Detecting Anosognosia from the Prodromal Stage of Alzheimer’s Disease
Abstract
Background:
Though not originally developed for this purpose, the Healthy Aging Brain Care Monitor (HABC-M) seems a valuable instrument for assessing anosognosia in Alzheimer’s disease (AD).
Objectives:
Our study aimed at 1) investigating the validity of the HABC-M (31 items), and its cognitive, psychological, and functional subscales, in discriminating AD patients from controls; 2) exploring whether the HABC-M discrepancy scores between the self-reports of patients/controls in these different domains and the respective ratings provided by their caregivers/informants correlate with an online measure of self-awareness; 3) determining whether the caregiver burden level, also derived from the HABC-M, could add additional support for detecting anosognosia.
Methods:
The HABC-M was administered to 30 AD patients and 30 healthy controls, and to their caregivers/informants. A measure of online awareness was established from subjects’ estimation of their performances in a computerized experiment.
Results:
The HABC-M discrepancy scores distinguished AD patients from controls. The cognitive subscale discriminated the two groups from the prodromal AD stage, with an AUC of 0.88 [95% CI: 0.78;0.97]. Adding the caregiver burden level raised it to 0.94 [0.86;0.99]. Significant correlations between the HABC-M and online discrepancy scores were observed in the patients group, providing convergent validity of these methods.
Conclusions:
The cognitive HABC-M (six items) can detect anosognosia across the AD spectrum. The caregiver burden (four items) may corroborate the suspicion of anosognosia. The short-hybrid scale, built from these 10 items instead of the usual 31, showed the highest sensitivity for detecting anosognosia from the prodromal AD stage, which may further help with timely diagnosis.
INTRODUCTION
The Healthy Aging Brain Care Monitor (HABC-M) [1, 2] was initially developed as an instrument to rapidly measure and track dementia symptoms in the clinical setting. It comprises two versions— the patient self-report and the informant/caregiver report— covering the same 27 items on three main domains of subjects’ symptoms (cognitive, six items: e.g. “remembering appointments”; functional, 11 items: e.g. “taking medications in the right dose at the right time”; behavioral/psychological, 10 items: e.g. “less interest or pleasure in doing things, hobbies or activities”), plus four items to assess the caregiver burden (corresponding to generic items related to the caregivers “quality of life domain”, such as concerns about their own “mental health” or “financial future”), to be answered solely by informants/caregivers [1]. A discrepancy score between the caregiver and the patient reports (namely, when the patient reports fewer difficulties than the caregiver observes) may suggest lack of awareness of one’s symptoms [2]. Therefore, while not originally developed for this purpose, evidence suggests that the HABC-M may be a valuable instrument for detecting anosognosia (i.e., the lack of awareness of one’s own deficits [3]) from the preclinical stages of AD [4]. But the HABC-M may be of further interest in the clinical context of anosognosia, because it also assesses the quality of life of caregivers. Conceptually, caregiver burden has been defined as a multidimensional reaction to the physical, emotional, social, and financial stressors associated with the caregiving experience. Anosognosia can be a major source of stress for caregivers, as it often delays disease diagnosis, affects compliance with treatment [5], and threatens patient safety [6]. For example, reduced self-awareness of cognitive and functional changes in AD patients can result in risky behaviors such as continued driving [7]. In line with this, decreased awareness of AD patients was significantly associated with higher caregiver burden [8, 9]. Also, the frequency of anosognosia has been found to increase considerably with the severity of dementia [10].
In neurological practice, the most commonly used methods for assessing anosognosia are the “clinician rating” and the “caregiver-patient discrepancy” questionnaire. The clinician rating method relies on the clinician’s judgment of the patient’s level of awareness, which is determined through interviews with both the patient and caregiver. On the other hand, the caregiver-patient discrepancy questionnaire involves contrasting the parallel patient and caregiver reports, in which both rate the patient’s abilities on distinct domains. These two approaches measure anosognosia in an offline way, covering declarative knowledge about one’s own abilities. There are also online measures of anosognosia, although more time consuming and less frequently used in the clinical setting [11]. Online measures involve comparing the patient’s actual performance on a cognitive task with their own performance prediction or postdiction, thereby capturing real-time “performance discrepancy” [12]. At present, only a few studies have directly compared offline with online methods in the same population, which points to the need for further research in this area [13].
A recent review encompassing 46 articles evaluated various instruments used to assess anosognosia in patients with dementia [14]. A total of 49 different methods were identified: 10 corresponded to clinical rating instruments, 25 to caregiver-patient discrepancy instruments, and 14 to performance discrepancy instruments, thus revealing a lack of consensus on the optimal strategy for detecting anosognosia in clinical practice. The HABC-M has not been included in this review, probably because it was not originally developed for this specific purpose. After comparing those 49 anosognosia assessment instruments, the authors recommended the following two for routine use: the Clinical Insight Rating scale (or CIRS; clinical judgment method [15]) and the Abridged Anosognosia Questionnaire— Dementia (or AAQ; patient-caregiver discrepancy method [16]). Both the CIRS and the AAQ are multi-domain instruments that offer practicality and efficiency, but none has been proven particularly sensitive in the early stages of AD. Moreover, neither the CIRS nor the AAQ specifically address caregiver quality of life, despite the established association between caregiver burden and anosognosia [8]. Supporting caregivers is crucial in the care of older adults with dementia [17]. Therefore, evaluating caregivers’ well-being in anosognosia assessment instruments may provide help to put in place medico-social measures to relieve families facing this harmful condition. Importantly, as explained in [1], “the HABC-Monitor assesses general caregiver quality of life, and does not require the caregiver to identify whether problems with their quality of life are due to patient symptoms or due to other sources”. Last, but not least, the detection of anosognosia in early AD stages holds significant relevance as it may lead to timely diagnosis, improved disease management, and reduced caregiver burden [18].
