Efficacy of Souvenaid® Combined with Acetylcholinesterase Inhibitors in the Treatment of Mild Alzheimer’s Disease
Abstract
Background:
Souvenaid® is a medical food that contains nutrients that can help synapse synthesis in Alzheimer’s disease (AD). The potential effectiveness of combination therapy of Souvenaid with cholinesterase inhibitors (AChEI) is currently not well-known.
Objective:
To look into the effect of combination therapy with Souvenaid plus AChEI in people with mild AD in the real-world.
Methods:
We carried out a retrospective analysis in mild AD patients attending a memory clinic. Three groups were studied according to the treatment they received: Souvenaid alone (n = 66), AChEI alone (n = 84), and Souvenaid+AChEI (n = 70). Treatment effects were evaluated at baseline, 6 and 12 months. Cognitive functioning was assessed by Mini-Mental State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT), Symbol Digit Modalities Test (SDMT), Boston Naming Test (BNT), Trail Making Test (TMT/A-B), Phonemic and Semantic Verbal Fluency Test (PVFT/SVFT); neuropsychiatric symptoms were evaluated by the Neuropsychiatric Inventory (NPI); functional capacity was assessed by the Bayer Activities Daily Living Scale (BAYER-S). A Mixed Model for Repeated Measures analysis was carried out to evaluate changes in outcome scores.
Results:
After 12 months Souvenaid+AChEI showed significant improvement in MMSE (p < 0.001), RAVLT (p < 0.0001), SVFT (p = 0.002), PVFT (p = 0.007), TMTA (p = 0.039), TMTB (p = 0.001), and NPI (p < 0.0001) compared to AChEI alone.
Conclusion:
Souvenaid showed cognitive and behavioral benefits in mild AD patients. These effects increased when Souvenaid and AChEI were used in combination. This study can serve as a model for the design of prospective controlled trials that help to support the combined use of Souvenaid and antidementia drugs in AD.
INTRODUCTION
Alzheimer’s disease (AD) is one of the leading causes of cognitive and functional impairment among older persons and has negative consequences for patients, caregivers, and society [1]. At present, no cure for AD is available and the results of several clinical trials conducted to find disease course-modifying therapies have failed [2, 3]. Current treatments only manage to slow down the progression of symptoms or delay institutionalization of the patient [4, 5]. Moreover, the available drugs are often administered too late, when the neuronal damage already affects some crucial brain areas. Hence, given the progressive course of AD there is an opportunity for treatment through the development of new therapeutic approaches. Along with amyloid-β aggregation and the tau protein hyperphosphorylation [6], another key pathophysiological characteristics in the early stages of AD is a reduction of synaptic connections [7]. Synaptic loss can lead to disruption of neuronal communication and is hypothesized to play a significant contribution in the course of the disease [8–10]. Moreover, some components needed to synthesize phospholipids, the main components of synaptic membrane, are depleted in AD [11]. Slowing synaptic loss while improving synapse function could help maintain neuronal communication and thus positively affect cognitive functioning. Therefore, reducing loss of synapses and improving synapse functionality offers a potential approach for intervention in AD [8, 12].
Souvenaid is a medical nutritional supplement that contains a combination of docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), uridine monophosphate, choline, phospholipids, selenium, B vitamins, vitamins C, E, and folic acid. Uridine monophosphate, choline, DHA, and EPA are precursors for the formation of neuron membranes and the proper function of neurons and their combination is aimed at helping the synapse formation and memory function in AD [13].
Findings from preclinical research show that this specific combination of nutrients contributes to the reduction of AD-related brain pathologies in a neuroprotective manner [14–16]. In addition, previous clinical trials evaluated Souvenaid in patients with mild cognitive impairment due to AD or dementia of the Alzheimer’s type and showed that Souvenaid benefited memory functioning in early AD patients taking no anti-dementia drugs [17–19]. On the contrary, the results of a recent Cochrane review states that there is no strong evidence that Souvenaid shows significant results for people with AD in the prodromal phase or in the mild and moderate phases of dementia [20].
The LipiDiDiet study showed no significant effect of Souvenaid treatment in people with prodromal AD on the Neuropsychological Test Battery primary outcome after 24-month intervention [21]. However, the results also showed a level of cognitive impairment lower than expected in these patients, implying that the primary endpoint is underpowered. Group differences on hippocampal atrophy were observed. In an extension of the intervention to a 36-month period, the LipiDiDiet clinical trial showed significant benefits on cognitive decline suggesting that Souvenaid effects on disease progression may be enhanced with longer-term use over time [22]. Recently, the recommendations of an expert consensus stated that there is evidence for Souvenaid to be considered as a therapeutic option in some patients with mild AD and those with mild cognitive impairment due to AD (MCI-AD) [23].
Currently cholinesterase inhibitors (AChEI) are the most frequently prescribed drugs to address early to moderate AD manifestations [24]. Nevertheless, studies that consider the potential efficacy of combining Souvenaid with AChEIs for the treatment of AD including prodromal AD are lacking. Recently, a prospective, non-interventional research showed that mild AD patients taking Souvenaid alone and the Souvenaid plus AChEI combination had significantly lower monthly increases in Clinical Dementia Rating Scale scores [25] than either those patients taking AChEIs alone or those receiving no treatment [26]. Therefore, since there appears to be a potential usefulness for adding Souvenaid to usual AChEI treatment in AD, further evidence supporting the use of combination therapy is worthwhile.
The objective of this research is to shed light on the potential synergistic effect of the combination of Souvenaid with AChEI in patients with mild AD in a real-world context. For this purpose, we conducted a review of data from AD patients who attended a memory clinic over a 12-month period.
METHODS
Patient selection
We retrospectively identified patients diagnosed with Alzheimer’s disease (AD) attending the Alzheimer Disease Center and Memory Clinic of the Instituto Andaluz de Neurociencia (IANEC), Málaga, Spain, between January 2017 and December 2020. We selected AD patients from the IANEC memory clinic records. For the diagnosis of AD, the criteria established by the National Institute on Aging Alzheimer’s Association workgroups (NIA/AA) were followed. To be included in the study AD patients had to be at stage 3 or 4 of the Reisberg Global Deterioration Scale (GDS) [27]. These GDS stage are a criterion to prescribe Souvenaid at the IANEC memory clinic.
