Background Music and Memory in Mild Cognitive Impairment: The Role of Interindividual Differences
Abstract
Background:
Recent research has shown that background music may improve memory consolidation and retrieval. Nevertheless, in the clinical conditions preceding dementia such as mild cognitive impairment (MCI), there is no current evidence speaking to what effect background music during memory tasks has on impaired cognition.
Objective:
Across three experiments, we investigated if background music is able to improve memory performance, the most impacted cognitive domain in amnestic MCI.
Methods:
We tested the effect of background music by using a face recognition memory task in patients with amnestic MCI. In Experiment 1, we tested the effect of background music on memory when it was played solely during an encoding phase. In Experiment 2, we explored effects of background music when played during both encoding and recognition phases. In Experiment 3, we explored the role of musically induced arousal on memory.
Results:
The main finding from these three experiments was that background music played during a memory task did not improve or worsen participant performance. However, when exposed to high-arousal music, memory performance was predicted by individual mood regulation. For low-arousal music conditions, there was a negative relationship between rating scores for music pleasantness and performance on the memory task.
Conclusion:
Our results suggest that the benefits of background music on memory in individuals with MCI are modulated by interindividual preferences towards music.
INTRODUCTION
A growing body of research has attempted to show the benefits of listening to music or practicing musical activities in order to compensate for symptoms of age-related disorders. However, more attention has been placed on the positive effects of music on mood and emotional dysregulation rather than its benefits for cognitive deficits [1].
In healthy individuals, the positive effects of background music on learning and memory recall are still controversial (known as the ‘Mozart effect’) [2], especially in young adults [3, 4]. Nevertheless, recent studies with older adults have added new evidence of the cognitive benefits of background music on learning and memory [5–7] (for reviews, see [8, 9]).
Considering the potential benefits of background music on cognition in healthy older adults, one compelling question becomes whether music might be used to boost the effects of the cognitive stimulation in people with age-related disorders.
This hypothesis has been explored very little in the literature and the benefits of music for patients’ cognitive functioning are often anecdotal [10]. The evidence to date comes primarily from studies with Alzheimer’s disease (AD) patients: the positive effects of background music have been shown in autobiographical memory [11, 12] and in lexical retrieval [13] within this clinical population. Nevertheless, in the clinical conditions preceding AD such as mild cognitive impairment (MCI), there is no current evidence speaking to the effect of background music on cognition. Musically induced cognitive benefits in this population could inform novel forms of cognitive intervention in the preclinical stages of dementia.
Addressing this, the present study aims to investigate whether music exposure may boost memory in people with amnestic MCI (aMCI). Patients with an amnestic form of MCI demonstrate a neuropsychological profile primarily characterized by episodic memory deficits. Building upon previous evidence of background music aiding specifically within the memory domain in healthy older adults [5–7], aMCI patients are an ideal clinical population for testing this study’s hypotheses.
Across three experiments, we investigated if background music is able to improve memory performance, the most impacted faculty in aMCI. In Experiment 1, we tested the effect of background music when played during an encoding phase. In Experiment 2, we expanded our scope to explore music-induced effects when background music is played during both encoding and recognition phases. In Experiment 3, we explored whether the effect of music on memory performance is mediated by musically induced arousal.
In the next section, we review the current evidence on the effects of music therapy and background music in aging and cognitive decline. In the last paragraph of the introduction, we detail the hypotheses and the expected results for each experiment.
Music therapy and background music in aging and cognitive decline
Music therapy is the clinical use of music aimed at treating cognitive and behavioral disorders. It may include a variety of therapeutic interventions based on listening, singing, playing instruments, or composing music. It has been widely used in aging and cognitive decline, but evidence of its efficacy is still controversial [1, 14]. The Cochrane review by Vink et al. [14] examined ten studies of patients with dementia undergoing music therapy; their review was inconclusive on the efficacy of music therapy because of the poor methodological quality in these studies. In a subsequent Cochrane review by van der Steen et al. [1], 22 studies were included. They concluded that at least five sessions of music-based intervention may produce benefits for depression and improve emotional being for individuals with dementia. However, both Cochrane reviews arrived at the conclusion that music therapy had little or no effect on cognition in this patient population. Conversely, a review by Moreno-Morales et al. [15], which included eight studies on patients with dementia, concluded that music-based therapies may improve cognition. Specifically, they found that shorter interventions (less than 20 weeks) and those based on listening to music improved general cognition outcomes in patients with mild to moderate dementia.
In MCI patients, strong conclusions on the positive effects of music-based interventions on cognition and behavioral symptoms cannot be drawn given the low quality of the methodology employed in the studies and the small effect sizes [16, 17]. Multitask movement music therapy seems to more beneficial for patients with MCI than single-training task as it has been shown to modulate the prefrontal cortex activity [18].
Finally, considering healthy older adults, a metanalysis conducted by Xu et al. [19] concluded that music-based therapies are moderately effective in improving cognitive outcomes, decreasing depressive symptomatology, and particularly effective for reducing disruptive behaviors. More robust effects for cognition were found for older adults who have had both short- and long-term musical training [20].
These reviews and metanalyses have analyzed data from music interventions conducted across multiple sessions and by comparing test scores pre- and post-intervention. An alternative way to investigate the effect of music on cognition is to use background music. In this condition, participants are passively listening to the music while they are performing a cognitive or behavioral task. A metanalysis by Kämpfe et al. [21] in older adults found that, based on a global analysis including 97 studies, there was no effect of background music on cognition. However, in a series of sub-analyses, these authors found different effects on other outcomes outside of cognition. Specifically, they found trending positive effect of background music on emotional experiences after music exposition and larger positive effects on sports performance. Additionally, one study by Reave et al. [22] reported negative effects of background music on associative memory. Older adults judged the background music played during the task as distracting and had poorer performed in recognizing previously learned face-name associations when exposed to music compared to silence.
Ferreri et al. [6] investigated the effect of background music on verbal learning (word lists) in older adults, finding it to be beneficial for the encoding. When older adults were exposed to background music, they committed less false alarms and showed less activation of the dorsolateral prefrontal cortex than in the silent condition. The authors interpreted the benefits of background music in memory as a product of creating a musical learning context with efficient strategies for binding items to one another in healthy older individuals. This interpretation fits with the associative deficit hypothesis, which proposes that older adults’ deficiencies in episodic memory are related to their inability to form and retrieve links among single bits of information (for a similar argument in patients, see [23]).
Zooming in to consider those older adults experiencing cognitive decline, the evidence of background music’s effects on cognition is limited [10]. Two studies have reported positive effects of background music on autobiographical memory [11, 12] and an another reported benefits in lexical retrieval [13] for patients with AD, but there is scarce evidence on episodic memory. In MCI patients, in addition to the therapeutic effects of music, research has focused mainly on musical memory (e.g., [24]) but not on the benefits of background music for episodic memory. Thus, our research focused on this cognitive domain since it is one of most frequently affected in patients with MCI.
How background music may improve memory
For the present study, we based our two main hypotheses on two theories that have been proposed to explain how background music influences memory performance: the ‘arousal-mood hypothesis’ and the ‘encoding specificity principle’ [4].
According to the ‘encoding specificity principle’, music could act as a contextual factor that cues the associated memory. This may function in two similar yet distinct ways. First, playing the same music during learning and testing phases improves memory because similar contextual cues assist memory retrieval (‘context-dependent memory’) [25–27]. Alternatively, memory may be significantly better when the same mood or arousal is experienced by participants at both encoding and retrieval (‘state-dependent memory’) and music can act as the contextual cue for these states [26, 28]. Supporting the overarching encoding specificity principle, it has been shown that participants have higher performance in memory recall if they are exposed to it during both encoding and recall that during encoding only [4].
The ‘arousal-mood hypothesis’ predicts that memory performance is modulated by background music by its ability to alter the listener’s mood and arousal states. Previous research has found that the emotional valence and the pleasantness of music are two factors that increase arousal and mood, and in turn are beneficial for improving cognitive performance when they are manipulated [29]. For instance, Thompson et al. [30] found that participants showed better performance in a spatial memory task when they were exposed to background music compared to when exposed to silence. However, all background music was not equal. Participants’ performance improved when the background music was considered pleasant (a Mozart sonata), but not if it was sad (an Albinoni adagio). Additionally, other evidence suggests that the combination of both mood and arousal is essential for improving memory performance. Greene et al. [31] manipulated these two dimensions orthogonally and found that the benefits of background music on memory were salient in positive mood-high arousal and negative mood-low arousal conditions, relative to positive-low arousal and negative-high arousal ones. Finally, Nguyen and Grahn [4] found that participants performed better on memory tasks when listening to low arousal music irrespective of valence, but only when music was presented at both encoding and recognition phases.
