Association of Vitamin E and Cognitive Decline in Older Adults with and without the APOEɛ4 Allele: A Biracial Population-Based Community Study
Abstract
Background:
The association of different types of tocopherols (vitamin E) with cognition might vary by the APOE ɛ4 allele status.
Objective:
We examined the association of dietary tocopherols with cognitive decline among participants with and without the APOE ɛ4 allele over a median of 12 years.
Methods:
2,193 participants from the Chicago Health and Aging Project were included in the analyses. Global cognition was assessed in three-year cycles. We used a 144-item FFQ to assess dietary intakes of tocopherols and hME Sequenom mass-array platform to assess APOE genotype. We used linear mixed effects models to examine the relationship between tocopherol from food sources and global cognitive decline.
Results:
The mean baseline age was 74.1 (SD = 5.9) years. Among APOE ɛ4 carriers, participants in the highest quintile of intakes of dietary vitamin E had a slower cognitive decline of 0.022 SDU (95% CI: 0.000, 0.043) compared to those in the lowest quintile. A higher intake of dietary α-tocopherol from food sources only was associated with slower cognitive decline in APOE ɛ4 carriers (p for trend 0.002) but not among the non-carriers (p for trend 0.937). Among APOE ɛ4 carriers, those in the highest quintile of intake of α-tocopherol had a 16.4% slower rate of decline of global cognition compared to those in the lowest quintile (β= 0.034, 95% CI: 0.013, 0.054).
Conclusions:
Individuals consuming high α-tocopherol from food sources had slower cognitive decline among APOE ɛ4 carriers. In older adults, different forms of vitamin E might moderate the relationship of APOE ɛ4 with global cognition.
INTRODUCTION
Cognitive decline and Alzheimer’s disease (AD) and related dementias are degenerative neurological conditions that significantly impact the quality of life and have adverse health outcomes in older adults [1]. Due to high metabolic activity, the brain is particularly vulnerable to oxidative damage. Given the growing evidence implicating oxidative process in the etiology of neurodegenerative diseases, nutrients with antioxidant capacities have been suggested to have protective effects on brain function [2, 3].
Vitamin E is a fat-soluble vitamin with antioxidant capacities [4]. Strong evidence from animal studies and epidemiological studies suggests that high intakes of vitamin E were correlated with a lower risk of dementia [3], AD [5], and cognitive decline [6, 7]. Long-term vitamin E sufficiency early in life was associated with a significantly lower chance of developing AD neuropathologic changes, thereby suggesting a beneficial effect of long-term vitamin E intake among adults aged 90 years and older [8]. However, findings from randomized controlled trials on vitamin E supplementation in delaying cognitive decline or lowering the risk of AD cognition remain inconclusive [4, 9–12].
The heterogeneity among previous findings might be partially contributed to 1) the different sources and the forms of vitamin E (dietary versus supplements, α-tocopherols versus other forms), 2) the duration of supplementation, and 3) apolipoprotein E polymorphisms (APOE ɛ4 allele carrier versus none) [5]. For instance, APOE ɛ4 allele is a known risk factor for AD. Moreover, ApoE also has a vital function in transportation and the homeostasis of fat-soluble vitamins, such as vitamin E [13].
Individuals with the APOE ɛ4 allele might respond differently to vitamin E. Nevertheless, the association of different forms of vitamin E on cognition among APOE ɛ4 carriers and non-carriers remains largely unknown. Our previous investigation suggested that not only α-tocopherol but higher intakes of other tocopherol forms like γ- and δ-tocopherols were associated with a lower risk of incident AD [14]. The present study aims to further examine the association of vitamin E from food sources, varying tocopherol forms, and the influence of the APOE ɛ4 risk allele on cognitive decline among older persons in a longitudinal bi-racial cohort.
MATERIALS AND METHODS
Study population
The Chicago Health and Aging Project (CHAP), initiated in 1993, is a longitudinal, biracial, population-based study with a census of individuals aged 65 years or older located on the south side of Chicago [15]. Of those identified, 6,158 individuals (79%) received an in-home interview. Data on participants’ demographic information, health outcomes, and current functioning were collected during the in-home interview. We also conducted tests examining physical and cognitive performance. The initial interview was conducted from 1993 to 1997, and subsequent interviews were repeated at three-year intervals, with up to six data collection cycles. The CHAP study enrolled 62% Black participants and had 89% follow-up of all surviving participants over the study period. In this analysis, we included participants with APOE genotype assessment, a completed food frequency questionnaire (FFQ), and a minimum of two cognitive assessments. The exclusion criteria were individuals with body mass index (BMI) < 14 or > 55, implausible caloric intakes (<500 kcal or > 3800 kcal for women, < 800 kcal or > 4200 kcal for men), or with FFQs with entire pages or > 50% of pages left blank. The quality of FFQs was screened by a trained research assistant [14]. FFQs were also considered invalid among individuals with Mini-Mental State Examination score < 10 at the baseline interview. We included 2,195 participants in the final analyses over a median of 12 years. This study was approved by Rush University Medical Center Institutional Review Board. Written informed consent was obtained from all study participants.
Dietary assessment
We used a self-administered semi-quantitative 144-item FFQ modified from the Harvard FFQ to assess dietary intakes [16]. The FFQ was collected at each cycle. We used vitamin E intakes from the first available FFQ in the present study. Information regarding the computation of nutrient and food intakes based on FFQs has been published previously elsewhere [17]. Vitamin E isoform intake was calculated using the USDA and Harvard University food composition databases. We used FFQs collected prior to 2002 cycle when intakes of individual types of tocopherol were processed and reported from USDA and Harvard database. Because our previous investigation showed no association with vitamin E supplements [5, 18], in the present study, we investigated the association of intakes of total vitamin E isoforms (from both diet and supplements) and vitamin E from dietary sources only. α-, β-, δ-, and γ-tocopherol from food sources (i.e., vegetable oils, vegetables, nuts, and seeds) were analyzed. We used the residual regression method [19] to perform gender-specific energy adjustments for these vitamins. In a validation study among a subset of randomly selected CHAP participants (n = 232), intakes of individual forms of vitamin E from FFQs (α-, β-, δ-, and γ-tocopherols) have a moderate correlation with intakes from multiple 24-h dietary recalls over 12 months [14].
