Association Between Olfactory Dysfunction and Cognitive Impairment in Dementia-Free Older Adults: A Prospective Cohort Study in Taiwan
Abstract
Background:
Previous studies assessing olfactory function and cognition have mostly been cross-sectional, and few have investigated the Asian geriatric population.
Objective:
To examine the relationships of olfaction with global or domain-specific cognitive function in Taiwanese community-dwelling older adults.
Methods:
This cohort study (2015–2019) is part of the Taiwan Initiative for Geriatric Epidemiological Research. The Taiwanese version of the Montreal Cognitive Assessment (MoCA-T) and a battery of neuropsychological tests were assessed at baseline and at a two-year follow-up. The cross-culture modified Sniffin’ Sticks Identification Test (SSIT) was utilized to measure olfactory function. Generalized linear mixed models were used to examine the association of olfaction with cognitive performance over two years.
Results:
Data were collected from 376 participants (55.1% women), with a mean age of 75.6 years. A one-point decrease in the SSIT score (worsening of olfaction) was associated with worse global cognition (MoCA-T:
Conclusions:
Odor identification deficits were associated with poor global or domain-specific cognitive function in a four-year cohort of community-dwelling older adults. Cognitive assessments should be conducted in dementia-free elderly individuals with impaired odor identification.
INTRODUCTION
The geriatric population is rapidly growing worldwide. Older people are at a greater risk of developing cognitive impairment. The prevalence of cognitive impairment is related to age in a nonlinear manner and increases more rapidly in later life. In the United States, 11.3% of people aged ≥ 65 years had Alzheimer’s disease (AD), with the percentage increasing from 5.3% of people aged 65–74 years to 34.6% of people aged ≥ 85 years [1]. In Taiwan, the age-adjusted prevalence rates of mild cognitive impairment (MCI) and all-cause dementia are 18.8% and 8.0%, respectively, with the dementia rate doubling for every 5-year increase in age [2]. On average, people live 4–8 years after a diagnosis of AD, but some patients live with the disease for 20 years [3]. The complexity and long course of the disease may have a substantial impact on the physical and mental health of the patients and their families.
The dementia process involves progressive cognitive decline, over several years or even decades. The clinical presentation of cognitive impairment is preceded by gradual pathological changes in the brain. One of the most well-known indicators of AD is the aggregation of tau proteins, which form neurofibrillary tangles in neurons. Tau protein lesions are found in the olfactory bulb and transentorhinal/entorhinal cortex in the early stages of AD [4], suggesting that tau protein formation plays a crucial role in the olfactory system.
Epidemiological data have shown that deficits in odors identification are associated with cognitive impairment [5–7] or decline [8–10] and mortality in older adults [11–13]. Several cross-sectional studies [7, 14–16] and case—control studies [5, 6] have shown that deficits in odor identification are associated with MCI. Memory is one of the most involved cognitive domains and has been associated with poor olfactory function [6, 17, 18]; one Korean study showed that impairment in the language domains was associated with poor olfactory function in elderly patients with dementia [19]. Two cohort studies reported inconsistent results, with impaired odor identification predicting cognitive decline over a 5-year period but not in a shorter period of 3 years [20, 21]. Similar to cognition, olfactory function declines with age [22]. “Sniffin’ Sticks” is a well-validated assessment of olfactory ability, including the detection threshold, odor determination, and odor identification [23, 24]. Odor identification has been proposed as a single reliable test that correlates with cognitive impairment in older people [14, 25, 26]. Although odor identification tests have been evaluated in Chinese and Japanese studies [5, 14, 27], their longitudinal association with cognitive function over time has not been established. Older age and female sex are well-known risk factors for dementia. In addition, poor odor identification has been identified in older men [28]. Deficits in odor identification in individuals carrying apolipoprotein E (APOE) ɛ4 alleles are known to predict cognitive decline [10]. Cerebrospinal fluid clearance in the peri-olfactory pathway is positively correlated with sleep quality and cognitive function [30]. Due to the potential effect of these factors on the relationship between olfactory function and cognitive performance, we performed stratified analyses to clarify this relationship.
We hypothesized that olfactory dysfunction is a prodrome of global or domain-specific cognitive impairment. In this study, we aimed to explore the association of odor identification with global and domain-specific cognitive function over time, controlling for covariates, in a cohort of dementia-free older adults in Taiwan over a four-year period.
MATERIALS AND METHODS
Study design and participants
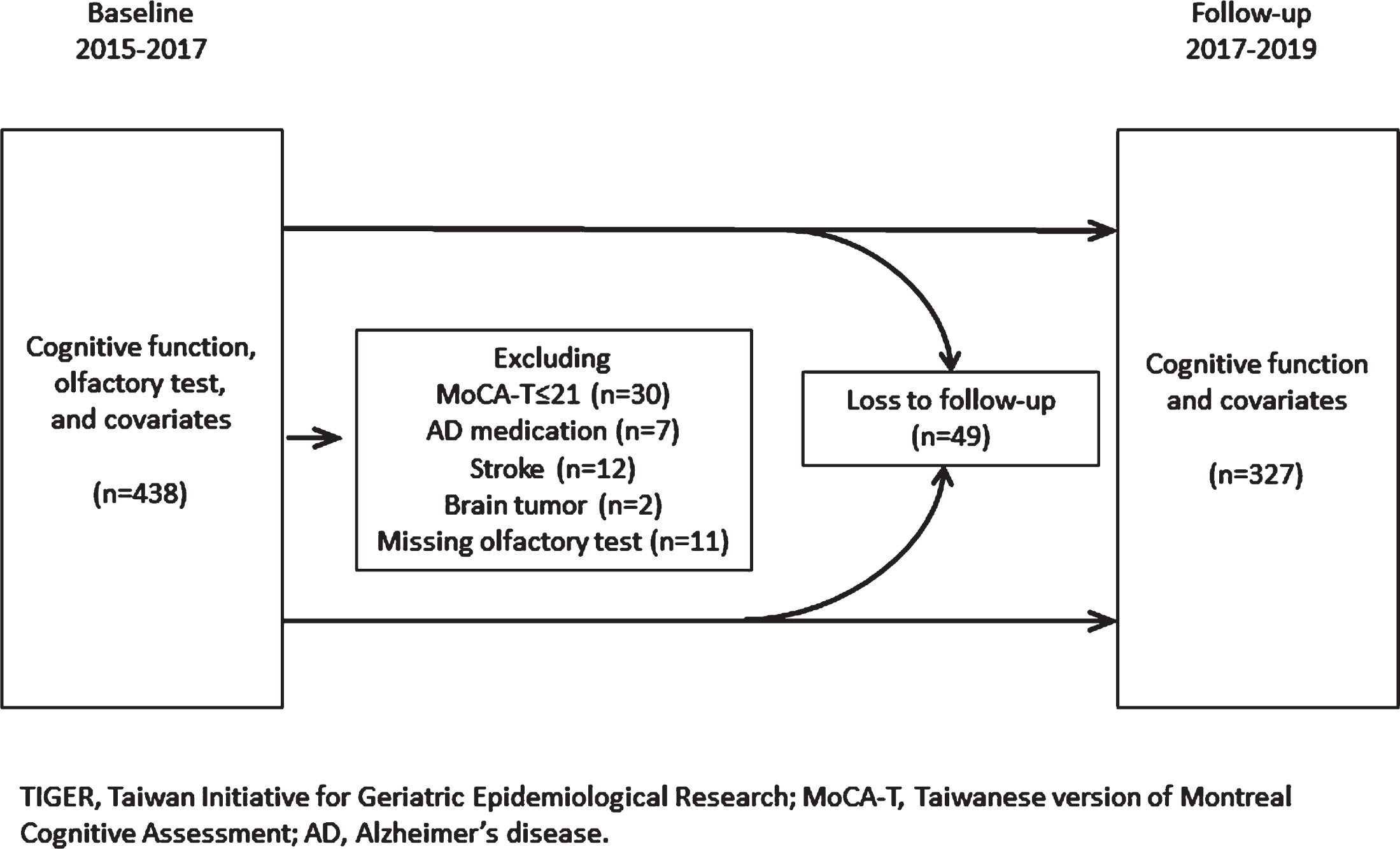
