Associations Between Parity and Cognition: Race/Ethnic Differences
Abstract
Background:
Race/ethnicity is associated with differences in reproductive history and cognition individually, yet it remains an understudied factor in the relationship between parity and later-life cognition.
Objective:
To evaluate if the association between parity and cognition differs between racial/ethnic groups.
Methods:
Participants included 778 older, postmenopausal women from the Health and Nutrition Examination Survey (Latina: n = 178, Non-Latino Black [NLB]: n = 169, Non-Latino White [NLW]: n = 431) who self-reported at least one birth. Cognitive outcomes included working memory, learning memory, and verbal fluency. Covariates included age, education, cardiovascular and other reproductive health factors, adult socioeconomic status (SES) and depressive symptoms. We fit a series of linear models to examine a) whether parity was associated with cognitive functioning, b) if this association varied by race/ethnicity through parity by race/ethnicity interactions, and c) individual parity with cognition associations stratified by race/ethnicity.
Results:
In the full sample, parity was significantly negatively associated with Digit Symbol Substitution Test (DSST) performance (b = –0.70, p = 0.024) but not Animal Fluency or word-list learning and memory. Tests of race/ethnicity-by-parity interactions were not statistically significant (ps > 0.05). However, stratified analyses by race/ethnicity showed a differential effect of parity on DSST performance, such that parity was significantly negatively associated with DSST performance (b = –1.66, p = 0.007) among Latinas but not in NLWs (b = –0.16, p = 0.74) or NLBs (b = –0.81, p = 0.191).
Conclusion:
Among Latina, but not NLB or NLW women, greater parity was associated with worse processing speed/executive functioning later in life. Further research is needed to understand the mechanisms driving racial/ethnic differences.
INTRODUCTION
Sex differences in age-related cognitive decline, as well as the incidence of Alzheimer’s disease and related dementias (ADRD) [1–5], have motivated researchers to examine potential underlying mechanisms, including female-specific factors. Pregnancy, in particular, is an experience unique to females that is accompanied by significant physiological changes [6–9]. Reproductive hormones (e.g., estradiol and estrogen), which are hypothesized to be neuroprotective [10, 11], rise drastically during pregnancy, increasing by as much as 300-fold across the 40-week gestational period [12–14]. Pregnancy is also associated with changes in immune functioning, risk of metabolic disorders, and brain physiology [15–19]. The impact of pregnancy on these physiological systems, however, is not always consistent over the life course. For example, studies suggest that parous, compared to nulliparous women, may have 20% less circulating estrogen [20], and this difference appears to persist after menopause [21]. Large epidemiological studies have shown that greater parity is associated with an increased risk of ADRD [6–9], reduced age of ADRD onset [22], greater density of neuritic plaques and neurofibrillary tangles [23], and reduced hippocampal volume [24].
In contrast to the literature on ADRD, evidence for the association between parity and cognition is more inconsistent, with some studies reporting that higher parity is associated with worse cognition [25–27], some reporting the opposite (i.e., higher parity is associated with better cognition) [28], and others reporting no significant associations [29]. Mental status (e.g., scores on the Mini-Mental State Exam [MMSE]) has repeatedly been associated with cognition: evidence of a detrimental effect of parity was detected in a large Chinese and smaller American cohort [27, 30], yet in the Women’s Health Study higher parity was associated with better mental status [28]. Parity has also been linked to cognitive performance in specific domains. For example, greater parity was associated with worse verbal memory on a list learning task [as assessed by Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) word learning (CERAD-WL) and delayed recall (CERAD-DL)] [27]. However, data from the Bogalusa Heart Study suggest that nulliparous, when compared to parous women, performed better on assessments of verbal story memory (Logical Memory 1 & 2) and executive functioning (Symbol Digit Coding Modality, Trails A & B, and Digit Span), yet higher parity was not associated with cognition [31]. The mixed nature of the literature on the parity-cognition association suggests other factors might be at play.
Race/ethnicity is a factor that remains understudied in the parity and cognition literature, despite the fact that race/ethnicity has been independently associated with various aspects of female reproductive history and cognitive performance. Compared to Non-Latino White (NLW) women, both Latina and Non-Latino Black (NLB) women have a younger mean age of menarche [32, 33], a younger mean age of first pregnancy [34], and have greater rates of grand multiparity (i.e., give birth to five or more children) [35]. Regarding race/ethnicity differences in cognitive performance, when compared to NLWs, Latinos/as, and NLBs experience increased rates of cognitive decline in older age, as well as higher rates of mild cognitive impairment and ADRD [36–40]. Differences in cognitive functioning and rate of decline are also present among women from different racial backgrounds. Avila et al. [41] showed that among community-living Medicare recipients, NLW women performed better than NLB and Latina women in cognitive tasks of memory, language, and visual-spatial ability. Also, compared to NLW women, NLB women showed a steeper decline in memory.
Socioeconomic (SES) status and education have been proposed as the main drivers of the disparities in cognitive outcomes between NLW, NLB, and Latinos/as; however, several studies have shown that adjusting for SES and education attenuates these disparities but does not eliminate them [40, 42–44]. SES, notably, has been shown to be significantly associated with pregnancy factors (e.g., lower early-life SES is associated with higher rates of pregnancy and at a younger age) [45, 46] and to also mediate the association between pregnancy history and later-life cognition in a racially/ethnically diverse sample in the United States [47]. Among Latinos/as, cognitive functioning may also be influenced by factors related to their degree of acculturation (e.g., length of residence in the US, foreign-or native-born, and English proficiency) [48–50]. Indeed, current research suggests that Latinos/as with higher acculturation performed better on cognitive tasks than those with less acculturation, but the rate of change in cognition over time tends to be similar across levels of acculturation [50, 51].
Considering that racial differences exist in both reproductive factors and cognition, it is plausible that racial differences would also exist in the parity and cognition association. To our knowledge, few studies have included Latinas and NLB women in their samples and none have explored the moderating effect of race/ethnicity in the parity and cognition association. Among women of Mexican heritage living in the United States and Mexico, parity has a more consistently detrimental association with cognition compared to more mixed findings for the parity and cognition association among NLW and Asian women [25, 26]. Namely, Saenz et al. [26] utilized the data of 11,380 Mexican men and women from the Mexican Health and Aging Study to examine the association between number of children and cognitive functioning. In this study, Saenz found that having 6 + children was associated with worse global cognition (as assessed by the Cross-Cultural Cognitive Examination), regardless of sex. Similarly, Rote and Angel [25] used the Hispanic Established Populations for the Epidemiologic Study of the Elderly sample (n = 2,779), which consisted of United States- and Mexican-born individuals living in the United States and found that women with 5 + children, compared to women with 2–4 children, are a higher risk for cognitive impairment and at a faster rate (as measured by MMSE). To the best of our knowledge, the previously mentioned studies performed by Harville et al. [30] and McLay et al. [31] are the only ones that included an adequate representation of NLB women in their sample (36.8% and 35% of the sample were NLB women, respectively). It remains unknown if the parity and cognition association differs between racial groups, as none of the aforementioned studies provided information on race/ethnicity -stratified or -moderation analyses.