The ‘arousal-mood hypothesis’ is based on evidence that cognitive performance is influenced by mood and arousal in a U-shaped fashion, with better performance associated with intermediate levels of arousal and poorer performance with either low or very high levels of arousal (also known as ‘Yerkes-Dodson law’, [29, 32]). At neurophysiological level, the changes in arousal and mood induced by music are associated with specific patterns of activity over the left and right frontal areas [33]. Specifically, participants showed greater electroencephalographic activity on the left frontal areas for musical excerpts with positive valence and greater activity on the right frontal areas for those with negative valence.
Perk et al. [10] proposed that the positive effects of music on cognition in patients may be driven by the change of the basal physiological arousal and/or the reactivation of the dopaminergic system induced by the exposure to music. Thus, the combination of cognitive and neurophysiological changes induced by music might explain difference in memory as related to levels of arousal. Fernandez et al. [34] found that background music modulated the performance of the participants while they performed an attentional task. Music with positive valence and high arousal was associated with faster reaction times and greater activation of the attentional neural network (fronto-parietal areas). Conversely, music with negative valence modulated the activation of the occipital areas and negatively impacted the performance in the attentional task.
The present study
Based on the above theories of ‘context-dependent memory’ and ‘arousal-mood hypothesis’ [4], we designed three experiments to test the effect of background music on memory performance in older adults with MCI.
First, in Experiment 1 and 2, we investigated the effect of ‘context-dependent memory’, as one of two definitions of the ‘encoding specificity principle’. In Experiment 1, background music was played during encoding only, whereas in Experiment 2, background music was included during both encoding and retrieval phases. Within each experiment, we compared memory performance following exposure to music versus a silence condition. Because Experiment 2 included the same music cues during encoding and retrieval phases, we expected better memory performance following the background music condition compared to the silence condition. These expected results and hypothesis were based on the findings reported by Nguyen and Grahn [4] showing better memory recall when exposed to music during both encoding and retrieval phases. As these authors observed better memory performance with low arousal music, we used this type of background music in Experiment 1 and 2.
Second, in Experiment 3, we tested the ‘arousal-mood hypothesis’, which predicts that memory performance is modulated by background music altering the listener’s mood and arousal states. Previous studies in healthy individuals have found mixed results on whether arousal induced by background music facilitates memory consolidation, and what level of arousal is ideal [4, 31, 35]. We explored this by exposing individuals to background music that induced low or high arousal, based on the methodology employed by Nguyen and Grahn [4]. Given the results of this study, we expected better memory performance with exposure to low arousal music compared to high arousal music.
Finally, we tested the role of interindividual differences within music-induced benefits on memory, considering a) interindividual preferences toward music with the Barcelona Music Reward Questionnaire (BMRQ) [36], b) subjective feelings towards music excerpts used in the experiments, and c) individual mood changes over the course of music exposure. All these variables may potentially influence the magnitude of music’s benefits on memory processes. Indeed, musical preferences and mood changes are the two main variables that modulate the benefits of music for behavioral disorder interventions in patients with AD [37] and for cognitive benefits in healthy individuals [29].
METHODS
Participants
Three separate groups of MCI patients were recruited to participate in these three experiments (Exp. 1: n = 20; Exp. 2: n = 20; Exp. 3: n = 25). Each patient participated only in one of the three experiments. Experiments were conducted at three different time points in sequential order. Sample size was determined based on the results of study by Ferreri et al. [6] which showed an effect size of background music on memory of about 10%. A power analysis run referencing Ferreri et al. [6] suggested an adequate sample size of 18 participants, with a power of 80% and an alpha of 0.05.
Participants were recruited from the Neuropsychology Unit of the Hospital de la Santa Creu i Sant Pau in Barcelona. All were diagnosed with MCI according to the recommendations from the National Institute on Aging Alzheimer’s Association [38], meeting the following criteria: a) subjective or informant-based cognitive decline, b) objective cognitive decline of episodic memory as measured by a neuropsychological assessment, c) the cognitive decline is not interfering with individual independence, d) CDR score = 0 suggesting absence of dementia, and e) onset after the age of 65 (usually in the late 70 s or thereafter). Exclusion criteria consisted of the following: a) the presence of psychiatric and neurological disorders other than MCI, b) clinically known hearing or vision impairment, c) a history of alcohol abuse, and d) documented history of cerebrovascular risk that may be indicative of a mixed variant of cognitive impairment. Additionally, we excluded participants who were musicians or had professional training in music.
Material and procedure
The experimental session of the three experiments included: a) a neuropsychological assessment; b) questionnaires on mood, music use and music preferences; and c) two face recognition memory tasks.
a. Neuropsychological assessment. The neuropsychological assessment included the following tests: Mini-Mental State Examination [39], Rey’s Auditory Verbal Learning Test [40], Forward and backward digit spans [41], Block Design Test [42], Poppelreuter test [43], Boston Naming Test [44, 45], semantic and phonemic fluency tasks [46]. See Table 1 for the neuropsychological test scores.
Table 1
Sociodemographic characteristics and neuropsychological test scores
| Experiment 1 | Experiment 2 | Experiment 2 | p | ||||
| Sex (Female/Male) | 12/8 | 13/7 | 16/9 | 0.94 | |||
| Means | (SD) | Means | (SD) | Means | (SD) | p | |
| Age (y) | 76.5 | 7.2 | 79.4 | 5.3 | 76.8 | 4.3 | 0.10 |
| Education (y) | 10.2 | 3.0 | 9.5 | 4.1 | 12.0 | 5.7 | 0.21 |
| MMSE | 25.7 | 1.9 | 25.8 | 1.9 | 26.0 | 1.8 | 0.90 |
| RAVLT- Trial I | 3.3 | 0.9 | 3.1 | 1.8 | 3.4 | 1.1 | 0.72 |
| RAVLT- Trial V | 6.0 | 1.4 | 5.6 | 1.9 | 5.7 | 2.0 | 0.78 |
| RAVLT- Total | 25.0 | 3.7 | 24.6 | 3.1 | 22.9 | 7.4 | 0.31 |
| RAVLT- Delayed recall | 2.1 | 1.6 | 2.1 | 2.1 | 2.8 | 2.8 | 0.38 |
| Forward digit span | 4.7 | 0.8 | 4.8 | 0.8 | 4.5 | 0.8 | 0.36 |
| Backward digit span | 3.3 | 0.9 | 3.0 | 0.9 | 3.1 | 0.9 | 0.83 |
| BNT | 47.4 | 6.9 | 44.6 | 9.2 | 47.4 | 5.2 | 0.49 |
| Phonemic fluency | 10.4 | 4.9 | 10.7 | 4.2 | 11.8 | 3.7 | 0.47 |
| Semantic fluency | 12.4 | 4.5 | 11.4 | 3.9 | 12.9 | 4.0 | 0.44 |
| Poppelreuter test | 9.9 | 0.2 | 9.9 | 0.3 | 9.8 | 0.3 | 0.99 |
| Block Design Test | 26.2 | 9.5 | 23.2 | 8.7 | 27.7 | 8.2 | 0.31 |
MMSE, Mini-Mental State Examination; RAVLT, Rey’s Auditory Verbal Learning Test; BNT, Boston Naming Test.
b. Questionnaires for mood and music use and preferences. Two self-report questionnaires were used across all studies, the Scale for Mood Assessment (EVEA) and the BMRQ [36] (http://brainvitge.org/z_oldsite/bmrq.php). The EVEA consists of a self-rating of emotional state and feelings (16 items, 10-point scale) and was administered before and after the memory task [47]. The items yield ratings for four emotional states: anxiety, hostility, sadness-depression and happiness.
The BMRQ was administered between the encoding and recognition phases of the memory task to assess musical preferences. It consists of 20 items and the answers range from 1 to 5 (from completely disagree to completely agree). The items and scores are grouped into six factors: music seeking, emotion evocation, mood regulation, sensorimotor, social, music reward.
c. Face memory task
Visual stimuli. A total of 100 colored pictures of unfamiliar faces, half males and half females, all of middle-age and older people, were downloaded from electronic datasets ‘FACES’ (https://faces.mpdl.mpg.de/imeji/) [48] and processed in Adobe Photoshop. The pictures were edited in order to obtain the same resolution and dimensions for all the pictures, and to add a uniform gray background. Ten different lists of 24 stimuli each were created in order to counterbalance the presentation order of trials across participants.
