Dementia Ideal Care: Ecosystem Map of Best Practices and Care Pathways Enhanced by Technology and Community
Abstract
Background:
Globally, much work has been done by nonprofit, private, and academic groups to develop best practices for the care of people living with dementia (PLWD), including Alzheimer’s disease. However, these best practices reside in disparate repositories and tend to focus on one phase of the patient journey or one relevant group.
Objective:
To fill this gap, we developed a Dementia Ideal Care Map that everyone in the dementia ecosystem can use as an actionable tool for awareness, policy development, funding, research, training, service delivery, and technology design. The intended audience includes (and not limited to) policymakers, academia, industry, technology developers, health system leaders, clinicians, social service providers, patient advocates, PLWD, their families, and communities at large.
Methods:
A search was conducted for published dementia care best practices and quality measures, which were then summarized in a visual diagram. The draft diagram was analyzed to identify barriers to ideal care. Then, additional processes, services, technologies, and quality measures to overcome those challenges were brainstormed. Feedback was then obtained from experts.
Results:
The Dementia Ideal Care Map summarizes the ecosystem of over 200 best practices, nearly 100 technology enablers, other infrastructure, and enhanced care pathways in one comprehensive diagram. It includes psychosocial interventions, care partner support, community-based organizations; awareness, risk reduction; initial detection, diagnosis, ongoing medical care; governments, payers, health systems, businesses, data, research, and training.
Conclusions:
Dementia Ideal Care Map is a practical tool for planning and coordinating dementia care. This visualized ecosystem approach can be applied to other conditions.
INTRODUCTION
Dementias, such as Alzheimer’s disease (AD), are widely recognized as a global issue with high prevalence. While more people are living longer [1] and exposed to cognitive risk factors, the rising prevalence of dementia has far-reaching consequences, not only for individuals living with dementia for potentially 5-20 years—it also has a profound impact on their extended family, friends, communities, workforce and society.
By 2030, worldwide there will be more than 75 million people living with dementia (PLWD), with an estimated annual global cost of care 2.8 trillion U.S. dollars [2]. However, currently roughly three-quarters (3/4) of PLWD are undiagnosed and an additional nine out of ten (9/10) people with mild cognitive impairment (MCI) are not aware of their condition [2] and not supported. Therefore, the affected individuals and their families do not know what is causing their symptoms/behaviors, and the lack of recognition denies them access to resources about what to expect, caregiver support, legal and financial planning, psychosocial interventions, and medical treatments during a vital phase when these could have the greatest impact on longer range outcomes [1, 3–6].
Much work has been done by patient advocacy organizations, nonprofits, academic researchers, and public and private sector institutions globally to develop best practices for the identification, care, and support of PLWD. However, these best practices are located in disparate repositories of information, tend to focus on one phase of the patient journey (such as diagnosis) or only on one relevant group (what is elsewhere referred to as a stakeholder group [7, 8], such as just physicians). Furthermore, care coordination across groups is lacking, and discrepancies persist between published best practices and the real-world care delivered to PLWD.
We aim to help fill these gaps by developing a Dementia Ideal Care Map that all relevant groups in the dementia ecosystem can use as an actionable tool to promote public awareness, start conversations, decrease stigma, develop policies, seek funding, conduct research, train staff, deliver services, increase access to resources, and design technology. The intended audience includes policymakers, public and private payers, researchers in academia and industry, businesses, technology developers, health system leaders, clinicians, social service workers, patient advocates, PLWD, their families, and communities at large.
The Dementia Ideal Care Map aims to summarize global best practices of “What to do” for risk reduction, detection, care, and quality of life promotion from both person-centered and ecosystem perspectives. For example, it goes beyond the care pathways for medical diagnosis [9] to include nonpharmacologic psychosocial interventions, the role of family/friend care partners, and the role of community, employers, and government. “Why and How” to do each best practice were out of scope for summarizing in this diagram and manuscript; those details are available in the references [1–140].
Furthermore, while there are existing technologies that are unfamiliar to most relevant groups, and new diagnostic and monitoring biomarkers and treatments yet to emerge—we aim to propose a range of technology solutions, enhanced care pathways, and quality measures that support improved care quality and care coordination for the “Ideal Care” of PLWD and their care partners. “Ideal Care” in this project means: What would be optimal dementia care in the near future if care was not limited by current constraints and feasibility? What would be the ideal PLWD experience and interventions before diagnosis and through ongoing care?
The Dementia Ideal Care Map’s goal is to be generalizable to most PLWD and a variety of organizations around the world, ultimately improving the care and quality of life of PLWD and their care partners.
METHODS
Population scope
The scope of this project includes best practices for PLWD, people with MCI, people with undiagnosed or preclinical brain changes, as well as the general public. Most of the recommendations are applicable to all settings—for PLWD, care partners, and care workers in PLWD’s homes, community centers, senior care homes, nursing facilities, clinics and hospitals.
Review of published literature
A search was conducted for published dementia care best practices worldwide: PubMed search for “dementia” to include clinical practice guidelines and relevant academic journal articles; and Google search for “dementia” to include toolkits, whitepapers, and resources created for patients, care partners, community organizations, policy and/or other non-academic audiences. The goal of the searches was to include both guidelines about physicians’ medical decisions and non-medical best practices that improve patient experience and quality of life for PLWD and their care partners.
The primary author read these sources and excerpted relevant lists and descriptions of best practices from each publication.
Additionally, a search was conducted for existing quality measures (QM) and Patient Reported Outcome Measures (PROM) by using search terms of (“quality measures” OR “PROM”) AND: “geriatrics”, “neurology”, “psychiatry”, “dementia”, “Alzheimer’s”, “cognitive”, or “behavior”. These quality measures were compiled, analyzed, and the most relevant ones highlighted for potential inclusion in the diagram.
Synthesis into diagram
The primary author synthesized the excerpts of best practices from the global published sources, identified key recommendations and themes, and summarized those in a visual diagram format adapted from capabilities architecture [10]. Best practices that could become potential quality measures were noted in the diagram with a “[QM]” designation.
Analysis of diagram and proposal of future state vision
Next, the primary author visually analyzed the initial draft diagram to identify current barriers to ideal care and gaps in existing best practices; then brainstormed additional processes, services, and quality measures to overcome those challenges. The primary author added technology solutions that complement traditional best practices and enable enhanced care pathways; these are based on her professional experience and knowledge of the technology market landscape, with most of the technologies already commercially available and technically feasible.
The technologies, new processes, services, and quality measures were added to the Ideal Care Map visual diagram to enhance previously published best practices and create a comprehensive vision for an ideal future state for dementia care.
Feedback from subject matter experts
To seek feedback from subject matter experts (SMEs) in dementia and care innovation, the primary author conducted semi-structured interviews via 1-on-1 video calls averaging 1.5 hours with each expert. Experts were asked:
• In your experience, what are the biggest barriers and gaps in dementia quality of life and care?
• Please share your screen showing the diagram draft, and feel free to explore whatever sections interest you. As you move around the diagram, please “think out loud” and share your reactions: What would you change? What would you add? What’s missing?
• Can you think of any other Quality Measures to improve barriers to Ideal care?
• Who do you feel is in the greatest position to act on what’s in this diagram? How can this diagram be disseminated and used to improve PLWD’s care and quality of life?
• Other resources or publications to consider?
The 11 SMEs interviewed are:
• Neurology physician and neuropsychologist specializing in dementia diagnosis and treatment
• Geriatrics physician specializing in clinical quality measures
• Geriatrics physician clinician in value-based care and diagnostics
• Geriatrics physician clinician in the community
• Global dementia care and innovation physician leader
• Geriatrics nurse and healthcare administration leader
• Public health nurse leader working with minoritized communities
• Occupational therapist specializing in training care partners and care workers
• Family care partner, who has experience with public health communications
• Patient advocacy leader specializing in policy
• Technology, enterprise architecture, and healthcare innovation leader
Several of these SMEs also shared recent personal experiences caring for family members with dementia. Because each SME contributed valuable feedback that changed and expanded the initial diagram draft to its current version, they were invited to be co-authors of this paper; some declined due to lack of time.
Group video calls with over a dozen other global experts in neurology and dementia care innovation provided additional feedback.
A person with no prior experience with dementia provided feedback as a young layperson.
Attempts were made to include feedback from PLWD. However, it was not feasible during this round of feedback due to regulatory compliance constraints. In light of this limitation, as a proxy, SMEs were chosen who have substantial experience directly interacting with PLWD in their roles as family care partners, dementia patient advocates, and/or leaders of community advisory boards. Viewpoints of PLWD were also included by referencing the PEPA-CEAFA Expert Panel of People with Alzheimer’s [11].
Revisions
Based on the feedback collected, the primary author revised the Ideal Care Map diagram layout and added more items. The second author provided additional specific feedback on diagram items that could potentially be developed into quality measures.
Note
No generative AI tools were used in this project. The primary author manually searched, read, and extracted the best practices; and then conceptualized, created, and revised the diagram. The manuscript was written based on the diagram contents and SMEs’ input.
This project does not involve experimentation on human subjects or animals.
RESULTS
Over two hundred (200) best practices and technology enablers were identified and summarized in the Dementia Ideal Care Map diagram (Fig. 1; please see online article to view and zoom-in on the image details).
The following text highlights some key observations about the Ideal Care Map and some best practices from each section; the text commentary below does not mention every best practice in the Ideal Care Map, so please refer to Figs. 1–9 to view the details and where each best practice is organized relative to the other concepts.
Figure 1: Dementia Ideal Care Map
Fig. 1
Dementia Ideal Care Map: Ecosystem Map of Best Practices and Care Pathways Enhanced by Technology and Community (diagram at a glance view). Click here for a full PDF of the figure.
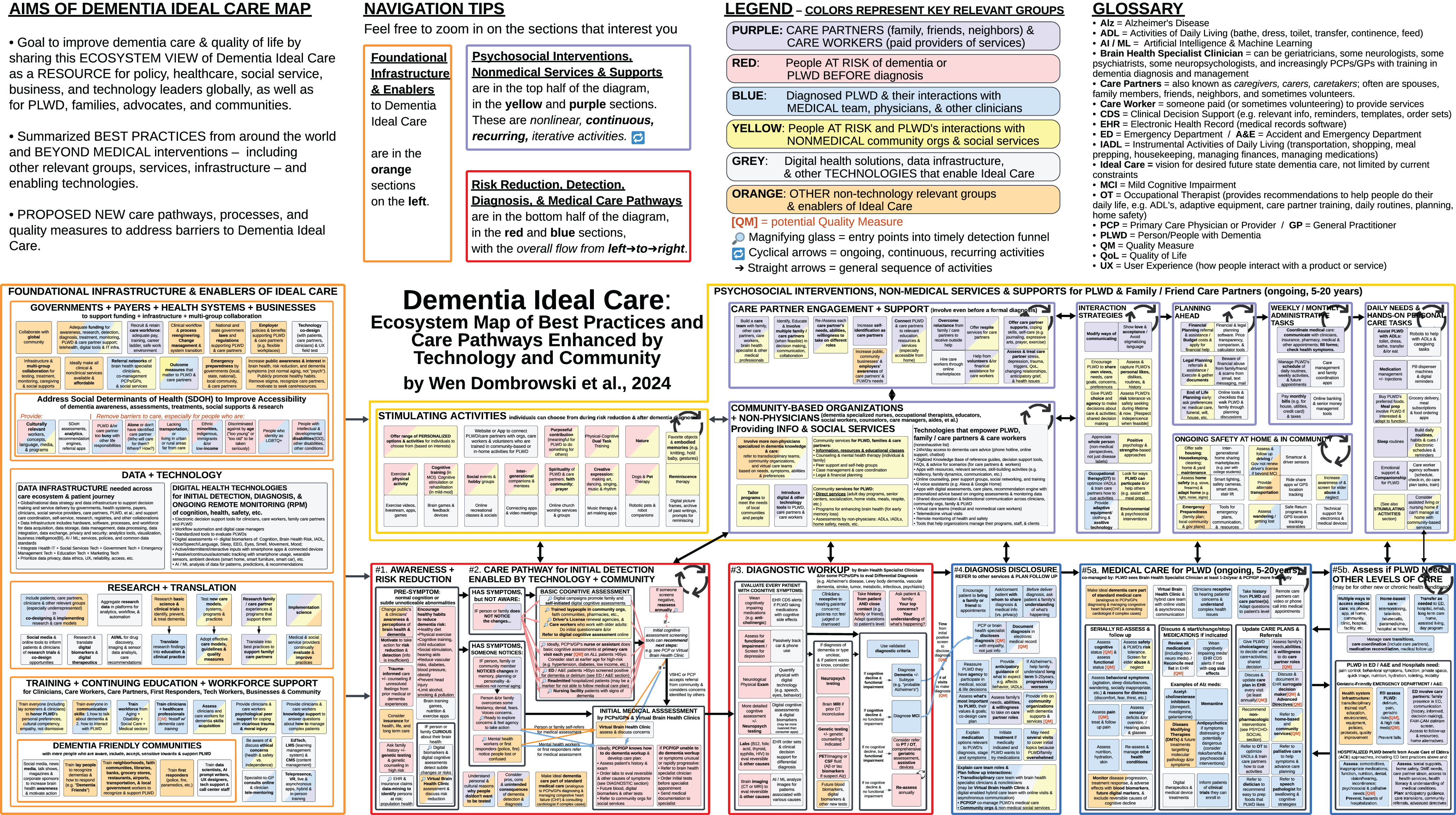
Legend: Diagram colors for relevant groups
The colors in the diagram represent the key relevant groups (elsewhere referred to as stakeholders [7, 8]) involved in each of the best practices:
Purple represents activities that involve care partners (who may be family, friends, or neighbors that provide some assistance to PLWD) and/or care workers (paid service providers or volunteers).
Red represents people who are either at elevated risk of developing dementia or PLWD before they are diagnosed. These are individuals who do not yet carry a clinical label of dementia.
Blue represents diagnosed PLWD and their interactions with the medical system through clinics, hospitals, and virtual care through telehealth.
Yellow represents people at risk and PLWD’s interactions with nonmedical community-based organizations and social services.
Grey represents digital health solutions, data infrastructure, and other technologies that enable Ideal Care. These are listed in the diagram as solution categories, not as names of specific vendors or products. The grey boxes are next to the best practice activities that each technology solution supports.
Orange represents other relevant groups that enable Ideal Care, such as government, employers, educators, and researchers.
Legend: Diagram icons
[QM] indicates potential Quality Measures to assess and improve the delivery of those activities.
Magnifying glass icon (🔎) indicates multiple entry points into an initial detection funnel where potential cognitive impairment or risk may be identified.
Cyclical arrows (🔁) signify ongoing, continuous, and/or recurring activities.
Straight arrows (➡) signify activities that typically happen in sequence, with items on the left occurring before those on the right.
Navigation tips: Overview of diagram sections
The diagram layout flows left to right, with many activities on the left side as precursors to those on the right. However, many activities on the left are ongoing or recurring throughout disease progression.
At the far left of the overall diagram, these primarily orange and grey sections (Figs. 8 and 9) reflect the Foundational Infrastructure and Enablers of Ideal Care such as governments, health systems, employers, technology, data infrastructure, research, and training. These capabilities are necessary to deliver the best practices throughout the Ideal Care Map.
The yellow and purple sections at the top middle and right side (Figs. 2–4) reflect Psychosocial Interventions, Non-Medical Services and Supports for PLWD and Care Partners. This includes stimulating activities, family/friend care partner engagement, and community-based organizations providing support. Arrows link subsequent subsections on the right to show care partner activities that increase with disease progression.
Red sections at the bottom middle (Figs. 5 and 6) reflect the initial increased awareness of brain health and actions for dementia risk reduction among children and young adults without dementia and adults with undiagnosed dementia. These are linked by arrows to care pathways for initial detection and diagnostic workup.
The blue sections at the bottom right (Figs. 6 and 7) reflect the patient journey in the medical care pathway from diagnosis disclosure to ongoing medical care, and determining when higher levels of care are needed.
Key observations from the overall diagram layout
This Dementia Ideal Care Map includes both Current State best practices and Future State innovations in processes, technology, terminology, and relevant groups. These are visually summarized in a format adapted from capabilities architecture [10] (with some subsections as care pathways), because the varied content did not fully fit into a conventional workflow flowchart, stakeholder map, or data flow diagram.
The nonlinear nature of best practices became apparent early in drafting this Ideal Care Map, especially for nonmedical activities and underlying infrastructure enablers, which are ongoing, continuous, and/or recurring. This differs from typical depictions of dementia medical care pathways that often show a straight line of sequential steps [6]. In real life, multiple activities happen simultaneously and with fluctuating intensity. While everyone’s journey with dementia is different [6], we summarized best practices to help PLWD, care partners, and other relevant groups prepare for the long journey ahead.
Currently, many PLWD and their families are unaware that dementia (and especially Alzheimer’s) is a chronic, progressive disease. People with Alzheimer’s dementia currently have a life expectancy of around 5 to 10 years after diagnosis, with a wide range of variability. The Ideal Care Map indicates a range of 5 to 20 years of life after dementia diagnosis because: 1) some PLWD have lived 20 years or more; 2) as more people are diagnosed earlier, their post-diagnosis survival period is extended; and 3) although innovations in public health [12, 13] and clinical treatments may reduce incidence and prevalence, delay onset, and slow or stop disease progression – it is important to communicate the wide range of survival and anticipatory guidance to PLWD and their care partners, so they can prepare for what to expect in upcoming years [14–18].