Cognitive function assessment
During an in-home interview at each cycle, we conducted four cognitive performance tests consisting of two measures of episodic memory [20, 21], one measure of perceptual speed [22], and the Mini-Mental State Examination [23]. Details on each test were reported in previous publication [17]. We used baseline population mean and standard deviation for each test to standardize the raw scores to z scores. We then created a composite z score by averaging the z scores from all four tests as the global cognitive score. In this case, the cognitive score was scaled in standard units and a higher score indicates better performance. Compared to analyzing the individual tests, the global measure had the advantages of reducing problems associated with measurement error, including floor and ceiling effects and having an approximately normal distribution.
APOE ɛ4 allele
We used two single nucleotide polymorphisms (SNPs), rs7412 and rs429358 to determine APOE ɛ4 genotypes. These SNPs were measured by the hME Sequenom MassARRAY platform (Sequenom, Inc., San Diego, CA) at the Broad Institute at Harvard University (Cambridge, MA) [24]. For SNP rs7412 and SNP rs429358, genotyping call rates were 100% and 99.8%, respectively. We created an indicator variable based on the two SNPs for participants with one or more copies of the APOE ɛ4 risk allele.
Assessment of demographic variables
We used the 1990 US Census questionnaire during an in-home interview to collect data on social and demographic characteristics [25]. History of medical conditions and medication use was self-reported. Weight (kg) and height (meters) were measured and used to compute BMI (kg/meter2).
Statistical analysis
The primary outcome was annual changes in the rate of cognitive decline. For continuous variables, data were presented as mean and standard deviations (SDs); for categorical variables, data were presented as frequency (%). We used Welch Two Sample t-test and Pearson’s Chi-square Test to compare baseline characteristics among APOE ɛ4 carriers versus non-carriers.
Linear mixed effects models were used to examine the association between intakes of vitamin E (total, and the individual tocopherols: α, γ, β, and δ) and the annual change in cognitive function. The random-effect model includes subject-specific intercept and slope. The random-effect model allows for individual variation in initial cognitive function and the change in annual cognitive function. We used multivariate model accounted for age (years), sex (F/M), education (years), calorie (kcal), smoking status (current, former), race (Blacks, Whites), APOE ɛ4 allele (any ɛ4 allele or none), and their respective interactions with time, and interaction term between vitamin E (total, and the individual tocopherols: α, γ, β, and δ), time, and APOE ɛ4 genotype. Based on a priori hypothesis, we performed stratification analyses of each model stratified by APOE ɛ4 allele carriership. Intakes were modeled as both continuous variables and categorical variables in quintiles. We used the lowest quintile as the referent category. We used median values of each quintile to calculate the p-value for the trend. When intakes were modeled as continuous variables, we used 5 mg as unit of exposure for total vitamin E (diet and supplements), α-, γ-tocopherol and 1 mg for β- and δ-tocopherols. SAS version 9.4 was used for data analysis with a type 1 error rate for significance at 0.05, and all tests were 2-sided.
RESULTS
The mean age of study participants was 74.0 (SD 5.9) years, with 62% female and 56% Black participants. Overall, the APOE ɛ4 allele frequency was higher among Black participants than White participants. Participants without the APOE ɛ4 allele were older and had a higher global cognition score at baseline assessment than those with the APOE ɛ4 allele (Table 1). Intakes of tocopherol from food sources were similar between participants with or without the APOE ɛ4 allele.
Table 1
Participants’ baseline characteristics
| Overall N = 2,193 | No APOE ɛ4 N = 1,501 | Any APOE ɛ4 N = 692 | P* | |
| Age | 74.0 (5.9) | 74.2 (6.0) | 73.5 (5.7) | 0.006 |
| Education | 12.6 (3.6) | 12.6 (3.6) | 12.5 (3.6) | 0.65 |
| Daily Calories | 1,738 (607) | 1,738 (598) | 1,738 (627) | >0.99 |
| Global cognition | 0.31 (0.67) | 0.34 (0.66) | 0.26 (0.67) | 0.009 |
| Total vitamin E* (mg) | 8.5 (5.2, 37.4) | 8.8 (5.2, 38.3) | 8.3 (5.1, 36.1) | 0.34 |
| Tocopherols from food sources (mg) | ||||
| α-tocopherol | 7.2 (5.5, 9.3) | 7.2 (5.6, 9.3) | 7.2 (5.5, 9.4) | 0.96 |
| β-tocopherol | 0.76 (0.54, 1.02) | 0.75 (0.54, 1.02) | 0.76 (0.54, 1.03) | 0.65 |
| δ-tocopherol | 3.5 (2.5, 5.0) | 3.6 (2.5, 5.0) | 3.4 (2.5, 4.9) | 0.63 |
| γ-tocopherol | 12.2 (8.8, 16.8) | 12.2 (8.8, 16.9) | 12.0 (8.8, 16.4) | 0.46 |
| Male (%) | 827 (38) | 544 (36) | 283 (41) | 0.041 |
| Black (%) | 1,236 (56) | 789 (53) | 447 (65) | <0.001 |
| Current smoker (%) | 264 (12) | 184 (12) | 80 (12) | 0.69 |
| Former smoker (%) | 862 (39) | 577 (38) | 285 (41) | 0.24 |
*sum of vitamin E from supplements and food sources; Data are mean (SD); Median (IQR); n (%); p-values were calculated using; * Welch Two Sample t-test; Wilcoxon rank sum test; Pearson’s Chi-squared test.