The goal of the present study was to better understand the associations between race/ethnicity, parity, and individual cognitive domains (memory, verbal fluency, and executive functioning) among NLW, NLB, and Latinas in the United States. Furthermore, we aimed to determine whether measurements of preferred language and acculturation affect the parity and cognition association among Latina women, as these factors are more likely to vary within this community.
METHODS
Study population
Data were obtained from the National Health and Nutrition Examination Survey (NHANES). The NHANES is a cross-sectional, population-based sample composed of a representative sample of adults and children living in the United States. The NHANES annually collects demographic, socioeconomic, dietary, and health-related information through a combination of surveys and medical examinations and makes these data freely available. The present study utilized the 2011–2012 and 2013–2014 waves as these were the only available waves with both reproductive history information and cognitive data. All participants provided informed consent prior to data collection. Data collection protocols were reviewed and approved by the Institutional Review Board. Additional details regarding the NHAMES can be found at: http://www.cdc.gov/nchs/nhanes.
Women self-identifying as Asian (N = 55) or other race/multi-race (N = 19) were excluded due to their small sample size. Power calculations were performed to explore if a sample of 55 would be sufficient to detect small (d = 0.2) [52] effect sizes, as those commonly reported in the parity and cognition literature. With a significance criterion of α= 0.05 and power = 0.80, and 10 covariates, the minimum sample size needed would be n = 91.
Nulliparous women were excluded from the main analyses (n = 117; sensitivity analyses including nulliparous women are described below) because data to properly characterize reason(s) for nulliparity were unavailable. Many reasons exist for women to decide not to have children, and nulliparity can be an indicator of infertility. Infertility in women can be an indicator of worse underlying health and may be caused by factors also associated with cognition (e.g., autoimmune disorders, endometriosis, primary ovary insufficiency) [53–55]. Alternatively, nulliparity can be a choice that does not reflect underlying health concerns for some women. Furthermore, nulliparous women could not be included in primary linear regression models as we included age at first birth as a covariate in all our models. Previous literature has shown that the timing of birth (e.g., age at first birth) is associated with cognitive outcomes and dementia risk [56–58]. Only 3 women reported having more than 10 live births; thus, they were also excluded.
Outcomes of interest: cognitive functioning
All cognitive examinations were performed face-to-face by trained interviewers in the participant’s preferred language of testing (e.g., English, Spanish). Cognitive functioning was assessed by 1) the CERAD-WL) and CERAD-DL subtests [59], 2) the Animal Fluency test (AF) [60], and 3) the Digit Symbol Substitution test (DSST) which reflect learning and memory, semantic fluency, and executive functioning cognitive domains, respectively [61]. In the CERAD-WL learning trials, participants are asked to read aloud ten unrelated words, one at a time, and then immediately recall them. The CERAD-DL delayed word recall occurred approximately 8–10 minutes from the start of the word learning trials. The maximum score possible on each trial is 10. In the AF test, participants are asked to name as many animals as possible in one minute. A point is given for each named animal. The DSST assesses several components of executive functioning (e.g., processing speed, sustained attention, and working memory). During this test, participants are asked to transcribe symbols to corresponding numbers according to a key located at the top of the page as fast as they can during a two-minute time frame. The score is calculated as the total number of correct matches. Raw cognitive scores were used as outcomes.
Exposure
Parity was defined as the number of deliveries resulting in a live birth (continuous variable, range 1–10). This data was obtained through self-report via the Reproductive Health Questionnaire. (more information about the questionnaire can be found here: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?BeginYear = 2011).
Reproductive health variables
Data on female reproductive history [oral contraceptive use (ever or never used), age at first pregnancy (continuous variable), and age at last menstrual period (continuous variable)] were obtained through self-report via the Reproductive Health Questionnaire.
Covariates
Demographic covariates were age at assessment and race/ethnicity (Latino, NLB, or NLW) which were self-reported during in-home interviews and in the participant’s preferred language (English or Spanish). SES covariates included education (less than 9th grade, less than a high school degree, high school/GED, some college/associates degree, college graduate or above) and family poverty-to-income ratio (PIR; smaller family PIR suggests a lower family income level). Health factor covariates included body mass index (BMI), self-reported history of diabetes (yes/no), history of hypertension (yes/no; indicated by either self-report, systolic blood pressure≥140, diastolic blood pressure≥90, or use of antihypertensive medication), and cardiovascular diseases (CVD; a composite score generated from summing the following self-reported health information: stroke, congestive heart failure, coronary heart failure, heart attack, angina pectoris). Depressive symptomatology over the past two weeks was measured using the Patient Health Questionnaire (PHQ), in which greater scores indicate higher risk of depression [62].
Level of acculturation in the Latina portion of the sample was assessed using the Multi-Ethnic Study of Atherosclerosis (MESA) criteria. The MESA criteria utilize information on three proxies of acculturation: nativity (United States-born or foreign-born), language spoken at home, and years in the United States to create a total acculturation score (0 to 5), where lower scores indicate lower levels of acculturation. Kandula and colleagues [49] provide specific details on calculating the MESA acculturation score. Previous literature has established a significant cross-sectional association between lower MESA acculturation scores and lower global cognition in a sample of diverse Latinos in the United States [48]. Preferred language during cognitive testing (English or Spanish) was also included as a covariate in the Latina-only secondary analyses (see below).
Data analyses
ANOVA and chi-square tests were used to examine differences in key demographic variables by race/ethnicity in our full sample. All linear regression models were fit using the stats package (version 3.6.2) [63] in R (Version 1.3.1093). In model set 1, linear models were used to determine associations between race/ethnicity (three-level categorical variable with Latinas as the reference group) and each of the raw cognitive scores (DSST, AF, CERAD-WL, CERAD-DL). All linear regression models covaried for oral contraceptive use, age at first pregnancy, age at last menstrual period, age at assessment, education, PIR, BMI, history of diabetes, history of hypertension, CVD, and PHQ.
Model set 2 was comprised of linear models that included all described terms plus a parity by race/ethnicity interaction term to examine whether the effect(s) of parity on cognition varied as a function of race/ethnicity. Further, in Model set 3, Model set 1 was repeated with the analysis stratified by race/ethnicity (Latina, NLB, NLW).
Secondary analyses were performed to evaluate the effect of acculturation and preferred language during cognitive testing in the parity and cognition association. ANOVA and chi-square tests were used to examine differences in key demographic variables by preferred language (English or Spanish) in our sample of only Latinas. For Model set 4, analyses were performed in a sample of Latina women for whom measures of were available (n = 156–171) to examine whether these factors affect the association between parity and cognition among Latinas living in the United States. In all models, statistical significance was regarded as two-sided p < 0.05.
Supplemental analyses
In order to examine the non-linear effects of parity on cognition, model sets 1, 3, and 4 were repeated with the addition of a quadratic parity term (See Supplemental model sets 1, 2, and 3, respectively). To assess the effect of including nulliparous women in our sample, model sets 1–4 as well as Supplemental model sets 1–3 were repeated (see Supplemental model sets 4–10). The regression models that included nulliparous women (Supplemental model sets 4–10) could not covary for age at first pregnancy, as this information is not available to them. All other covariates included in the main models were included.
