Lacticaseibacillus rhamnosus HA-114 and Bacillus subtilis R0179 Prolong Lifespan and Mitigate Amyloid-β Toxicity in C. elegans via Distinct Mechanisms
Abstract
Background:
Recent advances linking gut dysbiosis with neurocognitive disorders such as Alzheimer’s disease (AD) suggest that the microbiota-gut-brain axis could be targeted for AD prevention, management, or treatment.
Objective:
We sought to identify probiotics that can delay Aβ-induced paralysis.
Methods:
Using C. elegans expressing human amyloid-β (Aβ)1–42 in body wall muscles (GMC101), we assessed the effects of several probiotic strains on paralysis.
Results:
We found that Lacticaseibacillus rhamnosus HA-114 and Bacillus subtilis R0179, but not their supernatants or heat-treated forms, delayed paralysis and prolonged lifespan without affecting the levels of amyloid-β aggregates. To uncover the mechanism involved, we explored the role of two known pathways involved in neurogenerative diseases, namely mitophagy, via deletion of the mitophagy factor PINK-1, and fatty acid desaturation, via deletion of the Δ9 desaturase FAT-5. Pink-1 deletion in GMC101 worms did not modify the life-prolonging and anti-paralysis effects of HA-114 but reduced the protective effect of R0179 against paralysis without affecting its life-prolonging effect. Upon fat5 deletion in GMC101 worms, the monounsaturated C14:1 and C16:1 FAs conserved their beneficial effect while the saturated C14:0 and C16:0 FAs did not. The beneficial effects of R0179 on both lifespan and paralysis remained unaffected by fat-5 deletion, while the beneficial effect of HA-114 on paralysis and lifespan was significantly reduced.
Conclusions:
Collectively with clinical and preclinical evidence in other models, our results suggest that HA-114 or R0179 could be studied as potential therapeutical adjuncts in neurodegenerative diseases such as AD.
INTRODUCTION
Alzheimer’s disease (AD), a neurodegenerative syndrome impairing behavior, memory, and cognitive functions, is the most common form of dementia associated with aging. Despite considerable research efforts over the past decades, the full spectrum of pathological mechanisms driving AD onset and progression remains elusive. Curative treatments are still lacking, and current therapeutic approaches rely on mitigating the decline in cognitive functions and managing symptoms.1,2 A hallmark of AD and a traditional target for therapy is the accumulation of amyloid-β (Aβ) protein fragments into plaques on the surfaces of glial cells and neurons, which are implicated in altering neural cell structure, deforming the cellular membrane, hindering neurotransmission, and leading to inflammation, oxidative damage and neuronal cell death.3 However, attempts at improving clinical outcomes by disrupting or preventing the formation of these plaques have repeatedly failed over the past 20 years4 and the efficacy of this therapeutic strategy remains controversial despite the recent approval of aducanumab to treat AD patients.5–7 Hence, the exploration of other associated risk factors remains essential to identify effective treatments or diet and lifestyle changes that could delay or prevent the onset or progression of symptoms.8
In this context, a better understanding of the emerging link between the host intestinal microbiome and neurogenerative disorders via the gut-brain axis could lead to significant developments in the management of AD.9 In addition to the significant effect of diet on both the microbiota and AD pathology, referred to as the diet-microbiota-brain axis,10 lipid metabolism and mitochondrial health were also shown to link the microbiome and gut homeostasis to neurodegeneration.11,12 Impaired lipid metabolism was shown to affect production and clearance of Aβ and tau phosphorylation13 and was linked to mitochondrial dysfunction and neurodegenerative diseases.14,15 Neurodegeneration is characterized by the development of mitochondrial dysfunction over time, which involves a disruption of the regulated turnover of damaged mitochondria via mitophagy.16 Mitophagy stimulation was found to reverse memory impairment in a C. elegans Aβ-overexpressing model and reduced cognitive impairments in mouse models via microglial phagocytosis of extracellular Aβ plaques.17 Abnormal gut health, in addition to mitochondrial dysfunction and dyslipidemia, was also linked to neurodegeneration via exacerbating inflammation, decreasing neurotransmitter levels, oxidative stress, and apoptosis.9 Considering that each of these mechanisms represent potential targets of probiotics, microbiota-targeted interventions with probiotics could impact multiple key mechanistic factors surrounding the pathogenesis of AD.18
Accumulating preclinical evidence suggests a beneficial effect of probiotics (various single or multi-strain formulations) on cognitive outcomes in preclinical AD models,19 including C. elegans20,21 and D. melanogaster.22,23 Probiotics also demonstrated beneficial effects in individuals with AD or mild cognitive impairment in clinical studies,24–27 although only a few single probiotic strains were assessed in clinical trials, namely L. plantarum C29-fermented soybean (DW2009),28 Bifidobacterium breve A1,27 and L. rhamnosus HA-114.24 Other clinical trials in AD subjects assessed a mixture of B. bifidum BGN4 and B. longum BORI29 or a formulation containing selenium with L. acidophilus, B. bifidum, and B. longum.30 This growing knowledge base suggests that probiotic strain specificity and timing of administration before or after disease onset will be important factors to consider in future trials. Currently, however, the specific mechanisms of action behind the effect of probiotics on memory and other AD outcomes are not fully understood. The strain L. rhamnosus HA-114 was recently shown to reduce paralysis in C. elegans models of amyotrophic lateral sclerosis via the modulation of fatty acid metabolism.31
The aims of the current study were to characterize different probiotic strains for their effects on Aβ-induced paralysis in the GMC101 C. elegans model and gain further insight on their potential mechanism of action by specifically evaluating the contribution of two pathways previously linked to AD, namely fatty acid desaturation and mitophagy. The GMC101 C. elegans strain is a well-described Aβ toxicity model32 expressing the full-length human Aβ peptide in body wall muscles. We report that feeding these worms with Lacticaseibacillus rhamnosus HA-114 or Bacillus subtilis R0179 resulted in delay or reduction of paralysis through distinct mechanisms, involving the host fatty acid metabolism for HA-114 but not R0179. Conversely, the beneficial effect of R0179 but not HA-114 on Aβ-induced paralysis was abolished upon mitophagy impairment. Our results also provide support and mechanistic insights for the clinical benefits of HA-114 recently reported in AD patients,24 thereby strengthening the translational value of the GMC101 C. elegans AD model.32
MATERIALS AND METHODS
C. elegans strains and maintenance
The nematode strains wild type N2; GMC101 (dvIs100 [unc-54p::A-beta-1-42::unc-54 3′-UTR+mtl-2p::GFP]); RB2547 (pink1(ok3538) II.); and BX107 (fat-5(tm420) V.), as well as E. coli OP50 were obtained from the Caenorhabditis Genetics Center (CGC, Minneapolis, funded by NIH Office of Research Infrastructure Programs (P40 OD010440), USA). The strains GMC101;Δpink-1 [pink1(ok3538) II; dvIs100] and GMC101;Δfat-5 [fat-5(tm420) V.; dvIs100] were generated for this study. C. elegans strains were maintained according to standard laboratory practice.33 Probiotic strains were obtained from Lallemand Bacteria Culture collection (LBCC, Montreal, Canada). Worms were synchronized with bleach, hatched overnight, and L1 worms were transferred to fresh OP50 and incubated for 3 days at 15°C prior to initialization for paralysis (25°C) or lifespan assays (20°C).
