Incidence of Newly-Diagnosed Dementia After COVID-19 Infection versus Acute Upper Respiratory Infection: A Retrospective Cohort Study
Abstract
Background:
There is emerging evidence that coronavirus disease 2019 (COVID-19) is giving rise to seemingly unrelated clinical conditions long after the infection has resolved.
Objective:
The aim of this study is to examine whether COVID-19 is associated with an increased risk of dementia including Alzheimer’s disease.
Methods:
This retrospective cohort study is based on longitudinal data from the IQVIATM Disease Analyzer database and included patients aged≥65 with an initial diagnosis of COVID-19 or acute upper respiratory infection (AURI) from 1,293 general practitioner practices between January 2020 and November 2021. AURI patients were matched 1 : 1 with COVID-19 patients using propensity scores based on sex, age, index quarter, health insurance type, the number of doctor visits, and comorbidities associated with dementia risk. Incidence rates of newly-diagnosed dementia were calculated using the person-years method. Poisson regression models were used to compute the incidence rate ratios (IRR).
Results:
The present study included 8,129 matched pairs (mean age 75.1 years, 58.9% females). After 12 months of follow-up, 1.84% of the COVID-19 patients and 1.78% of the AURI patients had been diagnosed with dementia. The Poisson regression model resulted in an IRR of 1.05 (95% CI: 0.85–1.29).
Conclusion:
This study did not find any association between COVID-19 infection and one-year dementia incidence after controlling for all common risk factors for dementia. Because dementia is a progressive disease, which can be difficult to diagnose, a longer follow-up period might offer a better insight into a possible association between COVID-19 infection and an increased incidence of dementia cases in the future.
INTRODUCTION
Caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the respiratory infection coronavirus disease 2019 (COVID-19) has infected over 600 million people globally since the start of the global pandemic in late 2019 [1, 2]. Yet little is known about the short- and long-term negative health outcomes following COVID-19 infection [3]. There is increasing evidence to support the notion that long-term negative health outcomes following COVID-19 infection are common. A recent meta-analysis and systematic review suggests that 80% of patients will experience one or more specific symptoms after the acute phase of the illness [4]. While many of these symptoms, such as fatigue, headache, or cough, correspond to the clinical sequelae of the preceding COVID-19 infection, there is also a growing body of evidence indicating that COVID-19 infection can lead to the development of new-onset diseases that are seemingly unrelated to the index COVID-19 infection.
Increasing evidence suggests that the post-acute phase of COVID-19 may be associated with an increased incidence of new-onset type 2 diabetes [5, 6], new-onset hypertension [7], and new diagnosis of neuropsychiatric conditions such as depression, anxiety, insomnia, and memory and attention impairments [8–12]. While the mechanisms underlying these associations are not yet fully understood, this observation may have several important implications for health and disease beyond the index COVID-19 infection.
It is conceivable that shared underlying mechanisms exist that are driving the increased incidence of specific conditions following COVID-19. It is also notable that the specific conditions that appear to be upregulated following COVID-19 infection, as outlined above, may also lead to an increased risk of other negative health outcomes. For example, type 2 diabetes, hypertension, depression, anxiety, and cognitive impairment are well-established risk factors for developing dementia and may thus lead to an increased incidence of dementia following COVID-19 infection [11]. Taquet and colleagues analyzed a large US federated electronic health record database including 69.8 million patients and reported a significantly higher incidence rate for dementia in the 14–90 days beyond COVID-19 diagnosis compared to matched patient cohorts with influenza and other respiratory tract infections [13]. Freudenberg-Hua et al. (2022) reported an increased 1-year incidence rate of post-COVID dementia in adults aged 65 + who were hospitalized with COVID-19 in the US [13]. However, there is a need for further studies conducted in other countries.
Given the worldwide prevalence of COVID-19, even a small increase in the incidence of these negative health outcomes would lead to a substantial increase in patient numbers. This would in turn place a significant burden on patients and healthcare systems.
In summary, there is emerging evidence that COVID-19 is leading to seemingly unrelated clinical conditions long after the infection has resolved. This may have far-reaching health effects, which are largely unknown at the present time. The overall aim of this study is to examine whether COVID-19 is associated with an increased risk of new-onset dementia following COVID-19 infection.
