Hippocampal Volume and Episodic Associative Memory Identify Memory Risk in Subjective Cognitive Decline Individuals in the CIMA-Q Cohort, Regardless of Cognitive Reserve Level and APOE4 Status
Abstract
Background:
Subjective cognitive decline (SCD) was proposed to identify older adults who complain about their memory but perform within a normal range on standard neuropsychological tests. Persons with SCD are at increased risk of dementia meaning that some SCD individuals experience subthreshold memory decline due to an underlying progression of Alzheimer’s disease (AD).
Objective:
Our main goal was to determine whether hippocampal volume and APOE4, which represent typical AD markers, predict inter-individual differences in memory performance among SCD individuals and can be used to identify a meaningful clinical subgroup.
Methods:
Neuropsychological assessment, structural MRI, and genetic testing for APOE4 were administered to one hundred and twenty-five older adults over the age of 65 from the CIMAQ cohort: 66 SCD, 29 individuals with mild cognitive impairment (MCI), and 30 cognitively intact controls (CTRLS). Multiple regression models were first used to identify which factor (hippocampal volume, APOE4 allele, or cognitive reserve) best predicted inter-individual differences in a Face-name association memory task within the SCD group.
Results:
Hippocampal volume was found to be the only and best predictor of memory performance. We then compared the demographic, clinical and cognitive characteristics of two SCD subgroups, one with small hippocampal volume (SCD/SH) and another with normal hippocampal volume (SCD/NH), with MCI and CTRLS. SCD/SH were comparable to MCI on neuropsychological tasks evaluating memory (i.e., test of delayed word recall), whereas SCD/NH were comparable to CTRLS.
Conclusion:
Thus, using hippocampal volume allows identification of an SCD subgroup with a cognitive profile consistent with a higher risk of conversion to AD.
INTRODUCTION
It is now recognized that Alzheimer’s disease (AD) includes a long preclinical phase during which the disease develops without cognitive impact. This means that the point at which a person meets criteria for dementia occurs very late in the disease process, a situation that impairs understanding of the disease and implementation of early treatment. The concepts of mild cognitive impairment (MCI) and subjective cognitive decline (SCD) have been proposed to better understand the pre-dementia phase of AD. Both identify non-demented older adults who complain about their memory, but people with MCI perform below normative values on standardized neuropsychological tests, whereas those with SCD perform within normal range [1, 2]. SCD has gained considerable recent attention because memory complaint is believed to represent one of the earliest symptomatic manifestations of memory impairment in the prodrome of AD [3]. Thus, it is hypothesized that a proportion of people meeting criteria for SCD are in a pre-clinical phase of AD, before the onset of significant cognitive symptoms. A meta-analysis indicated that the risk of developing dementia is doubled in these persons compared to healthy controls [4] and that they have a greater risk of clinical progression to MCI [5]. At a very early phase, people would experience progressive cognitive problems that would remain undetected with current psychometric tools, while generating a sense of change and a complaint. Although SCD is a promising concept for identifying people in the early stage of AD, it is likely a heterogeneous group combining older adults in the prodrome of AD, worried well older adults, and people in whom the complaint relates to causes other than AD. More work is thus needed to validate and optimize this relatively recent classification [4, 6, 7].
One pragmatic approach to better understand the early AD phase is to combine current classification of SCD with low-cost biomarkers of AD [8–11] in their capacity to differentiate people with SCD. Magnetic resonance imaging (MRI) is widely accessible and the neurodegeneration of the hippocampal region is a recognized neuropathological marker of AD [12, 13]. This well-established AD marker could thus be useful to refine our knowledge on the cognition of people with SCD who are in the early phase of AD. Also, the presence of the ɛ4 allele of the apolipoprotein E (APOE4) is known as a major AD genetic risk factor [14, 15]. It has been well demonstrated that the presence of APOE4 significantly increases the risk of having late-onset AD ([16], for a meta-analysis).
Therefore, the goals of this study are to determine whether hippocampal volume and/or presence of APOE4 are associated with interindividual differences in associative memory within a group of people with SCD. We also included cognitive reserve (CR) in our analyses, as it is an important factor to consider when examining the relationship between biomarkers and memory performance within SCD individuals. Indeed, current biomarkers of aging or neurodegenerative diseases are imperfect indicators of cognitive status and the concept of CR has been proposed to explain why this is the case [17]. Psychosocial characteristics, such as education, premorbid IQ, or profession, moderate the relationship between pathology and symptoms [18–20] because people with higher CR proxies may endure more pathology while maintaining a given level of cognition [21] and/or experience a reduced risk of cognitive decline ([22], for a review; [23]). Such a protective effect was found to be greater in the pre-dementia phase [24] and in people positive for AD biomarkers [25]. For these reasons, this study proposes to assess the effect of hippocampal volume and APOE4 on the memory of persons with SCD, while also measuring the contribution of CR as a potential moderator of the relation between these two biomarkers and memory. Associative memory is used here as a predicted outcome because it relies on the integrity of the hippocampus and might start to show subthreshold decline at an early phase, while classical memory assessment is often not sensitive enough to reveal subtle deficits in an SCD population, an episodic associative memory task like the Face-name task might prove useful for detecting SCD cognitive deficits early on. Such greater sensitivity might be due to different characteristics of the task: first, it requires memorizing an association between a visual and verbal element [26]; second, the association between a face and a particular name is arbitrary; and finally, memorizing face-name associations does not typically rely on prior semantic knowledge. By evaluating the influence of these predictors on memory, this study proposes to define a way to classify an SCD subgroup more at risk of being affected by AD. If a significant predictor is observed, we will then identify and compare SCD subgroups based on the predictor to a group of cognitively intact older adults and a group of MCI on neuropsychological tasks. This will determine contribute to support the validity of this SCD classification proposed as more refined.