The Ideal Care Map design takes a person-centered, life journey perspective, instead of only focusing on the medical patient journey. With a person-centered view, psychosocial interventions, non-medical services and supports can begin before an individual enters a medical patient journey or medical care pathway. For example, the intellectually and socially Stimulating Activities in Fig. 2 can begin early in life to benefit overall cognitive, psychological, and physical health.
Similarly, when an individual or family member initially notices symptoms of cognitive impairment and begins to explore their concerns, they do not need to wait until after a definitive medical diagnosis to interact with patient advocacy associations and other community-based organizations (Fig. 3). In fact, during this early stage of their journey is when individuals and families greatly need emotional support, information about types of dementia, resources, cognitive assessments, and how to access dementia specialists for diagnostic workup. These organizations are valuable throughout the care and patient journey—from early symptoms to bereavement.
By looking at Ideal Care from a person-centered, life journey perspective, it became apparent that much of the risk reduction, initial detection, and after-diagnosis care workload can be shifted to multidisciplinary teams, virtual care teams, and/or nonphysician care workers from community-based organizations. Instead of expecting PCPs (primary care physicians)/GPs (general practitioners) or dementia specialist physicians to “do it all,” they can delegate to non-physician care workers trained in dementia to: assess and monitor non-medical needs; answer non-medical questions; educate, advise, and provide resources; provide direct care and social services. This is especially important considering the ongoing shortage of dementia specialists and the bottlenecks for PLWD to access dementia care. Distributing dementia care responsibilities can help physicians feel less overwhelmed, allow more time to focus on the diagnosis and medical management, and provide a more holistic suite of services to maximize quality of life for the PLWD and their care partners.
Technologies that augment human efforts can support clinical and non-clinical care workers, care partners, and PLWD. For example, decision support tools, personalized recommendation engines, and remote patient monitoring programs [15]. Technology solutions should bridge all stages of life and disease, and all relevant groups including PLWD, care partners, primary care, specialists, community-based organizations, and government-sponsored services.
Therefore, ideally, the impact of technologies (grey boxes) and cross-cutting best practices (orange boxes) could be depicted as wide horizontal bars across the entire diagram, across the whole person journey. However, due to practical limitations of diagram space and publication format, the bars across the diagram were changed to individual boxes within subsections.
Another example of a cross-cutting best practice highlighted by multiple experts is the importance of eliciting and understanding each PLWD’s personal and cultural concepts of dementia and health. How someone views dementia directly impacts what actions they take, if any. Someone who views dementia as an inevitable part of “normal aging” [1, 14, 18, 19] may be less likely to take steps to reduce risk, assess, or treat. In contrast, someone who views dementia as a preventable disease may be more likely to pursue risk reduction strategies, diagnosis, and treatment.
PSYCHOSOCIAL INTERVENTIONS, NON-MEDICAL SERVICES AND SUPPORTS
Figures 2–4 summarizes psychosocial, non-medical, nonpharmacologic interventions for PLWD, family/friend care partners, volunteers, and paid care workers. These are supported by community-based organizations and social service providers.
Figure 2: Stimulating activities
Fig. 2
Stimulating Activities that Individuals Can Choose from During Risk Reduction and After Dementia Diagnosis.
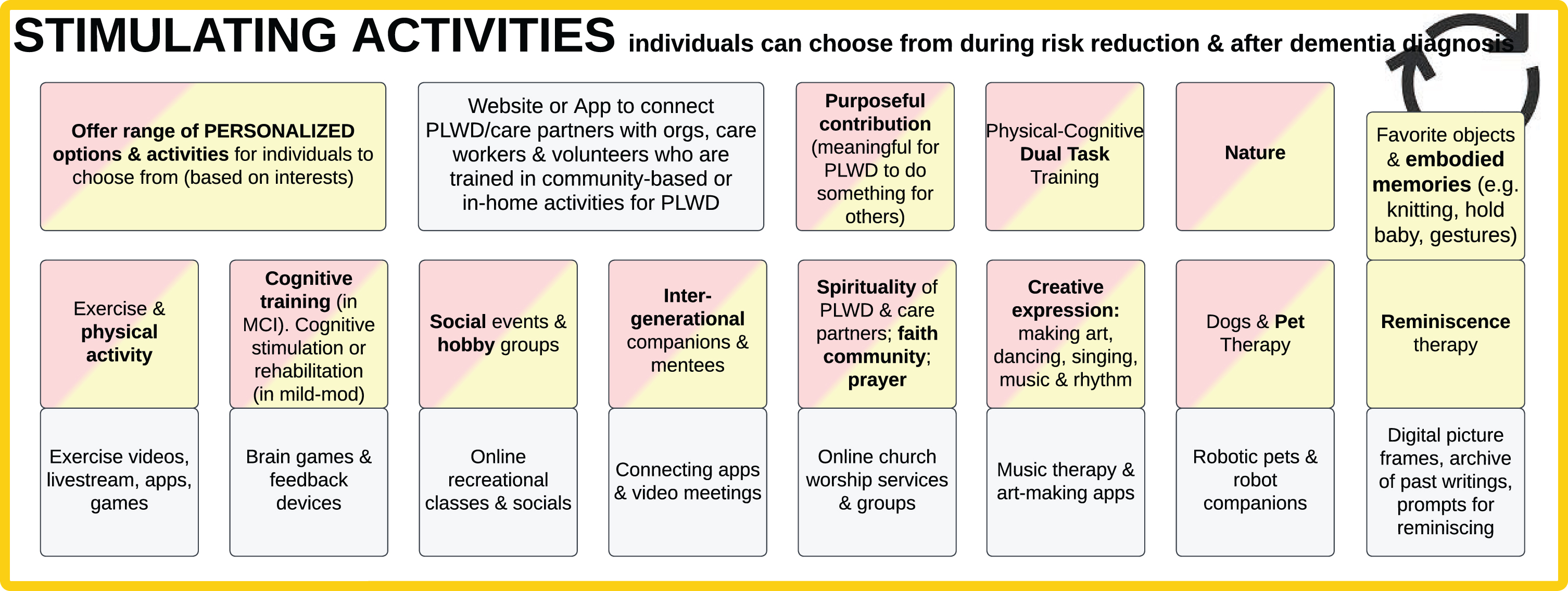
The section in Fig. 2 includes cognitively stimulating activities (such as physical exercise, cognitive training, making music, art therapy) [3, 4, 18, 20–23] to improve quality of life and primary and secondary risk reduction in children, adults at elevated risk, PLWD, and their care partners. Several of these can also be helpful to PLWD with behavioral and psychological symptoms of dementia (BPSD).
One category of activities with emerging evidence is Physical-Cognitive Dual-Task Training. This involves simultaneously performing a physical and mental task (dual-task), such as walking while talking [24, 25]. Recent studies show that “multimodal physical exercise training and multisensory cognitive stimulation in a dual-task paradigm improved cognitive performance on visual episodic memory, verbal episodic memory, sustained visual attention, and ... cardiorespiratory conditioning, lower limb strength resistance, functional mobility, gait speed ... and quality of life” [26].
Work or purpose-filled contribution helps both PLWD and non-PLWD experience meaning, hope, and less isolation [18, 27]. Thus, the Ideal Care Map emphasizes contributory activities in which all individuals can receive, give, or share. Especially in early and moderate stages of dementia, there are numerous ways PLWD can work in supported roles, volunteer, or make things that are enjoyable [21]. For example, a traditional, deficits-based medical model of dementia may limit PLWD’s kitchen activities due to safety concerns; in contrast, a strengths-based approach focuses on using retained skills in collaborative activities such as assisting with making salad or drying dishes. Engaging PLWD even in simple tasks, household chores, and asking their opinion about what a care partner is working on can be mutually beneficial for the PLWD, care partner, and their overall relationship.
Figure 3: Care partners and community-based organizations
Fig. 3
Care Partner Engagement and Support (top) and Community-Based Organizations Providing Information and Social Services (bottom).
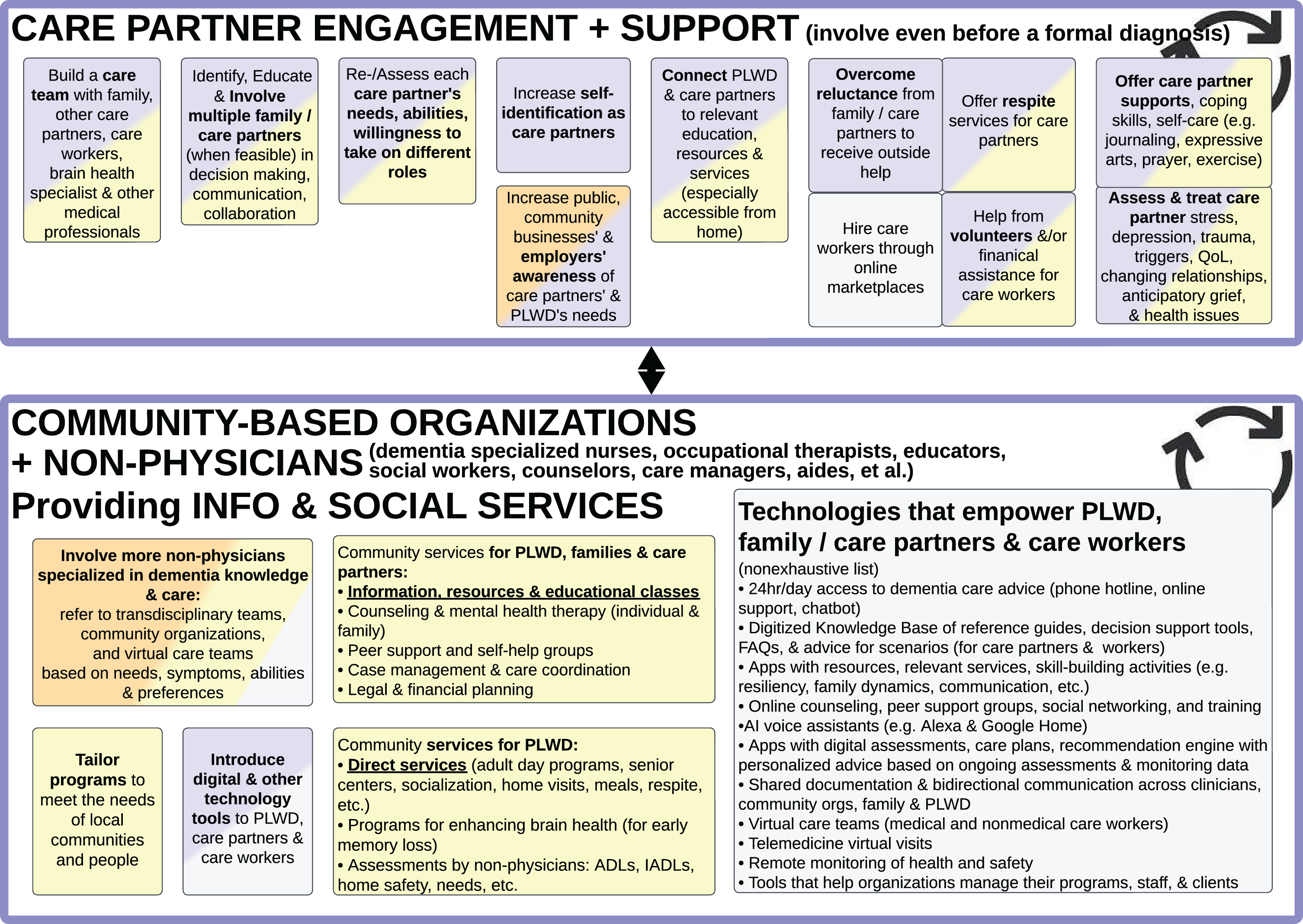
Care partner engagement and support
SMEs who work closely with PLWD recommended the term “care partners” (instead of caregivers or carers or caretakers) because “partnering” shifts the mindset from “giving care to” someone who passively receives, to “doing things with” someone. It shifts the approach to engaging PLWD in their personal agency to participate and make decisions [27]. There is a mutuality in the relationship, with both the PLWD and family and friends partnering to navigate life together [28–31].
Most societies and cultures presume family members will take on care partner and caregiving responsibilities [5, 13, 15], but oftentimes a family member may be unable or unwilling to assist a PLWD with all their needs, particularly as dementia progresses and spans years or decades. PLWD’s spouses may be physically frail, and PLWD’s adult children often have competing responsibilities of work, parenting, or their own health challenges [5, 32]. Some PLWD have also alienated their family or friends through historic or ongoing relationship issues.
Perhaps it is time to actively discourage one individual care partner from carrying all responsibilities alone. A network of family, friends, neighbors, volunteers and social service providers may be more capable of, and willing to, handle various responsibilities such as companionship, home cleaning and maintenance, medication supervision, medical care coordination, finances, and legal planning (Fig. 4) [5, 33].
SMEs emphasized the importance of medical and non-medical providers assessing (and reassessing) whether family/friends will take on and sustain care partner responsibilities and which responsibilities. As care demands and care partner availability change, it is important to discuss their level of interest, knowledge, skills, gaps, personal needs, expected roles, and need for outside help [4, 5, 20, 21, 31, 33–36]. Otherwise, the care partners may experience preventable emotional distress and physical injuries [4, 5, 15, 17, 21, 37, 38].
For gaps in knowledge and skills, community-based organizations can provide education and training to family/friend care partners [4, 5, 15, 18, 34, 39]. For the care tasks that family is unable or unwilling to support, they may be able to find volunteers and paid care workers [5]. This relates to the Funding and Infrastructure summarized in Fig. 8, where it would be transformative if governments and health systems would make all necessary services available and affordable [4, 5, 11, 40].
Furthermore, it would help to have governments, businesses, and the general public be more responsive to “caregiver burden” and proactively support care partners through flexible work policies and benefits (Fig. 8) [5, 13] and Dementia Friendly Communities (Fig. 9) [5, 15, 41–46].
Community-based organizations providing information and social services
Community-based organizations (CBOs; sometimes referred to as community organizations) typically are non-profit public or private organizations that serve community needs. Digital technologies increasingly allow many organizations to serve not only their local/regional communities, but also national and global communities (i.e., “glocal” reach). The size and structure can vary, from informal grassroots groups such as social media support groups and local faith communities to formally incorporated multinational organizations [5].
Each CBO offers a different range of services. Some CBOs offer educational information about dementia through publications, websites, online videos, and occasionally public outreaches [15, 31, 41, 47, 48]; this information is often provided to PLWD, care partners, and the general public as a unilateral interaction. There are also some CBOs that bi-directionally interact with PWLD and care partners through talking about advice and feelings—such as in-person or online interactive trainings, mental health counseling, peer support groups, care coordination, curated recommendations [35, 49], legal and financial planning services [5, 15].
This differs from the direct services provided by some CBOs—hands-on, physical support to PLWD and care partners—such as adult day programs, meal delivery, assistance with Activities of Daily Living (ADLs such as toileting, dressing, bathing, transferring, eating) [50], and respite care (when someone assists a PLWD while the care partner takes a break) [5].
Educational information is not enough to support PLWD and their care partners, especially in underserved communities and low-income countries where direct services may not exist and/or people aren’t aware of how to access those services [1, 5, 18]. It is important to fill these gaps by developing and bringing services to where people are in their communities (which relates to the Infrastructure capabilities described in Figs. 8 and 9).
Information, support, and direct services need to be tailored to the unique needs of individual PLWD, their care partners, and local communities. For example, considering cultural, spiritual, social, and geographic differences [1, 5, 15, 18, 31, 41, 42, 51].
SMEs emphasized directly engaging PLWD when providing information, resources, and support (instead of only focusing on helping care partners) [4]. Too often, advice is directed only at care partners and assumes PLWD are unable to participate. Especially during the earlier stages of dementia, PLWD can engage, learn, voice their preferences, and assert personal agency [11, 14, 27, 31, 32]. Similarly, instead of developing “caregiver apps”, mobile apps can be developed to empower both PLWD and their care partners.
The COVID pandemic demonstrated that PLWD and their care partners benefit from technology tools, including accessing digital resources, telehealth medical appointments [52, 53], and online counseling, training, and peer support meetings [5, 15, 18, 31, 54]. These technologies also expanded the reach of CBOs’ impact and increased access for PLWD and care partners who might otherwise be unable to travel to in-person services and meetings because of geography, lack of transportation, or inconvenience [4, 31].
This highlights the importance of engaging user experience (UX) designers and human factors engineers in collaboration with intended users (PLWD, care partners, care workers and clinicians)—from ideation through design, development, and marketing—so that websites, mobile apps, other software, and devices are optimized for relevance, ease of use and accessibility [33, 55, 56]. It can also be helpful for CBOs to involve “tech support” personnel or volunteers to assist PLWD and care partners with setting up their accounts, apps, video meetings, etc.
Figure 4: Interaction strategies, planning, safety, administrative tasks, and personal care
Fig. 4
Interaction Strategies (left); PLWD need increasing assistance with Planning Ahead (left of center), Ongoing Safety at Home and in Community (bottom), Weekly/Monthly Administrative Tasks (right of center), Daily Needs and Hands-On Personal Care Tasks (right).
