Costs During the Last Five Years of Life for Patients with Clinical and Pathological Confirmed Diagnosis of Lewy Body Dementia and Alzheimer’s Disease
Abstract
Background:
Little is known regarding healthcare expenditures for patients with dementia with Lewy bodies (DLB) during the end of life.
Objective:
This study estimated Medicare expenditures during the last 5 years of life in a decedent sample of patients who were clinically diagnosed with Alzheimer’s disease (AD) or DLB and had autopsy confirmed diagnosis.
Methods:
The study included 58 participants clinically diagnosed with mild dementia at study entry (AD: n = 44, DLB: n = 14) and also had autopsy-confirmed diagnoses of pure AD (n = 32), mixed AD+Lewy body (LB) (n = 5), or pure LB (n = 11). Total Medicare expenditures were compared by clinical and pathology confirmed diagnosis, adjusting for sex, age at death, and patient’s cognition, function, comorbidities, and psychiatric and extrapyramidal symptoms.
Results:
When pathology diagnoses were not considered, predicted annualized total Medicare expenditures during the last 5 years of life were similar between clinically diagnosed AD ($7,465±1,098) and DLB ($7,783±1,803). When clinical diagnoses were not considered, predicted expenditures were substantially higher in patients with pathology confirmed mixed AD+LB ($12,005±2,455) than either pure AD ($6,173±941) or pure LB ($4,629±1,968) cases. Considering clinical and pathology diagnosis together, expenditures for patients with clinical DLB and pathology mixed AD+LB ($23,592±3,679) dwarfed other groups.
Conclusion:
Medicare expenditures during the last 5 years of life were substantially higher in patients with mixed AD+LB pathology compared to those with pure-AD and pure-LB pathologies, particularly in those clinically diagnosed with DLB. Results highlight the importance of having both clinical and pathology diagnoses in examining healthcare costs.
1INTRODUCTION
Dementia with Lewy bodies (DLB) is a neurodegenerative disorder reported as being the second most common dementia subtype in older people following Alzheimer’s disease (AD) [1, 2]. Clinically, DLB is characterized by dementia with fluctuating cognition with deficits in the extrapyramidal motor system, hallucinations or other psychiatric symptoms, REM sleep behavior disorder, and autonomic dysfunction with syncope and falls [3]. Most [4–15], though not all [16–18], studies have reported that patients with DLB have worse outcomes than patients with AD, including more rapid cognitive and functional decline, increased risk of institutionalization, greater risk of falls and fractures, and shorter survival.
As the second most common form of dementia following AD, our understanding of healthcare utilization and costs in DLB continues to be limited. Several, though not all studies have reported a higher estimated cost of care associated with DLB compared to AD [19–26]. Part of these inconsistencies may be due to changes over time in clinical diagnosis guidelines [27]. Diagnostic uncertainties, missed diagnosis, delay in diagnosis, and misdiagnosis are common and also may have hampered these estimates [28–30].
Pathological confirmation is a gold standard of disease diagnosis. Approximately 50% of patients with Lewy body (LB) pathology at postmortem examination did not have characteristic clinical profile of DLB during life but had high levels of AD neuropathological change [31]. Similarly, between one third and one half of cases clinically diagnosed with AD show some degree of LB pathology at autopsy [31]. Whether clinical diagnosis and pathology-confirmed diagnosis are associated with healthcare costs has yet to be examined.
A recent study documenting end of life (EOL) experiences of individuals with DLB and their families reported several DLB-specific barriers to quality EOL care, including diagnostic challenges, lack of knowledge regarding DLB and resultant prescribing errors, and difficulty accessing resources due to behavioral changes in DLB [32]. Little is known regarding EOL expenditures for DLB patients. To fill some of these gaps, we explore in the current study healthcare expenditures in the last 5 years of life in patients clinically diagnosed with AD and DLB who have pathology confirmed diagnosis.
2METHODS
2.1Study participants and sample selection
The participants of the current study were from the Predictors 2 study, a cohort of patients who were clinically diagnosed with mild dementia-predominantly AD but also DLB [33]. Recruitment of this cohort was initiated in 1997 at three sites: Columbia University, Johns Hopkins University, and Massachusetts General Hospital. Participants were then followed up every 6 months with repeated clinical measurements including medical, neuropsychological, functional, and dependence measures. AD was clinically diagnosed according to NINCDS-ADRDA criteria (n = 221) [34] and DLB was diagnosed according to the 1996 Consensus Guidelines (n = 28) [35]. 78 patients donated brains and have pathological data available. Of these 78, 62 participants had autopsy-confirmed diagnosis of AD (n = 34), mixed AD+LB (n = 17), or LB (n = 11). Sixteen participants who did not have α-synuclein immunohistochemistry staining to confirm the presence of Lewy body disease/synucleinopathy, seven participants who did not have pathological features required for AD or DLB diagnosis, and one participant who had no follow-up visits to assess clinical trajectory were excluded from the current analysis. Pathological categorization for each case into AD, DLB, or AD-DLB was based on review of neuropathologic reports and slides if necessary and staging of AD and Lewy body pathology as outlined in the National Institute on Aging-Alzheimer’s Association (NIA-AA) pathologic assessment of AD and Lewy body disease [36]. Details of clinical and pathological diagnosis procedures were reported previously [15]. We further excluded four participants who did not have Medicare claims. Differences in participants’ age, sex, education, and clinical symptoms at study enrollment between excluded and included samples were not statistically significantly different. The project was approved by the Institutional Review Board at each study site. All patients and their proxy decision makers provided written informed consent.
2.2Medicare claims
Individuals were matched to Medicare Beneficiary Summary files using social security number and Medicare beneficiary ID. Medicare expenditures data were obtained from Medicare Standard Analytic Files (SAFs) and included all covered services (inpatient, outpatient, professional, emergency department, physician office visits, hospital outpatient visits, hospice, skilled nursing facility, home health, and durable medical supplies) in 6-month intervals from date of death to 5 years prior to death. Total expenditures in the last 5 years of life and average annual expenditures were summed. Expenditures were adjusted to 2021$ using the medical care component of the Consumer Price Index [37].