Probiotic plates, heat treatment, and cell-free supernatants preparation
Glycerol stocks were used to prepare individual colonies, which were then used to inoculate cultures for 24-h incubation at 37°C. E. coli, Lactobacilli, and B. subtilis were cultured in LB, MRS, and Tryptic Soy media, respectively. Following incubation, bacterial cell pellets were washed three times in M9 buffer, then bacterial cell concentration was determined by flow cytometry (BD Accuri C6) and diluted to 109 cells/mL in M9. 200 μL of bacteria were seeded onto NGM plates, then incubated overnight and stored at 4°C until use (within 1 week of preparation). For heat treatment of bacteria, dilutions were incubated at 80°C in a water bath for 20 min prior to seeding. For testing of bacterial secretome, culture supernatants were collected, filter sterilized (0.22 μm), and 1:1 mixture were prepared with washed, diluted OP50 to a final concentration of 109 cells/mL before seeding. For fatty acid supplementation, stocks were diluted in ethanol and added to molten NGM media following autoclaving.
Paralysis scoring
After 3 days at 15°C on OP50 following hatching, amyloidogenic worms were washed three times in M9, then approximately 30–40 worms for each replicate were transferred to their respective plates and incubated for 24 h at 20°C for initialization on probiotics. Each condition had at least three replicates. After 24 h, worms were transferred to fresh plates with probiotics, and the temperature was switched to the Aβ-inducing temperature of 25°C. Locomotion was assessed every 24 h for 3 days, and worms were scored as paralyzed if they failed to produce movement beyond their head region, either spontaneously or following provocation with touch. Worms that were scored non-paralyzed were transferred to fresh plates, repeating this process for 2 days for a total of 72 h at 25°C. Worms that crawled off or perished due to internal hatching were recorded but censored from data analysis. The C. elegans [pink1(ok3538) II; dvIs100] worms were slower to develop, so developing worms were incubated an additional 24 h on OP50 for a total of 96 h instead of 72 h, before proceeding to initialization for 24 h and paralysis scoring for 48 h at 25°C.
Staining and microscopy
Worms were fed with either OP50, L. rhamnosus HA-114, L. rhamnosus R0343, or B. subtilis R0179. Following 24 h at 25°C (to induce paralysis in the GMC101 strain), worms were collected and washed three times in PBS. X-34 dye (Sigma-Aldrich, SML1954) was used for visualization of amyloid deposits, as previously described.34 Briefly, day 1 adult worms were incubated in 100 μL X-34 working solution (1 mM in 10 mM Tris-HCL pH 8.0) for 2 h at 20°C in the dark. Worms were then washed three times in PBS before being placed on OP50-NGM to allow the worms to destain overnight at 20°C. Animals were immobilized in 5 mM levamisole dissolved in M9 and mounted on slides with 2% agarose pads. Deposits were visualized at 385 nm using a Zeiss Axio Imager M2 microscope, using a ×40 objective. The software used was AxioVs40 4.8.2.0. For each worm, the number of deposits in the head area was counted. At least 30 worms were scored per condition, over 3 distinct experiments.
Lifespan assays
To prepare for the lifespan assay on solid media, L1 worms were maintained at 20°C on OP50 for 3 days before they were washed 3 times in M9 and transferred to their respective probiotic plates. Worms were scored for viability every 24 h for 4 days, and live worms were transferred to fresh plates. Following the end of their fertility, worms were scored and transferred every 2–3 days. At least 30 worms were used for each replicate, with at least two replicates per condition. Worms that crawled off or perished due to internal hatching were recorded but censored from data analysis. The [pink1(ok3538) II; dvIs100] double mutant worms are sensitive at normally non-challenging 20°C temperature, so longevity assays were conducted at 15°C.
Fatty acids profiling by LC-MS/MS
For free fatty acid analysis, 150 μL of ice-cold methanol and 10 μL of isotope-labeled internal standard mixture was added to 50 μL of sample for overnight protein precipitation. Then it was centrifuged at 13000×g for 20 min. 50 μL of supernatant was loaded into the center of wells of a 96-deep well plate, followed by the addition of 3-nitrophenylhydrazine (NPH) reagent. After incubation for 2 h, Butylated hydroxytoluene (BHT) stabilizer and water were added before LC-MS injection.
Mass spectrometric analysis was performed on an ABSciex 4000 Qtrap® tandem mass spectrometry instrument (Applied Biosystems/MDS Analytical Technologies, Foster City, CA) equipped with an Agilent 1260 series UHPLC system (Agilent Technologies, Palo Alto, CA). The samples were delivered to the mass spectrometer by a LC method followed by a direct injection (DI) method. Data analysis was done using Analyst 1.6.2.
Statistical analyses
Graphpad Prism 9 was used to generate graphs and statistical analyses. Two-way ANOVA with multiple comparison testing were used to assess significance in paralysis assays (Dunnet’s) and gene expression assays (Tukey’s). Gehan-Breslow-Wilcoxon test was performed to determine statistical significance of survival differences between groups in lifespan assays.
RESULTS
Decrease in paralysis by probiotic bacteria is strain specific
Several probiotic strains were assessed for their impact on paralysis in the C. elegans strain GMC101, which expresses the human Aβ1–42 peptide in muscle walls resulting in age-dependent paralysis in a temperature-sensitive manner.32 Worms on OP50 showed progressive paralysis with a marked incidence at 48 and 72 h following the temperature upshift. Worms are scored as paralyzed when they cannot move away upon gentle touching, and only the head is mobile and surrounded with a halo devoid of bacteria (Fig. 1A). While the worms supplemented with the strains Lacticaseibacillus rhamnosus GG, Lacticaseibacillus paracasei L26, and Lactobacillus helveticus R0052 showed a progressive and marked incidence of paralysis comparable to the OP50 controls, those supplemented with Bacillus subtilis R0179 (R0179), L. rhamnosus HA-111, L. rhamnosus R0011 and L. rhamnosus HA-114 (HA-114) showed a significant delay and reduction in the incidence of paralysis compared to OP50 (Fig. 1B). Two of the most effective strains, HA-114 and R0179, were selected for further analyses. These strains did not reduce the abundance of Aβ deposits in the head region 24 h after the temperature upshift compared to the OP50 and L. rhamnosus GG (R0343) diets (Fig. 1C), as represented on X-34 staining images (Fig. 1D).
Fig. 1
The effect of several probiotic strains on paralysis in GMC101 worms is strain specific. A) Representation of the scoring method for paralyzed and non-paralyzed worms. B) Paralysis assay in GMC101 worms comparing the effect of a panel of probiotic strains (109 cells/mL) over 72 h following the temperature upshift (n = 3). Error bars indicate SEM. Two-way ANOVA with Dunnett’s multiple comparison test; p < 0.0001 versus OP50. C) Quantification of aggregates stained with X-34 at 24 h after the temperature upshift showing D) Aβ aggregates in the head region of GMC101 worms fed the indicated diet.