RESULTS
Descriptives
Of the 778 women who met the inclusion criteria for the present analyses, 178 (22.8%) described themselves as Latina, 431 (55.26%) as NLW, and 169 (21.66%) as NLB. On average, Latinas scored significantly worse on the CERAD-WL, CERAD-DL, AF, and DSST compared to NLB and NLW women. Latinas were also, on average, significantly younger, less educated, reported lower history of oral contraceptive use, higher parity, higher PHQ scores (indicating a higher risk for depression), lower history of hypertension, and lower PIR compared (indicating a lower family income) to NLW and NLB women. Latinas and NLB women, compared to NLW women, had higher rates of diabetes, higher BMI, and younger age of first pregnancy (Table 1).
Table 1
Characteristics of the participants stratified by race/ethnicity
| Continuous variables (mean, SD) | Latina n = 178 | Non-Latino White n = 431 | Non-Latino Black n = 169 | pa |
| Age at cognitive testing | 67.1 (6.0) | 71.7 (6.9) | 67.2 (6.2) | <0.001 |
| Age at first pregnancy | 21.9 (5.3) | 22.7 (4.4) | 21.3 (5.0) | 0.004 |
| Parity (Number of children) | 3.7 (2.2) | 2.9 (1.5) | 3.1 (1.9) | <0.001 |
| Age at last menstrual period | 46.3 (7.2) | 45.5 (8.4) | 44.9 (9.1) | 0.29 |
| BMI | 30.7 (6.7) | 29.2 (6.6) | 33.1 (9.0) | <0.001 |
| Cardiovascular disease composite scoreb | 0.3 (0.7) | 0.4 (0.9) | 0.3 (0.7) | 0.312 |
| Poverty income ratio (PIR) | 1.9 (1.4) | 2.7 (1.6) | 2.2 (1.5) | <0.001 |
| Depressive symptoms (PHQ) | 7.3 (5.8) | 4.7 (4.3) | 5.6 (4.8) | <0.001 |
| CERAD Word Learning | 18.2 (4.6) | 20.1 (4.6) | 19.4 (5.0) | <0.001 |
| CERAD Delayed Recall | 5.7 (2.3) | 6.4 (2.3) | 6.0 (2.3) | 0.005 |
| Animal Fluency | 15.5 (4.9) | 17.6 (5.4) | 14.3 (5.1) | <0.001 |
| Digit Symbol Substitution test | 39.3 (18.1) | 52.4 (15.9) | 44.4 (15.2) | <0.001 |
| Categorical variables (n, %) | ||||
| Education | <0.001 | |||
| Less than 9th grade | 60 (33.7) | 16 (3.7) | 11 (6.5) | |
| Less than a high school degree | 32 (18.0) | 59 (13.7) | 39 (23.1) | |
| High School/GED | 30 (16.9) | 115 (26.7) | 52 (30.8) | |
| Some college/Associates Degree | 47 (26.4) | 154 (35.7) | 47 (27.8) | |
| College graduate or above | 9 (5.1) | 87 (20.2) | 20 (11.8) | |
| Oral contraceptive use (Ever) | 97 (54.5) | 270 (62.6) | 130 (76.9) | <0.001 |
| Hypertension history (Yes) | 126 (70.8) | 316 (73.3) | 139 (82.2) | 0.031 |
| Diabetes history (Yes) | 55 (30.9) | 100 (23.2) | 57 (33.7) | 0.015 |
aANOVA and chi-square tests. Bold values denote statistical significance at the p < 0.05 level. bCardiovascular disease composite score = stroke+congestive heart failure+coronary heart failure+heart attack+angina pectoris.
Main associations between parity with cognition for the overall sample
In model set 1, parity was significantly and negatively associated with DSST scores (b = –0.70, p = 0.024) but not CERAD-WL (b = 0.70, p > 0.05), CERAD-DL (b = 0.00, p > 0.05) or AF (b = –0.05, p > 0.05). Race/ethnicity was also significantly associated with cognitive performance on all tasks. Namely, NLW, compared to Latinas, performed significantly better in the CERAD-WL (b = 1.21, p = 0.006), CERAD-DL (b = 0.59, p = 0.008), AF (b = 1.48, p = 0.002), and DSST tests (b = 8.80, p < 0.001). Furthermore, NLB women, compared to Latinas, performed significantly worse in the AF (b = –2.18, p < 0.001) test. No significant differences were found between NLB women and Latinas in CERAD-WL, CERAD-DL, or DSST scores (ps > 0.05) (Table 2).
Table 2
Main effect examination of the relationships between parity, race/ethnicity, and cognitive outcomes
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| Latinas (ref.) | ||||||||
| NLW | 1.21 | 0.006 | 0.59 | 0.008 | 1.48 | 0.002 | 8.80 | <0.001 |
| NLB | 0.04 | 0.934 | –0.10 | 0.687 | –2.18 | <0.001 | –0.96 | 0.514 |
| Parity | 0.07 | 0.467 | 0.00 | 0.955 | –0.05 | 0.650 | –0.70 | 0.024 |
| Observations | 780 | 780 | 776 | 746 | ||||
| R2/R2 adjusted | 0.251/0.234 | 0.183/0.165 | 0.303/0.287 | 0.509/0.498 | ||||
Model Set 1; NLW, Non-Latino White; NLB, Non-Latino Black. All models were adjusted for: age at cognitive testing, age at first pregnancy, age at last menstrual period, BMI, cardiovascular disease (CVD) composite score, poverty income ratio (PIR), depressive symptoms (PHQ), education, history of oral contraceptive use, hypertension history, diabetes history. Bold values denote statistical significance at the p < 0.05 level.