2.3Patient characteristics
Participants underwent detailed cognitive and clinical assessment at baseline and at approximately 6-month intervals until drop out or death. Global cognitive status was assessed with the Folstein Mini-Mental State Examination (MMSE) (0–30, a higher score indicating better cognitive performance). Functional capacity was reported by the patient’s reliable informant using the Blessed Dementia Rating Scale (BDRS) Activities of Daily Living (ADL) sub-score [38], including seven instrumental ADL (IADL) items and three basic ADL items. Total ADL score was the sum of scores on all 10 items (range: 0–16), with higher scores indicating worse functional capacity. The ADL scale has good reliability and validity, with reliability coefficients reported to be between 0.60 and 0.80 [38]. Columbia University Scale for Psychopathology in Alzheimer’s Disease (CUSPAD) was used to measure patients’ psychotic, behavioral, and depressive symptoms [39]. The Unified Parkinson’s Disease Rating Scale (UPDRS) [40] was used to measure extrapyramidal signs (EPS) and treated as a binary variable with 1 indicating severity rating of mild-to-moderate or greater on any item [41]. Patients’ age at death, sex, and highest level of education were recorded. An Elixhauser comorbidities index was constructed using all ICD-9-CM diagnosis codes in all Medicare SAFs for the last 5 years of life [42, 43].
2.4Statistical analyses
Demographic and clinical characteristics were summarized by mean and standard deviation (SD) for continuous variables and by frequency and proportions for categorical variables. The measures were compared among clinical diagnosis and autopsy-confirmed diagnosis groups using Kruskal-Wallis test for continuous variables and Chi-square test for categorical variables.
We estimated the association between average Medicare costs per year during the last 5 years of life and clinical and pathological diagnosis using generalized linear models (GLM). The main independent variables were the clinical diagnosis (AD versus DLB), confirmed pathology (pure AD, pure LB, and mixed AD+LB), and their interaction terms. We also estimated models adjusted for 1) demographics including age at death, sex, number of comorbidities, 2) demographics, MMSE, and BDRS 5 years prior to death, and 3) demographics, MMSE, BDRS, and extrapyramidal signs and psychotic symptoms 5 years prior to death. The Modified Park test (i.e., GLM family test), was used to identify the appropriate distribution, and the Pregibon Link test used to examine linearity of response on scale of estimation. The Modified Park test suggested that the normal (Gaussian) distribution with an identity link (i.e. ordinary linear regression models) function provided the best fit for the data.
All analyses were conducted using Stata 16.0. Due to the exploratory nature of this analysis, statistical significance level was defined as p < 0.05 a priori without corrections for multiple comparisons.
3RESULTS
3.1Patient characteristics
Half of the participants in the current study were female. Age at death was 80.2±8.8 (mean±SD) years, and years of education was 15±2.9 (Table 2). Five years prior to death, participants had an average of 2±1.9 comorbid conditions, average MMSE was 18.3±6.4, and BDRS was 10.9±4.5. Most had extrapyramidal signs (83%), and 52% had psychotic symptoms.
44 participants had a clinical diagnosis of AD (75.9%) and 14 (14.1%) had a clinical diagnosis of DLB (Table 1). At autopsy, 32 (55.2%) were confirmed to have AD pathology, 11 (19%) were confirmed to have DLB pathology, and 15 (25.9%) had mixed AD+LB pathology. Of the 44 participants with a clinical diagnosis of AD, 30 (68.2%) were confirmed to have pure AD pathology, 4 (9.1%) were confirmed to have pure LB pathology, and 10 (22.7%) had both AD and LB. Of the 14 participants with a clinical diagnosis of DLB, 7 (50%) were found to have pure LB neuropathologic changes in the autopsy, 2 were confirmed to have pure AD pathology, and 5 (35.7%) had both AD and LB pathology.
Table 1
Number of patients with clinical and pathology confirmed diagnosis
| Pathology confirmed diagnosis | ||||
| Clinical diagnosis | Pure AD | Pure LB | Mixed AD+LB | Total |
| AD | 30 | 4 | 10 | 44 |
| DLB | 2 | 7 | 5 | 14 |
| Total | 32 | 11 | 15 | 58 |
Table 2
Participant characteristics by clinical and pathology confirmed diagnosis
| Clinical diagnosis | Pathology confirmed diagnosis | ||||||||||||
| Variable | AD (n = 44) | DLB (n = 14) | p | Variable | pure AD (n = 32) | pure LB (N = 11) | mixed AD+LB (n = 15) | p | |||||
| Pathology diagnosis, N (%) | Clinical diagnosis, N (%) | ||||||||||||
| pure AD | 30 | 68.2% | 2 | 14.3% | < 0.001 | AD | 30 | 93.8% | 4 | 36.4% | 10 | 66.7% | < 0.001 |
| pure LB | 4 | 9.1% | 7 | 50.0% | DLB | 2 | 6.3% | 7 | 63.6% | 5 | 33.3% | ||
| mixed AD+LB | 10 | 22.7% | 5 | 35.7% | |||||||||
| Age at death, y, mean (SD) | 81.5 | (8.3) | 76.2 | (9.6) | 0.05 | Age at death, y, mean (SD) | 81.8 | (8.7) | 77.2 | (8.4) | 78.9 | (8.9) | 0.04 |
| Female, N (%) | 23 | 52.3% | 6 | 42.9% | 0.54 | Female, N (%) | 17 | 53.1% | 2 | 18.2% | 10 | 66.7% | 0.04 |
