Moderation of Amyloid-β Deposition on the Effect of Cholinesterase Inhibitors on Cognition in Mild Cognitive Impairment
Abstract
Background:
Clinical trial findings on cholinesterase inhibitors (ChEIs) for mild cognitive impairment (MCI) are inconclusive, offering limited support for their MCI treatment. Given that nearly half of amnestic MCI cases lack cerebral amyloid-β (Aβ) deposition, a hallmark of Alzheimer’s disease; this Aβ heterogeneity may explain inconsistent results.
Objective:
This study aimed to assess whether Aβ deposition moderates ChEI effects on amnestic MCI cognition.
Methods:
We examined 118 individuals with amnestic MCI (ages 55–90) in a longitudinal cohort study. Baseline and 2-year follow-up assessments included clinical evaluations, neuropsychological testing, and multimodal neuroimaging. Generalized linear models were primarily analyzed to test amyloid positivity’s moderation of ChEI effects on cognitive change over 2 years. Cognitive outcomes included Mini-Mental Status Examination score, the total score of the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery, and Clinical Dementia Rating-sum of boxes.
Results:
The analysis found no significant ChEI use x amyloid positivity interaction for all cognitive outcomes. ChEI use, irrespective of Aβ status, was associated with more cognitive decline over the 2-year period.
Conclusions:
Aβ pathology does not appear to moderate ChEI effects on cognitive decline in MCI.
INTRODUCTION
Numerous clinical trials have established the efficacy of cholinesterase inhibitors (ChEIs) in the treatment of Alzheimer’s disease (AD) dementia, leading to their approval by the US FDA for mild to moderate AD dementia.1 However, the application of ChEIs in the context of mild cognitive impairment (MCI), often considered a risk or prodromal stage of dementia, especially AD dementia,2 has yielded inconsistent findings, lacking strong support for their use in MCI patients.3,4
Conceptually, MCI represents a diverse clinical state resulting from a variety of etiological conditions.2 Notably, amnestic MCI is closely associated with AD5 and has been a focal point in most ChEI trials for MCI.6–8 Nonetheless, approximately half of amnestic MCI cases do not exhibit amyloid-β (Aβ) deposition, a fundamental AD pathology, in the brain.9,10 Given that amnestic MCI patients lacking Aβ deposition likely possess distinct pathophysiological characteristics from those with Aβ deposition, this heterogeneity in Aβ presence or the AD process could elucidate the inconsistencies and less favorable results in ChEI trials for MCI.
A previous study,11 retrospectively analyzing data from a memory clinic to assess the impact of 1-year ChEI treatment on cognitive decline in amnestic MCI patients while accounting for the presence of Aβ deposition, found no significant difference between ChEI users and non-users, irrespective of Aβ status. However, this study did not investigate whether the presence of Aβ modulates the ChEI effect on cognitive decline, and it’s worth noting that one year may not provide sufficient time to ascertain the effect of ChEI use.
Within this context, the aim of this study is to examine the hypothesis that the presence of Aβ deposition moderates the impact of ChEI treatment on cognition in individuals with amnestic MCI.
METHODS
Participants
This study is part of the ongoing Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE), initiated in 2014. As of June 2023, the study enrolled 118 participants with MCI who completed baseline and 2-year follow-up assessments, ranging in age from 55 to 90. Additional participant details are available in a previous study.12 All MCI participants met the current consensus criteria for amnestic MCI, which include: (i) a memory complaint validated by an informant, (ii) objective memory impairment, (iii) preserved global cognitive function, (iv) independence in functional activities, and (v) the absence of dementia.13 Notably, the criterion (ii) required age-,education-, and gender-adjusted z-scores for at least one of four episodic memory tests to be below –1.0. These memory tests consist of Word List Memory, Word List Recall, Word List Recognition, and Construction Recall tests, all part of the Korean versions of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-K) neuropsychological battery.14 The study protocol received approval from the Institutional Review Boards of Seoul National University Hospital (C-1401-027-547) and SNU-SMG Boramae Center (26-2015-60), Seoul, South Korea. The research adhered to the current Declaration of Helsinki guidelines, and all participants provided written informedconsent.