METHODS
Database
This study is based on data from the IQVIATM Disease Analyzer electronic medical record database. The Disease Analyzer database contains longitudinal prescription (Anatomical Therapeutic Chemical [ATC] classification system) and diagnosis (International Classification of Diseases, 10th revision [ICD-10]) data from a national sample of office-based physicians (general practitioners and specialists) in Germany. Coverage is about 3% of outpatient practices in Germany and the database also includes basic demographic variables about patients. IQVIA obtains this information in anonymous format and monitors the quality of the reported data on a regular basis using various criteria (e.g., completeness of documentation, linkage between diagnoses and prescriptions). In 2018, Rathmann et al. demonstrated the validity of the Disease Analyzer database and showed that it is representative of general and specialized practices in Germany [14]. The database has already been used in previous studies focusing on dementia [15–17] and COVID-19 [5, 18, 19]. Previous research has reported that GP consultations initially decreased in 2020 but this decline was compensated in 2021. The database was considered appropriate for studies on the effects of the pandemic on health outcomes [20].
Study population
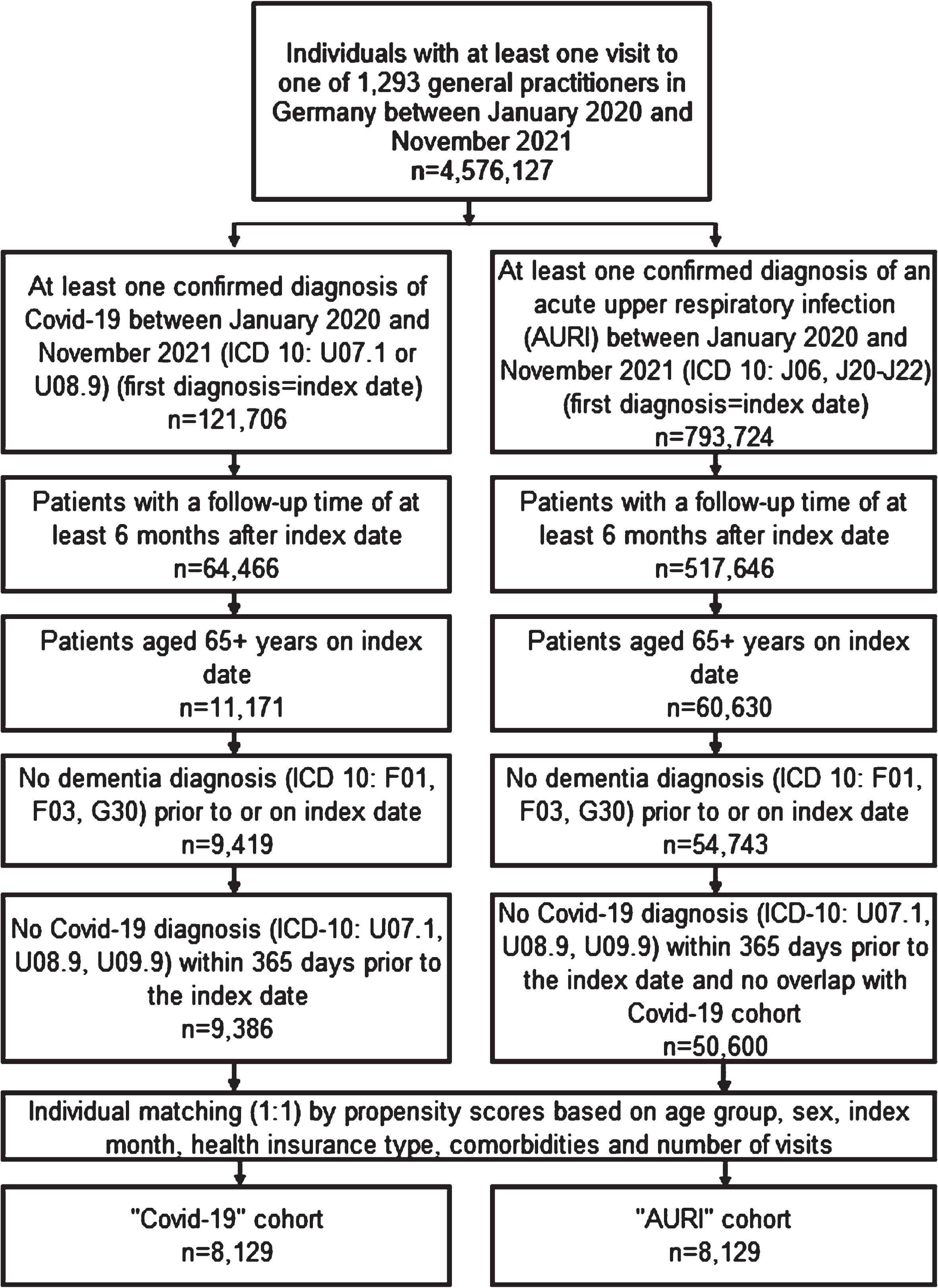
This retrospective cohort study included patients aged≥65 with an initial and confirmed diagnosis of COVID-19 (ICD-10: U70.1, U08.9) or with a confirmed diagnosis of acute upper respiratory infection (AURI) (ICD-10: J06, J20–J22) between January 2020 and November 2021. AURI patients were selected as positive controls in order to control for mechanisms associated with the acute infection episode. The maximum follow-up period was until May 2022. The EMR data used came from a total of 1,293 general practitioner (GP) practices in Germany (index date; Fig. 1). Several inclusion criteria were applied for both cohorts: First, patients had an observation period of at least 6 months after the index diagnosis in the database. Second, patients were aged 65 years or older on the index date. Third, patients had no record of dementia diagnosis prior to or on the index date. Finally, patients had not been diagnosed with COVID-19 within 365 days before the index date and there was no overlap between the COVID-19 group and the AURI group. Patients who met the inclusion criteria were assigned to their respective patient cohort. The index date and associated index quarter was the date when the COVID-19 or AURI diagnosis was made, respectively. Subsequently, patients in the AURI cohort were matched 1 : 1 with patients in the COVID-19 cohort using propensity scores based on sex, age group, index quarter (Q1 2020–Q4 2021), health insurance type (private health insurance or not), number of doctor visits, and comorbidities (diabetes [ICD10: E10, E11, E14], hypertension [I10], stroke including transient ischemic attack [I63, I64, G45], depression [F32, F33], intracranial injury [S06], and mild cognitive impairment (MCI) [F06.7]). The number of doctor visits is defined as the number of visits between the index date and dementia diagnosis or loss to follow-up. The comorbidities selected were conditions that are established risk factors which may give rise to an increased risk of developing dementia [21].
