Enlarged Perivascular Spaces Are Independently Associated with High Pulse Wave Velocity: A Cross-Sectional Study
Abstract
Background:
Recent studies have demonstrated an association between pulse wave velocity (PWV), cerebral small vessel disease (SVD), and cognitive impairment such as Alzheimer’s disease. However, the association between brachial-ankle PWV and enlarged perivascular spaces (EPVS), one component of cerebral SVD remains controversial.
Objective:
To investigate the relationship between brachial-ankle PWV and EPVS severity in participants without dementia.
Methods:
We performed a cross-sectional study of data of 74 participants from sub-analysis of ongoing research. We assessed cognitive function, brachial-ankle PWV, and brain magnetic resonance imaging (MRI) features. Using brain MRI, EPVS were separately assessed as basal ganglia (BG)-EPVS or centrum semiovale (CSO)-EPVS on the basis of their location. The relationship between EPVS severity and brachial-ankle PWV was evaluated using multivariable ordinal logistic regression analyses.
Results:
We analyzed 74 participants (women: 47%, mean age: 73 years, mild cognitive impairment [MCI]: 74%). Compared with participants with normal cognition, those with MCI were more likely to have both severe BG-EPVS and severe CSO-EPVS. In multivariable analyses, high brachial-ankle PWV and age were independently associated with BG-EPVS severity (odds ratio [95% confidence interval]: 1.19 [1.02–1.38], 1.09 [1.01–1.17], respectively), whereas only age was independently associated with CSO-EPVS severity. A causal mediation analysis under a counterfactual approach revealed a significant pure natural indirect effect of brachial-ankle PWV on MCI that was mediated by BG-EPVS (estimate: 1.04, 95% CI: 1.01–1.12, p = 0.006).
Conclusions:
Brachial-ankle PWV was associated with BG-EPVS severity. High PWV may cause cerebrovascular pulsatility, which accelerates BG-EPVS and may worsen cognitive impairment.
INTRODUCTION
Arterial stiffness, indicated by pulse wave velocity (PWV), is mainly caused by aging and hypertension, and is an important risk factor for cerebral small vessel disease (SVD).1,2 Cerebral SVD involves hypertensive vascular lesions that are detectable on brain magnetic resonance imaging (MRI) and is a major contributor to cognitive decline.3,4
Early stage cognitive decline is defined as mild cognitive impairment (MCI), involving an objective cognitive impairment in individuals with essentially normal functional activities who are not yet exhibiting dementia.5 Comprehensive clinical research has been conducted in Japan to examine approaches for reducing the risk of cognitive decline,6 because MCI occurs in up to 20% of older people and 5%–15% of individuals with MCI develop dementia annually.7
Enlarged perivascular spaces (EPVS) have been investigated as a hallmark neuroimaging manifestation of cerebral SVD, which may lead to cognitive decline.3,8,9 Recently, EPVS are drawing attention as a marker of subclinical cerebrovascular disease10 and impaired glymphatic flow on the Alzheimer’s disease.11 PVS are compartments surrounding cerebral blood vessels that become visible on MRI in the basal ganglia (BG) area (BG-EPVS) and in the centrum semiovale (CSO) area (CSO-EPVS) when enlarged.12,13
Previous studies have reported conflicting results regarding the associations between EPVS and pulse wave velocity (PWV),14,15 an indicator of aortic pulsatility.16 In a cohort of hypertensive individuals, carotid-femoral PWV was reported to be associated with BG-EPVS, but not with CSO-EPVS.14 Conversely, brachial-ankle PWV in stroke patients was associated with both BG-EPVS andCSO-EPVS.15
We are conducting an ongoing observational study of cognitive function and the gut microbiome. In the current study, by performing a sub-analysis of the data from this ongoing research, we aimed to examine the relationship between EPVS severity, brachial-ankle PWV in participants without dementia. We hypothesized that high brachial-ankle PWV would be associated with EPVS severity, particularly BG-EPVS rather than CSO-EPVS.
MATERIALS AND METHODS
Study design
We performed a cross-sectional sub-analysis of data from a hospital-based prospective cohort study, the Gimlet study, conducted at the National Center for Geriatrics and Gerontology (NCGG) in Japan.17–24 This study is an ongoing observational study of cognitive function and the gut microbiome. Briefly, patients who visited the Memory Clinic at the NCGG and agreed to undergo both a medical assessment of their cognitive function and a fecal examination were enrolled in the Gimlet study. Our previous analyses revealed that gut microbial dysregulation is associated with cognitive decline17,18,25 and cerebral SVD.23,24 The current sub-study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review boards of the NCGG (no. 1669) and the University of the Ryukyus Graduate School of Medicine (no. 2050). Written informed consent was obtained from all patients and their families before their participation in the Gimlet study. The Gimlet study is registered on the UMIN Clinical Trials Registry (UMIN000031851). Detailed information is provided in the Supplementary Materials.