Race/ethnicity modifications of the associations between parity with cognition
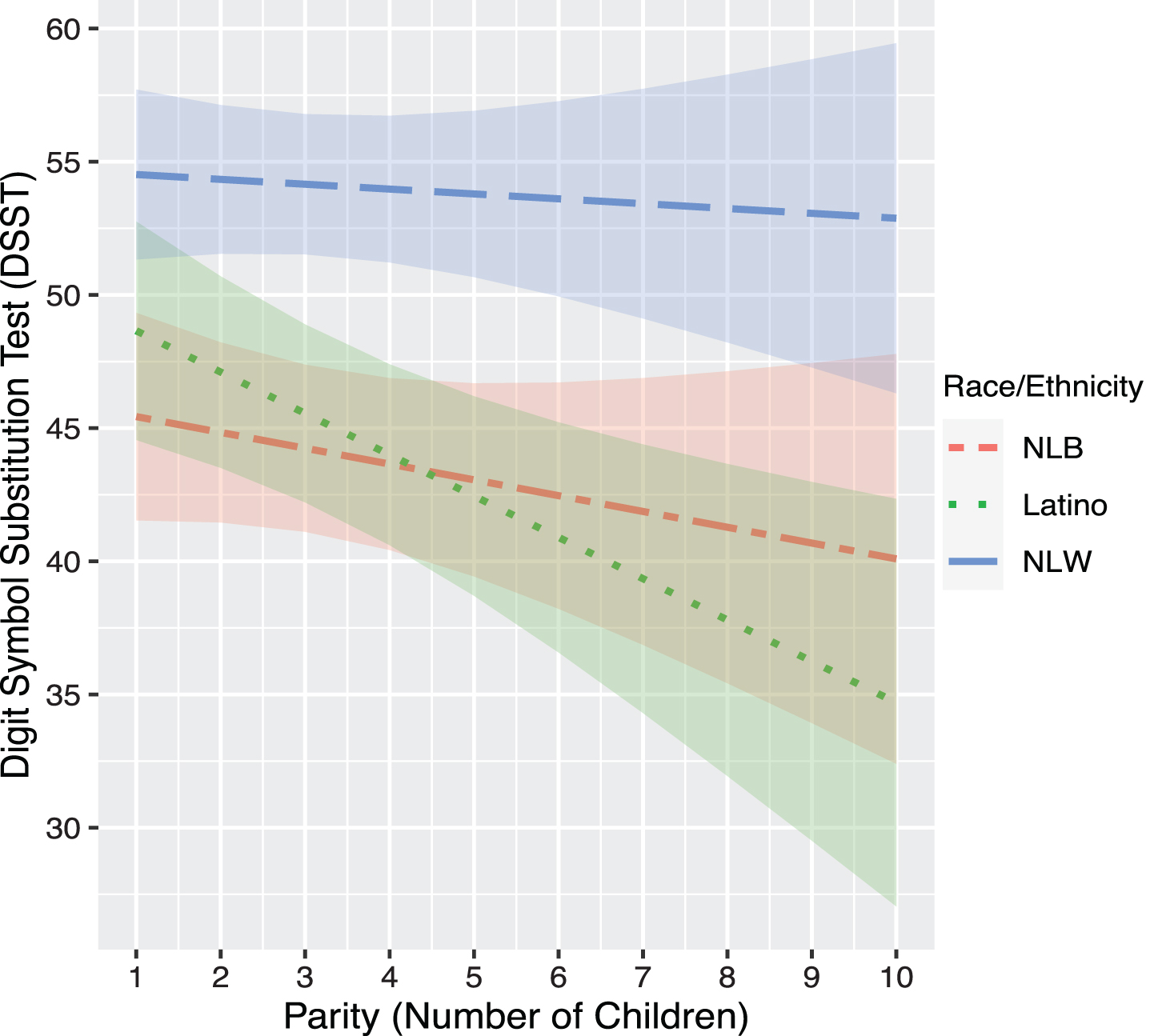
In model set 2, race/ethnicity-by-parity interactions were not significantly associated with any cognitive outcomes (ps > 0.05). However, planned stratified analyses by race/ethnicity showed a differential effect of parity on cognition by race/ethnicity. For Latinas, there was a significant negative association between parity and performance on DSST (b = –1.66, p = 0.007), which was not observed for NLW (b = –0.16, p > 0.05) or NLB women (b = –0.81, p > 0.05) (Table 3). Planned contrasts indicated that there was a differential effect of parity on DSST between Latina and NLW women (b = 1.37, p = 0.04), but not between Latina and NLB women (b = 0.96, p > 0.05), or NLW and NLB women (b = –0.41, p > 0.05) in DSST performance (Fig. 1).
Table 3
Race/ethnicity stratified analyses in the associations between parity with cognition
| Non-Latino White | ||||||||
| CERAD Word Learning | CERAD Delayed Recall | Animal Fluency | Digit Symbol | |||||
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| Parity | 0.06 | 0.681 | –0.01 | 0.850 | –0.16 | 0.337 | –0.16 | 0.742 |
| Observations | 431 | 431 | 428 | 421 | ||||
| R2/R2 adjusted | 0.225/0.197 | 0.188/0.159 | 0.314/0.289 | 0.421/0.400 | ||||
| Latina | ||||||||
| CERAD Word Learning | CERAD Delayed Recall | Animal Fluency | Digit Symbol | |||||
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| Parity | 0.06 | 0.742 | –0.04 | 0.649 | –0.15 | 0.482 | –1.66 | 0.007 |
| Observations | 179 | 178 | 177 | 162 | ||||
| R2/R2 adjusted | 0.415/0.361 | 0.294/0.228 | 0.249/0.179 | 0.639/0.602 | ||||
| Non-Latino Black | ||||||||
| CERAD Word Learning | CERAD Delayed Recall | Animal Fluency | Digit Symbol | |||||
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| Parity | 0.10 | 0.682 | 0.06 | 0.581 | 0.04 | 0.874 | –0.81 | 0.191 |
| Observations | 170 | 171 | 171 | 163 | ||||
| R2/R2 adjusted | 0.181/0.101 | 0.218/0.142 | 0.214/0.138 | 0.446/0.390 | ||||
Model set 3. All models were adjusted for: age at cognitive testing, age at first pregnancy, age at last menstrual period, BMI, cardiovascular disease (CVD) composite score, poverty income ratio (PIR), depressive symptoms (PHQ), education, history of oral contraceptive use, hypertension history, diabetes history. Bold values denote statistical significance at the p < 0.05 level.
Fig. 1
Association between Predicted DSST scores and Parity by Race/Ethnicity. NLB, Non-Latino Black; NLW, Non-Latino White. Models was adjusted for age at cognitive testing, age at first pregnancy, age at last menstrual period, BMI, cardiovascular disease (CVD) composite score, poverty income ratio (PIR), depressive symptoms (PHQ), education, history of oral contraceptive use, hypertension history, diabetes history.
Secondary analyses: Examinations of acculturation and language at testing in the parity and cognition associations among Latinas
Latinas who were tested in Spanish (n = 79) had less formal education, higher PHQ scores (indicating a higher risk for depression), higher parity, lower PIR (indicating a lower family income), lower MESA acculturation scores (indicating lower levels of acculturation), and scored significantly worse on the CERAD-WL, CERAD-DL, AF, and DSST tests compared to those that completed the assessments in English (n = 74; Table 4).
Table 4
Characteristics of the Latinas stratified by preferred language during cognitive testing
| Continuous variables (mean, SD) | English n = 74 | Spanish n = 79 | pa |
| Age at Cognitive Testing | 66.5 (6.1) | 66.3 (5.3) | 0.864 |
| Age at first pregnancy | 22.7 (6.1) | 21.2 (4.0) | 0.079 |
| Parity | 3.1 (1.7) | 4.1 (2.0) | 0.001 |
| Age at last menstrual period | 46.4 (7.9) | 46.2 (6.7) | 0.855 |
| BMI | 30.5 (6.1) | 30.6 (7.0) | 0.942 |
| Cardiovascular disease composite scoreb | 0.3 (0.8) | 0.2 (0.6) | 0.267 |
| Poverty income ratio (PIR) | 2.3 (1.4) | 1.6 (1.3) | 0.002 |
| Depressive symptoms (PHQ) | 6.3 (5.5) | 8.3 (5.8) | 0.033 |
| MESA acculturationc | 3.9 (0.9) | 2.0 (0.9) | <0.001 |
| CERAD Word Learning | 20.6 (4.1) | 17.3 (3.9) | <0.001 |
| CERAD Delayed Recall | 6.6 (2.3) | 5.4 (1.9) | <0.001 |
| Animal Fluency | 16.7 (4.9) | 15.5 (4.7) | 0.12 |
| Digit Symbol Substitution test | 49.9 (16.6) | 30.9 (13.8) | <0.001 |
| Categorical variables (n, %) | |||
| Education | <0.001 | ||
| Less than 9th grade | 5 (6.8) | 38 (48.1) | |
| Less than a high school degree | 12 (16.2) | 15 (19.0) | |
| High School/GED | 17 (23.0) | 13 (16.5) | |
| Some college/Associates Degree | 34 (45.9) | 10 (12.7) | |
| College graduate or above | 6 (8.1) | 3 (3.8) | |
| Oral contraceptive (Ever) | 51 (68.9) | 41 (51.9) | 0.047 |
| Hypertension history (Yes) | 55 (74.3) | 53 (67.1) | 0.421 |
| Diabetes history (Yes) | 22 (29.7) | 25 (31.6) | 0.935 |
aANOVA and chi-square tests. Bold values denote statistical significance at the p < 0.05 level. bCardiovascular disease composite score = stroke+congestive heart failure + coronary heart failure + heart attack + angina pectoris. cRange 0–5 where lower scores indicate lower levels of acculturation.
