Association of Anxiety and Unspecified Emotional Distress Obtained from a Medical Records Linkage System with Incident Cognitive Outcomes in a Population-Based Setting
Abstract
Background:
Studies that assess cognition prospectively and study in detail anxiety history in the participants’ medical records within the context of brain aging and Alzheimer’s disease are limited.
Objective:
To examine the associations of anxiety and unspecified emotional distress (UED) acquired throughout a person’s life with prospectively collected cognitive outcomes.
Methods:
Mayo Clinic Study of Aging participants who were cognitively unimpaired at baseline were included. Anxiety and UED data were abstracted from the medical record using the Rochester Epidemiology Project (REP) resources and were run separately as predictors in our models. The data were analyzed using Cox proportional hazards models for the outcomes of incident mild cognitive impairment (MCI) and dementia and using linear mixed effects models for the outcomes of global and domain specific cognitive z-scores and included key covariates.
Results:
The study sample (n = 1,808) had a mean (standard deviation) age of 74.5 (7.3) years and 51.4% were male. Anxiety was associated with increased risk of MCI and dementia and was associated with lower baseline cognitive z-scores and accelerated decline over time in the global, memory, and attention domains. UED was associated with faster decline in all domains except visuospatial but did not show evidence of association with incident cognitive outcomes. These results varied by medication use and timing of anxiety.
Conclusions:
Anxiety and UED both showed inverse associations with cognition. Utilization of anxiety and UED data from across the life course, as available, from the REP system adds robustness to our results.
INTRODUCTION
The association between anxiety and cognition in older adults has been previously reported. Studies have found that anxiety is associated with worse cognition over time1,2 and a higher risk of mild cognitive impairment (MCI)3,4 and dementia.5,6 The direction of causality between anxiety and cognitive decline remains elusive.7
A limiting factor in trying to elucidate this relationship is that most studies only have anxiety data collected from study visits, but have little, if any, knowledge of anxiety across a person’s life course. By abstracting anxiety data from the medical record throughout participants’ lives, as available, we were able to ascertain a more accurate history and onset of anxiety, compared to a person’s recollection. The study aimed to examine three research questions: 1) What is the association between anxiety, as abstracted from the medical record, and cognitive decline or impairment?; 2) How is anxiety with and without medication associated with cognitive outcomes?; and 3) Does the strength of the association of anxiety with cognitive outcomes vary by time of onset of anxiety? We studied these questions in a cohort of Mayo Clinic Study of Aging (MCSA) participants on whom we had abstracted anxiety data across their life course, as available, from the Rochester Epidemiology Project (REP) data resource.
Another novel aspect of our study, in addition to having this medical record abstracted anxiety data, is that we also collected unspecified emotional distress (UED) from the medical record. This allowed us to assess how emotional distress not reaching the level of anxiety or perhaps different in nature from anxiety is related to cognitive outcomes. A person may experience UED in the context of life events or psychosocial development with aging, and so, by studying UED here, we hope to shed new light on previous work that considered a model of development across the lifespan.8 We analyzed anxiety and UED in relation to cognition in two ways: using the categorical outcomes of incident MCI and dementia and using continuous cognitive composite z-scores.
MATERIALS AND METHODS
Study design: a prospective cohort study
The MCSA is a population-based study being carried out in Olmsted County, Minnesota, USA. Full details of the study have been published elsewhere.9 The study randomly samples individuals residing in this county using an age/sex stratification scheme. While the study focuses on persons that are mid-life and older (i.e., 50 + years in age), sampling does go down to the age of 30 years. If a person agrees to join the study, they participate in a baseline visit and then follow-up visits approximately every 15 months thereafter. The MCSA study approval was obtained from the Institutional Review Boards of the Mayo Clinic and Olmsted Medical Center in Rochester, Minnesota. Participants provided written informed consent before participation. In the case of participants with cognitive impairment sufficient to interfere with capacity, assent was obtained from a legally authorized representative.
In the present study, we included MCSA participants aged 60 years old and older at the time of the current study onset in 2018 (although some were slightly younger than 60 at MCSA baseline), who had undergone at least one neuroimaging study (brain MRI, PiB-PET, FDG-PET), were cognitively unimpaired at MCSA baseline, had neuropsychiatric assessment at baseline (at least one of Beck Anxiety Inventory [BAI], Beck Depression Inventory II [BDI-II], or Neuropsychiatric Inventory Questionnaire [NPI-Q]), and at least one follow-up, and who had not refused permission to use their medical records for research (Minnesota Research Authorization).10 The present study was approved by the institutional review boards of the Mayo Clinic and the Olmsted Medical Center in Rochester, Minnesota.
