Sociodemographic and Clinical Characteristics of People Living with Dementia and Their Associations with Unmet Healthcare Needs: Insights from the Baseline Assessment of the InDePendent Study
Abstract
Background:
The healthcare needs of People living with Dementia (PlwD) (such as Alzheimer’s disease) are often unmet. Information about the needs of community-dwelling PlwD and their association with sociodemographic and clinical characteristics is needed to fill the knowledge gap regarding factors influencing unmet needs among PlwD and to conduct a comprehensive needs assessment to develop tailored interventions.
Objective:
To describe sociodemographic and clinical characteristics of the InDePendent study population with particular reference to determinants of unmet needs.
Methods:
We analyzed baseline data of the multi-centre cluster-randomized controlled trial (InDePendent) using descriptive statistics to describe patients’ sociodemographic and clinical characteristics and Poisson regression models to predict unmet needs, separated by sex. Data were collected personally via face-to-face interviews.
Results:
Most of the n = 417 participating PlwD were mild to moderately cognitively impaired, were not depressed, had an average of 10.8 diagnoses, took 6.7 medications, and had, on average, 2.4 unmet needs (62% of PlwD had at least one unmet need) measured by the Camberwell Assessment of Need for the Elderly (CANE). Low social support, a high body-mass-index, a lower education, functional impairment, and worse health status were associated with more unmet needs, regardless of sex. In women, higher unmet needs were associated with more depressive symptoms, a poor financial situation, living alone and not being recently treated by a general practitioner. In males, unmet needs increased with the number of medications taken.
Conclusions:
PlwD had a broad array of unmet healthcare needs, indicating primary healthcare provision improvement potentials. The results underscore the significance of early assessment of patient’s clinical characteristics and unmet needs as a basis for individualized gender-sensible intervention strategies.∥ClinicalTrials.gov Identifier: NCT04741932, Registered on February 5, 2021
INTRODUCTION
Population aging is one of the most significant challenges healthcare systems face globally. This demographic change is associated with an increased prevalence of People living with Dementia (PlwD) (such as Alzheimer’s disease) [1, 2]. It is estimated that more than 55 million people worldwide are living with dementia, and according to forecasts, the number of PlwD is expected to rise to 139 million by 2050 [3]. In Germany, approximately 1.8 million individuals were living with dementia in 2021 and that number is expected to reach 2 million people aged 65 years and older by 2033 [1].
The German AgeCoDe study represents over 75-year-olds living in their own homes. Results show prevalences of cognitive impairment (23%), depression (8%), physical limitations (8%), and living alone (51%) [4]. Thyrian et al. [5] described the prevalence of socioeconomic parameters (e.g., 50% of PlwD live alone) and various clinical variables (e.g., 15% of PlwD suffer from depression) for PlwD in an ambulant setting in Germany (DelpHi MV Study, n = 516). Findings from the German IDemUck study (n = 235 community-living PlwD) demonstrate, among other things, that PlwD are predominantly married or in a partnership, had mild to moderate cognitive impairment, relatively high functional impairment, one-third receive antidementia medications, over half of the PlwD regularly consult a General Practitioner (GP), and that 58% of their caregivers would describe their quality of life as “good” [6]. The generalizability of these reported prevalences is unclear [7]. However, a comprehensive understanding of the sociodemographic and clinical characteristics of PlwD is vital to manage and improve their current situation [5]. We expanded our analyses to include more clinical and health-related variables (e.g., EQ-5D-5 L, body-mass-index, DEMMI, Timed-up-and-go, F-SozU, RUD, Zarit-Burden).
In many cases, PlwD have a complex and diverse need for healthcare and nursing because they often have several comorbidities, take various medications and need various types of support in their daily life. This complex situation causes unmet healthcare needs. The term “need” is based on the “ability to benefit” concept when a suitable intervention option is available that could address an unmet need [8]. Generally, unmet needs can be assessed by standardized instruments, like the Camberwell Assessment of Needs for the Elderly (CANE) [9], the John Hopkins Dementia Care Needs Assessment (JHDCNA) [10], or specifically developed Intervention Management Systems (IMS) [11]. Previous research has indicated that PlwD frequently experience various unmet healthcare needs, with their number ranging between 0.95 and 8.77 on average, depending on the respective questionnaire used [12–18].
Literature shows that unmet needs of PlwD are affected by a variety of factors [12–14, 17]. Eichler et al. [14] demonstrated that unmet needs were associated with worsening functional status. Black et al. [12] provided details about determinants, indicating that higher numbers of unmet needs among PlwD were significantly associated with lower income, less impairment in activities of daily living and more symptoms of depression. In addition, more unmet needs were significantly associated with lower quality of life [13]. The living situation among PlwD also determines unmet needs. Miranda-Castillo et al. [17] found that PlwD living in community networks with one to six members had significantly more unmet needs than individuals residing in communities with more than seven members. Individuals living alone show higher numbers of unmet needs than PlwD living in a partnership [17].
There is a need to study relationships, associations, and interactions between different variables in PlwD [5]. It is also necessary to conduct a comprehensive needs assessment to develop tailored interventions that address individual needs [14]. To our knowledge, the effects of clinical and sociodemographic parameters on unmet needs have yet to be considered separately to a large extent for women and men. A growing scientific literature documents that sex and gender have a differential impact on the risk, clinical presentation, and progression of dementia, and sex should, therefore, be considered as a factor in studies [19–21].
For example, it was found that neuropsychiatric abnormalities differed between the sexes [20]. A Canadian study also found that women received more supportive care but had higher unmet needs for home care than men [21]. As a result, these gender differences should be considered when developing programs, assessing the needs of care recipients and providing services [21], as these differences can impact health policy [20].
Therefore, this paper aims to describe the sociodemographic and clinical parameters of a baseline sample of community-dwelling PlwD, demonstrate the prevalence and types of unmet needs and identify associations between PlwD characteristics and needs stratified by sex.
METHODS
Study design
InDePendent (Interprofessional Dementia Care: Redistribution of tasks between physicians and qualified nurses in primary care) is a multi-centre, cluster-randomized, controlled trial with an intervention and a waiting control group to examine the efficacy of dementia-specific case and care management. The study protocol is published elsewhere [22]. Study participants were recruited in the primary care setting by GPs and medical specialists, e.g., neurologists and psychiatrists, who are members of one of five physician networks in three federal states in Germany (Mecklenburg-Western Pomerania, Brandenburg, and Hesse). Practitioners were informed about their randomization group after consenting to participate in the study, and they also informed their patients about the course of treatment and thus their study group upon inclusion in the study. Due to the nature of the intervention in this study, it is not possible to blind the study staff (nurses or practitioners) or the participants. The inclusion criteria for PlwD were: community-dwelling, formally diagnosed for dementia or positively screened for dementia (DemTect Score≤8 [23]), and provision of written informed consent. If the person could not provide written consent and had a legal guardian, then the guardian was asked to sign the consent form on his or her behalf. If a caregiver was available, he or she was also asked to provide written informed consent. The participating GPs received an allowance for each PlwD included in the study. The recruitment started in January 2021 and ended in December 2022.
The intervention is based on a collaborative dementia care model previously implemented and evaluated in the DelpHi-MV study [24], adapted for implementation in existing physician networks. Advanced care roles and substitution of medical activities enhanced the InDePendent intervention. This means that dementia-specifically qualified nurses, so-called Dementia Care Managers (DCM), collect all medical, pharmaceutical, psychosocial, social, and care needs using a self-developed computerized IMS at the patient’s home, and take over some of the tasks of the GPs with the aim of meeting or addressing all unmet needs within an intervention period of six months. The InDePendent study (funded by the German Innovationsfonds, ref. no.: 01NVF18034) aims to evaluate whether DCMs and the redistribution of tasks between nurses and physicians could significantly reduce the number of unmet needs of PlwD compared to routine care after six months.
The ethics committees of the University Medical Center Greifswald, the State Medical Council Brandenburg and the State Medical Council of Hesse approved the implementation of the InDePendent study (registration number: BB 144/20; AS 81(bB)/2020; 2020-2081-zvBO).
Study population
From n = 149 participating GPs and specialist practices, n = 471 PlwD and additionally, n = 188 informal caregivers provided their informed consent and participated in the study. Before baseline, n = 54 patients dropped out (11.5%), for example due to lack of interest, moving away or death. Finally, n = 417 PlwD started the baseline assessment (Fig. 1).
Fig. 1
CONSORT Diagram InDePendent study Baseline.