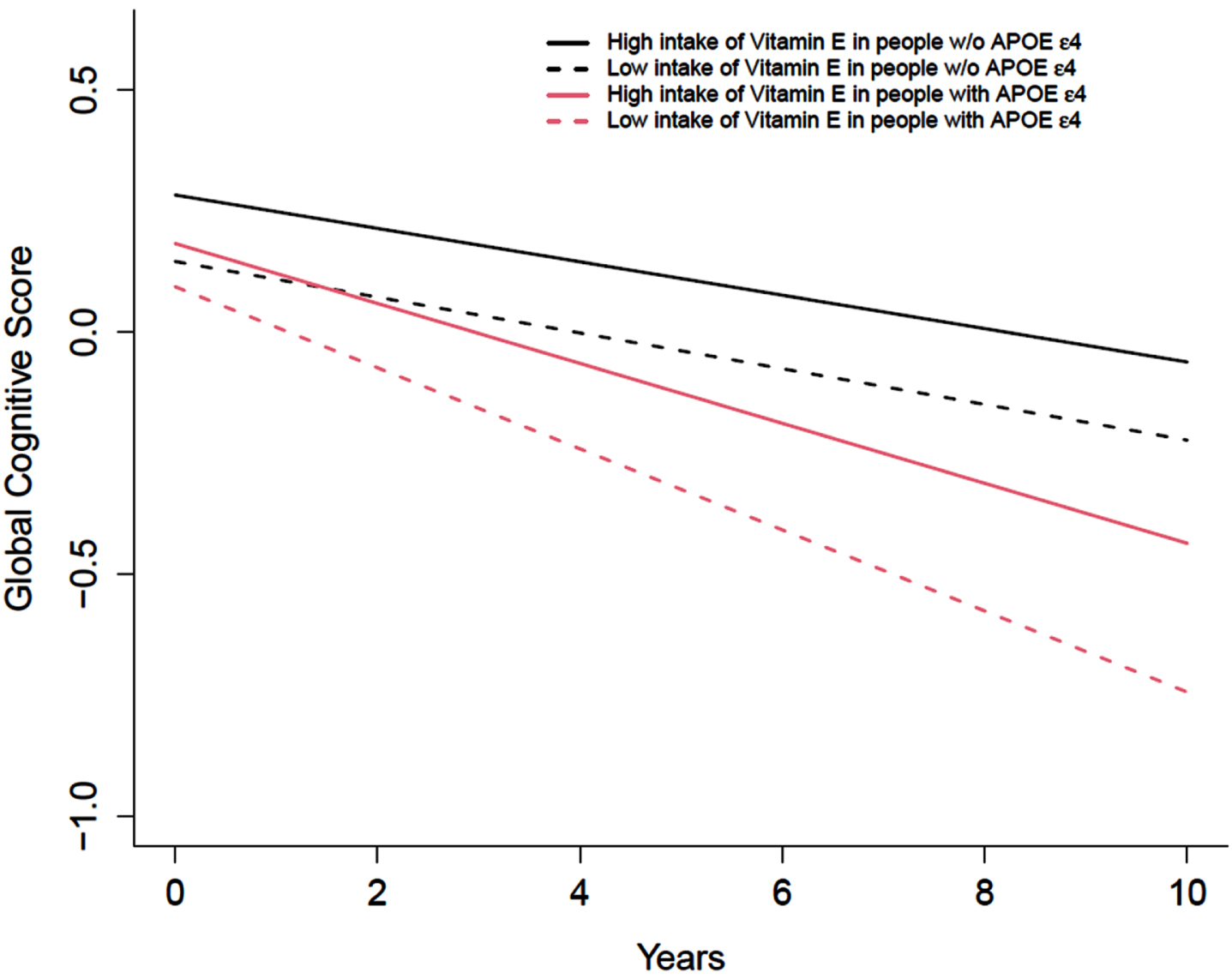
We first examined the association between total vitamin E intake and cognitive decline. We observed that vitamin E from food sources only was associated with a slower cognitive decline in those with the high-risk carrying APOE ɛ4 allele. Among APOE ɛ4 carriers, higher intakes of vitamin E from food sources were significantly associated with a slower cognitive decline (p for trend 0.02). Among APOE ɛ4 carriers, those in the highest quintile of intakes of vitamin E from food sources had a slower cognitive decline of 0.022 SDU (95% CI: 0.000 to 0.043) compared to those in the lowest quintile (Table 2). The differences were equivalent to a 22% slower annual rate of cognitive decline comparing the highest intake to the lowest intake of vitamin E among those with the APOE ɛ4 allele (Fig. 1). Vitamin E from food sources was not associated with the annual rate of cognitive decline in individuals without APOE ɛ4 allele.
Table 2
Association of total vitamin E intakes and intakes of vitamin E from non-supplementary sources with global cognition in participants with or without APOE ɛ4 allele
| Total Vitamin E * | |||||||
| Q1a | Q2 | Q3 | Q4 | Q5 | p for trend | Per unit increase† | |
| Median intakes, mg/d (min, max) | 4.43 (1.36,5.04) | 5.56 (5.05,6.19) | 6.90 (6.20, 8.59) | 24.20 (8.68,42.10) | 359.40 (42.20,1674.60) | ||
| All | –0.043 | 0.000 (–0.010, 0.010) | 0.005 (–0.005, 0.015) | 0.001 (–0.009, 0.011) | 0.006 (–0.003, 0.015) | 0.23 | 0.00 (0.00) |
| with APOE ɛ4 allele | –0.088 | –0.000 (–0.022, 0.021) | 0.01 (–0.011, –0.027) | 0.003 (–0.018, 0.024) | 0.016 (–0.005, 0.037) | 0.13 | 0.00 (0.00) |
| without APOE ɛ4 allele | –0.039 | 0.000 (–0.010, 0.010) | 0.003 (–0.008, 0.014) | 0.002 (–0.009, 0.012) | 0.002 (–0.008, 0.012) | 0.72 | 0.00 (0.00) |
| Vitamin E from food sources | |||||||
| Q1a | Q2 | Q3 | Q4 | Q5 | p for trend | ||
| Median intakes, mg/d (min, max) | 4.15 (1.51,4.63) | 5.01 (4.64,5.33) | 5.67 (5.34, 6.01) | 6.39 (6.02,6.89) | 7.78 (6.9, 43.45) | ||
| All | –0.048 | 0.01 (0.000, 0.019) | 0.0102 (0.000, 0.020) | 0.013 (0.004, 0.023) | 0.008 (–0.002, 0.017) | 0.18 | –0.002 (0.002) |
| with APOE ɛ4 | –0.099 | 0.012 (–0.009, 0.033) | 0.017 (–0.005, 0.038) | 0.03 (0.009, 0.051) | 0.022 (0.000, 0.043) | 0.02 | 0.005 (0.005) |
| without APOE ɛ4 | –0.043 | 0.008 (–0.002, 0.019) | 0.007 (–0.003, 0.017) | 0.005 (–0.005, 0.016) | 0.002 (–0.008, 0.013) | 0.94 | –0.002 (0.002) |
*Total intakes of vitamin E from foods and supplements. †Data are expressed as β (SD). β represents per 5 mg increased intakes of vitamin E. aCognitive score (in standard units) of participants in the first quintile. Data in Q2 to Q5 are differences in global cognitive scores compared to Q1. A higher score represents a slower rate of decline in global cognition. APOE, Apolipoprotein E. Model 2 was adjusted for age (years), sex (F/M), education (years), calorie (kcal), smoking status (current, former), race, and their respective interactions with time. The p value for the linear trend was tested by treating the median value of each quintile as a continuous variable.