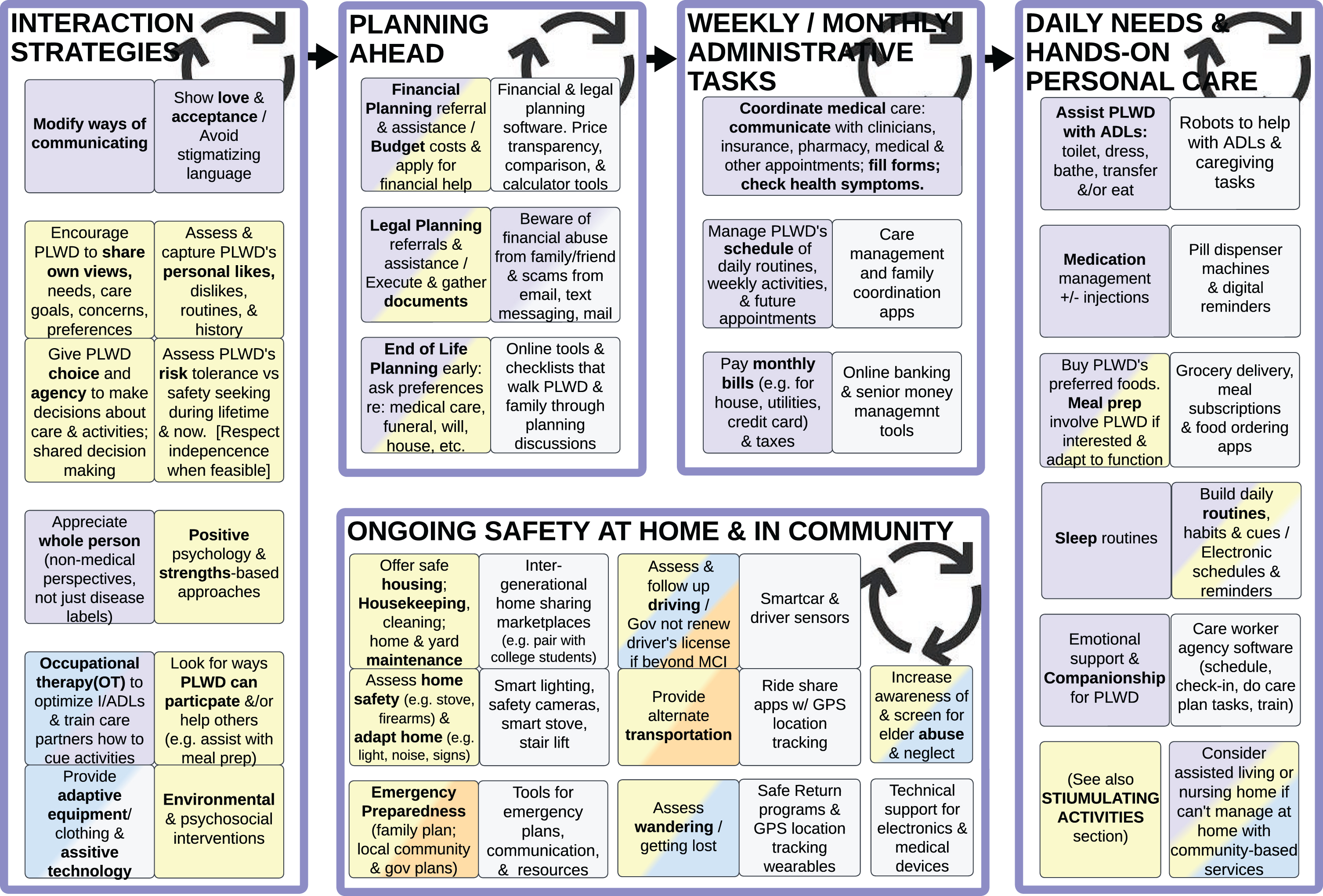
Figure 4 lists best practices that care partners and care workers can utilize to strengthen their relationship with and assist PLWD, beginning with Interaction Strategies and Planning Ahead from the onset of MCI and mild dementia. As disease progresses, PLWD’s needs increase from sporadic to more assistance with Safety at Home and in the Community, Administrative Tasks, and eventually Daily Personal Care Tasks [49].
Interaction strategies
SMEs recommend seeking opportunities for PLWD to lead and/or partner in matters concerning them and to grant PLWD with a degree of agency in their daily routines and decisions about longer-term plans, rather than imposing decisions made by others. The key is to acknowledge the person first (instead of a deficits-based medical model that focuses on disease), by using a person-centered approach that encourages each PLWD to share their own views, goals, preferences, likes and dislikes [4, 14, 15, 18, 20, 30–32].
Each PLWD’s prior lifestyle and personal preferences affect which best practices are applicable. For instance, if someone has historically been averse to taking medications or eating healthy foods, they may resist these as a PLWD. It is valuable to evaluate each PLWD’s historical risk tolerance, current safety preferences, and desire for independence. If a PLWD has always been a risk taker, but their care partners have different views on risk, efforts should be made to respect the PLWD’s autonomy whenever possible, as long as they do not pose a significant danger to others. What is feasible may vary as disease progresses and depend on the form of dementia (for example, frontotemporal degeneration typically impairs judgment earlier than Alzheimer’s disease).
To optimize successful and effective interactions, it is essential that care partners and care workers learn and use skills of modifying communication, showing acceptance, and having a strengths-based view of PLWD (instead of a medical deficits-based perspective) [20, 22, 27]. When a PLWD progresses to needing more assistance, care partners can learn strategies to simplify and cue (verbally, kinesthetically, and/or visually) when doing ADLs (Activities of Daily Living), IADLs (Instrumental Activities of Daily Living), other activities and routines [4, 21, 57].
Other helpful supports include modifications to clothing, set-ups, equipment, tasks, controls, and environments [4, 14, 20, 31, 58–60] to improve access, safety, and function. A good source of these interaction strategies and adaptations are occupational therapists (OT) [57, 61], gerontologists (non-physician specialists in older adults), and other care workers from community-based organizations who are trained in dementia support and care strategies [21].
Planning ahead
Longer term financial planning, legal planning, spiritual and end of life planning should be discussed early on while a PLWD has insight and capacity to contribute to decisions; these plans need to be updated periodically as PLWD’s needs or preferences change [1, 4, 5, 15, 18, 31, 34, 62].
Ongoing safety in home environment and community
This section of the Ideal Care Map summarizes what care partners need to consider to ensure that PLWD are safe while maximizing agency [35, 63, 64].
There is also the need to balance public health safety and individual autonomy. When an individual PLWD’s misuse of a car [18, 35, 45, 47, 65–69], stove, or gun can potentially hurt other people, this creates ethical dilemmas and the need to protect other people and protect PLWD themselves. Clinicians are often called upon to address these concerns [62]; training clinicians [20] and establishing systems are both needed to evaluate individual PLWD’s safety and risk profiles.
Other considerations for protecting PLWD that may limit their autonomy include enclosed homes, fences, and other environments where they are less prone to wander away [4, 21]. However, with GPS technologies now being ubiquitous, it is possible to use wearable devices that track a PLWD’s real-time location and send alerts to care partners, while providing PLWD more relative freedom of movement. Use of such devices may add technical support responsibilities to care partners.[5]
PLWD are vulnerable to physical, psychological, and financial abuse, neglect, and exploitation [15]. Unfortunately, it may be family or friends who are committing these abuses, so care workers from CBOs and medical professionals need to be vigilant in assessing this [15]. National and regional governments can actively share best practices and programs to combat abuse, neglect and fraud among PLWD [70].
Weekly/monthly administrative tasks
As each PLWD progresses in their dementia disease severity, they will need increasing help with managing administrative tasks and planning their monthly, weekly, and eventually daily routines. This can include organizing a schedule of activities, coordinating medical care (including accompanying PLWD to doctor’s appointments, filling forms, dealing with health insurance, paying medical bills), paying monthly utility bills, and providing technical support for devices [5, 32].
Daily needs and hands-on personal care tasks
Eventually, a PLWD’s needs go beyond periodic assistance to daily help with ADLs, taking medications, and even 24-hour care with disrupted sleep and behavioral and psychological symptoms of dementia (BPSD) [5, 32, 33]. SMEs emphasized that family/friend care partners often are unaware early in the journey that their roles and responsibilities will increase substantially when disease progresses. This can impose significant physical, psychological, and financial stress on family/friend care partners as they face increasingly intense roles [5, 15, 38, 39, 49].
In contrast, Ideal Care would include anticipatory guidance for care partners prior to their PLWD needing more care. This allows time for care partners to prepare mentally and emotionally, connect with community resources, recruit other care partners to share responsibilities, plan for potential changes in their work and life responsibilities, learn interaction strategies and adaptive modifications [5, 15, 31]. If a PLWD’s home safety, health, and/or personal care needs increase beyond what their care partners can support at home, they may need to consider transitioning to an assisted living or nursing home; this may alleviate some care partners’ mental and physical stress, but the costs may cause more financial stress [31].
AWARENESS AND MEDICAL ACTIVITIES
Figure 5 summarizes brain health Awareness, Risk Reduction, and an Enhanced Care Pathway for Initial Detection Enabled by Technology and Community. Figures 6 and 7 summarize the subsequent medically oriented activities: Diagnostic Workup, Diagnosis Disclosure, Ongoing Medical Care, and Assess If Need Other Levels of Care. All this can happen in parallel to the Psychosocial Interventions, Non-Medical Services and Supports described in Figs. 2–4.
Figure 5: Awareness, risk reduction, and detection
Fig. 5
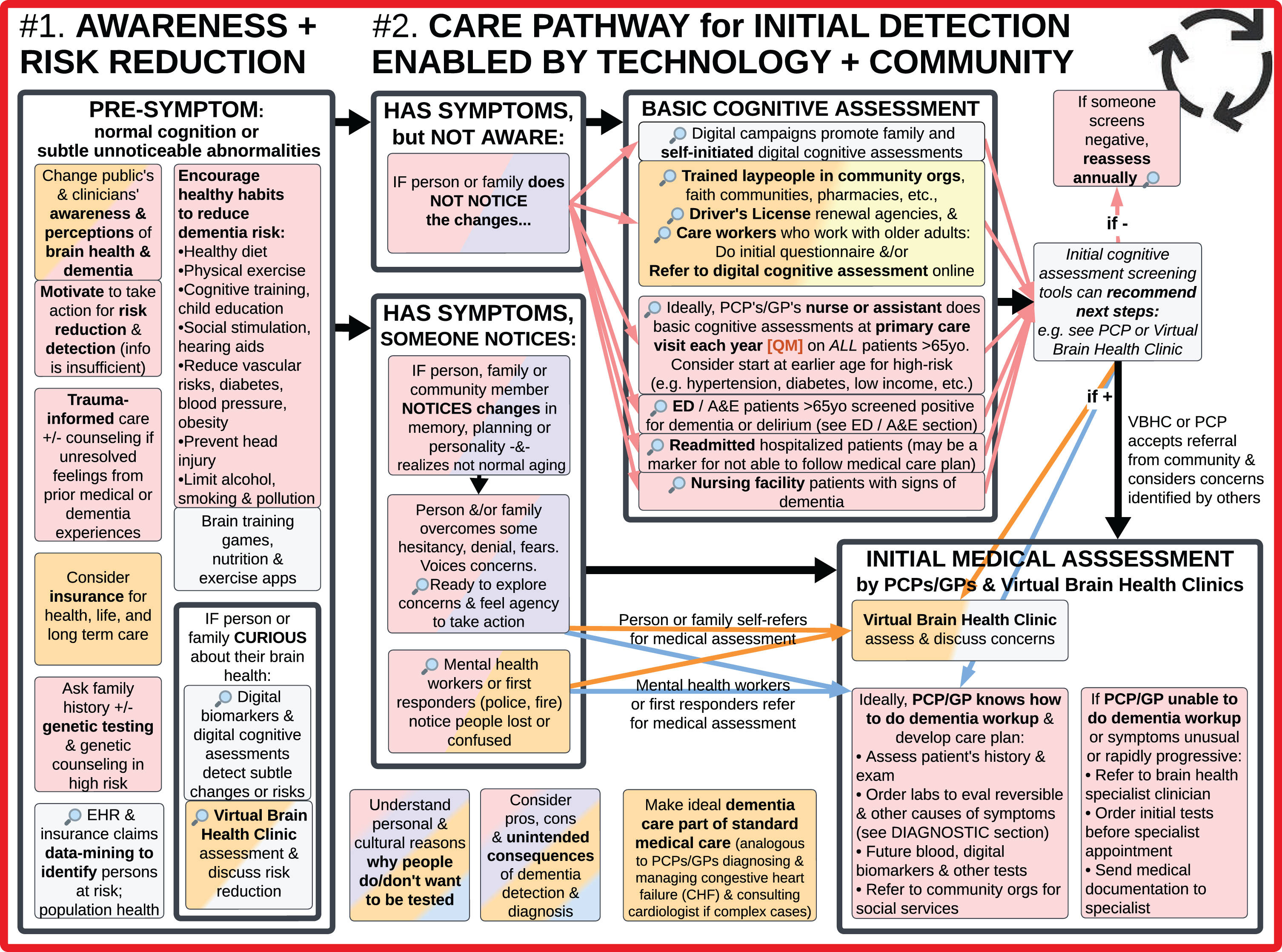
#1. Increase Awareness of Brain Health, Dementia, and Risk Reduction Among Individuals, Families, and Clinicians (left); #2. Enhanced Care Pathway for Initial Detection Enabled by Technology and Community (right).
#1. Increase awareness of brain health, dementia, and risk reduction among individuals, families, and clinicians
SMEs suggested national and local public awareness efforts analogous to obesity and women’s heart health campaigns [64, 71] to increase brain health awareness and motivate action to reduce dementia risk and improve care. This includes storylines within TV series, talk shows (radio, podcast, and vlog), news (broadcast, print, and electronic), health magazines, social media, influencers relevant to different target audiences, community outreach events, and creative grassroots efforts.
Awareness includes understanding lifetime strategies to optimize brain health, reduce risks for developing dementia, recognize dementia symptoms, seek diagnosis, access care, and improve quality of life with dementia [1, 15]. Increasing awareness among lay people and clinicians should begin before obvious symptoms and engage beyond traditional patient education and clinician education.
Lay people and clinicians can realize it’s possible to engage in dementia risk reduction and slow disease progression with nonpharmacologic lifestyle changes during early and mid-life [15, 21, 72–74], such as 1) “dietary counseling, physical exercise, cognitive training, and vascular and metabolic risk monitoring” studied in the FINGERS trials [18, 75]; 2) “evidence supports ... modifying 12 risk factors might prevent or delay up to 40% of dementias ... systolic BP of 130 mm Hg or less ... hearing aids ... Reduce exposure to air pollution ... Prevent head injury ... Limit alcohol use ... Avoid smoking ... Provide all children with primary and secondary education ... Reduce obesity ... Addressing other putative risk factors for dementia, like sleep, through lifestyle interventions.” [12]; and 3) the “Stimulating Activities” listed in Fig. 2.
Brain health and dementia risk reduction should be treated as life course activities, starting with optimizing prenatal health and regularly assessing brain health from birth onwards, especially in people with elevated risk factors (such as previously hit head playing football or has high blood pressure). “Prevention is about policy and individuals. Contributions to the risk and mitigation of dementia begin early and continue throughout life, so it is never too early or too late” [12]. The Infrastructure described in Figs. 8 and 9 (e.g., policy, technology, research translation, and training) is the foundation needed to enable the ideals in Fig. 5 (increase awareness and motivate action).
Awareness includes understanding that various forms of dementia including Alzheimer’s may show up not only as forgetfulness—dementia can manifest as a wide range of symptoms including behavioral issues, bad decisions, difficulty planning, confusion with familiar tasks, feeling lost, trouble with words, and/or personality changes [4, 5, 21, 73]; and recognizing dementia can occur at any age (even as early as 30 or 40 years old) [11].
However, policy and education about dementia are not sufficient: psychological safety and motivation are needed for individuals and communities to take an interest in these topics, pursue healthier lifestyle choices, and be willing to complete cognitive assessments. Efforts need to go beyond delivering information to address underlying fears about own health, grief from prior experiences with PLWD [5], and unresolved feelings from prior medical trauma, etc. It is also important for lay people and clinicians to develop skills in emotional self-regulation.
#2. Enhanced care pathway for initial detection enabled by technology and community
Addressing underlying psychological barriers and increasing awareness of brain health sets the stage for more people wanting to know about new dementia risk reduction measures, tests, and treatments as they become available.
Because the word “screening” has different connotations among different relevant groups and in different geographic regions, the word “detection” is used here to include the broad range of activities and situations where potential cognitive impairment or risk may be identified.
The visual analysis of the Ideal Care Map (Fig. 1) revealed that timely initial detection of dementia has the potential benefits of providing PLWD with earlier access to legal, financial, and spiritual planning, along with nonpharmacological interventions, care partner supports, secondary prevention measures, symptomatic and emerging disease modifying therapies (DMTs), and ultimately improve quality of life (QoL). Clinicians need to recognize these benefits of timely detection and not shy away from cognitive assessments when a patient or family requests it [3, 18, 38, 76]. To optimize access to the benefits of timely initial detection, ideally individuals with underlying disease would be identified even before they have noticeable symptoms and concerns [74].
