JAD: A Forum for Philosophy in Science
Abstract
The Journal of Alzheimer’s Disease (JAD) is already an established forum for cutting-edge science as well as ethical reflection. But I argue that beyond science and ethics, JAD is also a forum for philosophy in science, and that interdisciplinary researchers asking innovative questions about AD should publish their reflections and findings in JAD.
There is an urgent need for solutions for Alzheimer’s disease (AD). The Journal of Alzheimer’s Disease (JAD) and its sister online-only Open-Access journal, JAD Reports, are well-established interdisciplinary fora for cutting-edge science on AD. JAD also has a dedicated Ethics section, of which Dr. Allyson Rosen is the Editor, and which primarily features the Ethics Review and Response formats [1]. But I will argue that beyond science and ethics, JAD can also serve as a fruitful forum for philosophy within science to help find solutions to problems related to AD (Fig. 1).
Fig. 1
The Journal of Alzheimer’s Disease as a forum for philosophy in science between descriptive science on one hand, and normative philosophy on the other. While empirical science on AD is concerned with describing the condition, much philosophy and ethics related to AD is concerned with how clinicians and researchers should act and is “normative,” because it appeals to norms to assess actions. Philosophy in science uses a mix of descriptive and normative methods to tackle issues relevant to, as well as emerging from, scientific practice.
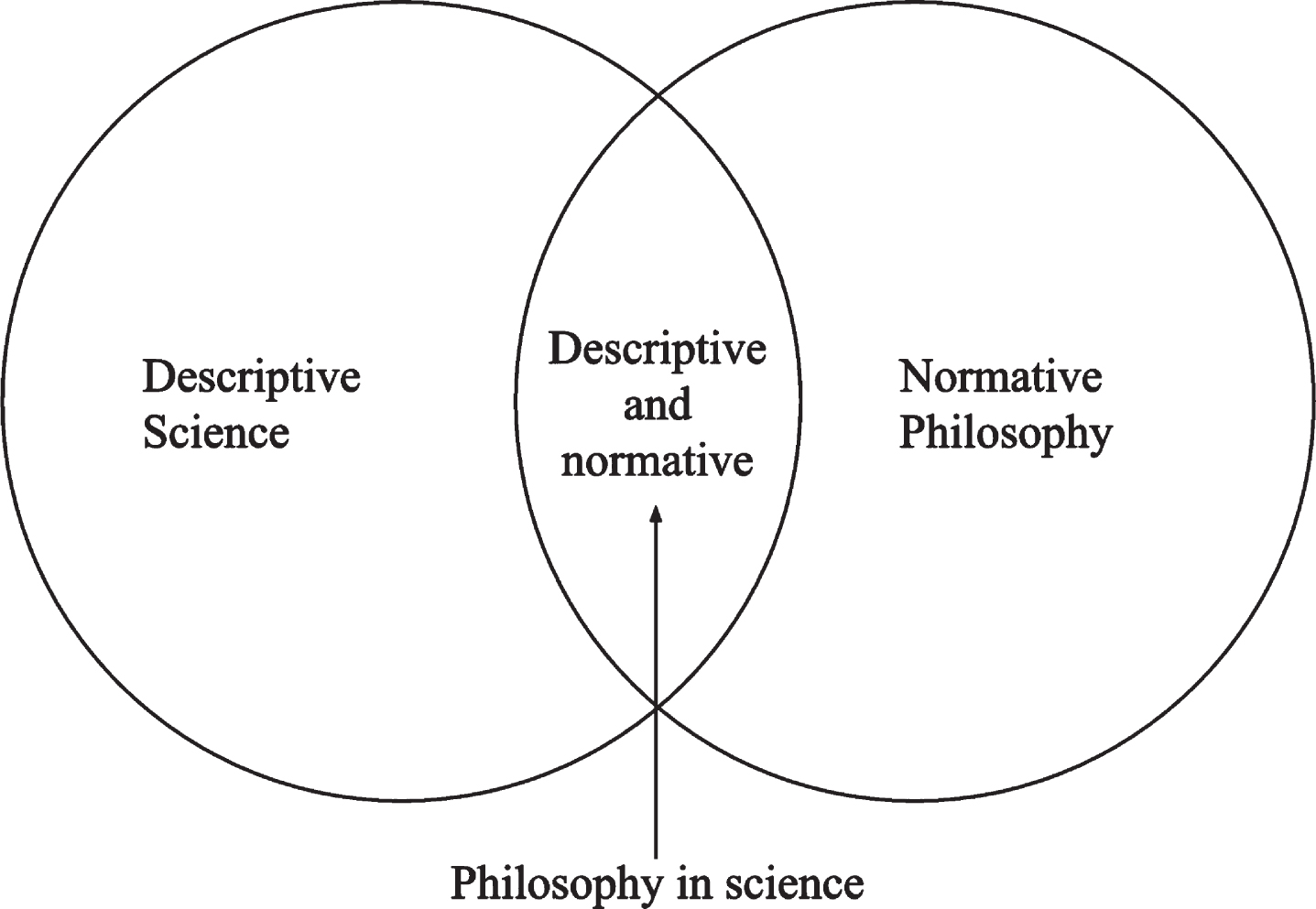
Generally speaking, normative philosophy tends to be distant from the practice of empirical science: philosophers may have more or less technical knowledge about the condition, and generally publish in philosophy rather than scientific journals. In other words, they generally perform philosophy on science. Between empirical and normative questions, we find philosophy in science or ‘PinS’ (pronounced “pins”), which uses philosophical tools to solve scientific problems [2]. PinS or “embedded philosophy” [3] has the benefit of being closely related to scientific practice but is not limited by wet lab methods or entirely descriptive goals.
