The Add Health Parent Study: A Biosocial Resource for the Study of Multigenerational Racial/Ethnic Disparities in Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias
Abstract
Background:
Alzheimer’s disease and Alzheimer’s disease related dementias (AD/ADRD) have increased in prevalence.
Objective:
This article describes the Add Health Parent Study (AHPS) Phase 2, a study of social, behavioral, and biological factors influencing healthy aging and risk for AD/ADRD, in a national sample of adults aged 58–90.
Methods:
Sample members are parents of the National Longitudinal Study of Adolescent to Adult Health (Add Health) cohort, initially interviewed in Add Health in midlife (1994-95). AHPS Phase 1 (2015–17) collected longitudinal data on a random subsample of parents and their spouse/partners, who were mostly Non-Hispanic (NH) White. AHPS Phase 2 will collect the same longitudinal socio-behavioral, and health survey data on all remaining NH Black and Hispanic parents (Black and Hispanic Supplement, BHS). Additionally, Phase 2 will collect cognitive and DNA data from AHPS Phase 1 and BHS sample parents and their current spouse/partners.
Results:
Funded by the National Institute on Aging, recruitment will occur between June 2025 and May 2026, producing an expected total AHPS sample of 5506 parents and their spouse/partners.
Conclusions:
The AHPS will be the first longitudinal cohort study powered to address multigenerational racial/ethnic disparities in AD/ADRD risk and protective factors across race/ethnic groups and socioeconomic strata.
INTRODUCTION
Significant knowledge gaps exist regarding intergenerational dimensions of cognitive aging and risk for Alzheimer’s disease and Alzheimer’s disease related dementias (AD/ADRD), and how these processes differ across race and ethnic groups.1 Racial/ethnic disparities in AD/ADRD are large and stem from inequitable social and economic conditions experienced over the life course. US Non-Hispanic (NH) Black and Hispanic adults have worse cognitive health than their NH White counterparts, including higher rates of AD/ADRD.2–6 Moreover, both Black and Hispanic older adults are approximately twice as likely to die due to dementia.7 These racial/ethnic disparities in cognitive function are not surprising given that current cohorts of older Black and Hispanic adults came of age during the Jim Crow era and have been exposed to overt and de jure racism as well as more contemporary subtle and de facto forms of racism across an array of societal domains—which have deleterious health consequences.3,4,6,8–10 Evidence suggests that structurally-rooted inequality via forms of residential segregation, differential access to socioeconomic resources, and exposure to stressors undergird racial disparities in cognitive impairment. To examine how contextual stressors (e.g., structural racism), and interpersonal stressors (e.g., discrimination and caregiving strain), affect AD/ADRD risk among US NH Black and Hispanic adults, multilevel (i.e., Census block group and tract, county, zip code and state) contextual data on structural racism and environmental exposures (e.g., pollution, noise, health inequality) must be combined with individual-level data on the social, behavioral, and biological factors influencing healthy aging and the development ofAD/ADRD.
However, the role of social conditions and other factors in AD/ADRD risk and the emergence of dementia and related health disparities across generations is unclear. Despite the growing literature on life course SES and AD/ADRD risk conducted in diverse racial and ethnic samples within a single generation in the US, many questions remain regarding what social conditions are transmitted across generations and how these generational advantages or disadvantages impact AD/ADRD risks and disparities.11–13 Linked data across two generations is needed to examine the key multilevel mechanisms by which disadvantage may become embedded biologically or psychologically and yield premature risk for disease and accelerated cognitive decline. Multigenerational data are also needed to further study the role of caregiving and caregiving stressors and strain on AD/ADRD risk among the three largest racial and ethnic groups in the US (i.e., NH White, NH Black, and Hispanic groups).
Research is growing on the genetic roots of AD/ADRD.14,15 but inadequate racial/ethnic diversity in genetic research participation contributes to bias in knowledge of these roots and hinders efforts to improve health equity. Existing genome-wide association study (GWAS) and epigenome-wide association study (EWAS) data are mostly from European-descent samples and drawn from socioeconomically-advantaged population segments.16,17 This selectivity biases GWAS and EWAS results and limits biomedical knowledge about racial/ethnic disparities.18–22 Moreover, datasets with parent-child genotyping, DNA methylation data, and high-quality measurement of the family environment across multiple racial/ethnic groups are rare. Multigenerational studies are needed to elucidate biosocial processes, which are hypothesized to be core features of intergenerational transmissions of health and wealth.23–25 Referred to as “genetic nurture,” these processes involve environmental mediation of genetic effects that originate in parents and impact offspring26 and necessitate a multigenerational design with parents and children drawn from a wider range of racial/ethnic populations and SES backgrounds.
The goal of the Add Health Parent Study (AHPS) Phase 2 is to recruit a national sample of 5506 adults aged 58–90 who are the parents or parent spouse/partners of participants in the National Longitudinal Study of Adolescent to Adult Health (Add Health). To address identified knowledge gaps, the AHPS data will be linked with rich longitudinal data on original Add Health participants to create and disseminate the first nationally representative multigenerational biosocial resource with cognitive, genomic, behavioral, and social data for the study of racial/ethnic disparities in cognitive aging and AD/ADRD risk. Multiple measures will be harmonized across the studies, including measures related to AD/ADRD risk and similar genomic markers to support innovative analysis of intergenerational predictors of AD/ADRD; the role of genomic processes in neurocognitive impairments and AD/ADRD risk; and intergenerational and lateral caregiving. Project specific aims are to:
1a. Recruit and interview additional sample of 2,505 NH Black and Hispanic parents with the AHPS survey.