The study might lead to new and important findings. It might reveal the factors necessary for identifying those among SCD individuals who exhibit features that are typically associated with AD. This is useful from a triple perspective: 1) first, clinicians may be able to establish earlier diagnostic leads for AD by identifying the most at-risk SCD individuals; 2) second, researchers may be benefited by enriching their inclusion criteria, thus forming less heterogeneous and more informative SCD groups; 3) finally, helping to establish a consistent profile as a clinically valid construct, at individual level, that may predict the progression from SCD to AD.
MATERIALS AND METHODS
Participants
The data used in this manuscript were obtained thanks to the Consortium for the Early Identification of Alzheimer’s Disease - Quebec (CIMA-Q). The main objective of CIMA-Q is the longitudinal characterization of an observational cohort of more than 350 elderly women and men, cognitively healthy, with subjective cognitive disorders, suffering from mild cognitive disorders, or suffering from dementia due to probable AD. CIMA-Q collects clinical, cognitive, biological, radiological and pathological data from these participants in order to 1) establish an early diagnosis of AD; 2) to provide the scientific community with a well-characterized cohort; 3) identify new therapeutic targets to prevent or slow down cognitive decline and AD; and 4) to support new clinical studies on these targets [27].
Participants were included if they were at least 65 years old, native French or English speakers, right-handed, and if they met the safety criteria for MRI. A telephone pre-screening interview was first conducted and the Telephone – Mini-Mental State Examination (T-MMSE; [28]) was administered to review exclusion/inclusion criteria. Then, participants were invited to a clinical diagnosis assessment by expert consensus (completed by a nurse and a physician) based on current clinical criteria.
Participants with SCD had to have (a) reported that their memory was not as good as it used to be and that it worried them; (b) normal education-adjusted scores on the Logical Memory II subtest of the Wechsler Memory Scale (WMS [29]; score of ≥3 for 0 to 7 years of education, ≥5 for 8 to 15 years, and ≥9 for 16 or more years); (c) a score of >26 on the Montreal Cognitive Assessment (MoCA; [30]); and (d) a score of 0 on the Clinical Dementia Rating Scale [31]. Participants with MCI had to meet clinical criteria for MCI based on the National Institute on Aging and the Alzheimer’s Association (NIA-AA; [32]): (a) a reported decline of their memory; (b) an objective memory impairment according to education-adjusted normative values on the Logical Memory II subtest of the WMS; (c) a score between 20 and 26 on the MoCA; and (d) a score of 0.5 on the Clinical Dementia Rating Scale. Finally, participants in the CTRLS group had to have: (a) no memory complaint or worry; (b) a normal score on education-adjusted normative values on the Logical Memory II subtest of the WMS; (c) a performance above 26/30 on the MoCA; and (d) a score of 0 on the Clinical Dementia Rating Scale.
To be able to ensure a follow-up of a longitudinal study, participants were not included if they were planning on moving outside of Quebec in the next 3 years. They were also excluded if they had any illness or condition that could compromise their participation in the study, such as a central nervous system disease; intracranial brain surgery; a history of addiction to alcohol, drugs, or narcotics; or a daily consumption of benzodiazepines.
For this specific study, the data from 125 participants who received a neuroimaging examination and provided a genetic sample were analyzed. The sample was composed of 66 SCD, 29 MCI and 30 CTRLS (Table 1). This sample was used in a previous CIMA-Q publication [33].
Table 1
Demographic, hippocampal and APOE4 data, and performance on selected neuropsychological measures, of participants with Subjective Cognitive Decline/Normal Hippocampus (SCD/NH), Subjective Cognitive Decline/Small Hippocampus (SCD/SH), Mild Cognitive Impairment (MCI), and cognitively healthy controls (CTRLS)
| p | ||||||||||