Participants’ assessment
All participants went through an exam. During the visit, they were seen by a study coordinator who administered multiple tests including the Beck Anxiety Inventory.11 The study coordinator also administered the Neuropsychiatric Inventory Questionnaire12 to the participant’s informant. A neurologic exam was performed by a behavioral neurologist. Neuropsychological tests were administered by psychometrists with supervision by neuropsychologists. These neuropsychological tests are grouped into four domains: memory13,14 (Wechsler Memory Scale – Revised: Logical Memory II Total Score, Wechsler Memory Scale – Revised: Visual Reproduction II Total Score, Auditory Verbal Learning Test: Half Hour Delay), attention/executive15,16 (Wechsler Adult Intelligence Scale – Revised: Digit Symbol Total Score, Trail Making Test Part B), language17,18 (Boston Naming Score, Category Fluency Total Score), and visuospatial15 (Wechsler Adult Intelligence Scale – Revised: Picture Completion Total Score, Wechsler Adult Intelligence Scale – Revised: Block Design Total Score). A z-score for each domain was computed by z-scoring (i.e., subtracting mean and dividing by standard deviation) the individual tests included in a given domain, averaging those z-scores, and then z-scoring that average. A global cognition z-score was computed by z-scoring the average of the four domain z-scores. A consensus diagnosis made by the behavioral neurologist, psychometrist, and study coordinator of cognitively unimpaired (CU),19 - 22 MCI according to the Mayo Clinic criteria for MCI,23,24 or dementia25 was assigned. This allowed us to assess the development of incident MCI or dementia. The above participant assessment details can also be found elsewhere with further detail.26,27 For those that drop out of the study, we were able to follow them passively through the medical record to see if dementia was developed after study discontinuation.
Medical record neuropsychiatric symptoms abstraction
The REP medical record linkage system10,28,29 is one of the unique resources in the world to investigate the incidence and natural course of several diseases and has been in existence since 1966 with foundations going back to 1907. Using this resource, we were able to abstract data on anxiety and UED. Nurse abstractors trained in extracting data from the REP reviewed and abstracted the data. Details of the REP data resources have been described in detail previously.30
A person was defined as having anxiety the first time an anxiety disorder diagnosis was documented in the patient record; in addition, an anxiety diagnosis was recorded by the abstractor if the patient did not have a documented diagnosis but had anxiety symptoms and received medication (see Supplementary Table 3 for a complete list of medications considered). For the present study, a person was defined as having anxiety the first time anxiety was documented on the record or the first time of medication use for anxiety, whichever was earlier. We also collected information on UED that was not formally diagnosed as a specific psychiatric disorder but was recorded in the medical record in the context of retirement or other life events [i.e., relationship problems, job loss, distress in the context of the daily hassles of life (i.e., conflict at work, social media stress), pet loss, other stress, personality traits (i.e., nervous or anxious personality traits, etc.), or repeated mention over time of stress-related events, etc.]. This allowed us to document emotional distress not reaching the level of the diagnosis of an anxiety disorder. Regarding the timing of UED, we used a similar approach as used with anxiety disorder i.e., a person was defined as having UED at the first time it was found in the medical record, or at the first time medication was used for UED, whichever was earlier. The latter was rarer in the case of UED. There were also cases for both anxiety and UED where exact time of diagnosis was unknown or unclear, and so, time of medication use was used for defining timing of onset. If the first diagnosis came after MCSA baseline, then, for the purposes of this analysis, these neuropsychiatric symptoms were defined as not present. Since we only had year of anxiety diagnosis and year of medication use available, we defined the exact date to be the middle day of the given year (i.e., July 2) for the purposes of comparing to the MCSA baseline date. If anxiety was present but both time of anxiety diagnosis and time of medication use for anxiety were missing, then anxiety was also set to missing for this analysis since we could not determine the timing of diagnosis relative to MCSA baseline. The same was done for UED. Only four had to be set to missing for anxiety and seven for UED. Medical record abstraction was stopped if a diagnosis of dementia was documented in the record.