Participants
Between 2016 and 2022, we enrolled 150 participants who had subjective memory impairment in the Gimlet study. Participants were divided into three groups: dementia, MCI, or normal cognition (NC). Participants in the Gimlet study were eligible for this MRI sub-study if they met the following criteria: (1) individuals underwent brain MRI; and (2) individuals were categorized as having either MCI or NC. Of the 150 participants in the Gimlet study, 56 participants with dementia and 20 participants with incomplete data were excluded. Thus, 74 participants were included in this sub-analysis (Fig. 1).
Fig. 1
Flow chart of study participants in the sub-analysis study. baPWV, brachial ankle pulse wave velocity; ABI, ankle brachial index.
Assessments
All participants underwent a comprehensive geriatric assessment of the following features: (1) demographic characteristics; (2) risk factors of cognitive impairment; (3) activities of daily living; (4) global cognitive function, assessed using the Mini-Mental State Examination (MMSE)26 and Clinical Dementia Rating (CDR) scale;27 (5) neuropsychological tests; (6) behavioral and psychological symptoms; (7) depression status; (8) laboratory parameters such as plasma neurofilament light chain (NfL; a disease-nonspecific biomarker of neural damage);21 (9) the ankle-brachial index (ABI) and brachial-ankle PWV, as indicators of arterial stiffness28 and the impact of pulse;1 and (10) nutritional and diet assessments.20 Clinical samples and data were provided by the NCGG Biobank, which collects clinical data for research.
Arterial stiffness
The ABI and brachial-ankle PWV were measured using an oscillometric device (Form PWV/ABI; Omron Colin Co., Ltd., Tokyo, Japan), which has been described in detail previously.16 After examinations had been performed on both the right and left sides, we subjected the lower ABI and the higher brachial-ankle PWV to analyses. A low ABI indicates advanced atherosclerotic changes in large arteries, and a high PWV indicates microvessel arteriosclerosis presenting with vascular endothelial dysfunction.16
Brain imaging
Patients underwent 1.5 T MRI (Philips Ingenia, Eindhoven, Netherlands) scanning of the brain. The obtained MRI scans included diffusion-weighted imaging, fluid-attenuated inversion recovery imaging (FLAIR), T2-weighted imaging, T2*-weighted gradient-echo imaging, and three-dimensional T1-weighted sagittal and axial coronal views. VSRAD software (Eisai Co., Ltd., Tokyo, Japan) was used to quantify cortical and hippocampal atrophy.29
MRI features of SVD
The presence and components of cerebral SVD, such as silent lacunar infarcts (SLI), white matter hyperintensities (WMH), cerebral microbleeds (CMB), and EPVS were categorized according to the Standards for Reporting Vascular Changes on Neuroimaging recommendations.30,31 We defined SLI as a focal lesion ≥3 mm in diameter that was hyperintense on T2-weighted imaging and hypointense on FLAIR images. We defined WMH as periventricular and deep white matter hyperintense lesions on T2-weighted and FLAIR images. Irregular periventricular hyperintensity (Fazekas grade ≥3) and/or early confluent or confluent separate deep white matter hyperintense lesions (Fazekas grade ≥2) was defined as severe WMH. We defined CMB as a focal area of signal loss in the brain parenchyma of <5 mm on a T2*-weighted gradient-echo imaging scan. We defined EPVS as small (<3 mm), punctate (if perpendicular to the plane of the scan), or linear (if longitudinal to the plane of the scan) hyperintensities on T2 images in the basal ganglia or centrum semiovale, as previously reported.32 BG-EPVS and CSO-EPVS were coded with the following scale, applied to standard axial images: 0 (no EPVS); 1 (≤10 EPVS); 2 (11–20 EPVS); 3 (21–40 EPVS); and 4 (>40 EPVS) (Supplementary Figure 1).32 Total cerebral SVD score was also calculated as the MRI SVD burden. On the basis of a previous study,33 we rated the total MRI burden of SVD on an ordinal scale ranging from 0 to 4 by summing the presence scores of each of the four features of cerebral SVD. In detail, the scoring system is as follows: (1) SLI, 1 point if present; (2) CMB, 1 point if present; (3) EPVS, 1 point if present in moderate-to-severe form in the basal ganglia [grades 2–4]); and (4) WMH, 1 point for the presence of either (early) confluent deep WMH (Fazekas score of 2 or 3) or irregular periventricular WMH extending into the deep white matter (Fazekas score of 3). In this sub-analysis, Cohen’s kappa coefficient was calculated on the basis of the MRI data of a randomly selected subset of 20 participants to assess the inter-rater reliability (for N.S. and Y.K.) and intra-rater reliability (for N.S.), as a measure of the quality of the assessment data for each component of cerebral SVD, as in the Gimlet study. Kappa values between 0.76 and 1.00 were interpreted as indicative of adequate agreement.34
Gut microbiome
The gut microbiome was analyzed using terminal restriction fragment-length polymorphism analysis.17 Each participant was categorized as representing one of three enterotypes: enterotype I, which included Bacteroides at >30%; enterotype II, which included Prevotella at >15%; and enterotype III, which comprised other combinations of microorganisms. The Firmicutes/Bacteroidetes (F/B) ratio was calculated because a high ratio is indicative of dysbiosis.17
Classification of cognitive function
Participants were categorized as having either MCI or NC. In accord with a previous study, MCI was defined as an MMSE score ≥20 and a CDR global score of 0.5, which indicates possible and very mild dementia and a higher risk of developing dementia.25 NC was defined as an MMSE score ≥20 and a CDR global score of 0.