1b. Consent all 5,506 AHPS members for participation in AD/ADRD Assessment and DNA data collection.
2a. Collect DNA and conduct single-nucleotide polymorphism (SNP) genotyping and DNA methylation analysis on AHPS sample.
2b. Develop an intergenerational genomic database to advance an understanding of the gene-environment interplay in the etiology of neurological impairments and AD/ADRD risk.
3a. Examine novel longitudinal and intergenerational social, health, and behavioral risk and preventive factors for AD/ADRD across racial/ethnic groups and social strata.
3b. Examine AHPS members’ caregiving experiences and the socioeconomic consequences of caregiving experiences related to AD/ADRD conditions or risks.
4. Document, disseminate, and promote use of AHPS data to the global scientific community.
The objective of this manuscript is to describe the design, sampling methods, and data collection procedures for AHPS Phase 2.
METHODS
Study participants
AHPS Phase 2 builds upon Add Health. Add Health used a school-based design to select 80 high schools and a paired feeder or middle school from a list of all schools in the US in 1994 and administered an in-school questionnaire to all students in grades 7–12 who attended these schools.27 From the school rosters, a grade- and sex-stratified sample was selected for a more extensive in-home interview with an adolescent and a caregiver (mainly biological mothers) during Wave I of Add Health in 1995. Based on responses to the in-school questionnaire, Add Health oversampled Black, Hispanic, and Asian adolescents; a “genetic” subsample consisting of 3000 pairs of identical and fraternal twins, full-siblings, half-siblings, and adolescents with no biological resemblance living in the same household; and physically disabled youth.28,29
The Wave I in-home sample included 20,745 Add Health Sample Members (AHSMs) ages 12–19. As part of Wave I, Add Health interviewed one of the AHSM’s parents or parent-figures, with preference for (biological, adoptive, or step) mothers by design. If mothers were not available, fathers or other guardians were interviewed. The parent who responded to this Wave I Parent Interview is the Wave I Parent (W1P). Currently ages 39–49, AHSMs have been re-interviewed in four subsequent waves (1996; 2001–02; 2008–09; 2016–18) and a sixth wave is currently in the field (Fig. 1).