| CTRLS | SCD/NH | SCD/SH | MCI | SCD/NH versus CTRLS | SCD/SH versus CTRLS | MCI versus CTRLS | SCD/NH versus SCD/SH | SCD/NH versus MCI | SCD/SH versus MCI | |
| Demographic | ||||||||||
| N | 30 | 47 | 19 | 29 | ||||||
| Sex (Male/Female) | 9/21 | 19/28 | 6/13 | 14/15 | – | – | – | – | – | – |
| Age | 71.9±5.7 | 72.0±5 | 73.5±5.4 | 76.3±5.3 | 1 | 1 | 0.009* | 1 | 0.004* | 0.416 |
| Education (y) | 16.1±3.8 | 15.2±3.1 | 15.4±3.5 | 15±3a | ns | ns | ns | ns | ns | ns |
| Clinical criteria tasks | ||||||||||
| MoCA | 28.5±1.4 | 27.9±1.4 | 27.8±1.3 | 24.8±2.1 | 0.78 | 1 | <0.001** | 1 | <0.001** | <0.001** |
| T-MMSE | 25.2±1 | 24.1±2.1 | 25.1±1 | 24.3±1.6 | 0.019* | 1 | 0.619 | 0.089 | 1 | 1 |
| Logical Memory | 14.7±4.6 | 13.3±4.6 | 13.7±3.9 | 9.6±4.3 | 1 | 1 | 0.003* | 1 | 0.06 | 0.091 |
| Predictors | ||||||||||
| Mean hippocampal volume | –0.1±0.9a | 0.3±0.7 | –1.5±0.6 | –1.2±1.2 | 0.275 | <0.001** | <0.001** | <0.001** | <0.001** | 1 |
| (Left and Right, z-score) | ||||||||||
| N APOE4 carriers | 6/30 | 6/47 | 7/19 | 12/29 | – | – | – | – | – | – |
| Cognitive reserve | 18±3.9 | 16.4±4.2 | 17.3±4.5 | 17.1±4a | ns | ns | ns | ns | ns | ns |
| Predicted variable | ||||||||||
| Face-Name delayed recall (/9) | 5.4±1.7 | 4.9±2.3b | 3.1±1.9a | 2.8±2.3 | 1 | 0.006* | 0.004* | 0.038* | 0.029* | 1 |
| Cognitive assessment | ||||||||||
| RAVLT delayed recall (/15) | 11.1±2.3d | 9.8±2.7 | 8.3±2.5b | 6.8±3.6 | 0.312 | 0.012* | <0.001** | 0.481 | 0.021* | 1 |
| Hayling inhibition test (/30) | 18.6±4.1c | 20.7±4.7b | 17.9±5.1c | 19±4.9d | ns | ns | ns | ns | ns | ns |
| Semantic Fluency test | 21.1±5 | 19.7±4.6 | 19.0±5 | 16.4±4 | 0.761 | 0.781 | 0.003* | 1 | 0.073 | 0.386 |
| Trail test Time B/ Time A | 1.9±0.6 | 2±0.5 | 2±0.9 | 2.8±1.9 | 1 | 1 | 0.083 | 1 | 0.099 | 0.244 |
| Depression and anxiety scales | ||||||||||
| GDS | 2.2±2.9 | 5.6±4.3 | 6.4±4.5 | 6.8±5.5 | 0.009* | 0.003* | 0.002* | 1 | 1 | 1 |
| GAI | 1.1±0.7a | 2.7±0.5 | 3.2±0.9 | 4.1±0.8 | ns | ns | ns | ns | ns | ns |
Note. Unless otherwise indicated, values are mean ± standard deviation. P values refer to significant analysis of variance (demographic, genetic and imaging data) and analysis of covariance models (neuropsychological tests controlled for age), followed by post hoc pairwise comparisons with Bonferroni correction; ns: non-significant analysis before post hoc comparisons; *p < 0.05, **p < 0.001; CTRLS: Cognitively healthy controls; SCD/NH: Subjective Cognitive Decline/Normal Hippocampus; SCD/SH: Subjective Cognitive Decline/Small Hippocampus; MCI: Mild Cognitive Impairment; MoCA: Montréal Cognitive Assessment; T-MMSE: Telephone – Mini-Mental-State-Examination; ApoE4: Apolipoprotein 4; RAVLT: Rey Auditory Verbal Learning Test; GDS: Geriatric Depression Scale; GAI: Geriatric Anxiety Inventory. aData missing for one subject, bfor two subjects, cfor three subjects, dfor five subjects.
Structural MRI
Participants received an anatomical MRI examination following the standardized Canadian Dementia Imaging Protocol parameters (https://www.cdip-pcid.ca/). The T1-weighted images were analyzed using the FreeSurfer 5.3.0 software (https://surfer.nmr.mgh.harvard.edu/). Hippocampal segmentation was done following the procedure described in Fischl et al.’s automatic parcellation study [34] and the raw left and right hippocampal volumes in standardized space were transformed into z-scores using normative data [35] adjusting for age, sex, estimated total intracranial volume, scanner type, and scanner strength. A mean of the left and right hippocampal z-score volumes was then computed to be used as a predictor.
Genotyping
The genomic DNA of the participants was extracted from the buffy coat fraction [27] and all the persons included in this study were genotyped for APOE4 alleles. Participants with at least one APOE4 allele were identified as positive.
Cognitive reserve
A proxy for cognitive reserve was quantified through the Cognitive Reserve Index questionnaire (CRIq; adapted in French by Eduardo Cisneros) [36], part of our CIMA-Q protocol, evaluating level of education, profession, stimulating activities, and physical activity with 15 questions (Maximum score = 30). The CRIq, while not evaluating a specific period of lifetime, illustrates the best personal time of functioning of the participants.