Statistical methods
Descriptive statistics were calculated and presented as mean (standard deviation [SD]) for continuous variables or frequencies (N) with percentages (%) for categorical variables. In our models, we used two different formulations of the anxiety and UED variables: presence before study entry in the medical record and considering medication use. The variables considering medication use have three levels: No anxiety, anxiety without medication use, anxiety with medication use. The variable considering medication use for UED was defined similarly. As was the case for defining the presence of anxiety and UED variables, the variables considering medication were not able to be computed in cases where medication was indicated but no year of medication use was available. This led to one additional missing value for analyses considering anxiety medication use and two additional missing values for analyses considering UED medication use. We also created a four-level variable for anxiety: No anxiety, anxiety before study entry only, anxiety at study baseline only, and anxiety at study baseline and before. This variable utilized the medical record abstracted anxiety data from before MCSA baseline as well as the BAI and the anxiety item from the NPI-Q collected at MCSA baseline in its calculation. For the BAI, scores 8 and higher were considered as having anxiety (i.e., more than minimal symptoms, indicating clinical anxiety), and presence of anxiety of any severity captured on the NPI-Q was considered anxiety. To be precise, the categories of the four-level variable were defined as follows: 1) No anxiety if anxiety was not found in the medical record before study entry and at least one of the BAI or the NPI-Q anxiety item were filled out at baseline and did not show anxiety; 2) Anxiety before study entry only if anxiety was found in the medical record before study entry and at least one of the BAI or the NPI-Q anxiety item were filled out at baseline and did not show anxiety; 3) Anxiety at study baseline only if no anxiety was found in the medical record prior to joining the study but either the BAI or the NPI-Q anxiety item indicated anxiety at baseline; 4) Anxiety at study baseline and before if anxiety was found in the medical record before study entry and either the BAI or the NPI-Q anxiety item indicated anxiety at baseline. This variable was not able to be computed for one person that was missing both BAI and NPI-Q anxiety data as well as for the four mentioned earlier for which medical record anxiety timing was indeterminate. An analogous variable for UED was not able to be computed as we do not have an in-study measure of UED.
To ascertain the relationship of anxiety and UED with the outcomes of incident MCI and incident dementia, Cox proportional hazards models with age as the time scale adjusting for sex, education, APOE ɛ4 allele status, and Charlson comorbidity index31 were run. Linear mixed effects models with participant-specific intercepts and slopes for time were run to estimate the associations of anxiety or UED with longitudinal trajectories of global and domain-specific cognitive z-scores. The models were adjusted for age at baseline, sex, education, APOE ɛ4 allele status, baseline Charlson comorbidity index, and whether this was the first time taking the cognitive test battery. Cox proportional hazards models and linear mixed effects models were also run additionally adjusting for study baseline BDI-II. These results were largely similar, and therefore, not presented, except for one notable difference, which is covered in the discussion. Statistical significance was defined using the usual alpha level of 0.05. Data preparation and statistical analysis was executed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Participant demographics
The sample consisted of 1,808 MCSA participants who were cognitively unimpaired at MCSA baseline. The sample had 930 males (51.4%) and 472 participants were APOE ɛ4 allele carriers (26.1%). The mean (SD) for age at MCSA baseline was 74.5 (7.3) years, and participants had a mean (SD) education of 14.5 (2.8) years and Charlson index of 3.0 (3.0). Anxiety was found in the medical record prior to study entry for 594 (32.9%) participants. This number for UED was 549 (30.5%). Among participants with anxiety, 404 (68.1%) took medication, and among those with UED, 81 (14.8%) took medication. When considering the timing of anxiety, 468 (26.0%) had anxiety before study entry only (as assessed by medical record review), 110 (6.1%) had anxiety at study baseline only (as assessed by BAI/NPI-Q anxiety item), and 125 (6.9%) had anxiety both before study entry and at study baseline (as assessed by medical record review and BAI/NPI-Q anxiety item, respectively). There were 490 (27.1%) individuals who progressed to MCI, and 292 (16.2%) that progressed to dementia. Table 1 contains a summary of the sample.
Table 1
Summary at MCSA baseline
| (N = 1,808) | |
| Age, mean (SD) | 74.532 (7.251) |
| Male (sex) | 930 (51.4) |
| APOE ɛ4 Allele carrier | 472 (26.1) |
| Education (y), mean (SD) | 14.542 (2.752) |
| Charlson comorbidity index, mean (SD) | 3.034 (2.989) |
| Anxietya | 594 (32.9) |
| Without medicationb | 189 (31.9) |
| With medicationb | 404 (68.1) |
| Timing of anxiety | |
| Before study only | 468 (26.0) |
| Study baseline only | 110 (6.1) |
| Prior to and at baseline | 125 (6.9) |
| Unspecified emotional distressc | 549 (30.5) |
| Without medicationd | 466 (85.2) |
| With medicationd | 81 (14.8) |
| Follow-up | |
| Incident MCI | 490 (27.1) |
| Incident dementia | 292 (16.2) |
Values presented are N (%) unless otherwise noted. SD, standard deviation; MCI, mild cognitive impairment; APOE, Apolipoprotein E. a4 missing for anxiety presence, 5 missing when considering medication use, 5 missing for anxiety timing. bPercentage computed relative to those with anxiety who had medication information available (n = 593). c7 missing for presence of unspecified emotional distress and 9 missing when considering medication use. dPercentage computed relative to those with unspecified emotional distress who had medication information available (n = 547).
Results on categorical outcomes of incident mild cognitive impairment and dementia
Participants with anxiety prior to study entry (HR = 1.425, 95% CI: 1.179 to 1.722) had an increased risk of developing MCI at any given time compared to those without anxiety. Presence of UED (HR = 1.160, 95% CI: 0.957 to 1.406) was not found to be associated with increased risk. Anxiety was associated with increased risk of MCI regardless of medication use. The results for the outcome of incident dementia were very similar.
