Risk of Major Types of Dementias Following Hospital-Diagnosed Infections and Autoimmune Diseases
Abstract
Background:
Population-based studies have shown an increased risk of dementia after infections, but weaker links were reported for autoimmune diseases. Evidence is scarce for whether the links may be modified by the dementia or exposure subtype.
Objective:
We aimed to investigate the association between infections and/or autoimmune diseases and rates of major types of dementias in the short- and long terms.
Methods:
Nationwide nested case-control study of dementia cases (65+ years) diagnosed in Denmark 2016–2020 and dementia-free controls. Exposures were hospital-diagnosed infections and autoimmune diseases in the preceding 35 years. Two groups of dementia cases were those diagnosed in memory clinics (MC) and those diagnosed outside memory clinics (non-memory clinic cases, NMC).
Results:
In total, 26,738 individuals were MC and 12,534 were NMC cases. Following any infection, the incidence rate ratio (IRR) for MC cases was 1.23 (95% CI 1.20–1.27) and 1.70 for NMC cases (1.62–1.76). Long-term increased rates were seen for vascular dementia and NMC cases. IRRs for autoimmune diseases were overall statistically insignificant.
Conclusions:
Cases with vascular dementia and not Alzheimer’s disease, and a subgroup of cases identified with poorer health have increased long-term risk following infections. Autoimmune diseases were not associated with any type of dementia. Notably increased risks (attributed to the short term) and for NMC cases may indicate that immunosenescence rather than de novo infection explains the links. Future focus on such groups and on the role of vascular pathology will explain the infection-dementia links, especially in the long term.
INTRODUCTION
Several previous epidemiological population-based studies have shown an increased risk of dementia following infections, and that the observed links were more prominent for vascular dementia (VaD) [1, 2], whereas there was either a small or no increased Alzheimer’s disease (AD) risk in some studies [3, 4]. Such findings can provide important insights into the potential underlying mechanisms linking infections to dementia, particularly because the infectious hypothesis has been principally directed towards AD pathology, prompted by findings that amyloid-β (Aβ) peptide, whose deposition is a hallmark of AD, has antimicrobial properties [5]. Recently, systemic inflammation has been suggested to explain the observed infection and dementia links [1, 4] and has also been proposed to be involved in mechanisms potentially linking autoimmune diseases to dementia [6]. The literature on autoimmune diseases has shown, similarly to infections, that the observed links (although overall close to null) are more prominent for VaD [7, 8].
In our previous study we leveraged the nationwide Danish registry data to investigate the associations between infections and autoimmune diseases and the risk of all-cause dementia, to explore potential shared signals between the two exposures [9]. Consistently with previous studies, we found increased dementia risk following hospital-diagnosed infections. In the same study we observed weak associations between autoimmune disease and dementia. However, we did not assess the risk for dementia subtypes because of low validity [10], a potential limitation in most population-based studies with dementia subtype data from electronic health records.
It therefore remains unknown whether the infection/dementia and/or autoimmune/dementia links vary by the dementia subtype. Such data would provide deeper insights and allow the generation of novel hypotheses regarding the roles of these conditions and the immune system in the development of dementia.
Here we utilize the nationwide Danish Quality Database for Dementia (DanDem) [11]. The nationwide database includes all dementia cases diagnosed in a memory clinic from 2016, providing clinically confirmed and valid subtype diagnoses.
The aim of this study is thus to investigate the short- and long-term associations between infections, autoimmune diseases, and subsequent incidence of major types of dementia in the Danish population.
METHODS
Data sources
Data sources were population-based Danish national registries: the National Patient Registry (NPR) includes inpatient data from 1977 in somatic wards whereas the Psychiatric Central Research Registry (PCRR) holds psychiatric ward records from 1969. In 1995, somatic and psychiatric in- and outpatients in all hospitals were included in the NPR. Diagnoses are registered according to International Classification of Diseases (ICD) 8th edition until 1993, and ICD 10th edition from 1994 onwards [12]. The National Prescription Registry (NPrR) contains individual-level information on all prescription drugs dispensed at Danish pharmacies from 1995 [12]. Finally, DanDem contains all dementia diagnoses given in a hospital-based memory clinic in Denmark from 2016 [11]. An individual’s lifelong personal identification number enabled precise individual cross-linkage across various registries.
Study design and population
We conducted a nested case-control study. The study population was the nationwide cohort of all residents of Denmark alive and in Denmark on 1 January 2016 and from their 65th birthday. We excluded people with a dementia record before age 65 years or 1 January 2016 and people with registered HIV infection. A dementia record was defined as the date of first dementia diagnosis (as defined in Supplementary Table 1 and Supplementary Methods) in the NPR, PCRR, or DanDem, or the date of a first redeemed anti-dementia medication in the NPrR.
Cases (outcome) and controls
Cases were individuals with incident dementia diagnosis (age 65+ years) between 2016 and 2020. Two groups of dementia cases were identified from the national registries: those diagnosed at a memory clinic, who are referred to as memory clinic (MC) dementia cases, and those diagnosed outside of the memory clinics, who are referred to as non-memory clinic (NMC) dementia cases.
MC cases were defined from the date of a first dementia record in DanDem. On that date, each case was matched by incidence density sampling to up to three controls matched by age (birthdate±90 days) and sex from the general population. Controls were eligible for matching provided that they had no record of dementia, were alive, and resided in Denmark. The matching date was assigned as the index date. Cases were assessed as (a) all-cause dementia (and further stratified by sex and age at index date), (b) AD (AD and mixed dementia), (c) VaD, (d) Other dementias (All other diagnoses in DanDem grouped together: other specific neurodegenerative disease, Lewy body dementia, Parkinson dementia, frontotemporal dementia, ParkinsonPlus –progressive supranuclear palsy/corticobasal degeneration/multiple system atrophy, normal pressure hydrocephalus, and Huntington’s chorea), and (e) unspecific dementia (a separate diagnosis recorded in DanDem).
NMC cases were defined from the date of a first dementia record in NPR, PCRR, or NPrR, and as not having any record in DanDem during the study period. We matched them to controls in a similar manner to the memory clinic cases. We did not use information on dementia etiology because subtypes generally have low validity in this data source [10]. Cases were assessed as all-cause dementia and further stratified by sex and age at index date.