History of ChEI use
Information regarding the current drug regimens, including the names of all drugs, was obtained from prescriptions in the KBASE cohort. In this study, the ChEI administration group consisted of participants currently taking ChEIs, including donepezil, rivastigmine, and galantamine. Additionally, the administration of memantine was investigated and used as a covariate.
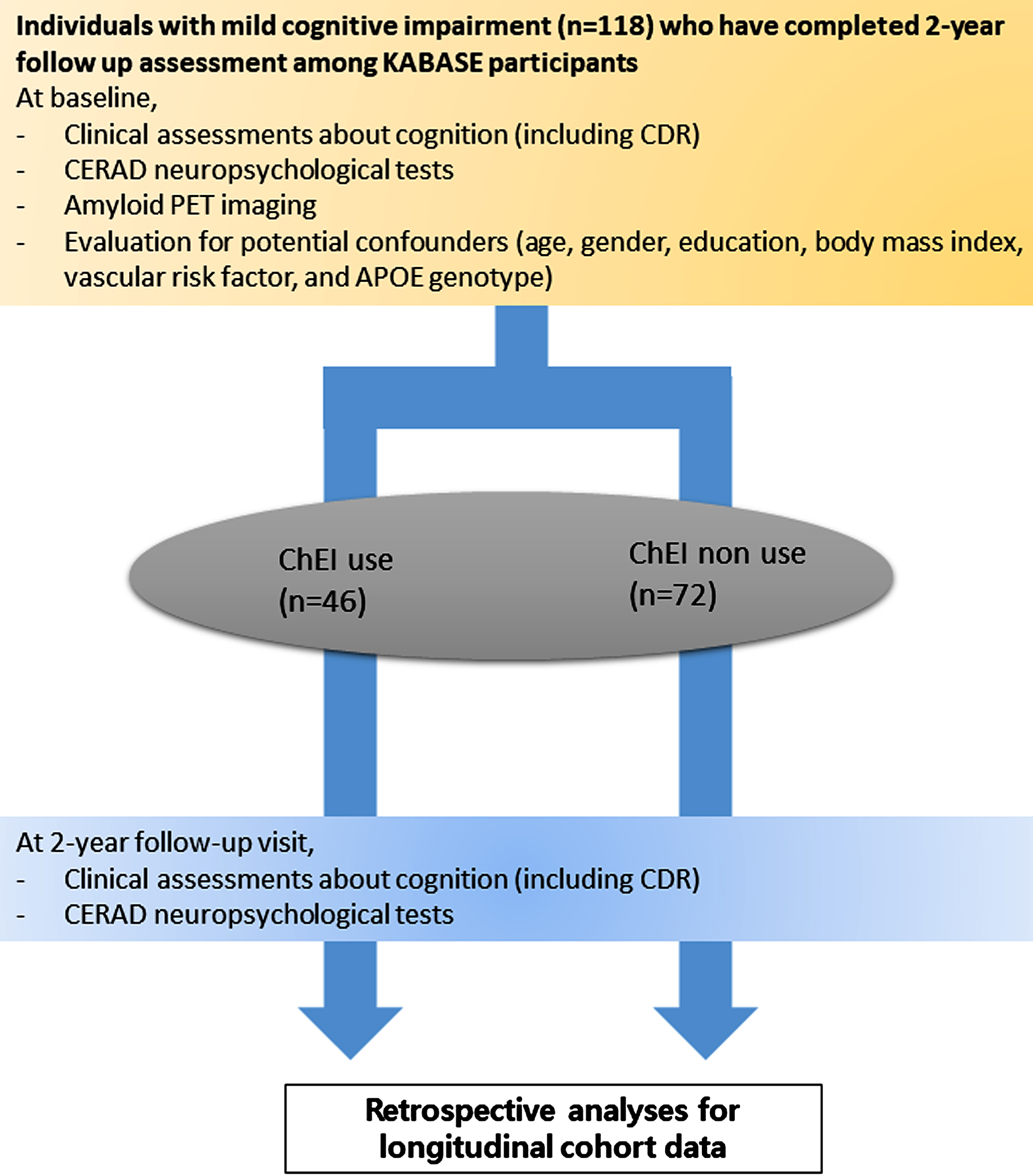
Clinical and neuropsychological assessment (Fig. 1)
At both baseline and the 2-year follow-up, participants underwent standardized clinical assessments conducted by trained board-certified psychiatrists. These assessments were based on the Korean Brain Aging Study’s clinical assessment protocol, incorporating the CERAD-K clinical assessment.15 The Clinical Dementia Rating (CDR) score was rated for all participants, evaluating the severity of cognitive impairment. The CDR-Sum-of-Box (CDR-SOB) score was computed as the sum of the CDR scores from all domains.16 Comprehensive neuropsychological assessments were administered at both time points by trained psychometrists, following a standardized protocol that included eight tests from the CERAD-K neuropsychological battery: the Mini-Mental State Examination (MMSE), Verbal Fluency (VF), 15-item Boston Naming Test (BNT), Word List Memory (WLM), Word List Recall (WLR), Word List Recognition (WLRc), Constructional Praxis (CP), and Constructional Recall (CR).14 The CERAD total score (CERAD-TS) was calculated by summing the scores of six tests from the CERAD-K neuropsychological battery (VF, BNT, WLM, WLR, WLRc, and CP).17,18
Fig. 1
Flow chart of current study. ChEI, cholinesterase inhibitor; CERAD, The Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease.