| Education, Mean, y (SD) | 15.2 | (3.0) | 15.0 | (2.7) | 0.85 | Education, Mean, y (SD) | 14.8 | (3.0) | 15.6 | (2.9) | 15.6 | (2.7) | 0.55 |
| Clinical characteristics 5 years prior to death | Clinical characteristics 5 years prior to death | ||||||||||||
| MMSE, mean (SD) | 17.8 | (6.5) | 19.9 | (5.9) | 0.31 | MMSE, mean (SD) | 18.0 | (6.5) | 21.4 | (5.1) | 16.4 | (6.7) | 0.11 |
| BDRS-ADL, mean (SD) | 10.7 | (3.8) | 11.3 | (6.9) | 0.82 | BDRS-ADL, mean (SD) | 11.1 | (3.6) | 8.2 | (5.6) | 12.2 | (5.2) | 0.10 |
| Extrapyramidal symptoms, N (%) | 35 | 78.6% | 14 | 100.0% | 0.10 | Extrapyramidal symptoms, N (%) | 24 | 80.0% | 8 | 100.0% | 11 | 78.6% | 0.37 |
| Any psychiatric symptom, N (%) | 20 | 45.2% | 11 | 75.0% | 0.07 | Any psychiatric symptom, N (%) | 14 | 46.7% | 7 | 77.8% | 7 | 46.7% | 0.23 |
| Delusion | 17 | 40.5% | 6 | 50.0 | 0.56 | Delusion | 11 | 36.7 | 5 | 55.6 | 7 | 46.7% | 0.56 |
| Hallucination | 5 | 11.9% | 8 | 66.7 | < 0.001 | Hallucination | 6 | 20.0 | 5 | 55.6 | 2 | 13.3 | 0.04 |
| Illusion | 2 | 4.9% | 3 | 25.0 | 0.04 | Illusion | 2 | 6.9 | 1 | 11.1 | 2 | 13.3 | 0.77 |
| Elixhauser comorbidities index, mean (SD) | 2.1 | (1.8) | 2.1 | (2.2) | 0.93 | Elixhauser comorbidities index, mean (SD) | 2.3 | (2.0) | 2.3 | (1.8) | 1.7 | (1.8) | 0.52 |
| Annualized total Medicare Expenditures during last 5 years of life, mean (SD) | 7,216 | (7,583) | 11,780 | (9,415) | 0.09 | Annualized total Medicare Expenditures during last 5 years of life, mean (SD) | 7,065 | (7,280) | 7,839 | (4,949) | 11,341 | (11,239) | 0.46 |
MMSE, Folstein Mini-Mental State Examination; BDRS, Blessed Dementia Rating Scale Between-group differences were tested using Kruskal-Wallis test for continuous variables and Chi-square tests.
Compared to patients clinically diagnosed with AD, those clinically diagnosed with DLB died younger (DLB: 76.2±9.6, AD: 81.5±8.3, p = 0.05), and were marginally more likely to have extrapyramidal symptoms (DLB: 100%, AD: 78.6%, p < 0.10) and psychiatric symptoms (DLB: 75.0%, AD: 45.2%, p = 0.07) five years prior to death. Hallucinations (DLB: 667%, AD 11.9%, p < 0.001) and illusions (DLB 25.0% AD: 4.9%, p = 0.04) were higher in DLB than AD patients.
Looking at pathology confirmed diagnosis, those with AD pathology were older at death (pure AD: 81.8±8.7, pure LB: 77.2±8.4; mixed AD+LB: 78.9±8.9, p = 0.04). Five years prior to death, hallucinations (pure AD: 20%, pure LB: 56%; mixed AD+LB: 13%, p = 0.04) were significant higher in those with DLB pathology.
3.2Medicare expenditures
During the last 5 years of life, unadjusted annualized total Medicare expenditures were higher in patients clinically diagnosed DLB ($11,780±9,415) than in those clinically diagnosed with AD ($7,216±7,583, p = 0.06). Unadjusted annualized total Medicare expenditures during the last 5 years of life were higher in patients with mixed AD+LB pathology ($11,341±11,239) than in those with pure AD ($7,065±7,280) and pure LB ($7,839±4,949) pathologies, but these differences were not statistically significant (Table 2, also Table 3, Models 1 and 2).
Table 3
Association between clinical and pathology diagnosis with average annual Medicare expenditures in the last 5 years of life
| Model 1 b (SE) | Model 2 b (SE) | Model 3 b (SE) | Model 4 b (SE) | Model 5 b (SE) | Model 6 b (SE) | |
| Clinical diagnosis (reference = AD) clinical DLB | 4,564 + (2,723) | –1,210 (1,606) | –4,443 * (2,113) | –7,783 * (2,790) | –8,134 * (3,166) | |
| Pathology diagnosis (reference = pure AD) pure LB | 774 (1,956) | 1,431 (2,454) | –723 (2,071) | –2,426 (2,696) | –3,242 (2,848) | |
| mixed AD+LB | 4,275 (3,159) | 33 (3,254) | 951 (3,000) | 740 (3,162) | 1,169 (3,360) | |
| Interaction between clinical and pathology diagnosis | ||||||
| Pathology confirmed pure LB, Clinical DLB | –844 (3,283) | 4,284 (3,866) | 3,338 (3,600) | 2,727 (3,728) | ||
| Pathology confirmed mixed AD+LB, Clinical DLB | 12,898 * (5,646) | 18,725 * (4,907) | 20,953 * (6,886) | 20,623 * (8,499) | ||
| Age at death | 9 (113) | 15 (126) | –39 (142) | |||
| Female | –4,098 * (1,966) | –4,381 + (2,346) | –3,593 (2,288) | |||
| Elixhauser comorbidities index | 1,753 * (641) | 1,823 * (781) | 2,318 * (831) | |||
| BDRS | 287 (172) | 260 (172) | ||||
| MMSE | –41 (263) | –158 (296) | ||||
| Extrapyramidal symptoms | 4,302 (3,653) | |||||
| Any psychiatric symptom | –1,476 (2,591) |
+p < 0.10 * p < 0.05 Independent variables included in Model 1 are indicator for clinical diagnosis (DLB versus AD) only. Model 2 includes indicators for confirmed pathology (pure AD, pure LB, and mixed AD+LB) only. Model 3 includes indicator for clinical diagnosis, indicators for confirmed pathology, and their interaction terms. Model 4 additionally included demographic characteristics, including age at death, sex, number of comorbidities. Model 5 additionally included dementia severity measures, MMSE, and BDRS 5 years prior to death. Model 6 (Fully adjusted model) additionally included extrapyramidal signs and psychotic symptoms 5 years prior to death.