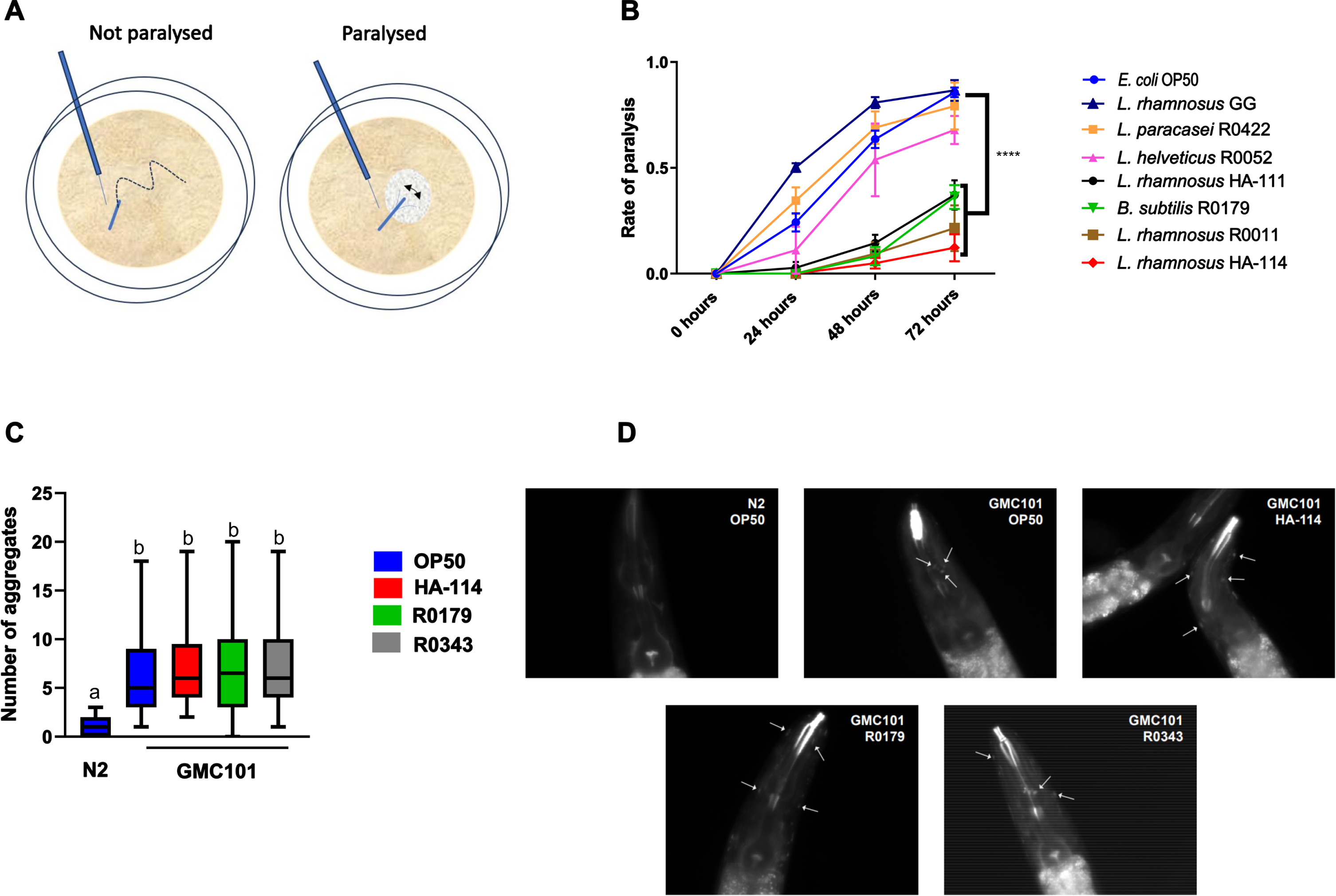
Effect of secretomes and heat-treated HA-114 and R0179 on paralysis
In contrast to live HA-114, the cell-free culture supernatant (Fig. 2A) or heat-treated HA-114 (Fig. 2B) did not protect against amyloidogenic paralysis. The R0179 supernatant did not confer any significant protection against paralysis (Fig. 2C), while heat treatment did not abolish the protection conferred by R0179 (Fig. 2D).
Fig. 2
Live HA-114 and R0179 confer maximal protection against paralysis. Paralysis assay in GMC101 worms comparing the effect of live HA-114 and R0179 (109 cells/mL) versus their corresponding cell-free supernatants (A and C, respectively; n = 3) or probiotic strains subjected to heat treatment (HT) at 80°C for 20 min prior to seeding (B and D, respectively; n = 3). Paralysis was scored over 72 h following the temperature upshift. Error bars indicate SEM. Two-way ANOVA with Dunnett’s multiple comparison test (versus OP50); *p < 0.05; **p < 0.01.
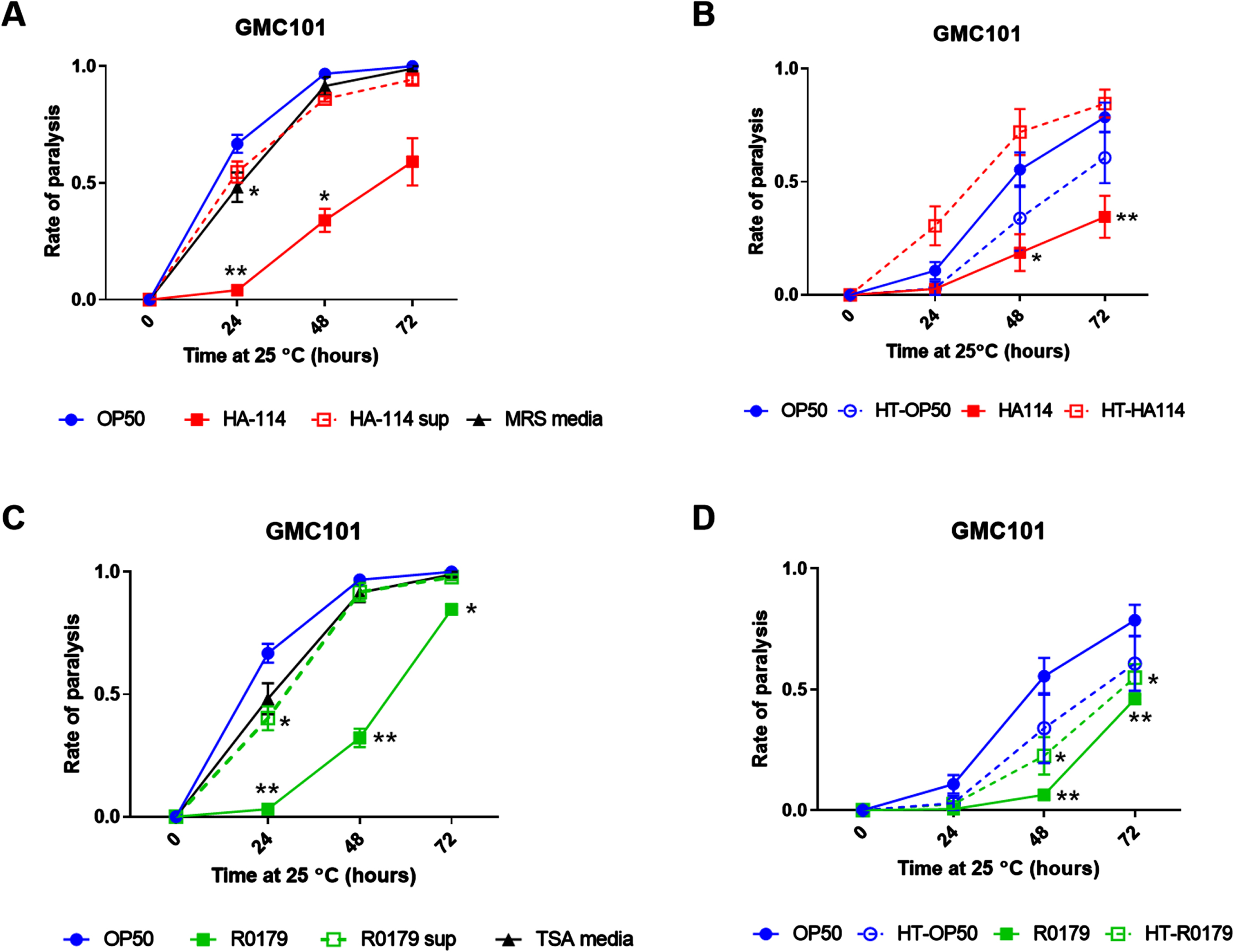
The effects of HA-114 on lifespan and paralysis are maintained in the absence of pink-1
To assess the potential contribution of mitophagy in the effects of HA-114 and R0179, we generated pink-1 null GMC101 worms (GMC101;Δpink-1). At 20°C, HA-114 significantly prolonged the lifespan of the wild-type N2 (Fig. 3A), GMC101 (Fig. 3B), and GMC101;Δpink-1 worms (Fig. 3C), while R0179 also extended the lifespan of these mutants significantly.
