Consequences and Perception of the COVID-19 Pandemic on Patients and Caregivers in an Austrian Memory Clinic Population One Year After Pandemic Onset
Abstract
Background:
The COVID-19 pandemic was associated with high mortality and negative consequences for patients with Alzheimer’s disease or dementia and their caregivers. Memory clinics play an important role in enabling early dementia diagnosis and providing support for patients and their caregivers.
Objective:
This study investigates the impact of the COVID-19 pandemic and its restrictions on patients of a memory clinic and their caregivers between March 2020 and March 2021.
Methods:
We conducted a prospective, single-center, questionnaire-based, observational study to assess consequences and perception of the COVID-19 pandemic on emotion, cognitive function, social living, areas of care, and information retrieval.
Results:
Results of 255 participants’ (mean age 76.78, SD 8.9; 12% cognitively intact, 33% mild cognitive impairment, 55% dementia) and 203 caregivers’ COVID-19 questionnaires (valid response rate 71%) could be included in the study. Participants reported a prevalence of psychological symptoms associated with the pandemic between 3-20%. Caregivers living outside compared to those living with the participant reported higher rates of new onset or worsening of neuropsychiatric symptoms in participants since pandemic onset. Patients with dementia showed the lowest use of digital communication before (15.7%) and after (17.1%) pandemic onset in the diagnostic groups.
Conclusion:
The COVID-19 pandemic frequently led to social isolation and reduced cognitive stimulation due to restrictions in elderly persons with cognitive deficits resulting in negative effects on emotional and social levels. We hypothesize that the implementation and sensitization with digital communication in clinical routine could provide a useful tool to counteract these negative effects.
INTRODUCTION
Starting in 2020, the total number of patients with the novel coronavirus disease (SARS-CoV-2) and the number of associated deaths was increasing all over the world. Older persons and in particular those with diseases such as dementia or cardiovascular-diseases were at highest risk of dying [1–5]. Already in February 2020, published correspondence stated that the COVID-19 pandemic can increase the risk of worsening of existing psychiatric symptoms and may impair daily functioning and cognition in the elderly [6]. The rapidly changing environments and newly created policies are not easy to understand and to follow for patients with dementia. Furthermore, there is increasing evidence that SARS-CoV-2 may cause multiple neurological complications particularly in elderly patients with dementia. In fact, patients with dementia are at a high risk for fatal disease outcome and worsening of cognitive and behavioral symptoms due to COVID-19 [7, 8]. In the course of the COVID-19 pandemic, an increasing number of publications already reported a worsening of cognitive functions and neuropsychiatric symptoms in patients with dementia [9–14]. Furthermore, SARS-CoV-2 may increase the risk for newly developing psychiatric and mental disorders such as dementia or depressive symptoms especially among the elderly population [15–17]. However, some reports found no direct or indirect association of the COVID-19 pandemic or a SARS-CoV-2 infection and the worsening of neurologic or psychiatric symptoms [18].
Close relationships within family members and their collaboration with professional caregivers are important aspects of well-being in the everyday life of persons with dementia [19, 20]. A higher degree of social interactions is associated with fewer neuropsychiatric symptoms in this population [21]. On the other hand, social isolation is an important factor that increases the risk of dementia in older people [22]. Older people and in particular patients with dementia have a high risk of negative consequences of the COVID-19 pandemic [17]. Reasons for this include the increasing risk for a fatal SARS-CoV-2 infection with age [14, 23] and the strict COVID-19 action plan including social and physical distancing, quarantine, and social isolation [24]. Another important aspect besides the lack of personal contact, is the limited access to alternative resources of medical and psychologic support such as telemedicine services or digital communication technologies. Old people without and especially those with dementia often live alone and less often use the internet or social media [25, 26]. Independent of the pandemic, it could be shown that usage of digital social technology among older people has a high potential to combat social isolation in late life [27]. Even though the restrictions of the governments most likely saved lives, potentially negative effects of these restrictions on personal networks and on other aspects of the well-being of older adults and patients with dementia are still not widely known. There is an urgent need for prospective and retrospective clinical studies to determine the impact of the COVID-19 pandemic on patients with dementia and other neurocognitive disorders in the short- and the long-term. This prospective observational study sheds light on the personal perceptions and caregiver view of outpatients of an Austrian memory clinic during the first year of the COVID-19 pandemic.