Statistical analysis
Continuous, ordinal, and categorical variables are expressed as means±standard deviations, medians and interquartile ranges, and frequencies and proportions (percentages), respectively. These data were compared using Student’s unpaired t-tests, Wilcoxon rank-sum tests, and χ2 tests, respectively. We first compared clinical characteristics between participants with NC and those with MCI. Second, we compared clinical characteristics between participants with severe BG-EPVS and those without, and participants with severe CSO-EPVS and those without. In detail, we prespecified a dichotomized classification of BG-EPVS, where a score ≥2 indicates severe BG-EPVS and a score <2 indicates non-severe BG-EPVS and of CSO-EPVS, where a score ≥3 indicates severe CSO-EPVS and a score <3 indicates non-severe CSO-EPVS according to the classification used in previous studies.35 Third, we used multivariable ordinal logistic regression models to identify independent associations between: (1) brachial-ankle PWV and BG-EPVS severity, and (2) brachial-ankle PWV and CSO-EPVS severity. The ordinal variables in this study were scores of BG-EPVS and CSO-EPVS. Potential confounding variables such as age, sex, brachial-ankle PWV, vascular risk factors such as hypertension and diabetes mellitus, and the gut microbiome (enterotype I) were entered simultaneously using the forced entry method of regression.23,36 Data were analyzed using the JMP 17.1 software package and SAS v9.4 (SAS Institute Inc., Cary, NC, USA). The odds ratio (OR) and 95% confidence intervals (CI) were calculated. All comparisons were two-tailed, and p < 0.05 was considered to indicate statistical significance. We also attempted a causal mediation analysis using a counterfactual approach based on logistic regression models to test the hypothesis that high PWV causes cognitive impairment through the presence of BG-EPVS. The exposure and outcome variables in the causal mediation analyses were brachial-ankle PWV and the presence of MCI, respectively. The putative mediator was BG-EPVS. We estimated the pure natural indirect effect and used 1,000 bootstrapped simulations to generate 95% CI using R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Participant characteristics
We analyzed 74 eligible participants (47.3% women; mean age: 73 years; mean MMSE score: 26). Of the 74 participants, 47 had hypertension (63.5%), 55 had MCI (74.3%), 14 had severe BG-EPVS (score ≥2; 18.9%), and 24 had severe CSO-EPVS (score ≥3; 32.4%, Supplementary Figure 2). Median brachial-ankle PWV of the 74 participants was 17.9 m/s. In the present study, 36 participants were enterotype I (48.6%), 4 were enterotype II (5.4%), and 34 were enterotype III (46.0%).
MCI versus NC
Compared with participants with NC, participants with MCI tended to be older (mean age; MCI versus NC; 77 versus 69 years, p = 0.002), and hypertensive (72.7% versus 36.8%, p = 0.011), with lower cognitive function and a higher likelihood of severe WMH (32.7% versus 5.3%, p = 0.030), severe BG-EPVS (score ≥2; 25.5% versus 0%, p = 0.015), severe CSO-EPVS (score ≥3; 40.0% versus 10.5%, p = 0.023), higher total SVD score (score ≥2; 30.1% versus 5.3%, p = 0.006, Supplementary Figure 3), plasma NfL (median value; 23.8 versus 19.0 pg/mL, p < 0.001), F/B ratio (median value; 1.21 versus 1.65, p = 0.048), and enterotype I (56.4% versus 26.3%, p = 0.033, Supplementary Table 1).
BG-EPVS ≥ 2 versus BG-EPVS <2
High brachial-ankle PWV was associated with severe BG-EPVS (with versus without BG-EPVS score ≥2; median value of brachial-ankle PWV: 20.6 versus 17.2 m/s, p = 0.022). Participants with BG-EPVS score ≥2 tended to be older (81 versus 74 years, p = 0.001), to have fewer years of education (9 versus 12 years, p = 0.002) and lower cognitive function, and to walk slowly (0.95 versus 1.16 m/s, p = 0.039), as well as being more likely to have SLI (15.2% versus 2.6%, p = 0.026), and severe CSO-EPVS (score ≥3; 57.6% versus 25.0%, p = 0.002, Table 1, Supplementary Table 2).