This prospective cohort study is part of the Taiwan Initiatives for Geriatric Epidemiological Research (TIGER), which recruited 605 community-dwelling older adults (aged ≥ 65 years) who participated in the senior health checkup program at National Taiwan University Hospital during 2011–2013. The TIGER was approved by National Taiwan University Hospital Research Ethics Committee (REC number: 201101039RB; 201112047RIB; 201312156RINC; 201412213RINC; 201712218RIN; 201712220RIN). Written informed consent was obtained from each participant before enrollment in 2011–2013. Figure 1 shows the recruitment procedure of our study. Because olfactory tests were performed in 2015–2017, we included 438 older adults who remained in the cohort at that time (the baseline in the present study) and underwent cognitive assessment. We excluded participants with a score of the Taiwanese version of the Montreal Cognitive Assessment (MoCA-T)≤21 (i.e., suspected dementia), AD medication use, history of stroke or brain tumor, or missing olfactory test data. Finally, 376 participants were analyzed. We followed up with the participants after two years (2017–2019), at which time 327 participants remained. The annual attrition rate was 3.8% on average.
Fig. 1
Flowchart of study population selection.
Assessment of olfactory function
We adopted a cross-culture modified version of the Sniffin’ Sticks Identification Test (SSIT, Burghart Messtechnik GmbH, Wedel, Germany) that has been validated for use in Taiwanese individuals to assess olfactory function [29]. The number of odors in the SSIT is the same as in the original version, but some of the descriptors have changed. Specifically, the odors “turpentine”, “cloves”, and “anise” were replaced with “tiger balm”, “wood”, and “star anise”, which are similar odors and are more familiar to Taiwanese people [29]. The SSIT is a 16-item multiple alternative forced choice procedure. The 16 odors are presented to the examinee in a specific order using an odor pen, and the examinee has to pick one of 4 potential choices. The total score is the number of items that the examinee has answered correctly. We further used the SSIT total scores to classify participants into three categories: SSIT scores ≥ 11 indicated normosmia, 8≤SSIT scores < 11 indicated hyposmia, and SSIT < 8 referred to anosmia.
Assessment of cognitive function
Cognitive functions were assessed at baseline (2015–2017) and at the two-year follow-up (2017–2019). Global cognitive function was assessed with the MoCA-T, and MoCA-T scores were used to classify individuals into three categories: ≥ 24 indicated normal cognitive function, 22 or 23 indicated MCI, and≤21 indicated suspected dementia [30]. Domain-specific cognitive function was assessed with the Wechsler Memory Scale-Third Edition for logical memory (immediate and delayed theme and free recall) and attention (digit span-forward and backward) [31], the Trail Making Test A and B for executive function [32], and the verbal fluency test (involving naming fish, vegetables, and fruit within 1 minute) [33]. The raw scores of cognitive domains were standardized into z scores by their baseline means and standard deviations (subtracting the mean and dividing by the standard deviation). The Trail Making Test A and B scores were multiplied by –1 such that lower scores were indicative of worse cognitive performance.
Other covariates
For each participant, a standardized questionnaire was administered by an experienced interviewer, which included sociodemographic characteristics (age, sex, years of formal education), lifestyle factors, and comorbidities. Cigarette smoking was recorded for self-reported current or former smokers. Alcohol consumption was recorded for self-reported current or former drinkers who consumed an average of more than 5 cc of wine per day. Hypertension, diabetes, dyslipidemia, and respiratory disease were determined by self-reported history or medication use. Depressive symptoms were considered present if any of the following criteria were met: self-reported history, medication use, or score on the 20-item Center for Epidemiologic Studies Depression (CES-D) scale ≥ 16 [34]. The Barthel index for activities of daily living (ADL) [35] and Lawton and Brody’s instrumental activities of daily living (IADL) [36] were used to assess independence in daily life. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) [37]; scores on 9 questions are summed to calculate the total score, which ranges from 0 to 21. A PSQI score > 5 indicates poor sleep quality. Body mass index (BMI) was derived by dividing body weight (kg) by squared body height (m2), these measurements were obtained by well-trained nurses in the health checkup program. APOE genotyping was performed by extracting genomic DNA from the buffy coat of blood samples using a QuickGene-Mini 80 system (Fujifilm, Tokyo, Japan). APOE ɛ4 status was determined by the presence of two single-nucleotide polymorphisms, rs429358 and rs7412, based on TaqMan genomic assays using an ABI 7900HT fast real-time PCR system (Applied Biosystems Inc., CA). The presence of at least one ɛ4 allele was taken to indicate that an individual was APOE ɛ4 positive [38]. All information on covariates was collected at baseline (2015–2017), with further information collected at the two-year follow-up (2017–2019). Age, years of education, BMI, ADL score, and IADL score were continuous variables, and other variables were classified as binary.