SMEs highlighted the importance of not waiting until the ages of 65 or 75 to assess cognitive function, as this will overlook people with MCI or younger-onset dementia [11]. Symptoms sometimes manifest earlier, even age 30 for those with specific gene mutations or diseases like frontotemporal degeneration. Ideally, initiatives for early detection should not treat everyone as a single group, but instead identify those at elevated risk to begin assessments at a younger age. For example, for people with risk factors such as lower socioeconomic status, hypertension, diabetes, intellectual disability [15, 65, 77], depression and/or family history of dementia – consider beginning cognitive assessments at 40–50 years old. Or at least qualitatively inquiring “If you compare yourself to last year, would you say your thinking abilities are about the same, worse, or better?” or similar questions [73].
These risk stratification and early detection efforts can be enhanced with technologies such as data-mining electronic health records (EHRs) [73, 78, 79]; analyzing data on social determinants of health (SDoH); and annually comparing scores from brain game apps, digital cognitive assessments, and emerging devices for remote monitoring of digital biomarkers [15, 55].
We recognize that because of stigma, fear, and concerns about losing their job or insurance—some people are reluctant to assess their cognition and would rather not know if they have a dementia diagnosis [1, 11, 15, 51, 80]. There are also some physicians who feel uncomfortable discussing a dementia diagnosis or assume patients don’t want to receive “bad news” [40]. However, in our clinical experience, many patients are frustrated with not knowing what condition they have—most would rather know what condition they have and how it will impact their life [5, 18, 81].
Community-based and technology-enabled dementia detection
A diagnosis from a trained and licensed medical professional is essential for dementia care. There is a critical need to determine how to detect the growing population of undiagnosed PLWD and acknowledge the limited number of medical professionals currently available for this. Scaling up timely dementia detection requires a combination of reconsidering the limits of current practices and enhancing alternatives to better serve diverse populations in non-cost prohibitive ways. Considering the existing barriers to and shortage of PCPs/GPs and neurologists to initiate dementia detection [19, 80], we propose an enhanced care pathway for detection that involves patients themselves, community lay people, and technology-enabled services to do the initial Basic Cognitive Assessments. These decentralized, multiple entry points into the broad funnel for detection are represented in the Ideal Care Map with a magnifying glass icon 🔎. Culturally sensitive models can bridge individuals who were resistant to testing into medical care [14, 15, 41].
For example, individuals who are curious about their own brain health and/or notice symptoms can directly access symptom questionnaires [21] and validated digital cognitive assessments through online consumer health websites and/or through “Virtual Brain Health Clinics” that provide telehealth services specialized in evaluating and managing dementia (described below).
Additionally, non-medical service providers who work with older adults, as well as lay people in community-based organizations, volunteer groups, and local pharmacies can be trained to provide awareness info about brain health and encourage people to complete basic questionnaires or validated digital cognitive assessments [1, 14, 31]. These local stores and groups are already strategically located throughout communities, frequently visited, and trusted. For example, Alter Dementia trains churches and other faith communities on recognizing dementia and sharing postcards that link individuals to a website with an initial screening questionnaire [41, 42, 82]. Government driver’s license renewal offices can also play a role in administering these questionnaires or basic validated digital cognitive assessments.
It is important that those with potentially concerning scores are connected to qualified medical professionals to review the results, perform formal diagnostic workup (Fig. 6), and decide the appropriate next steps of communication and suggested action [1, 4, 21]. Decision support content within the digital cognitive assessment applications can encourage people to see their PCP/GP, a neurologist, or Virtual Brain Health Clinic for Initial Medical Assessment; it can also provide the contact info of nearby brain health specialists and allow individuals to schedule these appointments online.
Another approach involves nurses and medical assistants at PCPs/GPs’ offices to administer yearly validated digital cognitive assessments to all patients over age 65 [3–5, 40, 73] (and at an earlier age for individuals in high-risk groups). This would offload cognitive testing from busy physicians/providers, standardize testing, and be automatically done without waiting for patients to recognize and ask about dementia symptoms. Patients with normal cognitive assessment results can repeat the assessment annually.
Emergency department (ED)/accident and emergency (A&E) and inpatient hospitalizations also play an important role in identifying potential PLWD by uncovering previously unnoticed declines in function in patients with frequent readmissions [83, 84], delirium [4, 12, 58], and/or positive cognitive screen (see “PLWD in ED/A&E and Hospitals” section in Fig. 7) [76].
Besides digital cognitive assessments that actively ask individuals to respond, other technologies on the horizon that assist with serial assessments include digital biomarkers of cognitive function based on passive monitoring of various variables, and future blood biomarkers [74, 85–90] that primary care PCPs/GPs can order to assess risk or progression of disease.
Brain health specialist clinicians and Virtual Brain Health Clinic care model
With any of the basic cognitive assessment and risk assessment tools mentioned above, individuals with potentially abnormal results need further evaluation, either in primary care if their PCP/GP knows how to do a dementia diagnostic workup and is willing to pursue it, or they can refer to a brain health specialist clinician [3, 4, 73]. Brain health specialist clinicians include current dementia/memory specialists such as neurologists, geriatricians, and geropsychiatrists [4, 21]; and considering the current specialist shortage and barriers to access to care, also may need to include other physicians, nurse practitioners, and physician assistants with specialized training in initiating diagnostic workup and subsequent monitoring of dementia [19, 74, 80].
To further improve access to care, we propose a new care model of Virtual Brain Health Clinics, which are telehealth practices that either provide hybrid digital plus in-person services, or are affiliated with physical dementia clinics, or refer to in-person brain health specialists. This Virtual Brain Health Clinic care model is based on the demonstrated feasibility of existing analogous models including: telemedicine programs that serve other neurologic and mental health conditions (such as Parkinson’s and depression) [91], disease-specific telemedicine programs for non-neurologic conditions, telehealth companies that screen for cognitive impairment and order blood tests, specialist video consultations occurring in senior care homes, and outpatient geriatrics teams that have adapted their clinical practice to virtual care during the COVID pandemic [92].
Specific services offered by Virtual Brain Health Clinics can include:
• Video visits with clinicians trained in cognitive assessment and management
• Promote brain health awareness and risk-reduction with lifestyle (smoking cessation) and medical management (manage blood pressure)
• Administer digital cognitive assessments and monitor remotely over time for changes from baseline measures
• Order initial blood and imaging tests for differential diagnosis
• May offer counseling and care coordination
• May prescribe some medications
• Refer to other clinicians for services beyond their capabilities
The expectation is not for Virtual Brain Health Clinics to assess and manage all patients, but rather to help initiate care, manage patients with straightforward symptoms, and/or supplement traditional services. This can also be helpful for people living in rural areas with difficult access to in-person care. In-person brain health specialists are needed for patients with more complicated diagnostic and treatment needs—for example, to examine reflexes, strength, and sensation to check for focal neurologic signs [91]; perform lumbar puncture for cerebrospinal fluid testing [65]; consider less common conditions besides Alzheimer’s; and administer onsite DMT infusions.
Figure 6: Diagnosis
Fig. 6
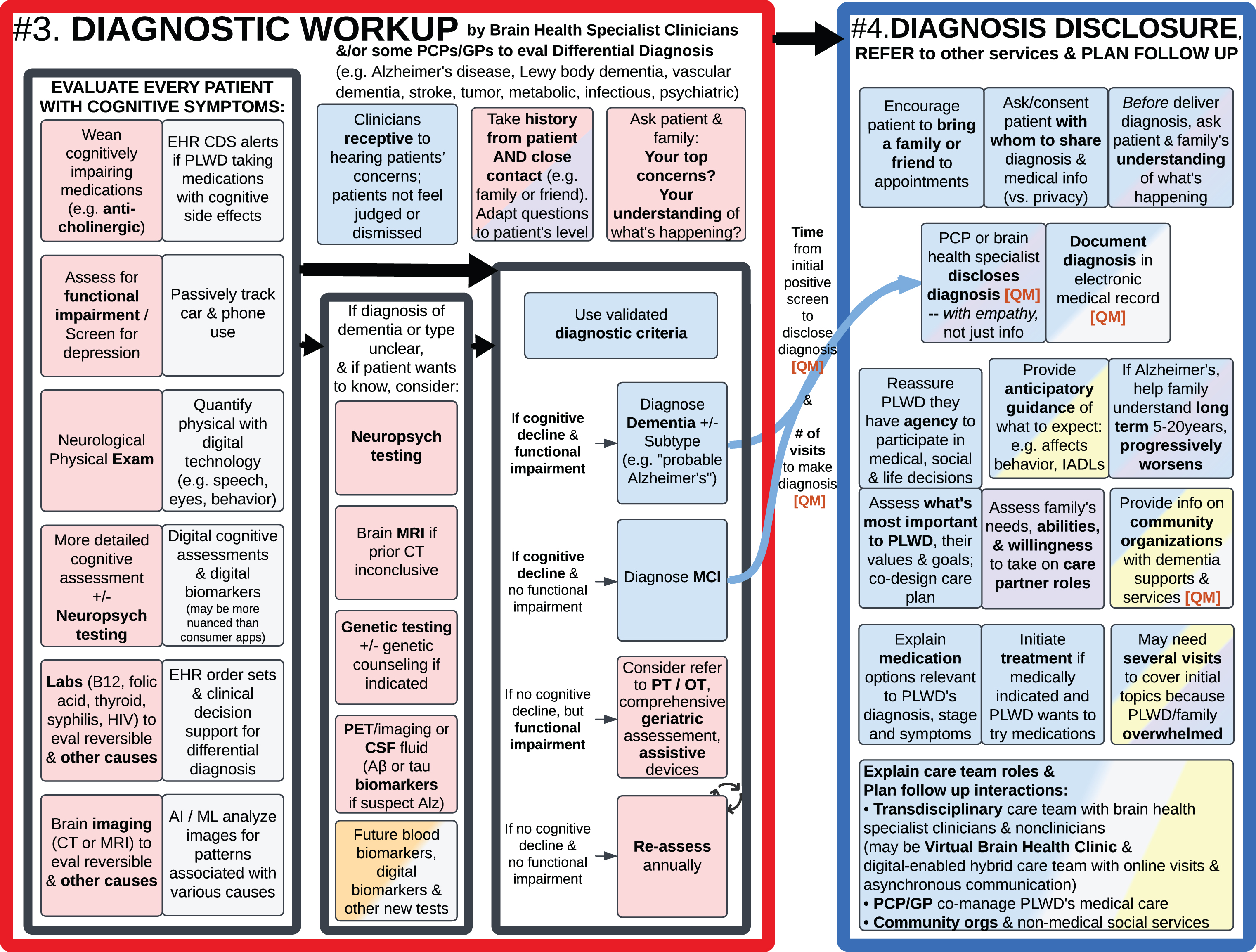
#3. Diagnostic Workup (left); #4. Disclose Diagnosis, Refer to Other Services, and Plan Follow Up (right).
#3. Diagnostic workup
After a basic cognitive assessment detects potential cognitive impairment and/or an individual or family member notices cognitive symptoms, brain health specialists and PCPs/GPs should comprehensively evaluate each patient [3] and make a differential diagnosis between various types of dementia (Alzheimer’s disease the most common, followed by vascular, Lewy body, and frontotemporal dementia) [4, 65, 93] and rule out dozens of non-dementia causes of cognitive impairment (such as medication side effects, depression, hearing loss, sleep apnea, vitamin deficiency, abnormal hormone levels, and infections) [3, 4, 20, 21]. This includes asking each patient and their family/friends about what they’ve noticed [3–5, 21, 94], assessing for functional impairment [3], performing a neurological physical exam, and neuropsychological tests that are more detailed than basic cognitive assessments [4]. Also weaning cognitively impairing medications [3, 15, 20, 21], doing blood tests (such as vitamin B12, folic acid, thyroid, syphilis, HIV; and emerging blood biomarkers [74, 85–90]) and brain imaging (PET, CT, or MRI) [65] can help identify the potential cause of cognitive impairment, including some treatable reversible causes. If the diagnosis is unclear, consider more comprehensive neuropsychological evaluation, genetic testing, and/or lumbar puncture for cerebrospinal fluid biomarkers.
Detailed clinical practice guidelines and research criteria for dementia diagnosis have been published elsewhere [3, 4, 65, 69, 93–99], so Fig. 6 here summarizes key existing best practices and emerging opportunities for Ideal Care.
Because of the developments in imaging, fluid, and blood biomarkers in recent years and the increasing commercial/clinical availability of such tests [85–90], the diagnosis of dementia has been and will continue to shift from symptom-based syndromic classifications to molecular pathology understandings of amyloid-β, tau, other molecules, and mixed pathologies.
The grey boxes in Fig. 6 highlight technology-enabled ways to enhance traditional, manual approaches to evaluating (and monitoring) patients with cognitive impairment. For example, clinical decision support (CDS) tools within EHRs [19, 20, 100] can alert clinicians to medications with cognitive side effects, what differential diagnosis to consider, what tests to order, and how to interpret the combination of results. Existing and emerging technologies can quantify various aspects of cognitive function, using methods beyond traditional paper tests to digitally assess multiple variables—such as quantifying response times, eye movements, body movements, voice, and other biometrics. Many of these high-tech measurement tools are no longer confined to academic medical research centers but are rather accessible for use in community and home settings.
In addition to high tech approaches to ideal dementia care, Fig. 6 highlights the importance of the human elements and psychology in care interactions. For example, developing clinicians who are receptive to hearing patients’ concerns and understanding complex health issues; and asking both the patient and family members for their perspectives [3, 14, 27, 73].
#4. Disclose diagnosis, refer to other services and plan follow up
In Fig. 6, #3. Diagnostic Workup and #4. Disclose Diagnosis are separately called out because the latter involves many psychosocial best practices that are not necessarily addressed during medical evaluation visits but are critically important for an ideal patient journey. SMEs pointed out it can feel overwhelming for someone to hear they have dementia and understand what to do about it [47]. Therefore, patients should be encouraged to bring a family member or friend to appointments and asked for consent to share their medical information with them [4, 18, 34, 62, 73].
As with the initial diagnostic journey, prior to delivering a diagnosis, clinicians should ask the patient and family/friend “What is your understanding of what is happening?” to elicit their personal and cultural framing of their condition, what concerns they’ve already noticed, and what words they are comfortable using (for example, “memory problems” versus “brain change” versus “I don’t know why I’m here” (anosognosia) versus “Why am I getting lost?”). Their understanding informs how the diagnosis is delivered and affects how the diagnosis might be received [18].
Delivering a dementia diagnosis goes beyond simply stating the name of a condition or disease label [34], to helping families understand this is a long-term condition that typically progressively worsens over years, providing anticipatory guidance about what to expect (such as planning for IADLs, ADLs, and how behavior may eventually be affected) [3, 16–18], and referring to local Alzheimer’s associations and other community-based organizations that offer dementia resources and services as summarized in Figs. 2–4 [4–6, 15, 18, 62]. If medically indicated, medication treatment options and their benefits and risks should be discussed and initiated if a PLWD wants to try it [4, 18].
It is important for clinicians to convey the diagnosis with compassion [18, 81], reassure PLWD that they have agency to make decisions about their life and care [11, 27], and reassure there are things they can do to live well with dementia. At the same time, it is helpful to assess family and/or friends’ willingness and ability to take on care partner roles [4, 5, 20, 21, 31, 33–36], instead of assuming a spouse, sibling, adult child, or friend will do and coordinate all the best practices summarized in Figs. 2–7 (see “Care Partner Engagement and Support” section above within Fig. 3).
PLWD and family’s initial reactions to receiving a dementia diagnosis can vary from feeling shock, disbelief, anger, anxiety, sadness, and/or even a sense of relief and empowerment in finding out the cause of concerns [18, 34, 47, 81]. Clinicians can explain to PLWD and care partners they may go through many mixed emotions in the coming months and years. During the diagnosis disclosure visit, clinicians should have empathy to determine how much information to provide now, versus focus on immediate emotional support and allow time to digest. Because physicians have limited time in clinic visits, nurses, social workers, and transdisciplinary team members can provide additional emotional support and information for PLWD and their family/friends, perhaps over several visits post-diagnosis, and explain the role of the various care team members and services [4, 18–20, 31, 34, 35, 40, 49, 62, 73, 101].