Fig. 3
Pink-1 is not essential for the effects of HA-114 on lifespan and paralysis in GMC101 worms. Lifespan of A) N2 (n = 4), B) GMC101 (n = 4), and C) GMC101;Δpink-1 (n = 2) fed the indicated diets. Gehan-Breslow-Wilcoxon test (versus OP50); ****p < 0.0001; ***p < 0.001; **p < 0.01. D) Paralysis of GMC101;Δpink-1 fed the indicated diets (n = 9) scored over 48 h following temperature upshift. Error bars indicate SEM. Two-way ANOVA with Dunnett’s multiple comparison test (versus OP50); ****p < 0.0001.
We next wanted to assess whether mitophagy was involved in the effect of HA-114 and R0179 on Aβ-induced paralysis. Considering their increased sensitivity to amyloid toxicity at the maintenance temperature of 20°C, GMC101;Δpink-1 worms were maintained at 15°C and reared for an additional 24 h on OP50 to reach the young adult stage before transferring to probiotic strains. Despite this adaptation in assay conditions to accommodate their slower development and increased sensitivity, the paralysis rate was higher than zero at the start of the experiment (all conditions, p > 0.05). Nevertheless, HA-114 retained its protective effect against paralysis in the GMC101;Δpink-1 worms (Fig. 3D), while R0179 completely lost its protective effect at 48 h.
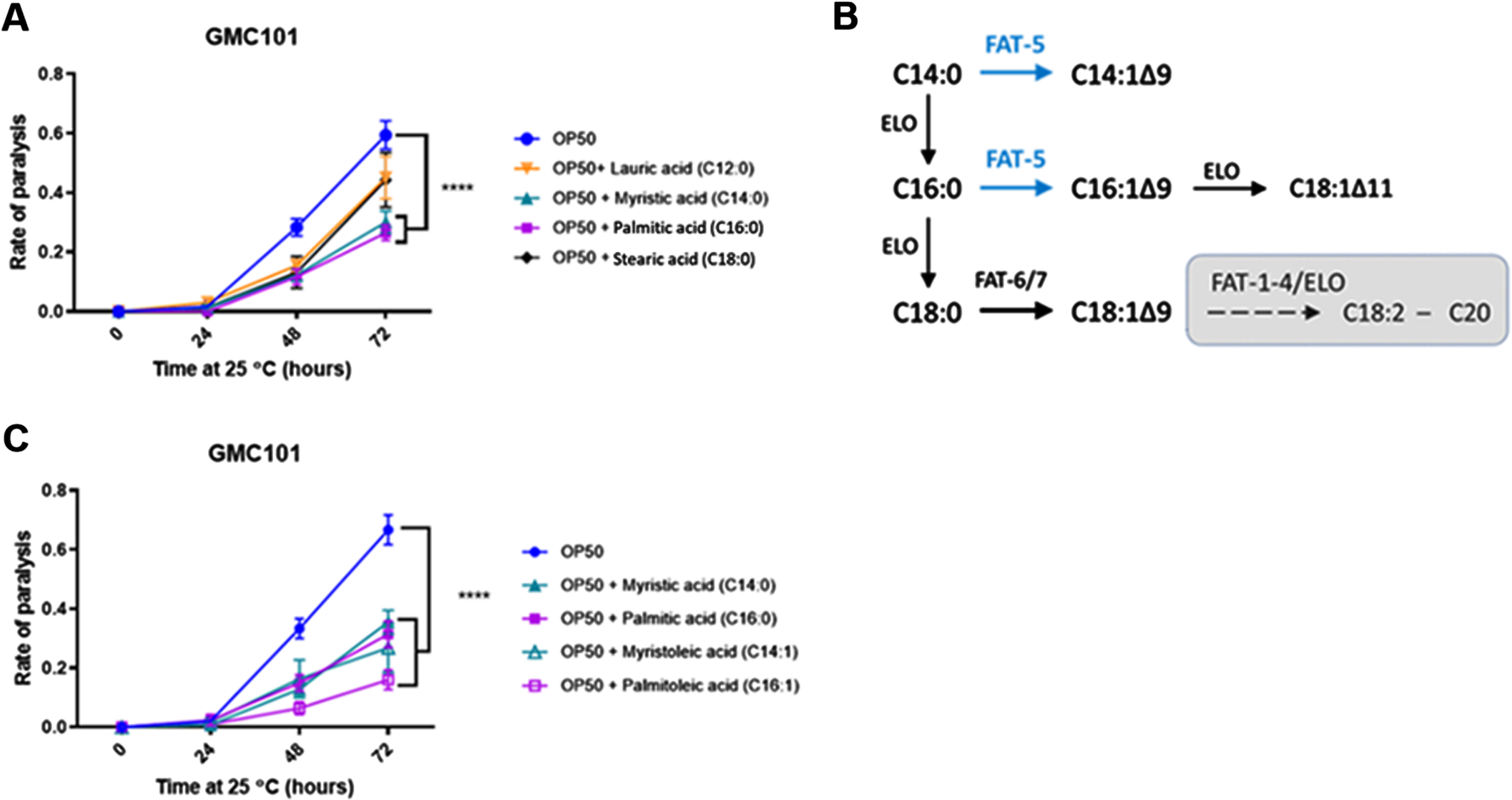
Supplemented fatty acids differentially affect paralysis
Next, we investigated the effect of supplementing a panel of common bacterial FAs (ranging from C12-C18) on the paralysis phenotype in GMC101 worms. Myristic acid (C14:0) and palmitic acid (C16:0) supplementation significantly reduced amyloidogenic paralysis at 72 h, while lauric acid (C12:0) and stearic acid (C18:0) only produced a mild non-significant amelioration at 72 h despite providing similar benefits at 48 h (Fig. 4A). C. elegans metabolism of the C16:0 and possibly C14:0 fatty acids can include their direct desaturation by the Δ9 desaturase fat-5 into the monounsaturated fatty acids (MUFAs) palmitoleic (C16:1) and myristoleic acid (C14:1), respectively (Fig. 4B). Interestingly, the main metabolites downstream of fat-5 also reduced paralysis in GMC101 worms, with 16:1 displaying a more pronounced effect (Fig. 4C).
Fig. 4
Fatty acids typically desaturated by fat-5 are more efficient at reducing paralysis. A) Paralysis assay in OP50-fed GMC101 worms comparing the effect of the indicated saturated fatty acids (200 μM; n = 3). B) Schematics of the fatty acid desaturation pathways in C. elegans (adapted from35). C) Paralysis assay in OP50-fed GMC101 worms comparing the effect of saturated and monounsaturated C14 and C16 fatty acids (200 μM; n = 6). Error bars indicate SEM. Two-way ANOVA with Dunnett’s multiple comparison test (versus OP50); ****p < 0.0001.
