Activity of Choline Alphoscerate on Adult-Onset Cognitive Dysfunctions: A Systematic Review and Meta-Analysis
Abstract
Background:
Choline alphoscerate (alpha glyceryl phosphorylcholine, α-GPC) is a choline-containing phospholipid used as a medicine or nutraceutical to improve cognitive function impairment occurring in neurological conditions including adult-onset dementia disorders. Despite its 1985 marketing authorization, there are still discrepancies between countries regarding its approval as a prescription medicine and discussions about its effectiveness.
Objective:
This study aimed to evaluate the efficacy of the α-GPC compound for treating cognitive impairment in patients with adult-onset neurological disorders.
Methods:
Relevant studies were identified by searching PubMed, Web of Science, and Embase. Studies that evaluated the effects of α-GPC alone or in combination with other compounds on adult-onset cognitive impairment reporting cognition, function, and behavior were considered. We assessed the risk of bias of selected studies using the Cochrane risk of bias tool.
Results:
A total of 1,326 studies and 300 full-text articles were screened. We included seven randomized controlled trials (RCTs) and one prospective cohort study that met our eligibility criteria. We found significant effects of α-GPC in combination with donepezil on cognition [4 RCTs, mean difference (MD):1.72, 95% confidence interval (CI): 0.20 to 3.25], functional outcomes [3 RCTs, MD:0.79, 95% CI: 0.34 to 1.23], and behavioral outcomes [4 RCTs; MD: –7.61, 95% CI: –10.31 to –4.91]. We also observed that patients who received α-GPC had significantly better cognition than those who received either placebo or other medications [MD: 3.50, 95% CI: 0.36 to 6.63].
Conclusion:
α-GPC alone or in combination with donepezil improved cognition, behavior, and functional outcomes among patients with neurological conditions associated with cerebrovascular injury.
INTRODUCTION
According to the United Nations report (2020), there are 727 million older persons (65 years or older) in the world, and this number is expected to more than double by 2050 (over 1.5 billion persons) [1]. As a result of this rapid demographic aging, disease and disability will be more prevalent. Among expected age-related disorders, cognitive impairment will take a relevant place [2]. Cognitive impairment leads to the progressive loss of learning and memory capabilities, resulting in an increase in dependency and social isolation [3]. Cognitive dysfunction ranges from mild deficits to dementia. There are many causes of adult-onset cognitive impairment, including Alzheimer’s disease (AD), vascular dementia, strokes, brain injury, Parkinson’s disease dementia, and other neurodegenerative disorders [4].∥It has been estimated that 10% of people diagnosed with mild cognitive impairment progress to dementia every year [5]. In 2019, 57.4 million people were living with dementia globally, and by 2050, this number will rise to 152.8 million [6]. There are many physical, psychological, social, and economic consequences associated with dementia disorders, not only for the individuals suffering from them but also for their families and society in general [7]. There is an urgent need to implement strategies to diagnose initial cognitive impairment and to stop or delay its progression into overt dementia. These efforts are aimed to mitigate this public health burden. Pharmacological treatments may have a relevant role in reducing the burden of cognitive impairment. In particular, they can contribute to delaying the transition from mild cognitive impairment into overt dementia. Choline alphoscerate (alpha glyceryl phosphorylcholine, α-GPC) is a choline-containing phospholipid with cognition enhancing capabilities, proposed for countering the cognitive impairment in AD, stroke, and other types of adult-onset dementias [8]. From a pharmacological point of view, α-GPC is considered as a parasympathetic agent, which is used both as a registered drug or as a nutraceutical in several countries. Preclinical studies have shown that α-GPC increases acetylcholine release and levels, as well as facilitates learning and memory [9]. It has been shown that α-GPC increases acetylcholine levels in the aging brain [8]. Acetylcholine is a neurotransmitter contributing to communication between nerve cells, and between neurons and their skeletal muscles and autonomic targets. It also plays a key role in the brain’s ability to store and recall information [10].∥In clinical studies, α-GPC was found to improve cognition, behavioral, and functional outcomes in patients with AD, stroke, and cerebral ischemic attacks [8, 11, 12]. The majority of studies have reported positive effects of the compound [13]. However, a study [14] conducted on a large sample size (12,008,977 participants) reported that α-GPC users had a higher risk for total stroke (adjusted hazard ratios [aHR], 1.43; 95% CI, 1.41–1.46), ischemic stroke (aHR, 1.34; 95% CI, 1.31–1.37), and hemorrhagic stroke (aHR, 1.37; 95% CI, 1.29–1.46). Although this paper presented a questionable and imprecise statistical analysis, it was the first one raising a safety issue for a molecule in general considered quite safe [13].∥In spite of the possible doubts about the preciseness and the correct statistical analysis of the above paper [14], the concerns raised suggest the need of a careful critical analysis about the clinical efficacy of α-GPC. To answer the question if α-GPC is effective in the treatment of some symptoms of adult-onset dementia disorders. We have therefore reviewed evidence from clinical studies on α-GPC on cognitive impairment of neurological origin, followed by a meta-analysis summarizing clinical data on the effects of α-GPC on cognitive function. The goal of this systematic review and meta-analysis was to determine the effects of α-GPC on cognition, functional, and behavioral symptoms assessed using different efficacy measures.
MATERIALS AND METHODS
This systematic review followed the Preferred Items for Systematic Review and Meta-analysis (PRISMA) checklists and diagrams to design and report the results [15]. A protocol for this systematic review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) and the registration number is CRD42022356965. It is available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=356965
Research questions
Does α-GPC improve cognitive function in terms of behavior, cognition, and the ability to perform basic daily activities in adults suffering from different pathologies with the common denominator of cognitive dysfunction?
Database search strategy and information sources
On August 2022, we conducted a comprehensive systematic search by PubMed, Web of Science, and EMBASE database using key terms of ‘L-Alpha glycerylphosphorylcholine*’, ‘alpha-GPC*’, ‘choline alphoscerate*’, ’Cereton*’, ‘α-Glycerylphosphorylcholine*’, ‘cognitive*’, ‘dementia disorders*’, ‘Alzheimer’s disease*’, ‘cognitive impairment’, ‘cognitive dysfunction’. To combine the search terms for outcome interest, we used Boolean operators such as “AND” and “OR”. Further relevant articles were manually reviewed from the retrieved study reference lists.
Eligibility criteria and study selection process
Analysis was limited to randomized controlled trials (RCT), cohort, or case-control studies in which α-GPC was used alone or in combination with cholinergic drugs versus placebo and/or another drug on cognitive function in patients with neurological disorders of different origin. Studies with no control group were not considered in this systematic review. We included studies that considered either male or female adult patients aged 50 years and older with the diagnosis of neurological disorders with cognitive impairment and using as efficacy measures the Mini-Mental State Examination (MMSE), or the Alzheimer’s Disease Assessment Scale-Cognition subscale (ADAS-Cog), Neuropsychiatric Inventory (NPI), Basic Activities of Daily Living (disability), and Instrumental activities of daily living (IADL) domains. We did not put restrictions on the route of administration, dose, duration of treatment, language, and publication date. Studies with unclear methodologies especially in terms of efficacy measurement and studies published only in abstracts and conference proceedings were excluded.