Auditory stimuli. The selection of the musical pieces was based on the ratings provided by a group of volunteers different from those participants that took part in the experiments. They were asked to rate two aspects of musical excerpts on a 10-point scale: a) arousal (from ‘relaxing’ to ‘exciting’), and b) mood (from ‘sad’ to ‘happy’). Participants also reported whether the music excerpt was known or not and, if it was familiar, whether they were able to recall the title of the piece. We asked participants to rate 20 excerpts taken from popular and traditional music of the Spanish culture (mainly from the 1950 s to 1970 s). We also included one classical excerpt from ‘Adagio’ in D minor by Johann Sebastian Bach in the rating process, as this music genre was used in previous studies with background music [6, 49, 50].
Based on participants’ ratings, we selected two musical excerpts for our studies: a) ‘Adagio’ in D minor by Johann Sebastian Bach (https://www.youtube.com/watch?v=2x-OHljZzHQ) (medium-low arousal: M = 3.4; medium-low mood: M = 4.6), and b) instrumental version of ‘Un rayo de sol’ by Los Diablos (https://www.youtube.com/watch?v=IownknoPwDw) (high arousal: M = 8.2; high mood: M = 8.5). Although the original version of the ‘Un rayo de sol’ includes lyrics, we used an instrumental version of it to make it comparable to the ‘Adagio,’ which does not contain lyrics. The use of music with or without lyrics may have differential effects on cognition [51].
The selected samples were edited using Audacity software, matched on volume, and transformed into MP3 files [50]. The use of these musical excerpts in experimental conditions is explained below.
Task procedure. The memory task was the same across the three experiments and was administered using DMDX software [52]. There were two phases, the encoding and recognition trials (see Fig. 1). Each participant was tested in an individual session, as opposed to group testing.
Fig. 1
Memory task and background conditions of the three experiments.
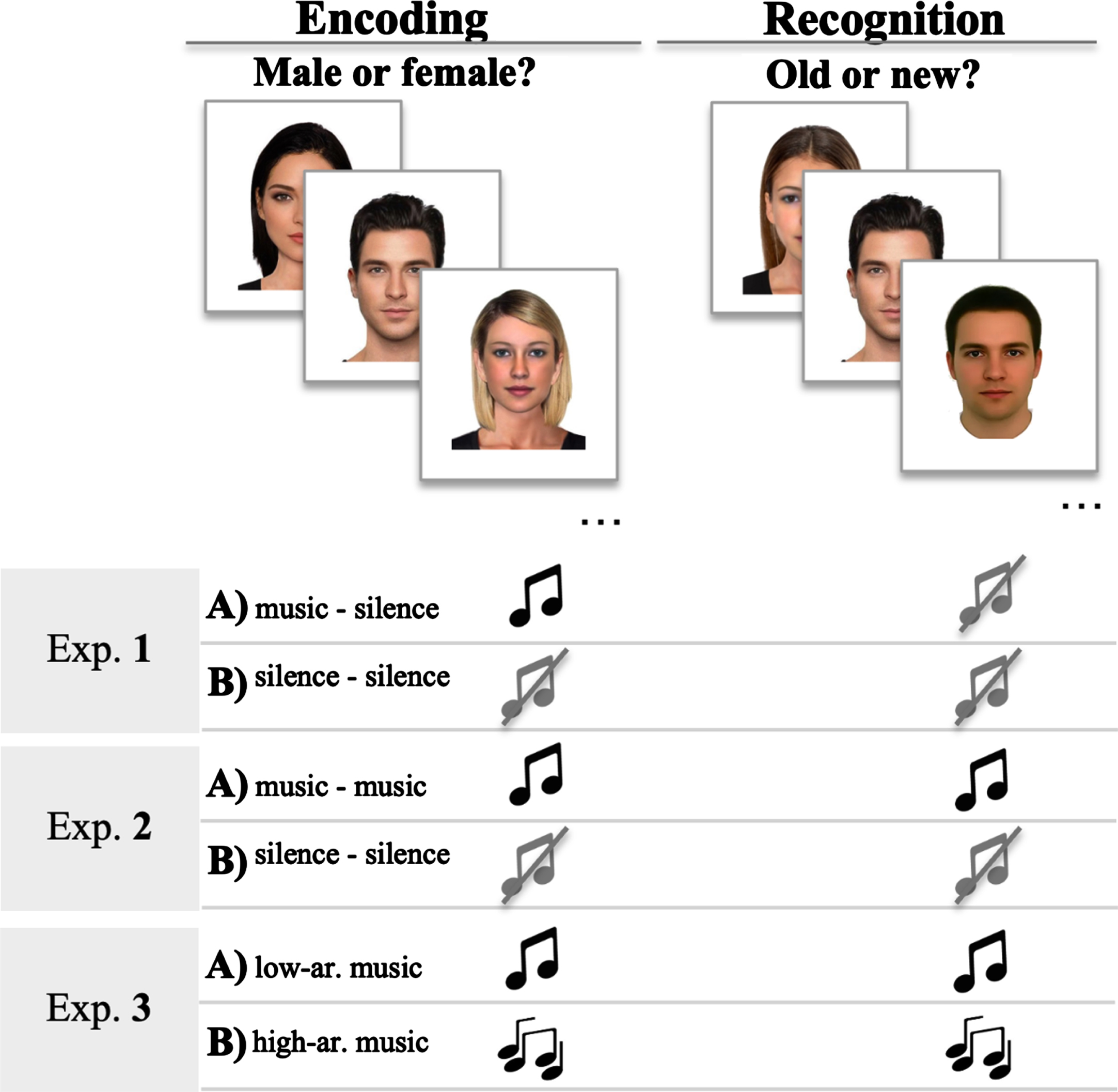
During the encoding phase, participants were presented with a series of 24 faces and were instructed to memorize them. Every trial started with a fixation point (a black cross) in the center of the screen displayed for 500 ms, followed by a picture for a maximum of 2500 ms. In order to promote task engagement, participants were instructed to indicate whether the faces were female or male by pressing one of two keys on the keyboard.
After a 10-min break, there was a recognition phase, where participants were presented with 24 faces that were previously presented and 24 faces that were new in random order. They were instructed to judge whether each face was ‘new’ (not presented during encoding) or ‘old’ (previously seen during encoding) by pressing one of two keys on the keyboard within a maximum of 2500 ms.
Experiments 1, 2, and 3. All experiments followed a within-subjects design, where participants performed the memory task twice over two distinct experimental conditions. Two sets of different pictures were used to avoid interference across conditions. The difference between experimental conditions was for the presence or absence of music (Experiments 1 and 2) or the use of two separate music excerpts inducing different levels of arousal (Experiment 3).
In Experiment 1, participants were exposed to ‘Adagio’ in D minor by Johann Sebastian Bach during the encoding phase in one condition (‘Music’) and they were not exposed to music in the other condition (‘Silence’).
In Experiment 2, participants were exposed to ‘Adagio’ in D minor by Johann Sebastian Bach during both encoding and recognition phases in one condition (‘Music’) and they were not exposed to music in the other condition (‘Silence’).
In Experiment 3, participants were exposed to ‘Adagio’ in D minor by Johann Sebastian Bach during the encoding phase of one condition (‘Low Arousal’) and they were exposed to ‘Un rayo de sol’ by Los Ladros in the other condition (‘High Arousal’). The order of presentation of the musical excerpts (i.e., Music-Silence versus Silence-Music) was balanced across participants.
The duration of the music excerpt was two minutes longer than the encoding phase of the Music conditions. The auditory presentation started after participants received task instructions, two minutes before the beginning of the experiment. This pre-task exposure was based on previous work determining that one to two minutes of music exposure is needed to elicit a change in mood and arousal [4].
The BMRQ [36] was administered between the encoding and recognition phases of the memory task. The EVEA [47] was completed before and after music exposure. For Experiments 1 and 2, EVEA was used for the Music condition and in Experiment 3 was employed in both Music conditions (Low and High Arousal). In Experiment 3, participants were also required to rate the music excerpts on three dimensions (happiness, arousal, pleasantness) on a 10-point scale (1 being low and 10 high in degree).
The procedures involving experiments were approved by the Ethics Committee of the Universitat Oberta de Catalunya for the proposal titled “The Mozart Effect on memory in patients with cognitive decline (MEM-COG)”.
Statistical analysis
Individual measures of sensitivity [d′= Z(Hits) - Z(False Alarms)] and response bias {c = - [z(Hits) + z(False Alarms)]/2} were calculated separately for each condition and experiment. Repeated measures ANOVA was performed to compare the d’ and c scores with condition (Experiment 1 and 2: Silence versus Music; Experiment 3: Low-Arousal Music versus High-Arousal Music) as a within-subject factor. The normality assumption was checked with the Shapiro-Wilk test and the results showed the data were normally distributed.