Statistical analysis
Baseline characteristics were compared across levels of SSIT scores (normosmia, hyposmia, and anosmia). Analysis of variance (ANOVA) was used to compare normally distributed continuous variables and the Kruskal—Wallis test was used to compare nonnormally distributed variables. The chi-squared test was used to compare categorical variables. The participants underwent repeated cognitive tests, so we adopted generalized linear mixed models (GLMMs) to account for heterogeneity across participants. The following GLMMs were used to examine the association of (1) olfactory scores (SSIT) and (2) olfactory levels (normosmia, hyposmia, and anosmia) with global and domain-specific cognitive function over time.
For the jth measurement (j = 1 the baseline, j = 2 the two-year follow-up) of the ith subject:
(1)
(2)
First, we included potential confounders that are clinically relevant to cognitive impairment and olfactory deficits, regardless of their statistical significance: age, sex, years of education, APOE ɛ4 status, depressive symptoms, sleep quality, respiratory disease, and smoking status. We also adjusted for the follow-up time and practice effects (i.e., number of cognitive tests completed). Finally, we introduced variables that led to a > 10% change in effect measures by using the backward “change-in-estimate” (CIE) approach [39]. In other words, we removed one covariate with the largest p value from the model each time. If the main effect measure changed by > 10%, the covariate was retained in the model. To visualize the effect of the interaction between olfactory performance and time on cognitive function, we used sliced fit plots to display the predicted cognitive score over time according to olfactory status (normosmia, hyposmia, and anosmia). Contour plots were used to illustrate the 3-dimensional relationship of the follow-up time (x-axis) and SSIT score (y-axis) with the predicted cognitive score represented by contours, and a contour line on the plot was the curve along which the cognitive score had a constant value. We further conducted stratified analysis in regard to age, sex, APOE ɛ4 status, and sleep quality. We carried out a separate hypothesis test to obtain stratum-specific effect estimates. Then we fit the model including the stratified variable × olfactory levels (or SSIT scores) interaction to obtain p values for the interaction. The statistical analysis was implemented in SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
During 2015–2017, 376 participants underwent olfactory testing and were included in the analysis. Their median (range) age was 75 (68–90) years, and 207 (55.1%) were women. Over half of the participants had normal olfaction, while 33.8% and 12.2% exhibited hyposmia and anosmia, respectively. The median (range) follow-up was 1.9 (1.0–3.0) years. Table 1 shows the sociodemographic characteristics, lifestyle factors, anthropometric measurements, physical performance, and comorbidities across the SSIT levels. Olfactory deficits were significantly associated with older age, deficits in IADL scores, and lower MoCA-T scores. Those with impaired olfaction were more likely to be men, have a smoking history, and have hypertension. We also summarized the differences in cognitive scores between the baseline (2015–2017) and the follow-up (2017–2019) in Supplementary Table 1.
Table 1
Baseline characteristics of participants according to scores on the odor identification test (n = 376)
| SSIT score | ||||||
| Missing | Total | 0–7 Anosmia | 8–10 Hyposmia | 11–16 Normosmia | p | |
| n | (n = 376) | (n = 46) | (n = 127) | (n = 203) | ||
| Mean (SD) | ||||||
| Age (y) | 0 | 75.6 (4.8) | 78.5 (5.2) | 76.4 (5.0) | 74.5 (4.2) | <0.001 |
| Education (y) | 0 | 13.9 (3.5) | 14.3 (3.7) | 13.8 (3.9) | 13.8 (3.1) | 0.704 |
| BMI (kg/m2) | 2 | 23.6 (3.1) | 23.6 (3.4) | 23.8 (2.7) | 23.6 (3.3) | 0.777 |
| ADL score | 3 | 99.3 (3.0) | 98.3 (5.8) | 99.5 (2.4) | 99.3 (2.3) | 0.231a |
| IADL score | 3 | 7.8 (0.7) | 7.7 (1.1) | 7.7 (0.9) | 8.0 (0.2) | 0.003a |
| MoCA-T score | 0 | 27.5 (1.9) | 26.4 (2.1) | 27.1 (2.0) | 27.9 (1.7) | <0.001 |
| Number (%) | ||||||
| Female sex | 0 | 207 (55.1) | 16 (34.8) | 58 (45.7) | 133 (65.5) | <0.001 |
| Cigarette smoking | 0 | 56 (14.9) | 7 (15.2) | 28 (22.1) | 21 (10.3) | 0.015 |
| Alcohol consumption | 0 | 91 (24.2) | 10 (21.7) | 34 (26.8) | 47 (23.2) | 0.694 |
| APOE ɛ4 carriers | 4 | 60 (16.1) | 5 (11.1) | 24 (19.1) | 31 (15.4) | 0.426 |
| Depressive symptom | 0 | 26 (6.9) | 7 (15.2) | 6 (4.7) | 13 (6.4) | 0.051 |
| Hypertension | 0 | 253 (67.3) | 39 (84.8) | 76 (59.8) | 138 (68.0) | 0.008 |
| Diabetes | 0 | 71 (18.9) | 9 (19.6) | 22 (17.3) | 40 (19.7) | 0.859 |
| Dyslipidemia | 0 | 232 (61.7) | 27 (58.7) | 74 (58.3) | 131 (64.5) | 0.473 |
| Respiratory disease | 1 | 60 (16.0) | 10 (22.2) | 25 (19.7) | 25 (12.3) | 0.099 |
| Poor sleep quality (PSQI score > 5) | 10 | 168 (45.9) | 26 (56.5) | 48 (39.3) | 94 (47.5) | 0.111 |
aThe Kruskal–Wallis test was used to compare nonnormally distributed continuous variables. Numbers in bold indicate statistically significant findings (p < 0.05). SSIT, Sniffin’ Sticks Identification Test; SD, standard deviation; BMI: body mass index; ADL, activity of daily living; IADL, instrumental activity of daily living; MoCA-T, Taiwanese version of Montreal Cognitive Assessment; APOE, apolipoprotein E; PSQI, Pittsburgh Sleep Quality Index.