In model set 4, including MESA acculturation scores and language of cognitive testing, resulted in a marginal improvement in the overall model predicting DSST performance R2 and R2 adjusted scores (0.639/0.602 versus 0.670/0.629, respectively). Parity remained significantly and negatively associated with DSST performance among Latina women after including MESA acculturation scores and language preference during cognitive testing (b = –1.54, p = 0.013) (Table 5).
Table 5
Examination of the effect of acculturation and preferred language during cognitive testing in the association between parity and cognitive outcomes among Latinas
| CERAD Word Learning | CERAD Delayed Recall | Animal Fluency | Digit Symbol | |||||
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| Parity | 0.18 | 0.302 | 0.04 | 0.696 | –0.19 | 0.394 | –1.54 | 0.013 |
| MESA Acculturation total score | 0.28 | 0.392 | 0.18 | 0.309 | 0.33 | 0.424 | 1.33 | 0.204 |
| Cog tests taken in English (Ref.) | ||||||||
| Cog tests taken in Spanish | –1.83 | 0.039 | –0.50 | 0.303 | 0.93 | 0.405 | –6.64 | 0.021 |
| Observations | 171 | 170 | 169 | 156 | ||||
| R2/R2 adjusted | 0.445/0.383 | 0.299/0.220 | 0.228/0.141 | 0.670/0.629 | ||||
Model set 4. All models were adjusted for: age at cognitive testing, age at first pregnancy, age at last menstrual period, BMI, cardiovascular disease (CVD) composite score, poverty income ratio (PIR), depressive symptoms (PHQ), education, history of oral contraceptive use, hypertension history, diabetes history. Bold values denote statistical significance at the p < 0.05 level.
Supplemental analyses: Associations between parity (quadratic) with cognition
There was no evidence of a non-linear association between parity and CERAD-WL or CERAD-DL in the overall sample, interactive models, and stratified analyses. However, in both the overall sample and the Latina-stratified samples, there was a curvilinear decrease in AF scores as a function of higher parity. In the Latina-only sample, the non-linear association between parity and AF remained significant after adjusting for MESA acculturation and preferred language during cognitive testing (Supplemental model set 3). A similar pattern emerged between parity and DSST but only in the NLW sample (Supplemental model set 2).
The inclusion of nulliparous women into the regression models only modestly changed the pattern of results. The association between parity and DSST became marginally significant in the overall sample and interactive models (Supplemental models 4 and 5) but remained significant in the Latina-only sample (Supplemental model 6). However, the association between parity and DSST in the Latina-only sample with nulliparous women was no longer significant when covarying for MESA acculturation scores and language preference (Supplemental model set 7). Similarly, the inclusion of nulliparous women attenuated the non-linear association between parity and DSST performance in the NLW-only sample (Supplemental model set 9).
DISCUSSION
In this racially/ethnically diverse community-dwelling sample of women, we found that greater parity was associated with worse processing speed/executive functioning, and this appeared to be driven by Latinas (predominantly from Mexican heritage), not NLW or NLB women. Among Latinas, each additional live birth, on average, resulted in a 1.66 decrease in DSST score. Additionally, the associations between parity and DSST performance among Latinas remained significant after adjusting for numerous sociodemographic and health factors in addition to preferred language during cognitive testing and acculturation level. No significant associations were found between parity, learning, and memory, suggesting parity might affect cognition in a domain-specific manner. Similarly, neuroimaging studies have shown that giving birth is associated with declines in the volume of brain areas that are part of the executive system (e.g., frontal and temporal brain regions) [64]. Our findings highlight the importance of race/ethnicity in the parity and cognition association and the need for future studies to include more diverse samples and examine culturally-relevant variables (e.g., language preference). Furthermore, our results also contribute to the larger body of work showing how risk factors for cognitive decline and dementia may affect different racial/ethnic groups [65].
Some of our findings are in agreement with previous literature supporting the overarching hypothesis of a detrimental effect of increased parity on cognition but provide a more nuanced perspective. Much of this literature has used brief cognitive screeners [7, 24–26, 66, 67], whereas we examined specific cognitive domains. In the present study, processing speed/executive functioning, but not learning and memory, appeared to be negatively impacted by higher parity, whereas other studies using domain-specific testing have found that learning and memory are equally or more sensitive. For instance, Heys and colleagues [27] reported significant associations between parity and verbal immediate and delayed verbal learning and memory, such that higher parity is associated with worse performance, while other studies found no association between parity and semantic fluency, verbal and working memory [29]. Direct comparisons with other studies, as well as incompatibility in results, could be due to several reasons, such as the sample country of origin (e.g., NHANES is a United States sample and may not be generalized to groups outside the United States) [27]; study design (cross-sectional versus longitudinal) and parity operationalization (continuous versus categorical variable with different levels) [24, 25, 28]. Regarding the latter, when we examined parity as a quadratic term, we observed a significant non-linear association between parity and verbal fluency, such that among Latina and NLW women, parity appears to be associated with lower scores on AF, particularly for women at the higher end of the parity range. Notably, this nonlinear association was only seen among the Latina and NLW women and may have been driven by individuals at the higher end of the parity spectrum. Our findings warrant further exploration within ethnic/racial groups with larger sample sizes.
Mixed findings between studies, more importantly, could also be due to demographic differences in the studies (e.g., race/ethnicity, education, age, etc.) [29] and the absence of race/ethnicity stratified or parity by race/ethnicity interactions [31]. In the present study, race/ethnicity differences in the association between parity and processing speed/executive functioning performance were small but present even after controlling for SES or health-related factors (e.g., cardiovascular disease). Another study in the NHANES cohort [47] observed that level of education and adult SES (aSES) attenuated the effect of parity on working memory/processing speed when controlling for race/ethnicity. However, when we examined this cohort by race/ethnicity grouping, parity remained a significant predictor of processing/executive functioning among Latinas when controlling for these sociodemographic factors, suggesting that other factors may be driving the parity and cognition association. Our study used the PIR variable as a proxy for aSES, as previous work done by our group has shown that PIR mediates the association between parity and cognition [47]. Although PIR has shown to be a valid proxy of aSES, it does not provide information on early-life SES (e.g., household income, parents’ educational attainment, and occupation), which may be critical for understanding race/ethnic differences in parity and cognition association, as on average, Latino and NLB households historically earn less than NLWs, and to this day earn about 50% less than NLW households [68]. Also, women with lower early-life SES, on average, have higher rates of pregnancy, have children at a younger age, and report higher rates of unintended pregnancies [45, 46]. Furthermore, having children at a younger age and an increased number of children are associated with lower levels of educational attainment [69]. Given that lower early-life SES is associated with lower education and aSES, two factors strongly associated with late-life cognitive function, it is plausible that early-life SES may also mediate the association between parity and cognition. Other non-pregnancy-related factors may also explain race/ethnic differences in the parity and cognition association. For example, parenting stress has been shown to affect cognition through chronic stress [70] and studies examining race differences in parenting stress have shown that, on average, parenting stress is highest among Latinas, followed by NLB and NLW women [71].
