda.gov/drugsatfda docs/label/2023/761269s000lbl.pdf (2023, accessed 16 April 2024). |
29. | Pang T , Chong EJY , Wong ZX , et al. Validation of the Informant Quick Dementia Rating System (QDRS) among older adults in Singapore. J Alzheimers Dis (2022) ; 89: : 1330–1323. |
30. | Ryu HJ , Moon Y , Kim M , et al. Validation of the Korean Quick Dementia Rating System (K-QDRS). J Alzheimers Dis (2021) ; 84: : 1645–1656. |
31. | Galvin JE . The Quick Dementia Rating System (QDRS): a rapid dementia staging tool. Alzheimers Dement (Amst) (2015) ; 1: : 249–259. |
32. | Ng A , Chew I , Narasimhalu K , et al. Effectiveness of Montreal Cognitive Assessment for the diagnosis of mild cognitive impairment and mild Alzheimer’s disease in Singapore. Singapore Med J (2013) ; 54: : 616–619. |
33. | Rosli R , Tan MP , Gray WK , et al. Cognitive assessment tools in Asia: a systematic review. Int Psychogeriatr (2016) ; 28: : 189–210. |
34. | Yang DW , Cho BL , Chey JY , et al. The development and validation of Korean Dementia Screening Questionnaire (KDSQ). J Korean Neurol Assoc (2002) ; 20: : 135–141. |
35. | Lee SJ , Han JH , Hwang JW , et al. Screening for normal cognition, mild cognitive impairment, and dementia with the Korean dementia screening questionnaire. Psychiatry Investig (2018) ; 15: : 384–389. |
36. | Lim L , Ng TP , Ong AP , et al. A novel language-neutral Visual Cognitive Assessment Test (VCAT): validation in four Southeast Asian countries. Alzheimers Res Ther (2018) ; 10: : 6. |
37. | Hampel H , Shaw LM , Aisen P , et al. State-of-the-art of lumbar puncture and its place in the journey of patients with Alzheimer’s disease. Alzheimer’s and Dementia (2022) ; 18: : 159–177. |
38. | Vance JM , Farrer LA , Huang Y , et al. Report of the APOE4 National Institute on Aging/Alzheimer Disease Sequencing Project Consortium Working Group: reducing APOE4 in carriers is a therapeutic goal for Alzheimer’s disease. Ann Neurol (2024) ; 95: : 625–634. |
39. | Ritchie M , Sajjadi SA and Grill JD . Apolipoprotein E genetic testing in a new age of Alzheimer disease clinical practice. Neurol Clin Pract (2024) ; 14: : e200230. |
40. | Hampel H , Au R , Mattke S , et al. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat Aging (2022) ; 2: : 692–703. |
41. | Hampel H , Elhage A , Cho M , et al. Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain (2023) ; 146: : 4414–4424. |
42. | Jeong SY , Suh CH , Shim WH , et al. Incidence of amyloid-related imaging abnormalities in patients with Alzheimer disease treated with anti-β-amyloid immunotherapy: a meta-analysis. Neurology (2022) ; 99: : e2092–e2101. |
43. | Cummings J , Apostolova L , Rabinovici GD , et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis (2023) ; 10: : 362–377. |