Table 1
Comparisons of background information between participants with and without severe enlarged perivascular spaces in the basal ganglia (BG-EPVS)
| BG-EPVS ≥2 | BG-EPVS <2 | p | |
| (n = 14) | (n = 60) | ||
| Demographics | |||
| Age, y | 81, 77–82 | 74, 67–78 | 0.001 |
| Sex, female, n (%) | 9 (64.3) | 26 (43.3) | 0.235 |
| Education, y | 9, 9–12 | 12, 11–14 | 0.002 |
| Risk factors | |||
| Hypertension, n (%) | 11 (78.6) | 36 (60.0) | 0.233 |
| Diabetes mellitus, n (%) | 3 (21.4) | 6 (10.0) | 0.358 |
| CKD, n (%) | 1 (7.1) | 20 (33.3) | 0.056 |
| Comprehensive geriatric assessment | |||
| DBDS | 12, 5–19 | 6, 3–14 | 0.049 |
| Gait speed, m/s | 0.95, 0.74–1.18 | 1.16, 1.00–1.31 | 0.039 |
| Cognitive function | |||
| MMSE | 25, 22–26 | 28, 24–29 | 0.034 |
| CDR-SB | 2.3, 0.9–3.1 | 1.0, 0.5–2.4 | 0.058 |
| RCPM | 24, 21–30 | 30, 26–33 | 0.013 |
| FAB | 10, 9–12 | 13, 10–14 | 0.008 |
| Brain MRI findings | |||
| Total SVD score ≥2, n (%) | 8 (57.1) | 5 (8.3) | <0.001 |
| SLI, n (%) | 5 (15.2) | 2 (2.6) | 0.026 |
| Severe WMH, n (%) | 9 (27.3) | 18 (23.7) | 0.810 |
| CMB, n (%) | 8 (24.2) | 11 (14.5) | 0.273 |
| CSO-EPVS ≥3, n (%) | 19 (57.6) | 19 (25.0) | 0.002 |
| VSRAD | 1.01, 0.77–1.21 | 0.84, 0.55–1.60 | 0.197 |
| Arterial stiffness | |||
| Ankle brachial index | 1.07, 1.04–1.14 | 1.11, 1.07–1.15 | 0.184 |
| PWV, m/s | 20.6, 17.8–25.5 | 17.2, 15.0–21.1 | 0.022 |
| Laboratory findings | |||
| NfL, pg/mL | 26.1, 21.2–31.9 | 20.9, 15.4–25.7 | 0.014 |
| F/B ratio | 1.80 (0.68–2.88) | 1.26 (0.81–1.96) | 0.482 |
| Enterotype I, n (%) | 5 (35.7) | 31 (51.7) | 0.377 |
Data are presented as medians, interquartile ranges, or number of patients (%). The Wilcoxon rank-sum test and χ2 test were used. Note that participants with severe BG-EPVS were defined as presenting with enlarged perivascular spaces in the basal ganglia (scores ≥2 on the basis of an MRI scan at the level of the basal ganglia). BG-EPVS, enlarged perivascular spaces in the basal ganglia; CDR-SB, Clinical Dementia Rating-Sum of Boxes; CKD, chronic kidney disease; CMB, cerebral microbleed; CSO-EPVS, enlarged perivascular spaces in the centrum semiovale; DBDS, Dementia Behavior Disturbance Scale; FAB, Frontal Assessment Battery; F/B ratio, Firmicutes/Bacteroidetes ratio; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; NfL, neurofilament light chain; PWV, pulse wave velocity; RCPM, Raven’s Coloured Progressive Matrices; SLI, silent lacunar infarct; SVD, small vessel disease; VSRAD, voxel-based specific regional analysis system for Alzheimer’s disease; WMH, white matter hyperintensity.
CSO-EPVS ≥ 3 versus CSO-EPVS <3
No significant differences were found between brachial-ankle PWV and severe CSO-EPVS (participants with versus without CSO-EPVS score ≥3; median value of brachial-ankle PWV: 20.4 versus 17.3 m/s, p = 0.125). Participants with CSO-EPVS score ≥3 tended to be older (78 versus 74 years, p = 0.006) and have lower cognitive function, and were more likely to have severe BG-EPVS (score ≥2; 37.5% versus 10.0%, p = 0.009, Supplementary Table 3).
Multivariable analyses
Multivariable ordinal logistic regression analyses revealed that high brachial-ankle PWV (per 1 m/s increase; OR: 1.19, 95% CI: 1.02–1.38, p = 0.025) and age (OR: 1.09, 95% CI: 1.01–1.17, p = 0.023) were independently associated with BG-EPVS severity (Table 2). We also attempted a causal mediation analysis using a counterfactual approach, which revealed a significant pure natural indirect effect of brachial-ankle PWV on MCI that was mediated by BG-EPVS (estimate: 1.04, 95% CI: 1.01–1.12, p = 0.006).
Table 2
Univariable and multivariable ordinal logistic regression analyses of the severity of enlarged perivascular spaces in the basal ganglia (BG-EPVS)
| Univariable | Multivariable | |
| PWV, by 1 m/s | 1.16 (1.02–1.30)* | 1.19 (1.02–1.38)* |
| Age, y | 1.09 (1.03–1.17)** | 1.09 (1.01–1.17)* |
| Sex, female | 1.68 (0.59–4.78) | 2.66 (0.83–8.53) |
| Hypertension | 1.43 (0.48–4.26) | 0.42 (0.11–1.55) |
| Diabetes mellitus | 1.98 (0.45–8.71) | 1.26 (0.26–6.17) |
| Enterotype I | 0.55 (0.19–1.60) | 0.33 (0.10–1.11) |
Data was presented as odds ratio (95% confidential interval). *p < 0.05, **p < 0.01. Adjusted for brachial-ankle PWV, age, sex, hypertension, diabetes mellitus and the gut microbiota (enterotype I). BG-EPVS, enlarged perivascular spaces in the basal ganglia; PWV, pulse wave velocity.