Mood changes after music exposition were analyzed by comparing the four subscale scores of the EVEA (sadness-depression, anxiety, anger-hostility, and happiness) with a repeated measures ANOVA, where time (pre versus post) served as a within-subject factor. We have applied the False Discovery Rate (FDR) with the Benjamini and Hochberg [53] procedure for multiple comparisons when necessary. This technique has the advantage of controlling for Type I errors and is applicable for both within- and between-subjects comparisons. The significance level is reported as a corrected p value.
The effect size was calculated as partial eta squared (
To analyze individual differences in the effect of music on memory, we ran a stepwise linear regression analysis with the d’ values as a dependent variable. For Experiments 1 and 2, the predictors were the subscale scores of the BMBQ (music seeking, emotion evocation, mood regulation, sensorimotor, social, and music reward) and the mood changes (sadness-depression, anxiety, anger-hostility, and happiness). For Experiment 3, the predictors were the subscale scores of the BMBQ, mood changes, and the music ratings for pleasantness, arousal, and happiness.
RESULTS
Experiment 1: background music at encoding versus silence
The d’ values were not significantly different between the Silence and Music conditions [F(1, 19) = 0.14, p = 0.51; Music: M = 0.82, SD = 0.81; Silence: M = 0.88, SD = 0.72]. Similarly, the c values were not significantly different between Silence and Music conditions in [F(1, 19) = 0.14, p = 0.71; Music: M = 0.005, SD = 0.05; Silence: M = 0.001, SD = 0.05].
Experiment 2: background music at both encoding and recognition versus silence
The d’ values were not significantly different between the Silence and Music conditions [F(1, 19) = 0.06, p = 0.81; Music: M = 0.73, SD = 0.51; Silence: M = 0.73, SD = 0.58]. Similarly, the c values were not significantly different between Silence and Music conditions [F(1, 19) = 0.005, p = 0.94; Music: M = –0.04, SD = 0.39; Silence: M = –0.04, SD = 0.45].
Experiment 3: Background music with high and low arousal
The d’ values were not significantly different between Low Arousal and High Arousal conditions [F(1, 24) = 1.30, p = 0.27; Low Arousal: M = 0.80, SD = 0.49; High Arousal: M = 0.69, SD = 0.56] (see Fig. 2 and Table 2). Similarly, the c values were not significantly different between Low- and High-Arousal conditions [F(1, 19) = 0.04, p = 0.85; Low Arousal: M = 0.14, SD = 0.44; High Arousal: M = 0.13, SD = 0.50].
Fig. 2
Memory performance (d’ values) for the three experiments separated by conditions (Experiments 1 and 2: Music versus Silence; Experiment 3: High-Arousal versus Low-Arousal Music).
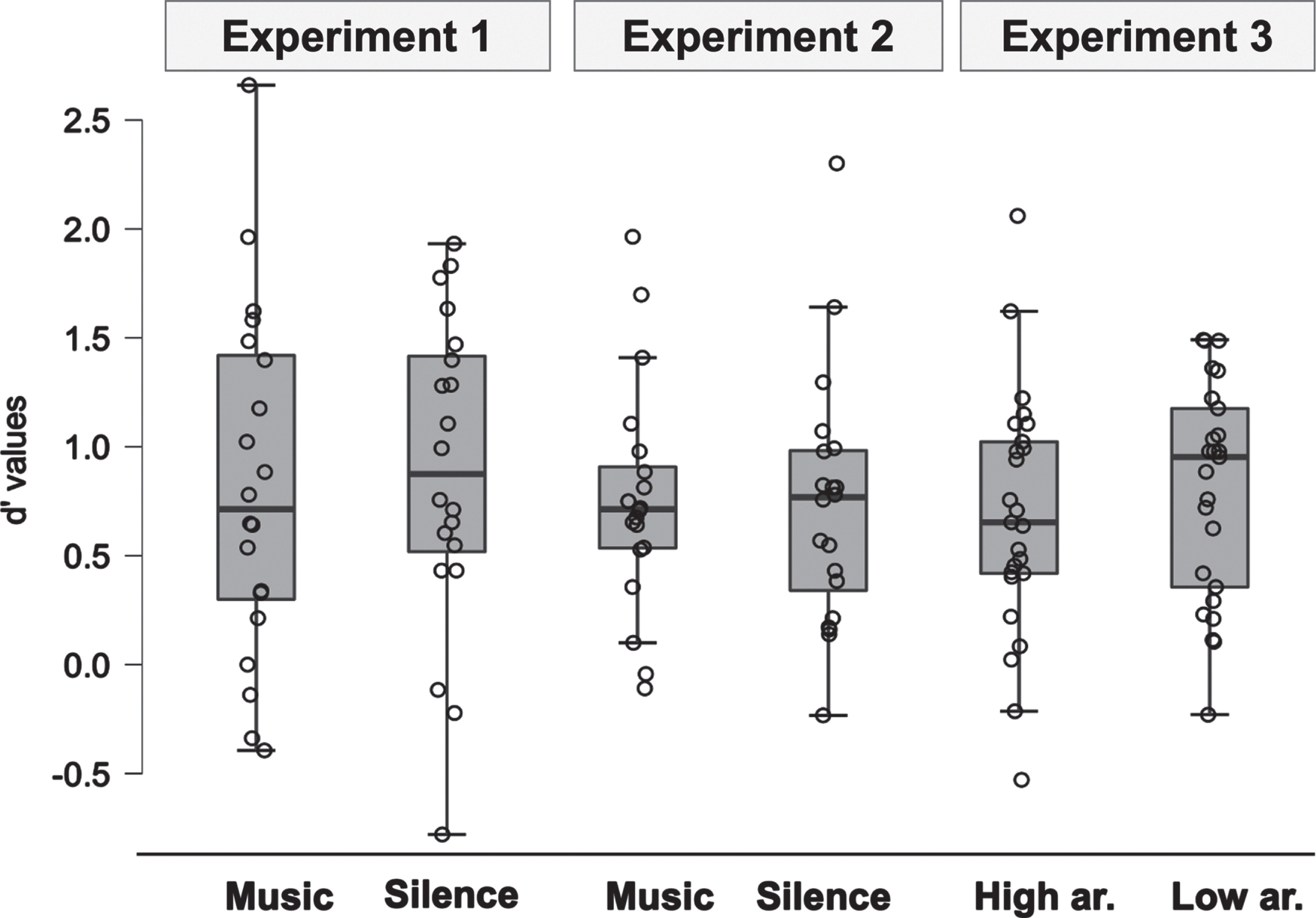
Table 2
ANOVAs results from memory performance (d’ and c values)
| D ’ values | ||||||
| Experiment 1 | ||||||
| Sum of Squares | df | Mean Square | F | p |
| |
| Music versus Silence | 1.806e-4 | 1 | 1.806e-4 | 0.14 | 0.71 | 0.007 |
| Residuals | 0.02 | 19 | 0.001 | |||
| Experiment 2 | ||||||
| Sum of Squares | df | Mean Square | F | p |
| |
| Music versus Silence | 1.806e-4 | 1 | 1.806e-4 | 0.14 | 0.71 | 0.007 |
| Residuals | 0.02 | 19 | 0.001 | |||
| Experiment 3 | ||||||
| Sum of Squares | df | Mean Square | F | p |
| |
| Low versus High arousal | 0.16 | 1 | 0.16 | 1.32 | 0.26 | 0.05 |
| Residuals | 0.88 | 24 | 0.12 | |||
| C values | ||||||
| Experiment 1 | ||||||
| Sum of Squares | df | Mean Square | F | p |
| |
| Music versus Silence | 1.806e-4 | 1 | 1.806e-4 | 0.14 | 0.71 | 0.007 |
| Residuals | 0.02 | 19 | 0.001 | |||
| Experiment 2 | ||||||
| Sum of Squares | df | Mean Square | F | p |
| |
| Music versus Silence | 2.970e-4 | 1 | 2.970e-4 | 0.005 | 0.94 | 2.651e-4 |
| Residuals | 1.12 | 19 | 0.06 | |||
| Experiment 3 | ||||||
| Sum of Squares | df | Mean Square | F | p |
| |
| Low versus High arousal | 0.005 | 1 | 0.005 | 0.04 | 0.85 | 0.004 |
| Residuals | 1.21 | 24 | 0.050 |
Background music and mood
In Experiment 1, there was no significant difference between pre- and post-music exposure for hostility [F(1, 19) = 0.55, p = 0.62], happiness [F(1, 19) = 0.02, p = 0.88]. The scores after music exposure was significantly lower for anxiety [F(1, 19) = 13.52, p = 0.008] and sadness-depression [F(1, 19) = 5.91, p = 0.04] as compared to pre-music assessment (see Fig. 3 and Table 3).