The Δ9 desaturase fat-5 is involved in the protective effect of HA-114 on paralysis
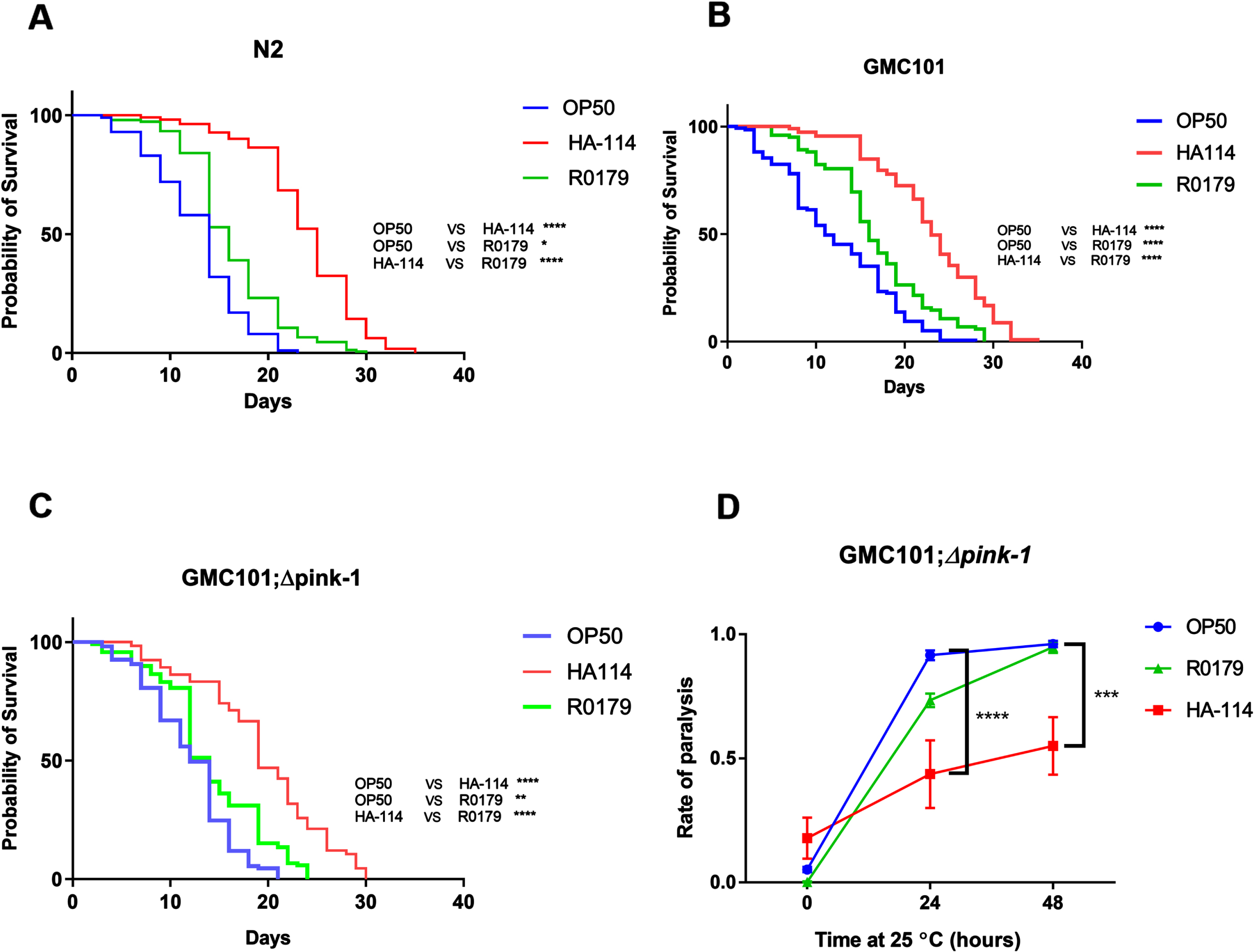
We next wanted to assess the importance of the host fatty acid Δ9 desaturase pathway in the protective effect of HA-114 and R0179. In GMC101;Δfat-5 worms, supplementation with the C16:1 and C14:1 MUFAs delayed the paralysis phenotype while the protective effect of C14:0 and C16:0 against paralysis was abolished (Fig. 5A). In these worms, the decrease in paralysis by HA-114 was significantly reduced compared to GMC101 while the rate of Aβ-induced paralysis was similar to GMC101 when the GMC101;Δfat-5 worms were fed OP50 or R0179 (Fig. 5B), suggesting that the host fat-5 desaturation pathway is involved in the protective effect of HA-114 but not R0179 against amyloidogenic paralysis. The deletion of fat-5 also reduced the pro-longevity effect of HA-114 (Fig. 5C) without affecting lifespan in worms fed OP50 or R0179. Indeed, HA-114 consistently yielded a lifespan prolongation of approximately 10–12 days, but this prolongation was shortened to 7 days in GMC101;Δfat-5 worms (Fig. 5D). Mean lifespan values are provided in Table 1. Considering the impact of fat-5 deletion on the protection conferred by HA-114 but not R0179, we explored the FA composition of HA-114 and R0179 in comparison to OP50. This analysis revealed a greater proportion of acetic acid in HA-114 and of palmitic acid (C16:0) in R0179 (Fig. 6).
Fig. 5
The effect of HA-114 on paralysis and lifespan requires fat-5. A) Paralysis assay in OP50-fed GMC101; Δfat-5 worms comparing the effect of the indicated saturated and monounsaturated C14 and C16 fatty acids (200 μM; n = 6). Error bars indicate SEM. Two-way ANOVA with Dunnett’s multiple comparison test (versus OP50); *p < 0.05. B) Paralysis assay in GMC101 (n = 4) and GMC101; fat-5 (n = 9) fed the indicated diets. Error bars indicate SEM. Two-way ANOVA with Tukey’s multiple comparison test; ***p < 0.001. C) Lifespan assay in GMC101 (n = 4) and GMC101;Δfat-5 (n = 4; n = 2 for OP50) fed the indicated diets. Gehan-Breslow-Wilcoxon test (versus GMC101 corresponding diet); **p < 0.0001; n.s., not significant. D) Bar graph of mean lifespan (n = 3; *p < 0.05 versus N2/HA-114). Dashed line at 12 days.
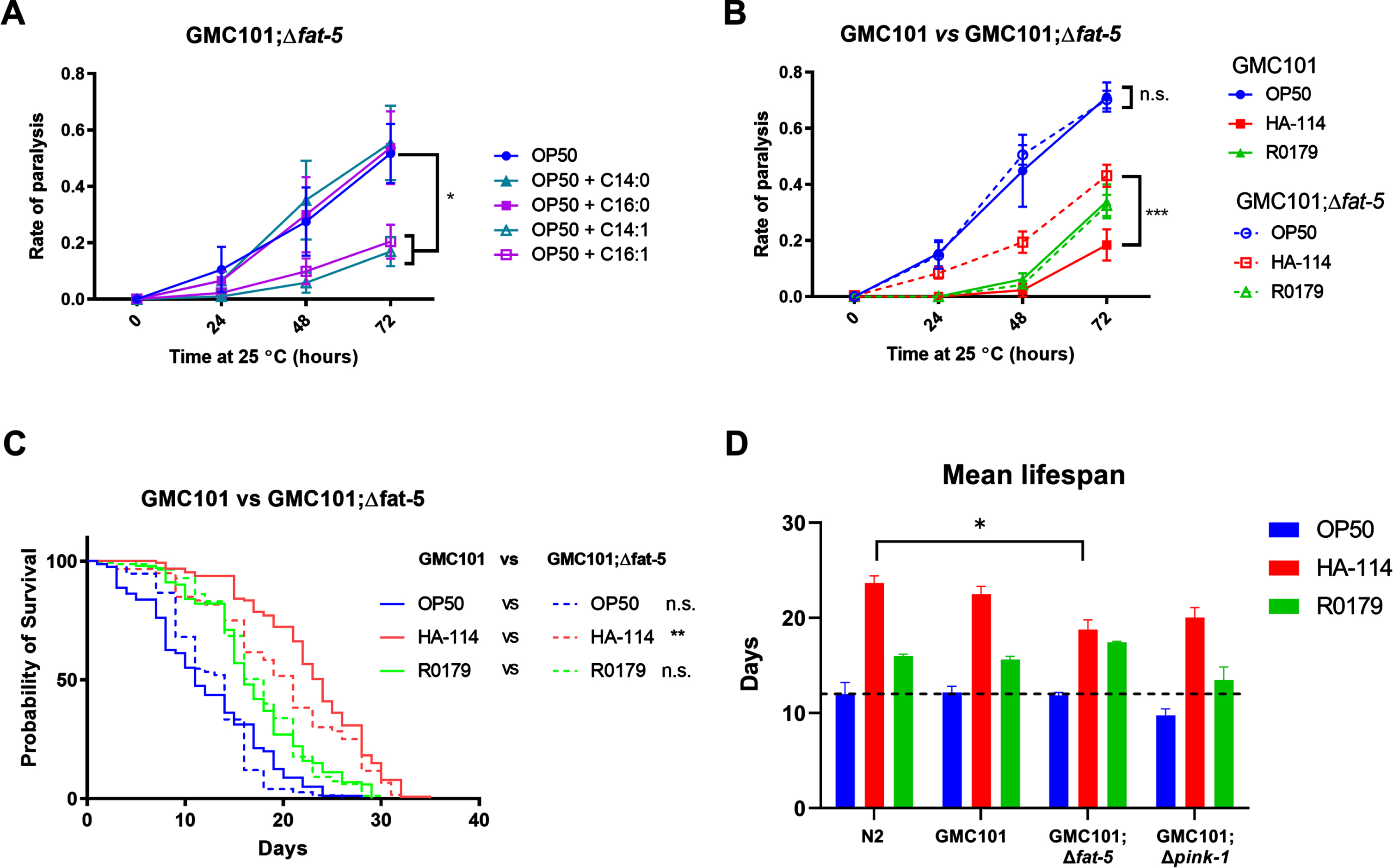
Fig. 6
Relative abundance of selected fatty acids in OP50, R0179, and HA-114. Doughnuts showing the relative abundance of A) short-chain fatty acids and B) C14-C18 fatty acids in OP50 (inner circle), R0179 (middle circle) and HA-114 (outer circle).