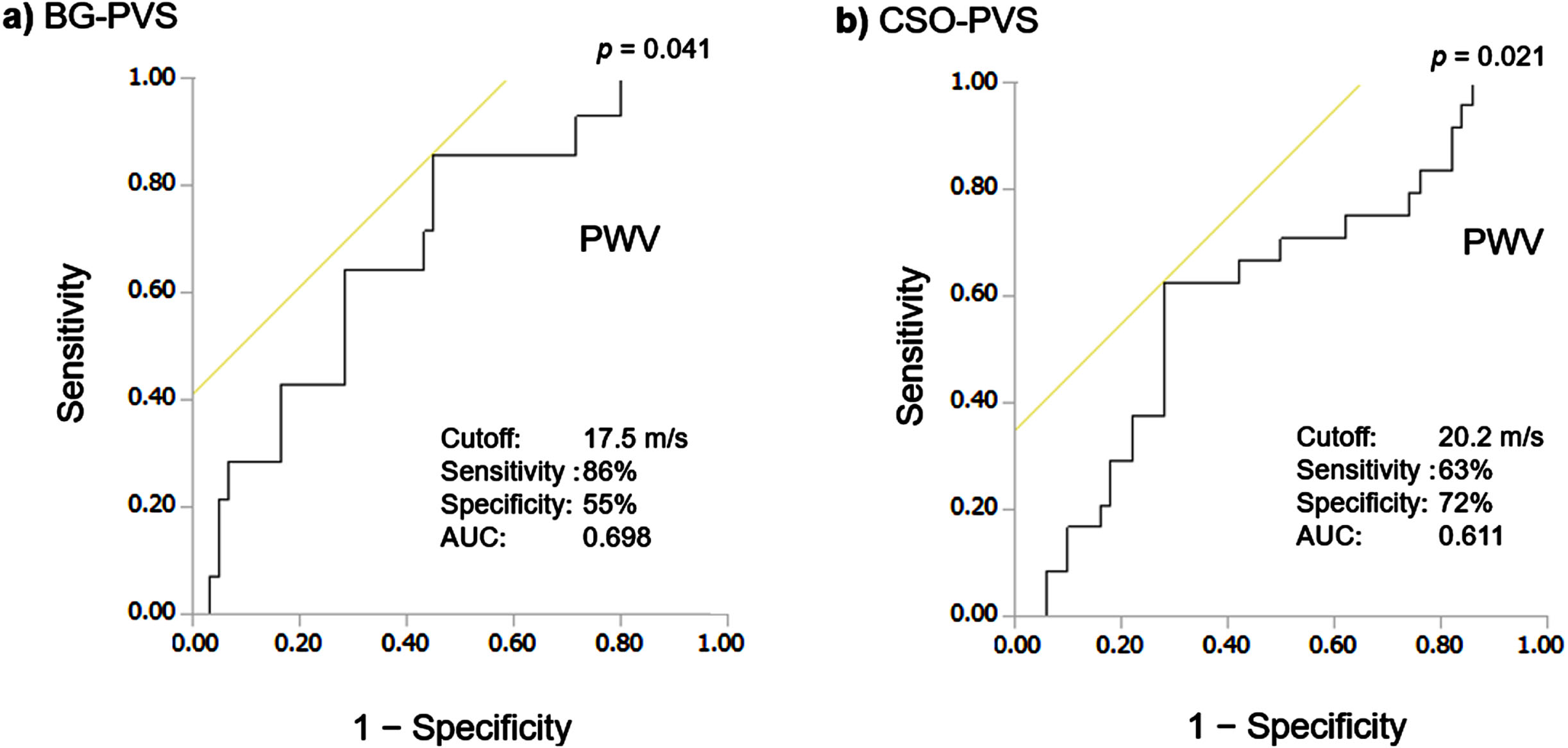
The brachial-ankle PWV cut-off value calculated according to the Youden index for the detection of BG-EPVS score ≥2 was 17.5 m/s (sensitivity 86%, specificity 55%, area under the receiver operating characteristic curve [AUC] = 0.70, p = 0.041, Fig. 2). In contrast, although age was independently associated with CSO-EPVS severity (OR: 1.10, 95% CI: 1.03–1.16, p = 0.002), brachial-ankle PWV did not show this association (Supplementary Table 4).
Fig. 2
Receiver operating characteristic curves of pulse wave velocity (PWV) for the detection of enlarged perivascular spaces in the basal ganglia (BG-EPVS) and in the centrum semiovale (CSO-EPVS). Here, the brachial-ankle pulse wave velocity (PWV) cut-off value calculated according to the Youden index for the detection of severe BG-EPVS (EPVS score ≥2; a) and severe CSO-(EPVS score ≥3; b) are shown. AUC: area under the receiver operating characteristic curve.
DISCUSSION
The main finding in the present study was that high brachial-ankle PWV was independently associated with BG-EPVS severity in participants without dementia. However, this factor was not associated with CSO-EPVS severity. Participants with severe BG-EPVS or CSO-EPVS had lower cognitive function. Additionally, the results revealed that age was associated with both BG-EPVS and CSO-EPVS severity. These findings suggest that both BG-EPVS and CSO-EPVS severity were associated with older age and cognitive decline. However, the etiologies of BG-EPVS and CSO-EPVS may be different, as indicated by the associations with brachial-ankle PWV.