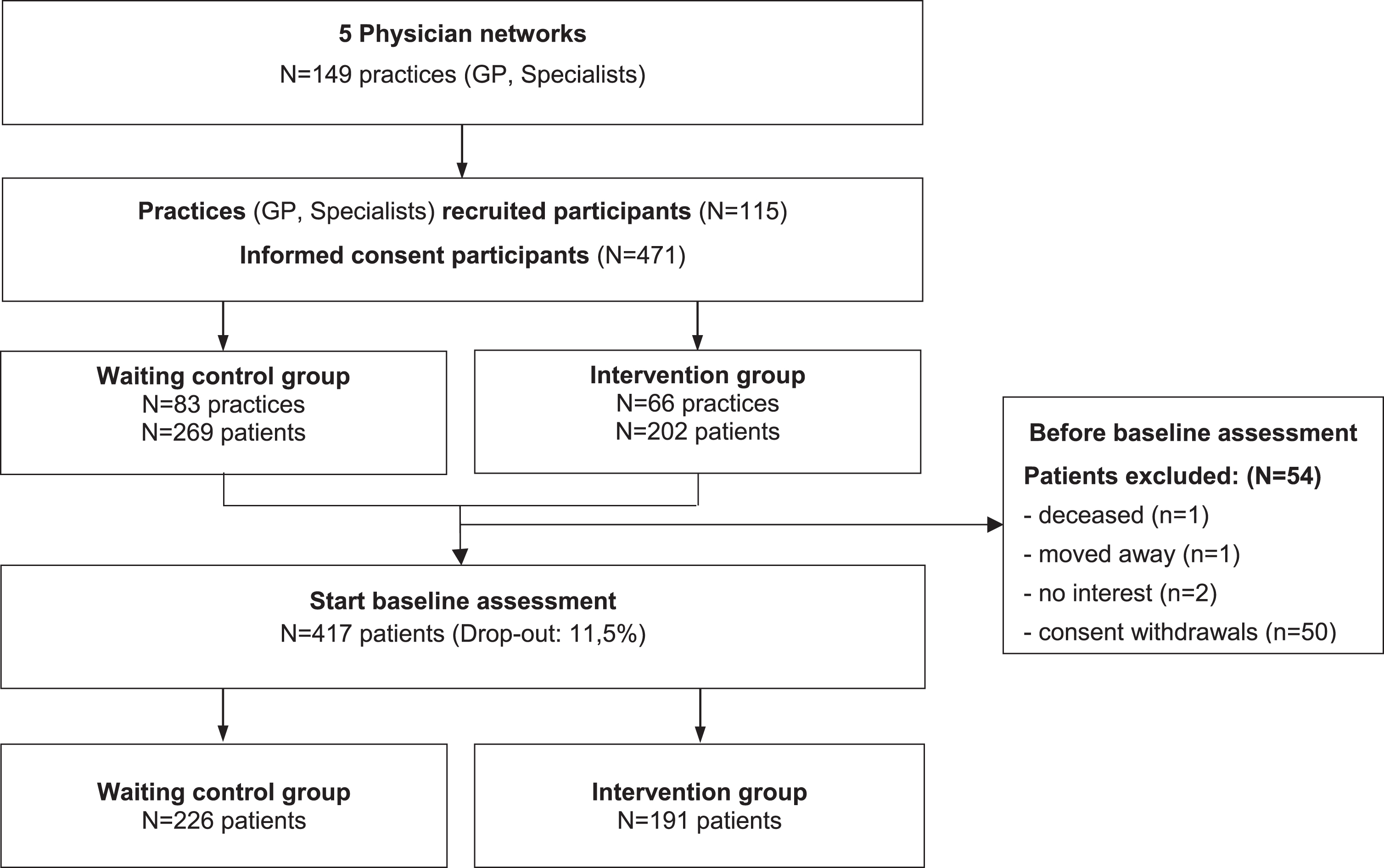
Differences between those who dropped out and those who started the baseline interview were tested using a logistic regression model controlled for age, sex, study group, and physician network membership.
Selection bias was found concerning the study group and physician network membership, as there was a highly significant difference between those who dropped out and those who started the baseline interview. The detailed analysis can be found in Supplementary Tables 1 and 2.
Data assessments
Upon enrolment in the study, all participants (PlwD and their caregivers) received a comprehensive computerized baseline assessment conducted by the DCMs in their homes. All data were collected as part of a personal face-to-face interview, in which the DCM read the questions separately to the PlwD and caregiver, if available [22]. The duration of the interviews varied depending on the condition of the interviewee and whether there was a caregiver who could also be interviewed. On average, the baseline interview could be conducted in 2.2 visits within about one month (mean = 27.8 days, SD = 50.9), and for example, CANE questionnaire lasted approximately 17.4 minutes (SD = 14.3). All instruments recorded the participant’s current condition unless stated otherwise below.
Sociodemographic factors
Sex, age, marital status (single, married, divorced, separated, widowed), self-assessed financial situation (good/not good), presence of children (yes/no), school education (primary education/ lower secondary education/ higher secondary education), living situation (alone/ not alone), and caregiver availability (yes/no) were assessed.
Clinical factors
Quality of life
Health-related quality of life was measured by the EQ-5D-5 L (self-rating instrument), covering five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) with five response options that range from 1 = “no problems” to 5 = “extreme problems” [25]. A completely healthy patient would, therefore, have a number combination of 11111. Based on this 5-digit number, a health index can be calculated using a special algorithm (the German value set by Ludwig et al. [26]), anchored between 0 (worst) and 1 (full) [27]. EQ-5D index values are derived from the general population using preference weights that reflect the severity of the corresponding health condition [28]. Such index values can then be used for country-specific economic evaluations of health measures and enable the calculation of quality-adjusted life years [27].
Functional status
Functional status was measured by the Bayer Activities of Daily Living Scale (B-ADL) (answered by caregiver), which scored 1 to 10, with 1 representing the best and 10 the worst functional status [29, 30]. To measure mobility status, the De Morton Mobility Index (DEMMI) was used, which covers the PlwD perspective and ranges from 0 to 100 points, with 100 points representing the best mobility [31]. Mobility was also assessed by administering the Timed “Up and Go” (TUG) test, wherein PlwD perform the task themselves. The TUG test measures the time to stand up from a chair, walk three meters, turn around, and sit down again. Less than 10 seconds represent “unrestricted mobility”, 10 to<20 seconds a “mobility impairment without everyday relevance”, 20 to<30 seconds a “restricted mobility with everyday relevance”, and more than 30 seconds a “pronounced restriction of mobility” [32, 33].
Depression
The Geriatric Depression Scale (GDS) (answered by PlwD) was used to detect depressive symptoms [34]. The score values are: 0–5 points, representing “no depressive symptoms”, 6–10 points = “indication for mild depression”, 11–15 points = “indication for severe depression” [35].
Cognition
Global cognitive performance was assessed by asking the PlwD using the Mini-Mental-Status-Test (MMST) [36], with scores categorized as 30 “no cognitive impairment”, 29–20 “mild cognitive impairment”, 19–10 “moderate cognitive impairment “ and ≤9 “severe cognitive impairment” [37].
Comorbidity and polypharmacy
All ICD-10 diagnoses listed in the treating practitioner’s file and all drugs taken (including over-the-counter medications) according to the medication plan were assessed by the DCM. Based on that data, we calculated the number of diagnoses and medicines taken and identified potentially inappropriate medications, according to the Priscus List [38] 1.0 1
Additional measures of health
We also looked for presence of a long-term care grade. A care grade (“Pflegegrad”) is a German classification for the level of care needed by an individual. It determines the amount of care and support a person receives due to his or her health condition or disabilities. The care grade ranges from 1 to 5, with one indicating some problems and five extreme problems. In Germany, the Medical Service of the Health Insurance Fund (MDK) decides on the classification into a care grade. This assessment is based on an appraisal of individual care needs by specially trained MDK assessors. We also assessed the Body-Mass-Index (BMI) of PlwD based on measurements of body length and weight.
Utilization of care services and informal care
The utilization of care services was measured using the Questionnaire for Health-related Resources in Older People (FIMA) by asking the PlwD [39]. Informal care provision by caregivers was recorded using the Resource Utilisation in Dementia (RUD) instrument, covering caregiver support (from the caregiver’s perspective) in hours per month for i) activities of daily living (ADL) (e.g., personal hygiene, eating, dressing), ii) instrumental ADL (e.g., shopping, meal preparation, housekeeping) and iii) supervision (such as preventing dangerous events) [40].
The following FIMA items have a recall period of three months: Utilization of GP, neurologist/psychiatrist, ambulatory care, physiotherapy, occupational therapy, speech therapy, ambulant nursing service, relatives and the following one a recall period of twelve months: semi-stationary care facility, day clinic, stationary treatment. In the case of RUD, the last 30 days were surveyed.
Social support
The F-SozU (reported by PlwD) is a questionnaire on social support, covering emotional support, practical support, social integration, and social stress with scores ranging from 1 (strongly agree) to 5 (not agree), where higher scores indicate higher social support [41].
The Zarit Burden questionnaire measures the subjective burden of PlwD’s caregivers, ranging from zero to 88 points, with higher scores representing a higher level of subjective caregiver burden [42].
Unmet needs
Unmet needs of PlwD were assessed using the German version of the Camberwell Assessment of Needs for the Elderly (CANE), which evaluates the met and unmet needs from the patients’ and the caregivers’ (proxy) perspectives. The CANE questionnaire includes 25 daily life domains (plus two caregiver needs items) to assess older people’s physical, psychological, social and environmental needs in various domains like household, nutrition, food, activities [9]. The CANE encompasses three inquiries for each designated domain: i) Does a need exist within this domain? ii) Is the need met or unmet? iii) Whom does the individual wish to engage as the person or service provider to address the unmet need? The result of the CANE is a total number of unmet needs of PlwD (minimum 0, maximum 27) [43]. For the regression, a joint CANE variable was created from the perspective of the PlwD and the caregiver (n = 414). To collect the CANE, either the PlwD or, if available, the caregiver was interviewed. In the rare cases (n = 10) where both answered the CANE, the caregiver version was chosen because PlwD typically report significantly fewer (unmet) needs than their caregivers, which could be due to a lack of awareness of difficulties, lack of knowledge about the existence of services, barriers to accessing services and unsatisfactory service provision [18, 44].
Since it is difficult to correctly record unmet needs within the complex home care situations for PlwD, a self-developed, algorithm-based, computerized needs assessment and intervention management system (IMS) was used in addition to the CANE, as it has a larger number of variables from various questionnaires for detecting unmet needs [11, 22]. The conceptualization of the IMS is published elsewhere [11], was tested in the DelpHi-study [11, 45] and further developed for use in care practice the InDePendent study. The results show that the IMS improves the systematic identification of unmet needs and the subsequent recommendation of measures to address these needs [11]. The IMS functions as a rule-based expert decision support system, utilizing a variety of validated questionnaires, tests, and customized queries (e.g., surveys on the housing situation, use of care aids) to align responses from the PlwD’s perspective to the computerized knowledge base. Unfulfilled needs are automatically identified through a standardized survey, employing algorithm-based trigger conditions preset by the system. Confirmation of these needs is carried out automatically by the system and validated by a DCM or additionally pinpointed by them. For each questionnaire, specific trigger conditions have been meticulously developed and defined, signifying distinct unmet needs. These unmet needs are categorized into the following types: a) medical care needs, b) medication care needs, c) nursing care needs, d) psychosocial care needs, and e) social–legal supply needs. In total, there are n = 115 predefined unmet needs.