We hypothesized that the reduction of cognitive, social, and physical activities due to the COVID-19 pandemic and the associated restrictions negatively impact patients with dementia. Especially these activities represent an important part of a non-pharmacological therapy concept for patients with dementia. Further, we assumed that the detailed evaluation of patients‘ and caregivers‘ reports via a questionnaire together with a clinical and neuropsychological examination at our memory clinic provides a comprehensive information of the vulnerable population of elderly people with cognitive decline. We aimed to gain a deeper understanding of what kinds of changes on personal social networks, emotional, cognitive, and physical symptoms elderly persons and patients with mild cognitive impairment (MCI) or dementia and their caregivers experienced. Further, this study aims to explore changes of the use of information sources and digital communication technologies related to the COVID-19 pandemic. The results of our study should improve crisis management strategies and identify difficulties especially in the use of such digital communication tools for patients with dementia and their caregivers.
MATERIALS AND METHODS
Study design
This study was designed as a prospective, single-center, questionnaire-based, observational trial. We assessed the subjective perception of the COVID-19 pandemic on emotion, cognitive function, social living, areas of care, and information retrieval using a newly developed questionnaire (participants and caregiver COVID-19 questionnaire). The study population consisted of cognitively intact elderly persons, patients with MCI or dementia, and their caregivers. We included all individuals and their caregivers with a scheduled appointment at the memory clinic (Department of Psychiatry) at the Medical University of Innsbruck for assessment of memory complaints or within their regular routine control between the reopening after the lockdown on 11 May 2020 and 23 May 2021. All participants and caregivers received the newly developed COVID-19 questionnaire by mail one week before the scheduled appointment and were asked to bring these filled out to the appointment. As part of the standard clinical procedure, participants completed a neuropsychological assessment, rating scales for everyday functioning and neuropsychiatric symptoms, and a clinical interview at their outpatient appointment. Information on somatic comorbidities, the APOE genotype, as well as current somatic and psychotropic medication including antidepressants, benzodiazepines, antipsychotics, and antidementive drugs was obtained. A cerebral imaging was scheduled if no current cerebral magnetic resonance imaging (MRI) was available.
Cognitively intact participants showed no objectifiable cognitive impairment in the neuropsychological assessments defined as not falling short of the threshold of 1 SD below the mean of scores corrected for age, sex and education and a Clinical Dementia Rating Scale (CDR) [28] score of 0.
MCI was diagnosed, according to the criteria of Petersen [29], if patients reported subjective memory complaints over the previous 6 months and showed an impaired memory function (verbal or figural) in the neuropsychological assessment of > 1.5 SD corrected for age, sex, and education and additionally had a CDR score of 0 or 0.5.
Dementia of any etiology was diagnosed in case of 1) presence of subjective memory complaints over the previous 6 months, 2) impaired neuropsychological function of > 2 SD corrected for age, sex, and education in one memory function (verbal or figural memory) and at least one other cognitive domain, 3) deficits in activities of daily living assessed with a clinical interview, and 4) a CDR score of 1 for mild, of 2 for moderate, and of 3 for severe dementia stage. Probable Alzheimer’s disease (AD) was diagnosed according to the criteria of McKhann [30] and mixed AD according to criteria of Dubois [31]. Dementia due to other etiologies was diagnosed according to ICD-10 criteria. For statistical analysis participants were divided into the diagnostic subcategories cognitive intact, MCI, and dementia.
Participants were excluded if they did not sufficiently understand the questionnaire due to severe cognitive impairment (moderate or severe dementia stage), language barrier, or unwillingness to answer the questions.
This prospective study was approved by the Ethics Committee of the Medical University of Innsbruck, Austria.
Newly developed COVID-19 questionnaire for participants and caregivers (COVID-19 questionnaire, Supplementary Table 1)
The questionnaire is divided into two parts: one for participants and one for caregivers. The time period of the survey refers to the change since the beginning of the COVID-19 pandemic and the start of its restrictions in Austria on 16 March 2020 and the scheduled visit at the memory clinic.