The primary objective of this study was to investigate several key aspects. First, we aimed to determine whether the discrepancy scores derived from the HABC-M could effectively differentiate patients with AD from healthy elderly controls. Second, we sought to evaluate the sensitivity of the cognitive, functional, and psychological subscales of the HABC-M in detecting anosognosia across different stages of AD, including prodromal, mild, and moderate stages. Third, we aimed to examine the correlation between the HABC-M discrepancy scores and an online measure of self-awareness, as well as with subjects’ level of cognition in several domains. Finally, we aimed to explore whether the level of caregiver burden, an exclusive caregiver/informant subscale of the HABC-M, could provide further insights into patients’ anosognosia.
MATERIAL AND METHODS
Participants
A total of 60 participants, comprising 30 AD patients (AD group) and 30 elderly healthy controls (CTRL group), were enrolled in the study. All had a study partner (caregiver or informant), who was their spouse (wife or husband) for 58 participants or their children for the other two participants. They knew their relatives well. Participants were native French speakers, ranging in age from 50 to 85 years, with normal or corrected-to-normal vision. The study was conducted at the Institute of Memory and Alzheimer’s Disease (IM2A), located within the Pitié-Salpêtrière Hospital in Paris, France. The research protocol was approved by the local ethics committee and conducted according to the Declaration of Helsinki. A written informed consent was obtained from all participants before their inclusion in the study.
The AD patients group (n = 30) fulfilled the diagnostic criteria outlined in [19], which primarily focus on a clinical core of early and significant episodic memory impairment. They were categorized into three AD stages, based on their Mini-Mental State Examination (MMSE) scores [20] and their level of autonomy in daily living activities. Specifically, we identified 12 patients with prodromal AD, with an MMSE score≥26 and fully autonomy; nine patients with mild AD, with an MMSE score≥22 and < 26 and partial autonomy; and nine patients with moderate AD, with an MMSE score≥18 and < 22 and partial autonomy.
The CTRL group (n = 30) consisted of elderly healthy individuals recruited from the general population, who were completely autonomous in their daily lives. Eligibility criteria further included normal scores on both the MMSE (i.e., a MMSE score≥27) and the Free and Cued Selective Reminding Test (i.e., a FCSRT total recall score≥42) [21].
Exclusion criteria were similar for both groups. Specifically, individuals were excluded from the study if they were illiterate or unable to read or count, had concomitant neurological disorders such as migraines, epilepsy, extrapyramidal signs, brain tumor, subdural hematoma, head trauma resulting in persistent neurological deficits, or had experienced a stroke within the past three months. Also excluded were individuals with a current or prior major psychiatric disorder, those taking potentially confounding medications, and those who did not meet the brain MRI inclusion criteria (i.e., to have a normal MRI, for CTRLs; or an MRI not indicative of another brain disorder, for AD patients).
Neuropsychological assessment
All participants underwent a comprehensive battery of neuropsychological tests to evaluate their brain functions. The following tests were administered to assess global and specific cognitive abilities: the MMSE, as a measure of global cognitive functioning; the free recall and total recall scores from the FCSRT, as measures of verbal episodic memory; the Audio-verbal and Visuo-spatial spans, the Trail Making Test (TMT) B-A score [22], the Frontal Assessment Battery (FAB) [23], and the lexical fluency as measures of executive functioning. For assessing mood and motivation, participants completed the Geriatric Depression Scale (GDS) [24] and the Starkstein Apathy Scale [25].
Anosognosia assessment
Offline measure. The HABC-M [2] was administered to all participants and their study partners (caregivers for the patients; informants for the CTRLs). In two similar versions of the questionnaire, the participants and their study partners were asked to estimate respectively their own or their partner’s frequency of difficulties encountered in three distinct domains (cognitive; functional; behavioral/psychological) during the past two weeks. Questions were only asked slightly differently, as for example: “Over the past two weeks, how often have you forgotten the correct month or year?” versus “Over the past two weeks, how often did your relative forget the correct month or year?”. Response options comprehend four ordinal categories, namely 0=“Not at all (0-1 day)”; 1=“Several days (2-6 days)”; 2=“More than half the days (7-11 days)”; 3=“Almost daily (12-14 days)”, according to difficulty levels for each item. Discrepancy scores were then calculated to assess the participants’ self-awareness in each domain as well as overall. A higher discrepancy score (partner minus participant) indicated an overestimation of the participants’ own abilities compared to their partner’s reports. The caregiver burden score was also measured using the same rating scale, ranging from 0 to 3, but it exclusively relied on informants/caregivers’ answers to four items evaluating “general quality of life”, asking, for example: “In the last two weeks, how often have you had concerns about . . . your mental health?”. A higher burden of care was indicated by a higher score.
Online measure. Participants performed a computer-based experiment in two sessions denoted as Session A and Session B. Each session comprised modified versions of the Go/NoGo task (90 trials; 7 min) and the Stroop task (90 trials; 7 min), resulting in a total duration of approximately 15 min per session. Importantly, instructions for the Go/NoGo task were similar between Session A and Session B, while they were reversed for the Stroop task, rendering the Stroop task in Session B more challenging than the Stroop task in Session A. It should be noted that this experiment was slightly tailored to each group according to their cognitive abilities, with the need for some adjustments at the very beginning of the protocol implementation. As a result, the final computerized experiment was performed by only 40 of the 60 participants (i.e., 20 CTRLs and 20 AD patients). After completing each task in both sessions, participants were asked to rate their performance based on the number of errors they thought they might have committed. An online awareness discrepancy score was then obtained by calculating the discrepancy between the actual number of errors made for a given task/session and the participant’s estimation (i.e., her/his postdiction).
Statistical analysis
To characterize our study population, we conducted comparisons between the overall CTRL and AD groups, as well as between the CTRL group and sub-groups of AD patients based on disease stage [prodromal (n = 12); mild (n = 9); and moderate (n = 9)]. These comparisons included demographic and neuropsychological scores. In the analysis of neuropsychological scores, we employed linear models (LMs) to adjust for the effects of age (years), sex (F/M), and education (years of education since intermediate school).