For the MC cases, some also had a prior dementia record outside of DanDem. These cases were categorized as MC cases with the date of dementia onset as the memory clinic diagnosis date. NMC cases were those who were only diagnosed outside of memory clinics with no records in DanDem. Therefore, the definition of MC and NMC cases was based on whether a case had a dementia record in DanDem (MC) or only outside of DanDem (NMC).
Exposures
Infections and autoimmune diseases were defined as any inpatient, outpatient, or emergency hospital contact with a primary or secondary discharge diagnosis of an infection or autoimmune disease recorded in the NPR (based on inpatient data before 1995). Exposure was assessed in the 35 years before the index date (ICD codes in Supplementary Table 2).
Exposure to infections was assessed as any infection, burden (number of new infections, as defined in [9]), and type (bacterial, viral, other including fungal, parasitic, and those unspecific to bacterial or viral alone, and a mixed group of co-infections from the listed categories). Exposure to autoimmune diseases was assessed as any disease and type (Organ-specific diseases, e.g., Crohn’s disease, systemic diseases, e.g., seropositive rheumatoid arthritis, and a mixed group of the two categories).
Owing to the limited period during which cases were available, we did not assess infection sites or number of autoimmune diseases.
Data analysis
Exposure to infections and autoimmune diseases was compared between cases and controls using conditional logistic regression analyses yielding dementia odds ratios interpreted as incidence rate ratios (IRR) using SAS statistical software version 9.4 (SAS Institute). Statistical significance was determined using 95% confidence intervals (CI) corresponding to a 5% significance level. Individuals were censored on the date of death, emigration, or the outcome of interest. Exposures and outcomes (cases) were analyzed as defined above. Exposures were analyzed with reference to no infection and no autoimmune disease. To test for reverse causality and to explore the risk in the short and long term, we additionally applied a lag period of 10 years before index date. Analyses with this period applied thus reflect the risk in the long term. This period meant that all exposures occurring during the 10-year period prior to index date were not counted in that analysis.
We adjusted for the following covariates assessed at index date or at the start of the 10-year lag period: highest attained educational level, stroke, myocardial infarction, hypertension, diabetes, and hypercholesterolemia (ICD and ATC codes in Supplementary Table 1).
We conducted a sensitivity analysis to test the robustness of our findings to any decisions we made. MC cases were defined from the first date of dementia in DanDem, assigned as the index date. However, 44% of the cases had a prior date outside DanDem. This discrepancy might arise because records in the NPR can be retrospectively closed leading to mismatched dates, or that a diagnosis was set before a diagnostic workup at the memory clinic. We repeated our main analyses after redefining the index date for these dementia cases as the first overall dementia record instead of the potentially later DanDem date.
RESULTS
Of 1,358,734 individuals in the cohort, we identified 39,272 cases with dementia (Fig. 1). Of these, 26,738 were MC dementia cases and were matched to 80,214 dementia-free controls. Of the cases, 71% were registered with AD, 13% with VaD, 10% with other dementias, and 6% with unspecific etiology. Their characteristics are presented in Table 1 and Supplementary Table 3.
Fig. 1
Population flow chart. Memory clinic dementia cases: Of all the controls, 75,430 were unique individuals (i.e., there were repeated controls), and 1,494 controls became cases after index date at which point, they were matched with 3 new controls (and are counted among the 26,738 cases). Non-memory clinic dementia cases: Of all the controls, 36,179 were unique individuals and 1,266 controls became cases after index date.
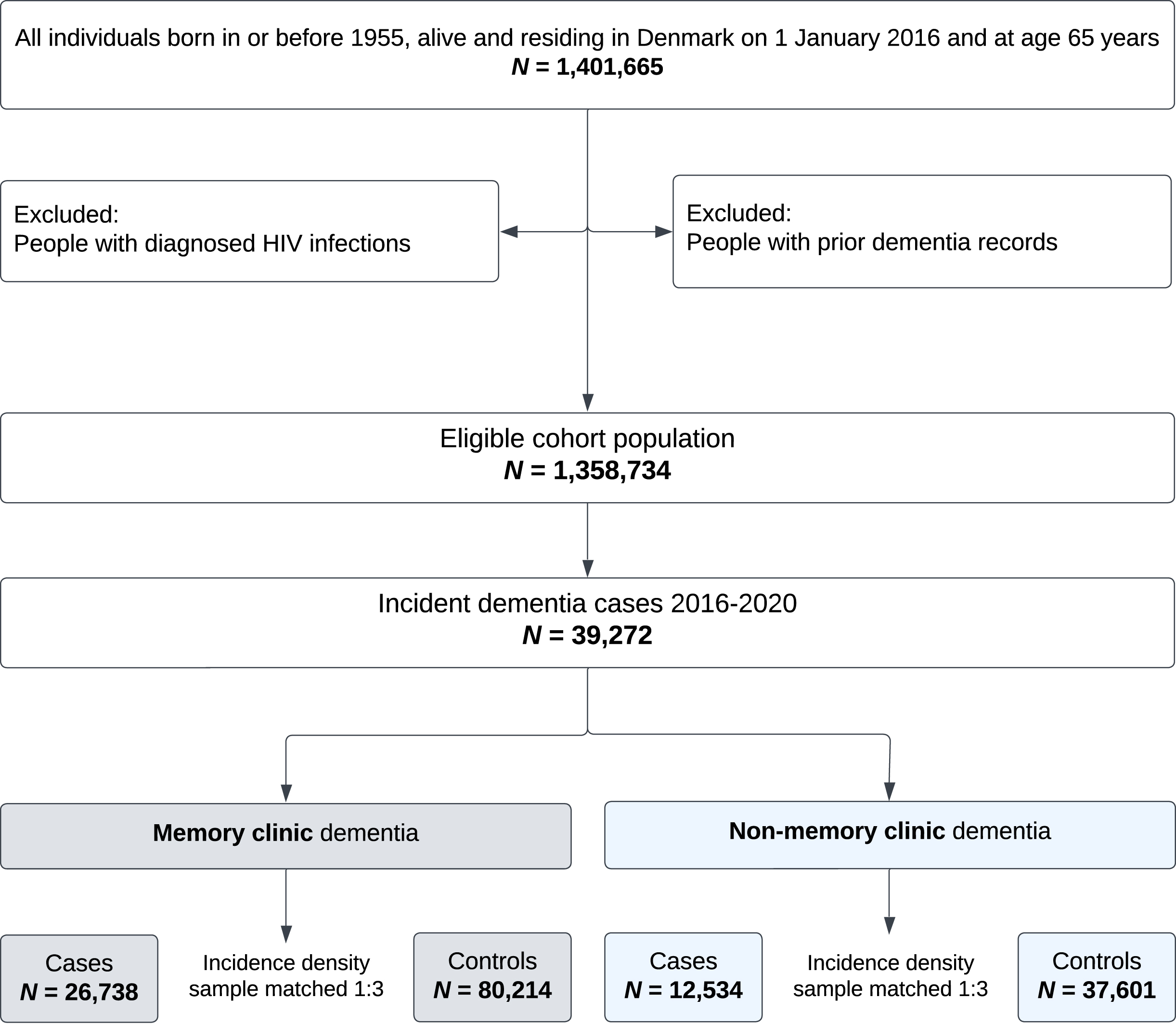
Table 1