Fig. 3
Pre- and post-music exposure mood ratings across the three experiments.
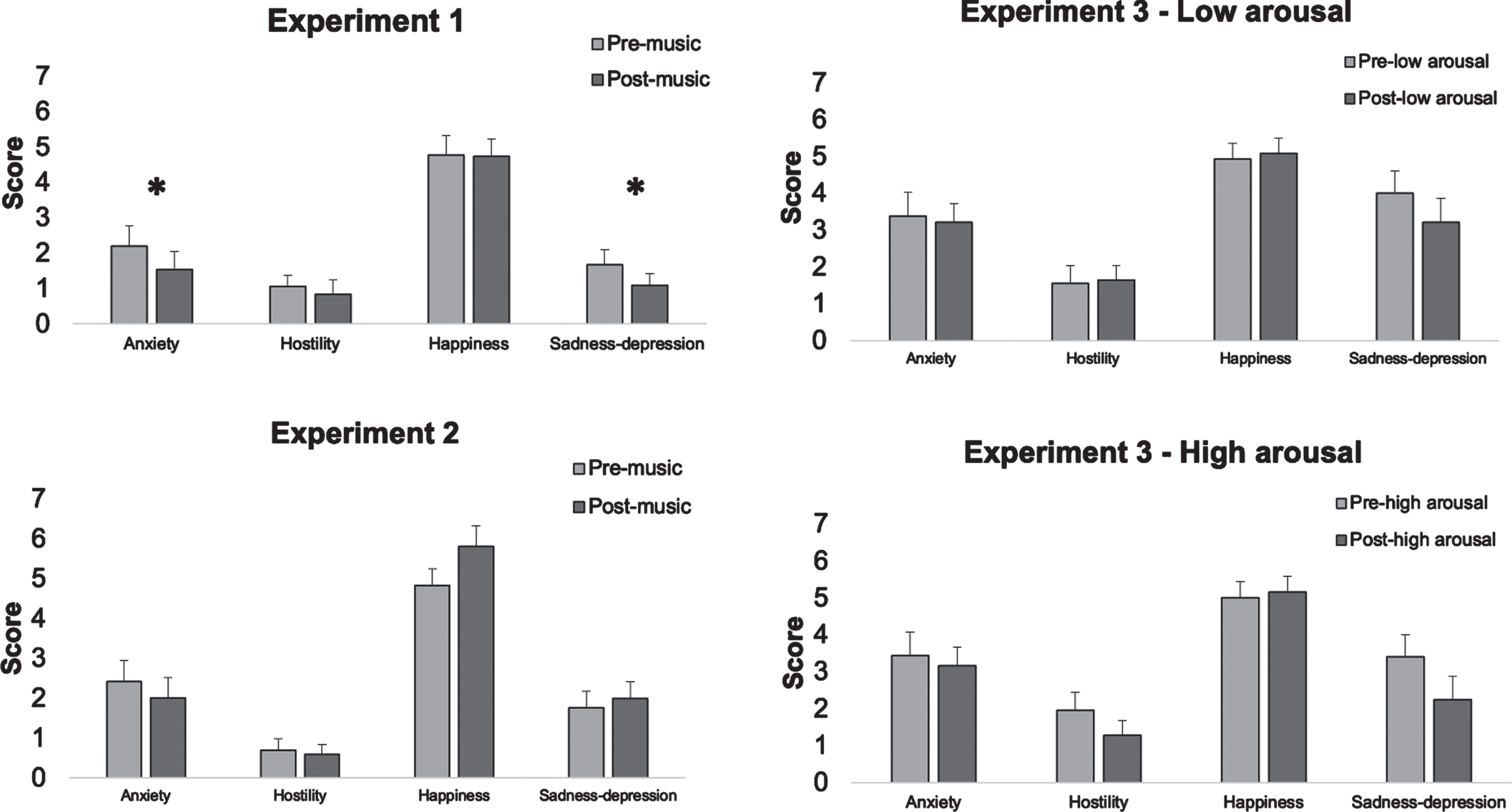
Table 3
Means and SD for the mood before and after music exposure
| Pre-music | Post-music | |||||
| Means | (SD) | Means | (SD) | p |
| |
| Experiment 1 | ||||||
| Hostility | 1.0 | 1.4 | 0.9 | 1.9 | 0.62 | 0.03 |
| Happiness | 4.8 | 2.5 | 4.7 | 2.2 | 0.88 | 0.001 |
| Anxiety | 2.2 | 2.5 | 1.5 | 2.3 | 0.008 | 0.41 |
| Sadness-depression | 1.7 | 1.9 | 1.1 | 1.5 | 0.04 | 0.24 |
| Experiment 2 | ||||||
| Hostility | 0.7 | 1.3 | 0.6 | 1.0 | 0.54 | 0.03 |
| Happiness | 4.8 | 2.0 | 5.8 | 2.3 | 0.16 | 0.21 |
| Anxiety | 2.4 | 2.4 | 2.0 | 2.3 | 0.52 | 0.07 |
| Sadness-depression | 1.8 | 2.0 | 2.0 | 1.9 | 0.54 | 0.02 |
| Experiment 3 | ||||||
| High-arousal | ||||||
| Hostility | 2.0 | 2.0 | 1.3 | 2.2 | 0.30 | 0.09 |
| Happiness | 5.0 | 2.6 | 5.2 | 2.8 | 0.81 | 0.001 |
| Anxiety | 3.4 | 2.7 | 3.2 | 2.6 | 0.79 | 0.01 |
| Sadness-depression | 3.4 | 2.8 | 2.2 | 2.7 | 0.16 | 0.17 |
| Low-arousal | ||||||
| Hostility | 1.5 | 2.4 | 1.6 | 2.0 | 0.99 | 0.001 |
| Happiness | 4.9 | 2.1 | 5.1 | 2.1 | 0.99 | 0.004 |
| Anxiety | 2.4 | 3.2 | 3.2 | 2.5 | 0.99 | 0.001 |
| Sadness-depression | 4.0 | 3.0 | 3.2 | 2.8 | 0.36 | 0.12 |
In Experiment 2, there was no significant difference between pre- and post-music exposure for hostility [F(1, 19) = 0.59, p = 0.54], happiness [F(1, 19) = 5.02, p = 0.16], anxiety [F(1, 19) = 1.37, p = 0.52], and sadness-depression [F(1, 19) = 0.39, p = 0.54].
In Experiment 3, the High-Arousal condition revealed no significant differences between pre- and post-music exposure for hostility [F(1, 24) = 2.21, p = 0.30], happiness [F(1, 24) = 0.06, p = 0.81], anxiety [F(1, 24) = 0.29, p = 0.79], and sadness-depression scores [F(1, 24) = 4.82,p = 0.16].
For the Low-Arousal condition, there was no significant difference between pre- and post-music exposure for hostility [F(1, 24) = 0.01, p = 0.99], happiness [F(1, 24) = 0.09, p = 0.99], anxiety [F(1, 24) = 0.12, p = 0.99], and sadness-depression [F(1, 24) = 3.05, p = 0.36].
Interindividual differences
In Experiment 1, we found that no variable significantly predicted the d’ scores for Music [F(10, 9) = 0.50, p = 0.83] and Silence conditions [F(10, 9) = 0.51, p = 0.84].
Likewise, in Experiment 2, we found that no variable significantly predicted the d’ scores for Music [F(10, 9) = 0.442, p = 0.90] and Silence conditions [F(10, 9) = 0.71, p = 0.69].