In addition to SSIT scores, we included clinically relevant covariates (age, sex, education years, APOE ɛ4 status, depressive symptoms, PSQI score, respiratory disease, smoking status, follow-up time, and practice effects) in the model. We further included hypertension and IADL score, which were statistically significant, as shown in Table 1. For covariates not measured at follow-up (2017–2019), we included the interaction terms between these variables and the follow-up time. Then we reduced the model by backward CIE, as mentioned above. Table 2 shows the GLMMs (slopes [β]; 95% confidence interval [95% CI]) of the association between olfaction and global or domain-specific cognitive function over time. Every one-point decrease in SSIT scores (worsening of olfaction) was associated with worse global cognitive function (MoCA-T:
Table 2
Association of olfactory function with global and domain-specific cognition over two years (n = 376)
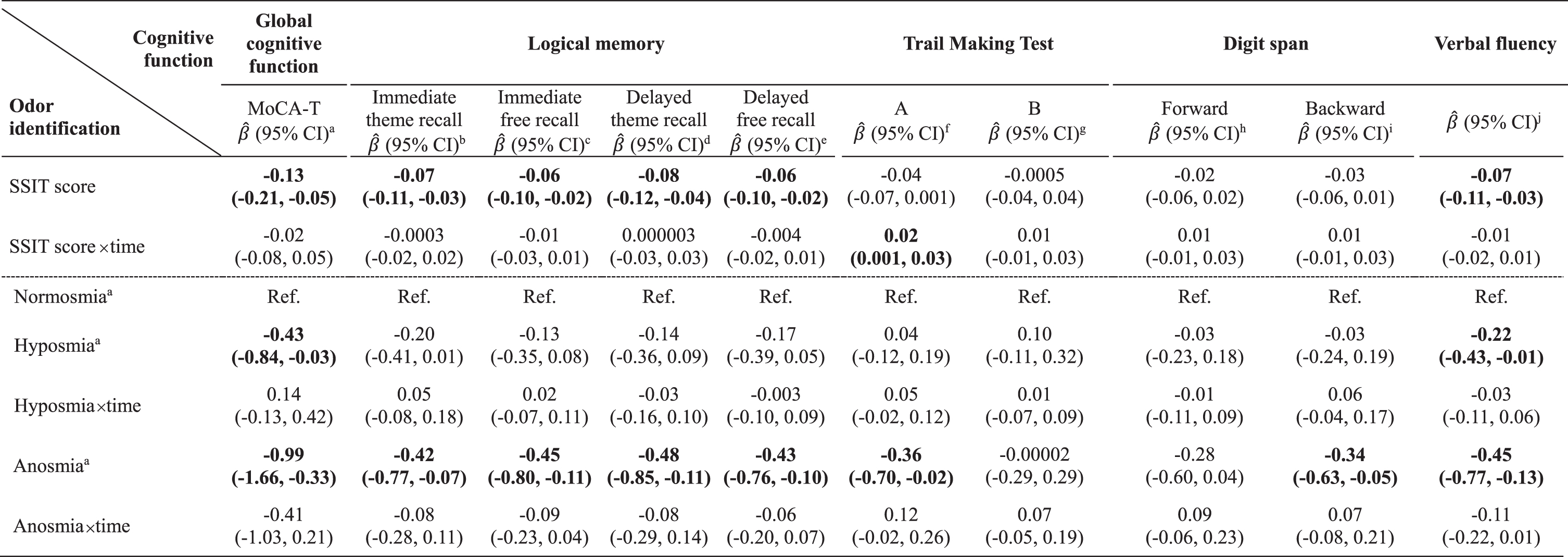 |
For continuous (SSIT scores) or categorical (normosmia, hyposmia, anosmia) olfactory function variables, GLMMs were used to estimate
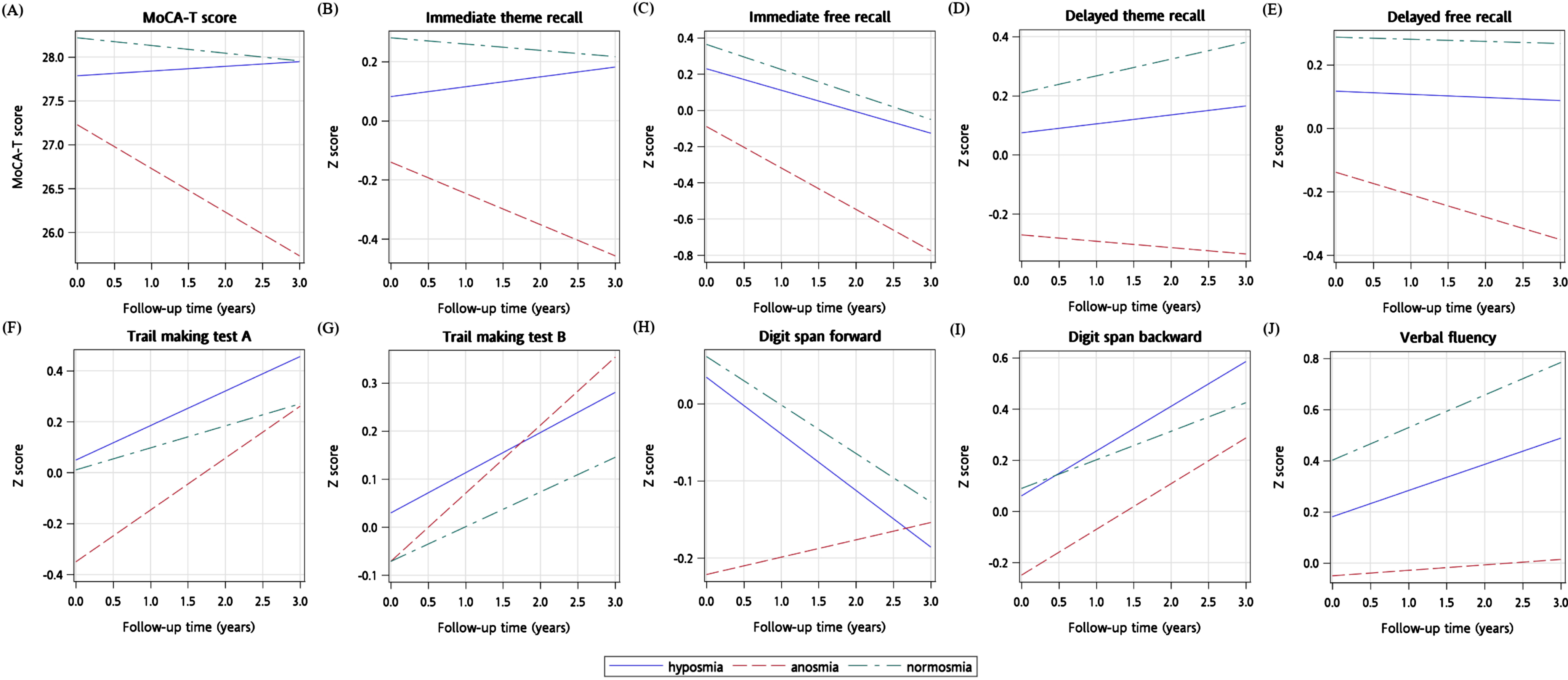
We explored the relationship of cognitive performance with time using multivariable models according to olfactory status using the sliced fit plots shown in Fig. 2. Each panel shows global or domain-specific cognitive performance over time across different olfactory levels, by conditioning on all other covariates. Some cognitive domains, such as executive function, attention (digit span backward), and verbal fluency, exhibited an improving trend at follow-up visits. In these visualizations, the cognitive function over time varied greatly with olfactory levels; however, no olfactory level by time interactions were statistically significant. Supplementary Figure 1 shows the relationship of SSIT scores with time in multivariable models with the predicted cognitive score represented by contours. Only the relationship between Trail Making Test A scores and SSIT scores was significantly attenuated at follow-up (
Fig. 2
The sliced fit plots (A to J) illustrating predicted cognitive scores over time by olfactory status (normosmia, hyposmia, and anosmia). Plots generated using multivariable adjusted models with the covariates listed in Table 2, Footnotes a-j.