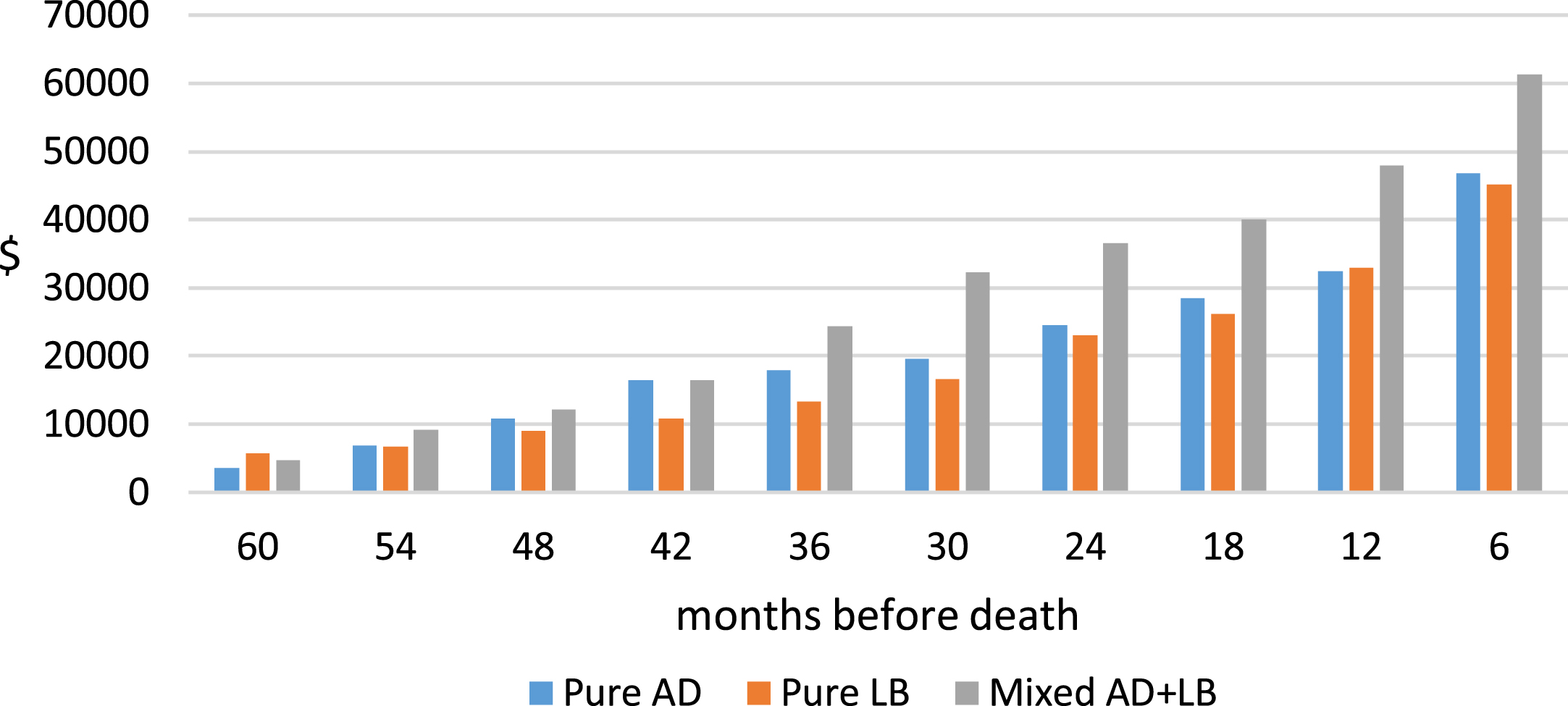
We computed total Medicare expenditures in 6-month intervals for the last 5 years of life by clinical and pathology diagnosis. For 8 of the 10 6-month intervals, Medicare expenditures for clinically diagnosed DLB patients were higher than those for AD patients. However, because there were substantial variations in expenditures during each 6-month intervals, differences between DLB and AD groups were not statistically significant in any individual 6-month interval. Cumulative Medicare expenditures during the last 5 years of life in 6-month increments are presented by clinical diagnosis (Fig. 1) and pathology confirmed diagnosis (Fig. 2).
Fig. 1
Unadjusted cumulative Medicare expenditures during the last 5 years of life, by clinical diagnosis. Medicare expenditures data were obtained from Medicare Standard Analytic Files (SAFs) and included all covered services (inpatient, outpatient, professional, emergency department, physician office visits, hospital outpatient visits, hospice, skilled nursing facility, home health, and durable medical supplies) in 6-month intervals from date of death to 5 years prior to death. Expenditures were adjusted to 2021$ using the medical care component of the Consumer Price Index.