As for the selection of the studies, the title and abstract were screened based on eligibility criteria by two authors independently. We retrieved the full-text articles from the databases based on the title and abstract screening, and three authors independently reviewed the full-text articles to select studies that met our inclusion criteria. The disagreements related to article selection were resolved through discussion if any occurred.
Data extraction
From selected studies, two authors collected the following information: name of the first author, publication year, participants in the study, treatment involved in both experimental groups and control groups with details of the treatment (name of the treatment, dose, route of administration, and duration of the treatment in days), the scales that were used to determine the efficacy of the treatment, and the study design. An Excel spreadsheet was used to collect pertinent data from the included studies that were used in the subsequent analysis.
Outcome measures
The purpose of this systematic review was to investigate the effect of α-GPC on cognitive dysfunction, using the following measures: 1) cognition, measured by the MMSE or the ADAS-Cog. MMSE is a common, validated screening instrument for assessing cognitive function (a lower score indicates greater impairment) and includes questions concerning basic temporal and spatial orientation, attention, language, calculation, memory fixing, and constructive practice [16]. An ADAS-Cog score is used to determine the severity of cognitive and noncognitive impairments in persons with AD (a single scale ranging from 0 to 70 was used for evaluating the patients’ performance on tasks, with higher scores indicating more severe impairments) [17]; 2) functional status, measured using the basic activities of daily living (BADL) [18] and IADL scales [18], and 3) behavior, measured by NP) [19].
Risk of bias assessments of selected studies
Our study has also investigated the risk bias in the RCTs of selected studies using the Cochrane Collaboration tool [20]. The risk of bias for the selected studies was independently assessed based on six domains by two authors. These domains include random sequence generation, allocation concealment, blinding personnel and participants, blinding outcome assessment, incomplete outcome data, selective reporting, and other biases. Based on each domain risk bias assessment, the studies were classified into three categories: low-risk bias, unclear-risk bias, and high-risk bias.
Statistical analysis
The data were entered into a Microsoft Excel spreadsheet and analyzed using R-statistical software (Version 4.1.1, The R Foundation for Statistical Computing, Vienna, Austria) [21]. We used R metafor package [22] along with different arguments to calculate effect size (i.e., mean difference). To calculate the effect size (mean difference) we used change from the baseline mean (i.e., before the interventions are administrated) to the post treatment mean (final value at the end of follow-up). The mean difference was used to estimate the amount by which the experimental intervention changes the outcome on average compared with the control (comparator) intervention. As a part of the pooled analysis, mean changes from baseline and post-intervention values along with standard deviations (SDs) of selected studies are required. For the studies that did not report SD of the mean change from the baseline, we considered the additional information from the included studies such as confidence intervals, p-values, t-values, F-values, and standard errors to determine the standard deviations based on the equation provided in Cochrane’s Handbook for systematic reviews of interventions [20]. In cases where the above information was not available, we contacted the corresponding author(s) and requested their datasets or the mean along with standard deviation changes between baseline and post-intervention. When corresponding authors did not provide their datasets or mean change along with SD from baseline values, the SD change value was calculated by adding 0.7 to the correlation coefficient (r) in the formula [
To generate the summary measures of effect in the form of mean difference (MD), random effects models with the inverse variance method were used [25]. Heterogeneity between studies was assessed using Cochrane’s Q test [26] and I2 test statistics [27]. The degree of heterogeneity was considered as low, moderate, and high based on I2 values of less than 25%, 25% to 75%, and more than 75%, respectively [28].
RESULTS
Literature search
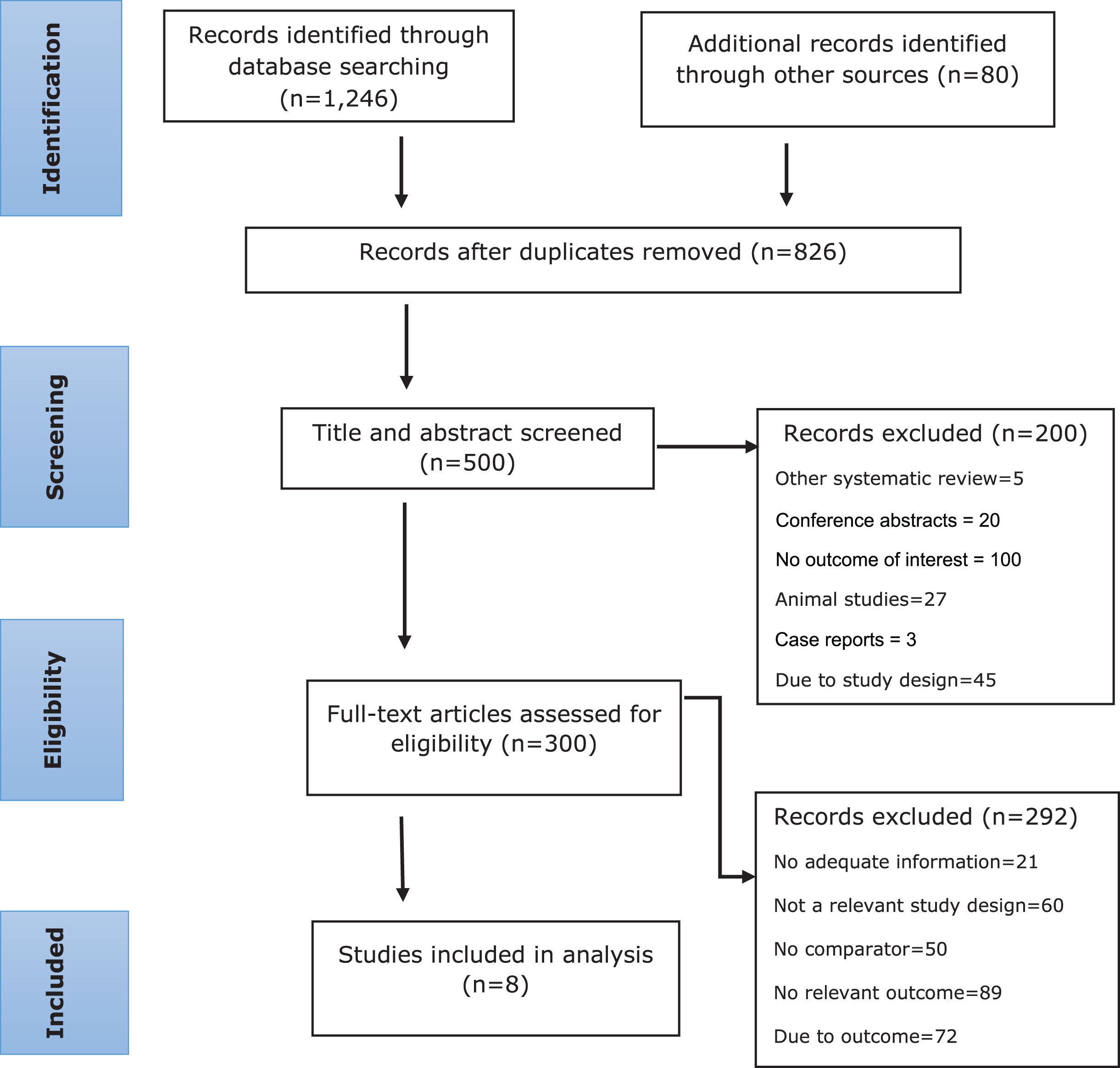
In our literature search, we found 1,326 records, of which 826 records were excluded because of duplicate records. As a result of title and abstract screening, 200 records were excluded. Three hundred full-text articles were found to be potentially relevant for inclusion. Based on the screening of full-text articles, 292 studies were excluded. We identified eight studies that met our eligibility criteria (Fig. 1). One of the included studies was a prospective cohort study [29], and the other seven were RCTs [30–36].