Measurement of potential confounders
Demographic information included age (in years), gender, and years of education. Participants were systematically assessed for vascular risk factors, including hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, transient ischemic attack, and stroke. A vascular risk score (VRS) reflecting vascular risk burden was determined.19 Genomic DNA was extracted from whole blood, and apolipoprotein E (APOE) genotyping was performed,20 with APOE4 positivity defined as the presence of at least one ɛ4allele.
Measurement of amyloid positivity
All participants underwent simultaneous three-dimensional (3D) [11C] Pittsburgh compound B (PiB) PET and 3D T1-weighted MRI at baseline. After intravenous administration of 555 MBq of 11C-PiB (range, 450–610 MBq), a 30-min emission scan was obtained 40 min after injection. The PiB PET image analysis followed previously described methods.21 PiB retention index as standardized uptake value ratio (SUVR) for each region of interest was calculated by dividing regional mean value by the individual mean cerebellar uptake values. The autonomic anatomic labeling algorithm and a region-combining method22 were applied to determine regions of interest (ROIs) to characterize the PiB retention level in the frontal, lateral parietal, posterior cingulate– precuneus, and lateral temporal regions. Each participant was classified as amyloid positive if the SUVR value was greater than 1.4 in at least one of the four regions of interest or as amyloid negative if the SUVR values of all four regions of interest was equal to or less than 1.4.22,23
Statistical analyses
Demographic, clinical, and neuroimaging data were compared between ChEI users and non-users using t-tests for continuous variables and chi-square tests (or Fisher’s exact tests) for categorical variables. Generalized linear model (GLM) analyses were performed to evaluate the effect of ChEI use and its interaction with amyloid positivity on cognition. GLM models included ChEI use as an independent variable and 2-year changes in MMSE, CERAD-TS, or CDR-SOB (outcome measures) as dependent variables. These models controlled for age, sex, education, APOE4 positivity, amyloid positivity, baseline values of corresponding outcome variables, VRS, and memantine use. To account for multiple comparisons in the GLM analyses for cognitive change (3 measures), the Bonferroni method was applied, setting the threshold for statistical significance at p < 0.017 (= 0.05/3). All statistical analyses were performed using the IBM SPSS Statistics 24 software (IBM Corp., Armonk, NY, USA).
RESULTS
Characteristics of participants
Table 1 presents the demographic, clinical, and neuroimaging characteristics of participants based on ChEI use. The ChEI use group exhibited a lower VRS (0.91±0.94 versus 1.35±1.06) and a higher frequency of APOE4 positivity (56.5% versus 20.8%) compared to the non-use group. Furthermore, the ChEI use group displayed higher baseline CDR-SOB scores (1.95±0.60 versus 1.17±0.46) and lower MMSE scores (21.24±3.25 versus 23.28±2.93) than the non-use group. These differences indicated that the ChEI use group included individuals with more advanced MCI in regard of cognitive impairment. Moreover, amyloid positivity ratio was higher in the ChEI use group (76.1% versus 26.4%).