The participants questionnaire gathers general information on the living situation of participants (living alone or together with a partner or family), marital status, and date of filling out the questionnaire. The questionnaire for caregivers collects anonymous information on living situation (lives with the participants, lives outside) and relationship to the participants. Participants and caregivers were asked for their opinion on the necessity (necessary and correct, partly necessary, not necessary, I do not know), comprehensibility (well understandable, understandable, not understandable, not sufficient, I do not know), and sources of information regarding the local COVID-19 restrictions (caregiver, friends, newspapers, TV-news, internet).
Further, the participants and the caregiver questionnaire include questions about pandemic associated changes on topics like medical care, emotional and social state, cognitive functioning, and digital communication (internet usage, video calls, short message use). Participants and caregivers were asked to rate every question on a three-part ordinal scale ranging from 1-3 (1 = absent, 2 = sometimes present, 3 = frequently present).
The caregiver questionnaire gathers additional information regarding change of caregiver and personal burden or stressful factors since the beginning of the COVID-19 pandemic in March 2020. Every question is rated on a three-point ordinal scale ranging from 1-3 (1 = no or never, 2 = slightly or occasionally, 3 = significantly or frequently). The occurrence of physical symptoms (pain, hypertension, sleep disturbances, vertigo, movement restrictions, appetite disturbances, other symptoms) and psychological symptoms (anxiety, sadness, loneliness, depressive mood, restlessness, irritability, fatigue) since the beginning of the COVID-19 pandemic were rated as “yes” or “no” items.
Neuropsychological assessment
All participants completed a neuropsychological assessment including subtests of the “Consortium to Establish a Registry for Alzheimer’s Disease” (CERAD) battery [32]. This battery includes subtests for the assessment of verbal memory and recognition (word list learning, word list delayed recall, and word list recognition), constructional praxis (figure drawing), figural memory (delayed recall), confrontational object naming (Boston Naming Test – short version), verbal fluency (animals/min, s-words/min), cognitive flexibility (Trail Making Test A and B) as well as the Mini-Mental State Examination (MMSE) [33]. Out of these measures age and education corrected z-scores were calculated.
Questionnaires and scales
The Neuropsychiatric Inventory (NPI) [34] was used to assess frequency (range: 0-4 points), severity (1-3 points), and emerging caregiver burden (0-5 points) of twelve neuropsychiatric and behavioral symptoms.
By the CDR scale, forgetfulness, difficulties in orientation, judgment and problem solving, community affairs, home and hobbies, and care were evaluated with a caregiver or informant. An algorithm results in scores ranging from 0 to 3 (0, normal cognition; 0.5, mild impairment; 1, mild; 2, moderate; 3, severe dementia).
Depressive symptoms were assessed using the 30-items version of the Geriatric Depression Scale (GDS) [35]. The GDS questions were answered with “yes” or “no”. The cumulative score is rated on a scoring grid. The grid sets a range of 0-9 as “not depressed”, 10-14 as “mildly depressed”, 15-20 as “moderately depressed”, and 21-30 as “severely depressed”.
Statistical methods
Demographic data are presented as frequencies or means (interquartile range) according to data distribution. Normal distribution of data were verified by the Shapiro-Wilk-Test. Spearman correlation was used to correlate means of results of ordinal-scaled questions with scores of NPI, MMSE, and/or GDS total score.
Gaussian distribution was confirmed by visual analysis of the Q-Q plots and the Kolmogorov-Smirnov test. Group differences of non-Gaussian distributed variables were analyzed by the Kruskal-Wallis one-way ANOVA by ranks, or Mann-Whitney U test.
RESULTS
In total, 358 participants and their caregivers came to their scheduled appointment at the memory clinic (Department of Psychiatry) at the Medical University of Innsbruck for assessment of memory complaints or within their regular routine control between 11 May 2020 and 23 May 2021. Out of these, 263 (73.5%) completed the COVID-19 questionnaire. Eight questionnaires had to be excluded completely from statistical analysis due to a high number of missing items (N > 15%). Finally, results of 255 participants (mean age 76.78, SD 8.9, range 41-91) and 203 caregivers could be included in the study. This resulted in a valid response rate of 71% of all participants (with or without caregivers) who visited our memory clinic within the survey period. Seventeen participant questionnaires (7%) had very few questions not completed but could be included in the analysis. For this reason the number of participants differed slightly between the diagnostic groups due to some missing values (cognitively intact: range 29-31, MCI: range 77- 84, dementia: range 132-140). Clinical and demographic data of participants and living situation of caregivers are summarized in Table 1. Of included participants, 31 (12%) were cognitively intact, 84 (33%) had MCI, and 140 (55%) dementia. In the group of people with dementia 62 (44%) suffered from probable AD and 43 (31%) from mixed AD. The remaining 35 (25%) had a dementia diagnosis due to other etiology. While gender distribution, GDS score and living situation were balanced between the diagnostic groups, educational level was highest in the cognitively intact group and result of MMSE (raw value for rough estimation of severity of cognitive impairment as well as age and education corrected z-scores) was lowest in the dementia group. Group comparison of NPI total scores showed a trend towards higher scores in the dementia group followed by MCI patients.