Fig. 1
Selection of study patients and patient flow.
Study outcomes and variables
The main outcome of the study was the incidence rate of dementia (vascular dementia, unspecified dementia, and Alzheimer’s disease, ICD-10: F01, F03, G30) in 1,000 person-years. In addition, the cumulative incidence of dementia within up to 12 months of the index date was computed.
Statistical analyses
Incidence rates of newly-diagnosed dementia were calculated using the person-years method. Kaplan-Meier curves and corresponding log rank tests were used to assess the cumulative incidence of dementia in patients with previous COVID-19 infection or with AURI diagnosis within up to 12 months of the index date. In addition, Poisson regression models were used to compute the incidence rate ratios (IRR). As part of this process, differential exposure times via offset were considered and marginal models were estimated based on the generalized estimating equations method to account for the correlation of observations within a matched pairs relationship. Additionally, we conducted a sensitivity analysis as Poisson regression was adjusted for physician ID to reflect the possible effect of physician on dementia diagnosis.
p-values < 0.05 were considered statistically significant. Analyses were carried out using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
The present study included a total of 16,258 matched patients, comprised of 8,129 patients with a documented COVID-19 diagnosis during the study period and 8,129 matched patients in the AURI control cohort (Table 1). After 1 : 1 propensity-matching, the baseline characteristics of the study cohorts, including patient demographics and comorbidities, were well balanced. The average patient age on the index date was 75.1 years (SD 7.8 years) in the COVID-19 cohort and 74.9 years (SD 7.6 years) in the AURI patient cohort.
Table 1
Baseline characteristics of COVID-19 patients and AURI patients after (1 : 1) matching
| Variable | Covid-19 cohort (n = 8,129) | AURI cohort (n = 8,129) |
| Age at baseline (Mean, SD) | 75.1 (7.8) | 74.9 (7.6) |
| Age 65–70 | 36.6% | 36.6% |
| Age 71–80 | 37.8% | 37.8% |
| Age 81+ | 25.6% | 25.6% |
| Female | 58.9% | 58.9% |
| Male | 41.1% | 41.1% |
| Private health insurance | 6.1% | 6.1% |
| Diagnoses prior to index date | ||
| Hypertension (I10) | 70.9% | 70.9% |
| Diabetes mellitus (E10, E11, E14) | 31.4% | 31.4% |
| Stroke incl. TIA (I63, I64, G45) | 5.5% | 5.5% |
| Depression (F32, F33) | 23.2% | 23.2% |
| Mild cognitive impairment (F06.7) | 0.2% | 0.2% |
| Intracranial injury (S06) | 0.4% | 0.4% |
| Epilepsy (G40, G41) | 0.5% | 0.5% |
| Index quarter of Covid-19 or AURI | ||
| Q1 2020 | 0.4% | 0.4% |
| Q2 2020 | 5.4% | 5.4% |
| Q3 2020 | 2.3% | 2.3% |
| Q4 2020 | 29.6% | 29.6% |
| Q1 2021 | 28.6% | 28.6% |
| Q2 2021 | 17.3% | 17.3% |
| Q3 2021 | 5.4% | 5.4% |
| Q4 2021 | 11.0% | 11.0% |
In terms of age categories, 36.6% of patients were aged 65–70 years on the index date, while 37.8% of patients were 71–80 years old. About one in four patients were 81 years or older on the index date. The number of female patients was higher in both cohorts (58.9%). The majority of patients were covered by statutory health insurance (93.9%).