Figure 7: Medical care
Fig. 7
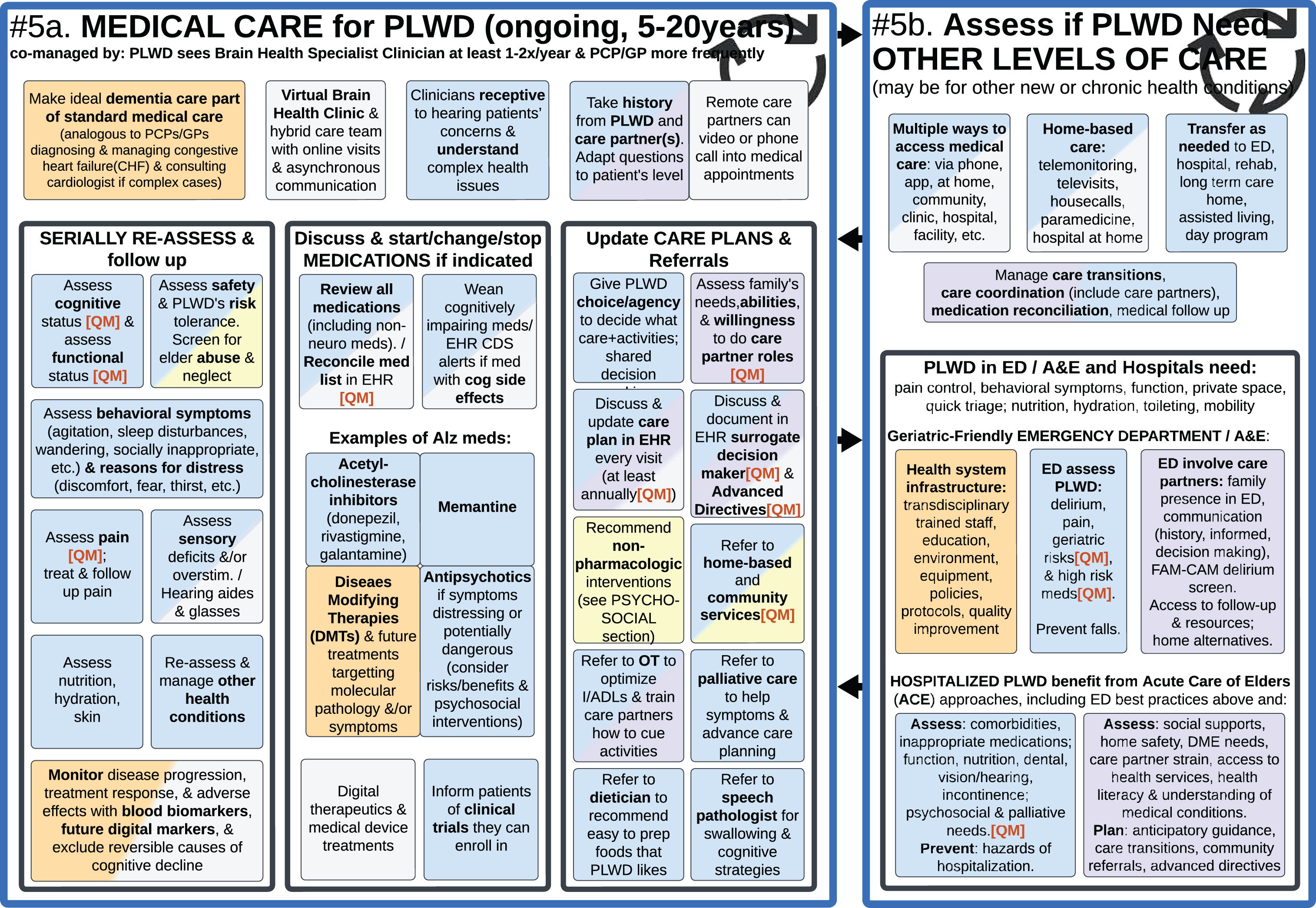
#5a. Medical Care for PLWD (ongoing, 5–20 years) (left); #5b. Assess if PLWD Need Other Levels of Care (right).
#5a. Medical care for PLWD (ongoing, 5–20 years)
After the Diagnosis Disclosure, PLWD need ongoing care throughout their lifetime. Holistic post-diagnostic care includes the Psychosocial Interventions, Non-Medical Services and Supports summarized above in Figs. 2–4, as well as Medical Care summarized in Fig. 7, both tailored to the needs of each PLWD and their care partners.
Because of the global shortage of neurologists, geriatricians, geriatric psychiatrists and other brain health specialists—ideally routine dementia care will become part of standard primary care, analogous to the current standard of care for congestive heart failure [15, 19, 40], with PCPs/GPs diagnosing and managing straightforward cases, while specialists are consulted and co-managing complex cases. PLWDs’ access to medical care can be further expanded with appropriately trained nurse practitioners, physician assistants, transdisciplinary hybrid care teams [14, 60, 101], and Virtual Brain Health Clinics (as described under Fig. 5, Section #2 above).
Ideally, the medical care team should follow up with each PLWD and their care partner(s) several times a year to assess for any changes in: cognition, function, safety, behavior (including BPSD), pain, sensory deficits, other health conditions, emerging biomarkers, quality of life, need for social care and care partner support [1, 3–5, 15, 18, 20, 21, 31, 62, 102]. Medications can be adjusted as needed based on progression of disease or improvement of symptoms with treatments. Figure 7 lists examples of U.S. Food and Drug Administration (FDA) approved medications used in people with Alzheimer’s, the most common cause of dementia; the pharmacological treatments differ for other causes of dementia. Guidelines for dementia medication management [4, 20, 39, 65, 93, 98, 99, 103] will continue to evolve as new medications become available that target various molecular pathology, DMTs, and/or reduce symptoms. PLWD can also be provided with information about enrolling in clinical trials researching new medications, lifestyle interventions, and monitoring tools [3, 4, 15, 41, 48, 73, 104].
In addition to medications, PLWD and their care partners can benefit from Risk Reduction lifestyle modifications (Fig. 5, Section #1); Stimulating Activities (Fig. 2); and referrals to: Community-Based Organizations and Social Services (Figs. 3 and 4) [5]; Occupational Therapist (OT) to optimize IADLs/ADLs and train care partners how to cue activities (Fig. 4) [61]; Speech-Language Pathologist for memory and communication strategies and swallow evaluation [18, 93]; Dietician/Nutritionist to recommend foods based on PLWD’s preferences; and Palliative Care for symptom management and/or end-of-life care planning [4, 18, 62].
Care plans should be updated regularly with psychosocial and medical recommendations that take into account each PLWD’s and care partner’s values, goals, preferences, needs and abilities; and then documented in the EHR for the PLWD, care partner, and care team members to reference [4, 15, 20, 21, 27, 31].
#5b. Assess if PLWD need other levels of care
Section 5b (in Fig. 7) is a visual reminder that not all medical care happens in traditional clinics and outpatient offices. Instead, there are multiple ways to access medical care, ranging from telehealth via apps and phone [53, 58, 105], to in-person home-based care, and facilities providing higher levels of long-term care or acute care [5]. It is important for brain health specialists, PCPs/GPs, and other clinicians to regularly assess PLWD’s evolving medical needs and personal preferences, to recommend higher levels of services in the current setting when possible [59] (to minimize transitions which are potentially traumatic) [20], or to transfer to other settings when needed [4, 15, 18, 31].
Sometimes the reason for needing more care is because of dementia-related symptoms, while other times the PLWD has other health conditions needing more attention [4]. Many PLWD may have difficulty looking after their own health or accessing care (potentially leading to intermittent health crises or frequent readmissions); and their other health conditions can negatively impact cognition and behavioral symptoms of dementia [12, 15, 20, 106].
This section of the Ideal Care Map also summarizes the best practices of Geriatric-Friendly Emergency Departments [53, 58, 59, 83, 106–108] and Acute Care For Elders (ACE) inpatient model of care for hospitalized older adults [109, 110]. These involve health systems infrastructure and protocols for addressing a wide range of factors that impact the health outcomes of older adults [4, 21, 31, 45, 60, 106, 111].
Regardless of the site of care, all care teams and care partners need to proactively manage care transitions [5, 15, 53, 112], coordinate care across settings [5, 6, 14, 31, 40], reconcile medication lists [20, 35, 58, 111], and set up medical follow up visits or calls [20, 53, 58].
FOUNDATIONAL INFRASTRUCTURE AND ENABLERS OF IDEAL CARE
The literature review and SMEs’ input identified Foundational Infrastructure and Enablers of Ideal Care (Figs 8 and 9) as necessary systems and structural interventions that go beyond the capacity of individual patients, families, clinicians, and social service providers.
Figure 8: Governments, health systems, businesses; data and technologies
Fig. 8
Foundational Infrastructure and Enablers of Ideal Care: Governments, Payers, Health Systems, and Businesses to Support Funding, Infrastructure, and Multi-Group Collaboration (top); Address Social Determinants of Health to Improve Accessibility (middle); Data Infrastructure and Digital Health Technologies for Initial Detection, Diagnosis, and Ongoing Remote Patient Monitoring (bottom).
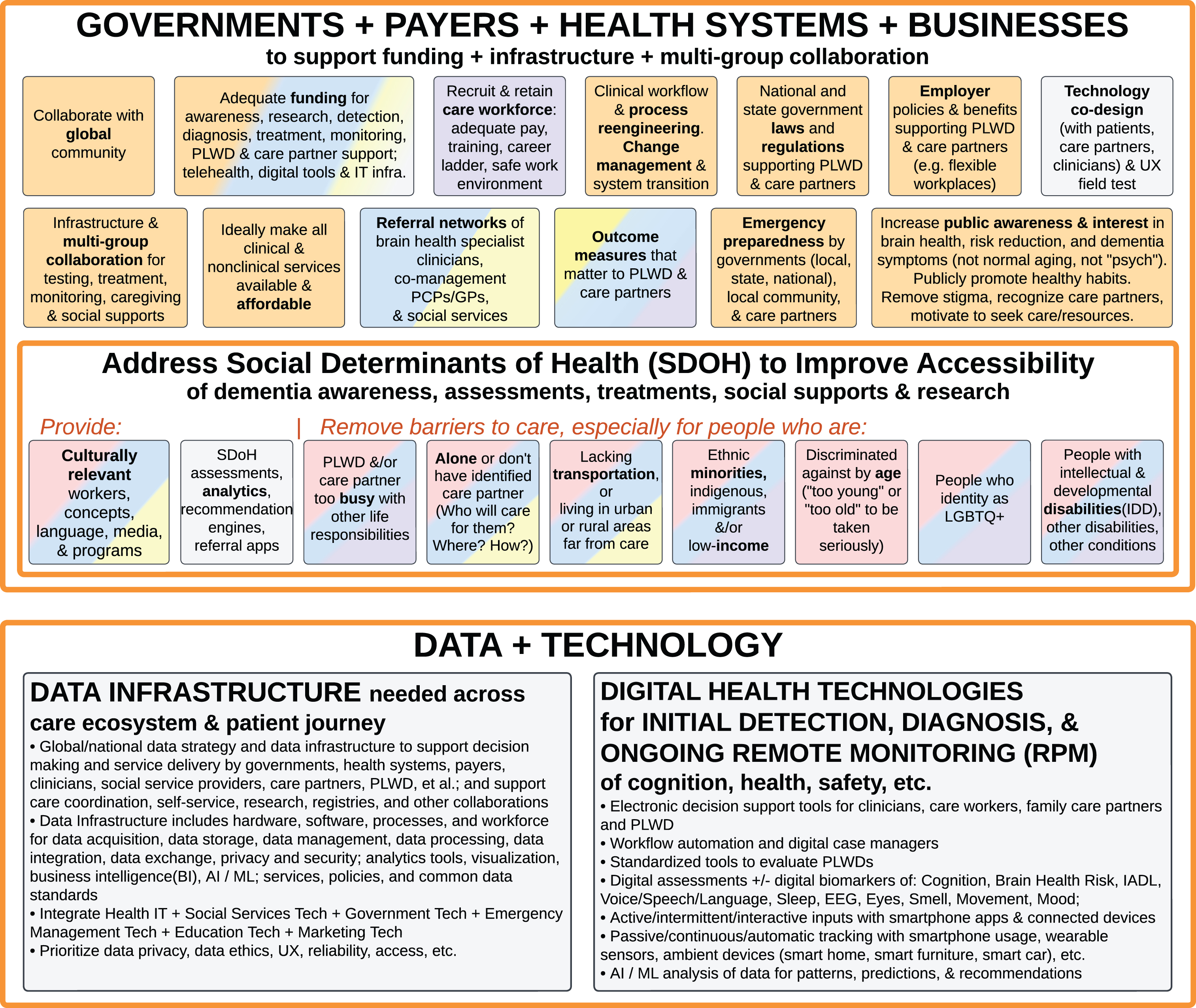
Governments, payers, health systems, and businesses to support funding, infrastructure, and multi-group collaboration
Governments [1, 11, 15, 40], policymakers, public and private health insurance payers, health system leaders [18, 35, 106, 111], researchers and innovators, professional societies, advocacy and social service organizations all play important roles in creating the conditions that are needed for the Ideal Care summarized in Figs. 2–7, including funding [11, 14], public awareness [5, 15, 72], training and workforce development [14, 15, 72], operational workflow redesign [14], quality management (see below), laws and regulations, cross sector collaborations [11, 74], policies that address social determinants of health [1, 4, 5, 12, 15, 20, 41, 42, 76, 81, 83, 106], and technology [14, 15].
For example, ideally all clinical and social services are made affordable to PLWD and their care partners [4, 5, 11, 13, 15, 40, 72]. Government and workplace policies can support PLWD and their family care partners, and protect them from discrimination [5, 11, 13, 15, 33]. Government and workplace policies can also support paid care workers with adequate pay, training, and safe work environments [5, 15, 20, 72]. Outcome measures used by health systems, payers and governments should address what matters to PLWD and their care partners (see below). Privacy and disability rights laws are needed to ethically protect PLWD’s genetic test results, diagnosis, and treatment plans.
Data infrastructure and digital health technologies for initial detection, diagnosis, and ongoing remote patient monitoring
Technology designers and developers of new hardware (medical devices and consumer electronics), software (enterprise systems and consumer apps), and algorithms (such as decision support, artificial intelligence (AI)/machine learning (ML)), as well as technical support and IT maintenance workforce play a critical role in enabling Ideal Care for PLWD [55].
For example, this Ideal Care Map summarizes Data Infrastructure that is needed across the care ecosystem and examples of Digital Health Technologies that enable Ideal Care (see Fig. 8 and the grey boxes throughout Figs. 1–9). This goes beyond simplistic apps and point solutions (software or product that only solves one problem), to national and enterprise IT (information technology for large organizations) approaches on data management and platform solutions [14, 15]. To leverage the potential of digital monitoring and/or AI/ML analysis of PLWDs’ data for patterns, predictions, and care recommendations—data from disparate sensors, apps, EHRs, and health systems need to be integrated through common data standards and data exchange protocols [15, 31, 55]. Data collection and data aggregation also needs to be done in an ethical, secure, and nonburdensome manner, not only for research or registry purposes [62, 74], but also for daily service operations [35], care coordination, quality improvement [58, 67, 113] (see below), and analytics for population health [6, 15, 51, 55, 78, 100].
Figure 9: Research and translation; training
Fig. 9
Foundational Infrastructure and Enablers of Ideal Care: Research and Translation (top); Training, Continuing Education, and Workforce Support (bottom).
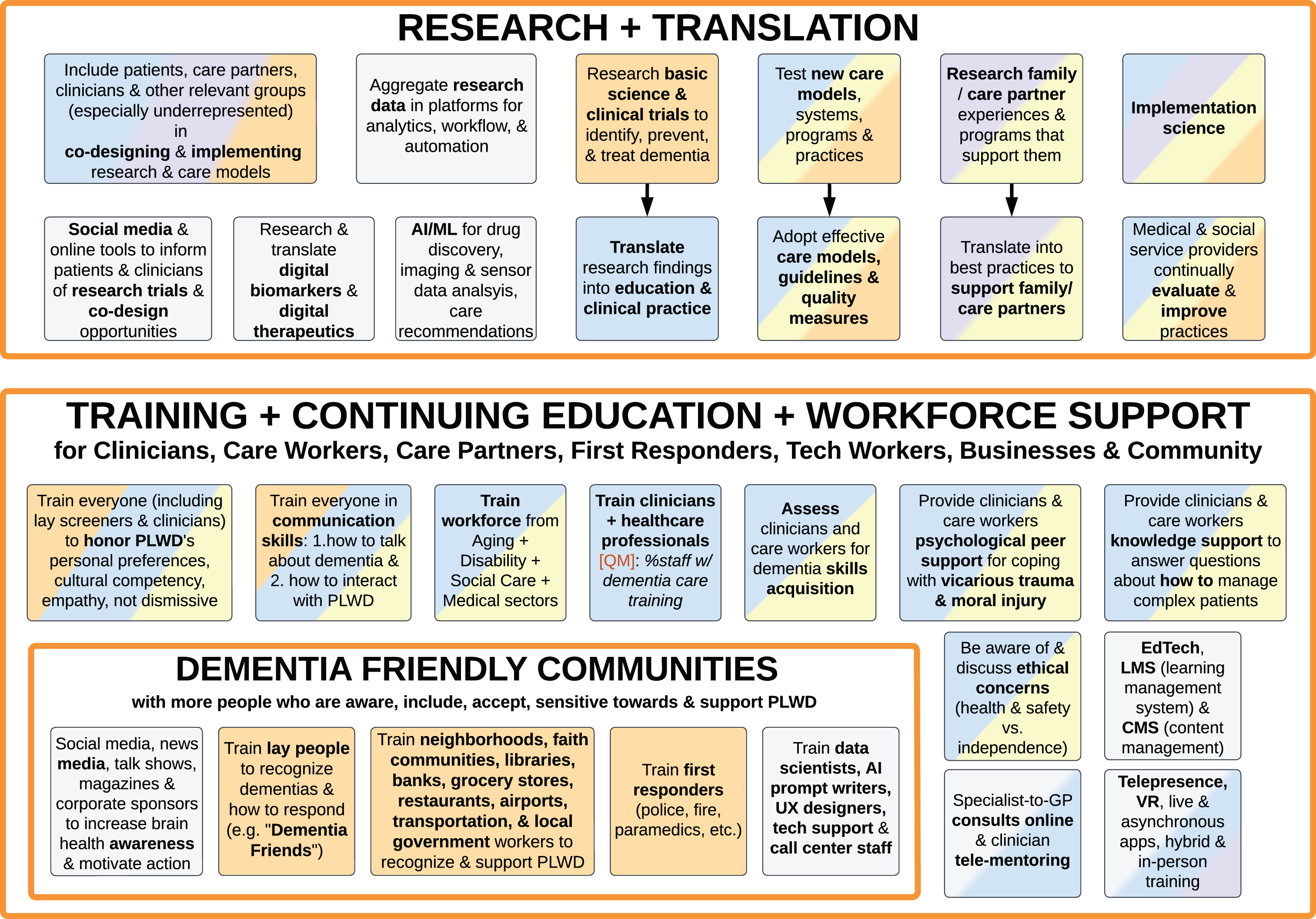
Research and translation
Additional infrastructure needed for Ideal Care includes Research and Translation, as well as Training, Continuing Education, and Workforce Support (Fig. 9). Besides research in basic science, diagnostics, and therapeutics, and clinical trials [74, 88–90, 93, 104], Ideal Care needs ongoing research of transdisciplinary care models that support PLWD and their family/friend care partners over their life course, and translation of best practices into accessible services using implementation science [4, 5, 14, 15, 33, 40–42, 53, 101, 106, 114, 115].