Considering timing of anxiety, participants with anxiety before study entry only, at study baseline only, and at both times all exhibited increased risk of developing MCI. The hazard ratio was the highest for those with anxiety at both times. These results were similar for the outcome of incident dementia with the exception that those with anxiety at study baseline only did not show evidence of increased risk of dementia. Median follow-up time for the outcome of incident MCI was 9 years, and for incident dementia, it was 10 years. All Cox proportional hazards model results can be found in Table 2.
Table 2
Cox proportional hazards models
| Incident MCI | Incident dementia | ||||||
| Predictor | N | MCI | HR (95% CI) | p | Dementia | HR (95% CI) | p |
| Anxiety | 1,804 | 487 | 1.425 (1.179, 1.722) | <0.001 | 291 | 1.628 (1.277, 2.075) | <0.001 |
| w/o Medication | 1,803 | 487 | 1.426 (1.084, 1.876) | 0.011 | 291 | 1.707 (1.218, 2.391) | 0.002 |
| w/ Medication | 1.431 (1.152, 1.779) | 0.001 | 1.593 (1.203, 2.110) | 0.001 | |||
| Before study only | 1,803 | 487 | 1.479 (1.198, 1.825) | <0.001 | 291 | 1.463 (1.116, 1.917) | 0.006 |
| Study baseline only | 1.456 (1.038, 2.041) | 0.029 | 0.829 (0.498, 1.380) | 0.471 | |||
| Both | 1.578 (1.125, 2.214) | 0.008 | 2.173 (1.448, 3.260) | <0.001 | |||
| UED | 1,801 | 484 | 1.160 (0.957, 1.406) | 0.130 | 289 | 1.245 (0.976, 1.587) | 0.078 |
| w/o Medication | 1,799 | 483 | 1.211 (0.991, 1.481) | 0.062 | 288 | 1.270 (0.984, 1.638) | 0.066 |
| w/ Medication | 0.882 (0.565, 1.378) | 0.582 | 1.072 (0.629, 1.825) | 0.799 | |||
Cox proportional hazards models with age as time scale and adjusted for sex, education, APOE ɛ4 allele status, and Charlson comorbidity index. MCI, mild cognitive impairment; HR, hazard ratio; CI, confidence Interval; w/o, without; w/, with; UED, unspecified emotional distress. All HR estimates are computed relative to not having the given neuropsychiatric symptom.
Results on continuous cognitive composite z-scores
The linear mixed effects models revealed that anxiety (Beta=– 0.138, 95% CI: – 0.219 to – 0.058) was associated with lower global cognition z-scores at baseline. Evidence of a baseline association with lower global cognition z-scores was not seen for UED. Participants without anxiety or UED decreased by about 0.09 SD per year in global cognition. Having anxiety or UED accelerated this decline. For example, those with anxiety declined in global cognition by 0.104 (beta for time+beta for interaction=– 0.088– 0.016) SD annually. This increased decline was seen for those not taking and those taking medication for anxiety, although the latter did not reach statistical significance. Increased decline over time was only exhibited for those not taking medication for UED. Anxiety was associated with a faster decline in global cognition for those with anxiety before study entry only and for those with anxiety prior to study and at study baseline, but not for those with anxiety indicated only at study baseline.