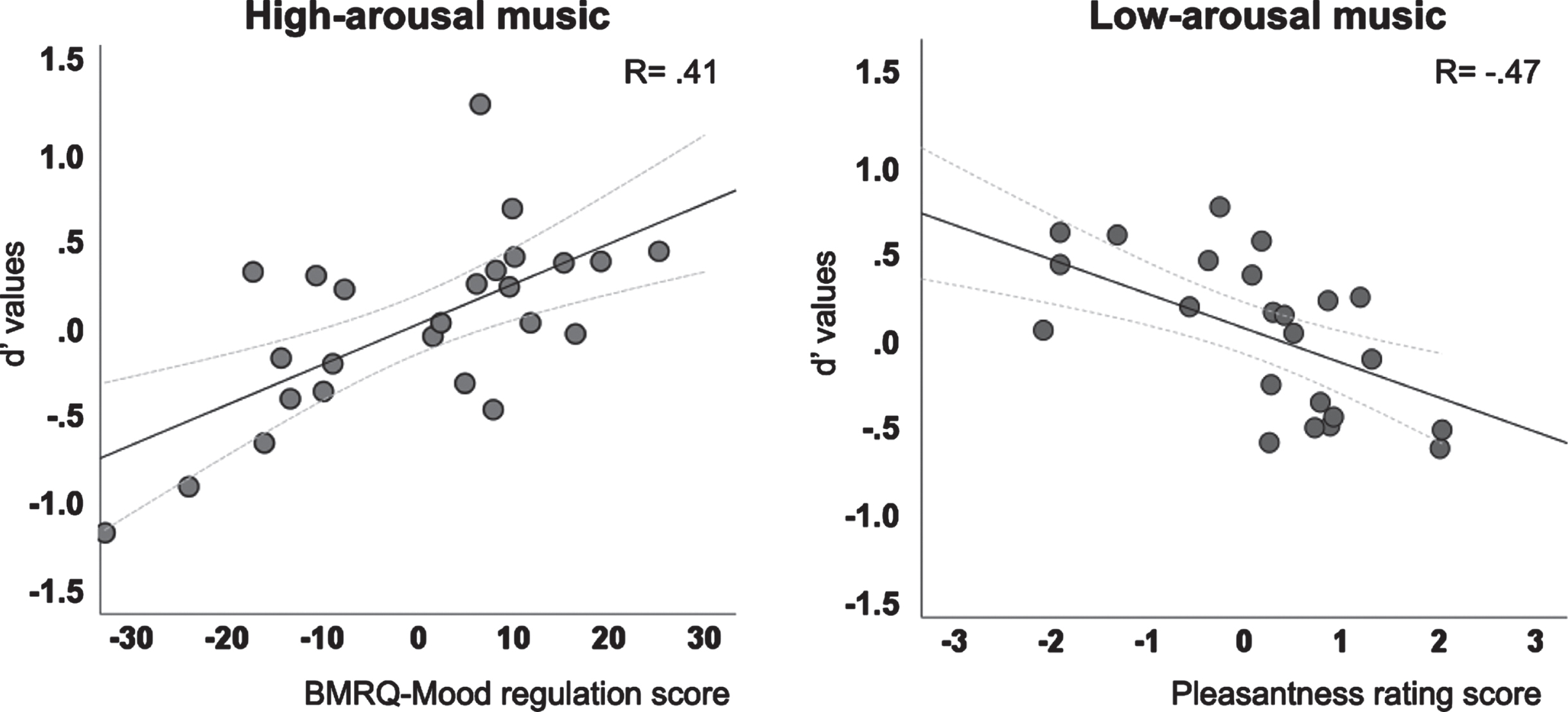
However, in Experiment 3, there were significant predictors of memory performance. For the Low-Arousal condition, pleasantness was a significant predictor of d’ values [B = –0.43; p = 0.02; F(1, 23) = 6.33, p = 0.02; R = 0.47], suggesting that lower scores of pleasantness were associated with better memory performance.
In contrast, for the High-Arousal condition, the Mood Regulation score of the BMRQ was a significant predictor of d’ values [B = 0.40; p = 0.04; F(1, 23) = 4.46, p = 0.04; R = 0.41], suggesting that the use of music as a strategy for mood regulation was associated with better memory performance (see Fig. 4).
Fig. 4
Partial regression plots for high-arousal music and BMRQ scores and for low-arousal music and pleasantness ratings scores.
DISCUSSION
In a series of experiments, we tested the effects of background music on memory in patients with amnestic MCI. In Experiments 1 and 2, participants were exposed to Music and Silence conditions, while in Experiment 3 they were exposed to Low- and High-Arousal Music conditions. Additionally, we collected data for mood changes after music exposure, individual experience toward music, and subjective ratings on the music excerpt played during the experiment.
The main finding from these three experiments was that background music played during a memory task did not improve or worsen participant performance.
In Experiments 1 and 2, we used low-arousal music since previous studies in healthy individuals showed that it has more probability to generate benefits on memory. For instance, Nguyen and Grahn [4] found that, despite background music having little influence on the memory performance of their young adult participants, mood and arousal in some conditions had some positive effects on memory recall. Specifically, they found that participants performed better when listening to low arousal music when music was presented at both encoding and the recognition phase. Greene et al. [31] found similar results for arousal and memory but through interaction with mood. They manipulated both dimensions orthogonally and their findings highlight that the benefits of music on memory were specific to exposure to positive mood-high arousal and negative mood-low arousal music types. Despite these previous findings, the low-arousal background music in our study did not enhance recognition memory performance. Likewise, the low-arousal music that we used was not optimal to boost memory consolidation. According to the Yerkes-Dodson law [29, 32] the cognitive performance is influenced by mood and arousal in a U-shaped fashion, with better performance at intermediate levels of arousal and poorer performance at low and very high level of arousal. Therefore, it is possible that our background music did not sufficiently increase arousal to reach an intermediate level that may be required to produce the best physiological conditions to consolidate new memories.
Across the first two experiments, we manipulated the exposure to music during encoding only (Experiment 1) and during both encoding and recognition phases (Experiment 2) to test the ‘context-dependent memory’ theory. According to this theory, playing the same music during encoding and recognition improves memory because similar contextual cues assist memory retrieval. For instance, if two different songs of the same genre are used as background music, memory recall is significantly reduced compared with when the same song is used [25] or tonality is kept constant [26]. As stated previously, ‘context-dependent memory’ is one component of a more general theory, the ‘encoding specificity principle’. A second aspect of this principle focuses on the effect of context for mood and arousal, known as ‘state-dependent memory’. According to this component of the theory, memory may be significantly better when the same mood or arousal induced by music is present at both encoding and retrieval. Hence, the music can act to elicit this same state in both phases [4, 26, 54]. Given that we did not observe any improvement of memory performance from Experiment 1 to 2, our results do not indicate a role for context-dependent memory in aiding memory retrieval. This is in line with the study by Murre [55], where he failed to replicate the classical context-dependent effects of the environment on memory.
In Experiment 3, we manipulated the arousal induced by the music. We included high-arousal music for this experiment since the results of the first two experiments suggested that low-arousal was not effective at inducing benefits in memory performance and other studies have reported that high-arousal music may boost performance in recognition memory tasks [31].
Participants rated the two music excerpts as having different degrees of arousal and the music that was rated as inducing high arousal was also more familiar to them (‘Un rayo de sol’). However, results in memory performance indicated a null effect for the level of arousal associated with the music, as found in Experiments 1 and 2. Similar to context-dependent memory, these findings do not provide evidence of state-dependent memory within this paradigm and clinical population.
In contrast to mood and arousal congruence at encoding and retrieval proposed by ‘state-dependent memory hypothesis’, the ‘arousal-mood hypothesis’ predicts that the effect of music on memory performance is modulated through altering the listener’s mood and arousal states. Indeed, Perk et al. [10] proposed that music may change the physiological arousal of an individual that is beneficial for attention in memory tasks.
To test this hypothesis, we collected self-ratings of mood before and after music exposure. The results showed that individuals experienced significant changes in sadness-depression and anxiety in Experiment 1. This suggests that background music may induce changes in mood for low arousal classical music. However, as shown by the findings of the regression analysis, mood was not a significant predictor of the memory performance. This leads us to conclude that background music may change an individual’s mood, but this change does not necessarily elicit a benefit in cognitive functioning, or at least not within learning and memory processes. Thus, our results do not completely support the ‘arousal-mood hypothesis’ for cognition, conflicting with previous studies [29, 56].
Despite our results suggesting that background music is not boosting memory consolidation and recognition at the group level, we found two results from Experiment 3 indicated that music benefits in memory are modulated by interindividual differences. For high-arousal music, memory performance was predicted by the Mood Regulation score of the BMRQ. Items within this subscore include experiencing music as relaxing and calming and feeling less alone when listening to music. Individuals who had higher scores on these items were more likely to demonstrate better performance when exposed to high-arousal music. Thus, the emotional aspects by which individuals experience music seem to be a crucial factor for inducing cognitive benefits. This is in line with the idea that the sensitivity for music reward and anhedonia is quite variable among individuals [36] and those who do not experience music as pleasant do not benefit from it [57]. Similarly, active engagement in music listening has been reported to be related to individual self-regulatory purposes for emotions and cognition [58].