Predicted variable
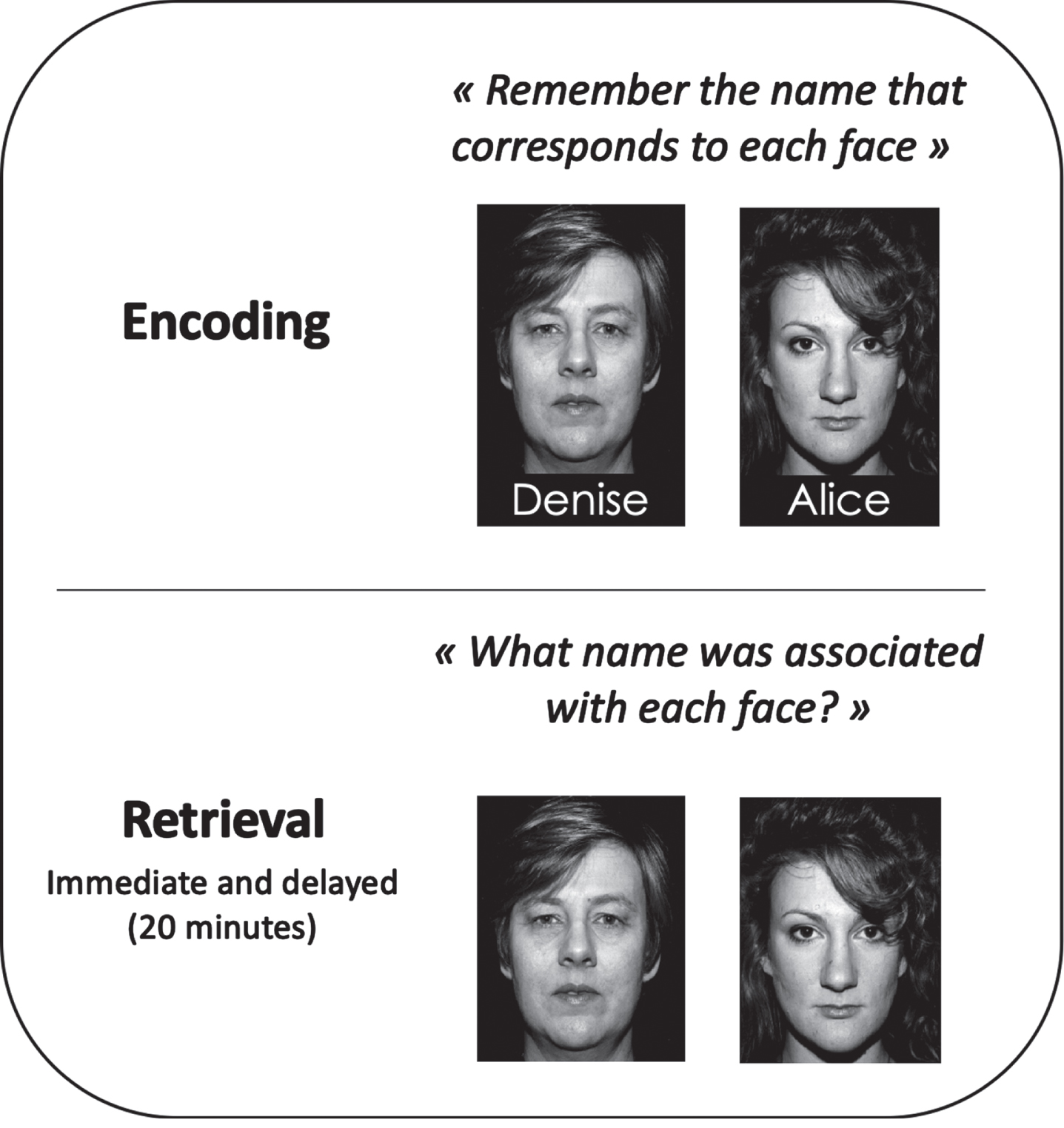
Associative memory was assessed through the delayed portion of the Face-name association (Brambati, for CIMA-Q), an associative memory task. Participants were instructed to remember the names of 9 faces of men and women during a presentation of eight seconds per face-name pairing on a computer screen. They then completed an immediate recall and delayed recall after 20 minutes (see Fig. 1). The delayed recall portion of the task presented the image of each face for a maximum of ten seconds and asked participants to give the associated name within this ten-second timeframe. The task provides a score out of 9 and was used as the predicted variable in our study, where a larger score represents better performance.
Fig. 1
Face-Name task (Brambati, for CIMA-Q).
Cognitive assessment to test group differences
A comprehensive neuropsychological assessment was also administered to all participants (for a detailed description, see [33]). It included memory and executive tests different from those used for inclusion criteria to avoid circularity issues. We selected and reported in this study the most sensitive tests to early AD according to a meta-analysis carried out in our research center [37]. Memory was measured with the delayed recall portion (after 20 min) of the Rey auditory verbal learning test (RAVLT), which is a 15-word list recall task (procedure adapted by CIMA-Q), and the Face-name association task described above as our predicted variable. Executive functions were measured with Category fluency [38] (i.e., one-minute fluency for the animal category), a computerized version of the Hayling test [39] where participants are asked to complete sentences using an unrelated and out-of-context word, and the Trail Making Test [40], using the ratio between completion times of Trail B and Trail A. For all these tests, a higher score indicated better performance, except for the Trail B/Trail A ratio, for which a lower score indicated better performance.
We also quantified the depressive state of participants through the Geriatric Depression Scale (GDS; [41]), a self-administered questionnaire in which participants answered by yes or no on 30 items about how they felt over the past week. The Geriatric Anxiety Inventory (GAI; [42]), a 20 item questionnaire about common anxiety symptoms (answer agree/disagree) was used to quantify the anxiety level of the participants.
Statistical analysis
All analyses were done with the Statistical Package for the Social Sciences (IBM-SPSS Statistics, version 25).
As a first step, progressive multiple linear regression analyses were used with the backward method to identify the predictors of associative memory in the SCD group [43]. The initial model included three independent variables (mean of hippocampal volumes, APOE4, and cognitive reserve score) in the forced regression. The regression removed the variables with the lowest contribution to the model if the variation of R2 was not significant. The procedure was repeated until all variables contributed significantly to the improvement of R2. The three independent variables were entered as predictors and the delayed Face-name task score as the dependent variable. Then, the model determined predictive values and explained variance. For this regression model, standardized residuals [43] were evaluated case by case to detect outliers. Because cognitive reserve was conceptualized as a moderating factor, a separate regression was computed to analyze the potential moderating effect of the reserve score on the relationship between face-name task performance and both hippocampal volume and APOE4 status.