The most common comorbidity in the study cohorts was hypertension (70.9%), followed by diabetes mellitus (31.4%) and depression (23.2%). About one in twenty patients had a history of stroke (5.5%). Epilepsy, intracranial injury, and MCI were less prevalent, occurring in 0.5%, 0.4%, and 0.2% of patients, respectively (see Table 1). Over half (58.2%) of COVID-19 infections were recorded between October 2020 and March 2021 (Q4 2020–Q1 2021) and a further 17.3% of cases in Q2 2021, making these three quarters the periods with the highest COVID-19 infection incidence rates. The mean follow-up time was 404 days (SD 140 days) in the COVID-19 cohort and 407 days (SD 142 days) in the AURI cohort. The number of doctor visits during follow-up (until dementia diagnosis or loss to follow-up) was 16.0 (SD 12.9) and 15.8 (SD 12.2) in the COVID-19 and AURI cohorts, respectively.
After 12 months of follow-up, 1.84% of the COVID-19 patients and 1.78% of the AURI patients had been diagnosed with dementia (Fig. 2). Poisson regression models were prepared, resulting in an IRR of 1.05 (95% CI: 0.85–1.29). Table 2 shows the results of the Poisson regression. The incidence rate of dementia in 1,000 person-years was 19.7 in the COVID-19 cohort and 18.8 in the AURI cohort. The models also showed that the incidence rate for dementia was higher for females in both study groups, but this effect was not significant. The incidence rate was higher in female patients with previous COVID-19 infection than in female patients with AURI (23.3 versus 19.8 in 1,000 person-years, IRR 1.17 (95% CI: 0.91–1.51)), but this correlation was reversed in men (14.6 versus 17.2 in 1,000 person-years, IR 0.85 (95% CI: 0.60–1.21)). The incidence rate was higher with increasing age in both study cohorts (Table 2).
Fig. 2
Cumulative incidence of dementia in patients with COVID-19 or AURI within 12 months of the index date.
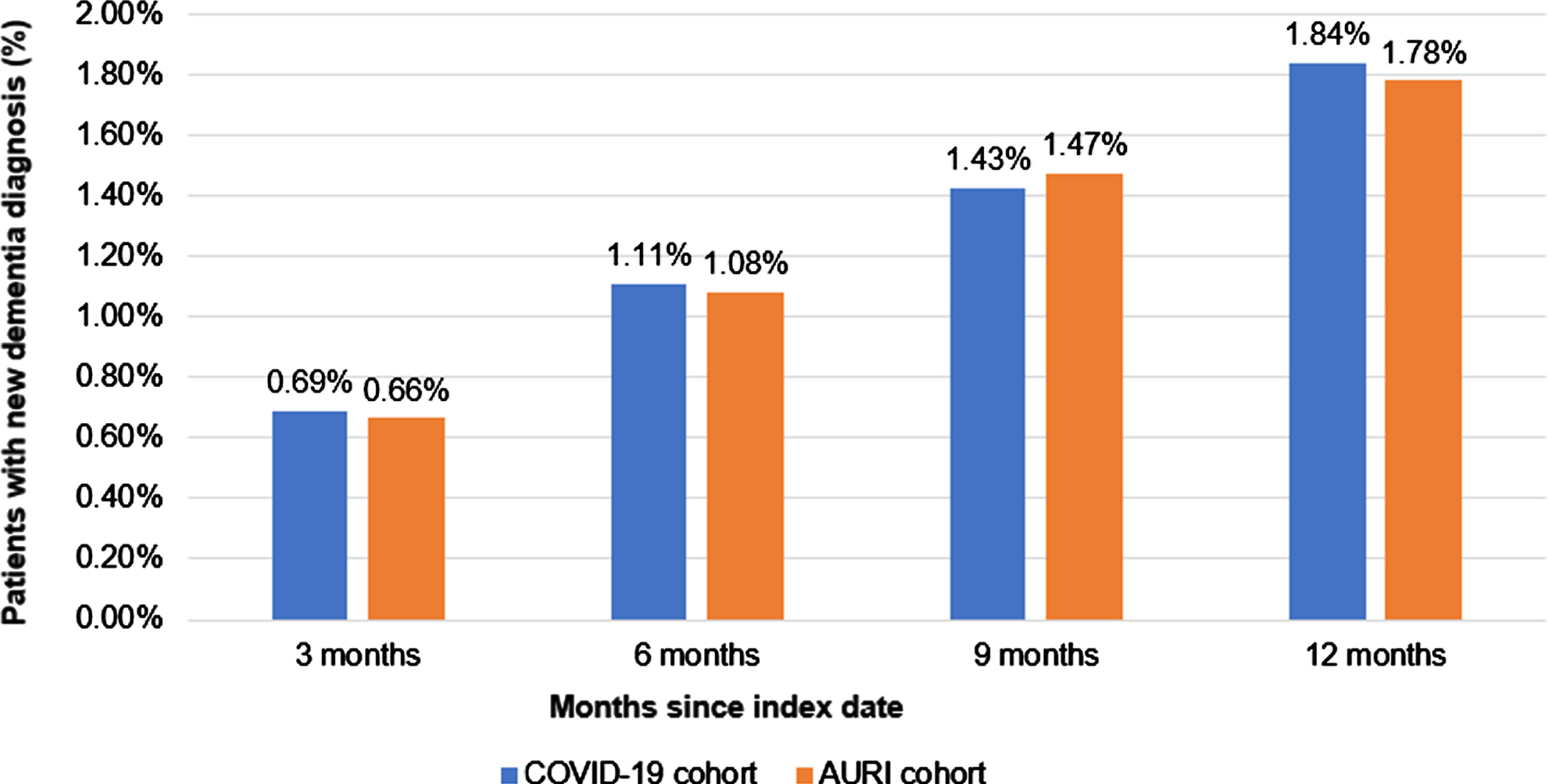
Table 2
Incidence rate ratio for dementia after COVID-19 infection or AURI and incidence rate of dementia in 1,000 person years
| Incidence rate in 1,000 person years | Univariable Poisson regression analysis | Poisson regression analysis adjusted for physician | ||||
| COVID-19 cohort | AURI cohort | Incidence rate ratio (95% CI) | p | Incidence rate ratio (95% CI) | p | |
| Dementia | 19.7 | 18.8 | 1.05 (0.85–1.29) | 0.653 | 1.03 (0.84–1.28) | 0.753 |
| By sex | ||||||
| Female | 23.3 | 19.8 | 1.17 (0.91–1.51) | 0.221 | 1.16 (0.89–1.50) | 0.274 |