Fig. 1
Selection of eligible studies.
Study characteristics
All of the included studies (eight studies) were conducted between 1993 and 2022 in Russia (one study) [29], Italy (six studies) [30–35], and Mexico (one study) [36] (Table 1). In four RCTs, α-GPC was combined with the cholinesterase inhibitor (ChE-I) donepezil [30, 31, 33, 35]. In one RCT, α-GPC was evaluated in combination with nimodipine [34], and the effects of α-GPC were examined in three RCTs without any combination of treatments [29, 32, 36]. In four RCTs, α-GPC and donepezil were compared to placebo and donepezil; in one RCT, α-GPC and nimodipine were compared to placebo and nimodipine. In three studies (two RCTs and one prospective cohort study), α-GPC was compared to either placebo or another active drug (Table 1). The number of participants in included studies ranged from 56 to 261.
Table 1
Characteristics of the included studies
| Study, year [Ref] | Disease | Number of participants | Treatment | Dose | Via | Duration | Efficacy measures | Study design |
| Selezneva et al., 2020 [29] | Alzheimer’s disease | N = 62 N = 30 [Intervention group (E)] n = 32 [Control group (C)] | choline alphoscerate (E) and placebo (C) | E = 1200 mg (CA) C = placebo | PO | 90 days | MMSE and MoCA | Prospective cohort |
| Salvadori et al., 2021 [34] | Cerebral small vessel disease | N = 62 n = 31(E) n = 31(C) | choline alphoscerate and nimodipine (E), nimodipine and placebo (C) | E = 1200 mg (CA) and NI = 90 mg (NI) C = 90 mg (NI) and placebo capsule (BID) | PO | 360 days | MoCA, ADL, IADL, DAD, RAVL | RCT |
| Parnetti et al., 1993 [32] | Dementia | N = 126 n = 65 (E) n = 61 (C) | choline alphoscerate (E) and ST200 (acetyl-L-carnitine) (C) | E = 1200 mg (CA) C = 1500 mg | PO | 180 days | GDS, SCAG, GBS, MMSE | RCT |
| Moreno et al., 2003 [36] | Alzheimer’s disease | N = 261 n = 132 (E) n = 129 (C) | choline alphoscerate (E) and placebo (C) | E = 1200 mg (CA) C = placebo capsule | PO | 180 days | MMSE, ADAS-Cog, CGI, GDS, ADAS-Behav, ADAS_Total | RCT |
| Amenta et al., 2012 [30] | Alzheimer’s disease | N = 91 n = 44 (E) n = 47 (C) | choline alphoscerate and donepezil (E), donepezil and placebo (C) | E = 1200 mg (CA) + 10 mg (DP) C = 10 mg (DP) + placebo | PO | 360 days | MMSE, ADAS-Cog, BADL, IADL, NPI | RCT |
| Amenta et al., 2014 [31] | Alzheimer’s disease + Cerebrovascular injury | N = 113 n = 57 (E) n = 56 (C) | choline alphoscerate and donepezil (E), donepezil and placebo (C) | E = 1200 mg (CA) + 10 mg (DP) C = 10 mg (DP) + placebo | PO | 720 days | MMSE, ADAS-Cog, BADL, IADL, NPI | RCT |
| Traini et al., 2020 [33] | Alzheimer’s disease | N = 56 n = 29 (E) n = 27 (C) | choline alphoscerate and donepezil (E), donepezil and placebo (C) | E = 1200 mg (CA) + 10 mg (DP) C = 10 mg (DP) + placebo | PO | 720 days | MMSE, ADAS-Cog, BADL, IADL, NPI | RCT |
| Carotenuto et al., 2022 [35] | Depression | N = 90 n = 45 (E) n = 45 (C) | choline alphoscerate and donepezil (E), donepezil and placebo (C) | E = 1200 mg (CA) + 10 mg (DP) C = 10 mg (DP) + placebo | PO | 720 days | MMSE NPI | RCT |
MoCA, Montreal Cognitive Assessment; ADL, activities of daily living; DAD, Disability Assessment in Dementia; RAVL, Rey Auditory-Verbal Learning Test; GDS, Global Deterioration Scale; SCAG, Sandoz Clinical Assessment Geriatric scale; GBS, Gottfries-Bfllne-Steen Rating Scale; CGI, Clinical Global Impression.
Effect of choline alphoscerate on cognition
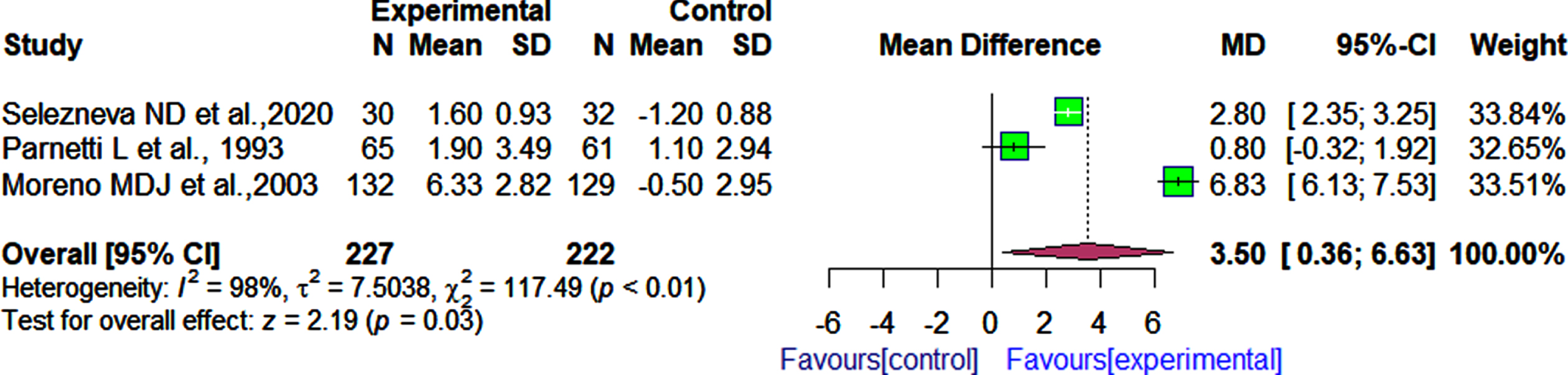
We identified three studies that evaluated the effects of α-GPC versus placebo or other drugs on cognitive function using the MMSE [29, 32, 36]. In these three RCTs, there were a total of 449 participants (n = 227 in the experimental group and n = 222 in the control group). Our meta-analysis showed that patients who received α-GPC had significantly better cognition as measured by the MMSE than those who received either placebo capsules or other medications (MD 3.50, 95% CI: 0.36 to 6.63, I2 = 98%; Fig. 2).