Two SCD subgroups were then identified using their characteristics on the significant predictor(s) to create 1) an at-risk SCD subgroup and 2) a subgroup composed of the remaining SCD participants.
Analyses of variance (ANOVAs) were then conducted to assess differences between the two SCD subgroups, CTRLS, and MCIs on demographics, clinical, and neuroimaging data. Analyses of covariance (ANCOVAs) were computed to assess group differences on cognitive variables, controlling for the demographic that showed group differences. When a main group effect was found, Bonferroni’s post-hoc comparisons identified the location of the group difference. Chi-squared analyses were performed for discrete variables (sex and APOE4).
RESULTS
Multiple regression models
Progressive multiple linear regression models are detailed in Table 2. A significant effect was found with an F(3.59) = 4.193, p = 0.009 and an adjusted R2 = 0.13, indicating that our model successfully predicted the delayed Face-name task performance. The model indicated a significant effect of the mean hippocampal volume (t = 2.914; p = 0.005), while the two other factors (APOE4 status and CR) did not contribute significantly to the model.
Table 2
Predicting effect of mean of hippocampal volumes, APOE4, and cognitive reserve score on Face-name task delayed score in the SCD group
| Predictor | n | B value | SE B value | β | t | p |
| Hippocampal volume | 63 | 0.769 | 0.264 | 0.352 | 2.914 | 0.005* |
| APOE4 | 63 | –0.256 | 0.747 | –0.042 | –0.343 | 0.733 |
| Cognitive Reserve | 63 | 0.117 | 0.065 | 0.213 | 1.796 | 0.078 |
Note. SE = Standard Error; *p < 0.05.
The moderation analysis showed that the relationship between mean hippocampal volume and Face-name task performance (t = 0.47; p = 0.640) and between APOE4 and Face-name task performance (t = 0.11; p = 0.916) was not moderated by the reserve score.
Group comparisons
Based on the significant predictor included in the first regression model, participants with smaller mean (left and right) hippocampal z-scores volumes (small hippocampus or SH) were identified as SCD/SH (N = 19). They were compared to the remaining SCD participants with normal hippocampal volumes (NH), identified as SCD/NH (N = 47), as well as to MCI and CTRLS. Smaller mean hippocampal z-scores volumes were defined as those that were one standard deviation below the mean of study CTRLS (corrected for intracranial volume). Group comparisons are presented in Table 1 (see the Supplementary Material for ranges).
The ANOVAs that assessed the group differences on demographics (Table 1) indicated no group effect for years of education, but a significant group effect for age, as MCI were older than the CTRLS and SCD/NH. Thus, age was controlled for subsequent analyses that compared the two SCD subgroups to CTRLS and MCI. Chi-squared test was non-significant for sex, but significant for APOE4 (p = 0.021).
There was a main group effect for the pooled mean left and right hippocampal volumes. By design, hippocampal volumes were smaller in SCD/SH than in CTRLS (p < 0.001). Interestingly, there was no significant hippocampal volume difference between SCD/SH and MCI (p = 1), whereas SCD/NH and CTRLS differed from MCI (p < 0.001). There were no differences between SCD/NH and CTRLS (p = 0.275). Table 1 shows scores and group comparisons on the Face-name task. Participants in the SCD/SH group showed lower performance than that of SCD/NH (p = 0.038) which is expected given the regression model on the Face-name delayed recall. However, it is interesting to note that SCD/SH showed lower performance than CTRLS (p = 0.006) and did not differ from MCI (p = 1) on the Face-name delayed recall. In contrast, SCD/NH were not impaired relative to CTRLS (p = 1), but differed from MCI, the latter showing lower performance (p = 0.029).
Table 1 also shows group comparisons on clinical and neuropsychological measures. Both MCI (p < 0.001) and SCD/SH (p = 0.012) showed lower performance than CTRLS on the RAVLT delayed recall. MCI also showed lower performance than SCD/NH on the RAVLT (p = 0.021). MCI showed lower performance than CTRLS on the Semantic Fluency test (p < 0.05) and while the SCD/SH also showed lower performance than CTRLS on that task, this did not reach significance. There was no significant difference when comparing SCD/SH and MCI on memory or executive tasks.
Finally, the two SCD subgroups and MCI showed larger scores on the GDS than the CTRLS (p < 0.05), but no significant difference was observed on the GAI between the four groups.
DISCUSSION
Despite colossal efforts for a better characterization of SCD persons over the past few years, this concept remains, in essence, quite heterogeneous in regard to brain characteristics, cognitive performance, and prognosis [1]. In the present study, we used a biomarker of neurodegeneration (hippocampal volume), APOE4, and a measure of cognitive reserve to identify the factors that explain most inter-individual differences in associative memory performance within SCD individuals. We then identified two subgroups of SCD based on hippocampal volume and compared them on clinical and demographic variables to CTRLS and persons meeting the criteria for MCI. The goal was to provide empirically based criteria outside of cognition that would reduce heterogeneity among the group of SCD. Since those criteria are predictors of individual differences in memory, they could contribute to identify a clinically meaningful subgroup of SCD and one closer to MCI in terms of cognitive profile.