| Male | 14.6 | 17.2 | 0.85 (0.60–1.21) | 0.362 | 0.83 (0.58–1.20) | 0.331 |
| By age group | ||||||
| Age 65–70 | 3.0 | 3.0 | 1.00 (0.42–2.41) | 0.995 | 0.93 (0.38–2.24) | 0.866 |
| Age 71–80 | 16.7 | 15.1 | 1.11 (0.77–1.59) | 0.583 | 1.08 (0.74–1.58) | 0.687 |
| Age 81+ | 48.2 | 46.9 | 1.03 (0.79–1.34) | 0.837 | 1.03 (0.79–1.35) | 0.804 |
DISCUSSION
Main findings
This retrospective cohort study examined the one-year incidence of newly-diagnosed dementia cases in patients with confirmed COVID-19 diagnoses following the index infection, compared with the AURI patient group. Our data do not support the hypothesis that COVID-19 infection leads to an increase in the incidence of dementia in the year following the index infection. This result is contrary to the findings of research conducted by Taquet et al. (2021). In their study, the increased risk of developing dementia was found to be two times higher in the COVID-19 group than in the control group. Taquet and colleagues propose that a proportion of the dementia diagnoses may represent misdiagnoses of patients who presented with delirium or short-term cognitive impairments [13]. Indeed, COVID-19 infection has repeatedly been reported to be associated with cognitive impairment [21], delirium [13], a dementia-like presentation [22], and so-called “brain fog” post-COVID [23]. This highlights the need to differentiate clearly between dementia-like cognitive symptoms, which are transient and reversible in nature, and a diagnosis of dementia, which is a progressive disease involving irreversible neurocognitive and behavioral changes. Diagnosis of the latter can be challenging and is clinically based on thorough assessment and FDG PET brain imaging.
This issue is further complicated by the fact that the COVID-19 pandemic and the related lockdown caused a significant disruption to routine health care processes and reduced patient utilization of healthcare offerings [24]. This had considerable effects on the number of admissions and on preventive and therapeutic care, and also led to a decline in new diagnoses, including new dementia diagnoses, at the height of the pandemic [25]. Consequently, the incidence of dementia appears to be declining in some countries such as Sweden [26] and Germany [25], with a respective decline of 20% (nationally in 2020) and 13% (February–May 2020 for general practitioners). Given that the worldwide prevalence of dementia cases is increasing, however, such trends are a misrepresentation of the actual disease burden.