The researchers collected data from the patients’ medical history. All selected participants were asked to allow data collection on their medical history. In addition to demographic and clinical characteristics, information on treatment with Souvenaid alone or AChEI alone or both substances combined, neurological and psychiatric data, neuropsychological evaluation, and magnetic resonance imaging (MRI) were collected. These evaluations are included in the routine examinations that patients undergo every six months at the IANEC.
We excluded patients who presented focal neurological signs, epileptic seizures, brain inflammation, uncontrolled psychiatric disorders, incomplete medical chart, and sensory impairments such as severe vision and hearing impairment disorders.
We considered as candidates those patients who had received treatment for 12 months with any of the following medications: Souvenaid (1 dose per day), donepezil (10 mg per day), galantamine (16 or 24 mg per day), or rivastigmine patch (9.5 or 13.3 mg per day). Patients were classified into three groups according to the treatment followed: Souvenaid alone, AChEI alone, or Souvenaid plus AChEI. Patients also were receiving stable doses of other classes of medications including antihypertensives (39.1%), anticoagulants (4.2%), calcium channel blockers (11.4%), diuretics (18.3%), lipid reducing agents (35.6%), and antidiabetic drugs (19.6%).
During the study period, a total of 332 patients with AD were identified. Of these 332 subjects, 28 potential participants were not included because they had no sufficient data in their medical charts, 20 were unable to be contacted, 17 declined or did not meet the inclusion criteria (n = 47).
As this was a retrospective study, the sample size was not determined a priori. The study was approved by the Málaga Local Ethics Committee and a written informed consent was signed by patients or their legal representative. The study was guided by the ethical standards adopted by the XVIII World Medical Assembly Declaration of Helsinki and subsequent revisions.
Assessment
Recorded data were collected on the effects of drugs studied on cognitive functioning, behavioral and psychological symptoms, and functional capacity. Evaluations were carried out at three points in time: at baseline (the day of assessment and treatment initiation), at 6 months and at 12 months.
Cognitive function
The Mini-Mental State Examination (MMSE) [28] is a 30-item questionnaire that is used broadly to assess cognitive impairment, where lower scores indicate poorer cognitive functioning.
The Rey Auditory Verbal Learning Test (RAVLT) [29] is designed as a word list learning task to evaluate verbal episodic memory and to follow changes in memory function over time. In this study, immediate recall was assessed using five repetitions of free-recall of a list of 15 nouns. The total encoding was obtained from the sum of the number of words recalled in the five trials.
The Symbol Digit Modalities Test (SDMT) [30] is frequently used for evaluation of visual-spatial processing and information processing speed. This consists of converting symbols in the form of meaningless geometric figures into oral or written responses in the form of a number, according to an established key. After being guided to complete the first 10 sample items, the number of responses the patient can give in 90 seconds is recorded.
The Boston Naming Test (BNT) [31] is a visual confrontational word retrieval test that includes black and white drawings of various animate and inanimate objects. The BNT is a widely used test for assessing lexical access ability. We used the reduced 15-item BNT.
The Trail Making Test (TMT) [32] is a tool designed to evaluate attention, flexibility of thought and visuospatial ability. The TMT has two parts: in the first part (TMT-A) it is necessary to quickly join the numbers with lines, these being randomly placed in numerical order and in the second part (TMT-B) it is necessary to join the numbers and letters with lines, these being randomly placed, for example joining the 1 with the A, the 2 with the B, etc. The patient is taught to complete both parts of the test as quickly and thoroughly as possible.
Semantic Verbal Fluency Test (SVFT) and Phonemic Verbal Fluency Test (PVFT) were used to assess verbal fluency [33]. Both activate multiple cognitive functions: working memory, sustained attention, executive functions, semantic memory, search and retrieval strategies for lexical items, among others. In the SVFT, all valid animal names evoked in 1 minute are counted. In the PVFT, subjects had to indicate as many words as possible beginning with the letter “P” for 1 minute.
Neuropsychiatric symptoms
The Neuropsychiatric Inventory (NPI) [34] was used to assess the most frequent behavioral and psychological symptoms of dementia (BPSD) in AD patients. The NPI is made up of 12 domains in which frequency of symptoms is scored from 1 to 4 points and their severity from 1 to 3 points. A composite score of up to 12 points for each domain and up to 144 points for the total NPI can be obtained.
Functional performance
The Bayer Activities of Daily Living Scale (BAYER-S) [35] was used to evaluate the functional capacity of the patients. The scale consists of 25 items that score from 1 to 10 to be answered by the patient’s family caregiver. Lower scores indicate better functional performance.
Statistical analysis
Demographic and clinical characteristics were obtained: mean, standard deviation in the case of quantitative variables; and number and percentage in the case of qualitative. Analyses of variance (ANOVA) for continuous variables or nonparametric tests for categorical variables were performed for comparisons between groups at baseline.
To study changes in cognitive, functional, and neuropsychiatric scores over follow-up, a Mixed Model for Repeated Measures (MMRM) analysis was carried out and the effects of time, treatment and their interaction were included in the analyses. Post hoc analyses were performed with a Bonferroni correction for group comparisons. Results were adjusted for the participants sex, age at baseline, level of education and AChEI doses. Cohen’s d standardized effect sizes were calculated and defined as small d = 0.20, medium d = 0.50 and large d = 0.80 [36].
All analyses were performed using SPSS Statistics (Version 25.0). A p-value ≤0.05 was considered statistically significant.
RESULTS
We recruited a total of 220 patients who met the inclusion criteria and whose medical data were available in the medical charts for analysis (131 female, 89 male). The mean age of the sample was of 75.77±5.60 years (range 64–96) and an average of 6.55±1.92 years of education (range 6–13). All patients were Caucasian. There was no difference at baseline between patients treated with Souvenaid alone (n = 66) and those treated with AChEI alone (n = 84) or with Souvenaid plus AChEI (n = 70) in relation to the clinical and demographic variables (Table 1).