The regression model identified that hippocampal volume was the only significant predictor of inter-individual variation in the associative memory task, with no effect of APOE4 status and level of CR. When using hippocampal volume to categorize the SCD group into SCD/SH and SCD/NH, we extended the finding observed with our regression by comparing these subgroups to MCI and CTRLS. We observed that the SCD/SH subgroup (i.e., those with smaller mean hippocampal volumes) performed similarly to MCI and was impaired compared to CTRLS on the Face-Name delayed recall. In contrast, the SCD/NH subgroup remained unimpaired compared to CTRLS on this task.
Interestingly, when this SCD categorization is used to compare the groups on classical neuropsychological tasks, SCD/SH were comparable to MCIs and more impaired than CTRLS on the delayed recall of the RAVLT, a memory test which measures delayed free recall of a word list. Note that, on this task, the difference between SCD/SH and SCD/NH was not significant, suggesting that there was more variability and/or smaller effect size for this task. In tasks evaluating executive functions, while not as impaired as those of the MCI group, SCD/SH still showed a trend of impairment when compared to CTRLS (e.g., Semantic fluency test). These results highlight two important elements: 1) dividing the SCD group according to participants’ hippocampal volume makes it possible to identify a subgroup (SCD/SH) that is closer to MCI in terms of memory performance; 2) an episodic associative memory task like the Face-name task was confirmed to be significantly useful for detecting SCD cognitive deficits early on. As mentioned above, this might be due to the special features of the face-name association task.
Our study is consistent with prior findings indicating that associative memory relies on the hippocampus [44] and is sensitive to early AD detection [45]. It shows that hippocampal volume is a good predictor of associative memory performance and suggests that identifying an SCD/SH subgroup where both of these dimensions are negatively affected increases our chances to flag individuals who may be at higher risk of converting to MCI. The Face-Name association task as our predicted variable proved useful in separating SCD/NH from SCD/SH, the latter being a seemingly more at-risk group and having characteristics closer to people with MCI. As such, associative memory may be used as a non-intrusive measure to identify at-risk individuals among people with a memory complaint, even at a very early and subtle level, which is promising for earlier AD detection and clinical intervention.
Persons who fall in the SCD/NH subgroup are interesting because these individuals are worried about their memory yet have very few signs of an underlying dementia. This subgroup most likely represents a heterogeneous set of individuals comprising non-AD older adults with conditions associated with memory concerns. Indeed, we know that certain health-related issues are associated with SCD in the elderly, such as psychiatric and mood disorders and certain medical conditions [9]. Noteworthily, we found no group differences on a measure of anxiety (GAI), but both SCD subgroups and MCI reported more depressive symptoms than controls, based on their GDS scores. Although a potential contribution of psychological symptoms in these early phases is recognized, this study validates the usefulness of SCDs and MCIs as risk groups that can be identified using AD-specific characteristics not driven by psychological symptoms. From a clinical standpoint, even if people meeting criteria for SCD do not stand in a preclinical phase of AD, their concerns are real and more research will be necessary to inform health care providers about the source of their concerns, as well as how to address them [46].
One limitation of our study is our sample size which may limit the scope of our interpretations and call for future work. Nevertheless, our results emphasize that persons with an SCD/SH profile differ from CTRLS and SCD/NH on a sensitive associative memory task, and this even in a modest sample size. Another limitation is that we restricted our biomarkers to hippocampal volume without validated staging tool and did not include AD pathology measures such as amyloid PET, or more recent biomarkers such as measures of inflammation [47]. However, the measures used in our study are more easily available and allow quicker implementation for research on this type of population.
Conclusion
Taken together, results from this study indicate that hippocampal volume is a relevant predictor of early performance degradation in episodic associative memory. Furthermore, using hippocampal volume as an AD biomarker to parse out heterogeneity in SCD identifies a subgroup with associative memory performance that is poorer than healthy controls and comparable to MCI individuals, which suggests a possible prodrome to AD. Sensitive tasks, such as the Face-Name association task, could be a precious tool in research but also in clinical practice to refine the criteria for categorizing SCD subjects. A longitudinal follow-up of this independent cohort could confirm whether the SCD/SH subcategory combined with associative memory performance is a valid predictor of a possible progression to AD.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The project was supported by a Canadian Institutes of Health Research (CIHR) Foundation Grant (to SB). CIMA-Q is supported by the Fonds de recherche du Québec – Santé (FRQ-S), Pfizer Innovation Program (to SB and CH), the Quebec Network for Research on aging, a network supported by the FRQ-S, the Fondation Courtois (Neuromod project; SB), the Consortium for the Neurodegeneration associated with Aging (CCNA/CCNV) to SB. SB holds a Canada Research Chair on Cognitive Neuroscience of Aging and Brain Plasticity. MC holds a post-doctoral fellowship grant from the CRIUGM and from the FRQ-S.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230131.