Statistical analysis
Descriptive statistics were used to demonstrate the sample characteristics, number, and types of unmet needs. We used Fisher’s exact test and Welch’s t-test, depending on the specific variable being examined, to detect any statistically significant differences between the sexes. Due to the Bonferroni correction [46], the significance level was adjusted to<0.001. To identify associations between sociodemographic and clinical factors and unmet needs, we used multiple Poisson regression models with random effects for treating GPs, representing the clusters of PlwD. The number of unmet needs (from IMS and CANE, respectively) were used as dependent variables and sociodemographic and clinical variables as independent variables. All models were stratified by sex. Missing data on covariates were imputed using multiple imputations by chained different imputation sets were created for each variable (GDS, F-Sozu, MMST, BMI, B-ADL). Therefore, a total of 20 data sets were created. Statistical analyses were performed using StataSE 16 (TX, USA: StataCorp. © 2019) and SPSS Statistics 29 (Armonk, NY, USA: IBM Corp. © 2022).
RESULTS
Significant differences between the sexes were found in relation to age, family status, living alone, caregiver sex, and relationship between caregiver and PlwD at a significance level of p < 0.001.
Sociodemographics
The participating PlwD (n = 417) were on average 80.6 (SD = 6.9) years old and 55.9% of the participants were women. Most PlwD (52.0%) were married or widowed (39.3%), and 44.6% had a caregiver participating in the study. More than half of the participants had children (87.5%), most had less than ten years of school education, and 40% lived alone. The caregivers were, on average, 67.9 years old (SD = 12.4), and 66.3% were female. Detailed statistics of the PlwD and caregiver characteristics are shown in Table 1 and Supplementary Table 3.
Table 1
Sociodemographic and clinical variables of PlwD, separated by sex
| Total sample | Men | Women | p | ||||
| (n = 417) | (n = 184) | (n = 233) | |||||
| Age, mean (SD) | 80.6 | 6.9 | 79.0 | 7.4 | 81.9 | 6.3 | <0.001 |
| Participating relative (yes), n (%) | 186 | 44.6% | 94 | 51.1% | 92 | 39.5% | 0.022 |
| Family Status | n = 414 | n = 183 | n = 231 | <0.001 | |||
| Single, n (%) | 13 | 3.1% | 9 | 4.9% | 4 | 1.7% | |
| Married, n (%) | 217 | 52.0% | 139 | 76.0% | 78 | 33.8% | |
| Divorced, n (%) | 17 | 4.1% | 4 | 2.2% | 13 | 5.6% | |
| Separated, n (%) | 3 | 0.7% | 2 | 1.1% | 1 | 0.4% | |
| Widowed, n (%) | 164 | 39.6% | 29 | 15.9% | 135 | 58.4% | |
| Living alone | n = 416 | n = 184 | n = 232 | <0.001 | |||
| yes, n (%) | 167 | 40.1% | 45 | 24.5% | 122 | 52.6% | |
| Having children | n = 416 | n = 183 | n = 233 | 0.654 | |||
| yes, n (%) | 365 | 87.7% | 159 | 86.9% | 161 | 84.7% | |
| Caregiver availability | n = 411 | n = 180 | n = 231 | 0.696 | |||
| yes, n (%) | 383 | 93.2% | 169 | 93.9% | 214 | 92.6% | |
| Graduation | n = 401 | n = 178 | n = 223 | 0.0032 | |||
| None, n (%) | 15 | 3.7% | 5 | 2.8% | 10 | 4.5% | |
| Up to 10 years, n (%) | 319 | 79.6% | 131 | 73.6% | 188 | 84.3% | |
| Over 10 years, n (%) | 61 | 15.2% | 39 | 21.9% | 22 | 9.9% | |
| Other, n (%) | 6 | 1.5% | 3 | 1.7% | 3 | 1.4% | |
| Financial situation | n = 402 | n = 177 | n = 225 | 1.000 | |||
| Good, n (%) | 324 | 80.6% | 143 | 80.8% | 181 | 80.4% | |
| Cognitive impairment (MMST) | n = 359 | n = 159 | n = 200 | 0.6325 | |||
| Score, mean (SD) | 18.6 | 6.5 | 18.8 | 6.4 | 18.5 | 6.6 | |
| None to mild, n (%) | 180 | 50.1% | 78 | 49.1% | 102 | 51.0 | |
| Moderate, n (%) | 141 | 39.3% | 69 | 43.4% | 72 | 36.0 | |
| Severe, n (%) | 38 | 10.6% | 12 | 7.6% | 26 | 13.0 | |
| Number of diagnosis | n = 412 | n = 182 | n = 230 | 0.7527 | |||
| Score, mean (SD) | 10.8 | 9.3 | 10.6 | 8.5 | 10.9 | 10.0 | |
| Number of medications | n = 412 | n = 183 | n = 229 | 0.0323 | |||
| Score, mean (SD) | 6.7 | 3.6 | 7.1 | 3.9 | 6.3 | 3.4 | |
| Medication | |||||||
| Antidementia treatment, n (%) | 129 | 31.3% | 60 | 32.8% | 69 | 30.1% | 0.594 |
| Antidepressant treatment, n (%) | 80 | 19.4% | 33 | 18.0% | 47 | 20.5% | 0.534 |
| Antipsychotic treatment, n (%) | 88 | 21.4% | 43 | 23.5% | 45 | 19.7% | 0.397 |
| Inappropriate medication (yes), n (%) | 50 | 12.1% | 18 | 9.8% | 32 | 14.0% | 0.1817 |
| Diagnosis | n = 412 | n = 182 | n = 230 | ||||
| Dementia (F01-F03), n (%) | 331 | 80.3% | 174 | 80.8% | 184 | 80.0% | 0.933 |
| Diabetes (E10-E14), n (%) | 129 | 31.3% | 58 | 31.9% | 71 | 30.9% | 0.831 |
| High blood pressure (I10-I15), n (%) | 277 | 67.2% | 124 | 68.1% | 153 | 66.5% | 0.752 |
| Cerebrovascular diseases (I60-I69), n (%) | 77 | 18.7% | 42 | 18.9% | 35 | 18.4% | 0.030 |
| Coronary heart diseases (I20-I25), n (%) | 83 | 20.2% | 43 | 23.6% | 34 | 14.8% | 0.048 |
| Body Mass Index | n = 397 | n = 175 | n = 222 | 0.0654 | |||
| Mean (SD) | 26 | 4,5 | 26.5 | 4.3 | 5.6 | 4.6 | |
| Having a caregrade (yes) | n = 417 | n = 184 | n = 233 | 1.000 | |||
| n, (%) | 279 | 66.9% | 123 | 66.9% | 156 | 67.0% | |
| Quality of life (EQ-5D-5 L) | n = 407 | n = 181 | n = 226 | 0.6264 | |||
| Score, mean (SD) | 0.74 | 0.01 | 0.73 | 0.23 | 0.75 | 0.23 | |
| Functional impairment (B-ADL) | n = 256 | n = 102 | n = 118 | 0.0233 | |||
| Score, mean (SD) | 5.4 | 2.3 | 5.0 | 2.4 | 5.8 | 2.2 | |
| Mobility impairment (DEMMI) | n = 350 | n = 162 | n = 188 | 0.3840 | |||
| Score, mean (SD) | 56.7 | 18.9 | 57.7 | 20.5 | 55.9 | 17.6 | |
| Timed-up-and-go | n = 348 | n = 157 | n = 191 | 0.8666 | |||
| Score, mean (SD) | 27.1 | 31 | 26.8 | 36.2 | 27.4 | 26.1 | |
| Unrestricted mobility, n (%) | 56 | 16.1% | 27 | 17.2% | 29 | 15.2% | |
| Mobility impairment without relevance to everyday life, n (%) | 124 | 35.6% | 60 | 38.2% | 64 | 33.5% | |
| Restricted mobility with relevance to everyday life, n (%) | 65 | 18.7% | 33 | 21.0% | 32 | 16.8% | |
| Pronounced mobility impairment, n (%) | 103 | 29.6% | 37 | 23.6% | 66 | 34.6% | |
| Depression (GDS) | n = 341 | n = 148 | n = 193 | 0.9833 | |||
| Score, mean (SD) | 3.6 | 3 | 3.6 | 3.0 | 3.6 | 3.1 | |
| No depressive symptoms, n (%) | 281 | 82.4% | 121 | 81.8% | 160 | 82.9% | |
| Mild depression, n (%) | 43 | 12.6% | 21 | 14.2% | 22 | 11.4% | |
| Severe depression, n (%) | 17 | 5.0% | 6 | 4.1% | 11 | 5.7% | |
| Utilization (FIMA) | |||||||
| Medical (last 3 months) | n = 400 | n = 175 | n = 225 | ||||
| General Practitioner, n (%) | 365 | 91.3% | 161 | 92.0% | 204 | 90.7% | 0.723 |
| n = 399 | n = 175 | n = 224 | |||||
| Neurologist/ Psychiatrist, n (%) | 127 | 31.8% | 64 | 36.6% | 63 | 28.1% | 0.083 |
| n = 402 | n = 177 | n = 225 | |||||
| Ambulatory care, n (%) | 28 | 7.0% | 13 | 7.3% | 15 | 6.7% | 0.845 |