Our study had several strengths, the most prominent being a racially/ethnically diverse sample that was well-representative of the United States population in key demographic variables such as education and income. Additionally, our study sample was well-powered to detect small to medium effect sizes (Cohen’s d = 0.2–0.5) [52], as those typically reported in the parity and cognition literature. Nonetheless, our study also had several limitations. First, the NHANES cognitive battery is relatively limited, and future studies should include a more comprehensive cognitive battery. Although limited, the NHANES research team took extensive measurements to ensure a proper and valid administration of the cognitive measurements used in this study. Of particular note is that the NHANES cognitive assessments were administered by bilingual interviewers in the participant’s preferred language. Second, the cross-sectional nature of our study does not allow us to differentiate if parity truly drives faster cognitive decline or if long-standing cognitive differences among women with different parity exist. Third, nulliparous women were not included in the main analyses as we didn’t have information on reasons for nulliparity (i.e., we cannot tell if not having children was a personal decision or due to health related reasons or infertility). This is of concern because infertility in women can be an indicator of worse underlying health and may be caused by factors also associated with cognition [53–55]. Nonetheless, the inclusion of nulliparous in the sensitivity analyses did not change our pattern of results. Fourth, due to the limited sample size, we were not able to include Asian women in our sample. The United States Asian community, similar to the Latino community, has a large immigrant presence; thus, future studies should evaluate if measurements of acculturation modify the parity and cognition association among Asian women.
Conclusion and future directions
The present study provides evidence that the parity and cognition association differs by racial/ethnic group and might be domain specific. Specifically, greater parity was significantly associated with worse processing speed/executive functioning among Latinas but not NLB or NLW women. No significant associations were seen among any race/ethnic group between parity and immediate and delayed verbal learning. To our knowledge, our study is the first to examine the role of race/ethnicity in the parity and cognition association, highlighting the need for future studies to include more diverse samples and examine culturally-relevant variables such as language preference and acculturation. Future studies would benefit from a more comprehensive cognitive battery and control for other important factors, such as early-life SES and parenting stress.
ACKNOWLEDGMENTS
We would like to thank the National Health and Nutrition Examination Survey for collecting this data and making it publicly available.
FUNDING
This work was supported by NIH/NIA AG063843, NIH/NIA R01AG05610, and NIH/NIA R21AG074212 awarded to Dr. Panizzon; NIH R01AG066088-01and CDPH-19-10613 awarded to Dr. Banks; NIH R01AG074221 and CDPH-19-10613 awarded to Dr. Sundermann; and NIH K08AG075351, L30AG074401, and U54CA267789 awarded to Dr. Stickel.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data used in this study is freely available on the National Health and Nutrition Survey website: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-221210.
REFERENCES
[1] | Alzheimer’s Association ((2019) ) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15: , 321–387. |
[2] | Andersen K , Launer L , Dewey M , Letenneur L , Ott A , Copeland J , Dartigues J , Kragh-Sorensen P , Baldereschi M , Brayne C ((1999) ) EURODEM Incidence Research Group: Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology 53: , 1992–1997. |
[3] | Gao S , Hendrie HC , Hall KS , Hui S ((1998) ) The relationships between age, sex, and the incidence of dementia and Alzheimer disease: A meta-analysis. Arch Gen Psychiatry 55: , 809–815. |
[4] | Miech RA , Breitner JCS , Zandi PP , Khachaturian AS , Anthony JC , Mayer L ((2002) ) Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology 58: , 209–218. |
[5] | Ott A , Breteler MMB , Harskamp F van , Stijnen T , Hofman A ((1998) ) Incidence and risk of dementia: The Rotterdam study. Am J Epidemiol 147: , 574–580. |
[6] | Bae JB , Lipnicki DM , Han JW , Sachdev PS , Kim TH , Kwak KP , Kim BJ , Kim SG , Kim JL , Moon SW , Park JH , Ryu S-H , Youn JC , Lee DY , Lee DW , Lee SB , Lee JJ , Jhoo JH , Skoog I , Najar J , Sterner TR , Scarmeas N , Yannakoulia M , Dardiotis E , Riedel-Heller S , Roehr S , Pabst A , Ding D , Zhao Q , Liang X , Lobo A , De-la-Cámara C , Lobo E , Kim KW , for Cohort Studies of Memory in an International Consortium (COSMIC) ((2020) ) Parity and the risk of incident dementia: A COSMIC study. Epidemiol Psychiatr Sci 29: , e176. |
[7] | Jang H , Bae JB , Dardiotis E , Scarmeas N , Sachdev PS , Lipnicki DM , Han JW , Kim TH , Kwak KP , Kim BJ , Kim SG , Kim JL , Moon SW , Park JH , Ryu S-H , Youn JC , Lee DY , Lee DW , Lee SB , Lee JJ , Jhoo JH , Yannakoulia M , Kosmidis MH , Hadjigeorgiou GM , Sakka P , Kim KW ((2018) ) Differential effects of completed and incomplete pregnancies on the risk of Alzheimer disease. Neurology 91: , e643–e651. |
[8] | Prince MJ , Acosta D , Guerra M , Huang Y , Jimenez-Velazquez IZ , Rodriguez JJL , Salas A , Sosa AL , Chua K-C , Dewey ME , Liu Z , Mayston R , Valhuerdi A ((2018) ) Reproductive period, endogenous estrogen exposure and dementia incidence among women in Latin America and China; A 10/66 population-based cohort study. PLoS One 13: , e0192889. |
[9] | Ptok U , Barkow K , Heun R ((2002) ) Fertility and number of children in patients with Alzheimer’s disease. Arch Womens Ment Health 5: , 83–86. |
[10] | Azcoitia I , Arevalo M-A , De Nicola AF , Garcia-Segura LM ((2011) ) Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab 22: , 467–473. |
[11] | Green PS , Simpkins JW ((2000) ) Neuroprotective effects of estrogens: Potential mechanisms of action. Int J Dev Neurosci 18: , 347–358. |