Associations between EPVS, particularly BG-EPVS, and carotid-femoral PWV in hypertensive individuals were reported previously.14 Recently, the association between aortic stiffness as indicated by cardiac MRI and EPVS was also investigated in participants without dementia.9 Our findings are in line with these reports,9,14 and widen the knowledge regarding cerebral SVD. Increased PWV indicates both aortic pulsatility and microvascular arterial stiffness. Furthermore, measurement of brachial-ankle PWV is useful for older adults because it is non-invasive and cost-effective. This is the first report to demonstrate the association between EPVS and brachial-ankle PWV in participants without dementia.
The etiology of EPVS has been proposed to involve several causal mechanisms: (1) arterial stiffening,14 (2) protein aggregation,37 (3) brain atrophy,36 and (4) other mechanisms such as inflammation or oxidative stress caused by perivascular macrophage activation.36 Arterial stiffness leads to loss of the cushioning function of the aorta, which predicts subsequent increase in pulsatility. We previously proposed that intracerebral microvascular pulsatility may be caused by the impact of PWV, resulting in a “tsunami effect in the brain”.1 Specifically, repetitive, increased pulsatile pressure and flow may directly lead to cerebral microcirculatory damage, resulting in damage to the cerebral parenchyma. Regarding BG-EPVS, the perforating arteries that feed the basal ganglia are particularly vulnerable to damage from increased pulsatility.36 On the basis of this theory,1 the association between high brachial-ankle PWV and BG-EPVS severity found in the present study appears to be reasonable.
Contrary to BG-EPVS, the etiology of CSO-EPVS is related to abnormal aggregation of proteins, such as amyloid β, and is recognized as a marker of cerebral amyloid angiopathy.36 The severity of cerebral amyloid angiopathy is reported to be associated with CSO-EPVS.37 Increased vascular amyloid β accumulation upstream has also been proposed to accelerate CSO-EPVS.36 The non-significant association between brachial-ankle PWV and CSO-EPVS severity found in the present study is in accord with these findings.36,37
Recent studies indicated that increased arterial stiffness is a novel risk factor for cognitive decline.38,39 It is reasonable that arterial stiffness, cerebral SVD, and cognitive decline are reciprocally linked. In our causal mediation analyses, BG-EPVS mediated the association between brachial-ankle PWV and MCI. Thus, BG-EPVS may have a causal relationship between the PWV and cognitive decline.
Another notable finding of the present study was that older age was associated with both BG-EPVS and CSO-EPVS severity. This finding appears to be reasonable because vascular risk factors increase with aging, and aging is one of the most significant risk factors for cerebral SVD.3 Second, the current findings revealed that participants with severe BG-EPVS or severe CSO-EPVS had lower cognitive function. This result is in accord with the findings of previous studies.40,41 Third, plasma NfL concentration was associated with participants with severe BG-EPVS and severe CSO-EPVS. Although this finding is novel, it is reasonable because components of cerebral SVD other than EPVS were found to be associated with NfL.42 Finally, the cut-off value of brachial-ankle PWV for the detection of BG-PVS (17.5 m/s) was similar to that reported for other subtypes of cerebral SVD, such as SLI (17.2 m/s),43 progressive acute lacunar stroke (18.2 m/s),44 and WMH (18.3 m/s).45 These findings appear to be reasonable because these factors are components of hypertensive cerebral SVD.1
The present study has several strengths. First, we revealed novel relationships between EPVS severity and brachial-ankle PWV in participants without dementia. Specifically, high brachial-ankle PWV was associated with BG-EPVS severity, suggesting that high PWV increases cerebrovascular pulsatility, thus accelerating hypertensive cerebral SVD. Second, a non-significant association between brachial-ankle PWV and CSO-EPVS severity suggests that the etiology of CSO-EPVS is more likely to be caused by cerebral amyloid angiopathy, rather than hypertensive SVD.36 The current findings strengthened this mechanism in addition to the robust association between cerebral hypertensive SVD and PWV.1,28,44,45 Concomitant assessment of arterial stiffness and brain MRI may be useful for healthcare in older adults in aging societies, potentially contributing to the prevention of dementia.
The present study involved several limitations that should be considered. The small number of participants and the large number of potential variables may have led to our study being statistically underpowered. Because this was a single hospital-based cohort study, selection bias may have occurred. A causal relationship between PWV and EPVS could not be established because of the present study’s cross-sectional design. In addition, we were unable to assess amyloid-β for the detection of Alzheimer’s disease in the current study because cerebrospinal fluid testing and positron emission tomography were not included in the Gimlet study.
The current results suggest that high PWV may cause cerebrovascular pulsatility. Subsequently, cerebrovascular pulsatility appeared to accelerate BG-EPVS and worsen cognitive impairment. Detailed assessment of the relationships between EPVS and PWV, and cognitive decline should be conducted in future studies to clarify the mechanisms underlying these effects.