Freudenberg-Hua et al. (2022) reported an increased 1-year incidence rate of post-COVID dementia in adults aged 65 + [13]. Their retrospective cohort analysis was based on electronic health records from a US integrated academic health system. The group reported a 1-year incidence rate of 12.7% for post-COVID dementia. This incidence rate exceeds the incidence rate reported by Taquet et al. who report an incidence rate of 2.7% [27] and 1.6% [28] for post-COVID dementia at 6 months and 12 months, respectively (in patients aged 65 + years). One possible explanation for the high incidence rate in the Freudenberg-Hua (2022) study may lie in their definition of a new diagnosis of dementia. Freudenberg-Hua and colleagues (2022) defined new dementia cases not just based on a record of a new dementia diagnosis, but also based on incident use of donepezil or other cognitive enhancers. Comparability between Freudenberg-Hua et al. (2022) and the research reported herein is further reduced by that fact that Freudenberg-Hua and colleagues only included patients who were hospitalized with COVID-19 and thus focused on a severely affected patient cohort. Due to these significant methodological differences, the results of Freudenberg-Hua et al. (2022) and Taquet et al. (2021) cannot be compared directly to our study.
The work of Freudenberg-Hua et al. (2022) raises the question of whether the severity of the COVID-19 infection modulates/plays a role in the context of dementia incidence. It may be hypothesized that a severe COVID-19 disease trajectory could exacerbate disease mechanisms which might increase the risk of developing dementia. The theory of a modulatory effect is indeed supported by Taquet and colleagues (2021). Using the same database described above, the group also investigated the 6-month incidence for several disease conditions, including dementia [27], compared to propensity-score matched patients with influenza and other respiratory diseases. Taquet et al. (2021) report a significantly increased 6-month incidence for dementia post-COVID compared to patients with influenza and with respiratory diseases (including influenza). Notably, dementia incidence was generally moderate, amounting to 2.7% in patients aged 65 + years within 6 months after index diagnosis. The group also investigated whether the risk of developing post-COVID dementia was modulated by the presence of one the following factors during the COVID infection: 1) hospitalization, 2) admission to intensive therapy unit (ITU), or 3) presentation with delirium. All three factors were significantly associated with increased hazard ratios for developing post-COVID dementia. The strongest effect was observed for patients who experienced delirium, followed by hospitalization and ITU admission.
In addition to the strengths of this retrospective cohort study, which include the large sample size, nationwide coverage, and the fact that there is no recall bias thanks to the use of EMR data, the work is also subject to several limitations, especially of the database, which also need to be mentioned. First, patients with COVID-19 infection who were not diagnosed by their GP, but instead in a COVID-19 test center or hospital were only captured if the infection was recorded by their GP. Second, the database does not include detailed information about hospitalizations, as the Disease Analyzer database only includes information recorded in the primary care setting of office-based physicians. Data from specialists or hospitals are only available if the GP records the data for the patients. This fact might have led to a strong information bias, as the proportion of COVID-19 patients treated in hospitals may be much higher than that of AURI patients. However, we assume that most of the study patients had a rather mild COVID-19 infection as they were diagnosed or followed in GP offices. Although many COVID-19 patients were included in the study, no information on SARS-CoV-2 infection severity was available. It has been suggested that the severity of SARS-CoV-2 infection could impact post-infection cognitive decline in COVID-19 survivors [29–31]. Furthermore, mild cognitive impairment diagnoses, which are known to be the main risk factor for dementia, are strongly underestimated in the health care sector [32, 33]. Although we cannot rule out the possibility that some patients have undiagnosed MCI, we assume that the proportion of such patients should not differ between the two study cohorts. Moreover, cognition level at baseline was not available in the database and could not be included in matching. Finally, the database contains electronic medical records from GPs only, meaning that no information was available on diagnosis methods.