Fig. 2
Comparison of effects of choline alphoscerate (1 r200 mg per day) and placebo or acetyl-L-carnitine (1 r500 mg per day) on patient cognition as measured by the MMSE after follow-up periods ranging from 90 days to 180 days.
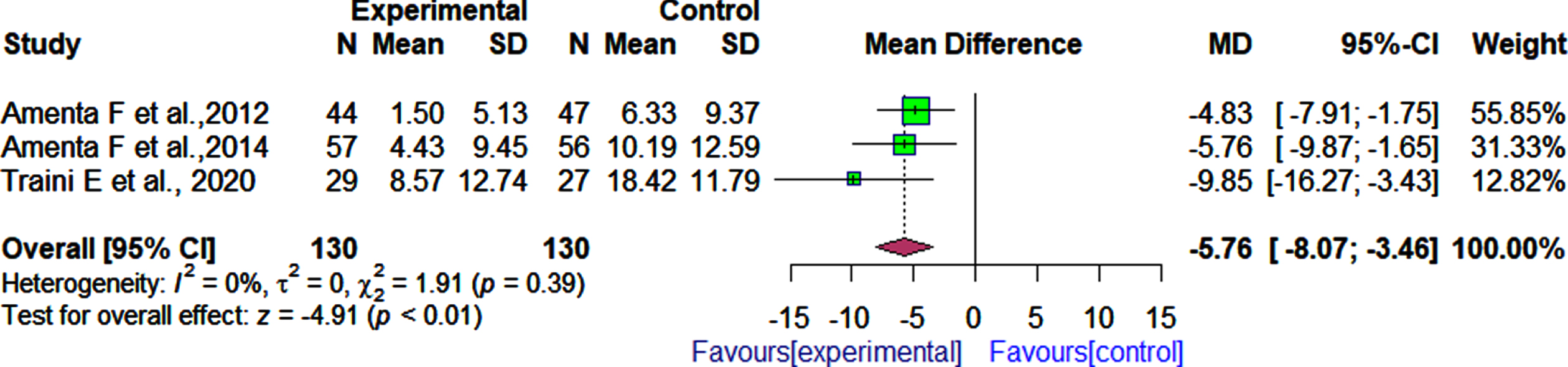
Four RCTs assessed the effectiveness of α-GPC combined with the ChEI donepezil on cognition as measured by the MMSE test [30, 31, 33, 35], while three RCTs used as a cognitive measure the ADAS-Cog test [30, 31, 33]. These four RCTs included 175 participants in the active treatment group (with α-GPC) and 175 participants in the control group. The pooled effect estimate showed that patients who received α-GPC and donepezil had significantly improved cognitive function than those who received donepezil and placebo as measured by MMSE (4 RCTs, MD: 1.72, 95% CI: 0.20 to 3.25, I2 = 61%, Fig. 3).Significant differences in cognition between the patients who received α-GPC and donepezil compared to the patients who received donepezil and placebo as measured by the ADAS-Cog were observed (3 RCTs, MD: –5.76, 95% CI: –8.07 to –3.46, I2 = 0.0%, Fig. 4). After 180 days of follow-up, a significant difference was observed between patients who received α-GPC and those who received placebo, in a single RCT [36] that assessed cognition outcomes using the ADAS-Cog (MD: –6.10, 95% CI: –7.51 to –4.69).
Fig. 3
Comparison of the effects of choline alphoscerate (1 r200 mg/day) and donepezil (10 mg/day) rand placebo and donepezil (10 mg/day) on patient cognition as measured by the MMSE after follow-up periods ranging from 360 days to 720 days.
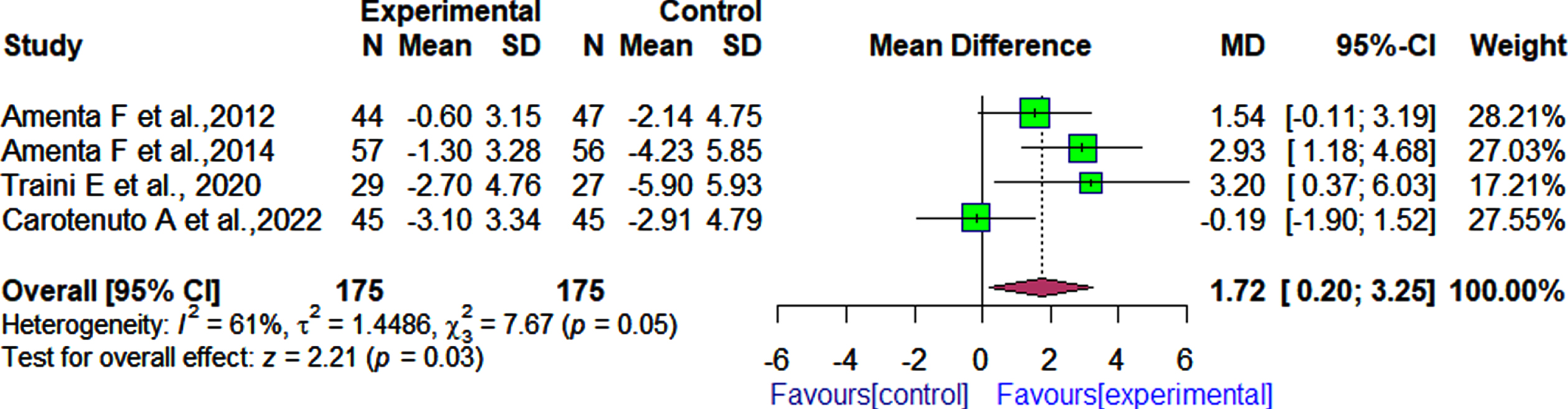
Fig. 4
Comparison of effects of choline alphoscerate (1 r200 mg/day) and donepezil (10 mg/day) rand placebo and donepezil (10 mg/day) on patient cognition as measured by the ADAS-Cog after follow-up periods ranging from 360 days to 720 days.