Fig. 1
Intakes of total vitamin E from food sources only and annual change of cognitive function among participants with or without APOE ɛ4 allele (n = 2,193). The intakes of total vitamin E from food sources were categorized into quintiles, with the lowest quintile as the referent group. Red dash line and the solid line represent the lowest and highest quintile of total vitamin E intake among participants with APOE ɛ4 allele. Black dash line and the solid line represent the lowest and highest quintile of total vitamin E intake among participants without APOE ɛ4 allele. Model was adjusted for age (years), sex (F/M), education (years), calorie (kcal), smoking status (current, former), race, and their respective interactions with time.
Subsequently, we examined the association of different forms of tocopherols from foods and their association with the annual rate of cognitive decline. Higher intakes of α-tocopherol were associated with slower cognitive decline (p for trend 0.012). The association of α-tocopherol and cognition was dependent on APOE ɛ4 allele carriership (p for interaction 0.046). Among individuals with APOE ɛ4 allele, intakes of α-tocopherol were associated with a slower annual change in cognitive function (p for trend 0.002). Per 5 mg increase in the intakes of α-tocopherol was associated with a slower annual change in global cognitive score by 0.008 SDU (SD 0.0048, p = 0.099). Participants with the APOE ɛ4 allele in the highest quintile of α-tocopherol intakes had a slower annual change in cognitive function by 0.033 SDU (95% CI: 0.013 to 0.054) (Table 3). The differences in cognitive function were equivalent to having a 31.6% slower annual cognitive decline between the highest versus the lowest quintile in those with APOE ɛ4 allele (Fig. 2). In contrast, among individuals without APOE ɛ4 allele, higher intakes of δ-tocopherol was associated with slower cognitive decline (p for trend = 0.03) (Table 3). There was a tendency of slower cognitive decline associated with higher intake of γ-tocopherol among APOE ɛ4 allele non-carrier (p = 0.07). Intakes of β-tocopherol was not associated with annual change of global cognition. We conducted a sensitivity analysis using α-tocopherol equivalents (ateq). Similar results were reported. APOE ɛ4 carriers, for global cognition, those in the highest quintile of ateq compared to those in the lowest quintile had a slower cognitive decline by β= 0.024±0.0107 (p = 0.03, data not shown).
Fig. 2
Intakes of α-tocopherol from food sources only and the rate of change in global cognitive score among participants with or without APOE ɛ4 allele (n = 2,193). The intakes of α-tocopherol from food sources were categorized into quintiles, with the lowest quintile as the referent group. Red dash line and the solid line represent the lowest and highest quintile of α-tocopherol intake among participants with APOE ɛ4 allele. Black dash line and the solid line represent the lowest and highest quintile of α-tocopherol intake among participants without APOE ɛ4 allele. Model was adjusted for age (years), sex (F/M), education (years), calorie (kcal), smoking status (current, former), race, and their respective interactions with time.
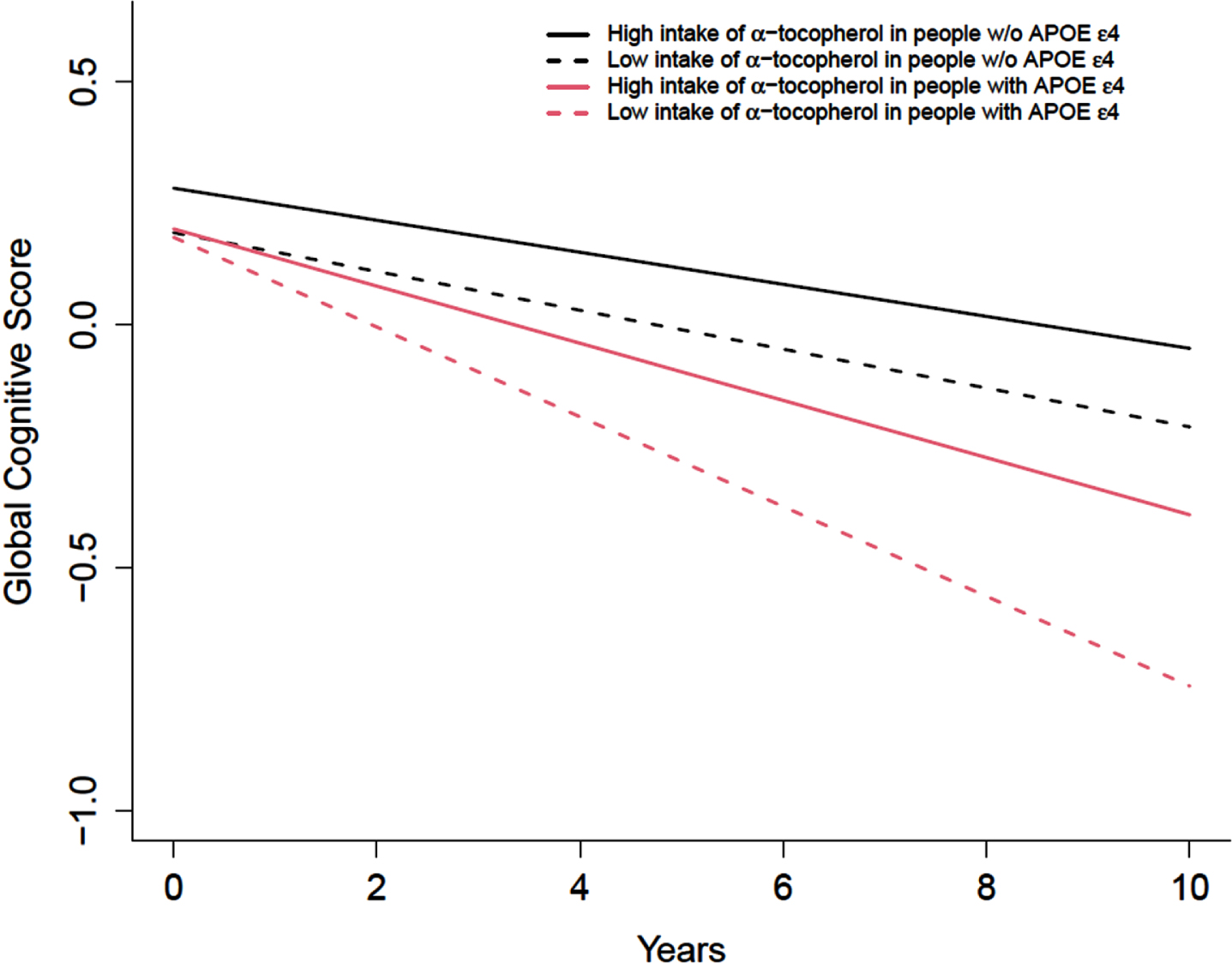
Table 3