Fig. 1
Intergenerational life course overlap of AHPS and add health interview timelines.
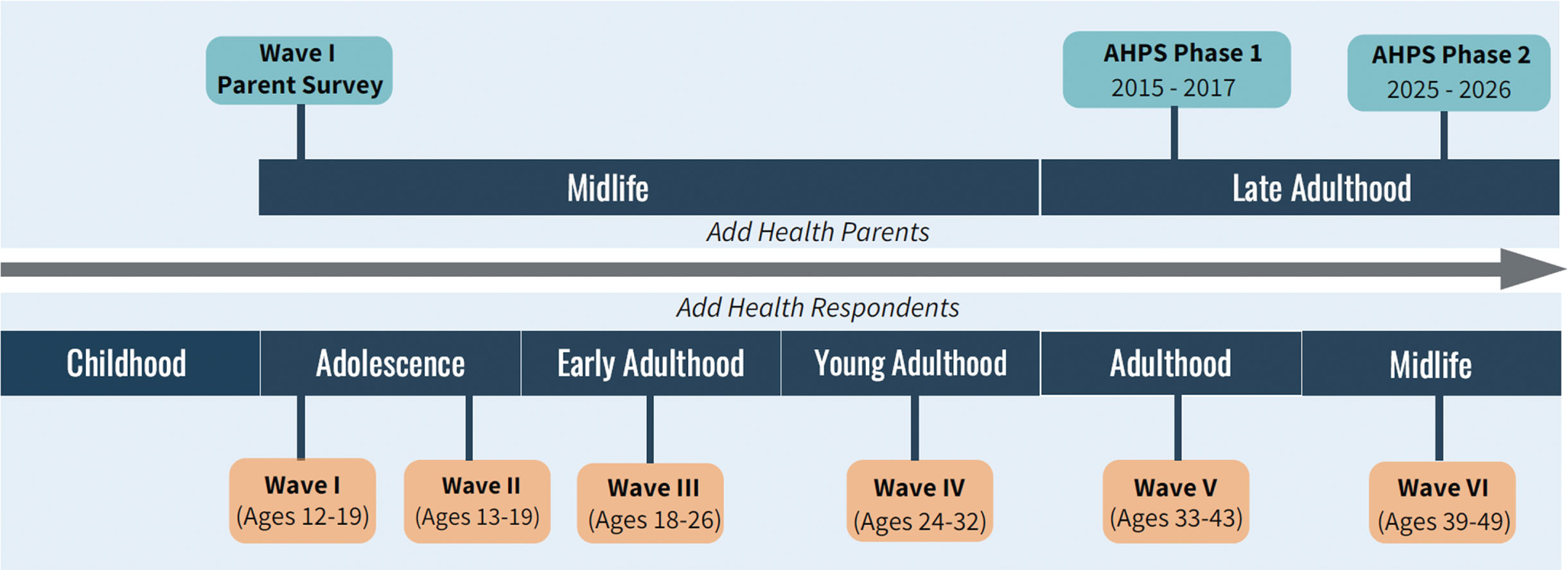
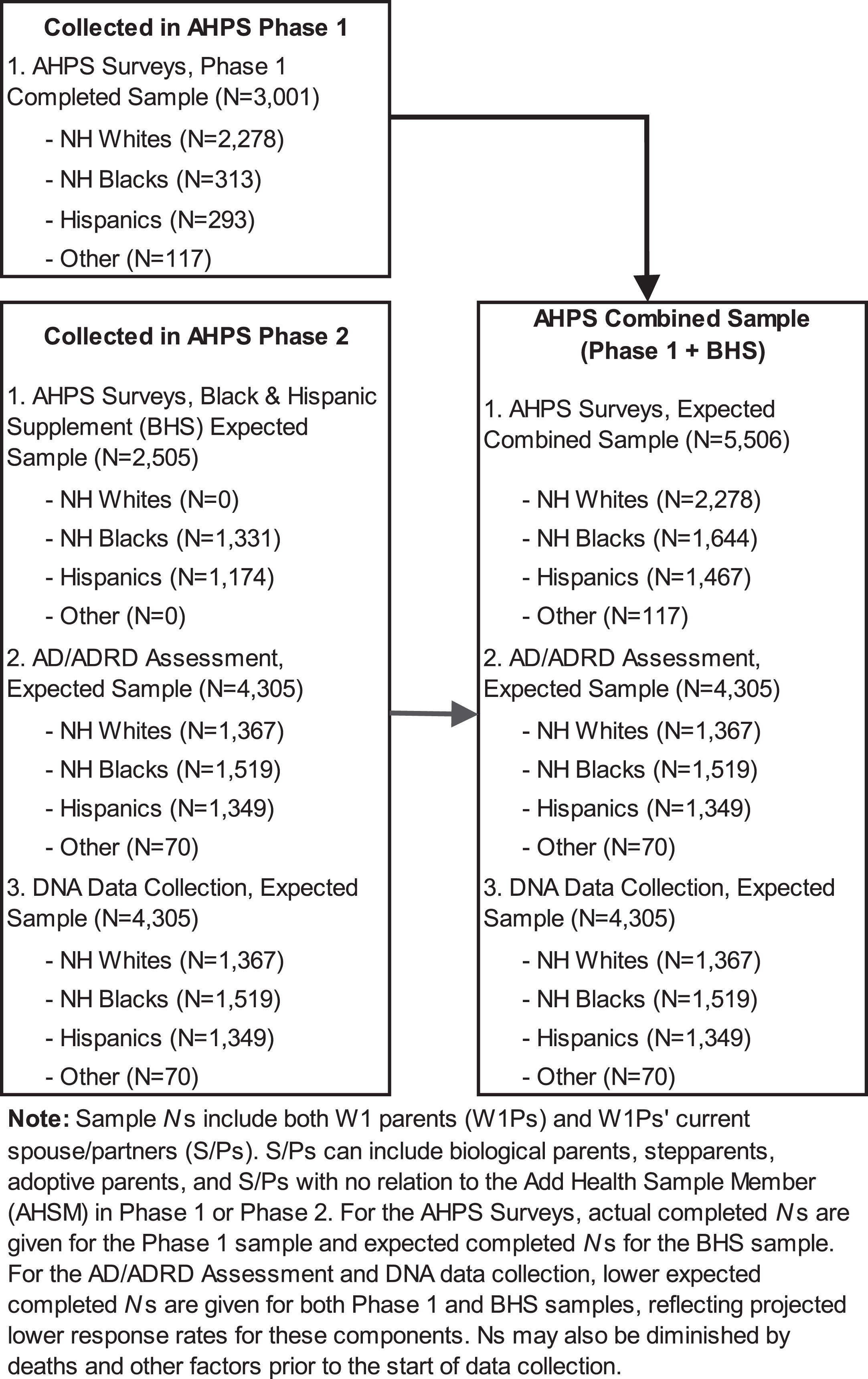
We aim to recruit 5506 Wave I parents (W1P) and their spouse/partners (S/Ps) to comprise the full AHPS sample. To be eligible for inclusion, the parent must be a living biological/adoptive/stepparent of one or more AHSMs, have completed the Add Health W1 Parent Survey, and be fluent in English or Spanish. Based on a screening protocol, W1Ps and S/Ps are excluded if they are unable to participate due to mental and/or physical incapacitation. AHPS Phase 1 parents are a random subsample of W1Ps and their spouse/partners (N = 3001), the majority (76%) of whom were NH White. AHPS Phase 2 participants will include all remaining NH Black and Hispanic W1Ps and their S/Ps (Black and Hispanic Supplement [BHS], N = 2505) and AHPS Phase 1 participants. With only a small number (n = 248) of AHSM identifying as American Indian, there was insufficient statistical power to justify additional recruitment of American Indian parents and their spouse/partners.
Recruitment procedures
At Add Health Wave I, parent interviews were completed for 85.2% of AHSMs; 72.7% of the interviews were with biological mothers and 4.6% with step or adoptive mothers. Anticipating the AHPS, AHSMs were asked at Wave IV (2008-09) if their W1P was still living and, if so, W1P locating information was requested; 88% of AHSMs participating in Wave IV had a living W1P. Among those with a living W1P (∼5% had died), the AHSMs provided locating information on 96.4%. During AHPS Phase 1, a random subsample of W1Ps and their current S/Ps completed the 60-min interviewer-administered AHPS survey and a 5-min Family Health History (FHH) that was left behind at the interview to be completed and returned.