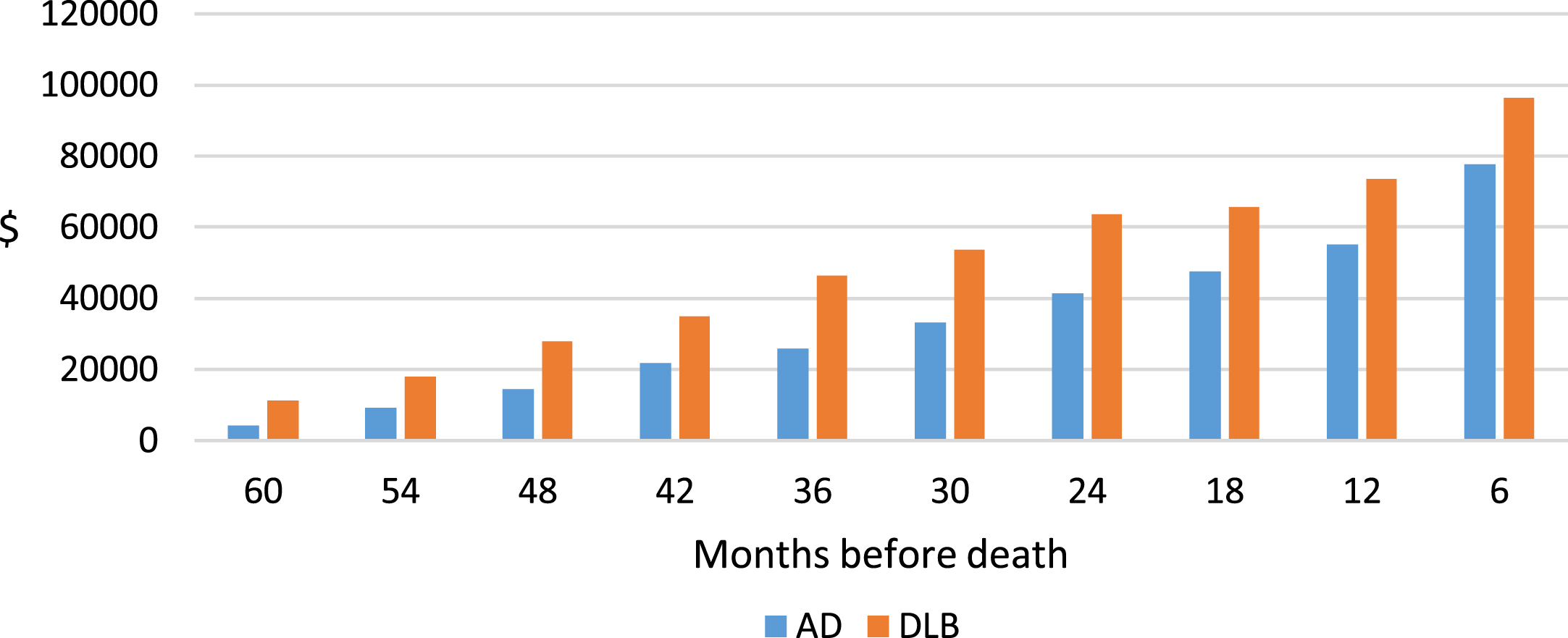
Fig. 2
Unadjusted cumulative Medicare A + B Payment, last 5 years of life, by pathology confirmed diagnosis. Medicare expenditures data were obtained from Medicare Standard Analytic Files (SAFs) and included all covered services (inpatient, outpatient, professional, emergency department, physician office visits, hospital outpatient visits, hospice, skilled nursing facility, home health, and durable medical supplies) in 6-month intervals from date of death to 5 years prior to death. Expenditures were adjusted to 2021$ using the medical care component of the Consumer Price Index.
Models 3–6 in Table 3 show estimated relationships between Medicare expenditures when both clinical and pathology-confirmed diagnoses are considered. Model 3 did not include any additional control variables, Model 4–6 additional controlled for demographics, dementia severity measures, and DLB specific clinical characteristics. Although the magnitude of the estimated coefficients varied across models, the directions of the estimates were consistent.
In the fully adjusted model (Table 3, model 6), the negative coefficient estimate (beta±SE = –8134±3166, p < 0.05) on clinical DLB suggests that in patients with pure AD (reference group), expenditures were lower in those clinically diagnosed as DLB than those clinically diagnosed with AD. However, such lower expenditures in clinically diagnosed DLB versus AD among pure AD cases was offset substantially if the patients carried mixed AD+LB pathologies rather than pure AD, as reflected by the significant positive coefficient on the interaction term ‘pathology confirmed mixed AD+LB * clinical DLB’ (beta±SE = 20,623±8,499, p < 0.05), suggesting that expenditures were higher for those clinically diagnosed with DLB than for those clinically diagnosed with AD among those with mixed AD+LB pathology. The coefficient on the interaction term “pathology confirmed pure LB * clinical DLB” (beta±SE = 2,727±3,728) was not statistically significant, suggesting that the lower expenditure of clinical DLB compared to clinical AD was similar in pure LB patients as in pure AD patients.
None of the coefficient estimates on the pathology diagnoses were statistically significant, suggesting that in patients clinically diagnosed with AD (reference group), differences in expenditures by pathology diagnosis were not statistically significant. The coefficient estimates on the interaction term on pathology confirmed mixed AD+LB and clinical DLB were statistically significant for all models, suggesting that for those clinically diagnosed with DLB, expenditures were substantially higher in those with mixed AD+LB pathology than in those with pure AD or pure LB.
Tables 4 shows predicted Medicare expenditures from Model 4, which includes clinical and pathology diagnosis and their interactions and demographic characteristics, but does not adjust for the subject’s clinical features that might drive costs of care (e.g., BDRS, MMSE, EPS, any psychotic symptoms), and the fully adjusted model (Model 6). Results show that, when pathology diagnoses were not considered, predicted expenditures were similar between clinically diagnosed AD ($7,465±1,098) and DLB ($7,783±1,803). When clinical diagnoses were not considered, predicted expenditures were substantially higher in patients with pathology confirmed mixed AD+LB ($12,005±2,455) than either pure AD ($6,173±941) or pure LB ($4,629±1,968) cases. Considering clinical and pathology diagnosis together, expenditures for patients with clinical DLB and pathology mixed AD+LB ($23,592±3,679) dwarfed other groups.