Table 1
Clinical and demographic participants characteristics and living situation of caregivers. Group comparison of diagnostic groups
| Participants characteristics | Diagnostic group of participants | Comparison | |||||
| Mean±SD or N (%)* | Test statistic | p | df | ||||
| Total | Cog. intact | MCI | Dementia | ||||
| N = 255 | N = 31 | N = 84 | N = 140 | ||||
| Age (y) | 76.78±8.9 | 70.58±10.73 | 72.64±8.91 | 80.62±6.24 | H = 57.91 | < 0.001a | 2 |
| Education (y) | 10.76±2.72 | 11.43±1.94 | 11.21±2.99 | 10.34±2.65 | —– | 0.004a | —– |
| Male | 89 (35) | 11(36) | 30(36) | 48 (34) | χ2= 0.52 | 0.974a | 2 |
| Female | 166 (65) | 20 (64) | 54 (64) | 92 (66) | |||
| MMSE total score | 23.33±5.71 | 29.10±0.91 | 26.70±2.45 | 20.01±5.52 | H = 139.62 | < 0.001a | 2 |
| MMSE z-scoreb | – 2.46±2.73 | 0.27±0.84 | – 1.45±1.42 | – 3.79±2.91 | H = 111.67 | < 0.001a | 2 |
| GDS | 9.15±5.78 | 9.09±6.33 | 10.61±6.45 | 8.33±5.11 | H = 5.04 | 0.080a | 2 |
| NPI total score | 8.01±8.42 | 4.67±6.36 | 7.57±6.53 | 9.02±9.56 | H = 5.79 | 0.055a | – 2 |
| Living situation: | |||||||
| Alone | 87 (34) | 11 (36) | 26 (31) | 50 (36) | χ2= 0.559 | 0.756 | 2 |
| P/F | 168 (66) | 20 (64) | 58 (69) | 90 (64) | |||
| Caregiver characteristics | Diagnostic group from the | Comparison | |||||
| participants cared for | |||||||
| N** (%)* | Test statistic | p | df | ||||
| Living situation | Total | Cog. intact | MCI | Dementia | |||
| N = 203 | N = 16 | N = 59 | N = 128 | ||||
| Same household | 98 (48) | 12 (75) | 34 (58) | 52 (41) | χ2= 9.643 | 0.008 | 2 |
| Lives outside | 105 (52) | 4 (25) | 25 (42) | 76 (59) | |||
aDue to deviations from a normal distribution the Kruskal-Wallis-Test was used, bage and education corrected z-scores. P/F, with partner or family; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; cog.intact, cognitively intact; MCI, mild cognitive impairment; N, number; SD, standard deviation, *percentages are rounded; **N, number of participants with valid caregiver assessment.
Caregivers who completed the questionnaire mostly did not live in the same household with the participant. This was especially true for caregivers of patients with dementia who were in 50% of cases children or grandchildren, in 34% spouses and in the remaining 16% professional caregivers or friends.
Analysis of non-responders and excluded questionnaires
Of the 358 participants who had a scheduled appointment at the memory clinic within the survey period, 95 participants and caregivers did not complete and 8 participants insufficiently completed the COVID-19 questionnaires but had their clinical and neuropsychological examination. Of the 8 participants who insufficiently completed the questionnaire, 4 were in a severe dementia stage, 2 participants stated that they did not sufficiently understand the questionnaire, and 2 participants did not want to answer most questions. The remaining 95 non-responding participants (6 cognitively intact, 26 MCI, 54 dementia, 9 other diagnosis) had a mean age of 74.8 years (SD 11.6, range 36-98) with a mean MMSE score of 22.3 (SD 5.9, range 3-30). Eleven participants did not have sufficient knowledge of German language to answer the questionnaire. Of the 54 patients with dementia (age 79.7±8.9 years, MMSE 15.3±4.3), 15 were in a moderate to severe dementia stage (CDR score 2 or 3). The mean NPI total score of non-responding dementia patients was 16.2±5.4 and the mean GDS score was 11.8±5.3. Within the clinical examination, participants expressed as the most common reasons for not completing the questionnaire that they forgot it, that they did not understand the questions, or the questions overwhelmed them. The minority was annoyed by the questionnaire.