Association of food sources α-, β-, δ-, and γ-tocopherol with global cognition in participants with or without APOE ɛ4 allele
| α-tocopherol | |||||||
| Q1a | Q2 | Q3 | Q4 | Q5 | p for trend | ||
| Median intakes, mg/d (min, max) | 5.52 (2.08,6.00) | 6.43 (6.01,6.75) | 7.09 (6.76, 7.44) | 7.82 (7.45,8.32) | 9.12 (8.33,45.2) | Per unit increase† | |
| All | –0.053 | 0.021 (0.011, 0.030) | 0.012 (0.003, 0.022) | 0.021 (0.012, 0.031) | 0.015 (0.006, 0.025) | 0.012 | –0.002 (0.002) |
| with APOE ɛ4 allele | –0.11 | 0.028 (0.007, 0.049) | 0.03 (0.010, –0.027) | 0.0423 (0.021, 0.064) | 0.034 (0.013, 0.054) | 0.002 | 0.008 (0.005)* |
| without APOE ɛ4 allele | –0.05 | 0.016 (0.006, 0.026) | 0.003 (–0.007, 0.014) | 0.012 (0.002, 0.022) | 0.007 (–0.003, 0.017) | 0.51 | –0.002 (0.002) |
| β-tocopherol | |||||||
| Median intakes, mg/d (min, max) | 0.5 (0.12,0.58) | 0.65 (0.59,0.70) | 0.76 (0.71, 0.81) | 0.88 (0.82,0.97) | 1.10 (0.98,2.91) | ||
| All | –0.048 | 0.000 (–0.009, 0.009) | –0.002 (–0.011, 0.007) | 0.001 (–0.008, 0.01) | 0.00 (–0.010, 0.010) | 0.91 | 0.007 (0.008) |
| with APOE ɛ4 allele | –0.099 | –0.013 (–0.033, 0.007) | –0.015 (–0.036, –0.027) | –0.0127 (–0.034, 0.009) | –0.015 (–0.037, 0.007) | 0.23 | –0.019 (0.013) |
| without APOE ɛ4 allele | –0.043 | 0.005 (–0.005, 0.015) | 0.003 (–0.007, 0.013) | 0.006 (–0.004, 0.016) | 0.007 (–0.004, 0.018) | 0.19 | 0.008 (0.007) |
| δ-tocopherol | |||||||
| Median intakes, mg/d (min, max) | 2.28 (0.33,2.66) | 2.98 (2.67,3.25) | 3.53 (3.26,3.83) | 4.17 (3.84,4.66) | 5.38 (4.67,9.76) | ||
| All | –0.048 | 0.015 (0.006, 0.024) | 0.006 (–0.003, 0.015) | 0.008 (–0.001, 0.017) | 0.010 (0.001, 0.02) | 0.17 | 0.002 (0.002) |
| with APOE ɛ4 allele | –0.087 | 0.026 (0.006, 0.047) | 0.003 (–0.016, –0.027) | –0.005 (–0.025, 0.015) | 0.008 (–0.013, 0.029) | 0.70 | –0.000 (0.003) |
| without APOE ɛ4 allele | –0.045 | 0.010 (0.000, 0.020) | 0.007 (–0.003, 0.017) | 0.013 (0.003, 0.023) | 0.011 (0.001, 0.021) | 0.03 | 0.002 (0.001) |
| γ-tocopherol | |||||||
| Median intakes, mg/d (min, max) | 8.17 (1.41,9.38) | 10.4 (9.39,11.25) | 12.1 (11.3, 13.0) | 14.1 (13.0,15.5) | 17.7 (15.5,30.2) | ||
| All | –0.045 | 0.006 (–0.004, 0.015) | 0.007 (–0.003, 0.016) | 0.006 (–0.004, 0.015) | 0.007 (–0.002, 0.017) | 0.21 | 0.003 (0.002) |
| with APOE ɛ4 allele | –0.085 | 0.009 (–0.011, 0.030) | 0.008 (–0.012, –0.027) | –0.0108 (–0.031, 0.010) | 0.008 (–0.014, 0.030) | 0.88 | 0.003 (0.005) |
| without APOE ɛ4 allele | –0.043 | 0.003 (–0.007, 0.013) | 0.006 (–0.005, 0.016) | 0.012 (0.002, 0.023) | 0.007 (–0.003, 0.018) | 0.07 | 0.003 (0.002) |
*p < 0.1. †Data are expressed as β (SD). β represents per 5 mg increased intakes of α-tocopherol, γ-tocopherol, and per 1 mg increased intakes of β-tocopherol, δ-tocopherol, respectively. aCognitive score (in standard units) of participants in the first quintile. Data in Q2 to Q5 are differences in global cognitive scores compared to Q1. A higher score represents a slower rate of decline in global cognition. APOE, Apolipoprotein E. Model was adjusted for age (years), sex (F/M), education (years), calorie (kcal), smoking status (current, former), race, and their respective interactions with time. The p value for the linear trend was tested by treating the median value of each quintile as a continuous variable.