| Therapeutic | n = 401 | n = 177 | n = 224 | ||||
| Physiotherapy, n (%) | 90 | 22.4% | 51 | 28.8% | 39 | 17.4% | 0.008 |
| n = 402 | n = 176 | n = 226 | |||||
| Occupational therapy, n (%) | 44 | 11.0% | 27 | 15.3% | 17 | 7.5% | 0.015 |
| Speech therapy, n (%) | 9 | 2.2% | 7 | 4.0% | 2 | 0.9% | 0.046 |
| n = 402 | n = 175 | n = 227 | |||||
| Ambulant nursing service, n (%) | 152 | 37.8% | 52 | 29.7% | 100 | 44.1% | 0.004 |
| n = 389 | n = 173 | n = 216 | |||||
| Relatives, n (%) | 243 | 62.5% | 99 | 57.2% | 144 | 66.7% | 0.059 |
| n = 401 | n = 176 | n = 225 | |||||
| Semi-stationary care facility, n (%) (last 12 months) | 50 | 12.5% | 25 | 14.2% | 25 | 11.1% | 0.365 |
| n = 404 | n = 177 | n = 227 | |||||
| Day clinic, n (%) | 14 | 3.5% | 6 | 3.4% | 8 | 3.5% | 1.000 |
| n = 406 | n = 178 | n = 228 | |||||
| Stationary treatment, n (%) | 80 | 19.7% | 34 | 19.1% | 46 | 20.2% | 0.803 |
| Resource Utilization (RUD) - relatives’ perspective | n = 138 | n = 73 | n = 65 | ||||
| ADL, mean (SD) | 60.4 | 69.8 | 63.2 | 74.4 | 57.2 | 64.6 | 0.6159 |
| n = 165 | n = 81 | n = 84 | |||||
| IADL, mean (SD) | 73.8 | 66.4 | 83.5 | 64.0 | 64.5 | 67.6 | 0.0665 |
| n = 100 | n = 49 | n = 51 | |||||
| Supervision, mean (SD) | 82.9 | 152.5 | 100.1 | 166.5 | 66.3 | 137 | 0.2709 |
| Social support (F-SozU) | n = 358 | n = 156 | n = 202 | 0.3694 | |||
| Mean (SD) | 3.9 | 0.5 | 3.9 | 0.5 | 3.8 | 0.5 | |
| Unmet needs PlwD (CANE) –PlwD perspective | n = 242 | n = 96 | n = 146 | 0.9927 | |||
| Mean (SD) | 2.3 | 2.6 | 2.3 | 2.6 | 2.3 | 2.6 | |
| Unmet needs PlwD (CANE) –relatives’ perspective | n = 184 | n = 92 | n = 92 | 0.1025 | |||
| Mean (SD) | 2.5 | 2.8 | 2.2 | 2.5 | 2.8 | 3.1 | |
| Unmet needs (CANE) –both perspectives | n = 414 | n = 182 | n = 232 | 0.4140 | |||
| Mean (SD) | 2.4 | 2.7 | 2.3 | 2.6 | 2.5 | 2.8 | |
| Total unmet needs | 985 | 505 | 480 | ||||
| Unmet needs PlwD (IMS) –PlwD perspective | n = 417 | n = 184 | n = 233 | 0.0238 | |||
| Mean (SD) | 13.9 | 5.0 | 14.5 | 5.0 | 13.4 | 5.0 | |
| Caregiver sex (female) | n = 188 | n = 94 | n = 94 | <0.001 | |||
| n, (%) | 125 | 66.5% | 89 | 94.7% | 36 | 38.3% | |
| Caregiver age | n = 185 | n = 91 | n = 94 | 0.0137 | |||
| Mean (SD) | 67.8 | 12.5 | 70.0 | 11.3 | 65.6 | 13.3 | |
| Caregiver burden (ZARIT) | n = 179 | n = 87 | n = 92 | 0.4120 | |||
| Mean (SD) | 24.0 | 13.8 | 24.9 | 13.8 | 23.2 | 13.9 | |
MMST, Mini-Mental-Status-Test, range 0–30, higher score indicates better cognitive functioning; B-ADL, Bayer Activities of Daily Living Scale, range 0–10, lower score indicates better performance; GDS, Geriatric Depression Scale, sum score 0–15, score≥5 indicates depression; F-SozU, range 0–5; higher score indicates better social support; ZARIT; range 0–88; higher scores indicates greater caregiver burden, EQ-5D-5 L; range 0-1; higher score indicates better health-related quality of life. RUD: examines 3 domains: ADL, activities of daily living (such as personal hygiene, eating, dressing), 2) instrumental ADL (such as shopping, meal preparation, housekeeping) and 3) supervision (such as preventing dangerous events), data in hours per month. p-values to test for significant differences between men and women.
Clinical characteristics
Half of the PlwD (50.1%) had an MMST score between 20 and 30, which indicates mild cognitive impairment. 39.3% had a moderate, and 10.6% severe cognitive impairment. The participants had, on average, 10.8 diagnoses (SD = 9.3). 66.5% of the PlwD were formally diagnosed with dementia (all others were positively screened for dementia without being formally diagnosed), 31.3% suffered from diabetes, 67.2% from hypertension, 18.7% from cerebrovascular disease and 20.2% from coronary heart disease. The PlwD took, on average, seven medications (SD = 3.6) regularly. At baseline, 31.3% of the participants received antidementia drugs, 19.4% antidepressants and 21.4% antipsychotics. According to the Priscus List, 12.1% of the PlwD received inappropriate medication. These administered medications include, for example, the antidepressant amitriptyline, the urological drug solifenacin or the antipsychotic diazepam.
The average EQ-5D-5 L index score was 0.74. Most PlwD have no mobility restriction relevant to everyday life (35.6%), closely followed by those with severely limited mobility (29.6%). According to the GDS score (3.6, SD = 3.0), most participants (73.0%) had no depression, 18% had mild depression, and 2.6% had severe depression.
Use of care services and informal care
In the three months before data assessment, 91.3% of the PlwD had consulted a GP and 31.8% a neurologist/psychologist. Almost a quarter of the participants visited a physiotherapist (22.4%), 11.0% received ergotherapy and 2.2% speech therapy. 37.8% of the PlwD received ambulant nursing service. Help from family members, friends, acquaintances or neighbours was received by 62.5% of the PlwD.
In the 30 days before data assessment, caregivers spent, on average, 60 hours per month (2 hours per day) supporting PlwD in their daily tasks, such as personal hygiene or eating. 74 hours (2.5 hours per day) were spent supporting PlwDs’ instrumental activities of daily living, such as shopping or housekeeping, and 83 hours (2.8 hours per day) supervising the PlwD.
PlwD perceived solid social support from their environment (mean F-Sozu = 3.9), and the caregivers had, on average, a relatively low subjective burden (mean Zarit Burden = 24.0).
Unmet needs
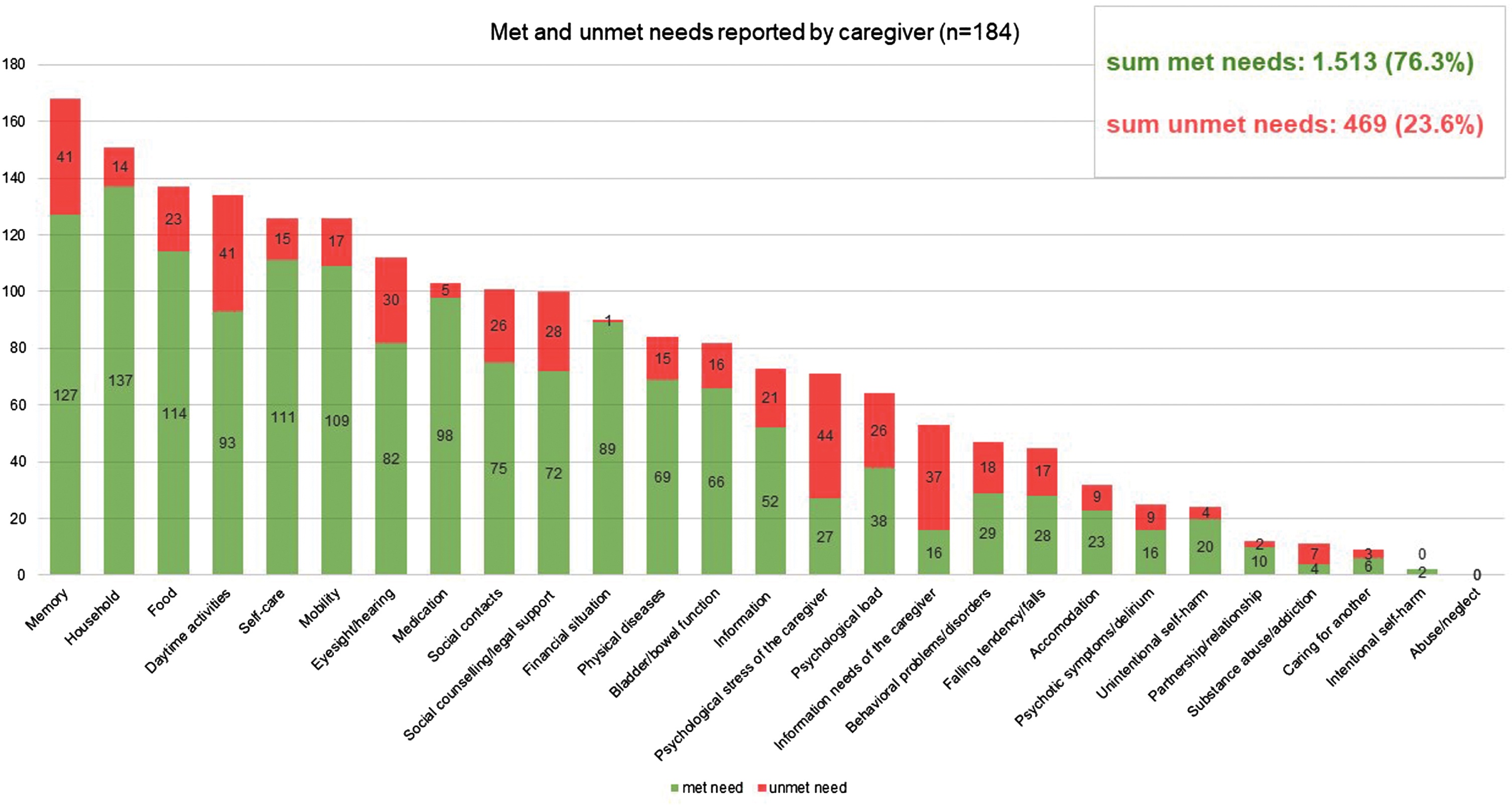
985 unmet needs among PlwD were detected using the CANE (sum of self-report by PlwD and proxy by caregivers). On average, 2.3 unmet needs (SD = 2.6) were assessed among n = 242 PlwD, and 2.5 unmet needs (SD = 2.8) among n = 184 caregivers. There are no significant differences between the sexes in terms of the number of unmet needs reported. The distribution of self- and proxy-assessed met and unmet needs set by CANE is shown in Figs. 2 and 3, respectively. If we look at the perspective PlwD and caregivers together and examine which three CANE areas result in the most unmet needs, then the areas “memory” and “daily activities” reveal the most unmet needs for both men and women—closely followed by the category “social contacts” for women and “social counselling/legal support” for men. 62,5% of PlwD had at least one unmet need. Referring to the total number of unmet and met needs across all domains calculated for the person level (mean = 7.7 reported by PlwD, mean = 10.7 reported by caregiver), unmet needs accounted for 27.2% from the PlwD perspective and 23.6% from the caregiver perspective of the total.