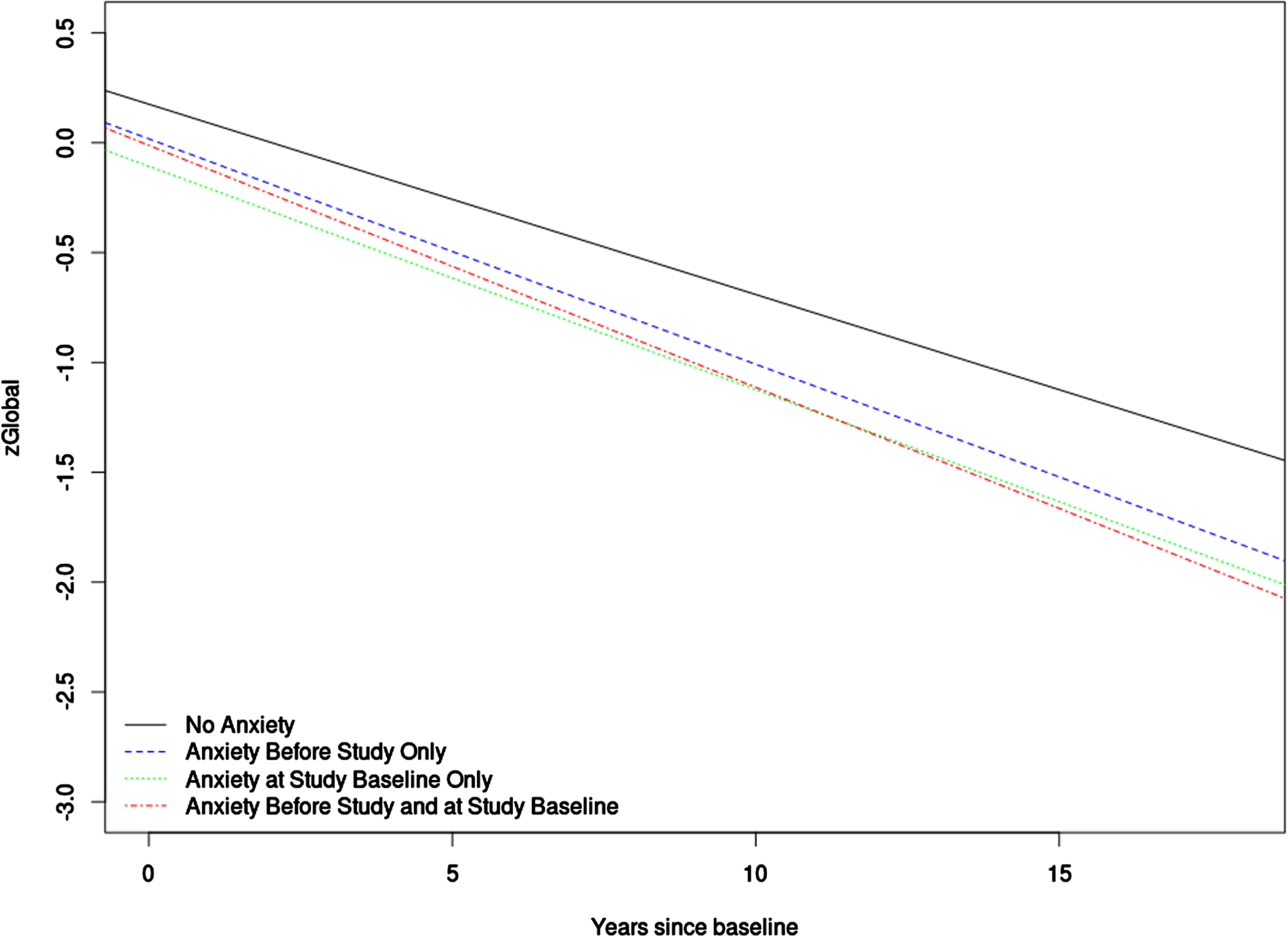
The results were largely similar for the outcomes of memory z-score and attention z-score. Anxiety was related to lower z-scores at baseline and faster decline over time, and UED only exhibited longitudinal association. The outcomes language z-score and visuospatial z-score showed some departures from this pattern as anxiety was not associated with faster decline in language or visuospatial z-scores, and UED did not show longitudinal association with visuospatial z-scores. Median follow-up time for the linear mixed effects models was 8 years. All linear mixed effects models results can be found in Table 3 and Supplementary Tables 1 and 2. Figure 1 contains a plot to help visualize the trajectories of the global z-score for the different anxiety timing groups.
Table 3
Linear mixed effects models
| zGlobal | ||
| Predictor | Na | Beta (95% CI) |
| Anxiety | 11,335 | – 0.138 (– 0.219, – 0.058) |
| Time | – 0.088 (– 0.095, – 0.081) | |
| Anxiety*time | – 0.016 (– 0.029, – 0.004) | |
| Anxiety w/o medication | 11,328 | – 0.135 (– 0.258, – 0.012) |
| Anxiety w/ medication | – 0.141 (– 0.233, – 0.049) | |
| Time | – 0.088 (– 0.095, – 0.081) | |
| Anxiety w/o medication*time | – 0.021 (– 0.040, – 0.002) | |
| Anxiety w/ medication*time | – 0.014 (– 0.028, 0.000) | |
| Anxiety before study only | 11,331 | – 0.158 (– 0.245, – 0.070) |
| Anxiety study baseline only | – 0.282 (– 0.440, – 0.125) | |
| Anxiety both | – 0.187 (– 0.337, – 0.038) | |
| Time | – 0.087 (– 0.094, – 0.079) | |
| Anxiety before study only*time | – 0.016 (– 0.030, – 0.002) | |
| Anxiety study baseline only*time | – 0.015 (– 0.041, 0.010) | |
| Anxiety both*time | – 0.024 (– 0.047, 0.000) | |
| UED | 11,318 | – 0.037 (– 0.118, 0.044) |
| Time | – 0.088 (– 0.096, – 0.081) | |
| UED*time | – 0.015 (– 0.028, – 0.002) | |
| UED w/o medication | 11,303 | – 0.034 (– 0.120, 0.052) |
| UED w/ medication | – 0.050 (– 0.229, 0.128) | |
| Time | – 0.088 (– 0.096, – 0.081) | |
| UED w/o medication*time | – 0.014 (– 0.027, 0.000) | |
| UED w/ medication*time | – 0.021 (– 0.049, 0.007) | |
Linear mixed effects models with participant-specific intercepts and slopes for time. All models adjusted for age at baseline, sex, education, APOE ɛ4 allele status, baseline Charlson comorbidity index, and whether this was the first time taking the cognitive test battery. aIncludes all longitudinal observations. CI, confidence interval; Time, years since study baseline; w/o, without; w/, with; UED, unspecified emotional distress. The reference level is not having the given neuropsychiatric symptom. Statistically significant results are bolded.