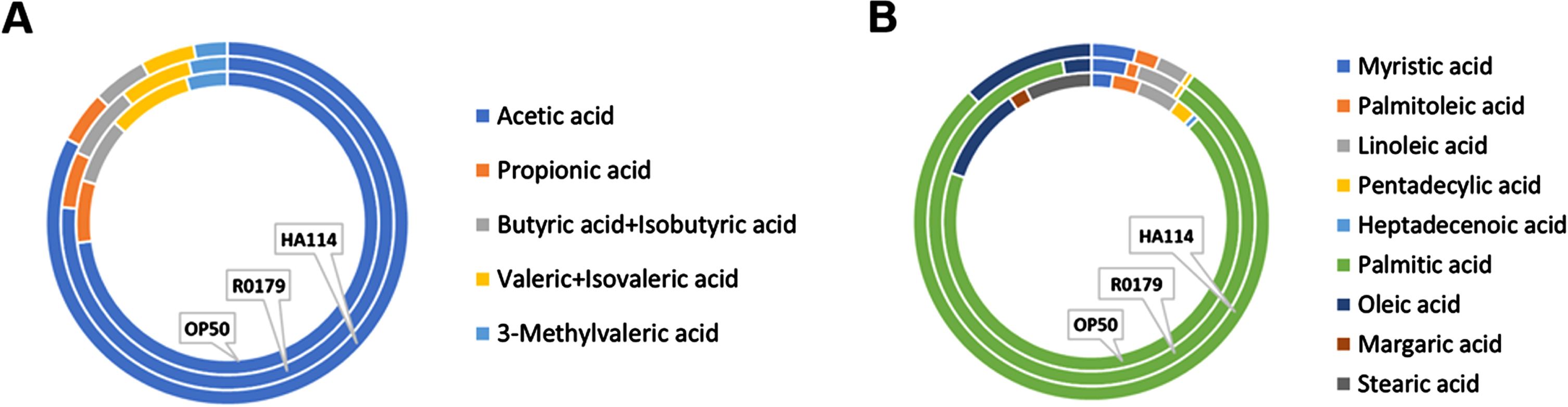
Table 1
Mean lifespan
| OP50 | HA-114 | R0179 | |||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| N2 | 12.0 | 2.1 | 3 | 23.7 | 1.3 | 3 | 16.0 | 0.4 | 3 |
| GMC101 | 12.2 | 1.1 | 3 | 22.5 | 1.4 | 3 | 15.6 | 0.5 | 3 |
| GMC101;Δfat-5 | 11.9 | 0.5 | 3 | 18.8 | 1.7 | 3 | 17.4 | 0.2 | 3 |
| GMC101;Δpink-1 | 9.8 | 1.2 | 3 | 20.0 | 1.8 | 3 | 13.5 | 2.3 | 3 |
DISCUSSION
Host-microbe interactions and the gut-brain axis are growing areas of focus for their roles in neurodegenerative diseases such as AD.36 Using the C. elegans human Aβ1–42 transgenic model of AD, we screened different probiotic strains for their effect on paralysis induced by the expression of a human Aβ1–42 transgene. Among the best candidates, L. rhamnosus HA-114 and B. subtilis R0179 exerted a marked protective effect with different requirements for the presence of live bacteria. Bacterial secretome supplementation was not sufficient to fully recapitulate the protective effect of either strain. Heat-treated HA-114 was unable to confer protection against paralysis but heat-treated R0179 conserved its effect. Importantly, our heat treatment of B. subtilis R0179 is not sufficient to kill spores, but only vegetative cells. This may be the reason behind the remaining protection conferred by heat-treated R0179. The heat treatment changes the spores:vegetative cells ratio, thereby reducing but not abolishing the protection. The conversion from spore to vegetative cells has been reported as important for the probiotic activity of B. subtilis.37 It is not possible in this experimental context to conclude whether the effect is mediated by the spores or the vegetative cells, but they support the hypothesis of a contribution of the germination process in the beneficial effect of B. subtilis R0179.
The beneficial effects of HA-114 on lifespan and paralysis were maintained after pink-1 deletion, while its effect on Aβ-induced paralysis but not lifespan was reduced by the deletion of fat-5. Contrary to HA-114, the anti-paralysis and pro-longevity effects of R0179 were conserved despite the absence of the Δ9 desaturase FAT-5 but were reduced following pink-1 deletion, which was previously shown to profoundly impair mitophagy in C. elegans.38 However, the effect of R0179 on mitophagy was not confirmed using additional mitophagy mutants. Nevertheless, these results suggest that both strains act via distinct mechanisms, involving host fatty acids metabolism in the case of HA-114 and probably mitochondrial function for R0179. These mechanisms appear to be independent of the aggregation process, with the same number of aggregates in all tested diets. It cannot be excluded that the probiotics could result in different aggregate conformations of lesser toxicity. While the number of aggregates that were enumerated was similar between the different probiotic diets, the conformation of the aggregates and the ‘conformation-related toxicity’ was not assessed and can differ. Indeed, FAs are known modulators of Aβ aggregation pathways, and it was recently shown that the different conformations of the aggregates in the presence of specific FAs appear to harbor different toxicity levels.39 Yet, in the model used herein, an aggregate-independent effect on the muscles or membranes fluidity cannot be excluded.