Fig. 2
Mean number of met and unmet needs per PlwD reported by PlwD; CANE questionnaire.
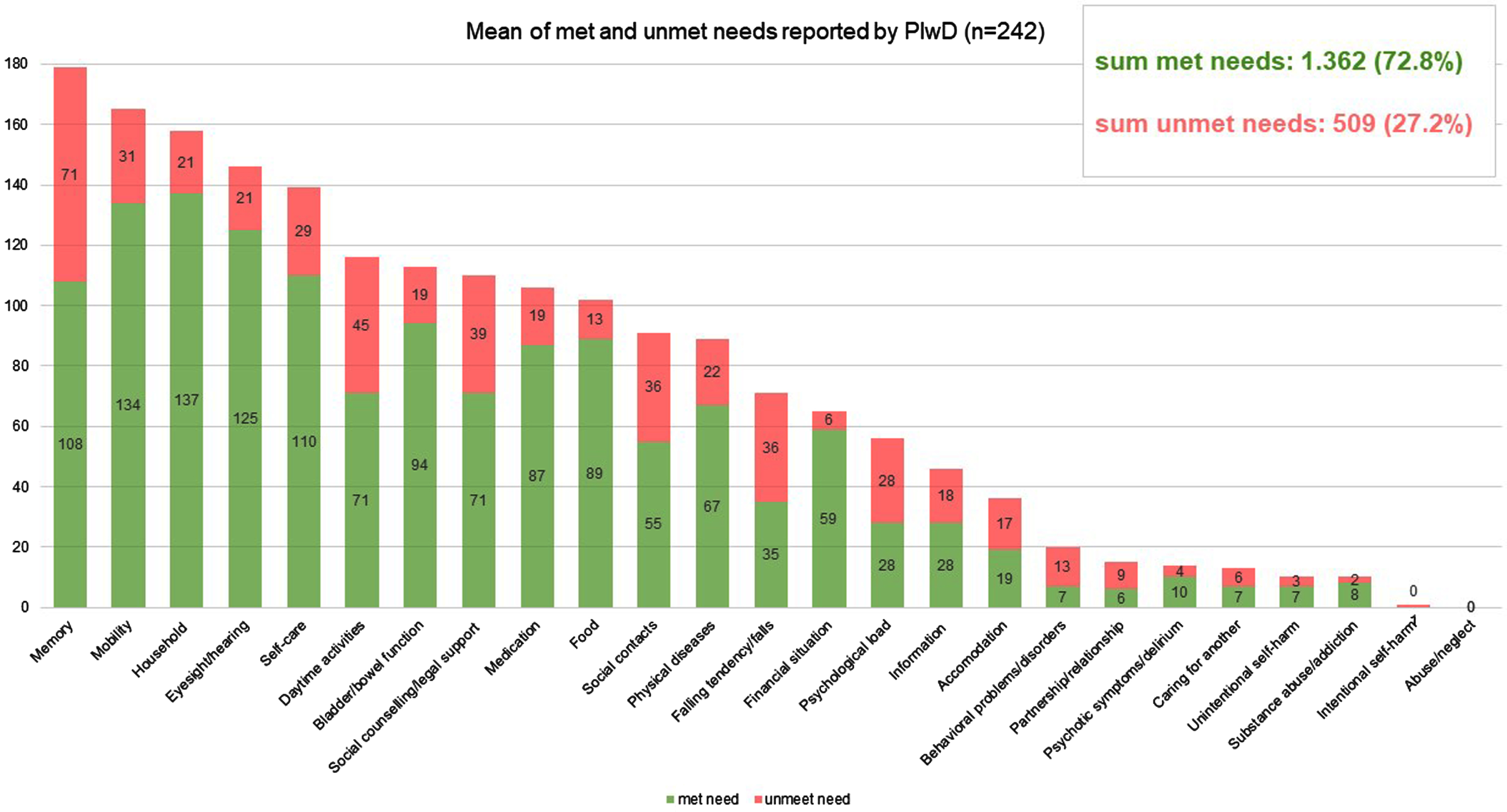
Fig. 3
Mean number of met and unmet needs per PlwD reported by caregiver; CANE questionnaire.
The IMS revealed an average of 13.9 unmet needs (SD = 5.0) for PlwD (PlwD perspective).
Characteristics associated with unmet needs
The results of the multivariate regression models are demonstrated in Table 2. Most variables used had low missing values (maximum 4% missing values). Imputations were carried out for the following variables as they had many missing values: BMI (5%), F-SozU (14%), MMST (14%), GDS (18%), and B-ADL (39%).
Table 2
Multiple regression models to identify determinants for unmet needs CANE and IMS, separately for men and women
| Unmet needs (CANE)* | Unmet needs (IMS)* | |||||||
| Female1 | Male2 | Female3 | Male4 | |||||
| β | SE | β | SE | β | SE | β | SE | |
| Patient age (years) | –0.01 | 0.01 | –0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 |
| Functional impairment (B-ADL) | 0.13*** | 0.03 | 0.12*** | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 |
| Social support (F-Sozu) | –0.23* | 0.11 | –0.41** | 0.13 | 0.02 | 0.05 | –0.11* | 0.05 |
| Depression (GDS) | 0.08*** | 0.02 | –0.04 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 |
| Cognitive impairment (MMST) | 0.02* | 0.01 | 0.04*** | 0.01 | –0.00 | 0.00 | 0.00 | 0.00 |
| Body Mass Index (BMI, Kg/m2) | –0.02 | 0.01 | –0.01 | 0.01 | 0.01** | 0.00 | 0.01* | 0.01 |
| Living alone (Ref. not alone) | 0.27* | 0.42 | 0.09 | 0.13 | 0.02 | 0.04 | –0.09 | 0.05 |
| Caregiver availability (Ref. No) | 0.24 | 0.10 | 0.26 | 0.26 | 0.04 | 0.08 | 0.08 | 0.10 |
| Lower sec. educ. (Ref. Primary education) | –0.55*** | 0.13 | –0.31* | 0.15 | 0.00 | 0.05 | –0.15** | 0.05 |
| Higher sec. educ. (Ref. Primary education) | –0.11 | 0.15 | –0.31* | 0.14 | 0.15* | 0.07 | –0.11 | 0.06 |
| Financial situation (Ref. not good) | 0.21 | 0.12 | –0.15 | 0.14 | –0.11* | 0.05 | 0.08 | 0.06 |
| GP visit last 3 months (Ref. no) | –0.48** | 0.14 | –0.29 | 0.17 | –0.05 | 0.06 | 0.06 | 0.07 |
| Neurologists visit last 3 months (Ref. No) | 0.42*** | 0.10 | 0.09 | 0.12 | –0.06 | 0.05 | –0.01 | 0.05 |
| General health (EQ-5D-5 L index) | –0.10 | 0.23 | –0.81** | 0.28 | –0.41*** | 0.09 | –0.50*** | 0.11 |
| Having a care grade (Ref. no) | –0.62*** | 0.10 | –0.47*** | 0.12 | –0.07 | 0.05 | 0.02 | 0.05 |
| Number of diagnoses | –0.01 | 0.01 | –0.02* | 0.01 | 0.01** | 0.00 | 0.01 | 0.00 |
| Number of drugs taken | 0.01 | 0.02 | 0.04* | 1.04 | 0.01 | 0.01 | –0.00 | 0.01 |
MMST, Mini-Mental-Status-Test, range 0–30, higher score indicates better cognitive functioning; B-ADL, Bayer Activities of Daily Living Scale, range 0–10, lower score indicates better performance; GDS, Geriatric Depression Scale, sum score 0–15, score≥5 indicates depression; F-SozU, range 0–5; higher score indicates better social support; ZARIT; range 0–88; higher scores indicates greater caregiver burden, EQ-5D-5 L; range 0-1; higher score indicates better health-related quality of life. RUD, examines 3 domains: ADL, activities of daily living (such as personal hygiene, eating, dressing), 2) instrumental ADL (such as shopping, meal preparation, housekeeping) and 3) supervision (such as preventing dangerous events). p* < 0.05; p** < 0.01; p*** < 0.001 tested for each independent variable with the dependent variable (CANE or IMS), separately by sex. Poisson regression models with random effects for treating General Practitioners, representing the clusters of PlwD. β, regression coefficient; SE, standard error; Model1: n = 206, Pseudo R2 = 0.1703, p < 0.001; Model2: n = 163, Pseudo R2 = 0.0934, p < 0.001; Model3: n = 206, Pseudo R2 = 0.0740, p < 0.001; Model4: n = 165, Pseudo R2 = 0.1005, p < 0.001.