Table 1
Demographic and clinical data at baseline of study participants
| Variable | Overall (n = 220) | Souvenaid (n = 66) | AChEI (n = 84) | Souvenaid+AChEI (n = 70) | p ANOVA/χ2 |
| Age | 75.77±5.60 | 75.92±5.96 | 75.50±5.22 | 75.94±5.77 | 0.857 |
| Sex | |||||
| Female | 131 (59.5) | 41 (62.1) | 52 (61.9) | 38 (54.3) | 0.554 |
| Male | 89 (40.5) | 25 (37.9) | 32 (38.1) | 32 (45.7) | |
| Marital status | |||||
| Married | 84 (38.2) | 28 (42.4) | 30 (35.7) | 26 (37.2) | 0.795 |
| Single/divorced | 20 (9.1) | 4 (6.1) | 8 (9.5) | 8 (11.4) | |
| Widowed | 116 (52.7) | 34 (51.5) | 46 (54.8) | 36 (51.4) | |
| Education, y | 6.55±1.92 | 6.50±1.92 | 6.61±2.06 | 6.51±1.75 | 0.932 |
| MMSE | 20.91±2.17 | 20.86±2.16 | 21.10±1.98 | 20.73±2.27 | 0.541 |
| RAVLT | 18.31±3.15 | 18.50±2.95 | 18.32±3.11 | 18.11±3.23 | 0.763 |
| SVFT | 8.38±1.88 | 8.42±1.74 | 8.44±1.68 | 8.27±1.18 | 0.450 |
| PVFT | 8.68±1.75 | 8.68±1.84 | 8.74±1.67 | 8.61±1.51 | 0.830 |
| TMTA | 163.7±38.52 | 163.0±39.14 | 163.31±36.19 | 164.81±21.37 | 0.819 |
| TMTB | 238.49±38.56 | 243.08±42.55 | 235.63±49.82 | 237.59±35.38 | 0.490 |
| SDMT | 23.48±9.76 | 22.08±10.62 | 25.26±9.12 | 22.67±9.48 | 0.098 |
| BNT | 9.60±1.90 | 9.64±1.59 | 9.39±1.93 | 9.83±2.12 | 0.364 |
| NPI | 24.21±5.67 | 23.26±5.12 | 24.35±5.48 | 24.96±6.31 | 0.210 |
| BAYER-S | 9.00±1.19 | 9.04±1.13 | 9.17±1.18 | 8.77±1.22 | 0.118 |
Values are mean±SD or number (%), ANOVA analysis of variance, χ2, Chi-square. MMSE, Mini-Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; SVFT; Semantic Verbal Fluency Test; PVFT; Phonemic Verbal Fluency Test; TMTA, Trail Making Test part A; TMTB, Trail Makin Test part B; SDMT, Symbol Digit Modalities Test; BNT Boston Naming Test; NPI, Neuropsychiatric Inventory; BAYER-S, Bayer Activities of Daily Living Scale.
Cognitive performance
With regard to the MMSE, the MMRM analysis showed a statistically significant time by treatment effect (F (2,217) = 40.657, p < 0.0001). The Souvenaid plus AChEI group had better results than the AChEI group at 12-month follow-up (+0.17 points, 95% CI: 0.15, 0.32; p < 0.001; Cohen’s d = 0.26) and showed an improvement already at 6 months. The Souvenaid plus AChEI group had better results than the Souvenaid group at 12-month follow-up (+0.90 points, 95% CI: 0.61, 0.14; p < 0.0001; Cohen's d = 0.62) and showed an improvement already at 6 months (Fig. 1).
Fig. 1
Significant results from the mixed model analysis at 12-month follow-up. T0, baseline; T1, 6 months; T2, 12 months. Numbers in the bars are 90% percentile. MMSE, Mini-Mental State Examination; RAVLT, Rey AuditoryVerbal Learning Test; SVFT, Semantic Verbal Fluency Test; PVFT, Phonemic Verbal Fluency Test; TMTA, Trail Making Test part A; TMTB, Trail Makin Test part B; SDMT, Symbol Digit Modalities Test; BNT, Boston Naming Test; NPI, Neuropsychiatric Inventory; BAYER-S, Bayer Activities of Daily Living Scale.