To assess whether the offline awareness scores, namely the discrepancy scores derived from the HABC-M scale and its subscales, are good measures of self-awareness, we first examined the associations between these scores and the online awareness measure. Associations between offline and online measures of self-awareness were investigated independently in each group. To account for the design involving two tasks (Go-NoGo/Stroop) and two sessions (Session A/Session B) of the online measure, we employed linear mixed models (LMMs) with the online measure as the dependent variable. One LMM was performed for each offline measure. The fixed effects included in the models were age, sex, education, the interaction of task*session*offline measure, and all lower-order interactions. To consider the individual variability, a random intercept based on the patient code number was included in the LMMs. Significance of the slopes was assessed to determine the association between online awareness and offline awareness. To control for multiple comparisons, we applied the Benjamini-Hochberg correction.
To explore the link between the offline awareness measure and cognition, we examined the associations between the HABC-M discrepancy scores and the scores obtained in the neuropsychological battery. These associations were assessed using LMs that accounted for age, sex, and education, with the neuropsychological scores as the dependent variable. Each neuropsychological score was analyzed separately in relation to the corresponding offline measure, within each group. To control for multiple comparisons, we applied the Benjamini-Hochberg correction.
To assess the discriminative ability of the offline awareness measure in distinguishing AD patients, including different AD stages (prodromal, mild, and moderate), from healthy CTRLs, we conducted group comparisons. LMs were used with the offline discrepancy scores, as the dependent variable, and group, as the independent variable, adjusting for age, sex, and education. The Benjamini-Hochberg correction was applied to control for multiple comparisons.
Receiver Operating Characteristic (ROC) curves were generated to assess the discriminative performance of the offline awareness discrepancy scores and caregiver burden score in differentiating (a) AD patients from CTRLs, and (b) prodromal AD patients from CTRLs. Optimal cut points and performance metrics, such as sensitivity, specificity, and area under the curve (AUC), were computed to evaluate the accuracy of the classification. The optimal cutoff value was defined as the point on the ROC curve that yielded the maximum Youden’s index (sensitivity+specificity-1). For further details, please see Supplementary Table 3.
Furthermore, the variation of the online awareness measure was examined using LMMs for each group independently. The fixed effects included age, sex, education, and the interaction of task (Go-NoGo / Stroop)*session (Session A / Session B)*group and all lower-order interactions. A random intercept was included for the patient code number in the LMMs. Both accuracy and discrepancy scores were analyzed as dependent variables.
All statistical analyses were conducted using R version 3.6.1 (R Development Core Team, 2019) and plots were generated with the ggplot2 package (v3.3.2, Wickham, 2016).
RESULTS
Sample description
For all healthy controls and almost all AD patients, the caregivers/informants were their spouses (wives or husbands), except for two patients whose informants were their children. Population description is presented in Table 1. The AD patients’ group was older, with more men than women compared to the CTRL group. No significant differences were found between the groups in years of education. The mean score on the GDS was slightly higher in AD patients compared to CTRLs, but it did not exceed the threshold for likely depression (i.e.,>4), and no significant differences were observed among the various stages of AD.
Table 1
Sample descriptions and between groups comparisons
| CTRL (a) | CTRL versus AD | CTRL versus AD stages | |||||
| N=30 (50%) | AD | p‡ | Prodromal AD (b) | Mild AD (c) | Moderate AD (d) | p‡ | |
| N=30 (50%) | N=12 (20%) | N=9 (15%) | N=9 (15%) | ||||
| Demographics | |||||||
| Sex (F) | 23 (76.67%) | 13 (43.33%) | 0.017* | 6 (50.00%) | 4 (44.44%) | 3 (33.33%) | 0.051 |
| Age | 68.70±6.30 b, d | 74.93±5.85 | <0.001* | 75.25±6.52 a | 74.11±6.60 | 75.33±4.58 a | 0.003* |
| Education | 15.90±3.54 | 15.10±3.27 | 0.367 | 15.25±2.56 | 16.67±2.83 | 13.33±3.94 | 0.158 |
| Motivation and Mood | |||||||
| Starkstein | 7.39±0.96 | 9.36±0.89 | 0.216 | 9.24±1.35 | 8.27±1.53 | 10.88±1.64 | 0.429 |
| GDS | 0.62±0.27 | 1.67±0.25 | 0.015* | 1.44±0.39 | 2.05±0.44 | 1.54±0.47 | 0.067 |
| Neuropsychological battery | |||||||
| MMSE | 29.13±0.82 b, c, d | 23.88±0.47 | <0.001* | 27.08±1.08 a, c, d | 23.33±1.41 a, b, d | 19.67±1.00 a, b, c | <0.001* |
| FCSRT Free Recall | 36.67±3.92 b, c, d | 10.73±1.19 | <0.001* | 12.45±7.79 a, d | 12.22±9.47 a | 5.22±4.60 a, b | <0.001* |
| FCSRT Total score | 47.23±1.28 b, c, d | 23.14±1.78 | <0.001* | 24.55±12.25 a | 26.78±16.05 a | 17.00±8.60 a | <0.001* |