During AHPS Phase 2, interviewers from Research Triangle Institute International (RTI) will re-contact Phase 1 participants and ask them to complete a neurocognitive assessment, with additional survey items on discrimination, caregiving, COVID-19, and other health-related items; and DNA data collection (15 min). RTI interviewers will also contact potential BHS participants, screen them for eligibility, and ask them to complete the same 60-min AHPS and 5-min FHH surveys previously completed by Phase 1 parents, as well as the same neurocognitive assessments with additional survey items on discrimination, caregiving, COVID-19 and other health measures; and DNA data collection completed by Phase 1 participants. Eligible Phase 1 or BHS participants who are not able to complete the AHPS survey, FHH survey, or additional survey items due to neurocognitive impairment will be able to nominate an individual (typically a S/P or adult caregiver) to complete components of each of the surveys as a proxy informant. AD/ADRD neurocognitive assessments will not be completed by proxy informants but proxy informants will be asked about the cognitive status of the primary respondent. BHS participants will receive a monetary incentive for completion of the AHPS-FHH surveys. Phase 1 participants and BHS participants will receive an additional monetary incentive for completion of the neurocognitive assessments and DNA data collection.
Figure 2 provides an overview of the AHPS study design. At Wave 1 of Add Health, data were collected from 17,670 W1P. Unweighted, 56.3% of the W1P were non-Hispanic white, 20.5% non-Hispanic Black, 14.8% Hispanic, and 8.4% non-Hispanic other (including both Asian and American Indians). The AHPS combined sample will include participants who are non-Hispanic white (41%), non-Hispanic Black (30%), Hispanic (21%) and non-Hispanic other (0.02%; including 55 American Indian adults and 62 Asian adults). Non-Hispanic Black and Hispanic W1Ps are purposefully oversampled at AHPS Phase 2 to have sufficient power for analyses within these racial/ethnic groups.
Fig. 2
Add Health Parent Study (AHPS) phase 2 study design.
RTI will utilize a Computer-Assisted In-Person Interview (CAPI) to administer the AHPS survey, neurocognitive assessment, and record DNA collection. To reduce respondent burden, participants will complete the paper-based FHH survey during or after the interview and return it via mail at their convenience (see below). Interviewers will also allow for breaks during the interview if participants express fatigue and may schedule a follow-up visit to complete the neurocognitive assessments and DNA collection.
Although we expect the majority of AHPS Phase 2 participants to complete the interview in-person, a telephone option will be available. When completed via telephone, some components of the neurocognitive assessment cannot be administered. For telephone-based interviews, DNA will be self-collected using kits mailed to the participant and returned to the lab in prepaid return packages. Similarly, the FHH will be mailed to participants and returned via mail to the research team. All components of the Phase 2 data collection can be completed in the participants’ preferred language, English or Spanish. During AHPS Phase 1, approximately 20% of Hispanic participants preferred Spanish.
Prior to calling potential AHPS Phase 2 participants, RTI will send them an initial contact letter/email in English and Spanish. The letter will describe the study and inform them that they will soon be contacted about study participation. In addition, the letter will contain a link to a 2-min informational video about the study to introduce the study team and promote participation, especially among Black and Hispanic participants.30,31 Participants can review the video prior to being contacted or at the time of their in-person interview.
Overview of AHPS-FHH survey measures and AD/ADRD assessment
The AHPS-FHH survey instruments were designed to: (1) include developmentally appropriate survey questions for the aging baby boom cohort of parents that AHPS represents; (2) leverage existing and validated survey items, tests, and measurement batteries in other surveys most relevant to the AHPS cohort, such as the Health and Retirement Study (HRS), National Social Life, Health, and Aging Project (NSHAP), and Panel Study of Income Dynamics (PSID); and (3) include validated survey, test, and measurement batteries from Add Health for comparable measures on both the parent and adult child generations.
The AHPS survey includes measures of (1) health and health behaviors, (2) neurocognitive function and personality, (3) family relationships and intergenerational support, (4) economic status and capacities, and (5) consent for administrative records links (Table 1). We will also obtain GPS data for current residence as well as data on participants’ primary country, U.S territory, or state of residence during childhood (ages 5–17). Given the geographic distribution of AHSMs in Add Health, we expect AHPS participants to be from all 50 U.S. states/DC or all 4 major U.S. Census regions (37% South, 24% Midwest, 15% Northeast, and 24% West). We also expect approximately 24% to be living in rural areas, 36% in suburban areas, and 40% in urban areas.