Unmet needs (CANE)
For both sexes, high social support (F-SozU), higher education, and having a care grade were associated with lower unmet needs in CANE. Similarly, a better cognitive status (MMST), a higher BMI, and increased functional limitation (B-ADL) were statistically significantly associated with higher unmet needs in both sexes.
In females living alone (β= 0.27, p < 0.05), the presence of depression (GDS, β= 0.08, p < 0.001) and visiting a neurologist within the last three months (β= 0.42, p < 0.001) were associated with higher unmet needs. In contrast, seeing a GP (β= –0.48, p < 0.005) emerged as a positive determinant among women.
In men, a better health-related quality of life (EQ-5D-5 L, β= –0.81, p < 0.005) and an increasing number of diagnoses (β= –0.04, p < 0.005) indicated fewer unmet needs, while a higher number of medications taken (β= 0.04, p < 0.05) was statistically significantly associated with an increased number of unmet needs.
Unmet needs (IMS)
Regarding the IMS, we also identified a better general health status (EQ-5D-5 L index) to be a positive determinant both in men (β= –0.41, p < 0.001) and women (β= –0.5, p < 0.001). Increased BMI is related to increased unmet needs for both women (β= 0.01, p < 0.01) and men (β= 0.01, p < 0.05).
In women, significant associations were found between the increasing number of diagnoses (β= 0.01, p < 0.01) and higher unmet needs; and a good financial situation (β= –0.11, p < 0.05) and lower unmet needs.
In males, there were associations between better social support (F-SozU, β= –0.11, p < 0.05) and higher education (β= –0.15, p < 0.005) and lower unmet needs.
DISCUSSION
This paper aimed to describe the InDePendent study sample of community-dwelling PlwD, their existing unmet needs and associations with clinical and sociodemographic parameters. The mean age was slightly over 80 years, and more than half of the participants were female, most had children, and were married or widowed. Most of the sample consisted of PlwD with no depression. Participants had an average of 10.8 diagnoses, and took an average of 6.7 medications. Using CANE, an average of 2.4 unmet needs (PlwD and caregiver perspective), and using the IMS (PlwD perspective), an average of 13.9 unmet needs were detected. Regardless of sex, a high BMI, low social support, a low education, not having a care grade, prevalent functional and cognitive impairment, and worse health were associated with more unmet needs. In women, depressive symptoms, increasing number of diagnoses, having visited a neurologist within the last three months, poor financial situation, and living alone were associated with higher unmet needs. In contrast, regular contact with a GP was associated with lower unmet needs. In men, however, unmet needs increased with more medications taken and decreased with more diagnoses.
Clinical characteristics
A large part of the sample consisted of PlwD with mild to moderate dementia (according to the MMST score), which is to be expected in primary care settings and is mainly in line with findings from Thyrian et al., who screened community-dwelling people aged over 70 years for dementia in GP practices [5]. Nevertheless, only two-thirds of study participants had a dementia diagnosis at baseline, potentially indicating an underestimation of dementia diagnoses and likely leading to a gap in healthcare for PlwD. On the other hand, this may also have been caused by the sensitivity and specificity of the MMST and the fact that people with mild cognitive impairment, in particular, may be less likely to be reliably detected by the test [47].
The results demonstrate that PlwD are often affected by multimorbidity and multiple medications, which is confirmed by the high utilization of healthcare services. Most PlwD had consulted a GP, almost one-third a neurologist/psychologist in the three months before the assessment, and nearly one-fifth had been hospitalized for inpatient treatment in the 12 months prior to the assessment, demonstrating a high burden of dementia disease on the medical system.
Unmet needs
Our findings confirm previous research [12–17, 48] and demonstrated a high disease load with a total number of 985 unmet needs in community-dwelling PlwD. PlwD (self-report, n = 242) had on average 2.3 unmet needs (CANE) and informal caregivers (n = 184) assessed on average 2.5 unmet needs (CANE) about their caregiver (in line with other study results [17, 18]). Nevertheless, the results differ from Khanassov et al. [16] and Kerpershoek et al. [13], who reported lower unmet needs among PlwD and Hancock et al. [49], who reported higher unmet needs. These variations in the results could be attributed to disparities in the characteristics of the samples used.
More than half of the participants (62.5%) showed at least one unmet need, which is a smaller proportion compared to what Black et al. [12] demonstrated in community-residing PlwD living in the US (99% had at least one unmet need). One possible explanation for this discrepancy could be related to the recruitment setting in our study through practices engaged in participating networks that likely have improved the communication among healthcare professionals. This could have resulted in more frequent and earlier recognition of unmet needs compared to an “average” GP practice.
In addition, we found unmet needs in almost every domain of the CANE (Figs. 2 and 3). This leads us to the conclusion that current primary care setting has potential to improve interdisciplinary care for PlwD. Eichler et al. [14] also found unmet needs (assessed with an earlier version of the IMS) in all predefined subcategories. Thus, PlwD and their caregivers are relevant target groups for individualized interventions.
Characteristics associated with unmet needs
The study demonstrated that unmet needs among PlwD and their caregivers are mainly predicted by predisposing, enabling and need factors.
Higher functional impairment (B-ADL score) was associated with a significant increase in unmet needs, which could be expected for the study population. In addition, 69.3% of the PlwD who are high functional impaired had a care grade. We created a contingency table between B-ADL and RUD and found that 81.8% of caregivers of severely physically impaired PlwD reported caring for their PlwD for many hours per month (at least 50 hours per month). Despite the relatively frequent availability of informal and formal care, increased physical decline is often accompanied by increasing unmet needs [17].
Better social support (F-SozU score) was positively associated with fewer unmet needs. These results agree with the findings of Miranda-Castillo et al. [17]. They found that PlwD living in small social networks (one to six members) had significantly more unmet needs than individuals living in high-community involvement networks (>seven members, locally integrated). This result demonstrates the high demand for social contact when coping with dementia.
Better cognitive functioning (MMST score) was associated with a significant increase of unmet needs, which may seem counterintuitive at first glance, but is in line with Black et al. [48]. One possible implication of this finding is that the needs of individuals with mild cognitive impairment, especially those in the early stages of dementia, are frequently overlooked or not adequately recognized. Many unmet needs often arise especially at the beginning of cognitive symptoms and shortly after a dementia diagnosis, such as the absence of a care grade. We found that only 57.8% of the PlwD in our sample with mild cognitive impairment had a care grade. When looking at informal care hours (RUD), we also found that these PlwD tended to be in the middle range and were rarely reported as very high for this group of PlwD. Therefore, unmet needs are likely not initially recognized even in our sample, and consequently, the right level of formal and informal support is not provided. Other reasons why formal support services are not used could include, for example, lack of availability or knowledge about their existence [17]. As the disease progresses, identifying unmet needs often becomes more challenging, as PlwD may no longer be able to express themselves. Therefore, early detection of dementia is essential to influence the quality of care positively [50].
Lower education among PlwD was identified as a characteristic associated with a significantly higher number of unmet needs, aligning with results reported in the literature [48, 51]. Moreover, the literature highlights that also a lower caregiver education significantly determines more unmet needs among PlwD [12, 48]. As a possible explanation, education could serve as an enabling factor that facilitates obtaining information about and access to dementia-related health services [12, 48].
Better general health (EQ-5D-5 L index) was found to be a positive determinant for a lower number of unmet needs, which is generally in line with the literature [52]. When overall health is poor, standardized assessments can quickly identify unmet needs. Although such assessments are not routinely conducted in healthcare, for instance, during doctor’s visits, they could be integrated into daily medical practice to swiftly identify patients experiencing issues.
The presence of a care grade proved to be an essential factor, as it was associated with lower unmet needs. This can be explained by the fact that the care grade is associated with support services (such as access to professional help, financial support, and consulting services) which can help to reduce the need for care.
The relationship between an increased BMI and higher numbers of unmet needs may be explained by the fact that overweight individuals have poorer scores in physical and social function, self-care, depression, life satisfaction and quality of life [53], and these multiple problems may lead to more needs that remain unmet.
Depression (GDS) is one of the most frequently observed comorbidities among PlwD [54, 55] and the elderly population in general [56]. Studies showed that depression was significantly associated with unmet needs among individuals living in the community [12, 15, 49]. However, we found depression as a significant determinant for unmet needs only in women. This could be explained by the fact that women with dementia live alone more often (female: 73.0%, male: 27.0%) and that living alone predicts depression [57]. Person-centred interventions demonstrate the potential to alleviate symptoms of depression effectively [58], and collaborative approaches are especially effective for women living alone to increase their quality of life [59]. Consequently, early detection of depression symptoms is crucial for managing the disease and should be emphasized in dementia-focused interventions.
The consultation of a GP within the last three months was also found to be a significant determinant for less unmet needs in women. A possible explanation might be that women tend to make more frequent doctor visits (possibly also because they live alone more often [59]) and generally display a higher level of health awareness than men [60, 61]. In addition, a correlation was shown between women living alone and higher unmet needs. Another study could only show the link between living alone and higher unmet needs for both sexes combined, but did not examine separately by sex [12].
The association between female PlwD who reported being in an excellent financial situation and lower unmet needs can be explained by the fact that income is a factor in obtaining information and accessing dementia-related services. The literature confirms this association when both sexes are considered together [12].
Only men show a correlation between the number of medications taken and increased unmet needs. Literature suggests that taking many medications leads to a high risk of drug interactions for PlwD [62]. This could lead to higher unmet needs.