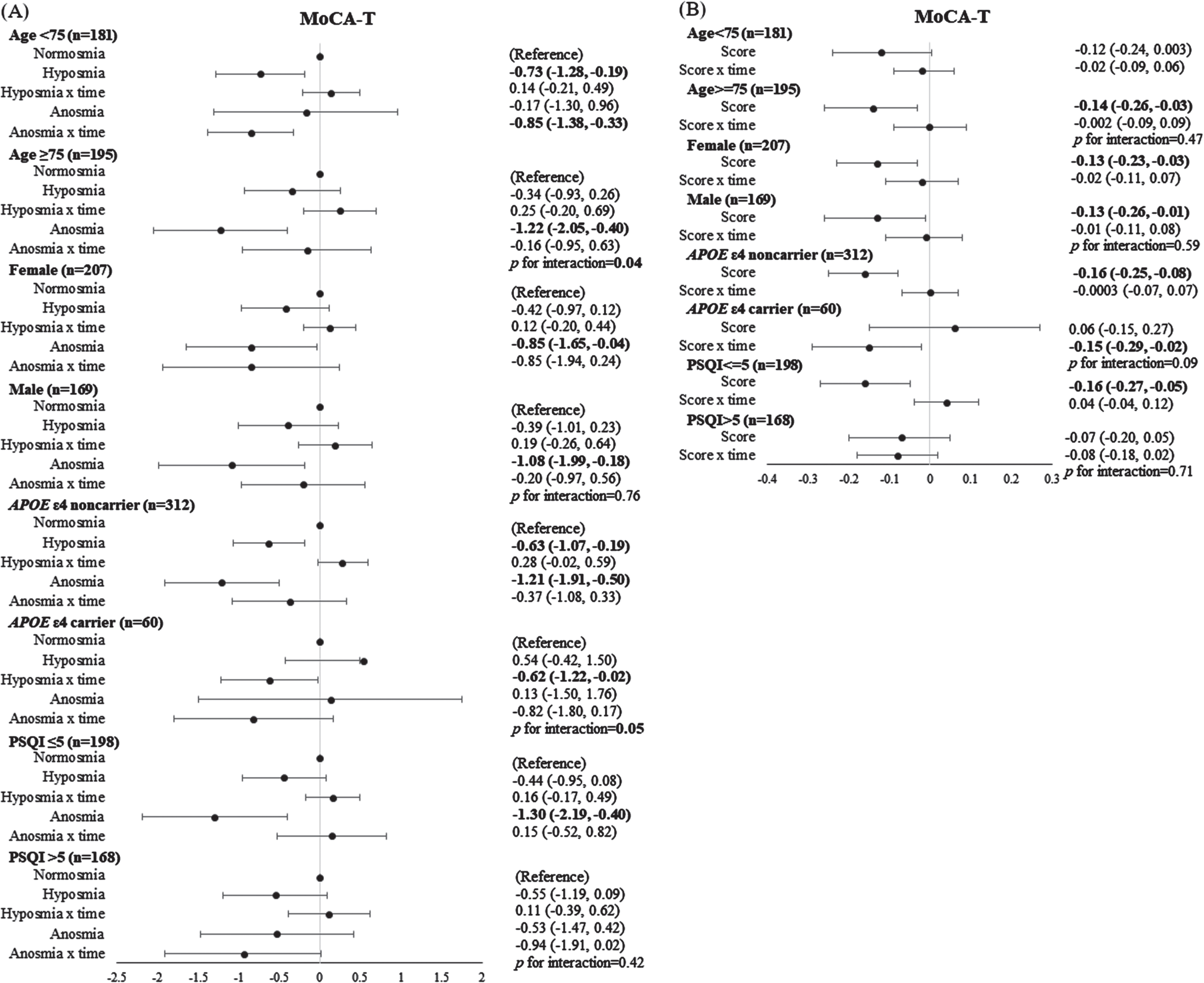
We conducted stratified analyses and evaluated the influences of age, sex, APOE genotype, and sleep quality. Figure 3 shows the association of olfactory levels and SSIT scores with global cognitive function within subgroups. Elderly individuals with anosmia or lower SSIT scores (worsening of olfaction) had significantly lower MoCA-T scores in subgroups of age ≥ 75 years, males and females, APOE ɛ4 noncarriers, and good sleep quality (PSQI score≤5). We also generated forest plots (shown in Supplementary Figures 2–10), showing the association with domain-specific cognitive function. Anosmia or lower SSIT scores were associated with impaired performance on logical memory tests in subgroups of age ≥ 75 years, males, and APOE ɛ4 noncarriers; impaired attention on the digit span backward task in APOE ɛ4 noncarriers; and impaired verbal fluency in subgroups of age ≥ 75 years, males, good sleep quality (PSQI scores≤5), and APOE ɛ4 carriers and noncarriers. The effect of the interaction of olfactory levels with age on MoCA-T scores (p = 0.04) and immediate free recall scores (p = 0.02) was significant. The effect of the interaction of olfactory levels with APOE ɛ4 status on MoCA-T (p = 0.05), immediate free recall (p = 0.02) and delayed theme recall (p = 0.01), and digit span forward (p < 0.01) was significant. When treating both olfaction and age as a continuous scale, the interaction of SSIT scores with age was significant for the memory domain (immediate and delayed theme and free recall scores: p = 0.05, 0.03, 0.001, and 0.01, respectively; data not shown).