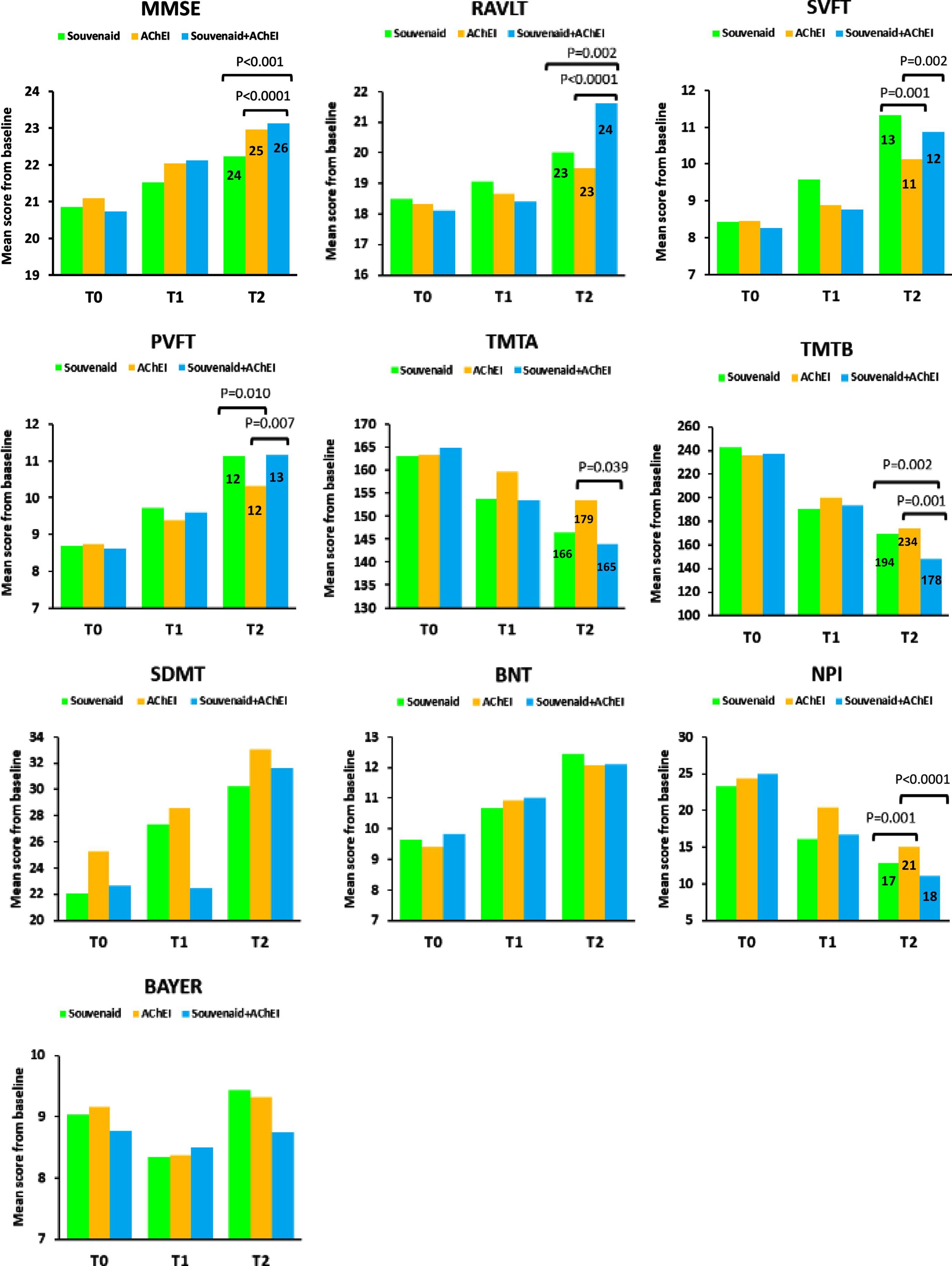
Concerning the RAVLT, the MMRM analysis showed a statistically significant time by treatment effect (F(2,217) = 54.522, p < 0.0001). The Souvenaid plus AChEI group yielded better results than the AChEI group at 12-month follow-up (+2.15 points, 95% CI: 1.07, 3.23; p < 0.0001; Cohen's d = 0.75). The Souvenaid plus AChEI group yielded better results than the Souvenaid group at 12-month follow-up (+1.61 points, 95% CI: 0.46, 2.76; p = 0.002; Cohen's d = 0.59) (Fig. 1).
In regard to the SVFT, the MMRM analysis showed a statistically significant time by treatment effect (F(2,217) = 16.054, p < 0.0001). The Souvenaid plus AChEI group rendered better results than the AChEI group at 12-month follow-up (+0.74 points, 95% CI: 0.22, 1.25; p = 0.002; Cohen's d = 0.38). The Souvenaid group rendered better results than AChEI group at 12-month follow-up (+1.20, 95% CI: 0.67, 1.72; p = 0.001; Cohen's d = 0.72) and had an improvement already at 6 months (Fig. 1).
Regarding the PVFT, the MMRM analysis showed a statistically significant time by treatment effect (F(2,217) = 9.837, p < 0.0001). The Souvenaid plus AChEI group had better results than the AChEI group at 12-month follow-up (+0.83 points, 95% CI: 0.18, 1.46; p = 0.007; Cohen’s d = 0.45). The Souvenaid group had better results than AChEI group at 12-month follow-up (+0.81, 95% CI: 0.15, 1.45; p = 0.01; Cohen’s d = 0.44). (Fig. 1).
With regard to the TMTA, the MMRM analysis showed a statistically significant time by treatment effect (F(2,217) = 36.976, p < 0.0001). The Souvenaid plus AChEI group yielded better results than the AChEI group at 12-month follow-up (–9.70 points, 95% CI: –19.05, –3.05; p = 0.039; Cohen’s d = 0.25) (Fig. 1).
Concerning the TMTB, the MMRM analysis showed a statistically significant time by treatment effect (F(2,217) = 12.836, p < 0.0001). The Souvenaid plus AChEI group had better results than the AChEI group at 12-month follow-up (–25.69 points, 95% CI: –39.74, –11.64; p = 0.001; Cohen’s d = 0.52). The Souvenaid plus AChEI group had better results than the Souvenaid group at 12-month follow-up (–21.12 points, 95% CI: –36.02, –6.22; p = 0.002; Cohen’s d = 0.41) (Fig. 1).
The Mixed Model analysis showed no statistically significant time by treatment effect neither for the SDMT (F(2,217) = 1.440, p = 0.239) nor for the BNT (F(2, 217) = 1.586, p = 0.207). There were no significant differences between-groups.
All three treatment groups showed statistically significant improvements on all measures at 12 months from baseline (Table 2).