Population characteristics for memory clinic and non-memory clinic all-cause dementia cases and controls
| Memory clinic all-cause | Non-memory clinic all-cause | |||||||
| dementia | dementia | |||||||
| Cases (%) | Controls (%) | Cases (%) | Controls (%) | |||||
| N total | 26,738 | 80,214 | 12,534 | 37,601 | ||||
| Sex | ||||||||
| Women | 15,432 | (58) | 46,296 | (58) | 6,964 | (56) | 20,891 | (56) |
| Men | 11,306 | (42) | 33,918 | (42) | 5,570 | (44) | 16,710 | (44) |
| Age at index date | ||||||||
| 65–74 y | 5,514 | (21) | 16,547 | (21) | 2,339 | (19) | 7,017 | (19) |
| 75–84 y | 13,459 | (50) | 40,409 | (50) | 5,062 | (40) | 15,159 | (40) |
| 85++ y | 7,765 | (29) | 23,258 | (29) | 5,133 | (41) | 15,425 | (41) |
| Median (IQR) | 81 (75–85) | 81 (76–85) | 83 (76–88) | 83 (76–88) | ||||
| Mean (min-max) | 80 (65–102) | 80 (64–102) | 82 (65–107) | 82 (64–107) | ||||
| Education at index date* | ||||||||
| Low | 21,292 | (80) | 63,158 | (79) | 9,993 | (80) | 29,560 | (79) |
| Medium | 3,714 | (14) | 11,751 | (15) | 1,524 | (12) | 5,071 | (13) |
| High | 1,111 | (4) | 3,469 | (4) | 497 | (4) | 1,562 | (4) |
| Comorbidities at index date | ||||||||
| Hypertension | 21,157 | (79) | 61,745 | (77) | 10,289 | (82) | 29,561 | (79) |
| Diabetes | 4,664 | (17) | 11,712 | (15) | 2,185 | (17) | 5,362 | (14) |
| Hypercholesterolemia | 15,031 | (56) | 40,101 | (50) | 6,222 | (50) | 17,996 | (48) |
| Myocardial Infarction | 2,237 | (8) | 6,509 | (8) | 1,184 | (9) | 3,336 | (9) |
| Stroke | 4,972 | (19) | 9,471 | (12) | 2,999 | (24) | 4,829 | (13) |
| Any infection | 14,441 | (54) | 38,287 | (48) | 8,012 | (64) | 18,614 | (50) |
| Age at first infection | ||||||||
| Median (IQR) | 70 (60–78) | 70 (60–78) | 73 (63–81) | 72 (62–81) | ||||
| Mean (min-max) | 69 (30–99) | 68 (30–99) | 71 (30–101) | 71 (30–106) | ||||
| Any autoimmune disease | 2,768 | (10) | 8,020 | (10) | 1,301 | (10) | 3,616 | (10) |
| Age at first autoimmune disease | ||||||||
| Median (IQR) | 70 (61–77) | 70 (61–77) | 71 (62–78) | 71 (63–78) | ||||
| Mean (min-max) | 68 (31–95) | 68 (31–97) | 69 (38–95) | 70 (31–101) | ||||
| Deaths within 1 year of registered diagnosis | ||||||||
| N | 2,488 | (9) | 4,170 | (6) | 3,786 | (30) | 2,830 | (8) |
| Nursing home before or within 1 year of registered diagnosis | ||||||||
| N | 7,603 | (28) | 4,659 | (6) | 5,792 | (46) | 3,241 | (9) |
*Missing education information for 621 memory clinic cases and 1,836 controls, and for 520 non-memory clinic cases and 1,408 controls. IQR, interquartile range.
We additionally identified 12,534 NMC dementia cases. These were matched with 37,601 dementia-free controls. Compared with the MC cases, NMC dementia cases had a higher median age at diagnosis and had more comorbidities at index date, especially notable for stroke and hypertension. These cases also had higher median age at first infection and autoimmune diagnosis. Importantly, there was a markedly higher proportion of deaths within 1 year of a registered dementia diagnosis in this case group (30%) compared to 9% of the MC cases (Table 1).
Of all MC cases, 54% had been registered with an infection during the 35-year study period (compared with 48% of the matched controls). Of the NMC cases, 64% had been registered with an infection (compared with 50% of the matched controls). For autoimmune diseases, 10% of MC cases (and their controls) and 10% of NMC cases (and their controls) had been registered with an autoimmune disease (Table 1).
Figures 2 and 3 show IRRs for all dementia groups following infections and by infection type, respectively. IRRs for MC dementia cases were increased following any infection with reference to no infection (adjusted IRR 1.23, 95% CI 1.20–1.27), and increased with increasing number of infections, but the confidence intervals overlapped between the exposure group IRRs (thus not a clear dose-response relationship). IRRs were similar in women and men and were highest among dementia cases diagnosed at ages 65–74 years. Similar trends were seen across the different dementia etiology groups, with the highest overall IRRs observed for VaD and unspecific etiology dementias.