| TMT B-A, time | 29.93±14.73 b, c, d | 98.56±10.03 | <0.001* | 70.91±42.05 a | 127.22±92.03 a | 102.75±54.18 a | <0.001* |
| FAB | 17.27±1.05 b, d | 15.12±0.35 | 0.001* | 15.92±1.16 a, d | 15.89±1.69 d | 13.11±2.57 a, b, c | <0.001* |
| Lexical fluency | 35.03±7.87 b, c, d | 17.64±1.43 | <0.001* | 19.64±5.30 a | 15.50±4.81 a | 15.33±5.92 a | <0.001* |
| AV-span | 10.63±2.50 | 10.01±0.47 | 0.456 | 9.80±1.62 | 10.78±2.22 | 9.22±1.79 | 0.518 |
| VS-span | 10.47±1.70 d | 9.30±0.39 | 0.100 | 10.10±1.97 d | 9.67±1.41 d | 7.78±1.72 a, b, c | 0.011* |
| HABC-M (Offline) scores | |||||||
| Cognitive discrepancy | -0.97±2.09 b, c, d | 6.04±0.71 | <0.001* | 3.75±3.98 a, d | 4.44±3.13 a, d | 10.89±3.10 a, b, c | <0.001* |
| Functional discrepancy | -0.73±1.53 b, c, d | 4.50±0.73 | <0.001* | 1.42±2.23 a, d | 3.67±3.00 a | 9.00±6.30 a, b | <0.001* |
| Psychological | |||||||
| discrepancy | -0.70±3.29 c, d | 2.82±0.73 | 0.004* | 0.42±2.50 d | 2.22±2.59 a | 6.22±4.82 a, b | <0.001* |
| Total discrepancy | -2.40±6.28 b, c, d | 13.36±1.97 | <0.001* | 5.58±7.27 a, d | 10.33±7.79 a, d | 26.11±13.17 a, b, c | <0.001* |
| Caregiver burden | 0.03±0.18 b, c, d | 3.41±0.40 | <0.001* | 2.42±2.11 a | 4.11±3.66 a | 3.78±3.07 a | <0.001* |
| Hybrid | -0.93±2.15 b, c, d | 9.45±0.95 | <0.001* | 6.17±5.47 a, d | 8.56±6.09 a, d | 14.67±5.55 a, b, c | <0.001* |
Data are given as mean±standard deviation for continuous variables and as count (percentages) for categorical variables. Estimated marginal means±standard errors were presented for each measure adjusted for age, sex and education (i.e., all measures except demographics). A lower case letter (a, b, c, d) has been added to indicate whether the CTRL group (letter “a”) and the AD subgroups (“b”, prodromal AD; “c”, mild AD; “d”, moderate AD) are significantly different from each other, by means of these letters in the respective columns. ‡ For demographics, Welch’s t-test was used to compare the two groups, and ANOVA test to compare the four groups (followed by Tukey HSD test) for numerical variables and Fisher’s exact test for categorical variables. To compare groups, for mood and motivation measures, neuropsychological measures and offline HABC-M scores, LMs were performed and were adjusted for age, sex and education. Benjamini Hochberg correction was applied in both sets of measures. AD, Alzheimer’s disease; AV, Audio-Verbal (forward plus backward span); CTRL, controls; FAB, Frontal Assessment Battery; FCSRT, Free and Cued Selective Reminding Test; GDS, Geriatric Depression Scale; HABC-M, Healthy Aging Brain Care Monitor; MMSE, Mini-Mental State Examination; TMT, Trail Making Test; VS, Visuo-Spatial (forward plus backward span).
As expected, significant differences between CTRL and AD groups were observed in several cognitive domains, mainly regarding verbal episodic memory and executive functioning. The AV-span was the only neuropsychological test without significant differences between the groups. VS-span mean was only significantly lower in the moderate AD stage.
Self-awareness profile
Anosognosia as assessed by the HABC-M (offline measure)
When compared to the CTRL group, AD patients presented low awareness for their own deficits in all domains, as revealed by significant higher discrepancy scores in the cognitive, functional, and psychological HABC-M subscales, as well as by the HABC-M total discrepancy score (as shown in the lower part of Table 1). Also, when considering AD patients subgroups, according to the stage of the disorder, the results were similar, except for the psychological subscale of the HABC-M which did not distinguish the subgroup of prodromal AD patients from the CTRL group.
To investigate further, we performed paired t-tests to assess the agreement between the participants and their study partners (Table 2). Within the AD patients group, as well in the mild AD and moderate AD subgroups, patients overestimated their abilities compared to their caregivers in all scores. In prodromal AD, similar results were found only for the total HABC-M score and the cognitive HABC-M score. Caregiver burden scores were also significant for all AD stages, but not for the CTRL group.
Table 2
Paired t-tests on the HABC-M discrepancy scores to test agreement between the participant and the study partner within each group
| CTRL | AD Patients | Prodromal AD | Mild AD | Moderate AD | |
| (N = 30) | (N = 30) | (N = 12) | (N = 9) | (N = 9) | |
| Cognitive HABC-M | t=-2.53 p = 0.043 | t=7.19 p < 0.001 | t=3.26 p = 0.019 | t=4.26 p = 0.010 | t=10.54 p < 0.001 |
| Functional HABC-M | t=-2.63 p = 0.043 | t=4.71 p < 0.001 | N.S. | t=3.67 p = 0.011 | t=4.28 p = 0.004 |
| Psychological HABC-M | N.S. | t=3.62 p = 0.001 | N.S. | t=2.58 p = 0.033 | t=3.88 p = 0.006 |
| Total | |||||
| HABC-M | N.S. | t=5.65 p < 0.001 | t=2.66 p = 0.037 | t=3.98 p = 0.010 | t=5.95 p = 0.001 |
| Caregiver burden‡ | N.S. | t=6.23 p < 0.001 | t=3.97 p = 0.011 | t=3.37 p = 0.012 | t=3.69 p = 0.006 |
T-statistics and p-values are presented for each test. P-values were corrected for multiple testing using Benjamini-Hochberg method. ‡ For caregiver burden, since it is evaluated only by the informant, one-sample t-test was performed. N.S. (not significant): p > 0.05.