Participants’ perception of COVID-19 restrictions and information provided by the government
Results of the evaluation of information quality provided by the Austrian government on COVID-19 restrictions showed significant differences between the diagnostic groups (cognitively intact, MCI, dementia), while the attitude towards the necessity of COVID-19 restrictions did not. Participants from both the MCI and the cognitively intact group rated the information on COVID-19 restrictions as “well understandable” or “mostly understandable”. About one third of the patients with dementia could not assess the quality of information regarding COVID-19 restrictions provided by informational sources (Table 2).
Table 2
Participants perception on COVID-19 restrictions and given information
| Group | Comparison | |||||
| Variable | N (%)* | |||||
| Cog. intact | MCI | dementia | Test | df | p | |
| N = 29 | N = 80 | N = 133 | statistic | |||
| Necessity of the COVID-19 restrictions | χ2= 9.826 | 6 | 0.132 | |||
| necessary and correct | 22 (76) | 48 (60) | 79 (59) | |||
| partly necessary | 6 (21) | 22 (28) | 24 (18) | |||
| Not necessary | 0 (0) | 1 (1) | 2 (2) | |||
| I do not know | 1 (3) | 9 (11) | 28 (21) | |||
| Information regarding COVID-19 restrictions | χ2= 24.318 | 8 | 0.002 | |||
| well understandable | 17 (59) | 29 (36) | 39 (30) | |||
| understandable | 10 (35) | 33 (41) | 50 (39) | |||
| not understandable | 1 (3) | 4 (5) | 4 (3) | |||
| Not sufficient | 0 (0) | 6 (8) | 2 (2) | |||
| I do not know | 1(3) | 8 (10) | 35 (27) | |||
cog. Intact, cognitively intact; MCI, mild cognitive impairment; N, number; *percentages are rounded and therefore do not necessarily add up to 100%.
Primary information source about the COVID-19 pandemic dependent on diagnosis reported by participants
Comparison of the use of different information sources about the COVID-19 pandemic between the diagnostic groups showed that patients with dementia primarily gathered information from caregivers or television news but very rarely from the internet. Participants from both the MCI and the cognitively intact group mostly used newspapers and the television news as information source (Table 3). Chi2 test indicated differences between the diagnostic groups regarding the sources of information (χ2= 19.781, df = 10, p = 0.031). Participants from the cognitively intact group used up to five different information sources while patients with MCI or dementia group mostly used only one information source with a maximum of three sources during the 13 months of assessment. The correlation between the number of used information sources and the duration of the pandemic in months (first month March 2020 until 13 months in March 2021) showed a positive correlation in patients with MCI (Pearson’s correlation, p = 0.041, r = 0.227). This increase in numbers of information sources used might be explained by an increased use of newspapers and news on TV in the course of the pandemic.