Fig. 2
Dementia IRRs following infections in memory clinic and non-memory clinic cases, and with a 10-year lag period. The figure presents the incidence rate ratios (IRRs) of dementia following infections for: non-memory clinic all-cause dementia cases (stratified by infection burden, sex, and age at index date), memory clinic all-cause dementia cases (stratified by infection burden, sex, and age at index date), and memory clinic Alzheimer’s disease (AD) cases, vascular dementia (VaD), other dementias, and unspecific etiology cases (further stratified by infection burden in each). The first (left) forest plot represents the adjusted IRRs for all dementia groups when no lag period was applied. The second (right) forest plot represents the adjusted IRRs for all dementia groups when a 10-year lag period was applied. IRRs were adjusted for highest attained educational level, stroke, myocardial infarction, hypertension, diabetes, and hypercholesterolemia (with age at index date and sex as matching variables, and the regression model accounted for this matching).
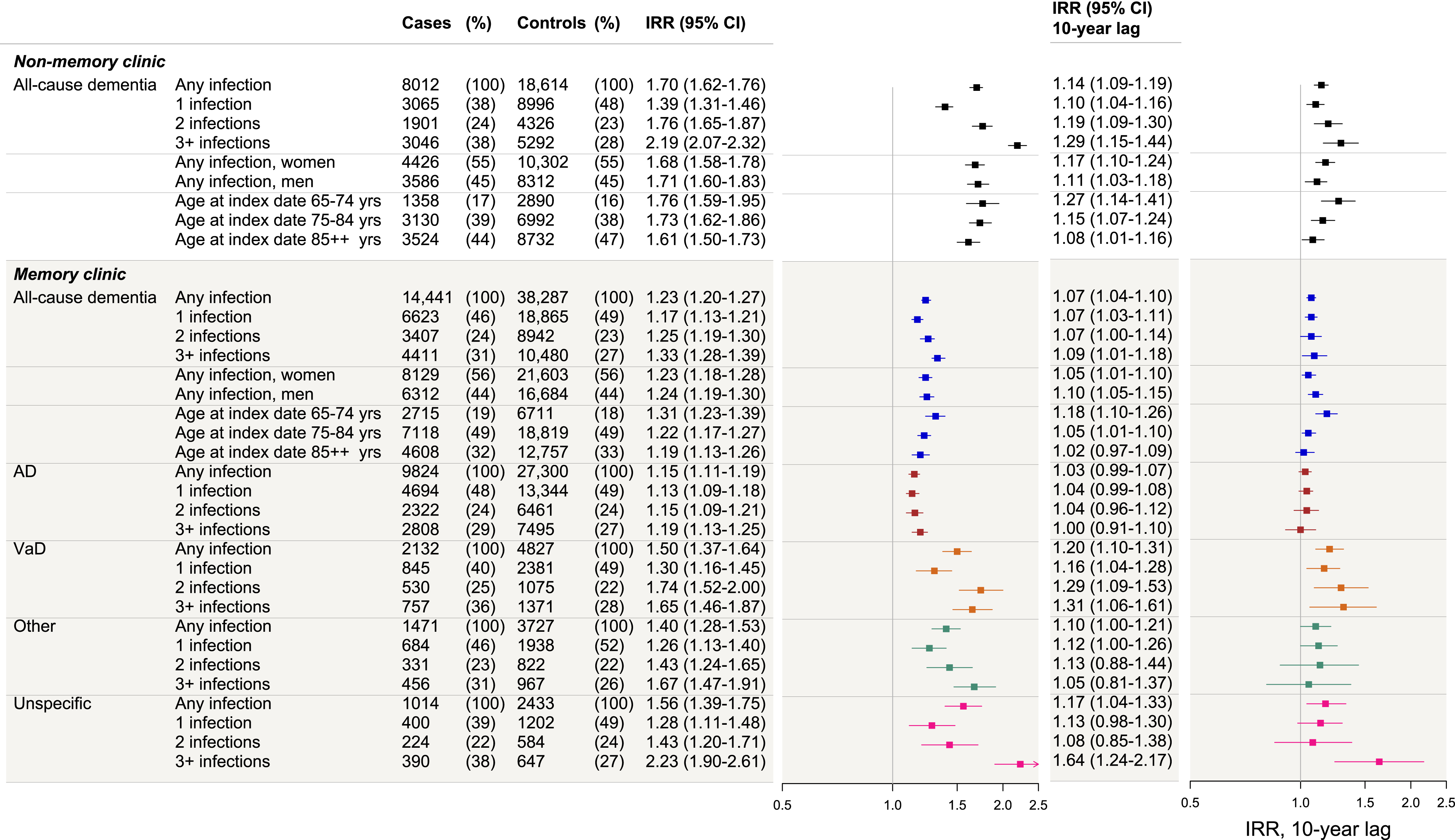
Fig. 3
Dementia IRRs following infections by type of infection in memory clinic and non-memory clinic cases, and with a 10-year lag period. The figure presents the incidence rate ratios (IRRs) of dementia following infections, stratified by infection type for: non-memory clinic all-cause dementia cases, memory clinic all-cause dementia cases, memory clinic Alzheimer’s disease (AD) cases, vascular dementia (VaD), other dementias, and unspecific etiology cases. “Other” category includes other types of infection than viral and bacterial and infections that were judged as not specific to either bacterial or viral alone. “Mixed” category includes cases and controls with co-infections of several types (e.g., bacterial and other). The first (left) forest plot represents the adjusted IRRs for all dementia groups when no lag period was applied. The second (right) forest plot represents the adjusted IRRs for all dementia groups when a 10-year lag period was applied. IRRs were adjusted for highest attained educational level, stroke, myocardial infarction, hypertension, diabetes, and hypercholesterolemia (with age at index date and sex as matching variables, and the regression model accounted for this matching).