Table 1
Add Health Parent Study (AHPS) and Family Health History (FHH) survey content overview
| Topic | Specific Measures |
| AHPS | |
| Health and Health Behaviors | Self-assessment of overall general health, and incidence and age-of-onset for provider-diagnosed chronic illnesses, self-reported depressive symptoms, self-perceived stress, experiences of discrimination, access to health care services, health insurance, medication log linked to associated diagnostic codes, smoking, drinking, substance use, diet, and physical activity, social relationships, social integration, and loneliness |
| Cognitive Functioning and Personality | Word recall test, the number series test, 20-item Mini-IPIP Big Five Personality factors, 2-item Grit Scale, 3-item self-control scale, and self-evaluation of mastery, optimism, anxiety, anger, and hostility |
| Family Relationships and Intergenerational Support | Roster of all of each Wave 1 Parent’s (W1Ps) children (including step and biological) with age, gender, education and marital status of each, length of the relationship and relationship quality (e.g., happiness and commitment) with current spouses/partners (S/P), parents’ living status and co-residential status, relationship quality (e.g., closeness and contact frequency) with the Add Health Sample Member (AHSM), recent and life-cycle transfers of time, money, and emotional support between the W1P and children (including AHSM), S/P and parents, and W1Ps’ perceptions and knowledge of the financial status and health of their AHSM children |
| Economic Status and Capacities | Income, financial assets, employment; labor earnings and unemployment benefits; disability status, workman’s compensation and disability benefits; and retirement status and pension and social security benefits |
| Consent for Administrative Record Links | Respondent’s permission to link to their Medicare and Medicaid records for all the years between 1991 and 2035 in which they were or will be participating, to geocode their home addresses to link multilevel contextual data. We will also link to the National Death Index (NDI) to confirm deaths uncovered during fieldwork and check whether W1Ps in our sample that we could not locate have died. |
| Geographic Data | GPS data for current residence; primary country, U.S territory, or state of residence during childhood (ages 5–17) |
| FHH | Whether biological parents are living and whether biological relatives (i.e. mother, father, siblings, aunts/uncles, or grandparents) were ever diagnosed with coronary heart disease, stroke, diabetes, hypertension, hyperlipidemia, cancer (including specifically breast cancer, colon cancer, ovarian cancer, prostate cancer, lung cancer, or other), depression, dementia or Alzheimer’s Disease, heavy alcohol use or alcoholism, obesity, asthma, or arthritis. |
The FHH survey combines features from other self-administered and web-based health histories that have shown the health history data collected about first- and second-degree relatives to be valid and reliable with acceptable sensitivity and specificity.32–35 These include questions on whether relatives ever had major health conditions corresponding to the leading causes of morbidity and mortality among US adults (e.g., cardiovascular disease, diabetes, cancers, AD/ADRD, depression, obesity) and the age at diagnosis or first occurrence for some conditions.36,37 Survey domains will also include experiences with chronic paid, long COVID, COVID-19 vaccination, discrimination and stigma, and caregiving stress collected as part of supplemental survey items included with the AD/ADRD neurocognitive risk assessments.
The in-home neurocognitive risk assessments will utilize the Add Health Wave VI Cognition Assessment, Physical, and Sensory function (Add CAPS) protocol.38 Many of the neurocognitive domains in Add CAPS are consistent with measures from other significant national aging studies, like the Health and Retirement Study and their Harmonized Cognitive Assessment Protocol (HRS-HCAP).39–41 The Add CAPS neurocognitive domains encompass verbal episodic memory, processing speed, executive function, working memory, language, and semantic fluency. Physical and sensory function measures under the Add CAPS protocol include grip strength and hearing. The majority of Add CAPS domains will be part of the AHPS home visit, ensuring that the measures are harmonized across the two related studies. Further insights into physical and sensory functions will be derived from supplemental survey items, including Instrumental Activities of Daily Living (IADLs) and Activities of Daily Living(ADLs).
Overview of DNA data collection and related data
The full AHPS sample (i.e., combined Phase 1 and BHS), will be consented for saliva and Dried-Blood Spot (DBS) for isolating DNA. DNA from saliva is collected for genome-wide analysis and DBS are collected for epigenetic data. Saliva collection will be conducted using the Oragene DNA kit following the protocol used successfully in Add Health Wave IV. DBS collection will be conducted using an automated DBS collection kit which collects four blood spots. The study lab (University of Texas at Houston) will receive saliva and DBS specimens, execute DNA extraction, conduct DNA array genotyping and calling of saliva specimens, complete DNA methylation (DNAm) analysis of DBS specimens, and transmit result and data quality datasets to the AHPS team. Whole-genome single-nucleotide polymorphism (SNP) genotype data will be generated using whole-genome SNP arrays. We plan to impute APOE genotypes from the genome-wide SNP data. Whole-genome DNA methylation data will also be generated.
The AHPS team will follow protocols established in the Add Health Study27–29 to analyze SNP genotype and DNAm datasets to compute SNP Principal Components, polygenic indices, and composite DNAm variables including epigenetic clocks.42–46 Analyses will result in matched datasets across parent-child generations. These matched datasets along with the primary SNP-genotype and DNAm data will form the Intergenerational Genomic AHPS-Add Health Database. Genomic data will include polygenic indices (PGIs) computed for AD/ADRD-related health conditions following established methods.44,45
Statistical power
A variety of analyses will be possible upon completion of AHPS Phase 2. To characterize the adequacy of the sample sizes of NH Whites, NH Blacks, and Hispanics for conducting analyses with the AHPS Survey data and the AD/ADRD and DNA data, we calculated Minimum Detectable Effect Sizes (MDESs) for analyses with our samples of W1Ps, the sampling unit in AHPS, for each of these groups and the total sample. MDESs are the smallest effect size for null hypotheses for tests of outcomes expressed in standard deviation units, given effective sample sizes, Type I errors (α), and power (1-β). We calculated MDES for tests of means (μ) and partial correlation coefficients (r) for adjusted effects on outcomes using Stata 17 for α= 0.05, 1-β= 0.80. The MDES calculated adjusted for unequal weighting effects (UWEs) of the combined Phase 1 and BHS samples that will have completed the AHPS survey and those who will have completed the AD/ADRD assessments and the DNA collection protocols during Phase 2. The resulting MDESs are presented in Table 2.