I consider myself to be a philosopher in science: I ask questions about how we can improve AD research, and publish with scientific colleagues in scientific journals, but I no longer perform wet lab research. Ideally, the impact of PinS is upstream of scientific experiments, i.e., asking questions before experiments get undertaken so as to improve the likelihood that future results are useful [2]. In part one of my PhD, I used a bibliometric study of citation practices of the amyloid cascade hypothesis [4] and an international expert survey promoted on Twitter [5] to get access to how researchers have invested their belief in amyloid-β and other therapeutic targets. Then in Part 2, I offered an analysis of the concept of association to try and guide a post-amyloid view of AD research [6], and in Part 3 I took a more explicitly ethical turn [7]. These papers were published in JAD and JAD Reports. Other colleagues undertaking embedded philosophy have published similar innovative research at the interface of scientific methods and ethical concerns [8, 9].
Broadly speaking, there are many unresolved philosophical issues related to empirical research and ethical questions emerging from AD research, that we might call “epistemological issues” because they relate to knowledge (from the Greek episteme) but are not necessarily solved merely by wet lab investigation. Three such issues strike me as particularly pressing for the future of AD research (Table 1).
Table 1
Three epistemological issues requiring interdisciplinary solutions in research into Alzheimer’s disease
| Epistemological issue in AD research | Associated ethical issues | Possible solutions |
| Over-reliance on the amyloid hypothesis to find a disease-modifying treatment for AD | Exposing people to anti-amyloid treatments with possibly harmful side effects [10] Overlooking viable etiological theories of AD [11] | Strict usage guidelines for anti-amyloid medicines [12] Improving the visibility and impact of alternatives to the amyloid hypothesis [13] |
| The unknown therapeutic and prognostic value of in vivo biomarkers of AD pathology | Premature over-medicalization of biomarkers before dementia [14] Stigma around the AD concept, even in the absence of dementia [15] | Underlining the limits of the biological definition of AD [16] Warning against the harms of over-screening of biomarkers and use of premature stigmatizing language [17] |
| Lack of integration of genetics and epidemiological approaches to dementia | How to spend limited research resources and assure equitable prevention [7] | Involving different stakeholders in debates around priority setting [5] |
Because of my scientific training, my PhD supervisor Yves Agid aptly quipped, “Tim, the problem with the acceptance of your work is that, to scientists, you’re a philosopher, but to philosophers, you’re a scientist.” Thanks to JAD, I have managed to overcome this unnecessary opposition between science and philosophy as a philosopher embedded in science.
I encourage other interdisciplinary researchers asking epistemological questions about AD to also publish in JAD using a PinS approach, or to draw on related approaches from human and social sciences including history of science [18], anthropology of medicine [19], and sociology [20]. It is vital that scholars across different fields continue to question AD research and its place in society so as to improve the likelihood that effective and equitable solutions be found.
ACKNOWLEDGMENTS
Timothy Daly thanks two anonymous reviewers for comments that improved the manuscript.
FUNDING
The author has no funding to report.
CONFLICT OF INTEREST
Timothy Daly is an Associate Editor of the Journal of Alzheimer’s Disease but was not involved in the peer-review process nor had access to any information regarding its peer-review. Timothy Daly has no other conflicts of interest to report.