FAT-5 acts as a palmitoyl-CoA-desaturase that initiates unsaturated fatty acid synthesis in C. elegans and generates the MUFA palmitoleic acid.35 MUFAs are critical components of phospholipids and triglycerides, and are important for membrane function, energy storage and signaling.40 Enzymes that generate MUFA’s like fatty acyl desaturases such as SCD’s (stearoyl-CO-A desaturase) are found widely across all living organisms, ranging from bacteria and yeast to humans.41 Alteration in the expression of SCD-5 expressed in human tissues has been associated with neurological and developmental diseases.42 The correlation between MUFA synthesis and increased longevity in C. elegans was previously reported.43 Specifically, levels of Δ9 desaturases were shown to be elevated in C. elegans mutants displaying a longer lifespan,44 and we found that the HA-114 diet significantly prolongs lifespan and moderately increased the levels of the Δ9 desaturase FAT-5 mRNA in N2 and GMC101 worms (data not shown). The deletion of fat-5 reduced the pro-longevity effect of HA-114 in the GMC101 background, suggesting that its effect on lifespan could be mediated via the same mechanism as its effect on paralysis. Indeed, the beneficial effect of HA-114 on amyloidogenic paralysis was markedly impaired by the deletion of fat-5. Of note, HA-114 produces more acetic acid than OP50 and R0179. Acetic acid was shown to prolong lifespan in C. elegans via the upregulation of DAF-16, a master metabolic regulator that controls FAT-5 expression.45,46
Probiotics have been shown to influence lipid metabolic profiles in other studies. Bifidobacterium animalis CECT 8145 was found to reduce total lipid and triglyceride content, oxidative stress, as well as modulate energy, lipid, and tryptophan metabolism in C. elegans.47 Supplementing mice on a high-fat, high-cholesterol diet along with Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 resulted in significant reductions of fat accumulation in adipose and liver tissue, cholesterol, hepatic triglycerides fatty acid synthetases expression and fatty acid oxidation activity compared to controls with no probiotic administration.48 However, the use of probiotics to normalize the host lipid metabolic profiles in the context of neurodegeneration is a relatively new area to be explored. In AD patients, the co-administration of a probiotic with selenium significantly reduced plasma triglycerides and normalized cholesterol levels in AD patients compared to selenium and placebo.30
Unlike HA-114, the effects of R0179 in the GMC101 worms do not seem dependent on FAT-5 activity but its protective effect on Aβ-induced paralysis was abrogated by pink-1 deletion. This suggests that R0179 may not be sufficient to overcome the mitochondrial dysfunction of the double mutant GMC101;Δpink-1 and that its effect may involve mitochondrial functions or the activation of mitochondrial stress response pathways. Indeed, as observed in the brains of AD patients, GMC101 worms display progressive mitochondrial function defects, and the activated mitochondrial unfolded protein response and mitophagy in these worms were shown essential to the phenotype amelioration caused by the mitochondrial stress response activation.49 In other studies with B. subtilis strains, benefits of the B. subtilis NCIB3610 on the AD C. elegans model CL2006 were associated to biofilm formation and production of the quorum sensing peptide CSF.20 These same properties were also shown to mediate its pro-longevity effect, via the DAF-2/DAF-16/HSF-1 signaling axis and the downregulation of the insulin-like signaling pathway.50 Several B. subtilis strains (PNX21, JH642, 168, and NCIB3610) were also shown to prolong lifespan and reverse α-synuclein aggregation and in a C. elegans model of α-synucleinopathy.21 In that model, the beneficial effect of B. subtilis PXN21 against alpha-synuclein aggregation was associated with its biofilm formation capacity and the modulation of host genes involved in the sphingolipid metabolism pathways.21
In C. elegans, physiological outcomes such as longevity and fat storage/metabolism can be significantly altered by nutritional composition of their bacterial foods.51 In addition, there are associated physiological defects between age-related decline and AD, including a progressive loss of mitochondrial function.52 Hence, the impacts of probiotics on longevity or Aβ-induced toxicity are not necessarily linked, and there could be tradeoffs between lifespan prolongation and other physiological outcomes of health span such as fertility or satiety, which were not explored in this study.53 In addition, we did not assess pharyngeal pumping or lawn thickness to compare the potential contribution of caloric intake between diets. Furthermore, lipid metabolism and its modulation by multiple transcriptional regulators and nutrient sensing pathways is complex,54–58 and this study did not comprehensively look at the metabolic and transcriptional responses to HA-114 or R0179 supplementation at the omics scale.
Conclusions
New studies suggest that the gut microbiome plays an increasingly important role in promoting overall health. However, there is a limited amount of research that thoroughly examines the specific mechanisms of action, particularly at the strain level, that impact the brain and the development of neurodegenerative diseases. Our results support the notion that supplementation L. rhamnosus HA-114 or B. subtilis R0179 could be an effective method for the mitigation of Aβ aggregates toxicity and warrant further investigations in mammalian AD models. A better understanding of probiotics mechanisms of action in vivo is necessary to explain the cognitive benefits of probiotics observed in clinical studies.
AUTHOR CONTRIBUTIONS
Stuart G. Foster (Conceptualization; Formal analysis; Investigation; Writing – original draft; Writing – review & editing); Shibi Mathew (Conceptualization; Data curation; Formal analysis; Investigation; Writing – original draft; Writing – review & editing); Audrey Labarre (Conceptualization; Formal analysis; Investigation; Writing – review & editing); J. Alex Parker (Conceptualization; Formal analysis; Supervision; Writing – review & editing); Thomas A. Tompkins (Conceptualization; Data curation; Formal analysis; Supervision; Writing – original draft; Writing – review & editing); Sylvie Binda (Conceptualization; Supervision; Writing – review & editing).
ACKNOWLEDGMENTS
We thank Dr. Annie Tremblay for her contribution to the scientific writing and editing of the manuscript.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Stuart G. Foster, Shibi Mathew, Thomas A. Tompkins, and Sylvie Binda are employed by Lallemand Health Solutions Inc., a company that manufactures and markets the tested probiotics to business clients but not to consumers. All other authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author.
REFERENCES
1. | Chakraborty A and Diwan A. Alzheimer and it’s possible therapy: a review. J Exp Neurol (2020) ; 1: (4): 115–122. |
2. | World Health Organization (2020) Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia (2020, accessed 22 July 2021). |
3. | Madav Y , Wairkar S and Prabhakar B. Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res Bull (2019) ; 146: : 171–184. |
4. | Bennett DA . Lack of benefit with idalopirdine for Alzheimer disease: Another therapeutic failure in a complex disease process. JAMA (2018) ; 319: : 123–125. |
5. | Walsh S , Merrick R , Milne R , et al. Aducanumab for Alzheimer’s disease? BMJ (2021) ; 374: : n1682. |
6. | Yang P and Sun F. Aducanumab: The first targeted Alzheimer’s therapy. Drug Discov Ther (2021) ; 15: : 166–168. |
7. | de la Torre JC and Gonzalez-Lima F. The FDA approves aducanumab for Alzheimer’s disease, raising important scientific questions. J Alzheimers Dis (2021) ; 82: : 881–882. |
8. | Bilbul M and Schipper HM. Risk profiles of Alzheimer disease. Can J Neurol Sci (2011) ; 38: : 580–592. |
9. | Westfall S , Lomis N , Kahouli I , et al. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci (2017) ; 74: : 3769–3787. |
10. | Kincaid HJ , Nagpal R and Yadav H. Diet-microbiota-brain axis in Alzheimer’s disease. Ann Nutr Metab (2021) ; 77: (Suppl 2): 21–27. |