We further investigated the association of dietary α-tocopherol with annual change in cognition among participants with different APOE ɛ4 risk alleles. We found that higher intakes of α-tocopherol were associated with slower annual rates of cognitive decline among participants with APOE ɛ3/ɛ4, and APOE ɛ4/ɛ4 risk alleles (Table 4). In individuals carrying APOE ɛ3/ɛ4 allele, those in the highest quintile, and the second highest intakes of dietary α-tocopherol was associated with a slower annual change in global cognition by 0.040 SDU (SD 0.012, p = 0.001) and 0.029 (SD 0.012, p = 0.012) compared to those in the lowest quintile, respectively. In individuals carrying APOE ɛ4/ɛ4 allele, individuals with highest α-tocopherol intakes had a slower annual change in global cognition by 0.084 (SD 0.032, p = 0.009).
Table 4
Association of food source α-tocopherol with global cognition among participants with different APOE genotype
| α-tocopherol | |||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| APOE ɛ2/ɛ4 (n = 77) | Ref | 0.054 (0.037) | 0.051 (0.034) | 0.043 (0.039) | –0.006 (0.039) |
| APOE ɛ3/ɛ4 (n = 552) | Ref | 0.016 (0.012) | 0.026 (0.012) | 0.040 (0.012)** | 0.029 (0.012)* |
| APOE ɛ4/ɛ4 (n = 63) | Ref | 0.092 (0.033)** | 0.045 (0.033) | 0.041 (0.038) | 0.084 (0.032)** |
| APOE ɛ2/ɛ2 (n = 22) | Ref | –0.184 (0.199) | –0.052 (0.127) | 0.045 (0.111) | –0.092 (0.132) |
| APOE ɛ2/ɛ3 (n = 297) | Ref | 0.025 (0.011) | 0.004 (0.011) | 0.014 (0.011) | 0.014 (0.011) |
| APOE ɛ3/ɛ3 (n = 1178) | Ref | 0.014 (0.006)* | 0.001 (0.006) | 0.008 (0.006) | 0.004 (0.006) |
Model was adjusted for age (years), sex (F/M), education (years), calorie (kcal), smoking status (current, former), race, and their respective interactions with time. Data are expressed as β (SD). *p < 0.05. **p < 0.01.
DISCUSSION
In our study, we found that the vitamin E from food sources was associated with a slower cognitive decline. Of the different tocopherol forms, dietary sources of α-tocopherol were associated with slower cognitive decline, specifically among individuals with APOE ɛ4 allele. Higher intakes of δ-tocopherol were associated with slower cognitive decline among individuals without APOE ɛ4 allele. These findings suggest that the protective association of vitamin E and brain health might depend on 1) the sources of vitamin E and 2) the APOE risk alleles.
Previous studies suggest that vitamin E intake was protective of risk of dementia [26] and AD [14]. Recent evidence suggests that the sources of vitamin E can be a contributing factor to brain function. Higher intakes of vitamin E were associated with a lower risk of dementia and AD, with vitamin E from dietary sources demonstrating a more robust association with lower risk [27]. Previous findings from our group showed that higher food intakes of α-tocopherol and γ-tocopherols were associated with slower cognitive decline [14]. The antioxidant capacities of vitamin E have been proposed to reduce oxidative stress, preserve neuronal integrity, and normal cell function, therefore, delaying cognitive decline and lowering risk of AD [28].
The recommended dietary allowance (RDA) for vitamin E for adults is 15 mg/day [29]. Although overt vitamin E deficiency is rare, previous evidence suggested that more than 60% of adults in the US have intakes below the recommended level [30]. It is worth noting that the average total vitamin E intake in the present study was 8.5 mg/day. The dietary intake of α-tocopherol from food was 7.2 mg/day, which aligns with previous findings among 18,063 American adults in the NHANES 2003–2006 [30], well below the RDA levels. The threshold effect cannot be sufficiently assessed, given that most participants had intakes below the RDA. Further investigation is warranted to examine the threshold effect and its association with cognition.
We observed that vitamin E and α-tocopherol from food sources only were associated with slower cognitive decline among participants with the APOE ɛ4 risk allele. Nutrients are not consumed in isolation but in the matrix of foods. Therefore, the synergistic effects of vitamin E with other nutrients from the diet may further benefit brain health. Common food sources of dietary tocopherols, i.e., nuts, fruit and vegetables, and vegetable oils [31, 32]. Alpha-tocopherol is the most biologically active form of vitamin E in most human and animal tissues and with the most potent antioxidant capacity [33]. Kinetic studies demonstrated that α-tocopherol has a longer retention rate in plasma and tissues, underscoring the concept that α-tocopherol can be maintained and accumulated in the tissue, whereas other forms of tocopherols are metabolized quickly [13]. In addition to its antioxidant properties, α-tocopherol enhances the activity of phosphoprotein phosphatase 2A, an enzyme implicated in AD pathophysiology [34].