Fig. 1
This figure shows a plot of the linear mixed effects model for anxiety timing predicting global z-score. For the purposes of plotting, average values were used for numeric covariates and 0.5 was used for dichotomous covariates.
DISCUSSION
While the association between anxiety and cognitive outcomes (both incident MCI/dementia and continuous scores of cognitive trajectories) has been well-examined as noted in the introduction, our study enriched this literature by utilizing anxiety data that was abstracted from the medical record, where available, in addition to data collected at the MCSA visits, and we employed the novel measure of UED. We found anxiety was associated with increased risk of developing MCI and dementia, lower baseline cognitive scores, and accelerated downward trajectory over time in global cognition, memory, and attention/executive z-scores. These results are in line with our previous work in the MCSA using anxiety data collected from study visits as we have previously seen anxiety to be associated with increased risk of MCI4 and with accelerated global cognitive decline.1 Some of our prior work also departs from the present results though. For example, we have previously found anxiety to not be statistically significantly associated with increased risk of incident dementia.32 It should be noted, too, that the above-mentioned study showing increased global decline for those with anxiety saw evidence for this association when using the NPI-Q but not when using the BAI. This underscores the importance of considering the anxiety collection tool (NPI-Q, BAI, abstraction from the medical record, etc.) when interpreting results. Participants with UED showed accelerated downward cognitive trajectory in most domains when compared to those without UED. Those with UED were not found to have statistically significantly higher risk of developing MCI and dementia. In models run not including Charlson comorbidity index as a covariate (not presented), those with UED showed higher risk of developing MCI and dementia suggesting that UED may be partially capturing distress related to worse comorbidity burden.
Publications from other groups have likewise shown mixed results relative to those presented here. In corroboration of our results, it was seen that anxiety was associated with decline in symbol digit modality,2 and anxiety was seen to be related to increased risk of dementia.5,6 On the other hand, one of the studies just noted showed that the results were no longer statistically significant after additionally adjusting for Clinical Dementia Rating.5 One of the latter studies also saw that anxiety was not associated with increased risk of MCI,6 and another study showed anxiety was not a predictor of later cognitive decline.33 Again, it is important to consider how the anxiety data was collected as well as how cognitive decline was quantified. Differences in sample composition could explain some of the differences in results too.
We further considered medication use in our analysis. Anxiety, regardless of medication use, was associated with increased risk for incident MCI and dementia. We observed that participants with anxiety showed increased decline in global cognition for both those taking and not taking medication relative to those without anxiety, although the former did not quite reach statistical significance. UED only exhibited accelerated decline for those not taking medication, which could be due to not many taking medication for UED. These associations were similar for the memory and attention domains, with the exception that anxiety only showed accelerated decline for those taking medication.
A key feature of our study was that we had anxiety data abstracted from the medical record, which allowed us to determine whether participants had anxiety before study entry. Anxiety was associated with increased risk of developing MCI regardless of whether it was first seen before study entry or at study baseline. It should be noted that the highest hazard ratio estimate for anxiety was for those with anxiety in the medical record before study entry that also had anxiety at study baseline. The results for the outcome of dementia were mostly similar with the exception being that those with anxiety at study baseline only did not exhibit increased risk of developing dementia. The linear mixed effects models showed accelerated decline in the global z-scores for those with anxiety before study entry only and for those with anxiety both before study entry and at study baseline. It should be noted that including BDI-II from MCSA baseline as a covariate in the Cox and linear mixed effects models yieled mostly similar results (not presented) across all models; the major exception being the model with anxiety timing as the predictor and incident MCI as the outcome. In this model, the associations for those with anxiety at study baseline, regardless of whether anxiety was in the medical record before study baseline, were attenuated and not statistically significant when also adjusting the models for BDI-II from study baseline. This suggests that co-occurring depression could be playing a role in these associations.