It is clear that understanding the relationship between COVID-19 infection and the risk of developing dementia is a topic that is of great importance for the global health burden. Dementia has become a global health challenge worldwide and is ranked among the top ten leading causes of death worldwide. In light of this fact, even a small increase in dementia incidence due to COVID-19 would have far-reaching effects on the health of millions of people as well as on healthcare systems and the global economy. To date, only a few studies have investigated post-COVID dementia incidence. While two research groups have reported an increased risk of developing dementia, the incidence rates reported vary considerably, and methodological differences make it difficult to delineate individual factors that might influence the reported change. The severity of the COVID-19 infection might have a modulatory role, and this topic needs to be examined further. The present study did not find any association between COVID-19 infection and new-onset dementia after controlling for all common risk factors for dementia. Because dementia is a progressive disease, which can be difficult to diagnose, a longer follow-up period might offer a better insight into a possible association between COVID-19 infection and an increased incidence of dementia cases in the future.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Karel Kostev is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
All other authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
REFERENCES
[1] | World Health Organization, WHO Coronavirus (COVID-19) Dashboard, https://covid19.who.int, Last updated October 21, 2023, Accessed on October 21, (2023) . |
[2] | Li Q , Guan X , Wu P , Wang X , Zhou L , Tong Y , Ren R , Leung KSM , Lau EHY , Wong JY , Xing X , Xiang N , Wu Y , Li C , Chen Q , Li D , Liu T , Zhao J , Liu M , Tu W , Chen C , Jin L , Yang R , Wang Q , Zhou S , Wang R , Liu H , Luo Y , Liu Y , Shao G , Li H , Tao Z , Yang Y , Deng Z , Liu B , Ma Z , Zhang Y , Shi G , Lam TTY , Wu JT , Gao GF , Cowling BJ , Yang B , Leung GM , Feng Z ((2020) ) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382: , 1199–1207. |
[3] | Gemelli Against COVID-19 Post-Acute Care Study Group ((2020) ) Post-COVID-19 global health strategies: The need for an interdisciplinary approach. Aging Clin Exp Res 32: , 1613–1620. |
[4] | Lopez-Leon S , Wegman-Ostrosky T , Perelman C , Sepulveda R , Rebolledo PA , Cuapio A , Villapol S ((2021) ) More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci Rep 11: , 16144. |
[5] | Rathmann W , Kuss O , Kostev K ((2022) ) Incidence of newly diagnosed diabetes after Covid-19. Diabetologia 65: , 949–954. |
[6] | Birabaharan M , Kaelber DC , Pettus JH , Smith DM ((2022) ) Risk of new-onset type 2 diabetes in 600 055 people after COVID-19: A cohort study. Diabetes Obes Metab 24: , 1176–1179. |
[7] | Akpek M ((2022) ) Does COVID-19 cause hypertension? Angiology 73: , 682–687. |
[8] | Vanderlind WM , Rabinovitz BB , Miao IY , Oberlin LE , Bueno-Castellano C , Fridman C , Jaywant A , Kanellopoulos D ((2021) ) A systematic review of neuropsychological and psychiatric sequalae of COVID-19: Implications for treatment. Curr Opin Psychiatry 34: , 420–433. |
[9] | Klaser K , Thompson EJ , Nguyen LH , Sudre CH , Antonelli M , Murray B , Canas LS , Molteni E , Graham MS , Kerfoot E , Chen L , Deng J , May A , Hu C , Guest A , Selvachandran S , Drew DA , Modat M , Chan AT , Wolf J , Spector TD , Hammers A , Duncan EL , Ourselin S , Steves CJ ((2021) ) Anxiety and depression symptoms after COVID-19 infection: Results from the COVID Symptom Study app. J Neurol Neurosurg Psychiatry 92: , 1254–1258. |
[10] | Renaud-Charest O , Lui LMW , Eskander S , Ceban F , Ho R , Di Vincenzo JD , Rosenblat JD , Lee Y , Subramaniapillai M , McIntyre RS ((2021) ) Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J Psychiatr Res 144: , 129–137. |
[11] | Rogers JP , Chesney E , Oliver D , Pollak TA , McGuire P , Fusar-Poli P , Zandi MS , Lewis G , David AS ((2020) ) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7: , 611–627. |
[12] | Bacaro V , Chiabudini M , Buonanno C , De Bartolo P , Riemann D , Mancini F , Baglioni C ((2020) ) Insomnia in the Italian population during Covid-19 outbreak: A snapshot on one major risk factor for depression and anxiety. Front Psychiatry 11: , 579107. |
[13] | Freudenberg-Hua Y , Makhnevich A , Li W , Liu Y , Qiu M , Marziliano A , Carney M , Greenwald B , Kane JM , Diefenbach M , Burns E , Koppel J , Sinvani L ((2022) ) Psychotropic medication use is associated with greater 1-year incidence of dementia after COVID-19 hospitalization. Front Med 9: , 841326. |