The associations we observed herein were genotype specific. APOE polymorphism plays a critical role in lipoprotein transport and lipid metabolism [35], and APOE ɛ4 genotype is one of the strongest risk factors for AD. In vitro, APOE protein shows antioxidant activity. Polymorphisms in APOE, such as APOE ɛ4, APOE ɛ3, or APOE ɛ2 have been reported to have decreased antioxidant capacity (increased oxidative stress), with APOE ɛ4 having the least antioxidant capacity, and APOE ɛ2 with the most potent antioxidant capacity [36–38]. In human studies, those with APOE ɛ4 versus non-APOE ɛ4 carriers showed decreased antioxidant status, and increased oxidized LDL [39], and increased oxidative stress [40]. ApoE determines the transportation of vitamin E into peripheral tissues through HDL binding to scavenger receptor class B type I (SR-BI) [41]. In ApoE-deficient mice, lower concentrations of α-tocopherol were observed in different brain regions, indicating the importance of APOE in transporting α-tocopherol to the brain [13]. Indeed, findings from animal studies suggested differences in the processing of vitamin E regarding APOE genotype with an impaired delivery of vitamin E to peripheral tissues observed in the APOE ɛ4 genotype [35]. This evidence indicate that APOE ɛ4 carriers have a significantly decreased antioxidant capacity either 1) because of the polymorphism of APOE ɛ4 and/or 2) the reduced capacity of APOE ɛ4 in transporting antioxidant nutrients like vitamin E. In the present study, we observed that tocopherols from food sources were associated with slower cognitive decline, particularly in participants with APOE ɛ4 allele, and δ-tocopherol was associated with slower cognitive decline in participants without APOE ɛ4 allele. We hypothesize that higher intakes of food α-tocopherol among participants with APOE ɛ4 genotype might associate with an increase in the uptake of α-tocopherol to the peripheral tissue, including the central nervous system, which may compensate for the reduced antioxidant capacity of ɛ4 allele that could therefore attenuate the cognitive decline.
Our study has limitations. Firstly, intakes of vitamin E were assessed using self-reported FFQ, which could be prone to recall error, and misclassification of dietary intake. However, our previous investigation demonstrated the validity of FFQ using objective biochemical markers in older adults from the CHAP study [42, 43]. We used FFQ data collected prior to 2002 when individual types of tocopherol were reported which decreased the sample size. We excluded participants with MMSE less than 10 to address the potential recall error related to cognitive decline. Second, we used vitamin E intakes from the first available FFQ, which might limit us to assessing the causal association of changes in intakes over time and the changes in cognitive function. There is a potential limitation in generalizing our findings to populations with different socio-economic statuses, given the study demographic represents older adults from the south side of Chicago. Although we have accounted for multiple potential confounders, we cannot completely rule out other residual confounding. We must be cautious against a causal interpretation of the results due to the study’s observational nature and lack of objective measurement of vitamin E. Given the relative smaller samples size of each risk allele among participants with APOE ɛ4 genotype, future studies of different racial groups with larger sample sizes are warranted to validate our findings, and to further investigate the causal role of vitamin E from dietary sources on cognition. The present study adds to the evidence base by examining intakes of vitamin E and cognitive function in a biracial population-based community study with a long-term follow-up among participants with APOE determinations.
Conclusion
Vitamin E from food sources was associated with slower cognitive decline in a high-risk population. Specific forms of vitamin E, i.e., α-tocopherol from food sources, was protective of cognitive decline among those with APOE ɛ4 allele. Our findings suggest that different forms of dietary vitamin E might have different associations with cognitive decline in older adults depending on their APOE ɛ4 allele status.
ACKNOWLEDGMENTS
We thank participants of the CHAP study for their dedication to research.
FUNDING
The present work was supported by Alzheimer’s Association Research Grant AARG-22-928311, research grants from National Institutes of Health R01AG03154, R01AG058679, R01AG073627, and UH2AG083289. CJF is a REC scholar at the UC Davis ADRC (P30AG072972).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data are not publicly available due to privacy or ethical restrictions. The data supporting the findings of this study can be requested at https://www.riha.rush.edu/dataportal.html pending approval of study proposal and DUA.
REFERENCES
[1] | United Nations, Department of Economic and Social Affairs (2019) World Population Ageing 2019: Highlights (ST/ESA/SER.A/430). |
[2] | Dominguez LJ , Barbagallo M , Muñoz-Garcia M , Godos J , Martinez-Gonzalez MA ((2019) ) Dietary Patterns and cognitive decline: Key features for prevention. Curr Pharm Des 25: , 2428–2442. |
[3] | Devore EE , Grodstein F , van Rooij FJ , Hofman A , Stampfer MJ , Witteman JC , Breteler MM ((2010) ) Dietary antioxidants and long-term risk of dementia. Arch Neurol 67: , 819–825. |
[4] | Farina N , Isaac MG , Clark AR , Rusted J , Tabet N ((2012) ) Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev 11: , CD002854. |
[5] | Morris MC , Evans DA , Bienias JL , Tangney CC , Bennett DA , Aggarwal N , Wilson RS , Scherr PA ((2002) ) Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 287: , 3230–3237. |
[6] | Masaki KH , Losonczy KG , Izmirlian G , Foley DJ , Ross GW , Petrovitch H , Havlik R , White LR ((2000) ) Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 54: , 1265–1272. |
[7] | Grodstein F , Chen J , Willett WC ((2003) ) High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am J Clin Nutr 77: , 975–984. |
[8] | Paganini-Hill A , Bukhari S , Montine TJ , Corrada MM , Kawas CH ((2023) ) Alzheimer’s disease neuropathologic change and vitamin supplement use decades earlier: The 90+ Study. Alzheimer Dis Assoc Disord 37: , 1–6. |
[9] | Sano M , Ernesto C , Thomas RG , Klauber MR , Schafer K , Grundman M , Woodbury P , Growdon J , Cotman CW , Pfeiffer E , Schneider LS , Thal LJ ((1997) ) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 336: , 1216–1222. |