Table 2
MDESs for AHPS total & subgroup samples of W1Ps1
| Samples & Subgroups | Actual N | Effective N2 | MDES for μ3 | MDESPartial r’s4 |
| Combined Phase 1 & BHS Sample Sizes for AHPS Survey Data: | ||||
| NH Whites | 1,484 | 1,178 | 0.082 | 0.081 |
| Hispanics | 1,011 | 532 | 0.122 | 0.121 |
| NH Blacks | 1,303 | 686 | 0.107 | 0.106 |
| Total Sample5 | 3,877 | 2,459 | 0.057 | 0.056 |
| Samples Sizes for AD/ADRD & DNA Data: | ||||
| NH Whites | 890 | 469 | 0.130 | 0.129 |
| Hispanics | 930 | 490 | 0.127 | 0.126 |
| NH Blacks | 1,204 | 633 | 0.112 | 0.111 |
| Total Sample5 | 3,072 | 1,617 | 0.070 | 0.070 |
1S/Ps are not included in the MDES calculation. 2Effective Ns adjust for unequal weighting effects for Phase 1 & Phase 2 sampling. 3MDESs for Tests of standardized Means (μ’s) 4MDESs for Tests of Partial Correlation Coefficients (r’s) 5Total Sample also includes other race/ethnic groups.
We evaluated these MDESs relative to criteria proposed by Cohen,47 where MDES ≤0.10 are considered “very small” and MDESs between 0.10 and 0.20 are considered “small.” All of the MDESs in Table 2 fall in the small range; some even fall in the very small range. Thus, with customary levels of significance, we will have at least 80% power to detect associations with standardized effect sizes ranging from 0.056 to 0.129. Our AD/ADRD and DNA sample sizes are smaller than the Phase 1+ BHS survey sample, given anticipated completion rates in our re-contacts of Phase 1 parents. For this sample, we benchmark against effect sizes in the existing research. For example, polygenic indices for many AD/ADRD-related risk factors have effect-sizes of r > 0.1;45 DNA methylation composite variables have effect sizes for prediction of cognition phenotypes of r > 0.2. The MDESs in Table 2 indicate that our AD/ADRD and DNA samples sizes are more than sufficient to detect effect sizes with at least 80% power for each racial/ethnic group separately, as well as for the total sample. For many analyses, the actual power is likely to be greater than 80%.
In analyses using AHPS data, it will be possible to control for a variety of confounds longitudinally and to evaluate non-response bias by also using data collected from the Add Health Parent Interview at Wave 1. The W1P interview collected data on a variety of parent characteristics including parents’ demographic factors, economic factors, neighborhoods, romantic relationships, health and health behaviors, current spouse/partners, and information on their AHSM child.
Dissemination plans
AHPS data will be distributed in two forms: public-use and restricted-use datasets. Public-use data contains a random subset of the Add Health cohort and their parents in linked longitudinal files and will be available through data repositories. Restricted-use datasets are limited to researchers who enter into data use agreements with Add Health. Genomic data will be deposited to both the database of Genotypes and Phenotypes (dbGaP) and the National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site. We will also disseminate the harmonized PGI library, DNAm clocks, and other DNAm composite measures through restricted data contracts.
DISCUSSION
This study addresses weaknesses in prior research on AD/ADRD risk by: (1) expanding the availability of longitudinal data on social conditions linked to AD/ADRD risk among Hispanic and NH Black individuals; (2) harmonizing the collection of neurocognitive assessments across adult children participating in Add Health Wave VI and their parents participating in AHPS Phase 2; and (3) providing genotyping and DNAm data linked to AD/ADRD risk on two generations (parent and child) across multiple races/ethnicities.
This study has four limitations. First, we have insufficient numbers of AHSMs identifying as Asian or American Indian to warrant recruiting their parents due to the lack of power to make statistically valid inferences about or comparisons with these two subgroups. Second, some participants may request phone interviews which would result in the loss of some neurocognitive assessments requiring in-person interviews. Thus, we follow best practices to reduce burden and enhance benefits to participants to maximize in-person interviews.48,49 Third, we collect blood samples through interviewer-collected DBS rather than phlebotomy. With no reduction in the DNA data, this substantially reduces participant burden and the cost of the study. Finally, the W1Ps are now between the ages of 58 and 90. While this places them at increased risk of AD/ADRD, it also places them at increased risk of institutionalization and death. Thus, completion of data collection for AHPS within the next few years is essential.
Despite the aforementioned limitations, the ability to conduct a cost-effective longitudinal study of AD/ADRD in the three largest racial/ethnic groups in the US and to link two generations—AHPS parents and AHSMs—is timely, significant, and innovative. AHPS Phase 2 data will advance multiple NIA Strategic Objectives by improving our understanding of AD/ADRD disparities and the biological and social conditions contributing to these disparities. In combination with the Add Health data, researchers will be able to address novel questions on race, ethnic, and socioeconomic (SES) status variation in (1) intergenerational correlations between cognitive health and impairment; (2) midlife risk and protective factors (e.g., employment, education, health behavior, parent-child relations, children’s behavior) for cognitive decline and AD/ADRD risk in old age; and (3) multigenerational met and unmet caregiving needs and expectations and their relationship with cognitive functioning in the development of AD/ADRD. Controlling for shared genetic variance across generations, researchers can also examine the role of social and physical environments in facilitating or constraining offspring caregiving of their parents with AD/ADRD risk factors and cognitive impairments. Moreover, the AHPS Phase 2 data will result in a diverse, national data source on AD/ADRD risk and protective factors that will be available to researchers world-wide, informing generations of scholarship and advancing the science of AD/ADRD.
Conclusions
The AHPS Phase 2 includes several new and important study design attributes. First, with increased sampling of participants from NH Black and Hispanic parents and their spouse or partners in Phase 2, AHPS will have enough statistical power to address, for the first time, the measurement of health, social, and behavioral differences in AD/ADRD risk and protective factors across racial/ethnic groups and socioeconomic levels. Second, AHPS 2 will offer the chance to evaluate harmonized neurocognitive, physical, and sensory measures by using the established Add CAPS protocol from Add Health Wave VI. Third, the AHPS Phase 2 study will introduce new biological measures of disease and aging risk, including genomic measures also available in the Add Health study. For all these reasons, AHPS will provide an unparalleled opportunity to evaluate the national-level impacts of social, caregiving support, environmental, and biological exposures on the health of an older population and their intergenerational transmission of crucial health risks.