Fig. 3
The association of olfactory levels (A) and SSIT scores (B) with global cognitive performance according to subgroups of age, sex, APOE ɛ4 status, and sleep quality. The confidence intervals have not been adjusted for multiple comparisons; hence, definitive effects for the subgroups cannot be inferred.
DISCUSSION
Our findings reveal that impaired olfactory identification ability was associated with poor global cognitive function, memory, and verbal fluency in dementia-free older adults in Taiwan. The associations above remained significant in subgroups of adults aged ≥ 75 years, males, and APOE ɛ4 noncarriers. Our study is among few prospective cohort studies to evaluate the longitudinal relationship between olfactory deficits and cognitive impairment in Asian populations, by administering a battery of neuropsychological tests that cover a wide range of cognitive functions.
Few studies have evaluated the association between olfactory identification and cognitive impairment in older Asian adults. Dong et al. conducted a cross-sectional study that included 4,481 participants aged 65 years and over living in rural areas of Shandong Province, China [27]. They found that a lower 16-item SSIT score or anosmia was associated with increased risks of all-cause dementia, AD, and vascular dementia. Liang et al. conducted a community-based cross-sectional study that included 1,782 dementia-free participants aged 65 years or older residing in downtown Shanghai, China [14]. They found that a lower score on the 12-item SSIT, a simplified version of the 16-item SSIT, was independently associated with the risk of MCI. Kouzuki et al. conducted a case—control study of 114 older Japanese patients and found that olfactory dysfunction was associated with MCI and AD [5]. In their study, olfaction was evaluated with the Odor Stick Identification Test for Japanese (OSIT-J), comprising 12 odors familiar to Japanese individuals. Our study, a prospective cohort study in an Asian population, yielded findings consistent with those of previous studies that showed a positive association between olfactory identification and cognitive function in the preclinical stage. We excluded patients with existing dementia, as olfactory areas are known to be affected by the early dementia process, and the inclusion of such cases could prevent proper causal inference.
Most previous studies with an average follow-up period of 3–5 years have shown that deficits in olfactory identification predict cognitive decline in old age [8, 20, 40]. For example, a cohort study of 589 cognitively intact older adults in the United States found that poor olfactory identification was associated with a more rapid decline in global cognitive function, development of MCI, and transition from MCI to AD, during 5 years of follow-up [20]. However, a cohort study in Australia recruiting 308 middle-aged to elderly participants showed that olfactory identification did not predict cognitive decline over 3 years of follow-up [21]. The discrepancy may be attributable to the inclusion of middle-aged participants. Although we did not assess the difference in cognitive function between baseline and follow-up, we incorporated longitudinal effects by utilizing GLMMs. We included only elderly participants, and found a positive association between olfactory identification and global cognitive function (assessed by MoCA-T, which can discriminate between normal cognitive function and MCI).
Domain-specific cognitive function has been evaluated in some previous studies. Makizako et al. recruited a total of 220 MCI patients and found that olfactory identification was associated with memory rather than attention or executive function [17]. Doorduijn et al. showed that memory, but not other cognitive domains, was associated with odor identification in patients visiting the Alzheimer Center [6]. Schiffman et al. found that patients with familial risk for AD had poor memory and verbal fluency performance over time [41]. Our findings are partly consistent with the above studies, in that both anosmia and lower SSIT scores (worsening of olfaction) were significantly associated with poor global cognitive function, memory, and verbal fluency over time. It is noteworthy that our study participants (dementia-free or with MCI) were quite different from those recruited in previous studies, and this may be the reason for the inconsistent results. Furthermore, these associations remained significant in those ≥ 75 years, males, and APOE ɛ4 noncarriers. Since most young-old individuals (aged 65–74 years) are in good health, the Japan Gerontological Society and the Japan Geriatrics Society have proposed categorizing the ages of 65 to 74 years as pre-old age and ≥ 75 years as old age [42]. Age ≥ 75 years has also been shown to predict olfactory function and its decline [43]. As neurologic degeneration occurs more frequently in old age, impairments in olfaction and cognitive function are exacerbated. This partly explains the stronger association between olfaction and cognitive function in the subgroup aged ≥ 75 years in our study. Although female superiority in olfaction is generally accepted, one meta-analysis revealed that the sex difference in olfactory identification was small [44]. In our analysis, we observed a greater sex difference in olfactory identification in the geriatric population, although we are not sure if most of the odors in the assessment tool are easily recognized by young adults of both sexes. We found that odor identification was associated with global cognitive function, memory, and verbal fluency in older men, and with global cognitive function in older women. While dementia is more prevalent in women at older ages, sex differences in MCI were minimized or even reversed in the oldest group [2]. The fact that women have superior olfaction and are more likely to have dementia attenuates the association between olfactory deficits and cognitive impairment. However, this attenuation is weakened in regard to MCI, a milder form of cognitive impairment. This may explain our observation that odor identification was associated with domain-specific cognitive function in males, and global cognitive function (i.e., MCI) in both sexes.
The association between olfactory identification and memory could be explained by the involvement of central olfactory structures inside the temporal lobe in the preclinical phase of dementia. Tau protein theory is a hallmark of the pathogenesis of AD, the most common type of dementia. Neuropathological evidence has shown correlations between the distribution of neurofibrillary tangles and the clinical course of AD. The first neurofibrillary tangles occur in pre-α cells of the transentorhinal cortex, an area of transition between the allocortical entorhinal cortex and the temporal isocortex, before the occurrence of MCI [45, 46]. It is worth noting that the association between cerebrospinal fluid biomarker levels and olfactory function was inconsistent [5, 6], implying that patients with dementia other than AD also tend to have difficulty in recognizing odors [47]. Each domain-specific cognitive function is correlated with different brain areas. The prefrontal cortex and medial temporal lobe have been associated with age-related memory impairments, including episodic memory and working memory [48]. The prefrontal cortex has also been related to verbal fluency in aging, which requires both executive and language function [49]. Various brain areas particularly the prefrontal region, white matter volume change, and brain structural and functional connectivity have all been related to the age-related decline in executive function [50].