To our knowledge, this is the first study that provided determinants for unmet needs (according to CANE and IMS, respectively) stratified by patient sex in community-dwelling PlwD in Germany. In general, some determinants differ between patient sex, which leads to the recommendation that interventions in PlwD pay sufficient attention to patient sex to provide gender-sensible strategies in the future. In conclusion, our results provide evidence and quantify the diverse needs (medical, financial, and social) of PlwD living at home and emphasize the need for innovative individualized approaches adressing these needs. The outcomes of our study provide empirical support for challenges in the current healthcare provision for PlwD. Our findings also highlight the urgent need for PlwD and their caregivers to communicate with experts concerning the coordination of dementia care. Future research should focus more on differentiating the individual areas of unmet need to identify areas that would otherwise go unrecognized to develop innovative treatments and personalized interventions.
Limitations
The study sample was recruited within five physician and dementia networks, which are likely characterized by a higher communication standard among participating healthcare professionals compared to routine healthcare. Due to these more effective cooperative structures within the physician networks, advantages in terms of provision of dementia-specific medications and referrals to specialists [6], it is likely that the number of unmet needs identified may be lower compared to PlwD and their caregivers who are treated in separate primary care practices. Thus, the generalizability of the results may be somewhat limited. In addition, all assessments are based on self-reports of PlwD or their caregiver, which could lead to an over- or underestimation concerning specific unmet needs and, therefore, would also limit the external validity of the results.
The significant differences between the drop-outs and the PlwD who started the baseline interview in terms of the physician network cluster and the randomization group can be explained, on the one hand, by the fact that a larger proportion of PlwD who are randomized to the waiting control group and thus learn that they will receive the intervention six months later drop out of the study early. Second, the specific physician network clusters may have influenced the number of early drop-outs, as recruitment behaviour, structures within the network, physician-patient relationships, and motivation within network physicians might differ across these. Due to the nature of the intervention, it was unfeasible to implement blinding among participating physicians. Consequently, we cannot exclude that some participating physicians may have altered their recruitment behaviour upon becoming aware of their assigned group.
Whether met or unmet, care needs indicate the importance of individualized and targeted care services. This is more than just a one-time task. Both the PlwD and their caregiver need to be prospectively monitored, as due to the progressive course of dementia, any need that has been fulfilled may later transform into an unfulfilled need again.
AUTHOR CONTRIBUTIONS
Annelie Scharf (Formal analysis; Writing – original draft); Fabian Kleinke (Formal analysis; Writing – original draft); Bernhard Michalowsky (Conceptualization; Methodology; Writing – review & editing; co-principal investigator); Anika Rädke (Project administration; Writing – review & editing; study coordinator); Stefanie Pfitzner (data monitoring); Franka Mühlichen (Writing – review & editing); Maresa Buchholz (Writing – review & editing); Neeltje van den Berg (Conceptualization; Writing – review & editing; Evaluation); Wolfgang Hoffmann (Conceptualization; Supervision; Writing – review & editing; principal investigator).
ACKNOWLEDGMENTS
We would like to thank all participating patients, their caregivers, and the participating general practitioners and specialists for their most valued collaboration.
FUNDING
The study is funded by the Innovation Fund at the Federal Joint Committee (G-BA Grand No: 01NVF18034). The funders have had no influence on the conceptualization and conduct of the study and will not have any role in the data analysis and publication of the results.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Information about the data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
{ label (or @symbol) needed for fn } The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-231173.
REFERENCES
[1] | Blotenberg I , Hoffmann W , Thyrian JR ((2023) ) Demenz in Deutschland: Epidemiologie und präventionspotenzial. Dtsch Arztebl Int 120: , 470–476. |
[2] | Prince M , Guerchet M , Prina M (2015) The Epidemiology and Impact of Dementia - Current State and Future Trends. WHO Thematic Briefing. |
[3] | Gauthier S , Webster C , Sernaes S , Morais JA , Rosa-Neto P (2022) World Alzheimer Report 2022. Life after diagnosis: Navigating treatment, care and support . Alzheimer’s Disease International, London. |
[4] | Jessen F , Wiese B , Bickel H , Eiffländer-Gorfer S , Fuchs A , Kaduszkiewicz H , Köhler M , Luck T , Mösch E , Pentzek M , Riedel-Heller SG , Wagner M , Weyerer S , Maier W , van den Bussche H ,AgeCoDe Study Group ((2011) ) Prediction of dementia in primary care patients. PLoS One 6: , e16852. |
[5] | Thyrian JR , Eichler T , Michalowsky B , Wucherer D , Reimann M , Hertel J , Richter S , Dreier A , Hoffmann W ((2016) ) Community-dwelling people screened positive for dementia in primary care: A comprehensive, multivariate descriptive analysis using data from the DelpHi-Study. J Alzheimers Dis 52: , 609–617. |
[6] | Köhler L , Meinke-Franze C , Hein J , Fendrich K , Heymann R , Thyrian JR , Hoffmann W ((2014) ) Does an interdisciplinary network improve dementia care? Results from the IDemUck-study. Curr Alzheimer Res 11: , 538–548. |
[7] | Thyrian JR ((2017) ) Menschen mit Demenz in der primärärztlichen Versorgung. Z Gerontol Geriatr 50: , 32–38. |
[8] | McIntyre D , Mooney G , Jan S (2009) Need: What is it and howdo we measure it? In Oxford Textbook of Public Health, 5th edition, Detels R, Beaglehole R, Lansang MA, Gulliford M, eds. Oxford University Press, pp. 1535-1548. |
[9] | Stein J , Dorow M , Liegert P , Pabst A , Riedel-Heller S (2019)Camberwell Assessment of Need for the Elderly —CANE. Handbuch füur die adaptierte deutsche Version, Leipzig. |
[10] | Black BS , Johnston D , Handel S , Morrison A , Robbins B , Rye R (2008) Manual for the Johns Hopkins Dementia Care Needs Assessment (JHDCNA). Baltimore, MD. |
[11] | Eichler T , Thyrian JR , Fredrich D , Köhler L , Wucherer D , Michalowsky B , Dreier A , Hoffmann W ((2014) ) The benefits of implementing a computerized intervention-management-system (IMS) on delivering integrated dementia care in the primary care setting. Int Psychogeriatr 26: , 1377–1385. |
[12] | Black BS , Johnston D , Rabins P V , Morrison A , Lyketsos C , Samus QM ((2013) ) Unmet needs of community-residing persons with dementia and their informal caregivers: Findings from the maximizing independence at home study. J Am Geriatr Soc 61: , 2087–2095. |
[13] | Kerpershoek L , de Vugt M , Wolfs C , Woods B , Jelley H , Orrell M , Stephan A , Bieber A , Meyer G , Selbaek G , Handels R , Wimo A , Hopper L , Irving K , Marques M , Gonçalves-Pereira M , Portolani E , Zanetti O , Verhey F ((2018) ) Needs and quality of life of people with middle-stage dementia and their family carers from the European Actifcare study. When informal care alone may not suffice. Aging Ment Health 22: , 897–902. |
[14] | Eichler T , Thyrian JR , Hertel J , Richter S , Wucherer D , Michalowsky B , Teipel S , Kilimann I , Dreier A , Hoffmann W ((2016) ) Unmet needs of community-dwelling primary care patients with dementia in Germany: Prevalence and correlates. J Alzheimers Dis 51: , 847–855. |
[15] | Stein J , Pabst A , Weyerer S , Werle J , Maier W , Heilmann K , Scherer M , Stark A , Kaduszkiewicz H , Wiese B , Mamone S , König H-H , Bock J-O , Riedel-Heller SG ((2016) ) The assessment of met and unmet care needs in the oldest old with and without depression using the Camberwell Assessment of Need for the Elderly (CANE): Results of the AgeMooDe study. J Affect Disord 193: , 309–317. |
[16] | Khanassov V , Rojas-Rozo L , Sourial R , Yang XQ , Vedel I ((2021) ) Needs of patients with dementia and their caregivers in primary care: Lessons learned from the Alzheimer plan of Quebec. BMC Fam Pract 22: , 186. |
[17] | Miranda-Castillo C , Woods B , Galboda K , Oomman S , Olojugba C , Orrell M ((2010) ) Unmet needs, quality of life and support networks of people with dementia living at home. Health Qual Life Outcomes 8: , 132. |
[18] | Bakker C , de Vugt ME , van Vliet D , Verhey FRJ , Pijnenburg YA , Vernooij-Dassen MJFJ , Koopmans RTCM ((2014) ) The relationship between unmet care needs in young-onset dementia and the course of neuropsychiatric symptoms: A two-year follow-up study. Int Psychogeriatr 26: , 1991–2000. |
[19] | Nebel RA , Aggarwal NT , Barnes LL , Gallagher A , Goldstein JM , Kantarci K , Mallampalli MP , Mormino EC , Scott L , Yu WH , Maki PM , Mielke MM ((2018) ) Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement 14: , 1171–1183. |
[20] | Eikelboom WS , Pan M , Ossenkoppele R , Coesmans M , Gatchel JR , Ismail Z , Lanctôt KL , Fischer CE , Mortby ME , van den Berg E , Papma JM ((2022) ) Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: A meta-analysis. Alzheimers Res Ther 14: , 48. |