REFERENCES
[1] | Jessen F , Amariglio RE , Van Boxtel M , Breteler M , Ceccaldi M , Chételat G , Dubois B , Dufouil C , Ellis KA , Van Der Flier WM , Glodzik L , Van Harten AC , De Leon MJ , McHugh P , Mielke MM , Molinuevo JL , Mosconi L , Osorio RS , Perrotin A , Petersen RC , Rabin LA , Rami L , Reisberg B , Rentz DM , Sachdev PS , De La Sayette V , Saykin AJ , Scheltens P , Shulman MB , Slavin MJ , Sperling RA , Stewart R , Uspenskaya O , Vellas B , Visser PJ , Wagner M ; Subjective Cognitive Decline Initiative (SCD-I) Working Group ((2014) ) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10: , 844–852. |
[2] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[3] | Warren SL , Reid E , Whitfield P , Moustafa AA ((2022) ) Subjective memory complaints as a predictor of mild cognitive impairment and Alzheimer’s disease. Discov Psychol 2: , 13. |
[4] | Mitchell AJ , Beaumont H , Ferguson D , Yadegarfar M , Stubbs B ((2014) ) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand 130: , 439–451. |
[5] | Buckley RF , Maruff P , Ames D , Bourgeat P , Martins RN , Masters CL , Rainey-Smith S , Lautenschlager N , Rowe CC , Savage G , Villemagne VL , Ellis KA ((2016) ) Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimer Dement 12: , 796–804. |
[6] | Jorm AF , Butterworth P , Anstey KJ , Christensen H , Easteal S , Maller J , Mather KA , Turakulov RI , Wen W , Sachdev P ((2004) ) Memory complaints in a community sample aged 60-64 years: Associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med 34: , 1495–1506. |
[7] | Mendonça MD , Alves L , Bugalho P ((2016) ) From subjective cognitive complaints to dementia. Am J Alzheimers Dis Other Demen 31: , 105–114. |
[8] | Jack CR , Knopman DS , Jagust WJ , Shaw LM , Aisen PS , Weiner MW , Petersen RC , Trojanowski JQ ((2010) ) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: , 119–128. |
[9] | Jessen F , Amariglio RE , Buckley RF , van der Flier WM , Han Y , Molinuevo JL , Rabin L , Rentz DM , Rodriguez-Gomez O , Saykin AJ , Sikkes SAM , Smart CM , Wolfsgruber S , Wagner M ((2020) ) The characterisation of subjective cognitive decline. Lancet Neurol 19: , 271–278. |
[10] | Stern Y ((2009) ) Cognitive reserve. Neuropsychologia 47: , 2015–2028. |
[11] | Visser PJ , Verhey F , Knol DL , Scheltens P , Wahlund LO , Freund-Levi Y , Tsolaki M , Minthon L , Wallin ÅK , Hampel H , Bürger K , Pirttila T , Soininen H , Rikkert MO , Verbeek MM , Spiru L , Blennow K ((2009) ) Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol 8: , 619–627. |
[12] | van der Flier WM , Scheltens P ((2009) ) Alzheimer disease: Hippocampal volume loss and Alzheimer disease progression. Nat Rev Neurol 5: , 361–362. |
[13] | Barnes J , Bartlett JW , van de Pol LA , Loy CT , Scahill RI , Frost C , Thompson P , Fox NC ((2009) ) A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol Aging 30: , 1711–1723. |
[14] | Chartier-hariln MC , Parfitt M , Legrain S , Pérez-tur J , Brousseau T , Evans A , Berr C , Vldal O , Roques P , Gourlet V , Fruchart JC , Delacourte A , Rossor M , Amouyel P ((1994) ) Apolipoprotein e, ɛ4 allele as a major risk factor for sporadic early and late-onset forms of Alzheimer’s disease: Analysis of the 19q13.2 chromosomal region. Hum Mol Genet 3: , 569–574. |
[15] | Liu CC , Kanekiyo T , Xu H , Bu G ((2013) ) Apolipoprotein e and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9: , 106–118. |
[16] | Xu X , Zhang B , Wang X , Zhang Q , Wu X , Zhang J , Bai Y , Gu X ((2021) ) A meta-analysis of Alzheimer’s disease’s relationship with human ApoE gene variants. Am J Transl Res 13: , 9974–9982. |
[17] | Stern Y , Arenaza-Urquijo EM , Bartrés-Faz D , Belleville S , Cantilon M , Chetelat G , Ewers M , Franzmeier N , Kempermann G , Kremen WS , Okonkwo O , Scarmeas N , Soldan A , Udeh-Momoh C , Valenzuela M , Vemuri P , Vuoksimaa E ; the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup ((2020) ) Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement 16: , 1305–1311. |
[18] | Bennett DA , Wilson RS , Schneider JA , Evans DA , Mendes de Leon CF , Arnold SE , Barnes LL , Bienias JL ((2003) ) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60: , 1909–1915. |
[19] | Stern Y ((2012) ) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11: , 1006–1012. |
[20] | Bessi V , Mazzeo S , Padiglioni S , Piccini C , Nacmias B , Sorbi S , Bracco L ((2018) ) From subjective cognitive decline to Alzheimer’s disease: The predictive role of neuropsychological assessment, personality traits, and cognitive reserve. A 7-year follow-up study. J Alzheimers Dis 63: , 1523–1535. |
[21] | Vemuri P , Weigand SD , Przybelski SA , Knopman DS , Smith GE , Trojanowski JQ , Shaw LM , Decarli CS , Carmichael O , Bernstein MA , Aisen PS , Weiner M , Petersen RC , Jack CR Jr ; Alzheimer’s Disease Neuroimaging Initiative ((2011) ) Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain 134: , 1479–1492. |
[22] | Nelson ME , Jester DJ , Petkus AJ , Andel R ((2021) ) Cognitive reserve, Alzheimer’s neuropathology, and risk of dementia: A systematic review and meta-analysis. Neuropsychol Rev 31: , 233–250. |