The strengths of our study include the prospective cohort design that enabled repeated measures and causal interpretation, assessment of both global and domain-specific cognitive function, and APOE genotype data. We excluded patients with dementia and found that olfactory deficits may be useful in detecting early cognitive impairment, which may provide a valuable contribution to efforts to identify effective preventive strategies. There are also some limitations. First, olfactory function was only measured at baseline; hence, we incorporated an olfaction by time interaction into GLMMs to assess how olfaction affected cognitive function over a two-year period. Second, improvement in some cognitive domains over time was observed, which attenuated the main effects. This may be explained by participation bias, in that those who attended the health checkup program tended to be healthier. As follow-up time increased, the health status of participants became similar to that of the general population. Since these participants were dementia-free, their cognitive impairment may be reversible. Another reason was that the practice effect outweighed the aging effect during the short follow-up period. Finally, the power of dichotomized global cognitive function (MoCA-T score 22/23 versus ≥ 24) as an outcome (data not shown) may be too low to detect significant differences due to the small number of events during two years of follow-up. This can only be overcome by enrolling more participants or observing the participants for a longer period. We also recognize that stratifying the sample resulted in small sample sizes in each individual stratum, which have reduced the statistical power of analyses.
We found that olfactory deficits were associated with cognitive impairment in dementia-free Taiwanese older adults. Olfactory deficits may serve as a marker for future cognitive impairment. Future larger studies with longer follow-up periods are needed to explore the longitudinal relationship between olfactory and cognitive function.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This study was supported by the Ministry of Science and Technology in Taiwan with the following grants: 100-2314-B-002-103, 101-2314-B-002-126-MY3, 104-2314-B-002-038-MY3, and 107-2314-B-002-186-MY3 (to YCC) and 103-2314-B-002-033-MY3 and 107-2314-B-002-230 (to JHC).
CONFLICT OF INTEREST
Yen-Ching Chen is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding authors, YCC and JHC. The data are not publicly available because they contain information that could compromise the privacy of research participants.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230319.
REFERENCES
[1] | Alzheimer’s Association ((2021) ) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17: , 327–406. |
[2] | Sun Y , Lee H-J , Yang S-C , Chen T-F , Lin K-N , Lin C-C , Wang P-N , Tang L-Y , Chiu M-J ((2014) ) A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS One 9: , e100303. |
[3] | Uddin MS , Ashraf GM (2018) Introductory chapter: Alzheimer’s disease–the most common cause of dementia. In Advances in Dementia Research, IntechOpen, London. |
[4] | Braak H , Del Tredici K ((2015) ) The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138: , 2814–2833. |
[5] | Kouzuki M , Suzuki T , Nagano M , Nakamura S , Katsumata Y , Takamura A , Urakami K ((2018) ) Comparison of olfactory and gustatory disorders in Alzheimer’s disease. Neurol Sci 39: , 321–328. |
[6] | Doorduijn A , De Van Der Schueren M , van de Rest O , De Leeuw F , Fieldhouse J , Kester M , Teunissen C , Scheltens P , Van Der Flier W , Visser M ((2020) ) Olfactory and gustatory functioning and food preferences of patients with Alzheimer’s disease and mild cognitive impairment compared to controls: The NUDAD project. J Neurol 267: , 144–152. |
[7] | Churnin I , Qazi J , Fermin CR , Wilson JH , Payne SC , Mattos JL ((2019) ) Association between olfactory and gustatory dysfunction and cognition in older adults. Am J Rhinol Allergy 33: , 170–177. |
[8] | Devanand D , Lee S , Manly J , Andrews H , Schupf N , Doty RL , Stern Y , Zahodne LB , Louis ED , Mayeux R ((2015) ) Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 84: , 182–189. |
[9] | Graves AB , Bowen J , Rajaram L , McCormick W , McCurry S , Schellenberg G , Larson E ((1999) ) Impaired olfaction as a marker for cognitive decline: Interaction with apolipoprotein E ɛ4 status. Neurology 53: , 1480–1487. |
[10] | Schubert CR , Carmichael LL , Murphy C , Klein BE , Klein R , Cruickshanks KJ ((2008) ) Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc 56: , 1517–1521. |
[11] | Devanand DP , Lee S , Manly J , Andrews H , Schupf N , Masurkar A , Stern Y , Mayeux R , Doty RL ((2015) ) Olfactory identification deficits and increased mortality in the community. Ann Neurol 78: , 401–411. |
[12] | Wilson RS , Yu L , Bennett DA ((2011) ) Odor identification and mortality in old age. Chem Senses 36: , 63–67. |
[13] | Pinto JM , Wroblewski KE , Kern DW , Schumm LP , McClintock MK ((2014) ) Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One 9: , e107541. |
[14] | Liang X , Ding D , Zhao Q , Guo Q , Luo J , Hong Z ((2016) ) Association between olfactory identification and cognitive function in community-dwelling elderly: The Shanghai aging study. BMC Neurol 16: , 199. |
[15] | Suzuki H , Sugiura S , Nakashima T , Teranishi M , Shimono M , Murotani K , Sakurai T , Uchida Y , Saji N ((2022) ) Cognitive impairment is correlated with olfactory identification deficits in older Japanese adults: A cross-sectional study using objective and subjective olfactory measures. Geriatr Gerontol Int 22: , 924–929. |
[16] | Yamamoto K , Shiota S , Yoshiiwa A , Chishima T , Takigami S , Miyazaki E ((2022) ) Cognitive function and olfactory impairment in community-dwelling older adults attending a salon. J Prim Care Community Health 13: , 21501319221117793. |