The role of technology in research is summarized in grey boxes – including data tools, digital biomarkers, digital therapeutics, and social media.
Beyond formal research studies, medical and social service providers can set up internal processes to continually evaluate the effectiveness of their practices and make changes to improve [31, 35].
Across all types of research and translation and continuous improvement, it is vital to include all relevant groups in co-designing protocols, products, and services, including input from PLWD, care partners, clinicians, and others [5, 32, 35, 40, 64, 116–119].
Training, continuing education, and workforce support
Ideal Care infrastructure requires training of a broad range of relevant groups to translate and implement best practices [14, 15, 58, 101], including clinicians and care workers in aging, disability, social care, and medical sectors, as well as care partners, employers, first responders and other public services (e.g., transit, libraries, health departments), local businesses, technology workers, and the general community at large (Fig. 9) [5, 15, 18, 31, 45, 120].
In addition to training specific relevant groups mentioned in this section, broad public awareness is needed for all stages of dementia, not only to recognize symptoms for initial detection, but also for risk reduction in early life (Fig. 5), supportive resources (Figs. 3 and 4), dispel myths that “this is just old age, this is normal aging”, help diagnosed PLWDs’ family and care partners understand the impact of these progressive conditions and how to promote quality of life [15, 31, 41]. Awareness campaigns can use various media as described in Fig. 5, Section #1 above.
Training should go beyond an overview of common dementia types. Ideally, all individuals in relevant groups should be trained in communication and interaction skills, how to honor personal preferences, cultural competency, empathy, and not be dismissive of PLWDs’ or care partners’ concerns [4, 5, 15, 19, 20, 27, 31, 35, 51, 57, 60, 80, 83]. For example, Dementia Friendly Communities is an approach that trains local businesses, grocery stores, restaurants, banks, legal providers, faith communities, healthcare and social services, libraries, airports and other transportation, local governments, and the general public to recognize and support PLWD [5, 15, 41–46, 120].
Clinicians, care workers, and the community workforce can also benefit from both knowledge support and personal psychological support from their peers and specialists, especially with the convenience of technology-mediated online consults, tele-mentoring (such as ECHO [19, 121–125]), online forums, live tele-presence, and asynchronous learning management systems (LMS) [5, 35, 92].
Clinicians, care workers, and organizations that promote themselves as “specialized in dementia” should be required to demonstrate relevant knowledge and skills (not just complete training) [41, 106]. Demonstrating this expertise is not meant to be a barrier to entry, but rather to assure care quality and accountability.
QUALITY MEASURES
Quality measures provide a way to assess if appropriate care is delivered [6, 15, 58, 62], and accountability when tied to payment [40, 74, 101, 126, 127]. Realistic QMs are based on data that can be electronically captured during care delivery, so as not to increase administrative burden on clinicians and care workers [20, 40, 67, 113].
Some potential quality measures that may improve dementia care are included in the Ideal Care Map with “[QM]” designation and listed in Table 1. These are based on reviewing previously published proposed measures related to dementia, geriatrics, psychiatry, or neurology [3, 15, 20, 53, 58, 62, 66–68, 113, 127–135], and then ideating new measures by visually analyzing the Ideal Care Map to identify current gaps and barriers to Ideal Care.
Table 1
Potential Process Measures for Ideal Care of People Living With Dementia (PLWD)
| Medical Care |
| •Administer basic cognitive assessment annually to all people over age 65 (or earlier age for individuals in high-risk groups). |
| •Assess older adults in hospitals using guidelines from Geriatric-Friendly Emergency Department (Geri-ED) and Acute Care of Elders (ACE) |
| •Perform diagnostic workup (would need to specify which essential components must occur, such as neurologic exam, which blood tests, imaging test) |
| •Disclose diagnosis to PLWD |
| •Document dementia diagnosis in medical record/EHR |
| •Assess family’s needs, abilities, and willingness to take on care partner role(s) |
| •Document care partner(s) and surrogate decision maker |
| •Provide info about support organizations to PWLD and/or care partner |
| •Refer to home-based and/or community services |
| •Assess cognitive status serially |
| •Assess functional status serially |
| •Assess pain serially |
| •Reconcile medications serially |
| •Discuss and update care plan serially |
| Social Services |
| •Document PLWD’s personal life goals, care goals, routines, likes and dislikes |
| •Complete dementia care training (% clinicians and care workers) |
| •Provide nutritious meals (e.g., if government is paying for someone to take care of PLWD) |
The perspectives of PLWD, care partners, and care workers are also important to measure, as self-reported and Patient Reported Outcome Measures (PROM) [5, 6, 20, 32, 36, 53, 74, 102]. Because the ultimate goal of this Ideal Care Map is to promote quality of life for PLWD and those who care for them, Table 2 lists examples of previously published dementia-specific PROM measuring quality of life [102, 136].
Table 2
Examples of Dementia-Specific Patient Reported Outcome Measures (PROM) of Quality of Life (QoL)
| •Alzheimer’s Disease-Related Quality of Life (ADRQL) |
| •Bath Assessment of Subjective Quality of Life in Dementia (BASQID) |
| •Dementia Quality of Life Measure (DEMQoL) |
| •Dementia Quality of Life (D-QoL) |
| •Family Quality of Life in Dementia (FQOL-D) |
| •Quality of Life in Alzheimer’s Disease (QoL-AD) |
| •Quality of Life in Late-Stage Dementia (QUALID) |
| •QUALIDEM |
In addition to further developing PROM and Process Measures (whether or not an action occurred), other types of measures that can be useful to promote ideal care of PLWD include: Structural Measures of systems and capacity [35, 126, 137, 138]; Outcome Measures at individual, organizational, and community level [3, 6, 41, 62, 67, 113, 117, 118]; and new Digital Measures can be developed as digital technologies and passive monitoring become more available [55, 139, 140].
Aside from various types of measures for Quality Management, accreditation of organizations and certification of care workers can serve a role in Quality Assurance for Ideal Care of PLWD.
DISCUSSION
From a person-centered and ecosystem perspective, this Dementia Ideal Care Map aims to comprehensively summarize global best practices for awareness, risk reduction, detection, diagnosis, care, and promotion of quality of life for PLWD. It also provides a landscape of technology-enabled use cases. Not all of these “ideal” practices are in place currently, and the gaps are opportunities for improvement.
We hope the current and future iterations of the Ideal Care Map will improve dementia care and quality of life by informing PLWD, their family/friend care partners, care workers, clinicians, as well as policymakers, health systems leaders, social services organizations, patient advocacy associations, researchers, technology developers, businesses, and other relevant groups – and inspiring them to work collaboratively.
Strengths and limitations
SMEs said this Ideal Care Map is informative to see best practices organized in one compact view (Fig. 1). This Ideal Care Map illustrates how dementia and many other complex conditions require a multifaceted life course and ecosystem approach—including not only clinicians focusing on medical care—also the important roles of affected individuals, family, friends, community, government, employers, technology developers, and more. Seeing the ecosystem in a single compact view reveals interdependencies and bottlenecks that need to be addressed; otherwise, if only some best practices are pursued, but the rest are not available or have capacity challenges, then the pursued best practices will have constrained benefits.
Over a hundred articles and resources were viewed to compile the global best practices for dementia care; these were primarily from Europe and the U.S. More emerging evidence and input from PLWD and care partners can be incorporated into future iterations of the Ideal Care Map, especially from low-income and middle-income countries [1, 14, 18], and other underrepresented or marginalized groups [5].
It is inherently complicated to visualize in one diagram over 200 best practices involving different relevant groups, different types and stages of dementia, and different phases of a life journey. Our aim is to be generalizable to most, yet detailed and comprehensive. Here we propose a starting point to build upon collaboratively. As with any summative framework, there may be items inadvertently omitted or opportunities for greater clarity; we encourage that feedback via email and welcome collaborators for iterating and disseminating future versions of the Ideal Care Map.
A key limitation is the static image format for print publication, compared to having the diagram in an interactive, online tool that allows users to link to relevant references, filter best practices pertinent to each relevant group, receive crowdsourced comments, publish iterative updates, and change visual accessibility preferences.
Applications and implications
SMEs noted that the Ideal Care Map contains valuable information for PLWD, care partners, and other lay people. It is helpful to know what solutions to consider at different phases of the long and complex care journey. Because the current version of the Ideal Care Map may be overwhelming for lay people, future versions can co-design with PLWD to adapt it into a personalized tool that walks people through where they are in their journey and empowers them for challenges they may face next, by providing specific subsets of information and resources in laymen’s terms to use as conversation starters and planning guides [5].
The Ideal Care Map can be a practical tool for system-level planning and coordinating what’s needed to enable PLWD to live their best life. It can be used by policymakers, health systems, social services organizations, communities, businesses, technology developers, educators, media, and other leaders as a high-level roadmap of what needs to be done for capacity building, service planning, interagency and intersectoral collaboration, etc. Beyond a visual checklist, the Ideal Care Map diagram can be annotated for gap analysis, pain points heatmapping, and capability maturity planning.
It can be used by individual PCPs/GPs, geriatricians, neurologists, emergency medicine providers, urgent care practitioners, rehabilitation therapists, social workers, other clinicians, care workers, and researchers to familiarize themselves with the breadth of interventions beyond their own roles and the transdisciplinary coordination needed for Ideal Care. Within each discipline, analysis of the Ideal Care Map can inform the further development of checklists, decision support tools, quality measures, digital measures, and patient reported outcomes.
Furthermore, future versions of the Ideal Care Map can be made for other relevant subgroups, to customize for geographic regions (such as for specific countries, states, health systems, languages), drill down into more levels of detail, and include more information specific to each type of dementia.
Beyond dementia, the Ideal Care Map’s visualized ecosystem approach can be adapted to other chronic or complex conditions, including other neurodegenerative disorders such as Parkinson’s disease and multiple sclerosis, common diseases such as diabetes, rare diseases, long COVID (also known as Post-Acute Sequelae of SARS-CoV-2 infection (PASC)), and general aging.
Comparison to other published findings
The initial draft of this Dementia Ideal Care Map was completed prior to the publication of “Designing the next-generation clinical care pathway for Alzheimer’s disease” [105].
Some differences in our Ideal Care Map diagram include:
• Lead time benefits of early awareness, early risk reduction, and stimulating activities.
• More detailed steps of the initial detection and diagnosis care pathway.
• Multiple entry points into the detection funnel, including involvement of community groups, direct-to-consumer self-initiated assessments, and people without overt symptoms but have subtle changes.
• Digital biomarkers including voice biomarkers and NLP, passive phone listening, passive phone keystroke tracking and text analysis, eye/gaze tracking, car/driver sensors, and machine learning of imaging and other data.
• Virtual brain health clinics, telehealth, and remote patient monitoring are used not only during post-diagnosis care, but also during initial assessment and diagnosis.
• Ecosystem of care including infrastructure enablers.
• The future directions opportunities discussed in the Applications and Implications section above.
While the Ideal Care Map is not intended to be a specific care model, there is inherent overlap with the summarized best practices and various care models. The Ideal Care Map can be used to inform new care model development and ongoing improvement. The scope of this paper did not review specific care models per se; future work could review care models globally to identify more best practices to add to the Dementia Ideal Care Map.
AUTHOR CONTRIBUTIONS
Wen Dombrowski (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Visualization; Writing – original draft; Writing – review & editing); Adrienne Mims (Validation; Writing – review & editing; Provided additional feedback on quality measures); Ian Kremer (Validation; Writing – review & editing); Pedro Cano Desandes (Validation; Writing – review & editing); Silvia Rodrigo-Herrero (Validation; Writing – review & editing); Fayron Epps (Validation; Writing – review & editing); Teepa Snow (Validation; Writing – review & editing); Myrna Gutierrez (Validation; Writing – review & editing); Anil Nasta (Validation; Writing – review & editing); Mikele Bunce Epperly (Funding acquisition; Writing – review & editing); Katrina Manaloto (Validation; Writing – review & editing); Jennie Chin Hansen (Validation; Writing – review & editing).
ACKNOWLEDGMENTS
Dr. Wen Dombrowski is grateful to God for the vision and stamina to create this diagram. We also thank Senior Associate Dean Maria Henke and the University of Southern California Leonard Davis School of Gerontology, Dr. Maria D’Souza for her feedback on the initial diagram draft, Jennifer Catherine Camaradou for sharing her extensive knowledge of neurology and health innovation resources, Nardo Manaloto for his expertise in emerging technologies and enterprise architecture, Supriya Yagnik and Jonathan Hirtz for coordinating the SME interviews, and the JAD Editorial Team, IOS Press, and Thomson Digital for their expertise.
FUNDING
This study was financially sponsored by F. Hoffmann-La Roche Ltd. The study approach, literature review, data synthesis, diagram, and manuscript were conceptualized and created by the primary author Wen Dombrowski, and not by the funder nor its affiliates.
CONFLICT OF INTEREST
Wen Dombrowski has received consulting fees and honoraria from F. Hoffmann-La Roche Ltd and Genentech, Inc.
Adrienne Mims has received honoraria from F. Hoffmann-La Roche Ltd and has received board member stipend from RRF Foundation for Aging; uncompensated board member of NCQA, uncompensated board chair of NAPA Advisory Council, uncompensated board vice chair of Georgia Council on Aging, uncompensated member of Georgia Alzheimer’s and Related Dementia (GARD) Council.
Pedro Cano Desandes is an employee of the Consorci Corporació Sanitaria Parc Taulí. No other honoraria.
Fayron Epps has received honoraria from F. Hoffmann-La Roche Ltd, is an uncompensated board member of Georgia Chapter Alzheimer’s Association, uncompensated advisory board member of James M. Dixon Foundation, and an uncompensated member of the board of directors for the Southern Gerontological Society; received grant funding from the National Institute on Aging (2020-Present), Alzheimer’s Association (2018-Present), RRF Foundation for Aging (2021–2023).
Teepa Snow has received honoraria from F. Hoffmann-La Roche Ltd, received honoraria from CVS Health for product endorsement (2022), received grant funding from the University of Southern Indiana Geriatrics Workforce Enhancement Program (2018–2023), is a shareholder advisory board member of Sensi-AI (2022-present), is an uncompensated board member of Snow Approach, Inc. (2022-present), is an uncompensated member of the Duke/University of North Carolina ADRD (Alzheimer’s Disease and Related Dementias) Research Education Component Core Advisory Committee (2022-present), and is an uncompensated member of the Professional Advisory Group of Dementia Alliance International (2019-present).
Ian Kremer is executive director of Leaders Engaged on Alzheimer’s Disease (LEAD Coalition). He has received honoraria from Biogen, Eisai, Iridium Continuing Education, Novo Nordisk, Roche, the UCLA Dementia Care Study, the CDC-funded NYU School of Medicine BOLD Public Health Center of Excellence on Early Detection of Dementia and University of Minnesota Public Health Center of Excellence on Dementia Caregiving, and the CDC National Healthy Brain Initiative Tribal Project (American Indian and Alaska Native Resource Center for Brain Health). Kremer serves or has served in the past three years without honoraria or other financial compensation on the Centers for Medicare and Medicaid Services (CMS) Medicare Evidence Development & Coverage Advisory Committee (MEDCAC), the Public Policy & Aging Report editorial board, and on steering and advisory committees for the National Institute on Aging (NIA) IMbedded Pragmatic AD/ADRD Clinical Trials (IMPACT) Collaboratory, the NIA-funded Hopkins’ Economics of Alzheimer’s Disease and Services (HEADS) Center and the WeCareAdvisor Study, the Alzheimer’s Disease Patient and Caregiver Engagement (AD PACE) initiative, the Dementia Friendly America initiative, the Alzheimer’s Association Dementia Care Navigation Roundtable, the Davos Alzheimer’s Collaborative Champions Cabinet, the CDC Healthy Brain Initiative’s (HBI) Leadership Committee developing the 2023–2027 Public Health Roadmap, the Food & Drug Administration (FDA) Prescription Drug User Fee Act Stakeholders Working Group for PDUFA VII, and as an external reviewer for the 2021 National Academies of Science, Engineering, and Medicine report, “Meeting the Challenge of Caring for Persons Living with Dementia and Their Care Partners and Caregivers: A Way Forward.”
Myrna Gutierrez has no conflict of interest to report.
Silvia Rodrigo Herrero has received honoraria from F. Hoffmann-La Roche Ltd.
Anil Nasta is an employee of Roche Diagnostics Corporation.