Table 4
Predicted average annual Medicare expenditures in the last 5 years of life, by clinical and pathology confirmed diagnosis
| Model 4 | Model 6 | |||
| Clinical diagnosis, disregarding pathology diagnosis | Predictive margin | Std. Err. | Predictive margin | Std. Err. |
| Clinical AD | 7,259 | (945) | 7,465 | (1,098) |
| Clinical DLB | 8,472 | (1,604) | 7,783 | (1,803) |
| Pathology diagnosis, disregarding clinical diagnosis | ||||
| Pathology confirmed pure AD | 6,078 | (835) | 6,173 | (941) |
| Pathology confirmed pure LB | 6,389 | (1,431) | 4,629 | (1,968) |
| Pathology confirmed mixed AD+LB | 11,549 | (2,506) | 12,005 | (2,455) |
| Clinical+Pathology confirmed diagnosis | ||||
| Clinical AD, Pathology pure AD | 7,151 | (1,166) | 7,466 | (1,302) |
| Clinical AD, Pathology pure LB | 6,427 | (1,527) | 5,415 | (2,146) |
| Clinical AD, Pathology mixed AD+LB | 8,101 | (2,824) | 8,694 | (2,917) |
| Clinical DLB, Pathology pure AD | 2,708 | (1,415) | 1,650 | (2,042) |
| Clinical DLB, Pathology pure LB | 6,268 | (2,833) | 1,878 | (3,071) |
| Clinical DLB, Pathology mixed AD+LB | 22,384 | (4,493) | 23,592 | (3,679) |
Estimates are derived from Model 4 (Table 3), which included indicators for clinical diagnosis, indicators for confirmed pathology, and their interaction terms, as well as demographic characteristics; and also from fully adjusted Model 6 (Table 3), which additionally included dementia severity measures, and extrapyramidal signs and psychotic symptoms 5 years prior to death.
Results from models 4–6 which additionally controlled for demographics, dementia severity measures, and DLB specific clinical characteristics showed that number of comorbidities was associated with higher Medicare expenditures. Being female was associated with lower expenditures, but the estimates were no longer statistically significant once dementia severity and DLB specific clinical characteristics were controlled for.
4DISCUSSION
In this study, we estimated Medicare expenditures during the last 5 years of life in a decedent sample of patients who were clinically diagnosed with AD or DLB and had autopsy confirmed diagnosis of pure-AD, pure-LB, or AD+LB. Consistent with much of the existing literature [19–26], our results showed that without considering the underlying pathology, patients clinically diagnosed with DLB had higher expenditures than those clinically diagnosed with AD. Results add to the current literature and further showed that expenditures differed by underlying pathology groups. Specifically, we found that expenditures were substantially higher in patients with mixed AD+LB pathology compared to those with pure-AD and pure-LB pathologies. A closer look at the interaction between clinical and pathology diagnoses showed that expenditures were particularly high in patients with mixed AD+LB pathologies, especially in those clinically diagnosed with DLB.
Our results should be considered in the context of our current understanding of costs associated with DLB. Healthcare expenditures vary tremendously. A number of recent studies have therefore reported on the cost of care associated with DLB as compared to AD using large administrative databases [19–26]. While administrative databases are a rich source of information on use and costs of healthcare use and costs, diagnosis codes documented in administrative claims are primarily for reimbursement purposes. Issues related to the substantial under-diagnosis, missed diagnosis, or mis-diagnosis of DLB, AD, and other dementias in the claims data that have been reported in the literature pose significant challenges in our understanding of healthcare costs when analyses rely solely on claims data. Compared to the claims-based studies, studies that relied on clinically diagnosed DLB patients remain small [19–21]. Our sample size, with 14 clinically diagnosed DLB patients and 44 clinically diagnosed AD cases, is on par with what has been reported in the literature to date (N = 15 [19], N = 34 [20]). The cohort included in the current analysis all have pathology confirmed diagnosis. To the best of our knowledge, this is the first study that examined cost of care in autopsy confirmed DLB patients.
Because of the small sample size of the cohort, our results are best considered as exploratory. Although our ability to examine healthcare costs in more detail or to perform additional subgroup analyses are limited, we explored the most frequent primary diagnoses in Part B claims for each group of patients. Among patients with pure AD pathology, AD was the most commonly reported primary diagnosis, accounting for 4.8% of all Part B claims in these patients; no other dementia diagnosis was among the top 10 primary diagnosis. Among patients with pure LB pathology, the most commonly reported primary diagnosis included AD, dementia in conditions classified elsewhere, and DLB, accounting for 3.0%, 2.9%, and 1.7% of all Part B claims in these patients. In patients with mixed AD+LB pathology, AD was the most commonly reported primary diagnosis, followed by vascular dementia, accounting for 4.7% and 1.8% of all Part B claims in these patients; DLB was rarely documented as a primary diagnosis (0.5% of all claims). Differences in these primary diagnoses raise the question of potential misdiagnosis in patients with mixed AD+LB pathology. Advances in PET, cerebrospinal fluid, and blood tests might lead to more accurate diagnosis. However, currently costs associated with these tests and their lack of availability are likely to make their use in clinical settings impractical.
Other limitations of the study include the clinic-based sample being predominantly white and highly educated, limiting the generalizability of the findings. We only examined Medicare, which does not cover nursing home care and personal care services often needed by patients with dementia. Patients with a clinical diagnosis of AD or DLB can have different distributions of underlying pathologies. This may in part lead to the variation in the clinical symptoms. Nevertheless, results from our study showed that it was the underlying pathologies that were most closely aligned with disease cost.
In conclusion, our results point to the gaps in our understanding of healthcare costs in DLB and highlight the importance of having both clinical and pathology diagnoses in examining healthcare costs. With the high prevalence of DLB in an aging population and extremely high societal burden of healthcare costs, it is critical to improve current understanding of costs of care among patients with DLB in order to inform public policies and clinical decision-making, as this will ultimately improve the quality of patient care.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This research was supported by grants from the National Institute on Aging (AG07370, AG037212). Dr. Zhu is also supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
CONFLICT OF INTEREST
Y Stern receives consulting fees from Eisai, Lilly, and Arcadia.