Table 2
Rating scales performance over 12-month follow-up
| Variable | T0 | T1 | T2 | Difference (T1-T0) | p | Difference (T2-T0) | p |
| MMSE | |||||||
| Souvenaid | 20.86±2.06 | 21.53±2.10 | 22.23±2.18 | +0.67 | <0.0001 | +1.37 | <0.0001 |
| AChEi | 21.10±1.92 | 22.04±1.96 | 22.96±2.75 | +0.94 | <0.0001 | +1.86 | <0.001 |
| Souvenaid+AChEI | 20.73±2.27 | 22.13±2.16 | 23.13±2.29 | +1.40 | <0.0001 | +2.40 | <0.0001 |
| RAVLT | |||||||
| Souvenaid | 18.50±2.95 | 19.05±2.76 | 20.02±2.63 | +0.55 | <0.0001 | +1.52 | <0.0001 |
| AChEi | 18.32±3.01 | 18.67±3.06 | 19.48±2.88 | +0.35 | <0.0001 | +1.16 | <0.0001 |
| Souvenaid+AChEI | 18.11±3.23 | 18.41±3.04 | 21.63±2.78 | +0.30 | <0.0001 | +3.52 | <0.0001 |
| SVFT | |||||||
| Souvenaid | 8.42±1.74 | 9.58±1.30 | 11.32±1.59 | +1.16 | <0.0001 | +2.90 | <0.0001 |
| AChEi | 8.44±1.68 | 8.89±1.94 | 10.12±1.71 | +0.45 | <0.0001 | +1.68 | <0.0001 |
| Souvenaid+AChEI | 8.27±1.68 | 8.77±1.72 | 10.86±1.96 | +0.50 | <0.0001 | +2.59 | <0.0001 |
| PVFT | |||||||
| Souvenaid | 8.68±1.34 | 9.73±1.13 | 11.14±1.91 | +1.05 | <0.0001 | +2.46 | <0.0001 |
| AChEi | 8.74±1.77 | 9.38±1.48 | 10.33±1.75 | +0.64 | <0.0001 | +1.59 | <0.0001 |
| Souvenaid+AChEI | 8.61±1.51 | 9.59±1.71 | 11.16±1.92 | +0.98 | <0.0001 | +2.55 | <0.0001 |
| TMTA | |||||||
| Souvenaid | 163.0±28.14 | 153.64±28.18 | 146.36±32.78 | –9.36 | <0.0001 | –16.64 | <0.001 |
| AChEi | 163.31±33.19 | 159.61±29.48 | 153.57±36.20 | –3.70 | <0.0001 | –9.74 | <0.001 |
| Souvenaid+AChEI | 164.81±28.37 | 153.47±29.59 | 143.87±42.10 | –11.34 | <0.0001 | –20.94 | <0.001 |
| TMTB | |||||||
| Souvenaid | 243.08±44.55 | 190.88±45.43 | 169.32±50.48 | –52.20 | <0.0001 | –73.76 | <0.001 |
| AChEi | 235.63±43.82 | 199.93±43.86 | 173.89±47.17 | –35.70 | <0.0001 | –61.74 | <0.001 |
| Souvenaid+AChEI | 237.59±45.38 | 193.19±38.90 | 148.20±51.57 | –44.40 | <0.0001 | –89.39 | <0.001 |
| SDMT | |||||||
| Souvenaid | 22.08±10.62 | 27.38±8.18 | 30.21±8.79 | +5.30 | <0.001 | +8.13 | <0.001 |
| AChEi | 25.26±9.12 | 28.57±9.69 | 33.08±9.60 | +3.31 | 0.002 | +7.82 | <0.001 |
| Souvenaid+AChEI | 22.67±9.48 | 22.46±10.41 | 31.63±10.93 | –0.21 | 0.192 | +8.96 | <0.001 |
| BNT | |||||||
| Souvenaid | 9.64±1.59 | 10.67±1.28 | 12.44±1.15 | +1.03 | <0.001 | +2.80 | <0.001 |
| AChEi | 9.39±1.93 | 10.93±1.21 | 12.07±1.33 | +1.54 | <0.001 | +2.68 | <0.001 |
| Souvenaid+AChEI | 9.83±2.12 | 11.00±1.35 | 12.10±1.32 | +1.17 | <0.001 | +2.27 | <0.001 |
| NPI | |||||||
| Souvenaid | 23.26±5.12 | 16.11±5.37 | 12.86±5.11 | –7.15 | <0.0001 | –10.40 | <0.0001 |
| AChEi | 24.35±4.48 | 20.45±5.94 | 14.98±5.28 | –3.90 | <0.0001 | –9.37 | <0.0001 |
| Souvenaid+AChEI | 24.96±6.31 | 16.73±5.26 | 12.06±5.33 | –8.23 | <0.0001 | –12.90 | <0.0001 |
| BAYER-S | |||||||
| Souvenaid | 9.04±1.13 | 8.34±1.61 | 9.43±0.51 | –0.70 | 0.422 | +0.39 | 0.310 |
| AChEI | 9.17±1.18 | 8.37±1.62 | 9.32±1.11 | –0.80 | 0.306 | +0.15 | 0.782 |
| Souvenaid+AChEI | 8.77±1.22 | 8.50±1.62 | 8.74±1.22 | –0.27 | 0.768 | –0.03 | 0.256 |
T0 baseline, T1 follow-up 6 months, T2 follow-up 12 months, Values are mean±SD. MMSE, Mini-Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; SVFT, Semantic Verbal Fluency Test; PVFT, Phonemic Verbal Fluency Test; TMTA, Trail Making Test part A; TMTB, Trail Makin Test part B; SDMT, Symbol Digit Modalities Test; BNT, Boston Naming Test; NPI, Neuropsychiatric Inventory; BAYER-S, Bayer Activities of Daily Living Scale.
Neuropsychiatric symptoms
In regard to the NPI, the MMRM analysis showed a statistically significant time by treatment effect (F(2,217) = 8.129, p < 0.0001). The Souvenaid plus AChEI group had better results than the AChEI group at 12-month follow-up (–2.92 points, 95% CI: –5.84, –1.99; p < 0.0001; Cohen’s d = 0.55) and showed an improvement already at 6 months. The Souvenaid group yielded better results than the AChEI group at 12-month follow-up (–2.12 points, 95% CI: –5.07, –1.15; p = 0.001; Cohen’s d = 0.41) and had an improvement already at 6 months (Fig. 1). All three treatment groups showed statistically significant differences at 12 months from baseline (Table 2).
Functional performance
With regard to the BAYER-S, the MMRM analysis showed no statistically significant time by treatment effect (F (2,217) = 1.586, p = 0.207). There were no significant differences between-groups. None of the three treatment groups showed statistically significant differences at 12 months from baseline (Table 2).
Safety analysis
Patients who experienced treatment-related adverse events were 18.6% in the Souvenaid group, 37.2% in the AChEI group, and 39.4% in the Souvenaid plus AChI group. Table 3 shows the adverse events that occurred in the three treatment groups; the most frequently reported were nausea (9.01%) and dizziness (6.06%) for Souvenaid group; nausea (8.33%), vertigo (8.33%), dizziness (7.14%), skin rash (5.95%), fatigue (5.95%), somnolence (5.95%), and constipation (5.95%) for the AChEI group and nausea (14.28%), dizziness (8.57%), headaches (7.14%), and skin rashes (7.14%) in the Souvenaid+AChEI group. None of the patients presented serious side effects and all of them were temporary and resolved spontaneously.