[23] | Soldan A , Pettigrew C , Cai Q , Wang J , Wang MC , Moghekar A , Miller MI , Albert M , BIOCARD Research Team ((2017) ) Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease HHS Public Access. Neurobiol Aging 60: , 164–172. |
[24] | Van Loenhoud AC , van der Flier WM , Wink AM , Dicks E , Groot C , Twisk J , Barkhof F , Scheltens P , Ossenkoppele R ((2019) ) Cognitive reserve and clinical progression in Alzheimer disease. Neurology 93: , e334–e346. |
[25] | McKenzie C , Bucks RS , Weinborn M , Bourgeat P , Salvado O , Gavett BE ((2020) ) Cognitive reserve predicts future executive function decline in older adults with Alzheimer’s disease pathology but not age-associated pathology. Neurobiol Aging 88: , 119–127. |
[26] | Mangels JA , Manzi A , Summerfield C ((2010) ) The first does the work, but the third time’s the charm: The effects of massed repetition on episodic encoding of multimodal face–name associations. J Cogn Neurosci 22: , 457–473. |
[27] | Belleville S , LeBlanc AC , Kergoat MJ , Calon F , Gaudreau P , Hébert SS , Hudon C , Leclerc N , Mechawar N , Duchesne S , Gauthier S ; Consortium for the Early Identification of Alzheimer’s disease-Quebec (CIMA-Q) ((2019) ) The Consortium for the early identification of Alzheimer’s disease–Quebec (CIMA-Q). Alzheimers Dementia (Amst) 11: , 787–796. |
[28] | Newkirk LA , Kim JM , Thompson JM , Tinklenberg JR , Yesavage JA , Taylor JL ((2004) ) Validation of a 26-point telephone version of the Mini-Mental State Examination. J Geriatr Psychiatry Neurol 17: , 81–87. |
[29] | Weschler D ((1997) ) Wechsler Adult Intelligence Scale (3rd ed.). Psychological Corporation, San Antonio, TX. |
[30] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[31] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412. |
[32] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[33] | Caillaud M , Hudon C , Boller B , Brambati S , Duchesne S , Lorrain D , Gagnon JF , Maltezos S , Mellah S , Phillips N , Belleville S ((2020) ) Evidence of a relation between hippocampal volume, white matter hyperintensities, and cognition in subjective cognitive decline and mild cognitive impairment. J Gerontol B Psychol Sci Soc Sci 75: , 1382–1392. |
[34] | Fischl B , Van Der Kouwe A , Destrieux C , Halgren E , Ségonne F , Salat DH , Busa E , Seidman LJ , Goldstein J , Kennedy D , Caviness V , Makris N , Rosen B , Dale AM ((2004) ) Automatically parcellating the human cerebral cortex. Cereb Cortex 14: , 11–22. |
[35] | Potvin O , Mouiha A , Dieumegarde L , Duchesne S ((2016) ) Normative data for subcortical regional volumes over the lifetime of the adult human brain. Neuroimage 137: , 9–20. |
[36] | Nucci M , Mapelli D , Mondini S ((2012) ) Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin Exp Res 24: , 218–226. |
[37] | Belleville S , Fouquet C , Duchesne S , Collins DL , Hudon C ((2014) ) Detecting early preclinical Alzheimer’s disease via cognition, neuropsychiatry, and neuroimaging: Qualitative review and recommendations for testing. J Alzheimers Dis 42: , S375–S382. |
[38] | Belleville S , Fouquet C , Hudon C , Zomahoun HTV , Croteau J ((2017) ) Neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer’s type dementia in older adults: A systematic review and meta-analysis. Neuropsychol Rev 27: , 328–353. |
[39] | Burgess PW , Shallice T ((1997) ) The Hayling and Brixton tests. Thames Valley Test Company Limited, Bury St Edmunds, UK. |
[40] | Strauss E , Sherman EMS , Spreen O , Spreen O ((2006) ) A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed.). Oxford University Press. |
[41] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1982) ) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: , 37–49. |
[42] | Pachana NA , Byrne GJ , Siddle H , Koloski N , Harley E , Arnold E ((2007) ) Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr 19: , 103–114. |
[43] | Field A ((2009) ) Discovering statistics using SPSS: (and sex and drugs and rock “n” roll) (3rd ed.). SAGE Publications. |
[44] | Mayes A , Montaldi D , Migo E ((2007) ) Associative memory and the medial temporal lobes. Trends Cogn Sci 11: , 126–135. |
[45] | Alegret M , Muñoz N , Roberto N , Rentz DM , Valero S , Gil S , Marquié M , Hernández I , Riveros C , Sanabria A , Perez-Cordon A , Espinosa A , Ortega G , Mauleón A , Abdelnour C , Rosende-Roca M , Papp KV , Orellana A , Benaque A , Tarraga L , Ruiz A , Boada M ((2020) ) A computerized version of the Short Form of the Face-Name Associative Memory Exam (FACEmemory®) for the early detection of Alzheimer’s disease. Alzheimers Res Ther 12: , 25. |
[46] | Gruters AAA , Ramakers IHGB , Verhey FRJ , Köhler S , Kessels RPC , De Vugt ME ((2019) ) Association between proxy- or self-reported cognitive decline and cognitive performance in memory clinic visitors. J Alzheimers Dis 70: , 1225–1239. |
[47] | Shen XN , Niu LD , Wang YJ , Cao XP , Liu Q , Tan L , Zhang C , Yu JT ((2019) ) Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry 90: , 590–598. |