Effect of choline alphoscerate on functional outcomes
In three RCTs [30, 31, 33] and one RCT [34], α-GPC was evaluated in combination with donepezil and nimodipine, respectively to determine its effect on the functional status in patients with cognitive dysfunction as assessed by the BADL and IADL. According to the pooled effect estimate, we observed no significant difference in functional outcomes, as measured by the BADL between patients who received α-GPC and donepezil when compared with those who received donepezil and placebo [3 RCTs rMD: 0.46 rI2 = 66% rFig. 5).
Fig. 5
Comparison of effects of choline alphoscerate (1 r200 mg/day) and donepezil (10 mg/day) rand placebo and donepezil (10 mg/day) on patient functional outcomes as measured by the BADL after follow-up periods ranging from 360 days to 720 days.
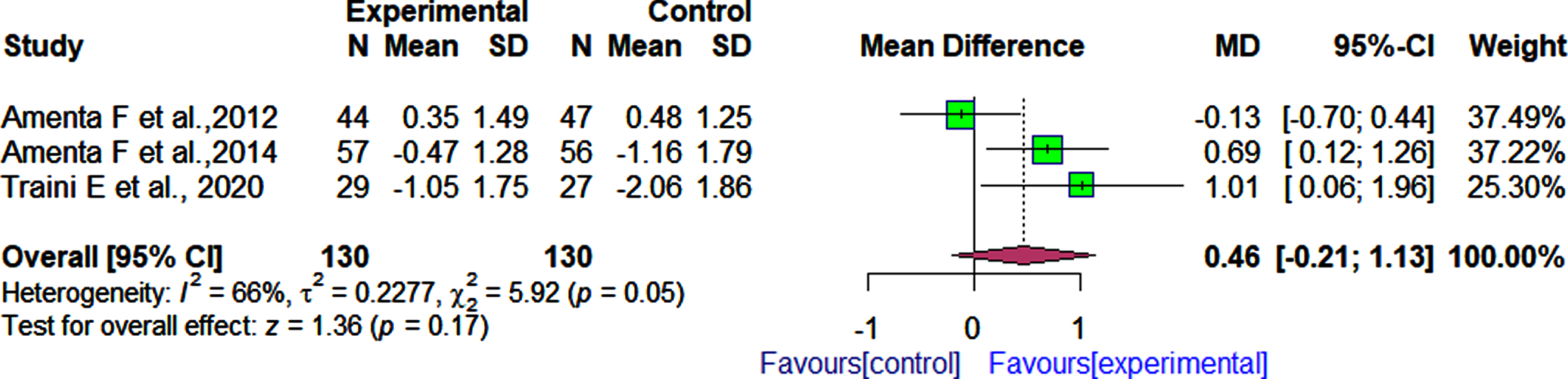
Similarly in the single RCT that reported functional outcomes based on both the activities of daily living and instrumental activities of daily living scales 34">3 RCTs, MD: 0.46, 95% CI: –0.21 to 1.13, I2 = 66%, Fig. 5).
Similarly, in the single RCT that reported functional outcomes based on both the activities of daily living and instrumental activities of daily living scales[34], after 360 days of follow-up, no significant difference was observed between patients who received α-GPC and nimodipine and those who received nimodipine and placebo as measured by the both ADL (MD:0.00, 95% CI: –0.51 to 0.51), and IADL (MD: –0.30, 95% CI: –1.54 to 0.94).
On the other hand, the pooled effect estimate showed that patients who received α-GPC and donepezil had significantly improved functional status than those who received donepezil and placebo as assessed by the IADL after follow-up periods ranging from 360 days to 720 days [3 RCTs rMD: 0.79 r95% CI: 0.34 to 1.23 rI2 = 0% Fig. 6).
Fig. 6
Comparison of effects of choline alphoscerate (1200 mg/day) and donepezil (10 mg/day) rand placebo and donepezil (10 mg/day) on patient functional outcomes as measured by the IADL after follow-up periods ranging from 360 days to 720 days.
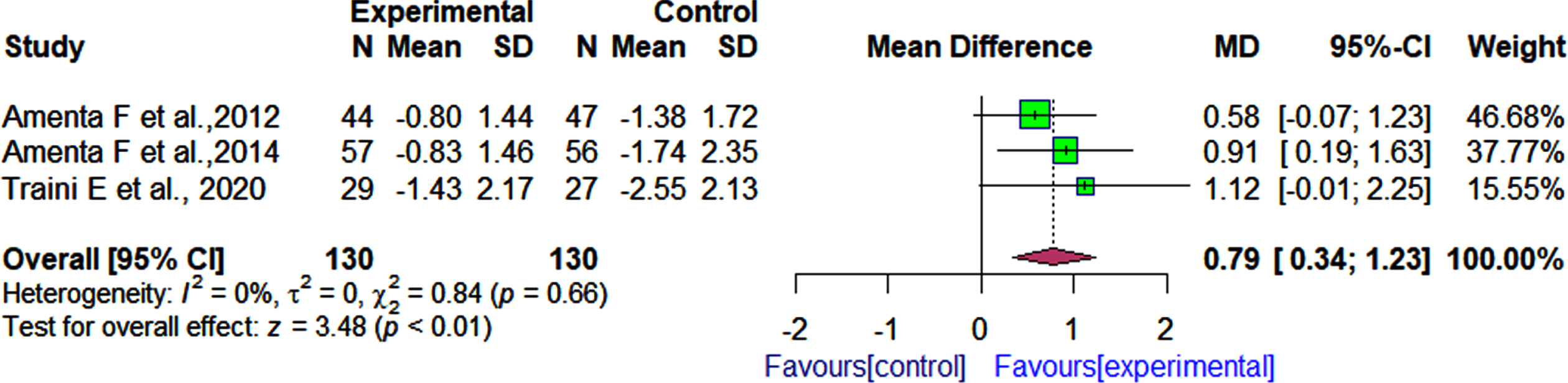
Effect of choline alphoscerate on behavioral outcomes
Four 30, 31, 33, 35] of the seven RCTs examined the effect of α-GPC in combination with donepezil on the behavioral status of patients with cognitive impairments using the NPI. The pooled effect estimate showed that patients treated with α-GPC and donepezil had a significantly reduced behavioral symptoms severity and caregiver distress as measured by the NPI, compared to those treated with donepezil and placebo after follow-up periods ranging from 360 days to 720 days (4 RCTs; MD: –7.61, 95% CI: –10.31 to –4.91, I2 = 42%, Fig. 7). Regarding the severity of behavioral symptoms (NPI-Fxs), we observed significant differences in patients who received α-GPC and donepezil, and those who received placebo and donepezil [MD: –7.74; 95% CI: –12.69 to –2.79, I2 = 52%, Fig. 7). In terms of caregiver distress, the pooled effect estimate indicated that caregiver distress (NPI-D) was reduced significantly in patients treated with donepezil and α-GPC in comparison to patients treated with placebo and donepezil [4 RCTs; MD: –7.37; 95% CI: –10.90 to –3.83, I2 = 46%].