Table 3
Treatment adverse events reported
| Souvenaid (n = 66) | AChEI (n = 84) | Souvenaid+AChEI (n = 70) | p Fisher’s Exact Test | |
| Nausea | 6 (9.0) | 7 (8.3) | 10 (14.3) | 0.31 |
| Headache | 3 (4.5) | 5 (5.9) | 5 (7.1) | 0.43 |
| Dizziness | 4 (6.1) | 6 (7.1) | 6 (8.6) | 0.15 |
| Constipation | 0 | 5 (5.9) | 4 (5.7) | 0.42 |
| Diarrhea | 1 (1.5) | 4 (4.8) | 4 (5.7) | 0.28 |
| Insomnia | 0 | 4 (4.8) | 4 (5.7) | 0.20 |
| Somnolence | 0 | 5 (5.9) | 2 (2.8) | 0.16 |
| Tremor | 0 | 3 (3.6) | 3 (4.3) | 0.11 |
| Fatigue | 0 | 5 (5.9) | 3 (4.3) | 0.13 |
| Vertigo | 0 | 7 (8.3) | 4 (5.7) | 0.26 |
| Agitation | 0 | 3 (3.6) | 4 (5.7) | 0.31 |
| Skin rashes | 0 | 5 (5.9) | 5 (7.1) | 0.05 |
| Dry mouth | 3 (4.5) | 3 (3.6) | 4 (5.7) | 0.17 |
Data are number (%).
DISCUSSION
The combination treatment of Souvenaid with AChEI for 12 months was well tolerated and showed significant improvement in cognitive functioning and behavioral symptomatology in patients suffering from early AD. These results, based on real clinical practice, suggest that combining two drugs whose actions are based on different mechanisms could provide greater efficacy in the treatment of early AD than using each drug separately. Moreover, these results may be useful for designing future prospective clinical trials to provide further evidence in support of combination therapy in patients with AD.
The combination of Souvenaid and AChEI yielded a statistically significant improvement over AChEI alone after the 12-month follow-up in MMSE, RAVLT, SVFT, PVFT, TMTA, TMTB, and NPI. Furthermore, the combined treatment also showed improvement already at 6 months in the MMSE and NPI. In the same line, the Souvenaid plus AChEI group showed a significant improvement compared to Souvenaid treatment alone at 12 months in MMSE, RAVLT and TMT-B. In addition, the combined treatment also showed an improvement already at 6 months in the MMSE. These results suggest that combined treatment may benefit performance in several cognitive areas: sustained attention, short-term memory, verbal episodic memory, working memory, linguistic competence, executive functioning, visuospatial ability, and semantic knowledge.
Regarding treatment with Souvenaid alone, a significant improvement over AChEI alone was seen in SVFT, PVFT, and NPI after the 12-month follow-up. In addition, the Souvenaid group also showed an improvement already at 6 months in SVFT and NPI. The positive effect observed in the Souvenaid group alone coincides with the findings presented by other studies showing the efficacy of Souvenaid in improving cognitive function in people with MCI and mild AD, while maintaining a good safety profile [17, 18, 21, 22, 37]. Overall, the results of these studies support the hypothesis that Souvenaid can benefit patients with mild AD. This multinutrient intervention showed beneficial effects on several cognitive functions, as well as on functional capacity, brain atrophy and disease advance. Interestingly, our findings also showed that along with the observed benefits on cognition, the Souvenaid treatment alone also yielded significant improvement on neuropsychiatric symptoms.
Regarding the benefit of the addition of Souvenaid to AChEIs, our findings showed a significant improvement at 12 months on most cognitive measures and also on neuropsychiatric measures compared to AChEI group alone or Souvenaid group alone. These superior benefits of the Souvenaid plus AChEI treatment group are especially relevant in episodic memory, as measured by the RAVLT, whose deficiency is one of the early manifestations in the initial stages of AD and is thought to be associated with synaptic dysfunction [38, 39]. The evidence of the improvement obtained by the combined treatment on cognitive function was reinforced by the positive results obtained in most of the tests used in the assessment, which seems to benefit performance not only in memory but also in other cognitive areas. Furthermore, our study also shows relevant benefits of combined treatment for neuropsychiatric symptoms compared to AChEI treatment alone.
Importantly, the clinical significance of the statistically significant effect observed with the combined treatment could be ascertained by the value of the effect sizes obtained, which reached a median Cohen’s d of 0.43 for the combined treatment compared to AChEI alone and 0.54 for Souvenaid alone. This is in agreement with data from randomized controlled trials of Souvenaid in AD that showed that effect sizes for cognitive, functional, and behavioral outcomes tend to confirm that Souvenaid can achieve clinically evident effects in patients with mild AD [40]. Nevertheless, given the retrospective nature of our study these results should be considered as indicative and exploratory and interpreted with caution.
Based on these findings, a potential synergistic effect based on the addition of different mechanisms of action exerted by both treatment groups should be considered. Since AD is a multifactorial disorder, it is of interest to investigate the association of different therapeutic approaches to address the different factors involved in its pathogenesis, including cholinergic deficit and synaptic dysfunction. The loss of the brain cholinergic neurons and the deterioration of neurotransmission related to reduced acetylcholinesterase activity are critical factors involved in AD pathogenesis [41, 42]. Therefore, improving brain acetylcholine levels by inhibiting acetylcholinesterase enzyme activity is one of the main therapeutic strategies. In this regard, the ability to restrain acetylcholinesterase action is the basis for the use of AChEIs, potentially restoring physiological levels of acetylcholine at the synapse. In this way, it would improve the functioning of the cholinergic system [42].
The specific nutrient combination of Souvenaid was specifically selected and designed to supply membrane precursors and cofactors that enhance the efficiency in phospholipid turn over, to preserve neuron membrane integrity and, consequently, extend the synaptic connectivity. This approach has been supported by the results of several studies. Based on the knowledge that the electroencephalography (EEG) signal reveals the synchronous activity of synaptic clusters and can thus be considered a derivate of underlying synaptic functionality, some EEG clinical trials have explored the effectiveness of Souvenaid on neural networks in mild AD patients [43]. The results of these studies show that Souvenaid contributes to the maintenance of brain connectivity in mild AD, which could potentially compensate for the gradual deterioration of neural networks as the disease progresses, thus supporting that Souvenaid influences connectivity and function of synapses [18, 44]. On the other hand, a cohort study of patients with MCI at risk for progression to AD used 18F-FDG PET scans as a direct indicator of synaptic function and organization. The results found that scans of patients taking Souvenaid showed less significant worsening of glucose metabolism compared to the significant worsening of those patients who did not take Souvenaid [37]. In line with findings in preclinical studies, proton magnetic resonance spectroscopy reveals that Souvenaid affects brain phospholipid metabolism in mild AD [45].