All other authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Geser F , Wenning GK , Poewe W , McKeith I ((2005) ) How to diagnose dementia with Lewy bodies: State of the art. Mov Disord 20 Suppl 12: , S11–20. |
[2] | Vann Jones SA , O’Brien JT ((2014) ) The prevalence and incidence of dementia with Lewy bodies: A systematic review of population and clinical studies. Psychol Med 44: , 673–683. |
[3] | McKeith IG , Boeve BF , Dickson DW , Halliday G , Taylor JP , Weintraub D , Aarsland D , Galvin J , Attems J , Ballard CG , Bayston A , Beach TG , Blanc F , Bohnen N , Bonanni L , Bras J , Brundin P , Burn D , Chen-Plotkin A , Duda JE , El-Agnaf O , Feldman H , Ferman TJ , Ffytche D , Fujishiro H , Galasko D , Goldman JG , Gomperts SN , Graff-Radford NR , Honig LS , Iranzo A , Kantarci K , Kaufer D , Kukull W , Lee VMY , Leverenz JB , Lewis S , Lippa C , Lunde A , Masellis M , Masliah E , McLean P , Mollenhauer B , Montine TJ , Moreno E , Mori E , Murray M , O’Brien JT , Orimo S , Postuma RB , Ramaswamy S , Ross OA , Salmon DP , Singleton A , Taylor A , Thomas A , Tiraboschi P , Toledo JB , Trojanowski JQ , Tsuang D , Walker Z , Yamada M , Kosaka K ((2017) ) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89: , 88–100. |
[4] | Stern Y , Albert M , Brandt J , Jacobs DM , Tang MX , Marder K , Bell K , Sano M , Devanand DP , Bylsma F , et al. ((1994) ) Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease: Prospective analyses from the Predictors Study. Neurology 44: , 2300–2307. |
[5] | Olichney JM , Galasko D , Salmon DP , Hofstetter CR , Hansen LA , Katzman R , Thal LJ ((1998) ) Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology 51: , 351–357. |
[6] | McKeith IG , Dickson DW , Lowe J , Emre M , O’Brien JT , Feldman H , Cummings J , Duda JE , Lippa C , Perry EK , Aarsland D , Arai H , Ballard CG , Boeve B , Burn DJ , Costa D , Del Ser T , Dubois B , Galasko D , Gauthier S , Goetz CG , Gomez-Tortosa E , Halliday G , Hansen LA , Hardy J , Iwatsubo T , Kalaria RN , Kaufer D , Kenny RA , Korczyn A , Kosaka K , Lee VM , Lees A , Litvan I , Londos E , Lopez OL , Minoshima S , Mizuno Y , Molina JA , Mukaetova-Ladinska EB , Pasquier F , Perry RH , Schulz JB , Trojanowski JQ , Yamada M ((2005) ) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 65: , 1863–1872. |
[7] | Stavitsky K , Brickman AM , Scarmeas N , Torgan RL , Tang MX , Albert M , Brandt J , Blacker D , Stern Y ((2006) ) The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol 63: , 1450–1456. |
[8] | Gill DP , Hubbard RA , Koepsell TD , Borrie MJ , Petrella RJ , Knopman DS , Kukull WA ((2013) ) Differences in rate of functional decline across three dementia types. Alzheimers Dement 9: , S63–71. |
[9] | Mueller C , Soysal P , Rongve A , Isik AT , Thompson T , Maggi S , Smith L , Basso C , Stewart R , Ballard C , O’Brien JT , Aarsland D , Stubbs B , Veronese N ((2019) ) Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: A meta-analysis of longitudinal studies. Ageing Res Rev 50: , 72–80. |
[10] | Price A , Farooq R , Yuan JM , Menon VB , Cardinal RN , O’Brien JT ((2017) ) Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: A retrospective naturalistic cohort study. BMJ Open 7: , e017504. |
[11] | Malek-Ahmadi M , Beach TG , Zamrini E , Adler CH , Sabbagh MN , Shill HA , Jacobson SA , Belden CM , Caselli RJ , Woodruff BK , Rapscak SZ , Ahern GL , Shi J , Caviness JN , Driver-Dunckley E , Mehta SH , Shprecher DR , Spann BM , Tariot P , Davis KJ , Long KE , Nicholson LR , Intorcia A , Glass MJ , Walker JE , Callan M , Curry J , Cutler B , Oliver J , Arce R , Walker DG , Lue LF , Serrano GE , Sue LI , Chen K , Reiman EM ((2019) ) Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease. PLoS One 14: , e0217566. |
[12] | Kramberger MG , Auestad B , Garcia-Ptacek S , Abdelnour C , Olmo JG , Walker Z , Lemstra AW , Londos E , Blanc F , Bonanni L , McKeith I , Winblad B , de Jong FJ , Nobili F , Stefanova E , Petrova M , Falup-Pecurariu C , Rektorova I , Bostantjopoulou S , Biundo R , Weintraub D , Aarsland D ((2017) ) Long-term cognitive decline in dementia with Lewy bodies in a large multicenter, international cohort. J Alzheimers Dis 57: , 787–795. |
[13] | Wilson RS , Capuano AW , Bennett DA , Schneider JA , Boyle PA ((2016) ) Temporal course of neurodegenerative effects on cognition in old age. Neuropsychology 30: , 591–599. |
[14] | Galasko DR , Gould RL , Abramson IS , Salmon DP ((2000) ) Measuring cognitive change in a cohort of patients with Alzheimer’s disease. Stat Med 19: , 1421–1432. |
[15] | Gu Y , Kociolek A , Fernandez KK , Cosentino SA , Zhu CW , Jin Z , Leverenz JB , Stern YB ((2022) ) Clinical trajectories at the end of life in dementia patients with Alzheimer disease and Lewy body neuropathologic changes. Neurology 98: , e2140–e2149. |
[16] | Lemstra AW , de Beer MH , Teunissen CE , Schreuder C , Scheltens P , van der Flier WM , Sikkes SA ((2017) ) Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 88: , 113–118. |
[17] | Breitve MH , Chwiszczuk LJ , Hynninen MJ , Rongve A , Brønnick K , Janvin C , Aarsland D ((2014) ) A systematic review of cognitive decline in dementia with Lewy bodies versus Alzheimer’s disease. Alzheimers Res Ther 6: , 53. |