Table 1
Baseline demographic, clinical and imaging characteristics of subjects
| Group | Total (n = 118) | ChEI + (n = 46) | ChEI – (n = 72) | p |
| Age (y) | 73.45±7.08 | 72.39±6.89 | 74.13±7.16 | 0.196 |
| Sex (Female %) | 75 (63.6%) | 32 (69.6%) | 43 (59.7%) | 0.279 |
| Education (y) | 10.03±4.53 | 10.20±4.58 | 9.93±4.53 | 0.758 |
| VRS | 1.18±1.04 | 0.91±.94 | 1.35±1.06 | 0.026* |
| BMI | 24.74±3.07 | 24.28±2.86 | 25.04±3.17 | 0.187 |
| APOE ɛ4 positivity (%) | 41 (34.7%) | 26 (56.5%) | 15 (20.8%) | <0.001* |
| Amyloid Positivity (%) | 54 (45.8%) | 35 (76.1%) | 19 (26.4%) | <0.001* |
| CDR-SOB | 1.47±0.64 | 1.95±0.60 | 1.17±0.46 | <0.001* |
| MMSE | 22.54±3.22 | 21.24±3.25 | 23.38±2.93 | <0.001* |
| CERAD-TS | 49.34±9.61 | 50.37±9.80 | 48.68±9.50 | 0.354 |
Mean values were presented as Mean±Standard deviation. Group was divided according to ChEI taking at baseline. *p < 0.05. ChEI, cholinesterase inhibitor; VRS, vascular risk score; BMI, body mass index; CDR-SOB, Clinical Dementia Rating Sum of Box; MMSE, Mini-Mental Status Examination; CERAD-TS, The Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Total score.
Effect of ChEI use and its interaction with amyloid positivity on cognitive outcome
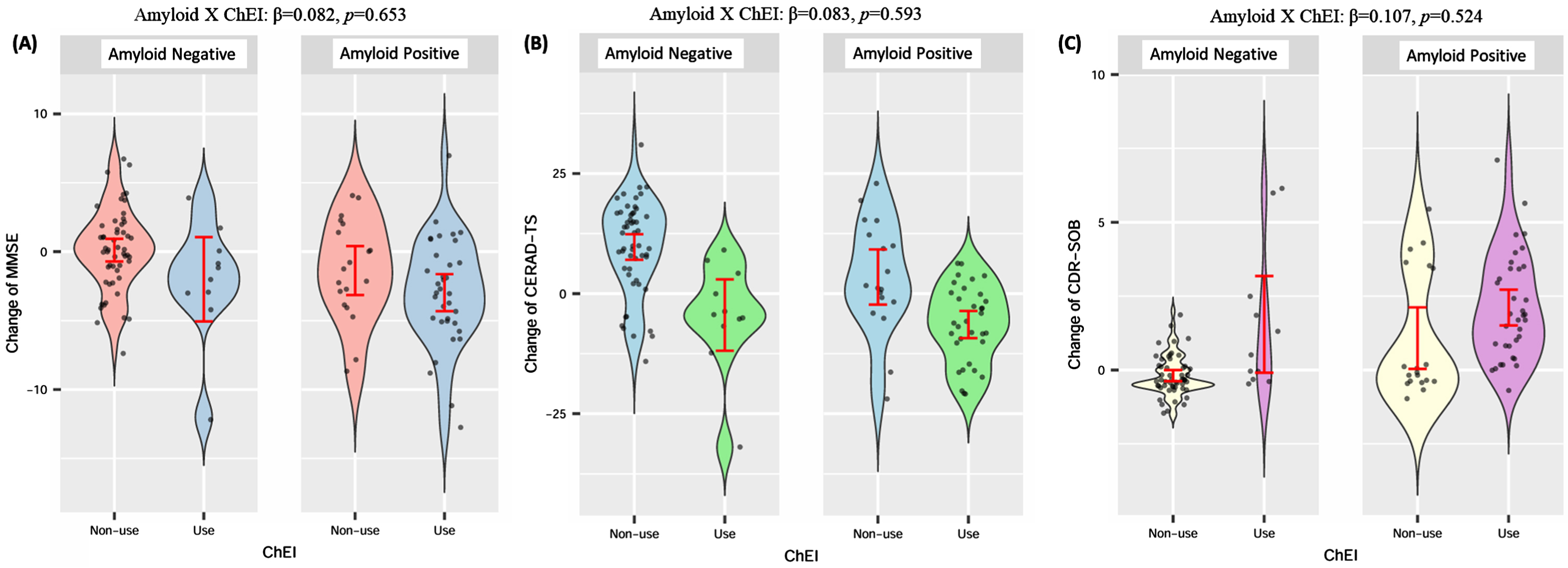
GLM analyses of the main effect only models demonstrated that ChEI use, regardless of amyloid positivity, had a significant negative impact on cognitive change over the 2-year period. Specifically, the ChEI use group exhibited greater declines in CERAD-TS (β= –0.478, p < 0.001) and MMSE scores (β= –0.302, p = 0.004), as well as greater increases in CDR-SOB scores (β= 0.299, p = 0.006), compared to the ChEI non-use group (Table 2). However, the analyses for the models including interaction terms did not reveal significant interaction effects between ChEI use and amyloid positivity on any cognitive outcome measures. Specifically, on MMSE (β= 0.082, p = 0.653) (Table 2 and Fig. 2A), CERAD-TS (β= 0.083, p = 0.593) (Table 2 and Fig. 2B), and CDR-SOB (β= –0.107, p = 0.524) (Table 2 and Fig. 2C).