Conclusion
Despite constituting a preliminary analysis, this sub-study provides evidence for the relationship between EPVS severity and brachial-ankle PWV. High brachial-ankle PWV was independently associated with BG-EPVS severity in participants without dementia, whereas brachial-ankle PWV was not associated with CSO-EPVS severity.
AUTHOR CONTRIBUTIONS
Yoshino Kinjo (Conceptualization; Formal analysis; Visualization; Writing – original draft); Naoki Saji (Conceptualization; Funding acquisition; Methodology; Project administration; Supervision; Writing – review & editing); Kenta Murotani (Formal analysis); Hirokuni Sakima (Writing – review & editing); Akinori Takeda (Writing – review & editing); Takashi Sakurai (Writing – review & editing); Yusuke Ohya (Supervision; Writing – review & editing); Kenya Kusunose (Supervision; Writing – review & editing).
ACKNOWLEDGMENTS
We would like to thank Yukie Ohsaki, Hana Saito, and Ayaka Suzuki (NCGG) for their technical and secretarial assistance, and the BioBank of NCGG for quality control of the clinical samples and data. We also thank Benjamin Knight, MSc. from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. Finally, we thank all of the participants who were involved in the Gimlet study, and their families.
FUNDING
This study was supported by JSPS KAKENHI (JP20k07861, JP24K10546), and by grants from the Research Funding of Longevity Sciences from the NCGG (19–24, 22-23), the NARO Bio-Oriented Technology Research Advancement Institution Project (Advanced Integration Research for Agriculture and Interdisciplinary Fields), the Danone Institute of Japan Foundation, and the Honjo International Scholarship Foundation.
CONFLICT OF INTEREST
Dr. Saji has received JSPS KAKENHI (JP20k07861, JP24K10546), the NARO Bio-Oriented Technology Research Advancement Institution Project (Advanced Integration Research for Agriculture and Interdisciplinary Fields), the Danone Institute of Japan Foundation, the Honjo International Scholarship Foundation, and the BMS/Pfizer Japan Thrombosis Investigator-Initiated Research Program. Drs. Saji, Takeda, and Sakurai have received research grants from the Research Funding of Longevity Sciences from the National Center for Geriatrics and Gerontology, and the Japan Agency for Medical Research and Development (AMED). Dr. Saji is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review. All other authors have no conflict of interest to report.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-240589.
REFERENCES
1. | Saji N , Toba K and Sakurai T. Cerebral small vessel disease and arterial stiffness: tsunami effect in the brain? Pulse (Basel) (2016) ; 3: : 182–189. |
2. | van Sloten TT , Protogerou AD , Henry RM , et al. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neurosci Biobehav Rev (2015) ; 53: : 121–130. |
3. | Ang PS , Zhang DM , Azizi SA , et al. The glymphatic system and cerebral small vessel disease. J Stroke Cerebrovasc Dis (2024) ; 33: : 107557. |
4. | Zhao B , Jia W , Yuan Y , et al. Effects of intensive blood pressure control on cognitive function in patients with cerebral small vessel disease. J Stroke Cerebrovasc Dis (2023) ; 32: : 107289. |
5. | Petersen RC . Mild cognitive impairment as a diagnostic entity. J Intern Med (2004) ; 256: : 183–194. |
6. | Saji N , Sakurai T , Suzuki K , et al. ORANGE’s challenge: developing wide-ranging dementia research in Japan. Lancet Neurol (2016) ; 15: : 661–662. |
7. | Dunne RA , Aarsland D , O’Brien JT , et al. Mild cognitive impairment: the Manchester consensus. Age Ageing (2021) ; 50: : 72–80. |
8. | Shi X , Zhou N , Sun B , et al. Perivascular space predicts brain hypometabolism of individuals with underlying amyloid pathology. J Alzheimers Dis (2022) ; 90: : 1329–1337. |
9. | Bown CW , Khan OA , Liu D , et al. Enlarged perivascular space burden associations with arterial stiffness and cognition. Neurobiol Aging (2023) ; 124: : 85–97. |
10. | Rundek T , Del Brutto VJ , Goryawala M , et al. Associations between vascular risk factors and perivascular spaces in adults with intact cognition, mild cognitive impairment, and dementia. J Alzheimers Dis (2022) ; 89: : 437–448. |
11. | Kim M , Song YS , Han K , et al. Impaired glymphatic flow on diffusion tensor MRI as a marker of neurodegeneration in Alzheimer’s disease: correlation with gray matter volume loss and cognitive decline independent of cerebral amyloid deposition. J Alzheimers Dis (2024) ; 99: : 279–290. |
12. | Doubal FN , MacLullich AM , Ferguson KJ , et al. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke (2010) ; 41: : 450–454. |
13. | Charidimou A , Boulouis G , Pasi M , et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology (2017) ; 88: : 1157–1164. |
14. | Riba-Llena I , Jimenez-Balado J , Castane X , et al. Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke (2018) ; 49: : 1279–1281. |
15. | Bae JH , Kim JM , Park KY , et al. Association between arterial stiffness and the presence of cerebral small vessel disease markers. Brain Behav (2021) ; 11: : e01935. |