AUTHOR CONTRIBUTIONS
Krista M. Perreira (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing); V. Joseph Hotz (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing); Naomi N. Duke (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing); Allison E. Aiello (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing); Daniel W. Belsky (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing); Tyson Brown (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing); Todd Jensen (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing); Kathleen Mullan Harris (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing).
ACKNOWLEDGMENTS
The Add Health Parent Study gratefully acknowledges the support of the National Institute on Aging of the National Institutes of Health under the following Awards to Kathleen Mullan Harris and V. Joseph Hotz: The Add Health Parent Study: Phase I (RO1AG042794) and Locating the Parents of Add Health (R21AG042663-01). We also acknowledge 27 years of support to Kathleen Mullan Harris for the Add Health Program Project (P01HD31921; 1994–2021) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations.
FUNDING
This research was funded by Grant R01AG084071 from the National Institute of Aging. The authors also express gratitude to the Carolina Population Center and its NIH Center grant (P2C HD050924) and to the Duke Center for Population Health and Aging and its NIA Center grant (5P30AG034424) for general support. Daniel W. Belsky is a fellow of the CIFAR CBD Network.
CONFLICT OF INTEREST
Authors have no conflicts of interest to report.
DATA AVAILABILITY
Information on how to obtain data from Phase 1 of the Add Health Parent Study is available at https://www.cpc.unc.edu/research-themes/projects/add-health-parent-study-phase-i/. Information on how to obtain the Add Health data is available on the Add Health website (http://www.cpc.unc.edu/addhealth).
REFERENCES
1. | National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Board on Behavioral, Cognitive, and Sensory Sciences; Committee on the Decadal Survey of Behavioral and Social Science Research on Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias. Reducing the Impact of Dementia in America: A Decadal Survey of the Behavioral and Social Sciences. Washington (DC): National Academies Press (US); July 26, 2021. |
2. | Farina MP , Hayward MD , Kim JK , et al. Racial and educational disparities in dementia and dementia-free life expectancy. J Gerontol B Psychol Sci Soc Sci (2020) ; 75: : e105–e112. |
3. | Glymour MM and Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev (2008) ; 18: : 223–254. |
4. | Weuve J , Barnes LL , Mendes De Leon CF , et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology (2018) ; 29: : 151. |
5. | Zeng Y , Lum TYS and Chen YC. The intersectionality of life course socioeconomic status, race, and cognitive decline: an 18-year follow-up. Int J Geriatr Psychiatry (2022) ; 37: : doi: 10.1002/gps.5774. |
6. | Zhang Z , Hayward MD and Yu YL. Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Soc Behav (2016) ; 57: : 184–199. |
7. | Stokes AC , Weiss J , Lundberg DJ , et al. Estimates of the association of dementia with US mortality levels using linked survey and mortality records. JAMA Neurol (2020) ; 77: : 1543–1550. |
8. | Caunca MR , Odden MC , Glymour MM , et al. Association of racial residential segregation throughout young adulthood and cognitive performance in middle-aged participants in the CARDIA study. JAMA Neurol (2020) ; 77: : 1000. |
9. | Chen R , Weuve J , Misra S , et al. Racial disparities in cognitive function among middle-aged and older adults: the roles of cumulative stress exposures across the life course. J Gerontol A Biol Sci Med Sci (2022) ; 77: : 357–364. |
10. | Garcia M , Saenz J , Downer B , et al. The role of education in the association between race/ethnicity/nativity, cognitive impairment, and dementia among older adults in the United States. Demogr Res (2018) ; 38: : 155–168. |
11. | Peterson RL , George KM , Gilsanz P , et al. Lifecourse socioeconomic changes and late-life cognition in a cohort of U.S.-born and U.S. immigrants: findings from the KHANDLE study. BMC Public Health (2021) ; 21: : 920. |
12. | Cha H , Farina MP , Hayward MD . Socioeconomic status across the life course and dementia-status life expectancy among older Americans. SSM Popul Health (2021) ; 15: : 100921. |
13. | Marden JR , Tchetgen Tchetgen EJ , Kawachi I , et al. Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol (2017) ; 186: : 805–814. |
14. | Sims R , Hill M and Williams J. The multiplex model of the genetics of Alzheimer’s disease. Nat Neurosci (2020) ; 23: : 311–322. |
15. | Calabrò M , Rinaldi C , Santoro G , et al. The biological pathways of Alzheimer disease: a review. AIMS Neurosci (2021) ; 8: : 86–132. |
16. | Fry A , Littlejohns TJ , Sudlow C , et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol (2017) ; 186: : 1026–1034. |
17. | Mills MC and Rahal C. A scientometric review of genome-wide association studies. Commun Biol (2019) ; 2: : 9. |
18. | Keyes KM and Westreich D. UK biobank, big data, and the consequences of non-representativeness. Lancet (2019) ; 393: : 1297. |