Table 2
Association between baseline ChEI use, amyloid positivity and change of primary outcome variables. (Main and interaction effects)
| Outcomes | Predictors | 95% CI of B | β | p | |
| Main effect only | ΔMMSE | Baseline ChEI | –2.289 (–3.815 – –0.764) | –0.302 | 0.004* |
| Amyloid positivity | –0.115 (–1.708 –1.478) | –0.016 | 0.886 | ||
| ΔCERAD-TS | Baseline ChEI | –11.722 (–15.825 – –7.620) | –0.479 | <0.001* | |
| Amyloid positivity | –1.841 (–6.068–2.387) | –0.077 | 0.390 | ||
| ΔCDR-SOB | Baseline ChEI | 1.117 (0.325–1.910) | 0.299 | 0.006* | |
| Amyloid positivity | 0.337 (–0.358–1.032) | 0.092 | 0.338 | ||
| Main &interaction effect | ΔMMSE | Baseline ChEI | –2.666 (–4.922––0.411) | –0.352 | 0.021 |
| Amyloid positivity | –0.355 (–2.270–1.560) | –0.048 | 0.714 | ||
| Baseline ChEI X Amyloid Positivity | 0.661 (–2.240–3.561) | 0.082 | 0.653 | ||
| ΔCERAD-TS | Baseline ChEI | –12.195 (–18.954––6.877) | –0.527 | <0.001* | |
| Amyloid positivity | –2.698 (–7.996–2.600) | –0.113 | 0.315 | ||
| Baseline ChEI X Amyloid Positivity | 2.175 (–5.877–10.226) | 0.083 | 0.593 | ||
| ΔCDR-SOB | Baseline ChEI | 1.377 (0.245–2.509) | 0.368 | 0.018 | |
| Amyloid positivity | 0.507 (–0.366–1.380) | 0.139 | 0.252 | ||
| Baseline ChEI X Amyloid Positivity | –0.427 (–1.750–0.896) | –0.107 | 0.524 |
Adjusted variables; Age, Sex, Education, APOE4 positivity, baseline outcome value, Vascular score, baseline memantine use. *p < 0.017; ChEI, cholinesterase inhibitor; CDR-SOB, Clinical Dementia Rating Sum of Box; MMSE, Mini-Mental Status Examination; CERAD-TS, The Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Total score.
Fig. 2
Change of outcome variables according to ChEI use and amyloid positivity (raw data). A) Change of MMSE according to ChEI use and amyloid positivity; B) Change of CERAD-TS according to ChEI use and amyloid positivity; C) Change of CDR-SOB according to ChEI use and amyloid positivity. Amyloid X ChEI interaction term was derived from GLM adjusted by age, sex, education, APOE4 positivity, baseline outcome value, vascular risk score, and baseline memantine use. The standardized coefficient and p value are represented. ChEI, cholinesterase inhibitor; VRS, vascular risk score; CDR-SOB, Clinical Dementia Rating Sum of Box; MMSE, Mini-Mental Status Examination; CERAD-TS, The Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Total score.