Anosognosia as assessed through the experimental task (online measure)
Consistent with the offline measure, our findings revealed contrasting profiles between the groups when examining self-awareness during the experimental task. Specifically, AD patients demonstrated reduced awareness for their errors, as evidenced by consistently positive mean discrepancy scores representing an overestimation of their performance (number of errors committed minus number of errors estimated after task completion > 0) (Fig. 1,d). This stands in stark contrast to CTRLs, who exhibited negative mean discrepancy scores (indicating an underestimation of their performance) (Fig. 1,c), although these scores were close to zero regardless of changes in performance accuracy between sessions (Fig. 1,a).
Fig. 1
a-d. Online awareness and accuracy of each group during the experimental task. Estimated marginal means as well as their 95% confidence interval extracted from LMM were presented. p-values were computed from post-hoc tests were performed on Task*Session interaction. Age, education, and gender were used for adjustment purposes. Significant differences are indicated by asterisks, Benjamini-Hochberg corrected: ***p<0.001, **0.001≤p<0.01, *0.01≤p<0.05.

Moreover, AD patients appeared to experience greater challenges in estimating their performance level when they committed more errors, namely in the Go/NoGo A and Stroop B tasks (Fig. 1,b).
Relationship between offline and online measures of self-awareness
The HABC-M, including the discrepancy scores for each subscale and the total HABC-M, as well as the caregiver burden score, exhibited significant associations with the discrepancy scores for online awareness derived from the Go/NoGo A task, indicating a shared neural mechanism (Supplementary Table 1). On the other hand, the existence of only one association between the functional HABC-M subscale and the Stroop B, the hardest task, suggests that task difficulty or ambiguity may also have an impact on error unawareness [26]. Conversely, when patients demonstrated high accuracy with minimal errors, namely while performing Stroop A (Fig. 1b), they also displayed heightened awareness of their performance, potentially explaining the lack of correlations between “performance discrepancy” scores in this task and the HABC-M scores.
The caregiver burden score also correlated significantly with the online awareness measure (namely, for the Go/NoGo A). This finding suggests that caregiver burden could serve as an indirect indicator of anosognosia in AD patients or provide additional support for its presence. Furthermore, it suggests that the observed correlations between the caregiver burden and the HABC-M discrepancy scores (all p < 0.004, Supplementary Table 2) might be partially independent of the caregiver burden level itself, despite some contradictory evidence [27].
Based on these findings, we conducted a ROC analysis combining the cognitive HABC-M (six items) and caregiver burden (four items) scores to evaluate the potential of a short-hybrid scale for the assessment of anosognosia. The next section presents this analysis. In particular, we anticipated that this scale, with a reduced number of items (10 instead of the usual 31), based on a combination of cognitive discrepancy scores and caregiver-exclusive quality of life scores, might be even more sensitive to anosognosia compared to individual HABC-M subscales or the total HABC-M scale.
ROC curves predicting early AD with HABC-M
In terms of differentiating between CTRLs and AD patients (whole group, n = 30) or CTRLs and prodromal AD patients subgroup (n = 12), the hybrid score (derived from the sum of the cognitive discrepancy score, six items, and the caregiver burden score, four items) performed best in the ROC analysis, followed by the cognitive discrepancy score (Fig. 2, below, and Supplementary Table 3). The hybrid score achieved an area under the receiver operating characteristic (AUROC) of 0.97 [0.94; 0.99] for CTRL group versus AD patients group discrimination, with a sensitivity of 0.93 [0.81; 1.00] and specificity of 0.90 [0.82; 1.00]. The cognitive discrepancy score achieved an AUROC of 0.94 [0.88;0.98], with a sensitivity of 0.83 [0.69; 0.97] and specificity of 0.83 [0.76; 1.00]. For CTRL group versus prodromal AD subgroup discrimination, the hybrid score achieved an AUROC of 0.94 [0.86; 0.99], with a sensitivity of 0.92 [0.71; 1.00] and specificity of 0.83 [0.76; 1.00], whereas the cognitive discrepancy score achieved an AUROC of 0.88 [0.78; 0.97], with a sensitivity of 0.83 [0.62; 1.00] and specificity of 0.80 [0.61; 0.97].
Fig. 2
ROC Curves of HABC-M scores between A) CTRL versus AD and B) CTRL versus prodromal AD.
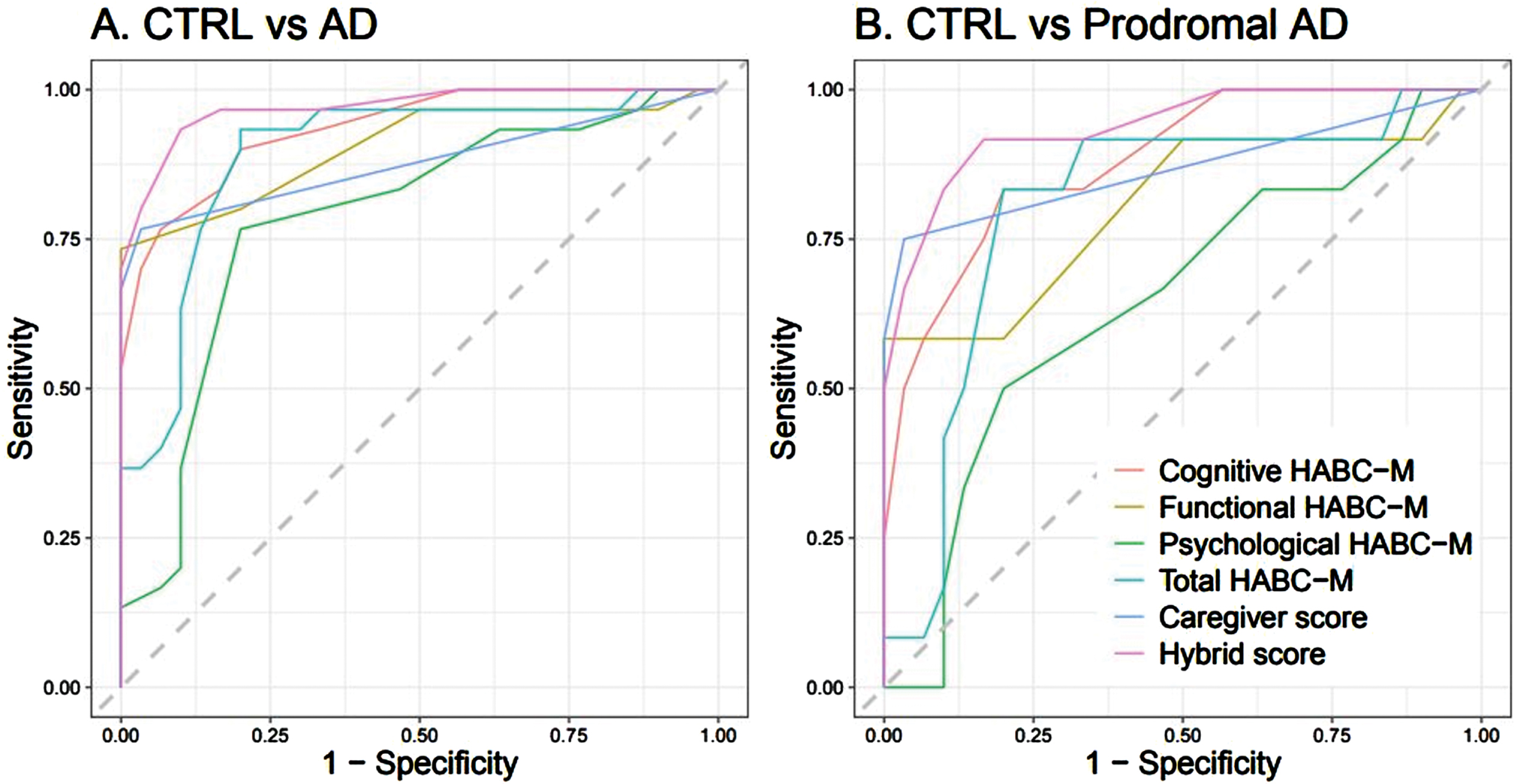
Nevertheless, although the ROC curve score for the short-hybrid scale was the highest in terms of sensitivity and specificity to discriminate CTRLs from AD patients (regarding either the whole group, n = 30, or the prodromal AD subgroup, n = 12), we observed wider variations in the confidence intervals of sensitivity and specificity for CTRLs versus prodromal AD patients (when compared to the whole AD patients group), which reflects the small sample size of this subgroup (n = 12) and should be considered as a limitation of this study.