REFERENCES
[1] | Rosen AC , Ashford JW , Perry G ((2014) ) Ethics review as a catalyst for progress. J Alzheimers Dis 40: , 233–235. |
[2] | Pradeu T , Lemoine M , Khelfaui M , Gingras Y ((2021) ) Philosophy in Science: Can philosophers of science permeate through science and produce scientific knowledge? Br J Philos Sci, doi: 10.1086/715518. |
[3] | Kaiser M , Kronfelder M , Meunier R ((2014) ) Interdisciplinarity in philosophy of science. J Gen Philos Sci 45: , 59–70. |
[4] | Daly T , Houot M , Barberousse A , Agid Y , Epelbaum S ((2020) ) Amyloid-β in Alzheimer’s disease: A study of citation practices of the amyloid cascade hypothesis between 1992 and 2019. J Alzheimers Dis 74: , 1309–1317. |
[5] | Daly T , Houot M , Barberousse A , Petit A , Epelbaum S ((2021) ) A proposal to make biomedical research into Alzheimer’s disease more democratic following an international survey with researchers. J Alzheimers Dis Rep 5: , 637–645. |
[6] | Daly T , Henry V , Bourdenx M ((2023) ) From association to intervention: The Alzheimer’s Disease-Associated Processes and Targets (ADAPT) ontology. J Alzheimers Dis 94: (1 Suppl), S87–S96. |
[7] | Daly T , Mastroleo I , Migliaccio R ((2022) ) Avoiding over-reliance on multi-domain interventions for dementia prevention. J Alzheimers Dis 90: , 989–992. |
[8] | Smedinga M , Tromp K , Schermer MHN , Richard E ((2018) ) Ethical arguments concerning the use of Alzheimer’s disease biomarkers in individuals with no or mild cognitive impairment: A systematic review and framework for discussion. J Alzheimers Dis 66: , 1309–1322. |
[9] | Tromp K , Smedinga M , Richard E , Perry M , Schermer MHN ((2021) ) Views on early diagnosis of Alzheimer’s disease among Dutch physicians: A qualitative interview study. J Alzheimers Dis 79: , 917–927. |
[10] | Atwood CS , Perry G ((2023) ) Playing Russian roulette with Alzheimer’s disease patients: Do the cognitive benefits of lecanemab outweigh the risk of edema, stroke and encephalitis? J Alzheimers Dis 92: , 799–801. |
[11] | Itzhaki RF , Lathe R , Balin BJ , Ball MJ , Bearer EL , Braak H , Bullido MJ , Carter C , Clerici M , Cosby SL , Del Tredici K , Field H , Fulop T , Grassi C , Griffin WS , Haas J , Hudson AP , Kamer AR , Kell DB , Licastro F , Letenneur L , Lövheim H , Mancuso R , Miklossy J , Otth C , Palamara AT , Perry G , Preston C , Pretorius E , Strandberg T , Tabet N , Taylor-Robinson SD , Whittum-Hudson JA ((2016) ) Microbes and Alzheimer’s disease. J Alzheimers Dis 51: , 979–984. |
[12] | Villain N , Planche V , Levy R ((2022) ) High-clearance anti-amyloid immunotherapies in Alzheimer’s disease. Part 2: Putative scenarios and timeline in case of approval, recommendations for use, implementation, and ethical considerations in France. Rev Neurol (Paris) 178: , 999–1010. |
[13] | Daly T ((2023) ) Learning from the amyloid hypothesis: Adaptability, brevity, and clarity of ideas. Neurol Sci 44: , 2177–2178. |
[14] | Schermer MHN , Richard E ((2019) ) On the reconceptualization of Alzheimer’s disease. Bioethics 33: , 138–145. |
[15] | Milne R , Karlawish J ((2017) ) Expanding engagement with the ethical implications of changing definitions of Alzheimer’s disease, Lancet Psychiatry 4: , e6–e7. |
[16] | Dubois B , Villain N , Frisoni GB , Rabinovici GD , Sabbagh M , Cappa S , Bejanin A , Bombois S , Epelbaum S , Teichmann M , Habert MO , Nordberg A , Blennow K , Galasko D , Stern Y , Rowe CC , Salloway S , Schneider LS , Cummings JL , Feldman HH ((2021) ) Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol 20: , 484–496. |
[17] | Le Couteur DG , Doust J , Creasey H , Brayne C ((2013) ) Political drive to screen for pre-dementia: Not evidence based and ignores the harms of diagnosis. BMJ 347: , f5125. |
[18] | Ballenger JF ((2006) ) Progress in the history of Alzheimer’s disease: The importance of context. J Alzheimers Dis 9: (3 Suppl), 5–13. |
[19] | Lock M ((2013) ) The Alzheimer Conundrum: Entanglements of Dementia and Aging, Princeton University Press, Princeton, NJ. |
[20] | Fletcher JR , Zubair M , Roche M ((2022) ) The neuropsychiatric biopolitics of dementia and its ethnicity problem. Sociol Rev 70: , 1005–1024. |