The present study has multiple strengths. First, it was carried out in a large sample from the MCSA, which is a study that has been in operation since 2004 and has amassed a rich database. Second, in contrast to most studies, we were able to abstract anxiety data from across the lifespan, as available, going back several decades, to provide a better and more complete picture of anxiety status. There is, of course, the limitation that we cannot capture time living away from area covered by the REP medical records linkage system. Although our study helps to expand the picture in terms of the relationship between anxiety and cognitive decline using the REP resources, the results should still be interpreted with caution, particularly in regard to considering causation. We acknowledge, too, that our definition of UED left room for some subjectivity on the part of the data abstractor, which limits replicability, but nevertheless, we find that UED is a valuable part of this study. Finally, more research in diverse populations is warranted as participants in the present study were 98.7% White, and 99.5% were not Hispanic or Latino. However, it has been shown that data from Olmsted County are generalizable to the U.S. population of Minnesota and the Upper Midwest.34
Overall, this study confirms that anxiety is associated with cognitive decline as well as development of MCI and dementia and demonstrated the need to consider timing of onset and potential persistence of anxiety. It also showed that UED is associated with cognitive decline. This might be an important finding that warrants further examination of neuropsychiatric symptoms different in nature and severity than traditionally collected neuropsychiatric symptoms. We hope that this study will spur future work examining more deeply the associations of anxiety and UED with cognitive decline both by our team and others.
AUTHOR CONTRIBUTIONS
Jeremy A. Syrjanen (Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing); Janina Krell-Roesch (Investigation; Methodology; Writing – review & editing); Walter K. Kremers (Formal analysis; Methodology); Julie A. Fields (Writing – review & editing); Eugene L. Scharf (Writing – review & editing); David S. Knopman (Funding acquisition; Writing – review & editing); Ronald C. Petersen (Funding acquisition; Writing – review & editing); Maria Vassilaki (Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – review & editing); Yonas E. Geda (Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing – review & editing).
ACKNOWLEDGMENTS
The authors thank the participants and staff at the Mayo Clinic Study of Aging.
FUNDING
Support for this research was provided by NIH grants: National Institute on Aging (R01 AG057708, U01 AG006786, P30 AG062677, R37 AG011378, R01 AG041851, R01 NS097495); the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program; the GHR Foundation; the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic; the Liston Award; the Schuler Foundation; the Mayo Foundation for Medical Education and Research; the Arizona Alzheimer’s Consortium; the Barrow Neurological Foundation; and used the resources of the Rochester Epidemiology Project (REP) medical records linkage system, which is supported by the National Institute on Aging (AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users.
CONFLICT OF INTEREST
Dr. Kremers receives research funding from NIH. Dr. Fields serves as a consultant for Medtronic, Inc. and receives research support from the NIH. Dr. Scharf is a consultant for Boston Scientific but receives no compensation. Dr. Knopman serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network Treatment Unit study. He was an investigator in Alzheimer clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California, both of which have ended, and is currently an investigator in a trial in frontotemporal degeneration with Alector. He has served as a consultant for Roche, AriBio, Linus Health, Biovie and Alzeca Biosciences but receives no personal compensation. He receives funding from the NIH. Dr. Petersen has consulted for Roche, Inc.; Genentech, Inc.; Eli Lilly, Inc.; Nestle, Inc. and Eisai, Inc.; a DSMB for Genentech, Inc. and receives royalties from Oxford University Press for Mild Cognitive Impairment and from UpToDate. His research funding is from NIH/NIA. Dr. Vassilaki consulted for F. Hoffmann-La Roche Ltd, unrelated to this manuscript; she currently receives research funding from NIH and has equity ownership in Amgen, Johnson and Johnson, Medtronic, and Merck. Dr. Geda receives funding from Roche, served on the Lundbeck advisory board, and receives research funding from the NIH. Drs. Geda and Vassilaki are Editorial Board Members of this journal but were not involved in the peer-review process nor had access to any information regarding its peer-review. The other authors report no disclosures.
DATA AVAILABILITY
The data used in this study is available to qualified researchers upon reasonable request.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-240213.