[12] | Berg FD , Kuss E ((1992) ) Serum concentration and urinary excretion of “classical” estrogens, catecholestrogens and 2-methoxyestrogens in normal human pregnancy. Arch Gynecol Obstet 251: , 17–27. |
[13] | Tal R , Taylor HS ((2000) ) Endocrinology of Pregnancy. In Endotext FeingoldKR, AnawaltB, BoyceA, ChrousosG, de HerderWW, DhatariyaK, DunganK, HershmanJM, HoflandJ, KalraS, KaltsasG, KochC, KoppP, KorbonitsM, KovacsCS, KuohungW, LaferrèreB, LevyM, McGeeEA, McLachlanR, MorleyJE, NewM, PurnellJ, SahayR, SingerF, SperlingMA, StratakisCA, TrenceDL, WilsonDP, eds. MDText.com, Inc., South Dartmouth (MA). |
[14] | Schock H , Zeleniuch-Jacquotte A , Lundin E , Grankvist K , Lakso H-Å , Idahl A , Lehtinen M , Surcel H-M , Fortner RT ((2016) ) Hormone concentrations throughout uncomplicated pregnancies: A longitudinal study. BMC Pregnancy Childbirth 16: , 146. |
[15] | Eid RS , Chaiton JA , Lieblich SE , Bodnar TS , Weinberg J , Galea LAM ((2019) ) Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging 78: , 1–17. |
[16] | Hillerer KM , Jacobs VR , Fischer T , Aigner L ((2014) ) The maternal brain: An organ with peripartal plasticity. Neural Plast 2014: , e574159. |
[17] | Li P , Shan Z , Zhou L , Xie M , Bao W , Zhang Y , Rong Y , Yang W , Liu L ((2016) ) Mechanisms in Endocrinology: Parity and risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur J Endocrinol 175: , R231–R245. |
[18] | Sanghavi M , Rutherford JD ((2014) ) Cardiovascular physiology of pregnancy. Circulation 130: , 1003–1008. |
[19] | Soma-Pillay P , Nelson-Piercy C , Tolppanen H , Mebazaa A ((2016) ) Physiological changes in pregnancy. Cardiovasc J Afr 27: , 89–94. |
[20] | Bernstein L , Pike MC , Ross RK , Judd HL , Brown JB , Henderson BE ((1985) ) Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst 74: , 741–745. |
[21] | Chubak J , Tworoger SS , Yasui Y , Ulrich CM , Stanczyk FZ , McTiernan A ((2004) ) Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Biomarkers Prev 13: , 1296–1301. |
[22] | Sobów TM ((2003) ) Modulation of age at onset in late-onset sporadic Alzheimer’s disease by estrogen-related factors: The age of menopause and number of pregnancies. German J Psychiatry, pp. 49–55. |
[23] | Beeri MS , Rapp M , Schmeidler J , Reichenberg A , Purohit DP , Perl DP , Grossman HT , Prohovnik I , Haroutunian V , Silverman JM ((2009) ) Number of children is associated with neuropathology of Alzheimer’s disease in women. Neurobiol Aging 30: , 1184–1191. |
[24] | Jung JH , Lee GW , Lee JH , Byun MS , Yi D , Jeon SY , Jung GJ , Joung H , Shin SA , Kim YK , Kang KM , Sohn C-H , Lee DY ((2020) ) Multiparity, brain atrophy, and cognitive decline. Front Aging Neurosci 12: , 159. |
[25] | Rote SM , Angel JL ((2021) ) Gender-based pathways to cognitive aging in the Mexican-origin population in the United States: The significance of work and family. J Gerontol B Psychol Sci Soc Sci 76: , e165–e175. |
[26] | Saenz JL , Díaz-Venegas C , Crimmins EM ((2021) ) Fertility history and cognitive function in late life: The case of Mexico. J Gerontol B Psychol Sci Soc Sci 76: , e140–e152. |
[27] | Heys M , Jiang C , Cheng KK , Zhang W , Yeung SLA , Lam TH , Leung GM , Schooling CM ((2011) ) Life long endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: The Guangzhou Biobank Cohort Study. Psychoneuroendocrinology 36: , 864–873. |
[28] | Zhou R , Liu H-M , Zou L-W , Wei H-X , Huang Y-N , Zhong Q , Gu S-Y , Chen M-F , Wang S-L , Sun H-X , Wu X-B ((2022) ) Associations of parity with change in global cognition and incident cognitive impairment in older women. Front Aging Neurosci 14: , 864128. |
[29] | Ilango SD , McEvoy LK , Laughlin GA , Bergstrom J , Barrett-Connor E , Kritz-Silverstein D ((2019) ) Pregnancy history and cognitive aging among older women: The Rancho Bernardo Study. Menopause 26: , 750–757. |
[30] | McLay RN , Maki PM , Lyketsos CG ((2003) ) Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci 15: , 161–167. |
[31] | Harville EW , Guralnik J , Romero M , Bazzano LA ((2020) ) Reproductive history and cognitive aging: The Bogalusa Heart Study. Am J Geriatr Psychiatry 28: , 217–225. |
[32] | Chumlea WC , Schubert CM , Roche AF , Kulin HE , Lee PA , Himes JH , Sun SS ((2003) ) Age at menarche and racial comparisons in US girls. Pediatrics 111: , 110–113. |
[33] | Deardorff J , Abrams B , Ekwaru JP , Rehkopf DH ((2014) ) Socioeconomic status and age at menarche: An examination of multiple indicators in an ethnically diverse cohort. Ann Epidemiol 24: , 727–733. |
[34] | Schempf AH , Branum AM , Lukacs SL , Schoendorf KC ((2007) ) Maternal age and parity-associated risks of preterm birth: Differences by race/ethnicity. Paediatr Perinat Epidemiol 21: , 34–43. |
[35] | Bornstein E , Eliner Y , Chervenak FA , Grünebaum A ((2020) ) Racial disparity in pregnancy risks and complications in the US: Temporal changes during 2007–2018. J Clin Med 9: , 1414. |
[36] | Díaz-Venegas C , Downer B , Langa KM , Wong R ((2016) ) Racial and ethnic differences in cognitive function among older adults in the USA. Int J Geriatr Psychiatry 31: , 1004–1012. |
[37] | González HM , Tarraf W , Schneiderman N , Fornage M , Vásquez PM , Zeng D , Youngblood M , Gallo LC , Daviglus ML , Lipton RB , Kaplan R , Ramos AR , Lamar M , Thomas S , Chai A , DeCarli C ((2019) ) Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: Study of Latinos-Investigation of Neurocognitive Aging results. Alzheimers Dement 15: , 1507–1515. |
[38] | Mayeda ER , Glymour MM , Quesenberry CP , Whitmer RA ((2016) ) Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 12: , 216–224. |
[39] | Miles TP , Froehlich TE , Bogardus ST Jr. , Inouye SK ((2001) ) Dementia and race: Are there differences between African Americans and Caucasians? J Am Geriatr Soc 49: , 477–484. |
[40] | Vásquez E , Botoseneanu A , Bennett JM , Shaw BA ((2016) ) Racial/ethnic differences in trajectories of cognitive function in older adults: Role of education, smoking, and physical activity. J Aging Health 28: , 1382–1402. |
[41] | Avila JF , Vonk JMJ , Verney SP , Witkiewitz K , Arce Rentería M , Schupf N , Mayeux R , Manly JJ ((2019) ) Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement 15: , 1516–1523. |
[42] | Zhang Z , Hayward MD , Yu Y-L ((2016) ) Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Soc Behav 57: , 184–199. |