[17] | Makizako M , Makizako H , Doi T , Uemura K , Tsutsumimoto K , Miyaguchi H , Shimada H ((2014) ) Olfactory identification and cognitive performance in community-dwelling older adults with mild cognitive impairment. Chem Senses 39: , 39–46. |
[18] | Wang Q , Chen B , Zhong X , Zhou H , Zhang M , Mai N , Wu Z , Huang X , Haehner A , Chen X ((2021) ) Olfactory dysfunction is already present with subjective cognitive decline and deepens with disease severity in the Alzheimer’s disease spectrum. J Alzheimers Dis 79: , 585–595. |
[19] | Cha H , Kim S , Son Y ((2022) ) Associations between cognitive function, depression, and olfactory function in elderly people with dementia in Korea. Front Aging Neurosci 13: , 799897. |
[20] | Wilson RS , Schneider JA , Arnold SE , Tang Y , Boyle PA , Bennett DA ((2007) ) Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry 64: , 802–808. |
[21] | Sohrabi H , Bates K , Weinborn M , Johnston A , Bahramian A , Taddei K , Laws S , Rodrigues M , Morici M , Howard M ((2012) ) Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl Psychiatry 2: , e118. |
[22] | Attems J , Walker L , Jellinger KA ((2015) ) Olfaction and aging: A mini-review. Gerontology 61: , 485–490. |
[23] | Hummel T , Sekinger B , Wolf SR , Pauli E , Kobal G ((1997) ) ‘Sniffin’sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22: , 39–52. |
[24] | Wolfensberger M ((2000) ) Sniffin’Sticks: A new olfactory test battery. Acta Otolaryngol 120: , 303–306. |
[25] | Eibenstein A , Fioretti A , Simaskou M , Sucapane P , Mearelli S , Mina C , Amabile G , Fusetti M ((2005) ) Olfactory screening test in mild cognitive impairment. Neurol Sci 26: , 156–160. |
[26] | Rezek DL ((1987) ) Olfactory deficits as a neurologic sign in dementia of the Alzheimer type. Arch Neurol 44: , 1030–1032. |
[27] | Dong Y , Wang Y , Liu K , Hou T , Han X , Cong L , Ren Y , Zhang Q , Tang S , Ekström I ((2022) ) Dementia screening in rural-dwelling Chinese older adults: The utility of a smell test and the self-rated AD8. J Am Geriatr Soc 70: , 1106–1116. |
[28] | Xu L , Liu J , Wroblewski KE , McClintock MK , Pinto JM ((2020) ) Odor sensitivity versus odor identification in older US adults: Associations with cognition, age, gender, and race. Chem Senses 45: , 321–330. |
[29] | Shu C-H , Yuan B-C , Lin S-H , Lin C-Z ((2007) ) Cross-cultural application of the “Sniffin’Sticks” odor identification test. Am J Rhinol 21: , 570–573. |
[30] | Tsai C-F , Lee W-J , Wang S-J , Shia B-C , Nasreddine Z , Fuh J-L ((2012) ) Psychometrics of the Montreal Cognitive Assessment (MoCA) and its subscales: Validation of the Taiwanese version of the MoCA and an item response theory analysis. Int Psychogeriatr 24: , 651–658. |
[31] | Wechsler D (1997) Wechsler Memory Scale–Third Edition manual, The Psychological Corporation, San Antonio, TX. |
[32] | Bowie CR , Harvey PD ((2006) ) Administration and interpretation of the Trail Making Test. Nat Protoc 1: , 2277–2281. |
[33] | Mok EHL , Lam LCW , Chiu HFK ((2004) ) Category verbal fluency test performance in Chinese elderly with Alzheimer’s disease. Dement Geriatr Cogn Disord 18: , 120–124. |
[34] | Lewinsohn PM , Seeley JR , Roberts RE , Allen NB ((1997) ) Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 12: , 277–287. |
[35] | Mahoney FI ((1965) ) Functional evaluation: The Barthel index. Md State Med J 14: , 61–65. |
[36] | Lawton MP , Brody EM ((1969) ) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: , 179–186. |
[37] | Buysse DJ , Reynolds CF III , Monk TH , Berman SR , Kupfer DJ ((1989) ) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: , 193–213. |
[38] | Chen Y-C , Jung C-C , Chen J-H , Chiou J-M , Chen T-F , Chen Y-F , Tang S-C , Yeh S-J , Lee M-S ((2017) ) Association of dietary patterns with global and domain-specific cognitive decline in Chinese elderly. J Am Geriatr Soc 65: , 1159–1167. |
[39] | Maldonado G , Greenland S ((1993) ) Simulation study of confounder-selection strategies. Am J Epidemiol 138: , 923–936. |
[40] | Wilson RS , Arnold SE , Tang Y , Bennett DA ((2006) ) Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 26: , 61–67. |
[41] | Schiffman S , Graham B , Sattely-Miller E , Zervakis J , Welsh-Bohmer K ((2002) ) Taste, smell and neuropsychological performance of individuals at familial risk for Alzheimer’s disease. Neurobiol Aging 23: , 397–404. |
[42] | Ouchi Y , Rakugi H , Arai H , Akishita M , Ito H , Toba K , Kai I ((2017) ) Redefining the elderly as aged 75 years and older: Proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int 17: , 1045–1047. |
[43] | London B , Nabet B , Fisher AR , White B , Sammel MD , Doty RL ((2008) ) Predictors of prognosis in patients with olfactory disturbance. Ann Neurol 63: , 159–166. |
[44] | Sorokowski P , Karwowski M , Misiak M , Marczak MK , Dziekan M , Hummel T , Sorokowska A ((2019) ) Sex differences in human olfaction: A meta-analysis. Front Psychol 10: , 242. |
[45] | Moloney CM , Lowe VJ , Murray ME ((2021) ) Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimers Dement 17: , 1554–1574. |
[46] | DeTure MA , Dickson DW ((2019) ) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14: , 1–18. |
[47] | Schiffman SS , Clark CM , Warwick ZS ((1990) ) Gustatory and olfactory dysfunction in dementia: Not specific to Alzheimer’s disease. Neurobiol Aging 11: , 597–600. |
[48] | Shing YL , Werkle-Bergner M , Brehmer Y , Müller V , Li S-C , Lindenberger U ((2010) ) Episodic memory across the lifespan: The contributions of associative and strategic components. Neurosci Biobehav Rev 34: , 1080–1091. |
[49] | Kahlaoui K , Di Sante G , Barbeau J , Maheux M , Lesage F , Ska B , Joanette Y ((2012) ) Contribution of NIRS to the study of prefrontal cortex for verbal fluency in aging. Brain Lang 121: , 164–173. |
[50] | Fjell AM , Sneve MH , Grydeland H , Storsve AB , Walhovd KB ((2017) ) The disconnected brain and executive function decline in aging. Cereb Cortex 27: , 2303–2317. |