[10] | Petersen RC , Thomas RG , Grundman M , Bennett D , Doody R , Ferris S , Galasko D , Jin S , Kaye J , Levey A , Pfeiffer E , Sano M , van Dyck CH , Thal LJ ((2005) ) Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352: , 2379–2388. |
[11] | Lloret A , Badía MC , Mora NJ , Pallardó FV , Alonso MD , Viña J ((2009) ) Vitamin E paradox in Alzheimer’s disease: It does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis 17: , 143–149. |
[12] | Dysken MW , Sano M , Asthana S , Vertrees JE , Pallaki M , Llorente M , Love S , Schellenberg GD , McCarten JR , Malphurs J , Prieto S , Chen P , Loreck DJ , Trapp G , Bakshi RS , Mintzer JE , Heidebrink JL , Vidal-Cardona A , Arroyo LM , Cruz AR , Zachariah S , Kowall NW , Chopra MP , Craft S , Thielke S , Turvey CL , Woodman C , Monnell KA , Gordon K , Tomaska J , Segal Y , Peduzzi PN , Guarino PD ((2014) ) Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 311: , 33–44. |
[13] | Vatassery GT , Lam C , Smith WE , Quach HT ((2006) ) Apolipoprotein E exerts selective and differential control over vitamin E concentrations in different areas of mammalian brain. J Neurosci Res 84: , 1335–1342. |
[14] | Morris MC , Evans DA , Tangney CC , Bienias JL , Wilson RS , Aggarwal NT , Scherr PA ((2005) ) Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr 81: , 508–514. |
[15] | Bienias JL , Beckett LA , Bennett DA , Wilson RS , Evans DA ((2003) ) Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis 5: , 349–355. |
[16] | Morris MC , Colditz GA , Evans DA ((1998) ) Response to a mail nutritional survey in an older bi-racial community population. Ann Epidemiol 8: , 342–346. |
[17] | Liu X , Dhana K , Barnes LL , Tangney CC , Agarwal P , Aggarwal N , Holland TM , Beck T , Evans DA , Rajan KB ((2022) ) A healthy plant-based diet was associated with slower cognitive decline in African American older adults: A biracial community-based cohort. Am J Clin Nutr 116: , 875–886. |
[18] | Morris MC , Evans DA , Bienias JL , Tangney CC , Wilson RS ((2002) ) Vitamin E and cognitive decline in older persons. Arch Neurol 59: , 1125–1132. |
[19] | Willett W , Stampfer MJ ((1986) ) Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol 124: , 17–27. |
[20] | Albert M , Smith LA , Scherr PA , Taylor JO , Evans DA , Funkenstein HH ((1991) ) Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 57: , 167–178. |
[21] | Wilson RS , Beckett LA , Barnes LL , Schneider JA , Bach J , Evans DA , Bennett DA ((2002) ) Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 17: , 179–193. |
[22] | Smith A (1982) Symbol Digit Modalities Test (SDMT): Manual. Western Psychological Los Angeles, CA. |
[23] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[24] | Rajan KB , McAninch EA , Wilson RS , Weuve J , Barnes LL , Evans DA ((2019) ) Race, APOEɛ4, and long-term cognitive trajectories in a biracial population sample. J Alzheimers Dis 72: , 45–53. |
[25] | Wilson RS , Bennett DA , Beckett LA , Morris MC , Gilley DW , Bienias JL , Scherr PA , Evans DA ((1999) ) Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci 54: , P155–160. |
[26] | De la Fuente M , Hernanz A , Guayerbas N , Victor VM , Arnalich F ((2008) ) Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res 42: , 272–280. |
[27] | Zhao R , Han X , Zhang H , Liu J , Zhang M , Zhao W , Jiang S , Li R , Cai H , You H ((2022) ) Association of vitamin E intake in diet and supplements with risk of dementia: A meta-analysis. Front Aging Neurosci 14: , 955878. |
[28] | Sopher BL , Fukuchi K , Kavanagh TJ , Furlong CE , Martin GM ((1996) ) Neurodegenerative mechanisms in Alzheimer disease. A role for oxidative damage in amyloid beta protein precursor-mediated cell death. Mol Chem Neuropathol 29: , 153–168. |
[29] | Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds (2000) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids Institute of Medicine, Washington, DC. |
[30] | Fulgoni VL , 3rd , Keast DR , Bailey RL , Dwyer J ((2011) ) Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr 141: , 1847–1854. |
[31] | Boccardi V , Baroni M , Mangialasche F , Mecocci P ((2016) ) Vitamin E family: Role in the pathogenesis and treatment of Alzheimer’s disease. Alzheimers Dement (N Y) 2: , 182–191. |
[32] | McLaughlin PJ , Weihrauch JL ((1979) ) Vitamin E content of foods. J Am Diet Assoc 75: , 647–665. |
[33] | Mustacich DJ , Bruno RS , Traber MG ((2007) ) Vitamin E. Vitam Horm 76: , 1–21. |
[34] | Voronkov M , Braithwaite SP , Stock JB ((2011) ) Phosphoprotein phosphatase 2A: A novel druggable target for Alzheimer’s disease. Future Med Chem 3: , 821–833. |
[35] | Huebbe P , Lodge JK , Rimbach G ((2010) ) Implications of apolipoprotein E genotype on inflammation and vitamin E status. Mol Nutr Food Res 54: , 623–630. |
[36] | Miyata M , Smith JD ((1996) ) Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet 14: , 55–61. |
[37] | Peroutka SJ , Dreon DM ((2000) ) The value of genotyping for apolipoprotein E alleles in relation to vitamin E supplementation. Eur JPharmacol 410: , 161–163. |
[38] | Gómez-Coronado D , Entrala A , Alvarez JJ , Ortega H , Olmos JM , Castro M , Sastre A , Herrera E , Lasunción MA ((2002) ) Influence of apolipoprotein E polymorphism on plasma vitamin A and vitamin E levels. Eur J Clin Invest 32: , 251–258. |
[39] | Talmud PJ , Stephens JW , Hawe E , Demissie S , Cupples LA , Hurel SJ , Humphries SE , Ordovas JM ((2005) ) The significant increase in cardiovascular disease risk in APOEepsilon4 carriers is evident only in men who smoke: Potential relationship between reduced antioxidant status and ApoE4. Ann Hum Genet 69: , 613–622. |
[40] | Dietrich M , Hu Y , Block G , Olano E , Packer L , Morrow JD , Hudes M , Abdukeyum G , Rimbach G , Minihane AM ((2005) ) Associations between apolipoprotein E genotype and circulating F2-isoprostane levels in humans. Lipids 40: , 329–334. |
[41] | Mardones P , Strobel P , Miranda S , Leighton F , Quiñones V , Amigo L , Rozowski J , Krieger M , Rigotti A ((2002) ) Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J Nutr 132: , 443–449. |
[42] | Morris MC , Tangney CC , Bienias JL , Evans DA , Wilson RS ((2003) ) Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 158: , 1213–1217. |
[43] | Tangney CC , Bienias JL , Evans DA , Morris MC ((2004) ) Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr 134: , 927–934. |