DISCUSSION
Amnestic MCI patients likely exhibit different pathophysiological characteristics based on the presence of amyloid pathology. We hypothesized that amyloid positivity moderates the effect of ChEI on cognitive decline. However, contrary to our hypothesis, we did not find evidence of such moderation by amyloid positivity for the ChEI effect on cognitive change. This suggests that the presence of brain Aβ deposition cannot explain the inconsistent and non-significant findings from previous ChEI trials in MCI patients. It also suggests that the action of ChEI, increasing cholinergic neurotransmission, may not be related to the presence of amyloid pathology. Cholinergic deficit, the target of ChEI, has been observed not only in AD dementia but also in other types of cognitive disorders, including vascular dementia and dementia with Lewy bodies.24–26
ChEI use was associated with more rapid cognitive decline (i.e., greater decreases in MMSE score and CERAD-TS and greater increases in CDR-SOB score over 2 years) regardless of amyloid positivity. This finding aligns with results from other studies that retrospectively analyzed data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort.27,28 The negative effect of ChEI treatment on cognitive decline observed in observational cohorts is likely due to differences in baseline disease severity. More severely impaired AD cases tend to progress more rapidly than less severe cases.29 In clinical practice for MCI, patients with more severe cognitive impairment are likely to receive ChEI treatment, leading to a selection bias.30 As shown in Table 1, the ChEI use group had more advanced cognitive impairment at baseline. Although the baseline values of each outcome variable were adjusted as covariates, it may not have been possible to fully control the influence of baseline disease severity on progression speed because the two groups could not be entirely matched in terms of severity, as is done in randomized controlled trials.
This study stands out by being the first to explore whether the presence of AD pathology, especially amyloid pathology, moderates the impact of ChEI use on cognitive change. However, several limitations require attention. First, this study did not rely on a randomized clinical trial but rather on retrospective analyses of observational cohort study data. Consequently, latent confounding variables could not be adequately controlled, and the comparison groups were not well-matched in terms of baseline characteristics. Thus, randomized controlled trials are still necessary to address the issues we have raised more rigorously. Second, the sample size of the study population may not be sufficiently large to conclusively establish the moderating effect of AD pathology.
In conclusion, our findings do not support the possibility that AD pathology, particularly Aβ deposition, moderates the effect of ChEI on cognitive decline in MCI.
AUTHOR CONTRIBUTIONS
Gihwan Byeon (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Software; Validation; Visualization; Writing – original draft; Writing – review & editing); Min Soo Byun (Funding acquisition; Investigation; Methodology; Project administration; Resources); Dahyun Yi (Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources); Hyejin Ahn (Data curation; Investigation; Project administration; Resources); Gijung Jung (Data curation; Methodology; Project administration; Resources); Yun-Sang Lee (Investigation; Methodology; Project administration; Resources); Yu Kyeong Kim (Investigation; Methodology; Resources); Koung Mi Kang (Investigation; Methodology; Project administration; Resources; Software); Chul-Ho Sohn (Methodology; Project administration; Resources; Software); Dong Young Lee (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing).
ACKNOWLEDGMENTS
We gratefully acknowledge the cooperation and patience of all individuals who participated in this study. We also appreciate Eisai Korea, Inc. for grant support.
FUNDING
This study was supported by a grant from the Ministry of Science and ICT, Republic of Korea (grant No: NRF-2014M3C7A1046042), a grant from the Ministry of Health & Welfare, Republic of Korea (HI18C0630 & HI19C0149), a grant from the Seoul National University Hospital, Republic of Korea (No. 3020200030), a grant from the National Institute on Aging, United States of America (U01AG072177), and a research grant from Eisai Korea, Inc. The funding source had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit it for publication.
CONFLICT OF INTEREST
Dong Young Lee received research funding from the company Eisai Korea, Inc. The other authors declare no conflict of interest.
DATA AVAILABILITY
The datasets generated and analyzed during the present study are not publicly available, owing to ethics considerations and privacy restrictions. Data might be obtained from the corresponding author after approval by the Institutional Review Board of the Seoul National University Hospital, South Korea.