REFERENCES
1. | Krell-Roesch J , Syrjanen JA , Machulda MM , et al. Neuropsychiatric symptoms and the outcome of cognitive trajectories in older adults free of dementia: The Mayo Clinic Study of Aging. Int J Geriatr Psychiatry (2021) ; 36: : 1362–1369. |
2. | Burhanullah MH , Tschanz JT , Peters ME , et al. Neuropsychiatric symptoms as risk factors for cognitive decline in clinically normal older adults: The Cache County Study. Am J Geriatr Psychiatry (2020) ; 28: : 64–71. |
3. | Wilson RS , Schneider JA , Boyle PA , et al. Chronic distress and incidence of mild cognitive impairment. Neurology (2007) ; 68: : 2085–2092. |
4. | Geda YE , Roberts RO , Mielke MM , et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry (2014) ; 171: : 572–581. |
5. | Rosenberg PB , Mielke MM , Appleby BS , et al. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry (2013) ; 21: : 685–695. |
6. | Kassem AM , Ganguli M , Yaffe K , et al. Anxiety symptoms and risk of dementia and mild cognitive impairment in the oldest old women. Aging Ment Health (2018) ; 22: : 474–482. |
7. | Geda YE , Krell-Roesch J , Sambuchi N , et al. Neuropsychiatric symptoms and neuroimaging biomarkers in Alzheimer disease: “Which is the cart and which is the horse?” Am J Geriatr Psychiatry (2017) ; 25: : 694–696. |
8. | Orenstein GA and Lewis L Eriksons Stages of Psychosocial Development. Treasure Island, FL: StatPearls Publishing, (2022) . |
9. | Roberts RO , Geda YE , Knopman DS , et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology (2008) ; 30: : 58–69. |
10. | St Sauver JL , Grossardt BR , Yawn BP , et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol (2011) ; 173: : 1059–1068. |
11. | Beck AT and Steer RA Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological CorHarcourt Brace Jovanovich, (1990) . |
12. | Kaufer DI , Cummings JL , Ketchel P , et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci (2000) ; 12: : 233–239. |
13. | Wechsler D . Wechsler memory scale-revised. New York, NY: The Psychological Corporation, (1987) . |
14. | Rey A . L’examen clinique en psychologie. Paris: Presses Universitaires de France, (1964) . |
15. | Wechsler D . Wechsler adult intelligence scale-revised. New York, NY: Psychological Corporation, (1981) . |
16. | Reitan RM . Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills (1958) ; 8: : 271–276. |
17. | Kaplan E , Goodglass H , Weintraub S . Boston Naming Test. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, (2001) . |
18. | Lucas JA , Ivnik RJ , Smith GE , et al. Mayo’s older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol (1998) ; 20: : 194–200. |
19. | Ivnik RJ , Malec JF , Smith GE , et al. Mayo’s older Americans normative studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol (1992) ; 6: (sup001): 1–30. |
20. | Ivnik RJ , Malec JF , Smith GE , et al. Mayo’s older Americans normative studies: WMS-R norms for ages 56 to 94. Clin Neuropsychol (1992) ; 6: (sup001), 49–82. |
21. | Ivnik RJ , Malec JF , Smith GE , et al. Mayo’s older Americans normative studies: updated AVLT norms for ages 56 to 97. Clin Neuropsychol (1992) ; 6: (sup001): 83–104. |
22. | Malec JF , Ivnik RJ , Smith GE , et al. Mayo’s older Americans normative studies: utility of corrections for age and education for the WAIS-R. Clin Neuropsychol (1992) ; 6: (sup001): 31–47. |
23. | Petersen RC . Mild cognitive impairment as a diagnostic entity. J Intern Med (2004) ; 256: : 183–194. |
24. | Winblad B , Palmer K , Kivipelto M , et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med (2004) ; 256: : 240–246. |
25. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders, DSM-IV. 4th edn. Washington, DC: American Psychiatric Association, (1994) . |
26. | Pink A , Krell-Roesch J , Syrjanen JA , et al. Interactions between neuropsychiatric symptoms and Alzheimer’s disease neuroimaging biomarkers in predicting longitudinal cognitive decline. Psychiatr Res Clin Pract (2023) ; 5: : 4–15. |
27. | Krell-Roesch J , Syrjanen JA , Vassilaki M , et al. Brain regional glucose metabolism, neuropsychiatric symptoms, and the risk of incident mild cognitive impairment: The Mayo Clinic Study of Aging. Am J Geriatr Psychiatry (2021) ; 29: : 179–191. |
28. | Rocca WA , Grossardt BR , Brue SM , et al. Data resource profile: expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol (2018) ; 47: : 368–368j. |
29. | Rocca WA , Yawn BP , St Sauver JL , et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc (2012) ; 87: : 1202–1213. |
30. | St Sauver JL , Grossardt BR , Yawn BP , et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol (2012) ; 41: : 1614–1624. |
31. | Deyo RA , Cherkin DC , Ciol MA . Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol (1992) ; 45: : 613–619. |
32. | Pink A , Stokin GB , Bartley MM , et al. Neuropsychiatric symptoms, APOE ɛ4, and the risk of incident dementia: a population-based study. Neurology (2015) ; 84: : 935–943. |
33. | Bierman EJ , Comijs HC , Rijmen F , et al. Anxiety symptoms and cognitive performance in later life: results from the longitudinal aging study Amsterdam. Aging Ment Health (2008) ; 12: : 517–523. |
34. | St Sauver JL , Grossardt BR , Leibson CL , et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc (2012) ; 87: : 151–160. |