16. | Tomiyama H . Vascular function: a key player in hypertension. Hypertens Res (2023) ; 46: : 2145–2158. |
17. | Saji N , Niida S , Murotani K , et al. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep (2019) ; 9: : 1008. |
18. | Saji N , Murotani K , Hisada T , et al. Relationship between dementia and gut microbiome-associated metabolites: a cross-sectional study in Japan. Sci Rep (2020) ; 10: : 8088. |
19. | Saji N , Hisada T , Tsuduki T , et al. Proportional changes in the gut microbiome: a risk factor for cardiovascular disease and dementia? Hypertens Res (2019) ; 42: : 1090–1091. |
20. | Saji N , Tsuduki T , Murotani K , et al. Relationship between the Japanese-style diet, gut microbiota, and dementia: A cross-sectional study. Nutrition (2022) ; 94: : 111524. |
21. | Saji N , Murotani K , Sato N , et al. Relationship between plasma neurofilament light chain, gut microbiota, and dementia: a cross-sectional study. J Alzheimers Dis (2022) ; 86: : 1323–1335. |
22. | Saji N , Saito Y , Yamashita T , et al. Relationship between plasma lipopolysaccharides, gut microbiota, and dementia: a cross-sectional study. J Alzheimers Dis (2022) ; 86: : 1947–1957. |
23. | Saji N , Murotani K , Hisada T , et al. The association between cerebral small vessel disease and the gut microbiome: a cross-sectional analysis. J Stroke Cerebrovasc Dis (2021) ; 30: : 105568. |
24. | Tabei KI , Saji N , Ogama N , et al. Quantitative analysis of white matter hyperintensity: comparison of magnetic resonance imaging image analysis software. J Stroke Cerebrovasc Dis (2022) ; 31: : 106555. |
25. | Saji N , Murotani K , Hisada T , et al. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Sci Rep (2019) ; 9: : 19227. |
26. | Folstein MF , Folstein SE and McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res (1975) ; 12: : 189–198. |
27. | Morris JC . The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology (1993) ; 43: : 2412–2414. |
28. | Saji N , Kimura K , Yagita Y , et al. Comparison of arteriosclerotic indicators in patients with ischemic stroke: ankle-brachial index, brachial-ankle pulse wave velocity and cardio-ankle vascular index. Hypertens Res (2015) ; 38: : 323–328. |
29. | Matsuda H , Mizumura S , Nemoto K , et al. Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer disease. AJNR Am J Neuroradiol (2012) ; 33: : 1109–1114. |
30. | Wardlaw JM , Smith EE , Biessels GJ , et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol (2013) ; 12: : 822–838. |
31. | Duering M , Biessels GJ , Brodtmann A , et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol (2023) ; 22: : 602–618. |
32. | Wardlaw JM , Benveniste H , Nedergaard M , et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol (2020) ; 16: : 137–153. |
33. | Staals J , Makin SD , Doubal FN , et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology (2014) ; 83: : 1228–1234. |
34. | McHugh ML . Interrater reliability: the kappa statistic. Biochem Med (Zagreb) (2012) ; 22: : 276–282. |
35. | Charidimou A , Meegahage R , Fox Z , et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry (2013) ; 84: : 624–629. |
36. | Bown CW , Carare RO , Schrag MS , et al. Physiology and clinical relevance of enlarged perivascular spaces in the aging brain. Neurology (2022) ; 98: : 107–117. |
37. | Charidimou A , Jaunmuktane Z , Baron JC , et al. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology (2014) ; 82: : 57–62. |
38. | Hirasawa A , Nagai K , Miyazawa T , et al. Relationship between arterial stiffness and cognitive function in outpatients with dementia and mild cognitive impairment compared with community residents without dementia. J Geriatr Cardiol (2022) ; 19: : 594–602. |
39. | Taniguchi Y , Fujiwara Y , Nofuji Y , et al. Prospective study of arterial stiffness and subsequent cognitive decline among community-dwelling older Japanese. J Epidemiol (2015) ; 25: : 592–599. |
40. | Wardlaw JM , Smith C and Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol (2019) ; 18: : 684–696. |
41. | Saji N , Kinjo Y , Murotani K , et al. High pulse wave velocity is associated with enlarged perivascular spaces in dementia with Lewy bodies. Sci Rep (2024) ; 14: : 13911. |
42. | Qu Y , Tan CC , Shen XN , et al. Association of plasma neurofilament light with small vessel disease burden in nondemented elderly: a longitudinal study. Stroke (2021) ; 52: : 896–904. |
43. | Saji N , Kimura K , Shimizu H , et al. Association between silent brain infarct and arterial stiffness indicated by brachial-ankle pulse wave velocity. Intern Med (2012) ; 51: : 1003–1008. |
44. | Saji N , Kimura K , Kawarai T , et al. Arterial stiffness and progressive neurological deficit in patients with acute deep subcortical infarction. Stroke (2012) ; 43: : 3088–3090. |
45. | Saji N , Shimizu H , Kawarai T , et al. Increased brachial-ankle pulse wave velocity is independently associated with white matter hyperintensities. Neuroepidemiology (2011) ; 36: : 252–257. |