[21] | Forbes DA , Jansen SL , Markle-Reid M , Hawranik P , Morgan D , Henderson S , Leipert B , Peacock S , Kingston D ((2008) ) Gender differences in use and availability of home and community-based services for people with dementia. Can J Nurs Res 40: , 39–59. |
[22] | Kleinke F , Michalowsky B , Rädke A , Platen M , Mühlichen F , Scharf A , Mohr W , Penndorf P , Bahls T , van den Berg N , Hoffmann W ((2022) ) Advanced nursing practice and interprofessional dementia care (InDePendent): Study protocol for a multi-center, cluster-randomized, controlled, interventional trial. Trials 23: , 290. |
[23] | Kalbe E , Kessler J , Calabrese P , Smith R , Passmore AP , Brand M , Bullock R ((2004) ) DemTect: A new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry 19: , 136–143. |
[24] | Thyrian JR , Fiß T , Dreier A , Böwing G , Angelow A , Lueke S , Teipel S , Fleßa S , Grabe HJ , Freyberger HJ , Hoffmann W ((2012) ) Life- and person-centred help in Mecklenburg-Western Pomerania, Germany (DelpHi): Study protocol for a randomised controlled trial. Trials 13: , 56. |
[25] | EQ-5D-5Lhttps://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. |
[26] | Ludwig K , Graf von der Schulenburg J-M , Greiner W ((2018) ) German Value Set for the EQ-5D-5L. Pharmacoeconomics 36: , 663–674. |
[27] | Gerlinger C , Bamber L , Leverkus F , Schwenke C , Haberland C , Schmidt G , Endrikat J ((2019) ) Comparing the EQ-5D-5L utility index based on value sets of different countries: Impact on the interpretation of clinical study results. BMC Res Notes 12: , 18. |
[28] | Marten O , Greiner W ((2021) ) EQ-5D-5L reference values for the German general elderly population. Health Qual Life Outcomes 19: , 76. |
[29] | Hindmarch I , Lehfeld H , de Jongh P , Erzigkeit H ((1998) ) The Bayer Activities of Daily Living Scale (B-ADL). Dement Geriatr Cogn Disord 9: (Suppl 2), 20–26. |
[30] | Erzigkeit H , Lehfeld H , Peña-Casanova J , Bieber F , Yekrangi-Hartmann C , Rupp M , Rappard F , Arnold K , Hindmarch I ((2001) ) The Bayer-Activities of Daily Living Scale (B-ADL): Results from a validation study in three European countries. Dement Geriatr Cogn Disord 12: , 348–358. |
[31] | Braun Christian TG ((2013) ) Assessment: De Morton Mobility Index (DEMMI) - Mobilitätstest in der Geriatrie. Ergopraxis 6: , 35–37. |
[32] | Podsiadlo D , Richardson S ((1991) ) The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39: , 142–148. |
[33] | Ortega-Bastidas P , Gómez B , Aqueveque P , Luarte-Martínez S , Cano-de-la-Cuerda R ((2023) ) Instrumented Timed Up and Go Test (iTUG)-more than assessing time to predict falls: A systematic review. Sensors (Basel) 23: , 3426. |
[34] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M ((1982) ) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: , 37–49. |
[35] | Shin C , Park MH , Lee S-H , Ko Y-H , Kim Y-K , Han K-M , Jeong H-G , Han C ((2019) ) Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J Affect Disord 259: , 370–375. |
[36] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[37] | Folstein MF , Folstein SE , McHugh PR (1990) Mini-Mental-Status-Test (MMST). Deutschsprachige Fassung von J. Kessler, P. Denzler und H. J. Markowitsch. Beltz Test. |
[38] | Holt S , Schmiedl S , Thürmann PA ((2010) ) Potentially inappropriate medications in the elderly: The PRISCUS list. Dtsch Arztebl Int 107: , 543–551. |
[39] | Seidl H , Bowles D , Bock J-O , Brettschneider C , Greiner W , König H-H , Holle R ((2015) ) FIMA - Fragebogen zur Erhebung von Gesundheitsleistungen im Alter: Entwicklung und Pilotstudie. Das Gesundheitswes 77: , 46–52. |
[40] | Wimo A , Jonsson L , Zbrozek A ((2010) ) The Resource Utilization in Dementia (RUD) instrument is valid for assessing informal care time in community-living patients with dementia. J Nutr Health Aging 14: , 685–690. |
[41] | Dunkel D , Antretter E , Fröhlich-Walser S , Haring C ((2005) ) [Evaluation of the short-form social support questionnaire (SOZU-K-22) in clinical and non-clinical samples]. Psychother Psychosom Med Psychol 55: , 266–277. |
[42] | Zarit SH , Reever KE , Bach-Peterson J ((1980) ) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20: , 649–655. |
[43] | Reynolds T , Thornicroft G , Abas M , Woods B , Hoe J , Leese M , Orrell M ((2000) ) Camberwell Assessment of Need for the Elderly (CANE). Development, validity and reliability. Br J Psychiatry 176: , 444–452. |
[44] | van der Roest HG , Meiland FJM , Comijs HC , Derksen E , Jansen APD , van Hout HPJ , Jonker C , Dröes R-M ((2009) ) What do community-dwelling people with dementia need? A survey of those who are known to care and welfare services. Int Psychogeriatr 21: , 949–965. |
[45] | Thyrian JR , Hertel J , Wucherer D , Eichler T , Michalowsky B , Dreier-Wolfgramm A , Zwingmann I , Kilimann I , Teipel S , Hoffmann W ((2017) ) Effectiveness and safety of dementia care management in primary care: A randomized clinical trial. JAMA Psychiatry 74: , 996–1004. |
[46] | Curtin F , Schulz P ((1998) ) Multiple correlations and Bonferroni’s correction. Biol Psychiatry 44: , 775–777. |
[47] | Arevalo-Rodriguez I , Smailagic N , Roqué-Figuls M , Ciapponi A , Sanchez-Perez E , Giannakou A , Pedraza OL , Bonfill Cosp X , Cullum S ((2021) ) Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 7: , CD010783. |
[48] | Black BS , Johnston D , Leoutsakos J , Reuland M , Kelly J , Amjad H , Davis K , Willink A , Sloan D , Lyketsos C , Samus QM ((2019) ) Unmet needs in community-living persons with dementia are common, often non-medical and related to patient and caregiver characteristics. Int Psychogeriatr 31: , 1643–1654. |
[49] | Hancock GA , Woods B , Challis D , Orrell M ((2006) ) The needs of older people with dementia in residential care. Int J Geriatr Psychiatry 21: , 43–49. |
[50] | Deutsche Gesellschaft f·ur Psychiatrie und Psychotherapie; Psychosomatik und Nervenheilkunde (DGPPN), Deutsche Gesellschaft f·ur Neurologie (DGN) (2016) S3-Leitlinie “Demenzen” (Lngversion - January 2016). |
[51] | Stein J , Pabst A , Luck T , Lühmann D , Heser K , Jessen F , Bickel H , Mösch E , Pentzek M , Fuchs A , Wiese B , Mamone S , König H-H , Brettschneider C , Werle J , Scherer M , Maier W , Weyerer S , Riedel-Heller SG ((2017) ) Unmet care needs in the oldest old primary care patients with cognitive disorders: Results of the AgeCoDe and AgeQualiDe Study. Dement Geriatr Cogn Disord 44: , 71–83. |
[52] | Handels RLH , Sköldunger A , Bieber A , Edwards RT , Gonçalves-Pereira M , Hopper L , Irving K , Jelley H , Kerpershoek L , Marques MJ , Meyer G , Michelet M , Portolani E , Røsvik J , Selbaek G , Stephan A , de Vugt M , Wolfs C , Woods B , Zanetti O , Verhey F , Wimo A ((2018) ) Quality of life, care resource use, and costs of dementia in 8 European countries in a cross-sectional cohort of the Actifcare Study. J Alzheimers Dis 66: , 1027–1040. |
[53] | Rambod M , Ghodsbin F , Moradi A ((2020) ) The association between body mass index and comorbidity, quality of life, and cognitive function in the elderly population. Int J Community Based Nurs Midwifery 8: , 45–54. |
[54] | Lee HB , Lyketsos CG ((2003) ) Depression in Alzheimer’s disease: Heterogeneity and related issues. Biol Psychiatry 54: , 353–362. |
[55] | Zhao Q-F , Tan L , Wang H-F , Jiang T , Tan M-S , Tan L , Xu W , Li J-Q , Wang J , Lai T-J , Yu J-T ((2016) ) The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord 190: , 264–271. |
[56] | Gühne Janine; , Riedel-Heller , Steffi US ((2016) ) Depression im Alter - Herausforderung langlebiger Gesellschaften TT - Depression in Old Age - Challenge of an Ageing Society. Psychiatr Prax 43: , 107–110. |
[57] | Stahl ST , Beach SR , Musa D , Schulz R ((2017) ) Living alone and depression: The modifying role of the perceived neighborhood environment. Aging Ment Health 21: , 1065–1071. |
[58] | Kim SK , Park M ((2017) ) Effectiveness of person-centered care on people with dementia: A systematic review and meta-analysis. Clin Interv Aging 12: , 381–397. |
[59] | Rädke A , Michalowsky B , Thyrian JR , Eichler T , Xie F , Hoffmann W ((2020) ) Who benefits most from collaborative dementia care from a patient and payer perspective? A subgroup cost-effectiveness analysis. J Alzheimers Dis 74: , 449–462. |
[60] | Tille F , Gibis B , Balke K , Kuhlmey A , Schnitzer S ((2017) ) Soziodemografische und gesundheitsbezogene Merkmale der Inanspruchnahme und des Zugangs zu haus- und fachärztlicher Versorgung - Ergebnisse einer deutschlandweiten Bevölkerungsbefragung von 2006 bis 2016. Z Evid Fortbild Qual Gesundhwes 126: , 52–65. |
[61] | Robert Koch-Institut (Hrsg) (2023) Gesundheitliche Lage der Frauen in Deutschland - wichtige Fakten auf einen Blick. Gemeinsam getragen von RKI und Destatis. Robert Koch- Institut. |
[62] | Oesterhus R , Aarsland D , Soennesyn H , Rongve A , Selbaek G , Kjosavik SR ((2017) ) Potentially inappropriate medications and drug-drug interactions in home-dwelling people with mild dementia. Int J Geriatr Psychiatry 32: , 183–192. |
Notes
1 The Priscus list 1.0 was used before the updated Priscus list 2.0, which contains 133 additional drugs, was released (2023).
2 According to the “International Statistical Classification of Diseases and Related Health Problems” (ICD-10-Codes). Dementia (F01-F03), Diabetes (E10-E14), High blood pressure (I10-I15), Cerebrovascular diseases (I60-I69), Coronary heart diseases (I20-I25)