All things considered, the benefits of combined treatment with Souvenaid and AChEIs showed in our study could be based on the potential sum of the acetylcholinesterase inhibitory result caused by AChEI added to the potential positive effect of Souvenaid on development and functioning of synapses.
A strength of this study was the one-year follow-up period, which may indicate that the clinical benefit of Souvenaid plus AChEI could be related to its long-term use. This is consistent with findings from a recent clinical trial showing the positive effects of Souvenaid after 36 months of treatment on prodromal AD [22]. In addition, all patients in the study went through a complete neuropsychological evaluation based on broadly used neuropsychological tests. Semiannual evaluations favored close monitoring of clinical changes.
This study has some limitations that should be kept in mind when reading the validity of the findings. Since this was a retrospective study, assignment of patients to treatment groups could not be randomized, which could affect the attained results reached. Furthermore, the observational nature of the study does not allow causality to be concluded. Moreover, as this was a single-center study, only a limited number of patients could be recruited. Moreover, we considered AChEIs as a whole without focusing specifically on any of them. Therefore, clinical differences could be found if the AChEIs were studied individually. Nevertheless, the available scientific evidence does not show superiority of any one single AChEI [46].
Conclusions
Treatment with Souvenaid yielded cognitive and behavioral benefits in patients with early AD, probably due to the positive effect exerted on functional neuronal connectivity. The combined use of Souvenaid and AChEI led to greater benefits, probably due to the combination of different pathophysiological mechanisms so that the combined treatment seems to be superior to the treatment with AChEI alone or Souvenaid alone. Importantly, the findings of the present study pave the way for future longitudinal controlled trials to provide further evidence on the efficacy of the combination of Souvenaid and other medications in MCI due to AD and in mild to moderate AD. In addition, this study could help clinicians in the pharmacological approach to the early stages of AD.
ACKNOWLEDGMENTS
The authors would like to thank the patients and their caregivers for their participation in the study. We would also like to thank additional recruiting centers, especially the Asociación AFADAR, Archidona; the Asociación ACEA, Campo de Criptana, all of them in Spain.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
[1] | World Health Organization ((2017) ) Global action plan on the public health response to dementia 2017–2025, WHO, Geneva. |
[2] | Doody RS , Thomas RG , Farlow M , Iwatsubo T , Vellas B , Joffe S , Kieburtz K , Raman R , Sun X , Aisen PS , Siemers E , Liu-Seifert H , Mohs R ((2014) ) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370: ,311–321. |
[3] | Salloway S , Sperling R , Fox NC , Blennow K , Klunk W , Raskind M , Sabbagh M , Honig LS , Porsteinsson AP , Ferris S , Reichert M , Ketter N , Nejadnik B , Guenzler V , Miloslavsky M , Wang D , Lu Y , Lull J , Tudor IC , Liu E , Grundman M , Yuen E , Black R , Brashear HR ((2014) ) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370: ,322–333. |
[4] | Corbett A , Pickett J , Burns A , Corcoran J , Dunnett SB , Edison P , Hagan JJ , Holmes C , Jones E , Katona C , Kearns I , Kehoe P , Mudher A , Passmore A , Shepherd N , Walsh F , Ballard C ((2012) ) Drug repositioning for Alzheimer’s disease. Nat Rev Drug Discov 11: ,833–846. |
[5] | Tan CC , Yu JT , Wang HF , Tan MS , Meng XF , WangC , Jiang T , Zhu XC , Tan L ((2014) ) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis 41: ,615–631. |
[6] | Hyman BT , Phelps CH , Beach TG , Bigio EH , Cairns NJ , Carrillo MC , Dickson DW , Duyckaerts C , Frosch MP , Masliah E , Mirra SS , Nelson PT , Schneider JA , Thal DR , Thies B , Trojanowski JQ , Vinters HV , Montine TJ ((2012) ) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8: ,1–13. |
[7] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr, , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: ,280–292. |
[8] | Selkoe DJ ((2002) ) Alzheimer’s disease is a synaptic failure. Science 298: ,789–791. |
[9] | Terry RD ((2006) ) Alzheimer’s disease and the aging brain. J Geriatr Psychiatr Neurol 19: ,125–128. |
[10] | Raskin J , Cummings J , Hardy J , Schuh K , Dean RA ((2015) ) Neurobiology of Alzheimer’s Disease: Integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr Alzheimer Res 12: ,712–722. |
[11] | Wurtman RJ , Cansev M , Sakamoto T , Ulus IH ((2009) ) Use of phosphatide precursors to promote synaptogenesis. Annu Rev Nutr 29: ,59–87. |
[12] | Nitsch RM , Blusztajn JK , Pittas AG , Slack BE , Growdon JH , Wurtman RJ ((1992) ) Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A 89: ,1671–1675. |
[13] | van Wijk N , Broersen LM , de Wilde MC , Hageman RJ , Groenendijk M , Sijben JW , Kampuis PJ ((2014) ) Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J Alzheimers Dis 38: ,459–479. |
[14] | de Wilde MC , Penke B , van der Beek EM , Kuipers AA , KamphuisPJ , Broersen LM ((2011) ) Neuroprotective effects of a specific multi-nutrient intervention against Abeta42-induced toxicity in rats. J Alzheimers Dis 27: ,327–339. |
[15] | Broersen LM , Kuipers AA , Balvers M , van Wijk N , Savelkoul PJM , de Wilde MC , van der Beek EM , Sijben JWC , Hageman RJJ , Kamphuis PJGH , Kiliaan AJ ((2013) ) A specific multi-nutrient diet reduces Alzheimer-like pathology in young adult AbetaPPswe/PS1dE9 mice. J Alzheimers Dis 33: ,177–190. |