[14] | Rathmann W , Bongaerts B , Carius H-J , Kruppert S , Kostev K ((2018) ) Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther 56: , 459–466. |
[15] | Kostev K , Bohlken J , Jacob L ((2019) ) Association between migraine headaches and dementia in more than 7,400 patients followed in general practices in the United Kingdom. J Alzheimers Dis 71: , 353–360. |
[16] | Bohlken J , Jacob L , Kostev K ((2017) ) Association between anti-dementia treatment persistence and daily dosage of the first prescription: A retrospective analysis in neuropsychiatric practices in Germany. J Alzheimers Dis 58: , 37–44. |
[17] | Zingel R , Bohlken J , Riedel-Heller S , Barth S , Kostev K ((2021) ) Association between low-density lipoprotein cholesterol levels, statin use, and dementia in patients followed in German general practices. J Alzheimers Dis 79: , 37–46. |
[18] | Jacob L , Rickwood S , Rathmann W , Kostev K ((2021) ) Change in glucose-lowering medication regimens in individuals with type 2 diabetes mellitus during the COVID-19 pandemic in Germany. Diabetes Obes Metab 23: , 910–915. |
[19] | Jacob L , Loosen SH , Kalder M , Luedde T , Roderburg C , Kostev K ((2021) ) Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in germany. Cancers (Basel) 13: , 408. |
[20] | Platen M , Bohlken J , Hoffmann W , Kostev K , Michalowsky B ((2022) ) The long-term impact of the COVID-19 pandemic on primary and specialized care provision and disease recognition in Germany. Front Public Health 10: , 1006578. |
[21] | Pinna P , Grewal P , Hall JP , Tavarez T , Dafer RM , Garg R , Osteraas ND , Pellack DR , Asthana A , Fegan K , Patel V , Conners JJ , John S , Silva ID ((2020) ) Neurological manifestations and COVID-19: Experiences from a tertiary care center at the Frontline. J Neurol Sci 415: , 116969. |
[22] | Varatharaj A , Thomas N , Ellul MA , Davies NWS , Pollak TA , Tenorio EL , Sultan M , Easton A , Breen G , Zandi M , Coles JP , Manji H , Al-Shahi Salman R , Menon DK , Nicholson TR , Benjamin LA , Carson A , Smith C , Turner MR , Solomon T , Kneen R , Pett SL , Galea I , Thomas RH , Michael BD , CoroNerve Study Group ((2020) ) Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 7: , 875–882. |
[23] | Asadi-Pooya AA , Akbari A , Emami A , Lotfi M , Rostamihosseinkhani M , Nemati H , Barzegar Z , Kabiri M , Zeraatpisheh Z , Farjoud-Kouhanjani M , Jafari A , Sasannia S , Ashrafi S , Nazeri M , Nasiri S , Shahisavandi M ((2022) ) Long COVID syndrome-associated brain fog. J Med Virol 94: , 979–984. |
[24] | Moynihan R , Sanders S , Michaleff ZA , Scott AM , Clark J , To EJ , Jones M , Kitchener E , Fox M , Johansson M , Lang E , Duggan A , Scott I , Albarqouni L ((2021) ) Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 11: , e045343. |
[25] | Michalowsky B , Hoffmann W , Bohlken J , Kostev K ((2021) ) Effect of the COVID-19 lockdown on disease recognition and utilisation of healthcare services in the older population in Germany: A cross-sectional study. Age Ageing 50: , 317–325. |
[26] | Axenhus M , Schedin-Weiss S , Tjernberg L , Wimo A , Eriksdotter M , Bucht G , Winblad B ((2022) ) Changes in dementia diagnoses in Sweden during the COVID-19 pandemic. BMC Geriatr 22: , 365. |
[27] | Taquet M , Geddes JR , Husain M , Luciano S , Harrison PJ ((2021) ) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 8: , 416–427. |
[28] | Taquet M , Luciano S , Geddes JR , Harrison PJ ((2021) ) Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 8: , 130–140. |
[29] | Liu Y-H , Chen Y , Wang Q-H , Wang L-R , Jiang L , Yang Y , Chen X , Li Y , Cen Y , Xu C , Zhu J , Li W , Wang Y-R , Zhang L-L , Liu J , Xu Z-Q , Wang Y-J ((2022) ) One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: A longitudinal cohort study. JAMA Neurol 79: , 509–517. |
[30] | He D , Yuan M , Dang W , Bai L , Yang R , Wang J , Ma Y , Liu B , Liu S , Zhang S , Liao X , Zhang W ((2023) ) Long term neuropsychiatric consequences in COVID-19 survivors: Cognitive impairment and inflammatory underpinnings fifteen months after discharge. Asian J Psychiatr 80: , 103409. |
[31] | Liu Y-H , Wang Y-R , Wang Q-H , Chen Y , Chen X , Li Y , Cen Y , Xu C , Hu T , Liu X-D , Yang L-L , Li S-J , Liu X-F , Liu C-M , Zhu J , Li W , Zhang L-L , Liu J , Wang Y-J ((2021) ) Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener 16: , 48. |
[32] | Bohlken J , Kostev K ((2019) ) Diagnostic behavior for mild cognitive impairment in general and neuropsychiatric practices in Germany. J Alzheimers Dis 68: , 925–930. |
[33] | Bohlken J , Riedel-Heller S , Steininger G , Kostev K , Michalowsky B ((2021) ) Trends in dementia and mild cognitive impairment prevalence and incidence in German general and specialist practices between 2015 and 2019. J Alzheimers Dis 79: , 1683–1690. |