Table 3
Primary information source about the COVID-19 pandemic reported by participants dependent on their diagnosis
| Group | Comparison | |||||
| Variable | N* | |||||
| Cog. intact | MCI | dementia | Test | df | p | |
| N = 31 | N = 82 | N = 134 | statistic | |||
| Information sourceb | N (%)** | |||||
| a. caregiver | 8 (26) | 18 (22) | 61 (46) | χ2= 13.765 | 2 | < 0.001 |
| b. friends | 2 (7) | 3 (4) | 9 (7) | χ2= 0.930 | 2 | 0.628 |
| c. newspapers | 17 (54) | 48 (59) | 55 (41) | χ2= 6.786 | 2 | 0.034 |
| d. television news | 24 (77) | 68 (83) | 82 (61) | χ2= 12.368 | 2 | 0.002 |
| e. internet | 4 (13) | 7 (9) | 2 (2) | χ2= 9.212 | 2 | 0.010 |
bMultiple selection possible; *N, number per group; N (%)**, number and rounded percentage of given responses per item. Due to the possibility of multiple selection of items (a-e), the numbers and percentages do not necessarily add up to 100%. cog. Intact, cognitively intact; MCI, mild cognitive impairment; N, number
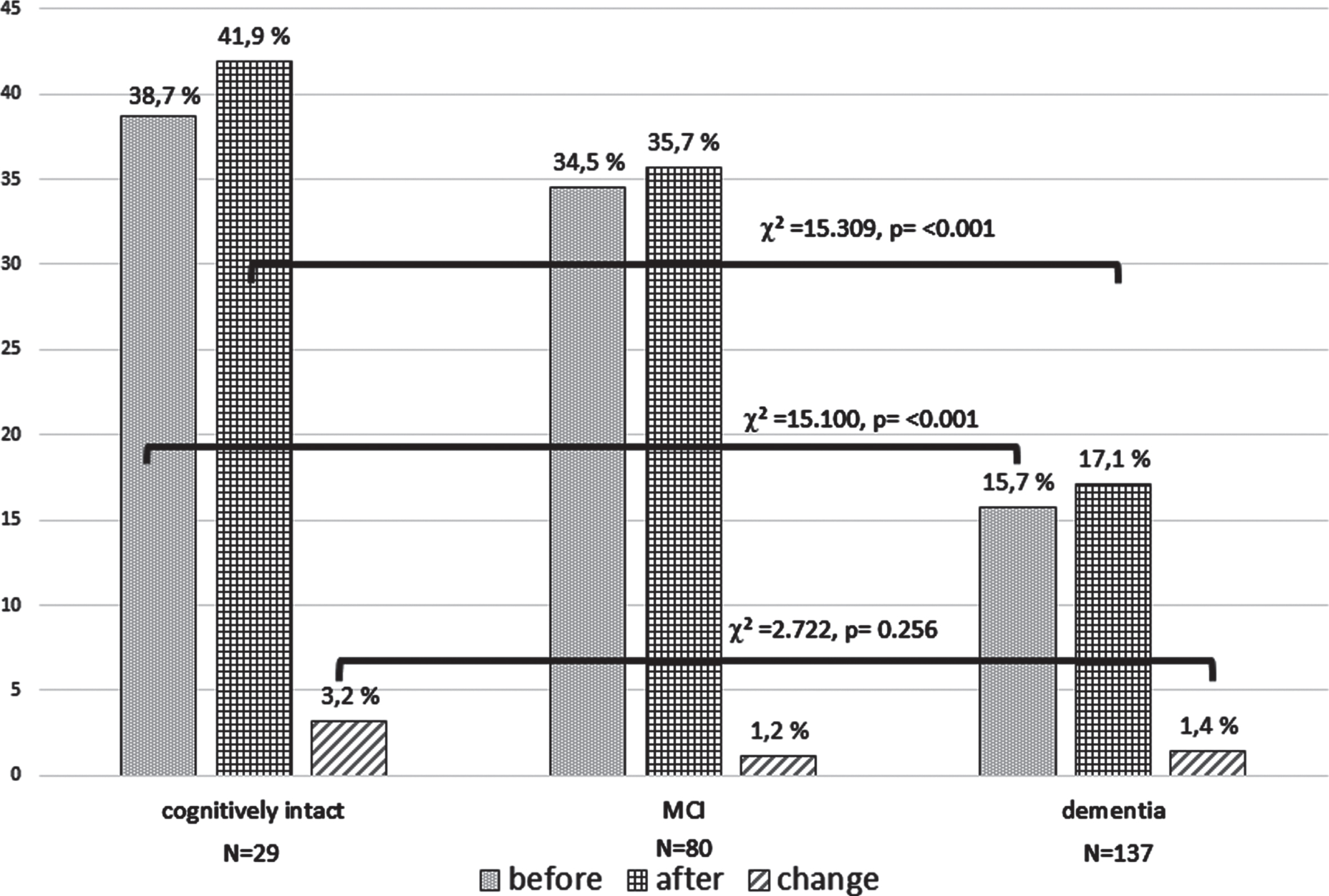
Use of digital communication before and after the beginning of the COVID-19 pandemic
Results on the use of digital communication of participants showed significant differences between the diagnostic groups before and after the pandemic onset (Fig. 1, Table 4). Participants from the cognitively intact group showed the highest use of digital communication followed by MCI patients and patients with dementia. Participants from the cognitively intact group and the MCI group had higher percentages of use of digital communication before the COVID-19 pandemic and a higher increase after pandemic onset when living with a partner. Presented results showed that patients with dementia did, even after the start of the COVID-19 pandemic, use digital communication technologies to a very low degree. However, the rate of use after the pandemic was slightly higher in participants living alone compared to participants living with a partner or with the family. Reported reasons for not starting digital communication after the onset of the COVID-19 pandemic were balanced between the three diagnostic groups for the predefined items “lack of interest” and “lack of opportunity”. Cognitive deficits as reason for not starting digital communication were most common in patients with dementia who lived with a partner.