Mikele Bunce Epperly is an employee of and owns stocks in F. Hoffmann-La Roche Ltd.
Katrina Manaloto is an intern at the University of California Berkeley Neurotech Collider Lab.
Jennie Chin Hansen has received honoraria from F. Hoffmann-La Roche Ltd and is a board member of SCAN Health Plan.
DATA AVAILABILITY
No numeric datasets were generated or analyzed during this study.
REFERENCES
[1] | Ferri CP , Jacob KS ((2017) ) Dementia in low-income and middle-income countries: Different realities mandate tailored solutions. PLOS Med 14: , e1002271-e1002271. |
[2] | ADI, Dementia statistics, Alzheimer’s Disease International, https://www.alzint.org/about/dementia-facts-figures/dementia-statistics, Accessed June 25, 2023. |
[3] | Petersen RC , Lopez O , Armstrong MJ , Getchius TSD , Ganguli M , Gloss D , Gronseth GS , Marson D , Pringsheim T , Day GS , Sager M , Stevens J , Rae-Grant A ((2018) ) Practice guideline update summary: Mild cognitive impairment report of the guideline development, dissemination, and implementation. Neurology 90: , 126–135. |
[4] | NICE, Dementia: Assessment, management and support for people living with dementia and their carers [ng97], National Institute for Health and Care Excellence, https://www.nice.org.uk/guidance/ng97, June 20, 2018. |
[5] | RAISE Family Caregiving Advisory Council, Recognize, assist, include, support, & engage (RAISE) family caregivers act: Initial report to congress, Administration for Community Living, https://acl.gov/RAISE/report, September 22, 2021. |
[6] | ihub, Change package for a dementia care co-ordination improvement programme (post-diagnostic support and care co-ordination), Healthcare Improvement Scotland, https://ihub.scot/improvement-programmes/dementia/dementia-care-co-ordination, October 2022. |
[7] | CDC, Preferred terms for select population groups & communities, Centers for Disease Control and Prevention, https://www.cdc.gov/healthcommunication/Preferred_Terms.html, November 3, 2022. |
[8] | Reed M, Should we banish the word “stakeholder”?, Fast Track Impact, https://www.fasttrackimpact.com/post/why-we-shouldn-t-banish-the-word-stakeholder, March 27, 2023. |
[9] | Volpe U , Amin H , Ayinde OO , Burns A , Chan WC , David R , Dejanovic SD , Djokic G , Eraslan D , Fischer GAL , Gracia-García P , Hamdani SU , Han C , Jafri H , Kallivayalil RA , Kriekaart RL , Kua EH , Lam LCW , Lecic-Tosevski D , Leroi I , Lobo A , Mihai A , Minhas FA , Mistry H , Ogundele AT , Olde Rikkert MGM , Olivera J , Palumbo C , Parker A , Pejuskovic B , Riese F , Robert P , Semrau M , Stoppe G , Sudhakar S , Tirintica AR , Tofique S , Tsoi C , Wolski L , Yalug I , Wang H , Yu X , Sartorius N ((2020) ) Pathways to care for people with dementia: An international multicentre study. Int J Geriatr Psychiatry 35: , 163–173. |
[10] | TOGAF, Business capabilities, version 2, The Open Group, https://pubs.opengroup.org/togaf-standard/business-architecture/business-capabilities.html, April 2022. |
[11] | Fernández Fernández I, Senén Rodríguez J, García García JA, García Del Moral J, Martínez Alborg S, Sobrino Fernández S, De la reivindicación a la acción. Dependencia y Alzheimer, CEAFA (Confederación Española de Asociaciones de Familiares de Personas con Alzheimer y Otras Demencias), https://www.ceafa.es/es/que-comunicamos/noticias/el-pepa-panel-de-expertos-de-personas-con-alzheimer-de-ceafa-pasa-a-la-accion-compartiendo-sus-reivindicaciones, 2023. |
[12] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[13] | Spillman BC, Allen EH, Favreault M, Informal caregiver supply and demographic changes: Review of the literature, U.S. Department of Health and Human Services, https://aspe.hhs.gov/reports/informal-caregiver-supply-demographic-changes-review-literature, December 2020. |
[14] | WHO, Integrated care for older people (ICOPE) implementation pilot programme: Findings from the ‘ready’ phase, World Health Organization, https://iris.who.int/handle/10665/353553, April 25, 2022. |
[15] | NAPA, National plan to address Alzheimer’s disease: 2021 update, https://aspe.hhs.gov/reports/national-plan-2021-update, December 2021. |
[16] | Shafir A , Ritchie CS , Garrett SB , Bernstein Sideman A , Naasan G , Merrilees J , Widera E , Flint L , Harrison KL ((2022) ) “Captive by the uncertainty”-experiences with anticipatory guidance for people living with dementia and their caregivers at a specialty dementia clinic. J Alzheimers Dis 86: , 787–800. |
[17] | Harrison KL , Garrett SB , Halim M , Bernstein Sideman A , Allison TA , Dohan D , Naasan G , Miller BL , Smith AK , Ritchie CS ((2022) ) “I didn’t sign up for this”: Perspectives from persons living with dementia and care partners on challenges, supports, and opportunities to add geriatric neuropalliative care to dementia specialty care. J Alzheimers Dis 90: , 1301–1320. |
[18] | Gauthier S, Webster C, Servaes S, Morais JA, Rosa-Neto P, World Alzheimer report 2022: Life after diagnosis: Navigating treatment, care and support, Alzheimer’s Disease International, https://www.alzint.org/resource/world-alzheimer-report-2022, September 21, 2022. |
[19] | Lam J , Mattke S ((2021) ) Memory care approaches to better leverage capacity of dementia specialists: A narrative synthesis. Neurodegener Dis Manag 11: , 239–250. |
[20] | APA, The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia, American Psychiatric Association, https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890426807, May 2016. |
[21] | WHO, Integrated care for older people (ICOPE): Guidance for person-centred assessment and pathways in primary care, World Health Organization, https://www.who.int/publications/i/item/WHO-FWC-ALC-19.1, (January 1, 2019). |
[22] | Clarke C , Wolverson E , Bryden C ((2016) ) Positive psychology approaches to dementia. Jessica Kingsley Publishers. |
[23] | Liu Q , Wang F , Tan L , Liu L , Cheng H , Hu X ((2023) ) Comparative efficacy of various art therapies for patients with dementia: A network meta-analysis of randomized controlled trials. Front Psychiatry 14: , 1072066. |
[24] | Nam S-M , Kim S-g ((2021) ) Dual-task training effect on cognitive and body function, β-amyloid levels in Alzheimer’s dementia patients: A randomized controlled trial. J Korean Phys Ther 33: , 136–141. |
[25] | Jin Y , Ju Y , Sanghun N , Bae S , Hong I ((2022) ) The effects of dual-task training for older adults with cognitive impairment: A systematic review. Am J Occup Ther 76: , 7610510210p7610510211-7610510210p7610510211. |
[26] | Jardim NYV , Bento-Torres NVO , Costa VO , Carvalho JPR , Pontes HTS , Tomás AM , Sosthenes MCK , Erickson KI , Bento-Torres J , Diniz CWP ((2021) ) Dual-task exercise to improve cognition and functional capacity of healthy older adults. Front Aging Neurosci 13: , 33–33. |
[27] | Post SG ((2013) ) Hope in caring for the deeply forgetful: Enduring selfhood and being open to surprises. Bull Menninger Clin 77: , 349–368. |
[28] | Feurich V, Caregiver vs. Care partner: Why you need to know the difference, Postive Approach to Care, https://teepasnow.com/blog/caregiver-vs-care-partner-why-you-need-to-know-the-difference, July 12, 2021. |
[29] | Levine C, Caregiver, care partner, companion? Why language matters, Next Avenue, https://www.nextavenue.org/caregiver-care-partner-companion-why-language-matters, August 2, 2022. |
[30] | Fazio S , Pace D , Flinner J , Kallmyer B ((2018) ) The fundamentals of person-centered care for individuals with dementia. Gerontologist 58: , S10–S19. |
[31] | Fazio S , Pace D , Maslow K , Zimmerman S , Kallmyer B ((2018) ) Alzheimer’s Association dementia care practice recommendations. Gerontologist 58: , S1–S9. |
[32] | McCleary KK, Paving the path for family-centered design: A national report on family caregivers’ roles in medical product development, https://www.caregiving.org/wp-content/uploads/2020/05/NAC_LEAD-Coalition_Paving-the-Path_Overview_May-2019.pdf, May 2019. |
[33] | Mehta R, Nafus D, Atlas of caregiving pilot study report, Family Caregiver Alliance, https://atlasofcaregiving.com/pilot-study, April 2016. |
[34] | NICE, Dementia – discussing and planning support after diagnosis, National Institute for Health and Care Excellence, https://www.nice.org.uk/about/nice-communities/social-care/quick-guides/dementia-discussing-and-planning-support-after-diagnosis, November 2018. |
[35] | UCSF, Care ecosystem toolkit, UCSF Memory and Aging Center, https://memory.ucsf.edu/research-trials/professional/care-ecosystem, February 2018. |
[36] | Reuben DB , Romero T , Evertson LC , Jennings LA ((2022) ) Overwhelmed: A dementia caregiver vital sign. J Gen Intern Med 37: , 2469–2474. |
[37] | ((2022) ) 2022 Alzheimer’s disease facts and figures. Alzheimers Dement 18: , 700–789. |
[38] | Tan YL , Lo YKJ , Ho CSH ((2024) ) Psychological and social impacts of frontotemporal dementia on caregivers and family members – a systematic review. Gen Hosp Psychiatry 86: , 33–49. |
[39] | Rabins PV , Rovner BW , Rummans T , Schneider LS , Tariot PN ((2017) ) Guideline watch (October 2014): Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Focus (Am Psychiatr Publ) 15: , 110–128. |
[40] | Ling S, A national perspective on healthcare quality, ACT on Alzheimer’s Health Care Leadership Summit, September 29, 2016. |
[41] | Epps F , Moore M , Chester M , Gore J , Sainz M , Adkins A , Clevenger C , Aycock D ((2022) ) The Alter program: A nurse-led, dementia-friendly program for African American faith communities and families living with dementia. Nurs Admin Q 46: , 72–80. |
[42] | Gore J , Toliver J , Moore MA , Aycock D , Epps F ((2022) ) A mixed-methods formative evaluation of a dementia-friendly congregation program for black churches. Int J Environ Res Public Health 19: , 4498. |
[43] | ADI, Dementia friendly community resources, Alzheimer’s Disease International, https://www.alzint.org/resource/dementia-friendly-community-resources, April 21, 2016. |
[44] | ADI, Dementia friendly communities, Alzheimer’s Disease International, https://www.alzint.org/what-we-do/policy/dementia-friendly-communities, 2023. |
[45] | DFA, Sector guides — Dementia Friendly America, Dementia Friendly America, https://www.dfamerica.org/-guides, (2023) . |
[46] | DFA, What is a dementia friendly community? Dementia Friends USA, https://dementiafriendsusa.org/what-dementia-friendly-community 2023. |
[47] | Australia Dementia, The dementia guide, edition 3, Dementia Australia, https://www.dementia.org.au/resources/the-dementia-guide, December 2022. |
[48] | Alzheimer’s Association, Help and support, https://www.alz.org/help-support, Accessed March 9, 2023. |
[49] | NAS, Families caring for an aging America, National Academies Press, https://nap.nationalacademies.org/catalog/23606/families-caring-for-an-aging-america, November 8, 2016. |
[50] | Katz S , Downs TD , Cash HR , Grotz RC ((1970) ) Progress in development of the index of ADL. Gerontologist 10: , 20–30. |
[51] | Ty D, Ahuja R, Chin Hansen J, One size does not fit all: Asian Americans and dementia risk, American Society on Aging, https://generations.asaging.org/asian-americans-and-dementia-risk-not-homogenous, January 10, 2023. |
[52] | Basil G , Luther E , Burks JD , Govindarajan V , Urakov T , Komotar RJ , Wang MY , Levi AD ((2021) ) The focused neurosurgical examination during telehealth visits: Guidelines during the covid-19 pandemic and beyond. Cureus 13: , e13503. |
[53] | Gettel CJ , Falvey JR , Gifford A , Hoang L , Christensen LA , Hwang U , Shah MN ; GEAR 2.0-ADC Network ((2022) ) Emergency department care transitions for patients with cognitive impairment: A scoping review. J Am Med Dir Assoc 23: , 1313.e1-1313.e13. |
[54] | NCDM, Our groups, National Council of Dementia Minds, https://dementiaminds.org/dementia-minds, Accessed February 15, 2023. |
[55] | NAS, Harnessing mobile devices for nervous system disorders: Proceedings of a workshop, The National Academies Press, https://nap.nationalacademies.org/read/25274/chapter/1, November 19, 2018. |
[56] | Wethington E , Eccleston C , Gay G , Gooberman-Hill R , Schofield P , Bacon E , Dombrowski W , Jamison R , Rothman M , Meador L , Kenien C, Pillemer K, Löckenhoff C, Reid MC ((2018) ) Establishing a research agenda on mobile health technologies and later-life pain using an evidence-based consensus workshop approach. J Pain 19: , 1416–1423. |
[57] | Snow T, Positive approach to care, https://teepasnow.com, Accessed June 14, 2022. |
[58] | Rosenberg MS , Carpenter CR , Bromley M , Caterino JM , Chun A , Gerson L , Greenspan J , Hwang U , John DP , Lichtman J , Lyons WL , Mortensen B , Platts-Mills TF , Ragsdale LC , Rispoli J , Seaberg DC , Wilber ST ((2014) ) Geriatric emergency department guidelines. Ann Emerg Med 63: , e7–25. |
[59] | Dresden SM , Mph ZT , Serina P , Mph MD , Kennedy M , Wescott AB , Hogan T , Shah MN , Hwang U ((2022) ) Optimal emergency department care practices for persons living with dementia: A scoping review. J Am Medical Dir Assoc 23: , 1314.e1–1314.e29. |
[60] | Sloane PD ((2022) ) The geriatric-focused emergency department: Opportunities and challenges. J Am Med Dir Assoc 23: , 1288–1290. |
[61] | Bennett S , Laver K , Voigt-Radloff S , Letts L , Clemson L , Graff M , Wiseman J , Gitlin L ((2019) ) Occupational therapy for people with dementia and their family carers provided at home: A systematic review and meta-analysis. BMJ Open 9: , e026308. |
[62] | Schultz SK , Llorente MD , Sanders AE , Tai WA , Bennett A , Shugarman S , Roca R ((2020) ) Quality improvement in dementia care: Dementia management quality measurement set 2018 implementation update. Neurology 94: , 210–216. |
[63] | NHS, How to make your home dementia friendly, National Health Service, https://www.nhs.uk/conditions/dementia/living-with-dementia/home-environment, January 12, 2022. |
[64] | Lenz Lock S, Noritake R, Impacts of dementia friendly initiatives, World Dementia Council, https://worlddementiacouncil.org/sites/default/files/2020-12/DFIs%20-%20Paper%202_V15.pdf, December 2020. |
[65] | SEN, Guías diagnósticas y terapéuticas de la Sociedad Española de Neurología 2018. 5. Guía oficial de práctica clínica en demencias, Sociedad Española de Neurología, https://www.sen.es/profesionales/guias-y-protocolos, November 16, 2018. |
[66] | AAN, Summary of quality measurement set for clinicians – updated dementia management quality measures, American Academy of Neurology, https://www.aan.com/siteassets/home-page/policy-and-guidelines/quality/quality-measures/geriatric-neurology/16dementiameasurecliniciansummary_pg2.pdf, (2016) . |
[67] | Sanders AE, Roca R, Dementia management - quality measurement set update – 2018 implementation update, American Academy of Neurology Institute and American Psychiatric Association. https://www.aan.com/siteassets/home-page/policy-and-guidelines/quality/quality-measures/2018-dementia-management-measures.pdf, November 8, 2018. |
[68] | NQF, Priority setting for healthcare performance measurement: Addressing performance measure gaps for dementia, including Alzheimer’s disease, National Quality Forum, https://www.qualityforum.org/priority_setting_for_healthcare_performance_measurement_alzheimers_disease.aspx, October 15, 2014. |
[69] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EG , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini ML , Rosen H , Prioleau-Latham CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: , 2456–2477. |
[70] | Elder Justice Initiative (EJI), U.S. Department of Justice, https://www.justice.gov/elderjustice, Accessed October 17, 2023. |
[71] | Long T , Taubenheim AM , Wayman J , Temple S , Ruoff BA ((2008) ) The heart truth: Using the power of branding and social marketing to increase awareness of heart disease in women. Soc Mar Q 14: , 3–29. |
[72] | NICE, Dementia, disability and frailty in later life – mid-life approaches to delay or prevent onset [ng16], National Institute for Health and Care Excellence, https://www.nice.org.uk/guidance/ng16, October 20, 2015. |
[73] | GSA, GSA KAER toolkit for primary care teams: Supporting conversations about brain health, timely detection of cognitive impairment, and accurate diagnosis of dementia, Gerontological Society of America, https://gsaenrich.geron.org/brain-health, July 2023. |