Fig. 7
Comparison of effects of choline alphoscerate (1,200 mg/day) and donepezil (10 mg/day), and placebo and donepezil (10 mg/day) on patient behavioral outcomes as measured by the NPI after follow-up periods ranging from 360 days to 720 days.
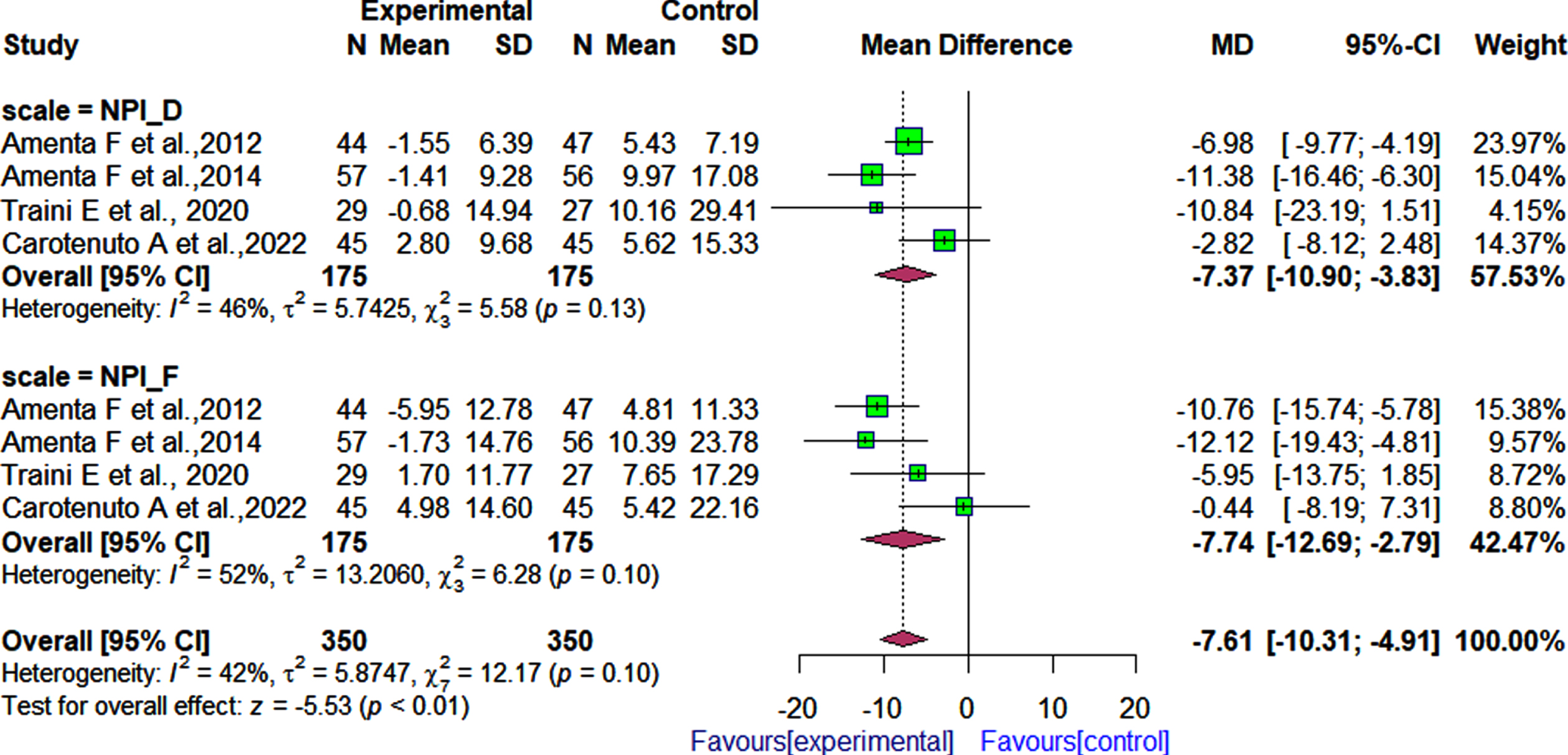
Methodological quality of included studies
In accordance with the Cochrane risk of the bias assessment tool, five studies were evaluated as having a low risk of bias on six of seven items (Table 2). In the remaining studies, at least two criteria were unclear regarding the risk of bias (Table 2).
Table 2
Assessment of risk of bias of the included studies using the Cochrane risk-of-bias tool
| Study | Risk of bias | ||||||
| Random sequence generation | Allocation concealment | Blinding of participants, personnel | Blinding of outcome assessors | Incomplete outcome data | Selective reporting | Others | |
| Selezneva et al., 2020 [29] | Unclear | Low | Unclear | Unclear | Low | Unclear | Unclear |
| Salvadori et al., 2021 [34] | Low | Low | Low | Low | Low | Low | Unclear |
| Parnetti et al., 1993 [32] | Low | Low | Low | Unclear | Unclear | Low | Unclear |
| Moreno et al., 2003 [36] | Low | Unclear | Low | Low | Low | Low | Low |
| Amenta et al., 2012 [30] | Low | Low | Low | Unclear | Low | Low | Low |
| Amenta et al., 2014 [31] | Low | Unclear | Low | Low | Low | Low | Unclear |
| Traini et al., 2020 [33] | Low | Unclear | Low | Low | Low | Low | Low |
| Carotenuto et al., 2022 [35] | Low | Low | Low | Unclear | Low | Low | Low |
Low, low risk of bias; unclear, unclear about the risk of bias.
DISCUSSION
In the present systematic review and meta-analysis, the effects of α-GPC alone or in combination with other drugs on cognitive function in patients with adult-onset dementia disorders of neurological origin caused by different pathologies compared to a placebo and/or other drugs were evaluated.
We synthesized eight studies (seven RCTs and one a prospective cohort study) with a total of 861 study participants (433 in the intervention group and 428 in the control group) between 1993 and 2022 that met the eligibility criteria in order to evaluate the effects of α-GPC on cognitive, functional, and behavioral domains in patients with neurological conditions.