Table 4
Use of digital communication before and after the beginning of the COVID -19 pandemic in alone living compared to in partnership/with family living participants reported by caregivers
| Variable | Group | |||||||||||
| N (%) | ||||||||||||
| Cog. intact | MCI | dementia | df | Test statistic | p | |||||||
| N = 29 (%) | N = 80 (%)* | N = 137 (%)* | ||||||||||
| Alone | P/F | Alone | P/F | Alone | P/F | Alone | P/F | Alone | P/F | |||
| Use of digital communicationa before the COVID-19 pandemic | ||||||||||||
| yes | 2 (7) | 10 (34) | 8 (10) | 21 (26) | 4 (3) | 18 (13) | 2 | χ2= 8.505 | χ2= 9.565 | 0.014 | 0.008 | |
| no | 8 (28) | 9 (31) | 14 (18) | 37 (46) | 45 (33) | 70 (51) | Total: χ2= 15.100 | Total: < 0.001 | ||||
| Start of use of digital communication after the onset of the COVID-19 pandemic | ||||||||||||
| yes | 0 (0) | 3 (10) | 1 (1) | 5 (6) | 4 (3) | 1 (1) | 2 | χ2= 1.094 | χ2= 8.245 | 0.579 | 0.016 | |
| no | 10 (35) | 16 (55) | 21 (26) | 53 (66) | 45 (33) | 87 (64) | Total: χ2= 2.722 | Total: 0.256 | ||||
| Reason for not starting to use digital communicationb | N (%)** | |||||||||||
| a. Cognitive deficits | yes | 0 (0) | 0 (0) | 5 (9) | 4 (8) | 22 (18) | 28 (23) | χ2= 1.446 | χ2= 12.381 | 0.485 | 0.002 | |
| no | 3 (21) | 11 (79) | 9 (17) | 38 (72) | 24 (20) | 49 (41) | Total: χ2= 14.422 | Total: < 0.001 | ||||
| b. Lack of interest | yes | 1 (7) | 7 (50) | 4 (8) | 19 (36) | 15 (13) | 32 (27) | 2 | χ2= 2.164 | χ2= 1.207 | 0.339 | 0.547 |
| no | 0 (0) | 6 (43) | 10 (19) | 23 (43) | 41 (34) | 45 (38) | Total: χ2= 2.659 | Total: 0.265 | ||||
| c. Lack of opportunity | yes | 0 (0) | 1 (7) | 3 (6) | 9 (17) | 18 (15) | 17 (14) | χ2= 2.024 | χ2= 1.224 | 0.364 | 0.542 | |
| no | 1 (7) | 11 (79) | 11 (21) | 33 (62) | 28 (23) | 60 (50) | Total: χ2= 3.241 | Total: 0.198 | ||||
aDigital communication include internet usage, video calls or short message use, bmultiple selection possible, partnership/with family living (P/F), *the percentages are rounded and therefore do not add up to 100%, **percentages are rounded, due to the possibility of multiple selection of answers a-c numbers and percentage deviate from number of participants per group and do not add up to 100%. cog. Intact, cognitively intact; MCI, mild cognitive impairment; N, number
Fig. 1
Comparison of the percentage and changes of use of digital communication before and after the COVID-19 pandemic onset between the three diagnostic groups. The y-axis shows percentage of patients, using digital communication (internet usage, video calls or short message use) before the COVID-19 pandemic (before March 2020), after the start of the COVID-19 pandemic (March 2020 until March 2021), and change of use of digital communication (March 2020 until March 2021).