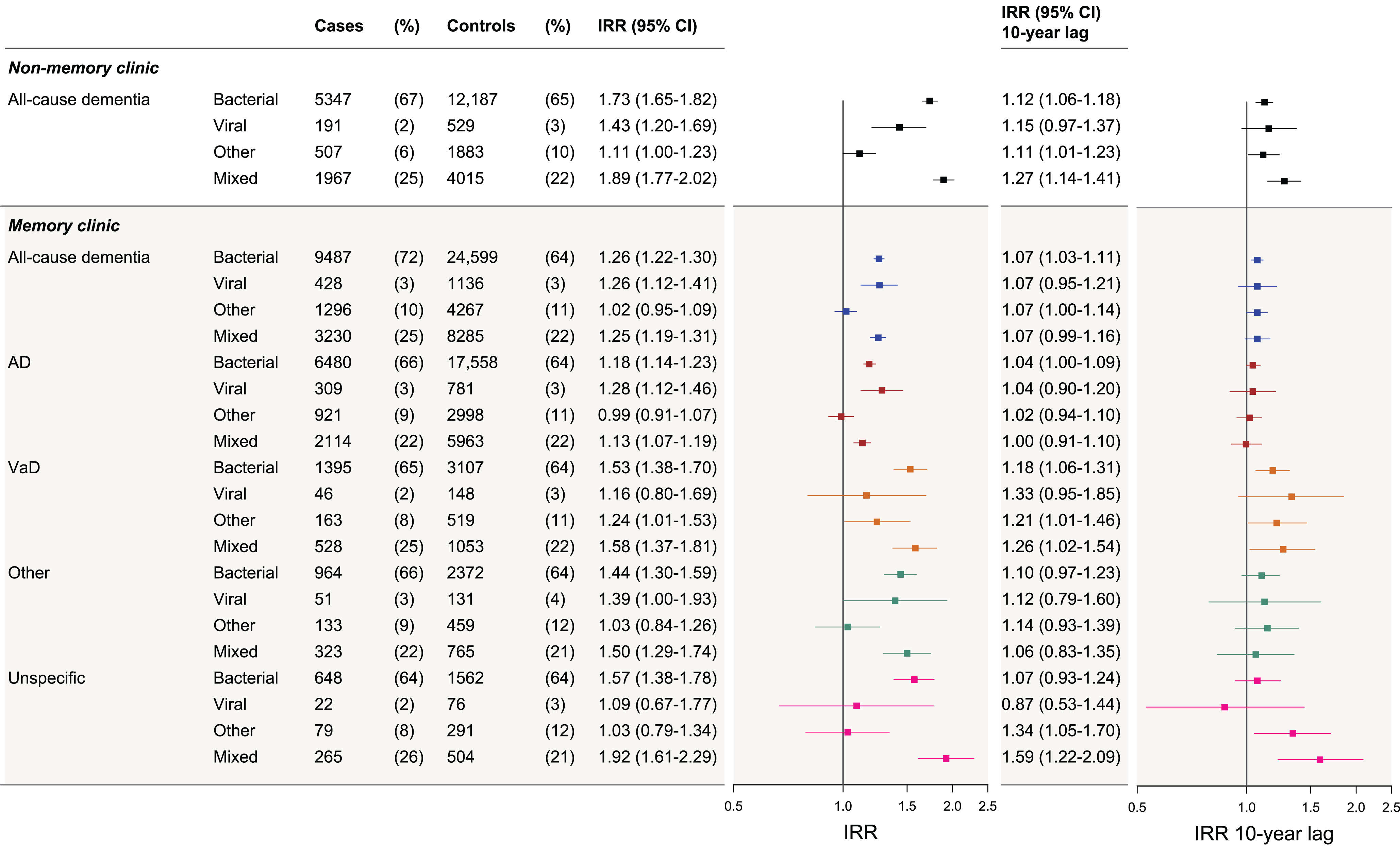
NMC cases showed markedly higher IRRs than any other group (adjusted IRR following any infection = 1.70, 95% CI 1.62–1.76), and a clear dose-dependent association with increasing number of infections was observed. Bacterial infections and the mixed infection group were associated with statistically significant increased IRRs in all assessed dementia groups and were highest for the NMC cases. Viral infections were associated with statistically significant increased IRRs in MC all-cause dementia, AD, and in NMC all-cause dementia.
IRRs attenuated after applying a 10–year lag period (analysis reflects risks in the long term) but remained statistically significantly increased across several groups; however, this was most notable for VaD among the MC cases and for NMC dementia cases. In this analysis, 27% of all MC cases had been registered with an infection (after removing exposures in the 10-year prior to index date), compared with 25% of their matched controls). For NMC cases, 29% of the cases had been registered with an infection compared with 26% of their matched controls (data not shown). Regarding infection types, after applying a 10–year lag period, few associations remained statistically significant. For all dementia cases, no clear dose-dependent association was observed with increasing number of infections after applying a 10–year lag period.
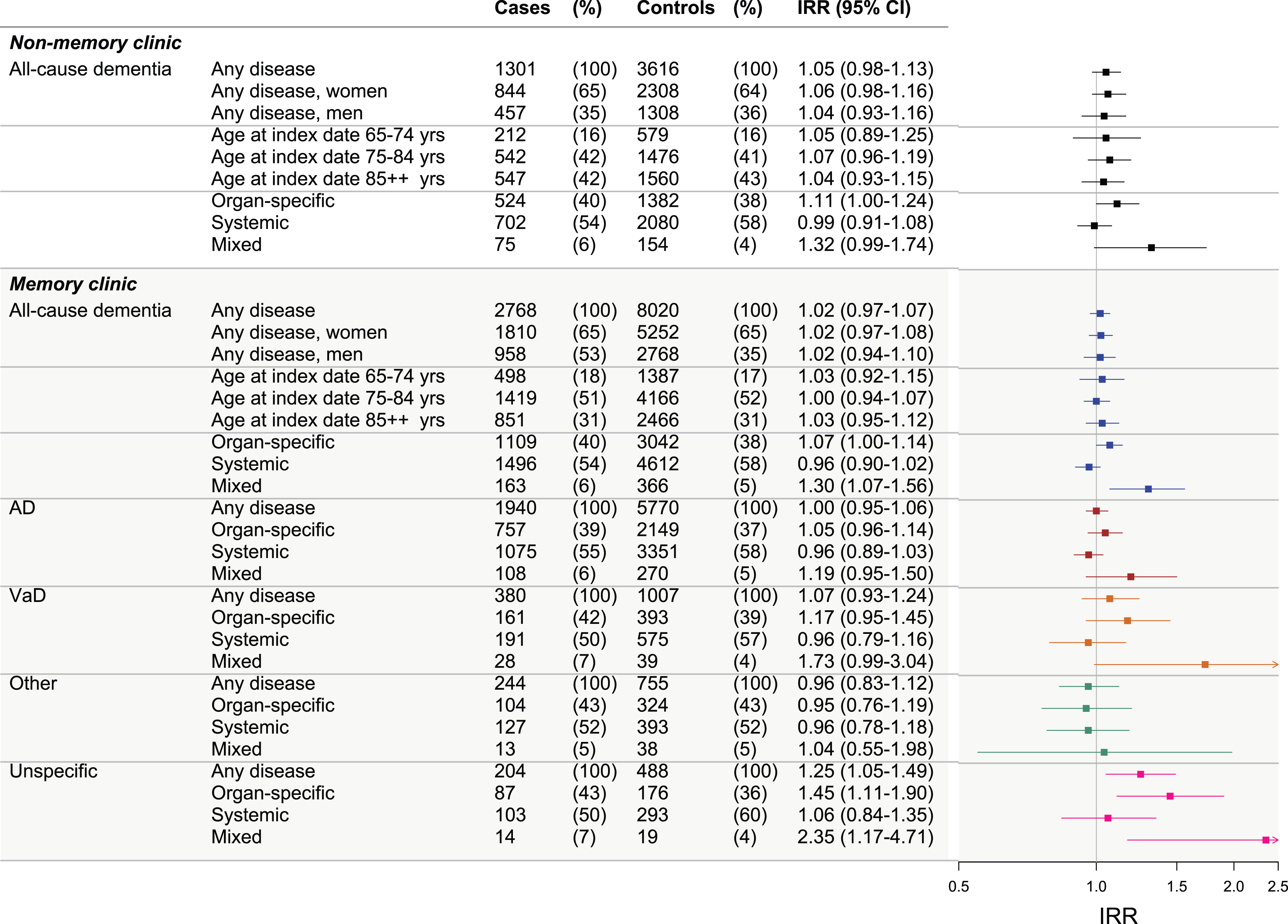
Figure 4 shows IRRs for all dementia groups following autoimmune diseases. Generally, only few statistically significantly increased IRRs were observed between autoimmune diseases and any of the dementia groups (small IRRs). For unspecific dementia, and particularly for the mixed disease group, IRRs were notably increased. However, this was based on very small numbers (33 individuals) and the IRRs were no longer statistically significant in the sensitivity analysis. The adjusted IRR for any autoimmune disease among MC cases was 1.02 (95% CI 0.97–1.07) and in NMC cases the IRR was 1.05 (95% CI 0.98–1.13). Crude estimates for all analyses are shown in Supplementary Tables 4 to 6. Sensitivity analysis showed robustness of our findings (Supplementary Figure 1).
Fig. 4
Dementia IRRs following autoimmune diseases in memory clinic and non-memory clinic cases. Figure presents the incidence rate ratios (IRRs) of dementia following autoimmune diseases for: non-memory clinic all-cause dementia cases (stratified by sex, age at index date, and disease type), memory clinic all-cause dementia cases (stratified by sex, age at index date, and disease type), and memory clinic Alzheimer’s disease (AD) cases, vascular dementia (VaD), other dementias, and unspecific etiology cases (further stratified by disease type in each). The ‘mixed’ category includes cases and controls with both organ-specific and systemic diseases. IRRs were adjusted for highest attained educational level, stroke, myocardial infarction, hypertension, diabetes, and hypercholesterolemia (with age at index date and sex as matching variables, and the regression model accounted for this matching).
DISCUSSION