Relationship between the HABC-M and cognitive function
The HABC-M discrepancy scores, reflecting patients’ self-awareness, showed negative associations with global cognition (p < 0.01), episodic memory (p < 0.05), and executive function (p < 0.05), indicating that lower self-awareness was related to greater cognitive deficits (Supplementary Table 4). No significant correlations were found between the caregiver burden level and patients’ cognitive deficits. In the CTRL group, no significant associations were observed.
DISCUSSION
An essential component of normal cognition is awareness of our own performance, which gives us the ability to recognize our limitations and adapt our behavior appropriately [28]. The opposite behavior, i.e., the lack of awareness of our own deficits, errors, or limitations, is referred to as anosognosia [3, 29]. It can manifest in several domains, including cognitive deficits, psychological changes or difficulties in daily living activities, with growing evidence indicating that unawareness of memory deficits may be a prodrome of AD [30, 31]. However, studies exploring the prevalence of anosognosia in AD have yielded heterogeneous findings [29], which may in part reflect variations in assessment methodologies. So it is key to establish a “gold standard” for detecting this intriguing syndrome from the early stages of AD, as it could lead to timely diagnosis and better management of this devastating neurodegenerative disorder [18].
In their seminal report, Monahan et al. [2] proposed that a discrepancy score between patient and caregiver versions of the HABC-M could indicate the patients’ inability to recognize their own deficits. Subsequent research has supported this perspective, with evidence that the cognitive HABC-M subscale can detect reduced awareness of memory decline in older individuals at risk of AD [4]. Nevertheless, these studies have not directly compared the cognitive subscale with other HABC-M subscales or alternative methods for assessing anosognosia. As a result, the reliability and effectiveness of this potential “anosognosia instrument” required further validation.
Our findings underscore the significance and practical utility of the HABC-M as a clinical tool for detecting anosognosia in AD from its very early stages. Notably, we identified significant correlations between the offline (HABC-M) and online anosognosia discrepancy scores, providing evidence for the convergent validity of these two methods for the assessment of anosognosia. Importantly, as this phenomenon can affect any type of deficits, we sought to dissociate anosognosia from memory impairment by using a “performance discrepancy” measure based on two executive tasks (Go/NoGo and Stroop) to assess online anosognosia.
Consistent with previous research, our results suggest that anosognosia is particularly linked to errors or core deficits [32]. Specifically, we observed a significant correlation between higher discrepancy scores in most HABC-M scales, except one, and episodic memory loss, but also with lower accuracy in the computerized experiment. These findings suggest that the commission of errors, resulting from deficits in domains such as episodic memory or executive functions, or eventually from any other cognitive or functional domain, may be at the root of anosognosia due to a failure in the error-monitoring system. Expanding on this perspective, we recently proposed a dual-path hypothesis for the emergence of anosognosia in AD. The core of our rationale is that anosognosia in AD would emerge from a critical failure in the neural system responsible for error awareness, affecting patients’ ability to be aware of their own errors (or deficits). In line with this view, we have recently published preliminary results on error-related potentials in AD patients, which indicate a synaptic failure in the error-monitoring system during a word memory recognition task, along with a decline of awareness for cognitive difficulties, at the time of diagnosis [33]. According to our dual-path hypothesis, this could result from direct and/or indirect damage to that system. For example, an impairment in emotional processing could have an indirect impact on the error-monitoring system, by preventing patients from monitoring the internal milieu for relevant errors (or deficits), resulting in unawareness of their own deficits (for more details, please see [34]). Additional support for this view comes from previous evidence of error-monitoring impairments in AD, as revealed by the inability of AD patients to differentiate between correct and incorrect responses in a memory recognition task, even when their memory performance was comparable to that of healthy controls by additional exposure (of patients) to the study material [35].