11. | Stefano G , Pilonis N , Ptacek R , et al. Gut, microbiome, and brain regulatory axis: Relevance to neurodegenerative and psychiatric disorders. Cell Mol Neurobiol (2018) ; 38: : 1197–1206. |
12. | Gentile F , Doneddu PE , Riva N , et al. Diet, microbiota and brain health: Unraveling the network intersecting metabolism and neurodegeneration. Int J Mol Sci (2020) ; 21: : 7471. |
13. | Sato N and Morishita R. The roles of lipid and glucose metabolism in modulation of β-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front Aging Neurosci (2015) ; 7: : 199. |
14. | Proitsi P , Kim M , Whiley L , et al. Association of blood lipids with Alzheimer’s disease: A comprehensive lipidomics analysis. Alzheimers Dement (2017) ; 13: : 140–151. |
15. | Wang W , Zhao F , Ma X , et al. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol Neurodegener (2020) ; 15: : 30. |
16. | Fivenson EM , Lautrup S , Sun N , et al. Mitophagy in neurodegeneration and aging. Neurochem Int (2017) ; 109: : 202–209. |
17. | Fang EF , Hou Y , Palikaras K , et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci (2019) ; 22: : 401–412. |
18. | De la Fuente M . The role of the microbiota-gut-brain axis in the health and illness condition: A focus on Alzheimer’s disease. J Alzheimers Dis (2021) ; 81: : 1345–1360. |
19. | de Rijke TJ , Doting MHE , van Hemert S , et al. A systematic review on the effects of different types of probiotics in animal Alzheimer’s disease studies. Front Psychiatry (2022) ; 13: : 879491. |
20. | Cogliati S , Clementi V , Francisco M , et al. Bacillus subtilis delays neurodegeneration and behavioral impairment in the Alzheimer’s disease model Caenorhabditis elegans. J Alzheimers Dis (2020) ; 73: : 1035–1052. |
21. | Goya ME , Xue F , Sampedro-Torres-Quevedo C , et al. Probiotic Bacillus subtilis protects against α-synuclein aggregation in C. elegans. Cell Rep (2020) ; 30: : 367–380.e7. |
22. | Tan FHP , Liu G , Lau SA , et al. Lactobacillus probiotics improved the gut microbiota profile of a Drosophila melanogaster Alzheimer’s disease model and alleviated neurodegeneration in the eye. Benef Microbes (2020) ; 11: : 79–89. |
23. | Liu G , Tan FH , Lau SA , et al. Lactic acid bacteria feeding reversed the malformed eye structures and ameliorated gut microbiota profiles of Drosophila melanogaster Alzheimer’s Disease model. J Appl Microbiol (2022) ; 132: : 3155–3167. |
24. | Akhgarjand C , Vahabi Z , Shab-Bidar S , et al. Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, and placebo-controlled study. Front Aging Neurosci (2022) ; 14: : 1032494. |
25. | Asaoka D , Xiao J , Takeda T , et al. Effect of probiotic Bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: Results of a 24-week randomized, double-blind, placebo-controlled trial. J Alzheimers Dis (2022) ; 88: : 75–95. |
26. | Xiao J , Katsumata N , Bernier F , et al. Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J Alzheimers Dis (2020) ; 77: : 139–147. |
27. | Kobayashi Y , Kuhara T , Oki M , et al. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef Microbes (2019) ; 10: : 511–520. |
28. | Hwang Y-H , Park S , Paik J-W , et al. Efficacy and safety of lactobacillus plantarum C29-fermented soybean (DWin individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients (2019) ; 11: : 305. |
29. | Kim CS , Cha L , Sim M , et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci (2021) ; 76: : 32–40. |
30. | Tamtaji OR , Heidari-Soureshjani R , Mirhosseini N , et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin Nutr (2019) ; 38: : 2569–2575. |
31. | Labarre A , Guitard E , Tossing G , et al. Fatty acids derived from the probiotic Lacticaseibacillus rhamnosus HA-114 suppress age-dependent neurodegeneration. Commun Biol (2022) ; 5: : 1340. |
32. | McColl G , Roberts BR , Pukala TL , et al. Utility of an improved model of amyloid-beta (Aβ 1-42) toxicity in Caenorhabditis elegans for drug screening for Alzheimer’s disease. Mol Neurodegener (2012) ; 7: : 57. |
33. | Stiernagle T . Maintenance of C. elegans. In: Hope I (ed) . C. elegans: a practical approach. Oxford: Oxford University Press, (1999) , pp. 51–67. |
34. | Link CD , Johnson CJ , Fonte V , et al. Visualization of fibrillar amyloid deposits in living, transgenic Caenorhabditis elegans animals using the sensitive amyloid dye, X-34. Neurobiol Aging (2001) ; 22: : 217–226. |
35. | Brock TJ , Browse J and Watts JL. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics (2007) ; 176: : 865–875. |
36. | Hill JM , Bhattacharjee S , Pogue AI , et al. The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front Neurol (2014) ; 5: : 43. |
37. | Bernardeau M , Lehtinen MJ , Forssten SD , et al. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J Food Sci Technol (2017) ; 54: : 2570–2584. |
38. | Cooper JF , Machiela E , Dues DJ , et al. Activation of the mitochondrial unfolded protein response promotes longevity and dopamine neuron survival in Parkinson’s disease models. Sci Rep (2017) ; 7: : 16441. |
39. | Rana P , Dean DN , Steen ED , et al. Fatty acid concentration and phase transitions modulate Aβ aggregation pathways. Sci Rep (2017) ; 7: : 10370. |
40. | Brock TJ and Watts JL. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet (2006) ; 2: : e108. |
41. | Igal RA and Sinner DI. Stearoyl-CoA desaturase 5 (SCD5), a Δ-9 fatty acyl desaturase in search of a function. Biochim Biophys Acta Mol Cell Biol Lipids (2021) ; 1866: : 158840. |
42. | Astarita G , Jung KM , Vasilevko V , et al. Elevated stearoyl-CoA desaturase in brains of patients with Alzheimer’s disease. PLoS One (2011) ; 6: : e24777. |
43. | Han S , Schroeder EA , Silva-García CG , et al. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature (2017) ; 544: : 185–190. |
44. | Murphy CT , McCarroll SA , Bargmann CI , et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature (2003) ; 424: : 277–283. |
45. | Chuang MH , Chiou SH , Huang CH , et al. The lifespan-promoting effect of acetic acid and Reishi polysaccharide. Bioorg Med Chem (2009) ; 17: : 7831–7840. |
46. | Zečić A and Braeckman BP. DAF-16/FoxO in Caenorhabditis elegans and its role in metabolic remodeling. Cells (2020) ; 9: : 109. |
47. | Martorell P , Llopis S , Gonzalez N , et al. Probiotic strain Bifidobacterium animalis subslactis CECT reduces fat content and modulates lipid metabolism and antioxidant response in Caenorhabditis elegans. J Agric Food Chem (2016) ; 64: : 3462–3472. |
48. | Yoo SR , Kim YJ , Park DY , et al. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-Induced obesity. Obesity (2013) ; 21: : 2571–2578. |
49. | Sorrentino V , Romani M , Mouchiroud L , et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature (2017) ; 552: : 187–193. |
50. | Donato V , Ayala FR , Cogliati S , et al. Bacillus subtilis biofilm extends Caenorhabditis elegans longevity through downregulation of the insulin-like signalling pathway. Nat Commun (2017) ; 8: : 14332. |
51. | Stuhr NL and Curran SP. Bacterial diets differentially alter lifespan and healthspan trajectories in C. elegans. Commun Biol (2020) ; 3: : 653. |
52. | Xia X , Jiang Q , McDermott J , et al. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell (2018) ; 17: : e12802. |
53. | Saul N , Möller S , Cirulli F , et al. Health and longevity studies in C. elegans: The “healthy worm database” reveals strengths, weaknesses and gaps of test compound-based studies. Biogerontology (2021) ; 22: : 215–236. |
54. | Nomura T , Horikawa M , Shimamura S , et al. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr (2010) ; 5: : 17–27. |
55. | Van Gilst MR , Hadjivassiliou H and Yamamoto KR. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A (2005) ; 102: : 13496–13501. |
56. | Antebi A . Nuclear receptor signal transduction in C. elegans. In: WormBook: The Online Review of C. elegans Biology [Internet]. Pasadena (CA): WormBook; 2005–2018. Available from https://www.ncbi.nlm.nih.gov/books/NBK19758/. |
57. | Taubert S , Hansen M , Van Gilst MR , et al. The Mediator subunit MDT-15 confers metabolic adaptation to ingested material. PLoS Genet (2008) ; 4: : e1000021. |
58. | Littlejohn NK , Seban N , Liu CC , et al. A feedback loop governs the relationship between lipid metabolism and longevity. Elife (2020) ; 9: : e58815. |