Two key observations emerge from this nested case–control study of dementia cases diagnosed in individuals aged 65 years or above between 2016 and 2020 in Denmark. First, infections, but not autoimmune diseases, in the preceding 35 years were associated with increased rates of dementia and were particularly attributed to risks in the short term (discussed below). In all analyses, the associations were not clearly specific for a particular type of infection, and nearly all investigated infection exposure groups in our study were associated with an increased risk of all types of dementia in the short term. After applying a 10-year lag period (reflecting risks in the long term), associations attenuated for all dementia cases but remained statistically significantly increased for VaD (of the MC cases) as well as for the NMC cases (discussed further below).
Second, we report markedly stronger associations between infections and dementia for the NMC than for the MC cases. Indeed, there was a clear dose–response association between dementia risk and increasing number of infections in the NMC cases. The difference between MC and NMC cases is not understood, but NMC could have a different risk profile. In support, a larger proportion of the NMC cases died within 1 year of diagnosis (30% versus 9% for the MC cases) or were institutionalized, potentially indicating a more vulnerable and ill population group. The potential waiting time for MC referral could possibly mean that cases of rapidly progressing dementia are unable to attend for MC evaluation, whereas MC cases might represent more slowly progressive disease. Indeed, NMC individuals may not have been referred for MC evaluation because diagnostic workup might have been deemed unnecessary, for instance because of advanced dementia or proximate end of life. However, this remains speculative, and further studies will be necessary to determine the factors underlying the distinction between MC and NMC cases.
Regarding the type of infection, we did not observe clear trends for a specific infection type, partly hindered by small sample sizes in some groups. Evidence from previous studies is mixed on whether the associations between infection and dementia are specific for particular types of infection or dementia. Two previous studies suggested that the infection and dementia links were not infection/pathogen-specific [1, 4]. Sipilä et al. investigated all infection types and the major dementia subtypes in one population and showed overall higher risks for VaD than for AD [1]. Similar trends were seen by Chu et al. following pneumonia [2]. A recent study investigating different viral infections showed overall equally increased risks of VaD and AD [13]. Although previous studies have linked viral [13, 14] and bacterial infections [2, 15] to increased dementia risk, others reported null findings [16, 17]. These previous studies suggested that the infection and dementia links might be explained by general systemic inflammation. However, in this and our previous study we found null or very weak associations between autoimmune/inflammatory disease and any of the major dementias (generally consistent with other studies with some mixed reports [7–9, 18]). Based on this, in our previous study we hypothesized that the explanation for the infection and dementia links is likely not through common systemic inflammation [19] or an inflammatory state induced by various factors (infections and autoimmune diseases), and that infection-specific processes are involved [9].