Finally, the significant correlations we observed between the caregiver burden level and both offline and online discrepancy measures, along with its ability to differentiate caregivers of prodromal AD patients from informants of CTRLs, suggest that the level of caregiver burden can provide support for the presence of anosognosia. These findings underline the importance of developing a short-hybrid version of the HABC-M, combining six cognitive items with four caregiver-exclusive quality of life items, as a valuable tool for detecting anosognosia in AD patients with a high sensitivity (>90%). Most notably, the short-hybrid scale achieved an AUROC score of 0.97 [0.94; 0.99] for discriminating CTRLs from AD patients (whole group), with a sensitivity of 0.93 [0.81; 1.00] and specificity of 0.90 [0.82; 1.00]; and it achieved an AUROC score of 0.94 [0.86; 0.99] for discriminating CTRLs from the prodromal AD subgroup, with a sensitivity of 0.92 [0.71; 1.00] and specificity of 0.83 [0.76; 1.00]. The wider confidence intervals observed for sensitivity and specificity in the latter case are due to the small sample size of the AD subgroup (n = 12). This limitation should be acknowledged as the primary constraint of the present study, necessitating further investigation in larger cohorts of patients with Alzheimer’s at various stages of the disease.
Conclusion
The findings of this study provide strong evidence for the high sensitivity of the HABC-M, particularly its cognitive (six items) and caregiver burden (four items) subscales, in detecting anosognosia across different stages of AD, effectively distinguishing CTRLs from AD patients. The development of a short-hybrid scale, comprising these 10 items instead of the usual 31-item HABC-M, holds significant practical implications in the clinical setting for timely AD diagnosis and screening of patients for trials targeting early-stage AD. Moreover, by shedding light on the challenges experienced by caregivers, such scale can facilitate the implementation of supportive medico-social measures to relieve families facing this condition. On the whole, our results suggest that this short-hybrid version of the HABC-M has the potential to serve as a “gold standard” for detecting anosognosia from the prodromal stage of AD, which may also contribute for more timely diagnosis, although further validation in larger cohorts is necessary.
ACKNOWLEDGMENTS
We thank all the participants and their study partners, without whom this study could not have occurred. We also wish to thank Professors Bruno Dubois and Richard Lévy for their interest in our research and for their support. Our acknowledgment extends to the research team of the IM2A.
FUNDING
This study was supported by a grant from the ANR (Agence Nationale de la Recherche): NOT_AWARE: N° ANR-17-CE37-0017-01 (to KA), and the National Institute of Aging of the National Institutes of Health under award numbers RF1AG074204 and RF1AG079324 (to DP).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
AUTHOR CONTRIBUTIONS
KA: Design and implementation of the study; Conceptualization of the Short-hybrid scale; Funding acquisition; Research supervision; Clinical data acquisition and management; Interpretation and discussion of the results; Manuscript writing (first draft and final version).
TG: Data acquisition and analyses; Discussion of the results; Artwork of the final manuscript. Contribution to the writing of the final manuscript; Supplementary material.
RL: Neuropsychological data acquisition; Literature review, contribution to the writing of the final manuscript.
MH: Statistical methods and analyses; Critical review and contribution to the writing of the final manuscript; Supplementary material.
SR: Data acquisition and contribution to the writing of the final manuscript.
FXL: Statistical methods and contribution to the writing of the final manuscript.
AK, GD, TM: Critical review and contribution to the writing of the final manuscript.
DP: Statistical analyses; Interpretation and discussion of the results; Critical review and major contribution to the writing of the final manuscript.
EK: Co-supervision of the study; Interpretation and discussion of the results; Critical review of the final manuscript.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230552.
REFERENCES
[1] | Monahan PO , Boustani MA , Alder C , Galvin JE , Perkins AJ , Healey P , Chehresa A , Shepard P , Bubp C , Frame A , Callahan C ((2012) ) Practical clinical tool to monitor dementia symptoms: The HABC-Monitor. Clin Interv Aging 7: , 143–157. |
[2] | Monahan P , Alder C , Khan B , Stump T , Boustani M ((2014) ) The Healthy Aging Brain Care (HABC) Monitor: Validation of the Patient Self-Report Version of the clinical tool designed to measure and monitor cognitive, functional, and psychological health. Clin Interv Aging 9: , 2123–2132. |
[3] | Babinski J ((1914) ) Contribution a l’etude des troubles mentaux dans l’ hemiplegie organique cerebrale (anosognosie). Rev Neurol 27: , 845–848. |
[4] | Cacciamani F , Tandetnik C , Gagliardi G , Bertin H , Habert MO , Hampel H , Boukadida L , Révillon M , Epelbaum S , Dubois B ; INSIGHT-PreAD study group ((2017) ) Low cognitive awareness, but not complaint, is agood marker of preclinical Alzheimer’s disease. J Alzheimers Dis 59: , 753–762. |
[5] | Cosentino S , Metcalfe J , Cary MS , De Leon J , Karlawish J ((2011) ) Memory awareness influences everyday decision making capacity about medication management in Alzheimer’s disease. Int J Alzheimers Dis 2011: , 483897. |