[16] | Grimm MOW , Michaelson DM , Hartmann T ((2017) ) Omega-3 fatty acids, lipids, and apoE lipidation in Alzheimer’s disease: A rationale for multi-nutrient dementia prevention. J Lipid Res 58: ,2083–2101. |
[17] | Scheltens P , Kamphuis PJGH , Verhey FRJ , Olde Rikkert MGM , Wurtman RJ , Wilkinson D , Twisk JWR , Kurz A ((2010) ) Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement 6: ,1–10.el. |
[18] | Scheltens P , Twisk JWR , Blesa R , Scarpini E , von Arnim CAF , Bongers A , Harrison J , Swinkels SHN , Stam CJ , de Waal H , Wurtman RJ , Wieggers RL , Vellas B , Kamphuis PJGH ((2012) ) Efficacy of Souvenaid in mild Alzheimer’s disease: Results from a randomized, controlled trial. J Alzheimers Dis 31: ,225–236. |
[19] | Olde Rikkert MG , Verhey FR , Blesa R , von Arnim CAF , Bongers A , Harrison J , Sijben J , Scarpini E , Vandewoude MFJ , Vellas B , Witkamp R , Kamphuis PJGH , Scheltens P ((2015) ) Tolerability and safety of Souvenaid in patients with mild Alzheimer’s disease: Results of multi-center, 24-week, open-label extension study. J Alzheimers Dis 44: ,471–480. |
[20] | Burckhardt M , Watzke S , Wienke A , Langer G , Fink A ((2020) ) Souvenaid for Alzheimer’s disease. Cochrane Database Syst Rev 12: ,CD011679. |
[21] | Soininen H , Solomon A , Visser PJ , Hendrix SB , Blennow K , Kivipelto M , Hartmann T , LipiDiDiet clinical study group ((2017) ) 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): A randomised, double-blind, controlled trial. Lancet Neurol 16: ,965–975. |
[22] | Soininen H , Solomon A , Visser PJ , Hendrix SB , Blennow K , Kivipelto M , Hartmann T , LipiDiDiet clinical study group ((2021) ) 36-month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer’s disease. Alzheimers Dement 17: ,29–40. |
[23] | Cummings J , Passmore P , McGuinness B , MokV , Chen C , Engelborghs S , Woodward M , Manzano S , Garcia-Ribas G , Cappa S , Bertolucci P , Chu LW ((2019) ) Souvenaid in the management of mild cognitive impairment: An expert consensus opinion. Alzheimers Res Ther 11: ,73. |
[24] | Sharma K ((1997) ) Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep 20: ,1479–1487. |
[25] | Morris JC ((1997) ) Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9: (Suppl 1),S173–S176. |
[26] | Viñuela F , Barroa A ((2021) ) Assessment of a potential synergistic effect of Souvenaid® in mild Alzheimer’s disease patients on treatment with acetylcholinesterase inhibitors: An observational, non-interventional study. J Alzheimers Dis 80: ,1377–1382. |
[27] | Reisberg B , Ferris SH , de Leon MJ , Crook T ((1982) ) The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 139: ,1136–1139. |
[28] | Folstein MF , Folstein SE , McHugh PR ((1975) ) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: ,189–98. |
[29] | Rey A ((1958) ) L'Examen Clinique in Psychologie, Presses Universitaires de France, Paris. |
[30] | Smith A ((1982) ) Symbol digit modalities test: Manual, Western Psychological Services, Los Angeles. |
[31] | Kaplan E , Goodglass H , Weintraub S , ((2002) ) The Boston Naming Test. 2nd ed, Lippincott Williams & Wilkins, Philadelphia. |
[32] | Reitan RM , Wolfson D ((1983) ) The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2nd ed, Neuropsychology Press, Tucson. |
[33] | Peña-Casanova J ((2002) ) Test Barcelona Revisado, Masson, Barcelona. |
[34] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: ,(Suppl 6),S10–S16. |
[35] | Hindmarch I , Lehfeld H , Jongh P , Erzigkeit H ((1998) ) The Bayer Activities Daily Living Scale (B-ADL). Dement Geriatr Cogn Disord 9: ,(Suppl 2),S20–S26. |
[36] | Cohen J ((1988) ) Statistical Power Analysis for the Behavioral Sciences, Routledge Academic, New York. |
[37] | Manzano Palomo MS , Anaya Caravaca B , Balsa Bretón MA , Muñiz Castrillo S , de la Morena Vicente A , Castro Arce E , Alves Prez MT ((2019) ) Mild cognitive impairment with a high risk of progression to Alzheimer’s disease dementia (MCI-HR-AD): Effect of Souvenaid® treatment on cognition and 18F-FDG PET scans. J Alzheimers Dis Rep 3: ,95–102. |
[38] | Jack CR Jr , Knopman DS , Jagust WJ , Shaw LM , Aisen PS , Weiner MW , Petersen RC , Trojanowski JQ ((2010) ) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: ,119–128. |
[39] | Scheff SW , Price DA , Schmitt FA , Mufson EJ ((2006) ) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27: ,1372–1384. |
[40] | Cummings J , Scheltens P , McKeith I Blesa R , Harrison JE , Bertolucci PHF , Rockwood K , Wilkinson D , Wijker W , Bennett DA , Shah RC ((2017) ) Effect size analyses of Souvenaid in patients with Alzheimer’s disease. J Alzheimers Dis 55: ,1131–1139. |
[41] | Bartus RT , Dean RL III , Beer B and Lippa AS ((1982) ). The cholinergic hypothesis of geriatric memory dysfunction. Science 217: ,408–414. |
[42] | Ibach B , Haen E ((2004) ) Acetylcholinesterase inhibition in Alzheimer’s disease. Curr Pharm Des 10: ,231–51. |
[43] | Siegel M , Donner TH , Engel AK ((2012) ) Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci 13: ,121–34. |
[44] | de Waal H , Stam CJ , Lansbergen MM , Wieggers RL , Patrick J G H KamphuisPJGH , Scheltens P , Maestú F , van Straaten ECW ((2014) ) Souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: A randomised controlled study. PLoS One 9: ,e86558. |
[45] | Rijpma A , van der Graaf M , Lansbergen MM , Meulenbroek O , Cetinyurek-Yavuz A , Sijben JW , Heerschap A , Olde Rikkert MGM ((2017) ) The medical food Souvenaid affects brain phospholipid metabolism in mild Alzheimer’s disease: Results from a randomized controlled trial. Alzheimers Res Ther 9: ,51. |
[46] | Matsunaga S , Fujishiro H , Takechi H ((2019) ) Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: A systematic review and meta-analysis. J Alzheimers Dis 71: ,513–523. |