Analysis of the effects of α-GPC on cognitive dysfunction was focused on scales measuring cognition such as MMSE and ADAS-Cog. Our work has demonstrated a positive effect of α-GPC on MMSE. Patients receiving α-GPC had significantly improved cognition compared to those treated with placebo or other drugs [3 RCTs; MD:3.50, 95% CI: 0.36 to 6.63]. This positive improvement was found in patients affected by AD. In three RCTs, α-GPC was administered orally at a dose of 1,200 mg per day for follow-up periods ranging from 90 to 180 days [29, 32, 36]. In two of three trials, α-GPC was compared to placebo [29, 36], whereas in one study it was compared to ST200 (acetyl-L-Carnitine) [32]. The results of this study are in line with the conclusions of the review of Parnetti and co-workers [8] on the effects of α-GPC on cognitive decline as measured by the MMSE. This study has shown that α-GPC improved the cognitive function of patients with degenerative dementia disorders in terms of orientation, memory, and language [8]. Our findings are consistent with previous studies which reported that α-GPC was beneficial in improving cognitive performance compared to placebo [29, 36] and acetyl-L-Carnitine [32].
Evidence gathered from the four RCTs in this meta-analysis indicates that α-GPC in association with the ChEI donepezil was more effective in terms of cognition as measured by the MMSE versus donepezil and placebo in patients with AD, AD disease with depression and cerebrovascular injury [4 RCTs, MD: 1.72, 95% CI: 0.20 to 3.25]. In general, the results of this study confirm the findings of a previous study, which indicated that α-GPC has significant cognitive effects with a good safety and tolerability profile [13]. We have also found significant differences between patients treated with α-GPC and donepezil as assessed by the ADAS-Cog and those treated with donepezil and placebo regarding cognition outcomes [3RCTs; MD: –5.76, 95% CI: –8.07 to –3.46]. In a single study, we observed that α-GPC significantly improved the cognitive function in patients with mild to moderate AD compared to patients treated with placebo as assessed by ADAS-Cog [MD: –6.10, 95% CI: –7.51 to –4.69].
The present systematic review has found that α-GPC was effective either in combination with donepezil or alone in improving cognitive function in patients with adult-onset dementia disorders as measured by both MMSE and ADAS-Cog when compared to those treated with either ChE-I or placebo. In terms of cognitive function outcomes our results are consistent with those of a previous review, which concluded that α-GPC improved cognitive performance in patients suffering from dementia disorders of neurodegenerative or vascular origin either in combination with ChEIs or alone [8, 37].
Analysis of functional outcomes included three randomized controlled trials with 260 participants divided into two groups (130 in the experimental group and 130 in the control group). These three studies evaluated the effects of α-GPC in combination with donepezil on the functional status of patients with AD and AD associated with cerebrovascular injury using both BADLs and ADLs. Our pooled analysis found no significant difference between patients treated with α-GPC plus donepezil compared to patients treated with donepezil and placebo as measured by the BADL [3 RCTs, MD: 0.46, 95% CI: –0.21 to 1.13]. From the functional point of view, our conclusion contradicted the findings of previous review, which reported that α-GPC could improve functional status in patients affected by AD or other forms dementia disorders of neurological origin in combination with other active treatments or as an independent treatment [8]. This inconsistency could be due to methodological differences and different efficacy measurement approaches. Our study used meta-analysis and the BADL to measure the effects of α-GPC, whereas the study by Parnetti and his colleagues [8], to which one of the co-authors of the present work has contributed was a review analyzing examined studies using the Matthew’s scale to measure functional outcomes.
We have also found a single randomized controlled trials, reporting no significant differences between patients receiving α-GPC in combination with nimodipine and those who received nimodipine and placebo as assessed by both the ADL [MD:0.00, 95% CI: –0.51 to 0.51] and IADL [MD: –0.30, 95% CI: –1.54 to 0.94] after 12 months follow-up periods. As measured by the IADL, however, there was a significant difference between patients treated with α-GPC plus donepezil and those treated with donepezil plus placebo [3 RCTs, MD: 0.79, 95% CI: 0.34 to 1.23). In terms of functional outcomes assessed by the IADL, our results are consistent with those of previous reviews on the effects of α-GPC on cognitive dysfunction 8, 13, 37].
As for the effect on NPI, we observed a significant reduction of behavioral symptoms severity and caregiver distress for patients treated with the combination α-GPC and donepezil when compared to those treated with placebo and donepezil after 12 to 24 months of follow-up periods [4 RCTs; MD: –7.61, 95% CI: –10.31 to –4.91]. These findings are consistent with those of the study conducted by Rea and his colleagues, which assessed the severity of apathy, behavioral symptoms among 113 study participants with mild-moderate AD randomized to receive α-GPC plus donepezil or donepezil plus placebo [38]. Based on follow-up results after 360 and 720 days, the authors found that patients treated with a combination (donepezil and α-GPC) experienced less apathy than those treated with donepezil and placebo [38]. Our study reached the same conclusion of previous randomized controlled trials [38, 39].
Strength and weakness of the present study
To our knowledge, this is the first systematic review with meta-analysis to evaluate the effects of cholinergic precursor α-GPC in combination with the ChE-I donepezil or alone in patients with adult-onset dementia disorders interfering with cognitive function. Considering this, it is essential to note that the results obtained through this systematic review will assist in making evidence-based decisions regarding the safety of α-GPC in patients with neurological disorders. Regarding the weakness of the study, we considered an assigned value of 0.7 in the formula to calculate the SD change for two studies. This may limit the certainty of our pooled findings.
Conclusion
In conclusion, the results of this study support the use of α-GPC in combination with the ChE-I donepezil or alone to improve cognition, functional, and behavioral status, but not BADL of patients with AD and other dementia disorders of neurological origin. Based on our pooled analysis from randomized controlled trials, we can conclude that α-GPC is effective in improving cognitive function in patients with adult-onset dementia disorders associated with cerebrovascular involvement. The above findings suggest the need of further and larger studies to confirm the interest of α-GPC in the treatment of the pathologies in which the compound has shown promising results.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The present work was supported by the institutional funding of the University of Camerino.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
All relevant data are included in the article or provided as supplementary materials. All extracted data are available upon request from the corresponding author.