For low-arousal music, we found that the rating scores for pleasantness negatively predicted performance on the memory task. This is quite surprising since we would expect, if anything, a positive correlation between these two variables. We propose two alternative explanations for this unexpected result. First, this music excerpt (‘Adagio’) might have shifted individuals’ focus during the task. That is, the participants who rated this excerpt as unpleasant could have “tuned out” the music and were more attentive to the task. Conversely, those who rated it as pleasant were possibly less attentive to the task. If this were the case, those who shifted their attention to task (by tuning out the music) would demonstrate better memory performance than those who were engaged in listening to the music. This interpretation is in line with the split-attention effect [59]. According to this effect, when learners have to acquire new information that requires the integration of different type of materials or modalities, there is an increase in cognitive load. It is known that cognitive load is increased by the difference of the temporal characteristics between the two modalities (auditory and visual) and/or by the complexity of the background music (e.g., number of musical instruments) [60]. Therefore, it is possible that participants tended to reduce the cognitive load by either focusing their attention on the task or listening to the background music.
As a second possible explanation, pleasantness ratings might be related to arousal. In the Low-Arousal condition, the rating scores for pleasantness and arousal were significantly correlated (r = 0.43, p = 0.03), where participants who rated the music as highly pleasant also rated it as highly arousing. Conversely, the correlation between pleasantness and arousal in the High-Arousal condition was not significant (r = 0.04, p = 0.85). High levels of arousal are known to disrupt hippocampal and prefrontal function, precluding memory binding [61]. Thus, although it was intended to elicit less arousal, high pleasantness ratings for the ‘Adagio’ piece may indicate that it modulated arousal among some individuals more than the music in the High-Arousal condition and ultimately disrupted memory consolidation for them in both conditions.
More broadly, interindividual differences are relevant for determining the benefits of background music in cognitive tasks. For instance, a review article by Küssner [62] discussed the evidence in favor and against the influence of extraversion in background music’s effect on cognition when comparing introverts and extraverts. Some studies have reported that introverts generally performed poorer with background music than extraverts on memory and comprehension tasks [63, 64], although this association was weak in other studies [62]. The role of extraversion in background music and cognition is based on the concept that personality traits are associated with different levels of cortical arousal, with extraverts demonstrating less arousal than introverts. Adopting this perspective, background music could be detrimental or beneficial in memory tasks as a function of the level of cortical arousal associated with interindividual traits such as extroversion.
Finally, we acknowledge that our study has some limitations. One limitation is having used a within-subject design for the Music conditions. While this is usually suggested as an optimal design because it allows researchers to compare the same individuals in different experimental conditions, this may not have been the case in our study. As music-induced effects on arousal take time, it is possible that effects of music persisted across conditions. A further limitation could be that the music excerpts were not effective at inducing a sufficient amount of change in arousal and mood to result in memory task improvement. Although mood was rated as better after music exposure, the magnitude of mood change might not have been large enough to improve memory performance. These limitations may have contributed to the lack of replicating previous findings of background music benefits in cognition within older adults [5–7]. Finally, we have to acknowledge that the ratings of music excerpts on arousal, pleasantness, and happiness on the same scale might be problematic. Arousal and pleasantness come from a dimensional approach, whereas happiness can be conceptualized as a basic emotional approach. Therefore, mixing these two approaches may not have been the optimal method to measure these dimensions in our participants. Finally, we have to acknowledge that the experimenters were aware of the general purpose of the study, despite they were not about the specific hypothesis of each experiment.
In conclusion, our results suggest that the benefits of background music on memory are modulated by interindividual preferences towards music within older adults with MCI. Specifically, an individual’s use of music as an emotional regulator predicts their performance in recognition memory. Building upon this finding, further research is needed to continue to explore the role of interindividual preferences and attitudes toward music in patients with MCI. Moreover, it would be useful to extend the effects of background music to procedural memory and other learning contexts. The more we know about how background music shapes cognitive processes, the better music can be used as a therapeutic tool in cognitive stimulation efforts.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This study is supported by the grant ‘The Mozart Effect on memory in patients with cognitive decline’ (reference: PID2020-118672RB-I00) funded by MCIN/AEI/ 10.13039/501100011033.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author (Marco Calabria, [email protected], Faculty of Health Sciences, Universitat Oberta de Catalunya, Barcelona, Spain).
REFERENCES
[1] | van der Steen JT , Smaling HJA , van der Wouden JC , Bruinsma MS , Scholten RJPM , Vink AC ((2018) ) Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev 2018: , CD003477. |
[2] | Giannouli V , Kolev V , Yordanova J ((2019) ) Is there a specific Vivaldi effect on verbal memory functions? Evidence from listening to music in younger and older adults. Psychol Music 47: , 325–341. |
[3] | de la Mora Velasco E , Hirumi A ((2020) ) The effects of background music on learning: A systematic review of literature to guide future research and practice. Educ Technol Res Dev 68: , 2817–2837. |
[4] | Nguyen T , Grahn JA ((2017) ) Mind your music: The effects of music-induced mood and arousal across different memory tasks. Psychomusicology Music Mind Brain 27: , 81–94. |
[5] | Bottiroli S , Rosi A , Russo R , Vecchi T , Cavallini E ((2014) ) The cognitive effects of listening to background music on older adults: Processing speed improves with upbeat music, while memory seems to benefit from both upbeat and downbeat music. Front Aging Neurosci 6: , 284. |
[6] | Ferreri L , Bigand E , Perrey S , Muthalib M , Bard P , Bugaiska A ((2014) ) Less effort, better results: How does music act on prefrontal cortex in older adults during verbal encoding? an fNIRS study. Front Hum Neurosci 8: , 301. |
[7] | Mammarella N , Fairfield B , Cornoldi C ((2007) ) Does music enhance cognitive performance in healthy older adults? The Vivaldi effect. Aging Clin Exp Res 19: , 394–399. |
[8] | Fang R , Ye S , Huangfu J , Calimag DP ((2017) ) Music therapy is a potential intervention for cognition of Alzheimer’s disease: A mini-review. Transl Neurodegener 6: , 2. |
[9] | Ferreri L , Moussard A , Bigand E , Tillmann B ((2019) ) Music and the aging brain. In The Oxford Handbook of Music and the Brain pp.622–644. |
[10] | Peck KJ , Girard TA , Russo FA , Fiocco AJ ((2016) ) Music and memory in Alzheimer’s disease and the potential underlying mechanisms. J Alzheimers Dis 51: , 949–959. |
[11] | Irish M , Cunningham CJ , Walsh JB , Coakley D , Lawlor BA , Robertson IH , Coen RF ((2006) ) Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer’s disease. Dement Geriatr Cogn Disord 22: , 108–120. |
[12] | El Haj M , Postal V , Allain P ((2012) ) Music enhances autobiographical memory in mild Alzheimer’s disease. Educ Gerontol 38: , 30–41. |
[13] | Thompson RG , Moulin CJA , Hayre S , Jones RW ((2005) ) Music enhances category fluency in healthy older adults and Alzheimer’s disease patients. Exp Aging Res 31: , 91–99. |
[14] | Vink AC , Bruinsma MS , Scholten RJ ((2003) ) Music therapy for people with dementia. Cochrane Database Syst Rev CD003477. |
[15] | Moreno-Morales C , Calero R , Moreno-Morales P , Pintado C ((2020) ) Music therapy in the treatment of dementia: A systematic review and meta-analysis. Front Med (Lausanne) 7: , 160. |