[74] | Bradley P , Akehurst R , Ballard C , Banerjee S , Blennow K , Bremner J , Broich K , Cummings J , Dening K , Dubois B , Klipper W , Leibman C , Mantua V , Molinuevo JL , Morgan S , Muscolo LAA , Nicolas F , Pani L , Robinson L , Siviero P , Van Dam J , Van Emelen J , Wimo A , Wortmann M , Goh L ((2015) ) Taking stock: A multistakeholder perspective on improving the delivery of care and the development of treatments for Alzheimer’s disease. Alzheimers Dement 11: , 455–461. |
[75] | Kivipelto M , Mangialasche F , Snyder HM , Allegri R , Andrieu S , Arai H , Baker L , Belleville S , Brodaty H , Brucki SM , Calandri I , Caramelli P , Chen C , Chertkow H , Chew E , Choi SH , Chowdhary N , Crivelli L , Torre RDL , Du Y , Dua T , Espeland M , Feldman HH , Hartmanis M , Hartmann T , Heffernan M , Henry CJ , Hong CH , Håkansson K , Iwatsubo T , Jeong JH , Jimenez-Maggiora G , Koo EH , Launer LJ , Lehtisalo J , Lopera F , Martínez-Lage P , Martins R , Middleton L , Molinuevo JL , Montero-Odasso M , Moon SY , Morales-Pérez K , Nitrini R , Nygaard HB , Park YK , Peltonen M , Qiu C , Quiroz YT , Raman R , Rao N , Ravindranath V , Rosenberg A , Sakurai T , Salinas RM , Scheltens P , Sevlever G , Soininen H , Sosa AL , Suemoto CK , Tainta-Cuezva M , Velilla L , Wang Y , Whitmer R , Xu X , Bain LJ , Solomon A , Ngandu T , Carrillo MC ((2020) ) World-wide FINGERS network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 16: , 1078–1094. |
[76] | Nowroozpoor A , Dussetschleger J , Perry W , Sano M , Aloysi A , Belleville M , Brackett A , Hirshon JM , Hung W , Moccia JM , Ohuabunwa U , Shah MN , Hwang U ; GEAR 2.0-ADC Network ((2022) ) Detecting cognitive impairment and dementia in the emergency department: A scoping review. J Am Med Dir Assoc 23: , 1314.e31–1314.e88. |
[77] | McCallion P, Knowles M, Gould E, Intellectual and developmental disabilities and dementia: Practical strategies for professionals, National Alzheimer’s and Dementia Resource Center, Administration for Community Living, https://nadrc.acl.gov/details?search1=169, July 31, 2019. |
[78] | Taylor B , Barboi C , Boustani M ((2023) ) Passive digital markers for Alzheimer’s disease and other related dementias: A systematic evidence review. J Am Geriatr Soc 71: , 2966–2974. |
[79] | Miled ZB , Haas K , Black CM , Khandker RK , Chandrasekaran V , Lipton R , Boustani MA ((2020) ) Predicting dementia with routine care EMR data. Artif Intell Med 102: , 101771. |
[80] | Williams R, Experience mapping the dementia diagnosis journey, Alzheimer’s Society, https://www.alzheimers.org.uk/blog/experience-mapping-dementia-diagnosis-journey, August 23, 2017. |
[81] | Gauthier S, Rosa-Neto P, Morais JA, Webster C, World Alzheimer Report 2021: Journey through the diagnosis of dementia, Alzheimer’s Disease International, https://www.alzint.org/resource/world-alzheimer-report-2021, September 21, 2021. |
[82] | Kenny B ((2022) ) Free innovative tool to address memory concerns and brain health for all communities. Alzheimers Dement 18: , e061986. |
[83] | Carpenter CR , Leggett J , Bellolio F , Betz M , Carnahan RM , Carr D , Doering M , Hansen JC , Isaacs ED , Jobe D , Kelly K , Morrow-Howell N , Prusaczyk B , Savage B , Suyama J , Vann AS , Rising KL , Hwang U , Shah MN ; GEAR 2.0-ADC Network ((2022) ) Emergency department communication in persons living with dementia and care partners: A scoping review. J Am Med Dir Assoc 23: , 1313.e15–1313.e46. |
[84] | Ma C , Bao S , Dull P , Wu B , Yu F ((2019) ) Hospital readmission in persons with dementia: A systematic review. Int J Geriatr Psychiatry 34: , 1170–1184. |
[85] | Hansson O , Edelmayer RM , Boxer AL , Carrillo MC , Mielke MM , Rabinovici GD , Salloway S , Sperling R , Zetterberg H , Teunissen CE ((2022) ) The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement 18: , 2669–2686. |
[86] | Brand AL , Lawler PE , Bollinger JG , Li Y , Schindler SE , Li M , Lopez S , Ovod V , Nakamura A , Shaw LM ((2022) ) The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: A literature review. Alzheimers Res Ther 14: , 195. |
[87] | Janelidze S , Bali D , Ashton NJ , Barthélemy NR , Vanbrabant J , Stoops E , Vanmechelen E , He Y , Dolado AO , Triana-Baltzer G , Pontecorvo MJ , Zetterberg H , Kolb H , Vandijck M , Blennow K , Bateman RJ , Hansson O ((2022) ) Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain 146: , 1592–1601. |
[88] | Gifford A , Praschan N , Newhouse A , Chemali Z ((2023) ) Biomarkers in frontotemporal dementia: Current landscape and future directions. Biomark Neuropsychiatry 8: , 100065. |
[89] | Gibson LL , Abdelnour C , Chong J , Ballard C , Aarsland D ((2023) ) Clinical trials in dementia with lewy bodies: The evolving concept of co-pathologies, patient selection and biomarkers. Curr Opin Neurol 36: . |
[90] | Abdelmoaty MM , Lu E , Kadry R , Foster EG , Bhattarai S , Mosley RL , Gendelman HE ((2023) ) Clinical biomarkers for Lewy body diseases. Cell Bioscie 13: , 209. |
[91] | Chirra M , Marsili L , Wattley L , Sokol LL , Keeling E , Maule S , Sobrero G , Artusi CA , Romagnolo A , Zibetti M , Lopiano L , Espay AJ , Obeidat AZ , Merola A ((2019) ) Telemedicine in neurological disorders: Opportunities and challenges. Telemed J E Health 25: , 541–541. |
[92] | Moo L, Hoang-Gia D, Practical tips for tele-dementia care: Communication, assessment, and caregiver education, VA Geriatric Scholars Learning Community, https://www.gerischolars.org/mod/page/view.php?id=1117, April 23, 2021. |
[93] | Antonioni A , Raho EM , Lopriore P , Pace AP , Latino RR , Assogna M , Mancuso M , Gragnaniello D , Granieri E , Pugliatti M , Di Lorenzo F , Koch G ((2023) ) Frontotemporal dementia, where do we stand? A narrative review. Int J Mol Sci 24: , 11732. |
[94] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , Dekosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[95] | Jack CR , Bennett DA , Blennow K , Carrillo MC , Feldman HH , Frisoni GB , Hampel H , Jagust WJ , Johnson KA , Knopman DS , Petersen RC , Scheltens P , Sperling RA , Dubois B ((2016) ) A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87: , 539–547. |
[96] | Dubois B , Villain N , Frisoni GB , Rabinovici GD , Sabbagh M , Cappa S , Bejanin A , Bombois S , Epelbaum S , Teichmann M , Habert M-O , Nordberg A , Blennow K , Galasko D , Stern Y , Rowe CC , Salloway S , Schneider LS , Cummings JL , Feldman HH ((2021) ) Clinical diagnosis of Alzheimer’s disease: Recommendations of the international working group. Lancet Neurol 20: , 484–496. |
[97] | Jack CR , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R , Elliott C , Masliah E , Ryan L , Silverberg N ((2018) ) NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[98] | Shaji KS , Sivakumar PT , Rao GP , Paul N ((2018) ) Clinical practice guidelines for management of dementia. Indian J Psychiatry 60: , S312-S312. |
[99] | O’Brien JT , Holmes C , Jones M , Jones R , Livingston G , McKeith I , Mittler P , Passmore P , Ritchie C , Robinson L , Sampson EL , Taylor JP , Thomas A , Burns A ((2017) ) Clinical practice with anti-dementia drugs: A revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol 31: , 147–168. |
[100] | Reuben DB , Rosenstein HL , Chen K , Pillai A , Lee DR , Meshkat SD , Chen GI ((2023) ) A population-based, electronic health record-guided approach to improve the quality of dementia care. J Am Geriatr Soc 71: , 927–934. |
[101] | Super N, Ahuja R, McDermott M, Scaling comprehensive dementia-care models, Milken Institute, https://milkeninstitute.org/report/dementia-care-models-scaling-comprehensive, November 2021. |
[102] | Ayton DR , Gardam ML , Pritchard EK , Ruseckaite R , Ryan J , Robinson SJ , Brodaty H , Ward SA , Ahern S ((2021) ) Patient-reported outcome measures to inform care of people with dementia—a systematic scoping review. Gerontologist 61: , e185–e194. |
[103] | NICE, Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease [TA217], National Institute for Health and Care Excellence, 2018. |
[104] | Alzheimers.gov, Find clinical trials, https://www.alzheimers.gov/clinical-trials, Accessed May 11, 2023. |
[105] | Hampel H , Au R , Mattke S , van der Flier WM , Aisen P , Apostolova L , Chen C , Cho M , De Santi S , Gao P , Iwata A , Kurzman R , Saykin AJ , Teipel S , Vellas B , Vergallo A , Wang H , Cummings J ((2022) ) Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat Aging 2: , 692–703. |
[106] | Carpenter CR , Dresden SM , Shah MN , Hwang U ((2022) ) Adapting emergency care for persons living with dementia: Results of the geriatric emergency care applied research network scoping review and consensus conference. J Am Med Dir Assoc 23: , 1286–1287. |
[107] | Carpenter CR , Bromley M , Caterino JM , Chun A , Gerson LW , Greenspan J , Hwang U , John DP , Lyons WL , Platts-Mills TF , Mortensen B , Ragsdale L , Rosenberg M , Wilber S ((2014) ) Optimal older adult emergency care: Introducing multidisciplinary geriatric emergency department guidelines from the American College of Emergency Physicians, American Geriatrics Society, Emergency Nurses Association, and Society for Academic Emergency Medicine. Acad Emerg Med 21: , 806–809. |
[108] | Tarabay R , Perry A , Al Aridi R , Malone M ((2021) ) Can an emergency department adequately address an older adult who has complex needs? J Geriatr Emerg Med 2: , 6. |
[109] | Palmer RM ((2018) ) The Acute Care for Elders unit model of care. Geriatrics (Basel) 3: , 59. |
[110] | Malone ML , Capezuti EA , Palmer RM ((2014) ) Acute Care for Elders: A model for interdisciplinary care, Springer, New York. |
[111] | IHI, Age-friendly health systems, Institute for Healthcare Improvement, https://www.ihi.org/initiatives/age-friendly-health-systems, Accessed June 27, 2022. |
[112] | Ashbourne J , Boscart V , Meyer S , Tong CE , Stolee P ((2021) ) Health care transitions for persons living with dementia and their caregivers. BMC Geriatr 21: , 285. |
[113] | Sanders AE, Roca R, Dementia management quality measurement set update, American Academy of Neurology Institute and American Psychiatric Association., https://www.aan.com/siteassets/home-page/policy-and-guidelines/quality/quality-measures/15dmmeasureset_pg.pdf, July 28, 2016. |
[114] | Reuben DB , Evertson LC , Jackson-Stoeckle R , Epstein-Lubow G , Spragens LH , Haggerty KL , Serrano KS , Jennings LA ((2022) ) Dissemination of a successful dementia care program: Lessons to facilitate spread of innovations. J Am Geriatr Soc 70: , 2686–2694. |
[115] | Gitlin LN , Marx K , Stanley IH , Hodgson N ((2015) ) Translating evidence-based dementia caregiving interventions into practice: State-of-the-science and next steps. Gerontologist 55: , 210–210. |
[116] | Majid T , Paulsen R , Callahan LF , Potashman M , Lee D , Hartry A , Wunderlich G , Hoffman D , Wieberg D , Kremer IN , DiBenedetti DB ((2020) ) The importance of care partner input in Alzheimer’s disease (AD) drug development. Alzheimers Dement 16: , e0400999. |
[117] | Hauber B , Paulsen R , Krasa HB , Vradenburg G , Comer M , Callahan LF , Winfield J , Potashman M , Hartry A , Lee D ((2023) ) Assessing what matters to people affected by Alzheimer’s disease: A quantitative analysis. Neurol Ther 12: , 505–527. |
[118] | DiBenedetti DB , Menne H , Paulsen R , Krasa HB , Vradenburg G , Comer M , Callahan LF , Winfield J , Potashman M , Heithoff K ((2023) ) Technical review of clinical outcomes assessments across the continuum of Alzheimer’s disease. Neurol Ther 12: , 571–595. |
[119] | Gil H , Shivers S , Savage B , Taylor G , Taylor J , DeFrancesco E , O’Connell M , Lepore M ((2020) ) Empowering partnerships among researchers, persons living with dementia, and caregivers. Innov Aging 4: , 729–729. |
[120] | Alzheimer’s Society, Guidance for dementia-friendly organisations, Alzheimer’s Society, https://www.alzheimers.org.uk/get-involved/dementia-friendly-resources/organisations/resources, Accessed February 13, 2023. |
[121] | Arora S , Kalishman SG , Thornton KA , Komaromy MS , Katzman JG , Struminger BB , Rayburn WF ((2017) ) Project ECHO: A telementoring network model for continuing professional development. J Contin Educ Health Prof 37: , 239–244. |
[122] | Catic A , Mattison M , Lipsitz L ((2013) ) ECHO-AGE: A video-consultation program to bring geriatric expertise to long-term care. J Am Geriatr Soc 61: (April SI), S108. |
[123] | Knoefel J , Herman C ((2015) ) Dementia care training for primary care providers: Project ECHO. Neurology 84: (14Supp), P6.182. |
[124] | Alzheimer’s Association, The Alzheimer’s and dementia care ECHO program for clinicians, Alzheimer’s Association, https://www.alz.org/professionals/health-systems-medical-professionals/echo-alzheimers-dementia-care-program. |
[125] | Alzheimer’s Association, The Alzheimer’s and dementia care ECHO program for professional care providers, Alzheimer’s Association, https://www.alz.org/professionals/professional-providers/the-alzheimers-and-dementia-care-echo. |
[126] | NQF, ABCs of measurement, National Quality Forum, https://www.qualityforum.org/Measuring_Performance/ABCs_of_Measurement.aspx, 2010. |
[127] | Medicare Drug Benefit and C & D Data Group, Antipsychotic use in part d enrollees with dementia, Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/Antipsychotic-Use-in-Part-D-Enrollees-with-Dementia-v12092015.pdf, November 16, 2015. |
[128] | Schnitker LM , Martin-Khan M , Burkett E , Beattie ERA , Jones RN , Gray LC ((2015) ) Process quality indicators targeting cognitive impairment to support quality of care for older people with cognitive impairment in emergency departments. Acad Emerg Med 22: , 285–298. |
[129] | RAND, ACOVE quality indicators applicable to medical records and administrative data, RAND Corporation, https://www.rand.org/health-care/projects/acove/acove3.html, 2007. |
[130] | NICE, Dementia: Quality standard [QS184], National Institute for Health and Care Excellence, https://www.nice.org.uk/guidance/qs184, June 28, 2019. |
[131] | NICE, Suspected neurological conditions: Recognition and referral quality standard [QS198], National Institute for Health and Care Excellence, https://www.nice.org.uk/guidance/qs198 January 8, 2021. |
[132] | NCQA, HEDIS MY 2022 measure descriptions, National Committee for Quality Assurance, https://www.ncqa.org/wp-content/uploads/2021/12/HEDIS-MY-2022-Measure-Descriptions.pdf, 2021. |
[133] | NQF, Priority setting for healthcare performance measurement: Addressing performance measure gaps in care coordination, National Quality Forum, https://www.qualityforum.org/Publications/2014/08/Priority_Setting_for_Healthcare_Performance_Measurement__Addressing_Performance_Measure_Gaps_in_Care_Coordination.aspx, October 15, 2014. |
[134] | NQF, Memo re: Voting draft report: National voluntary consensus standards for neurology, phase ii, National Quality Foundation, http://www.qualityforum.org/Projects/n-r/Neurology_Endorsement_Maintenance/Voting_Memo_-_Phase_II.aspx, January 14, 2013. |
[135] | NQF, Quality positioning system (QPS), National Quality Foundation, https://www.qualityforum.org/QPS, Accessed July 15, 2022. |
[136] | Rose KM , Williams IC , Anderson JG , Geldmacher DS ((2021) ) Development and validation of the family quality of life in dementia scale. Gerontologist 61: , e260–e268. |
[137] | AHRQ, Types of health care quality measures, Agency for Healthcare Research and Quality, https://www.ahrq.gov/talkingquality/measures/types.html, July, 2015. |
[138] | AAN, Quality measurement manual 2022 update, American Academy of Neurology, https://www.aan.com/siteassets/home-page/policy-and-guidelines/quality/quality-measures/how-measures-are-developed/22measuredevprocman.pdf February 18, 2022. |
[139] | DiMe, The playbook: Digital clinical measures, Digital Medicine Society, https://playbook.dimesociety.org, October 23, 2023. |
[140] | Manta C , Patrick-Lake B , Goldsack JC ((2020) ) Digital measures that matter to patients: A framework to guide the selection and development of digital measures of health. Digit Biomark 4: , 69–77. |