Regarding the time course of heightened risk, the associations between infection and dementia attenuated after applying a 10–year lag period (long term risk), and only a few remained statistically significant, predominantly for VaD and NMC cases. This indicates that associations observed in analyses without the 10-year lag period reflect risks in the short term. Several explanations are possible. One is reverse causality (consistent with previous studies) [1, 13]. Increased rates of hospitalization for infection have been reported in people with dementia that might reflect an association between immunosenescence and dementia [20]. Because dementia pathology likely starts years before clinical presentation, infection rates may be increased in individuals with preclinical dementia. Ascertainment/detection bias is another potential explanation because increased hospital contacts could increase the likelihood that dementia is noted and diagnosed. In addition, associations in the short (but not long) term could indicate an acute role for infections in triggering and/or accelerating dementia pathology, and this could potentially be especially relevant in a subgroup of individuals such as NMC cases.
In the current study, we further build on these observations and find that the dementia–infection link is predominantly seen in the short term, and potentially in a more ill subgroup of dementia cases (NMC cases), and in VaD in the long term. These interpretations may indicate that episodes of infection may be a marker of a declining immune system/immunosenescence [21] rather than reflecting de novo infection, consistent with the idea that immunosenescence predisposes to dementia development [21]. The differences between the NMC and MC cases in our study suggest that an association between dementias and infections is particularly relevant for a subgroup of cases who might be predisposed to both clinical infections and dementia development. However, NMC cases may differ from MC cases in other unmeasured factors, some of which may explain the observed risks and subsequently provide indications for potential underlying mechanisms. It is possible that genetics, lifestyle factors, or a combination determines the rates immune system decline, and potentially the rate of dementia progression. This can be better understood by further investigation and profiling of the population subgroups at risk, further focus on the involvement of vascular pathology in the infection and dementia links, and by replication of our findings in other populations.
Strengths and limitations
Our study has several strengths. First, this was a nationwide study of all dementia cases recorded in Denmark during the study period. We utilized several population-based registries, each boasting nationwide coverage, negligible loss-to-follow-up, and high validity. These registries are not dependent on consent, which ensures consistent patient data. We assessed exposures during a retrospective period of 35 years, allowing important conclusions regarding reverse causality and assessment of exposures from age 30 years. The 35-year study period also ensured assessment of incident dementia where records were available throughout a long period to exclude pre-existing dementia records. Moreover, etiology diagnoses in MC cases can be assumed to be highly valid, and NMC case all-cause dementia diagnoses were also highly valid [10]. We assessed exposures by types of infection/disease and by burden, and linked those to subtypes of dementia, therefore allowing insights into potential risk trends.
Important limitations to consider include the small number of individuals in some of our exposure groups (e.g., viral infections), and statistically insignificant estimates should therefore be interpreted cautiously. For infection types, we isolated viral and bacterial infections from each other, and infection diagnoses that may have been either viral or bacterial were categorized in the “other infections” group; associations in this group should therefore be interpreted considering this. We defined our exposures using hospital diagnoses, and less severe events which did not require a hospital contact were not investigated due to the lack of specific diagnostic codes from the primary care setting in our registries, and because our focus was on the more severe hospital-diagnosed exposures (consistent with our previous study [9] and other population-based studies we refer to and follow-up on [4, 22, 23]). Individuals with infections and autoimmune diseases diagnosed only in the primary care may thus be part of our reference group. If such exposures are also associated with increased risk of dementia, this may have led to under-estimation of our results. In the NMC dementia cases, it should be noted that comorbidities may also be under-registered owing to a possible decrease in healthcare seeking, which might indicate differential misclassification of the covariates and possibly inadequate adjustment. We cannot exclude residual confounding from lifestyle factors, which are likely important when we speak of phenotyping subpopulations, but no data on these were available. Finally, it has been shown that COVID-19 had an impact on rates of hospitalization and generally on healthcare utilization [24]. However, we repeated our analyses excluding the year 2020 from our study period and findings remained unchanged (data not shown).
In conclusion, our nested case–control study of all dementia cases diagnosed between 2016 and 2020 in Denmark showed associations between infections and increased risk of all dementia types (attributed to the short term), and strongest for VaD and for a more ill subgroup of dementia cases diagnosed outside memory clinics. Autoimmune diseases were not associated with the risk of any dementia type. Such findings (particularly risks attributed to the short term) suggest a role for immunosenescence rather than de novo infection for dementia risk. Long-term associations persisted, particularly for the subgroup of dementia cases diagnosed outside memory clinics and for VaD among the other cases. We encourage future focus on vascular pathology and on isolating and profiling subgroups of dementia cases, which will better explain the increased risk of dementia following infections, especially in the long term.
AUTHOR CONTRIBUTIONS
Janet Janbek (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing); Thomas Munk Laursen (Conceptualization; Data curation; Formal analysis; Methodology; Software; Validation; Writing – review & editing); Niels Frimodt-Møller (Conceptualization; Methodology; Validation; Writing – review & editing); Melinda Magyari (Conceptualization; Methodology; Validation; Writing – review & editing); Jürgen G. Haas (Conceptualization; Methodology; Validation; Writing – review & editing); Richard Lathe (Conceptualization; Methodology; Validation; Writing – review & editing); Gunhild Waldemar (Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Validation; Writing – review & editing).
ACKNOWLEDGMENTS
We thank the Danish Clinical Quality Program – National Clinical Registries (RKKP) for granting access to data from the Danish Quality Database for Dementia.
FUNDING
The Danish Dementia Research Centre is supported by the Danish Ministry of Health which had no role in the current study.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data included in the current study are all derived from the Danish public health registries and cannot be shared with the public. The data are collected and stored by Danish authorities and access to raw individual-level data can be granted via standard rules and regulations outlined by the Danish Data Protection Agency and the Danish Health Data Authority.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-231349.