REFERENCES
[1] | UN DESA Population Division (2020) World Population Ageing 2020 Highlights - Ten key messages. United Nations. |
[2] | Crimmins EM , Kim JK , Langa KM , Weir DR ((2011) ) Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 66 Suppl 1: , 162–171. |
[3] | Chertkow H ((2008) ) Diagnosis and treatment of dementia: Introduction. Introducing a series based on the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. CMAJ 178: , 316–321. |
[4] | Roh JH , Lee J-H ((2014) ) Recent updates on subcortical ischemic vascular dementia. J Stroke 16: , 18. |
[5] | Busse A , Angermeyer MC , Riedel-Heller SG ((2006) ) Progression of mild cognitive impairment to dementia: A challenge to current thinking. Br J Psychiatry 189: , 399–404. |
[6] | Nichols E , Steinmetz JD , Vollset SE , Fukutaki K , Chalek J , Abd-Allah F , Abdoli A , Abualhasan A , Abu-Gharbieh E , Akram TT , et al. ((2022) ) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7: , e105–e125. |
[7] | Dementia, https://www.who.int/news-room/fact-sheets/detail/dementia |
[8] | Parnetti L , Amenta F , Gallai V ((2001) ) Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: An analysis of published clinical data. Mech Ageing Dev 122: , 2041–2055. |
[9] | Govoni S ((1990) ) Effects of alpha-GFC on rat passive avoidance behavior and on acetylcholine levels. Basi Raz 20: , 55–60. |
[10] | Hasselmo ME ((2009) ) The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16: , 710–715. |
[11] | Sangiorgi GB , Barbagallo M , Giordano M , Meli M , Panzarasa R ((1994) ) α-glycerophosphocholine in the mental recovery of cerebral ischemic attacks. Ann N Y Acad Sci 717: , 253–269. |
[12] | Perri R Di , Coppola G , Ambrosio LA , Grasso A , Puca FM , Rizzo M ((1991) ) A multicentre trial to evaluate the efficacy and tolerability of α-glycerylphosphorylcholine versus cytosine diphosphocholine in patients with vascular dementia. J Int Med Res 19: , 330–341. |
[13] | Traini E , Bramanti V , Amenta F ((2013) ) Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent. Curr Alzheimer Res 10: , 1070–1079. |
[14] | Lee G , Choi S , Chang J , Choi D , Son JS , Kim K , Kim SM , Jeong S , Park SM ((2021) ) Association of L-α glycerylphosphorylcholine with subsequent stroke risk after 10 years. JAMA Netw Open 4: , e2136008. |
[15] | Moher D , Liberati A , Tetzlaff J , Altman DG , Altman D , Antes G , Atkins D , Barbour V , Barrowman N , Berlin JA , Clark J , Clarke M , Cook D , D’Amico R , Deeks JJ , Devereaux PJ , Dickersin K , Egger M , Ernst E , Gøtzsche PC , Grimshaw J , Guyatt G , Higgins J , Ioannidis JPA , Kleijnen J , Lang T , Magrini N , McNamee D , Moja L , Mulrow C , Napoli M , Oxman A , Pham B , Rennie D , Sampson M , Schulz KF , Shekelle PG , Tovey D , Tugwell P ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: , e1000097. |
[16] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[17] | Rosen WG , Mohs RC , Davis KL ((1984) ) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141: , 1356–1364. |
[18] | Katz S , Akpom CA ((1976) ) 12. Index of ADL. Med Care 14: , 116–118. |
[19] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[20] | Higgins J , Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions. |
[21] | R Core Team (2019) R: A Language and Environment for Statistical, Computing Foundation for Statistical Computing, Vienna, Austria. |
[22] | Wolfgang Viechtbauer ((2010) ) Conducting meta-analyses in R with the metafor package. J Stat Softw 36: , 1–48. |
[23] | McGirr A , Berlim MT , Bond DJ , Neufeld NH , Chan PY , Yatham LN , Lam RW ((2015) ) A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: Efficacy and tolerability. J Psychiatr Res 62: , 23–30. |
[24] | Papadopoulos VP , Apergis N , Filippou DK ((2020) ) Nocturia in CPAP-treated obstructive sleep apnea patients: A systematic review and meta-analysis. SN Compr Clin Med 2: , 2799–2807. |
[25] | DerSimonian R , Laird N ((1986) ) Meta-analysis in clinical trials. Control Clin Trials 7: , 177–188. |
[26] | Cochran WG ((2016) ) The combination of estimates from different experiments. Biometrics 10: , 101–129. |
[27] | Grant J , Hunter A ((2006) ) Measuring inconsistency in knowledgebases. J Intell Inf Syst 27: , 159–184. |
[28] | Goto A , Arah OA , Goto M , Terauchi Y , Noda M ((2013) ) Severe hypoglycaemia and cardiovascular disease: Systematic review and meta-analysis with bias analysis. BMJ 347: , f4533. |
[29] | Selezneva ND , Kolykhalov IV , Gavrilova SI ((2020) ) A comparative prospective multidisciplinary research of efficiency of choline alphoscerate in prevention of cognitive deficiency progressing in relatives of patients with Alzheimer’s disease. Psychiatry (Moscow) 18: , 6–15. |
[30] | Amenta F , Carotenuto A , Fasanaro AM , Rea R , Traini E ((2012) ) The ASCOMALVA trial: Association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in Alzheimer’s disease with cerebrovascular injury: Interim results. J Neurol Sci 322: , 96–101. |
[31] | Amenta F , Carotenuto A , Fasanaro AM , Rea R , Traini E ((2014) ) The ASCOMALVA (Association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in Alzheimer’s disease) Trial: Interim results after two years of treatment. ,. J Alzheimers Dis 42: , S281–S288. |
[32] | Parnetti L , Abate G , Bartorelli L , Cucinotta D , Cuzzupoli M , Maggioni M , Villardita C , Senin U ((1993) ) Multicentre study of l-α-glyceryl-phosphorylcholine vs ST200 among patients with probable senile dementia of Alzheimer’s type. Drugs Aging 3: , 159–164. |
[33] | Traini E , Carotenuto A , Fasanaro AM , Amenta F ((2020) ) Volume analysis of brain cognitive areas in Alzheimer’s disease: Interim 3-year results from the ASCOMALVA Trial. J Alzheimers Dis 76: , 317–329. |
[34] | Salvadori E , Poggesi A , Donnini I , Rinnoci V , Chiti G , Squitieri M , Tudisco L , Fierini F , Melone A , Pescini F , Pantoni L ((2021) ) Efficacy and safety of the association of nimodipine and choline alphoscerate in the treatment of cognitive impairment in patients with cerebral small vessel disease. The CONIVaD Trial. Drugs Aging 38: , 481–491. |
[35] | Carotenuto A , Fasanaro AM , Manzo V , Amenta F , Traini E ((2022) ) Association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in the treatment of depression in patients with Alzheimer’s disease. J Alzheimers Dis Rep 6: , 235–243. |
[36] | Moreno Moreno MDJ ((2003) ) Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clin Ther 25: , 178–193. |
[37] | Scapicchio PL ((2013) ) Revisiting choline alphoscerate profile: A new, perspective, role in dementia? . Int J Neurosci 123: , 444–449. |
[38] | Rea R , Carotenuto A , Traini E , Fasanaro AM , Manzo V , Amenta F ((2015) ) Apathy treatment in Alzheimer’s disease: Interim results of the ASCOMALVA Trial. J Alzheimers Dis 48: , 377–383. |
[39] | Carotenuto A , Rea R , Traini E , Fasanaro AM , Ricci G , Manzo V , Amenta F ((2017) ) The effect of the association betweendonepezil and choline alphoscerate on behavioral disturbances inAlzheimer’s disease: Interim results of the ASCOMALVA Trial. JAlzheimers Dis 56: , 805–815. |