[6] | Starkstein SE , Jorge R , Mizrahi R , Adrian J , Robinson RG ((2007) ) Insight and danger in Alzheimer’s disease. Eur J Neurol 14: , 455–460. |
[7] | Cotrell V , Wild K ((1999) ) Longitudinal study of self-imposed driving restrictions and deficit awareness in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 13: , 151–156. |
[8] | Turró-Garriga O , Garre-Olmo J , Vilalta-Franch J , Conde-Sala JL , de Gracia Blanco M , López-Pousa S ((2013) ) Burden associated with the presence of anosognosia in Alzheimer’s disease. Int J Geriatr Psychiatry 28: , 291–297. |
[9] | Kelleher M , Tolea MI , Galvin JE ((2016) ) Anosognosia increases caregiver burden in mild cognitive impairment. Int J Geriatr Psychiatry 31: , 799–808. |
[10] | Starkstein SE , Jorge R , Mizrahi R , Robinson RG ((2006) ) A diagnostic formulation for anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 77: , 719–725. |
[11] | Sunderaraman P , Cosentino S ((2017) ) Integrating the constructs of anosognosia and metacognition: A review of recent findings in dementia. Curr Neurol Neurosci Rep 17: , 27. |
[12] | Martyr A , Nelis SM , Clare L ((2014) ) Predictors of perceived functional ability in early-stage dementia: Self-ratings, informant ratings and discrepancy scores. Int J Geriatr Psychiatry 29: , 852–862. |
[13] | Hannesdottir K , Morris RG ((2007) ) Primary and secondary anosognosia for memory impairment in patients with Alzheimer’s disease. Cortex 43: , 1020–1030. |
[14] | de Ruijter NS , Schoonbrood AMG , van Twillert B , Hoff EI ((2020) ) Anosognosia in dementia: A review of current assessment instruments. Alzheimers Dement 12: , e12079. |
[15] | Ott BR , Lafleche G , Whelihan WM , Buongiorno GW , Albert MS , Fogel BS ((1996) ) Impaired awareness of deficits in Alzheimer disease. Alzheimer Dis Assoc Disord 10: , 68–76. |
[16] | Turró-Garriga O , Garre-Olmo J , López-Pousa S , Vilalta-Franch J , Reñé-Ramírez R , Conde-Sala JL ((2014) ) Abridged scale for the screening anosognosia in patients with dementia. J Geriatr Psychiatry Neurol 27: , 220–226. |
[17] | Zarit SH , Reever KE , Bach-Peterson J ((1980) ) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20: , 649–655. |
[18] | Clare L ((2004) ) Awareness in early-stage Alzheimer’s disease: A review of methods and evidence. Br J Clin Psychol 43: , 177–196. |
[19] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[20] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[21] | Grober E , Buschke H ((1987) ) Genuine memory deficits in dementia. Dev Neuropsychol 3: , 13–36. |
[22] | Amieva H , Le Goff M , Stoykova R , Lafont S , Ritchie K , Tzourio C , Fabrigoule C , Dartigues JF ((2009) ) Trail Making Test A et B (versionsans correction des erreurs): Normes en population chez des sujetsâgés, issues de l’étude des trois Cités. Rev Neuropsychol 1: , 210–220. |
[23] | Dubois B , Slachevsky A , Litvan I , Pillon B ((2000) ) The FAB: A Frontal Assessment Battery at bedside. Neurology 55: , 1621–1626. |
[24] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1982) ) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: , 37–49. |
[25] | Starkstein SE , Mayberg HS , Preziosi TJ , Andrezejewski P , Leiguarda R , Robinson RG ((1992) ) Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 4: , 134–139. |
[26] | Klein T , Danielmeier C , Ullsperger M ((2013) ) Editorial for E-Book: Error awareness— insights from cognitive neuroscience, psychiatry and neurology. Front Hum Neurosci 7: , 830. |
[27] | Perales J , Turró-Garriga O , Gascón-Bayarri J , Reñé-Ramírez R , Conde-Sala JL ((2016) ) The longitudinal association between a discrepancy measure of anosognosia in patients with dementia, caregiver burden and depression. J Alzheimers Dis 53: , 1133–1143. |
[28] | Vidal F , Burle B , Hasbroucq T ((2019) ) Errors and action monitoring: Errare humanum est sed corrigere possibile. Front Hum Neurosci 13: , 453. |
[29] | Starkstein SE ((2014) ) Anosognosia in Alzheimer’s disease: Diagnosis, frequency, mechanism and clinical correlates. Cortex 61: , 64–73. |
[30] | Therriault J , Ng KP , Pascoal TA , Mathotaarachchi S , Kang MS , Struyfs H , Shin M , Benedet AL , Walpola IC , Nair V , Gauthier S , Rosa-Neto P ((2018) ) Anosognosia predicts default mode network hypometabolism and clinical progression to dementia. Neurology 90: , e932–e939. |
[31] | Bastin C , Giacomelli F , Miévis F , Lemaire C , Guillaume B , Salmon E ((2021) ) Anosognosia in mild cognitive impairment: Lack of awarenessof memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry 12: , 631518. |
[32] | Leicht H , Berwig M , Gertz HJ ((2010) ) Anosognosia in Alzheimer’s disease: The role of impairment levels in assessment of insight across domains. J Int Neuropsychol Soc 16: , 463–473. |
[33] | Razafimahatratra S , Guieysse T , Lejeune FX , Houot M , Medani T , Dreyfus G , Klarsfeld A , Villain N , Pereira FR , La Corte V , George N , Pantazis D , Andrade K ((2023) ) Can a failure in the error-monitoring system explain unawareness of memory deficits in Alzheimer’s disease? Cortex 166: , 428–440. |
[34] | Andrade K , Guiyesse T , Medani T , Koechlin E , Pantazis D , Dubois B (2023) The dual-path hypothesis for the emergence of anosognosia in Alzheimer’s disease. arXiv, https://doi.org/10.48550/arXiv.2302.05723. |
[35] | Dodson CS , Spaniol M , O’Connor MK , Deason RG , Ally BA , Budson AE ((2011) ) Alzheimer’s disease and memory-monitoring impairment: Alzheimer’s patients show a monitoring deficit that is greater than their accuracy deficit. Neuropsychologia 49: , 2609–2618. |