19. | Martin AR , Gignoux CR , Walters RK , et al. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet (2017) ; 100: : 635–649. |
20. | Martin AR , Kanai M , Kamatani Y , et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet (2019) ; 51: : 584–591. |
21. | Salas LA , Peres LC , Thayer ZM , et al. A transdisciplinary approach to understand the epigenetic basis of race/ethnicity health disparities. Epigenomics (2021) ; 13: : 1761–1770. |
22. | Godbole N , Kwon SC , Beasley JM , et al. Assessing equitable inclusion of underrepresented older adults in Alzheimer’s disease, related cognitive disorders, and aging-related research: a scoping review. Gerontologist (2023) ; 63: : 1067–1077. |
23. | Belsky DW and Harden KP. Phenotypic annotation: using polygenic scores to translate discoveries from genome-wide association studies from the top down. Curr Dir Psychol Sci (2019) ; 28: : 82–90. |
24. | Cawley J , Han E , Kim J , et al. Testing for family influences on obesity: the role of genetic nurture. Health Econ (2019) ; 28: : 937–952. |
25. | Willoughby EA , McGue M , Iacono WG , et al. The role of parental genotype in predicting offspring years of education: evidence for genetic nurture. Mol Psychiatry (2021) ; 26: : 3896–3904. |
26. | Kong A , Thorleifsson G , Frigge ML , et al. The nature of nurture: effects of parental genotypes. Science (2018) ; 359: : 424–428. |
27. | Harris KM , Halpern CT , Whitsel EA , et al. Cohort profile: The National Longitudinal Study of Adolescent to Adult Health (Add Health). Int J Epidemiol (2019) ; 48: : 1415–1415k. |
28. | Harris KM , Halpern CT , Hussey J , et al. Social, behavioral, and genetic linkages from adolescence into adulthood. Am J Public Health (2013) ; 103: : S25–S32. |
29. | Harris KM , Levitt B , Gaydosh L , et al. Sociodemographic and lifestyle factors and epigenetic aging in US young adults. JAMA Network Open (2024) ; in press. |
30. | Dye T , Li D , Demment M , et al. Sociocultural variation in attitudes toward use of genetic information and participation in genetic research by race in the United States: implications for precision medicine. J Am Med Inform Assoc (2016) ; 23: : 782–786. |
31. | Kerath SM , Klein G , Kern M , et al. Beliefs and attitudes towards participating in genetic research – a population based cross-sectional study. BMC Public Health (2013) ; 13: : 114. |
32. | Milne BJ , Caspi A , Crump R , et al. The validity of the family history screen for assessing family history of mental disorders. Am J Med Genet B Neuropsychiatr Genet (2009) ; 150B: : 41–49. |
33. | Milne BJ , Caspi A , Harrington H , et al. Predictive value of family history on severity of illness. Arch Gen Psychiatry (2009) ; 66: : 738. |
34. | Higgins M , Province M , Heiss G , et al. NHLBI family heart study: objectives and design. Am J Epidemiol (1996) ; 143: : 1219–1228. |
35. | Arar N , Seo J , Abboud HE , et al. Veterans’ experience in using the online Surgeon General’s family health history tool. Per Med (2011) ; 8: : 523–532. |
36. | Heron M . National vital statistics reports - deaths: leading causes for 2017, https://www.cdc.gov/nchs/products/index.htm. (2019, accessed 12 November 2023). |
37. | Johnson NB , Hayes LD , Brown K , et al. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors–United States, 2005-2013. MMWR Suppl (2014) ; 63: : 3–27. |
38. | Aiello A and Hummer R. Add Health Wave VI cognition assessment, physical, and sensory function (Add CAPS) protocol. 2024 Add Health Users Conference. Chapel Hill NC, June 17, 2024. https://addhealth.cpc.unc.edu/wp-content/uploads/2024/07/2024-AH-Users-Conference_Add-Health-Cognitive-Assessment.Physical-and-Sensory-Function_Aiello.pdf. (2024, accessed 18 July 2024) |
39. | Weir D , Langa K and Ryan L. 2016 harmonized cognitive assessment protocol (HCAP) study protocol summary. https://hrs.isr.umich.edu/publications/biblio/9950. (2018, accessed 18 July 2024). |
40. | Crimmins EM , Kim JK , Langa KM , et al. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci (2011) ; 66B: i162–i171. |
41. | Langa KM , Ryan LH , McCammon RJ , et al. The health and retirement study harmonized cognitive assessment protocol project: study design and methods. Neuroepidemiology (2020) ; 54: : 64–74. |
42. | Price AL , Patterson NJ , Plenge RM , et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet (2006) ; 38: : 904–909. |
43. | Lehne B , Drong AW , Loh M , et al. A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol (2015) ; 16: : 37. |
44. | Choi SW and O’Reilly PF. PRSice- Polygenic Risk Score software for biobank-scale data. Gigascience (2019) ; 8: : giz082. |
45. | Privé F , Arbel J and Vilhjálmsson BJ. LDpred better, faster, stronger. Bioinformatics (2021) ; 36: : 5424–5431. |
46. | Becker J , Burik CAP , Goldman G , et al. Resource profile and user guide of the Polygenic Index Repository. Nat Hum Behav (2021) ; 5: : 1744–1758. |
47. | Cohen J . Statistical Power Analysis for the Behavioral Sciences. New York: Routledge, (2013) . |
48. | Dillman D , Smyth J and Christian L. Internet, phone, mail, and mixed-mode surveys: the tailored design method. 4th ed. Hoboken, NJ: Wiley, (2014) . |
49. | Einarsson H , Shlomo N and Cernat A. Reducing respondent burden with efficient survey invitation design. Surv Res Methods (2021) ; 15: : 207–233. |