REFERENCES
[1] | Sipilä PN , Heikkilä N , Lindbohm J V , Hakulinen C , Vahtera J , Elovainio M , Suominen S , Väänänen A , Koskinen A , Nyberg ST , Pentti J , Strandberg TE , Kivimäki M ((2021) ) Hospital-treated infectious diseases and the risk of dementia: A large, multicohort, observational study with a replication cohort. Lancet Infect Dis 21: , 1557–1567. |
[2] | Chu C-S , Liang C-S , Tsai S-J , Bai Y-M , Su T-P , Chen T-J , Chen M-H ((2022) ) Bacterial pneumonia and subsequent dementia risk: A nationwide cohort study. Brain Behav Immun 103: , 12–18. |
[3] | Douros A , Santella C , Dell’Aniello S , Azoulay L , Renoux C , Suissa S , Brassard P ((2021) ) Infectious disease burden and the risk of Alzheimer’s disease: A population-based study. J Alzheimers Dis 81: , 329–338. |
[4] | Sun J , Ludvigsson JF , Ingre C , Piehl F , Wirdefeldt K , Zagai U , Ye W , Fang F ((2022) ) Hospital-treated infections in early- and mid-life and risk of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis: A nationwide nested case-control study in Sweden. PLoS Med 19: , e1004092. |
[5] | National Institute on Aging, Division of Neuroscience (2021) Infectious Etiology of Alzheimer’s Disease: Is there a Causative Role for Infectious Agents in Alzheimer’s Disease?https://www.nia.nih.gov/sites/default/files/2022-03/workshopsummaryinfectious-etiology-adfinal.pdf |
[6] | Booth MJ , Kobayashi LC , Janevic MR , Clauw D , Piette JD ((2021) ) No increased risk of Alzheimer’s disease among people with immune-mediated inflammatory diseases: Findings from a longitudinal cohort study of U.S. older adults. BMC Rheumatol 5: , 48. |
[7] | Zhang Y-R , Yang L , Wang H-F , Wu B-S , Huang S-Y , Cheng W , Feng J-F , Yu J-T ((2022) ) Immune-mediated diseases are associated with a higher incidence of dementia: A prospective cohort study of 375,894 individuals. Alzheimers Res Ther 14: , 130. |
[8] | Wotton CJ , Goldacre MJ ((2017) ) Associations between specific autoimmune diseases and subsequent dementia: Retrospective record-linkage cohort study, UK. J Epidemiol Community Health (1978) 71: , 576–583. |
[9] | Janbek J , Laursen TM , Frimodt-Møller N , Magyari M , Haas JG , Lathe R , Waldemar G ((2023) ) Hospital-diagnosed infections, autoimmune diseases, and subsequent dementia incidence. JAMA Netw Open 6: , e2332635. |
[10] | Phung TKT , Andersen BB , Høgh P , Kessing LV , Mortensen PB , Waldemar G ((2007) ) Validity of dementia diagnoses in the Danish Hospital Registers. Dement Geriatr Cogn Disord 24: , 220–228. |
[11] | Johannsen P , Rühmann B , Olesen AM , Andersen BG , Holm E , Waldorff FB , Gottrup H , Andersen K , Jørgensen LM , Jakobsen S , Hare-Bruun H ((2017) ) [P2–534]: First year data from the Danish National Dementia Clinical Quality Database. Alzheimers Dement 13: (Suppl 7), P846–P847. |
[12] | Schmidt M , Schmidt SAJ , Adelborg K , Sundbøll J , Laugesen K , Ehrenstein V , Sørensen HT ((2019) ) The Danish health care system and epidemiological research: From health care contacts to database records. Clin Epidemiol 11: , 563–591. |
[13] | Levine KS , Leonard HL , Blauwendraat C , Iwaki H , Johnson N , Bandres-Ciga S , Ferrucci L , Faghri F , Singleton AB , Nalls MA ((2023) ) Virus exposure and neurodegenerative disease risk across national biobanks. Neuron 111: , 1086–1093.e2. |
[14] | Wu D , Wang C , Pang P , Kong H , Lin Z , Wang H , Chen X , Zhao J , Hao Z , Zhang T , Guo X ((2020) ) The association between herpes simplex virus type 1 infection and Alzheimer’s disease. J Clin Neurosci 82: , 63–70. |
[15] | Muzambi R , Bhaskaran K , Brayne C , Davidson JA , Smeeth L , Warren-Gash C ((2020) ) Common bacterial infections and risk of dementia or cognitive decline: A systematic review. J Alzheimers Dis 76: , 1609–1626. |
[16] | Khairan P , Shirai K , Shobugawa Y , Cadar D , Saito T , Kondo K , Sobue T , Iso H ((2022) ) Pneumonia and subsequent risk of dementia: Evidence from the Japan Gerontological evaluation study. Int J Geriatr Psychiatry 37: , 1–11. |
[17] | Warren-Gash C , Forbes HJ , Williamson E , Breuer J , Hayward AC , Mavrodaris A , Ridha BH , Rossor MN , Thomas SL , Smeeth L ((2019) ) Human herpesvirus infections and dementia or mild cognitive impairment: A systematic review and meta-analysis. Sci Rep 9: , 4743. |
[18] | Li X , Sundquist J , Zöller B , Sundquist K ((2018) ) Dementia and Alzheimer’s disease risks in patients with autoimmune disorders. Geriatr Gerontol Int 18: , 1350–1355. |
[19] | Parikh NS , Merkler AE , Iadecola C ((2020) ) Inflammation, autoimmunity, infection, and stroke. Stroke 51: , 711–718. |
[20] | Janbek J , Frimodt-Møller N , Laursen TM , Waldemar G ((2021) ) Dementia identified as a risk factor for infection-related hospital contacts in a national, population-based and longitudinal matched-cohort study. Nat Aging 1: , 226–233. |
[21] | Lathe R , St. Clair D ((2023) ) Programmed ageing: Decline of stem cell renewal, immunosenescence, and Alzheimer’s disease. Biol Rev 98: , 1424–1458. |
[22] | Sipilä PN , Heikkilä N , Lindbohm J V , Hakulinen C , Vahtera J , Elovainio M , Suominen S , Väänänen A , Koskinen A , Nyberg ST , Pentti J , Strandberg TE , Kivimäki M ((2021) ) Hospital-treated infectious diseases and the risk of dementia: A large, multicohort, observational study with a replication cohort. Lancet Infect Dis 21: , 1557–1567. |
[23] | Wotton CJ , Goldacre MJ ((2017) ) Associations between specific autoimmune diseases and subsequent dementia: Retrospective record-linkage cohort study, UK. J Epidemiol Community Health (1978) 71: , 576–583. |
[24] | Michalowsky B , Hoffmann W , Bohlken J , Kostev K ((2021) ) Effect of the COVID-19 lockdown on disease recognition and utilisation of healthcare services in the older population in Germany: A cross-sectional study. Age Ageing 50: , 317–325. |