[18] | Kraybill ML , Larson EB , Tsuang DW , Teri L , McCormick WC , Bowen JD , Kukull WA , Leverenz JB , Cherrier MM ((2005) ) Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 64: , 2069–2073. |
[19] | Murman DL , Kuo SB , Powell MC , Colenda CC ((2003) ) The impact of parkinsonism on costs of care in patients with AD and dementia with Lewy bodies. Neurology 61: , 944–949. |
[20] | Bostrom F , Jonsson L , Minthon L , Londos E ((2007) ) Patients with Lewy body dementia use more resources than those with Alzheimer’s disease. Int J Geriatr Psychiatry 22: , 713–719. |
[21] | Zhu CW , Scarmeas N , Stavitsky K , Albert M , Brandt J , Blacker D , Sano M , Stern Y ((2008) ) Comparison of costs of care between patients with Alzheimer’s disease and dementia with Lewy bodies. Alzheimers Dement 4: , 280–284. |
[22] | Vossius C , Rongve A , Testad I , Wimo A , Aarsland D ((2014) ) The use and costs of formal care in newly diagnosed dementia: A three-year prospective follow-up study. Am J Geriatr Psychiatry 22: , 381–388. |
[23] | Tahami Monfared AA , Meier G , Perry R , Joe D ((2019) ) Burden of disease and current management of dementia with Lewy bodies: A literature review. Neurol Ther 8: , 289–305. |
[24] | Mueller C , Perera G , Rajkumar AP , Bhattarai M , Price A , O’Brien JT , Ballard C , Stewart R , Aarsland D ((2018) ) Hospitalization in people with dementia with Lewy bodies: Frequency, duration, and cost implications. Alzheimers Dement (Amst) 10: , 143–152. |
[25] | Chen Y , Wilson L , Kornak J , Dudley RA , Merrilees J , Bonasera SJ , Byrne CM , Lee K , Chiong W , Miller BL , Possin KL ((2019) ) The costs of dementia subtypes to California Medicare fee-for-service, 2015. Alzheimers Dement 15: , 899–906. |
[26] | Espinosa R , Davis M , Johnson S , Cline S , Weintraub D ((2020) ) Direct medical costs of dementia with Lewy bodies by disease complexity. J Am Med Dir Assoc 21: , 1696–1704 e1695. |
[27] | Yamada M , Komatsu J , Nakamura K , Sakai K , Samuraki-Yokohama M , Nakajima K , Yoshita M ((2020) ) Diagnostic criteria for dementia with Lewy bodies: Updates and future directions. J Mov Disord 13: , 1–10. |
[28] | Galvin JE , Duda JE , Kaufer DI , Lippa CF , Taylor A , Zarit SH ((2010) ) Lewy body dementia: The caregiver experience of clinical care. Parkinsonism Relat Disord 16: , 388–392. |
[29] | Thomas AJ , Taylor JP , McKeith I , Bamford C , Burn D , Allan L , O’Brien J ((2017) ) Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: Feasibility study within the DIAMOND Lewy study. Int J Geriatr Psychiatry 32: , 1280–1304. |
[30] | Rizzo G , Arcuti S , Copetti M , Alessandria M , Savica R , Fontana A , Liguori R , Logroscino G ((2018) ) Accuracy of clinical diagnosis of dementia with Lewy bodies: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 89: , 358–366. |
[31] | Outeiro TF , Koss DJ , Erskine D , Walker L , Kurzawa-Akanbi M , Burn D , Donaghy P , Morris C , Taylor JP , Thomas A , Attems J , McKeith I ((2019) ) Dementia with Lewy bodies: An update and outlook. Mol Neurodegener 14: , 5. |
[32] | Armstrong MJ , Alliance S , Corsentino P , Maixner SM , Paulson HL , Taylor A ((2020) ) Caregiver-reported barriers to quality end-of-life care in dementia with Lewy bodies: A qualitative analysis. Am J Hosp Palliat Care 37: , 728–737. |
[33] | Stern Y , Folstein M , Albert M , Richards M , Miller L , Bylsma F , Lafleche G , Marder K , Bell K , Sano M , et al. ((1993) ) Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord 7: , 3–21. |
[34] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–944. |
[35] | McKeith IG , Galasko D , Kosaka K , Perry EK , Dickson DW , Hansen LA , Salmon DP , Lowe J , Mirra SS , Byrne EJ , Lennox G , Quinn NP , Edwardson JA , Ince PG , Bergeron C , Burns A , Miller BL , Lovestone S , Collerton D , Jansen EN , Ballard C , de Vos RA , Wilcock GK , Jellinger KA , Perry RH ((1996) ) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 47: , 1113–1124. |
[36] | Montine TJ , Phelps CH , Beach TG , Bigio EH , Cairns NJ , Dickson DW , Duyckaerts C , Frosch MP , Masliah E , Mirra SS , Nelson PT , Schneider JA , Thal DR , Trojanowski JQ , Vinters HV , Hyman BT ((2012) ) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 123: , 1–11. |
[37] | Bureau of Labor Statistics, Consumer price index, http://www.bls.gov/cpi/home.htm. |
[38] | Blessed G , Tomlinson BE , Roth M ((1968) ) The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 114: , 797–811. |
[39] | Devanand DP ((1997) ) Use of the Columbia University Scale to assess psychopathology in Alzheimer’s disease. Int Psychogeriatr 9: (Suppl 1), 137–142; discussion 143-150. |
[40] | Louis ED , Lynch T , Marder K , Fahn S ((1996) ) Reliability of patient completion of the historical section of the Unified Parkinson’s Disease Rating Scale. Mov Disord 11: , 185–192. |
[41] | Richards M , Marder K , Bell K , Dooneief G , Mayeux R , Stern Y ((1991) ) Interrater reliability of extrapyramidal signs in a group assessed for dementia. Arch Neurol 48: , 1147–1149. |
[42] | Elixhauser A , Steiner C , Harris DR , Coffey RM ((1998) ) Comorbidity measures for use with administrative data. Med Care 36: , 8–27. |
[43] | Quan H , Sundararajan V , Halfon P , Fong A , Burnand B , Luthi JC , Saunders LD , Beck CA , Feasby TE , Ghali WA ((2005) ) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: , 1130–1139. |