REFERENCES
1. | Lanctôt KL , Herrmann N , Yau KK , et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. Can Med Assoc J (2003) ; 169: : 557–564. |
2. | Gauthier S , Reisberg B , Zaudig M , et al. Mild cognitive impairment. Lancet (2006) ; 367: : 1262–1270. |
3. | Russ TC and Morling JR . Cholinesterase inhibitors for mild cognitive impairment Cochrane Database Syst Rev (2012) ; 9: : CD009132. |
4. | Tricco AC , Soobiah C , Berliner S , et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. Can Med Assoc J (2013) ; 185: : 1393–1401. |
5. | He J , Farias S , Martinez O , et al. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol (2009) ; 66: : 1393–1399. |
6. | Petersen RC , Thomas RG , Grundman M , et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med (2005) ; 352: : 2379–2388. |
7. | Winblad B , Gauthier S , Scinto L , et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology (2008) ; 70: : 2024–2035. |
8. | Feldman HH , Ferris S , Winblad B , et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol (2007) ; 6: : 501–512. |
9. | Wolk DA , Price JC , Saxton JA , et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol (2009) ; 65: : 557–568. |
10. | Kim JY , Lim JH , Jeong YJ , et al. The effect of clinical characteristics and subtypes on amyloid positivity in patients with amnestic mild cognitive impairment. Dement Neurocogn Disord (2019) ; 18: : 130–137. |
11. | Pyun JM , Ryoo N , Park YH , et al. Change in cognitive function according to cholinesterase inhibitor use and amyloid PET positivity in patients with mild cognitive impairment. Alzheimers Res Ther (2021) ; 13: : 10. |
12. | Byun MS , Yi D , Lee JH , et al. Korean Brain Aging Study for the early diagnosis and prediction of Alzheimer’s disease: methodology and baseline sample characteristics. Psychiatry Investig (2017) ; 14: : 851–863. |
13. | Albert MS , DeKosky ST , Dickson D , et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement (2011) ; 7: : 270–279. |
14. | Lee DY , Lee KU , Lee JH , et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J Int Neuropsychol Soc (2004) ; 10: : 72–81. |
15. | Lee JH , Lee KU , Lee DY , et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci (2002) ; 57: : P47–53. |
16. | Morris JC . The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology (1993) ; 43: : 2412–2414. |
17. | Chandler MJ , Lacritz LH , Hynan LS , et al. A total score for the CERAD neuropsychological battery. Neurology (2005) ; 65: : 102–106. |
18. | Seo EH , Lee DY , Lee JH , et al. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry (2010) ; 18: : 801–809. |
19. | DeCarli C , Mungas D , Harvey D , et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology (2004) ; 63: : 220–227. |
20. | Park JC , Han SH , Cho HJ , et al. Chemically treated plasma Aβ is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Ther (2017) ; 9: : 20. |
21. | Park JC , Han SH , Yi D , et al. Plasma tau/amyloid-β1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain (2019) ; 142: : 771–786. |
22. | Reiman EM , Chen K , Liu X , et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A (2009) ; 106: : 6820–6825. |
23. | Choe YM , Sohn BK , Choi HJ , et al. Association of homocysteine with hippocampal volume independent of cerebral amyloid and vascular burden. Neurobiol Aging (2014) ; 35: : 1519–1525. |
24. | Wang J , Zhang HY and Tang XC . Cholinergic deficiency involved in vascular dementia: possible mechanism and strategy of treatment. Acta Pharmacol Sin (2009) ; 30: : 879–888. |
25. | Tiraboschi P , Hansen LA , Alford M , et al. Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Arch Gen Psychiatry (2002) ; 59: : 946–951. |
26. | Hirano S , Shinotoh H , Shimada H , et al. Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain (2010) ; 133: : 2058–2068. |
27. | Schneider LS , Insel PS and Weiner MW . Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch Neurol (2011) ; 68: : 58–66. |
28. | Kennedy RE , Cutter GR , Fowler ME , et al. Association of concomitant use of cholinesterase inhibitors or memantine with cognitive decline in Alzheimer clinical trials: a meta-analysis. JAMA Netw Open (2018) ; 1: : e184080. |
29. | Zahodne LB , Wall MM , Schupf N , et al. Late-life memory trajectories in relation to incident dementia and regional brain atrophy. J Neurol (2015) ; 262: : 2484–2490. |
30. | Haneuse S . Distinguishing selection bias and confounding bias in comparative effectiveness research. Med Care (2016) ; 54: : e23–e29. |