[43] | Schwartz BS , Glass TA , Bolla KI , Stewart WF , Glass G , Rasmussen M , Bressler J , Shi W , Bandeen-Roche Karen ((2004) ) Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ Health Perspect 112: , 314–320. |
[44] | Zahodne LB , Sharifian N , Kraal AZ , Zaheed AB , Sol K , Morris EP , Schupf N , Manly JJ , Brickman AM ((2021) ) Socioeconomic and psychosocial mechanisms underlying racial/ethnic disparities in cognition among older adults. Neuropsychology 35: , 265–275. |
[45] | Iseyemi A , Zhao Q , McNicholas C , Peipert JF ((2017) ) Socioeconomic status as a risk factor for unintended pregnancy in the Contraceptive CHOICE Project. Obstet Gynecol 130: , 609–615. |
[46] | Penman-Aguilar A , Carter M , Snead MC , Kourtis AP ((2013) ) Socioeconomic disadvantage as a social determinant of teen childbearing in the U.S. Public Health Rep 128: , 5–22. |
[47] | Giudicessi AJ , Saelzler UG , Shadyab AH , Posis AIB , Sundermann EE , Banks SJ , Panizzon MS ((2022) ) The mediating role of socioeconomic status on the relationship between pregnancy history and later-life cognition. Climacteric 25: , 627–633. |
[48] | Stickel AM , Tarraf W , Gonzalez KA , Breton J , Keamy AJ , Morlett A , Gallo LC , Medina LD , Cai J , Pirzada A , Daviglus ML , Isasi CR , Kaplan R , Wassertheil-Smoller S , Lamar M , Gonzalez HM ((2021) ) Links between acculturation and level and change in cognition among middle-aged and older Hispanics/Latinos: Findings from the HCHS/SOL and SOL-INCA. Alzheimers Dement 17: (Suppl 7), e051196. |
[49] | Kandula NR , Diez-Roux AV , Chan C , Daviglus ML , Jackson SA , Ni H , Schreiner PJ ((2008) ) Association of acculturation levels and prevalence of diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 31: , 1621–1628. |
[50] | Mendoza L , Garcia P , Duara R , Rosselli M , Loewenstein D , Greig-Custo MT , Barker W , Dahlin P , Rodriguez MJ ((2022) ) The effect of acculturation on cognitive performance among older Hispanics in the United States. Appl Neuropsychol Adult 29: , 163–171. |
[51] | Lamar M , Barnes LL , Leurgans SE , Fleischman DA , Farfel JM , Bennett DA , Marquez DX ((2021) ) Acculturation in context: The relationship between acculturation and socioenvironmental factors with level of and change in cognition in older Latinos. J Gerontol B Psychol Sci Soc Sci 76: , e129–e139. |
[52] | Cohen J ((1992) ) Statistical power analysis. Curr Dir Psychol Sci 1: , 98–101. |
[53] | Silva CA , Yamakami LYS , Aikawa NE , Araujo DB , Carvalho JF , Bonfá E ((2014) ) Autoimmune primary ovarian insufficiency. Autoimmun Rev 13: , 427–430. |
[54] | Khizroeva J , Nalli C , Bitsadze V , Lojacono A , Zatti S , Andreoli L , Tincani A , Shoenfeld Y , Makatsariya A ((2019) ) Infertility in women with systemic autoimmune diseases. Best Pract Res Clin Endocrinol Metab 33: , 101369. |
[55] | McGlacken-Byrne SM , Conway GS ((2022) ) Premature ovarian insufficiency. Best Pract Res Clin Obstet Gynaecol 81: , 98–110. |
[56] | Gemmill A , Weiss J ((2022) ) The relationship between fertility history and incident dementia in the U.S. Health and Retirement Study. J Gerontol Ser B 77: , 1118–1131. |
[57] | Ryan J , Carrière I , Scali J , Ritchie K , Ancelin M-L ((2009) ) Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology 34: , 287–298. |
[58] | Fu C , Hao W , Ma Y , Shrestha N , Virani SS , Mishra SR , Zhu D ((2023) ) Number of live births, age at the time of having a child, span of births and risk of dementia: A population-based cohort study of 253,611 U.K. women. J Womens Health (Larchmt) 32: , 680–692. |
[59] | Lamberty GJ , Kennedy CM , Flashman LA ((1995) ) Clinical utility of the CERAD word list memory test. Appl Neuropsychol 2: , 170–173. |
[60] | Clark LJ , Gatz M , Zheng L , Chen Y-L , McCleary C , Mack WJ ((2009) ) Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am J Alzheimers Dis Other Demen 24: , 461–468. |
[61] | Jaeger J ((2018) ) Digit Symbol Substitution Test: The case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 38: , 513–519. |
[62] | Kroenke K , Spitzer RL , Williams JBW ((2001) ) The PHQ-9. J Gen Intern Med 16: , 606–613. |
[63] | R Core Team ((2013) ), R: A language and environment for statistical computing. |
[64] | Hoekzema E , Barba-Müller E , Pozzobon C , Picado M , Lucco F , García-García D , Soliva JC , Tobeña A , Desco M , Crone EA , Ballesteros A , Carmona S , Vilarroya O ((2017) ) Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci 20: , 287–296. |
[65] | Chen C , Zissimopoulos JM ((2018) ) Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y) 4: , 510–520. |
[66] | Li F-D , He F , Chen T-R , Xiao Y-Y , Lin S-T , Shen W , Wang X-Y , Zhai Y-J , Shang X-P , Lin J-F ((2015) ) Reproductive history and risk of cognitive impairment in elderly women: A cross-sectional study in Eastern China. J Alzheimers Dis 49: , 139–147. |
[67] | Rasgon NL , Magnusson C , Johansson ALV , Pedersen NL , Elman S , Gatz M ((2005) ) Endogenous and exogenous hormone exposure and risk of cognitive impairment in Swedish twins: A preliminary study. Psychoneuroendocrinology 30: , 558–567. |
[68] | Aladangady A , Forde A ((2021) ) Wealth Inequality and the Racial Wealth Gap. FEDS Notes. Board of Governors of the Federal Reserve System,Washington, October 22, 2021. https://doi.org/10.17016/2380-7172.2861 |
[69] | Kim MK , Lee SM , Bae S-H , Kim HJ , Lim NG , Yoon S-J , Lee JY , Jo M-W ((2018) ) Socioeconomic status can affect pregnancy outcomes and complications, even with a universal healthcare system. Int J Equity Health 17: , 2. |
[70] | Sapolsky RM ((1999) ) Glucocorticoids, stress, and their adverse neurological effects: Relevance to aging. Exp Gerontol 34: , 721–732. |
[71] | Nam Y , Wikoff N , Sherraden M ((2015) ) Racial and ethnic differences in parenting stress: Evidence from a statewide sample of new mothers. J Child Fam Stud 24: , 278–288. |
[72] | Centers for Disease Control and Prevention (CDC (2013) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). https://wwwn.cdc.gov/nchs/nhanes/default.aspx |
[73] | Centers for Disease Control and Prevention (CDC (2011) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). https://wwwn.cdc.gov/nchs/nhanes/default.aspx |