[16] | Jordan C , Lawlor B , Loughrey D ((2022) ) A systematic review of music interventions for the cognitive and behavioural symptoms of mild cognitive impairment (non-dementia). J Psychiatr Res 151: , 382–390. |
[17] | Dorris JL , Neely S , Terhorst L , VonVille HM , Rodakowski J ((2021) ) Effects of music participation for mild cognitive impairment and dementia: A systematic review and meta-analysis. J Am Geriatr Soc 69: , 2659–2667. |
[18] | Shimizu N , Umemura T , Matsunaga M , Hirai T ((2018) ) Effects of movement music therapy with a percussion instrument on physical and frontal lobe function in older adults with mild cognitive impairment: A randomized controlled trial. Aging Ment Health 22: , 1614–1626. |
[19] | Xu B , Sui Y , Zhu C , Yang X , Zhou J , Li L , Ren L , Wang X ((2017) ) Music intervention on cognitive dysfunction in healthy older adults: A systematic review and meta-analysis. Neurol Sci 38: , 983–992. |
[20] | Román-Caballero R , Arnedo M , Triviño M , Lupiáñez J ((2018) ) Musical practice as an enhancer of cognitive function in healthy aging - A systematic review and meta-analysis, PLoS One 13: , e0207957. |
[21] | Kämpfe J , Sedlmeier P , Renkewitz F ((2011) ) The impact of background music on adult listeners: A meta-analysis. Psychol Music 39: , 424–448. |
[22] | Reaves S , Graham B , Grahn J , Rabannifard P , Duarte A ((2016) ) Turn off the music! Music impairs visual associative memory performance in older adults. Gerontologist 56: , 569–577. |
[23] | El Haj M , Kessels RPC ((2013) ) Context memory in Alzheimer’s disease. Dement Geriatr Cogn Dis Extra 3: , 342–350. |
[24] | Kerer M , Marksteiner J , Hinterhuber H , Mazzola G , Kemmler G , Bliem HR , Weiss EM ((2013) ) Explicit (semantic) memory for music in patients with mild cognitive impairment and early-stage Alzheimer’s disease. Exp Aging Res 39: , 536–564. |
[25] | Standing LG , Bobbitt KE , Boisvert KL , Dayholos KN , Gagnon AM ((2008) ) People, clothing, music, and arousal as contextual retrieval cues in verbal memory. Percept Mot Skills 107: , 523–534. |
[26] | Mead KML , Ball LJ ((2007) ) Music tonality and context-dependent recall: The influence of key change and mood mediation. Eur J Cogn Psychol 19: , 59–79. |
[27] | Mishra J ((2012) ) Context-dependent memory: Implications for musical performance. Updat Appl Res Music Educ 20: , 27–31. |
[28] | Balch WR , Bowman K , Mohler LA ((1992) ) Music-dependent memory in immediate and delayed word recall. Mem Cognit 20: , 21–28. |
[29] | Husain G , Thompson WF , Schellenberg EG ((2002) ) Effects of musical tempo and mode on arousal, mood, and spatial abilities. Music Percept 20: , 151–171. |
[30] | Thompson A , Aguero A , Lany J ((2020) ) Statistical learning mechanisms in infancy. In Neural Circuit and Cognitive Development, Academic Press, pp. 319–333. |
[31] | Greene CM , Bahri P , Soto D ((2010) ) Interplay between affect and arousal in recognition memory. PLoS One 5: , e11739. |
[32] | Sarason IG ((1980) ) Test Anxiety: Theory, Research, and Applications. Lawrence Erlbaum Associates, Hillsdale, NJ. |
[33] | Schmidt LA , Trainor LJ ((2001) ) Frontal brain electrical activity (EEG) distinguishes valence and intensity of musical emotions. Cogn Emot 15: , 487–500. |
[34] | Fernandez NB , Trost WJ , Vuilleumier P ((2020) ) Brain networks mediating the influence of background music on selective attention. Soc Cogn Affect Neurosci 14: , 1441–1452. |
[35] | Thompson WF , Schellenberg EG , Husain G ((2001) ) Arousal, mood, and the Mozart effect. Psychol Sci 12: , 248–251. |
[36] | Mas-Herrero E , Marco-Pallares J , Lorenzo-Seva U , Zatorre RJ , Rodriguez-Fornells A ((2013) ) Individual differences in music reward experiences. Music Percept 31: , 118–138. |
[37] | Garrido S , Stevens CJ , Chang E , Dunne L , Perz J ((2018) ) Music and dementia: Individual differences in response to personalized playlists. J Alzheimers Dis 64: , 933–941. |
[38] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[39] | Folstein MF , Folstein SE , McHugh PR ((1975) ) Mini-mental state: A practical method for grading the mental state of patients for clinicians. J Psychiatr Res 12: , 189–198. |
[40] | Rey A (1958) L’ examen clinique en psychologie. [The clinical examination in psychology.]. |
[41] | Peña-Casanova J , Quiñones-Úbeda S , Quintana-Aparicio M , Aguilar M , Badenes D , Molinuevo JL , Torner L , Robles A , Barquero MS , Villanueva C , Antúnez C , Martínez-Parra C , Frank-García A , Sanz A , Fernández M , Alfonso V , Sol JM , Blesa R , Team for the NS ((2009) ) Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Verbal Span, Visuospatial Span, Letter and Number Sequencing, Trail Making Test, and Symbol Digit Modalities Test. Arch Clin Neuropsychol 24: , 321–341. |
[42] | Wechsler D (2012) The Wechsler Adult Intelligence Scale (Spanish version). University of Barcelona. |
[43] | Poppeireuter W ((1917) ) Die Psychischen Schaedungendurch Kopfschussin Kriege, pp. 1914–1916. |
[44] | Kaplan EF , Goodglass H , Weintraub S ((2001) ) The Boston Naming Test (ed. 2). Lea Febiger, Philadelphia. |
[45] | Peña-Casanova J , Quiñones-Úbeda S , Gramunt-Fombuena N , Aguilar M , Casas L , Molinuevo JL , Robles A , Rodríguez D , Barquero MS , Antúnez C , Martínez-Parra C , Frank-García A , Fernández M , Molano A , Alfonso V , Sol JM , Blesa R ((2009) ) Spanish multicenter normative studies (NEURONORMA project): Norms for boston naming test and token test. Arch Clin Neuropsychol 24: , 343–354. |
[46] | Casals-Coll M , Sánchez-Benavides G , Quintana M , Manero RM , Rognoni T , Calvo L , Palomo R , Aranciva F , Tamayo F , Peña-Casanova J ((2013) ) Spanish normative studies in young adults (NEURONORMA young adults project): Norms for verbal fluency tests. Neurologia 28: , 33–40. |
[47] | Sanz J ((2001) ) An instrument to evaluate the efficacy of mood induction procedures: The Scale for Mood Assessment. Análisis y Modif Conduct 27: , 71–110. |
[48] | Ebner NC , Riediger M , Lindenberger U ((2010) ) FACES-a database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav Res Methods 42: , 351–362. |
[49] | Ferreri L , Aucouturier JJ , Muthalib M , Bigand E , Bugaiska A ((2013) ) Music improves verbal memory encoding while decreasing prefrontal cortex activity: An fNIRS study. Front Hum Neurosci 7: , 779. |
[50] | Proverbio CAAM , Nasi VL , Arcari LA , De Benedetto F , Guardamagna M , Gazzola M , Zani A ((2015) ) The effect of background music on episodic memory and autonomic responses: Listening to emotionally touching music enhances facial memory capacity. Sci Rep 5: , 15219. |
[51] | de Groot AMB , Smedinga HE ((2014) ) Let The Music Play!: A short-term but no long-term detrimental effect of vocal background music with familiar language lyrics on foreign language vocabulary learning. Stud Second Lang Acquis 36: , 681–707. |
[52] | Forster KI , Forster JC ((2003) ) DMDX: A Windows display program with millisecond accuracy. Behav Res Methods Instruments Comput 35: , 116–124. |
[53] | Benjamini Y , Hochberg Y ((1995) ) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: , 289–300. |
[54] | Balch WR , Myers DM , Papotto C ((1999) ) Dimensions of mood in mood-dependent memory. J Exp Psychol Learn Mem Cogn 25: , 70–83. |
[55] | Murre JMJ ((2021) ) The Godden and Baddeley (1975) experiment on context-dependent memory on land and underwater: A replication. R Soc Open Sci 8: , 200724. |
[56] | He WJ , Wong WC , Hui ANN ((2017) ) Emotional reactions mediate the effect of music listening on creative thinking: Perspective of the arousal-and-mood hypothesis. Front Psychol 8: , 1680. |
[57] | Zatorre RJ ((2015) ) Musical pleasure and reward: Mechanisms and dysfunction. Ann N Y Acad Sci 1337: , 202–211. |
[58] | Chin TC , Rickard NS ((2012) ) The music USE (MUSE) questionnaire: An instrument to measure engagement in music. Music Percept 29: , 429–446. |
[59] | Mayer RE , Moreno R ((1998) ) A split-attention effect in multimedia learning: Evidence for dual processing systems in working memory. J Educ Psychol 90: , 312–320. |
[60] | de la Mora Velasco E , Hirumi A , Chen B ((2021) ) Improving instructional videos with background music and sound effects: A design-based research approach. J Form Des Learn 5: , 1–15. |
[61] | Mather M ((2007) ) Emotional arousal and memory binding: An object-based framework. Perspect Psychol Sci 2: , 33–53. |
[62] | Küssner MB ((2017) ) Eysenck’s theory of personality and the role of background music in cognitive task performance: A mini-review of conflicting findings and a new perspective. Front Psychol 8: , 1991. |
[63] | Cassidy G , Macdonald RAR ((2007) ) The effect of background music and background noise on the task performance of introverts and extraverts. Psychol Music 35: , 517–537. |
[64] | Dobbs S , Furnham A , Mcclelland A ((2011) ) The effect of background music and noise on the cognitive test performance of introverts and extraverts. Appl Cogn Psychol 25: , 307–313. |