Occurrence of physical and psychological symptoms since the beginning of the COVID-19 pandemic in participants of the memory clinic
Independently of diagnostic group, 1-23% of participants reported the onset or a worsening of physical symptoms and 10-24% of psychological symptoms within the first year of the pandemic. Physical symptoms such as pain, falls, dyspnea, headache, or tachycardia were most frequently reported in cognitively intact participants. The incidence of newly occurred or worsened psychological compared to physical symptoms was higher but comparable between the diagnostic groups (Table 5).
Table 5
Chi-square test of participant reported number of physical and psychological symptoms due to the COVID-19 pandemic in the diagnostic groups within the assessment period
| Variable | Group | |||||
| Question: Did one or more physical and/or psychological symptoms since the beginning of the COVID-19 pandemic in March 2020 newly occur? (yes/no) | Cog. intact | MCI | dementia | Test | df | p |
| N = 30 | N = 77 | N = 132 | statistic | |||
| N (%)* | ||||||
| Physical symptoms (yes) | ||||||
| a. pain | 2 (6.5)b↓ | 7 (8.3) | 7 (5.0) | χ2= 0.994 | 2 | 0.608 |
| b. hypertension | 2 (6.5) | 1 (1.2) | 4 (2.9) | χ2= 2.362 | 2 | 0.307 |
| c. sleep disorders | 7 (22.6) | 13 (15.5) | 10 (7.1) | χ2= 7.489 | 2 | 0.024 |
| d. vertigo | 4 (12.9) | 7 (8.3) | 15 (10.7) | χ2= 0.607 | 2 | 0.738 |
| e. motor disturbances | 3 (9.7) | 9 (10.7) | 17 (12.1) | χ2= 0.207 | 2 | 0.902 |
| f. loss of appetite | 1 (3.2)b↓ | 7 (8.3)b↓ | 10 (7.1) | χ2= 0.904 | 2 | 0.636 |
| g. Other symptomsa | 4 (12.8) | 4 (4.8) | 4 (2.8) | χ2= 40.601 | 2 | 0.018 |
| Psychological symptoms (yes) | ||||||
| a. anxiety | 3 (9.7) | 14 (16.7) | 13 (9.3) | χ2= 3.030 | 2 | 0.220 |
| b. sadness | 5 (16.1) | 11 (13.1) | 12 (8.6) | χ2= 2.056 | 2 | 0.358 |
| c. loneliness | 3 (9.7) | 20 (23.8) | 20 (14.3)b↓ | χ2= 4.696 | 2 | 0.096 |
| d. depressive mood | 6 (19.4)b↑ | 17 (20.2)b↓ | 22 (15.7) | χ2= 0.810 | 2 | 0.667 |
| e. agitation | 6 (19.4) | 17 (20.2) | 17 (12.1) | χ2= 2.960 | 2 | 0.228 |
| f. irritability | 3 (9.7) | 10 (11.9)b↓ | 15 (12.1) | χ2= 0.137 | 2 | 0.934 |
| g. fatigue | 6 (19.4) | 20 (23.8) | 19 (13.6) | χ2= 3.857 | 2 | 0.145 |
*N, number of selected items (a-g), multiple selection possible, due to small values percentages are rounded to one decimal place, due to the possibility of multiple selection of answers a-g numbers and percentage deviate from number of participants per group and do not add up to 100% afalls, dyspnoe, headache, tachycardia; bsignificant results of Chi-square test of number of physical and psychological symptoms with month in the course of the assessment period (1st month March 2020 until last month March 2021) in the diagnostic groups. ↓significant decrease, ↑significant increase. cog. Intact, cognitively intact; MCI, mild cognitive impairment; N, number.
Changes of physical and psychological symptoms within the first year of the COVID-19 pandemic (March 2020 until March 2021)
Within the first year of the COVID-19 pandemic, the presence of physical and psychological symptoms was associated with duration of the pandemic. Cognitively intact participants showed a higher prevalence of pain (χ2= 31.00, df = 12, p = 0.001) and loss of appetite (χ2= 31.00, df = 12, p = 0.001) in the first month of the pandemic with a decrease until March 2021. Contrary to this, in these participants the prevalence of depressive mood (χ2= 20.322, df = 12, p = 0.041) increased in the course of the first year of the pandemic.
In patients with MCI, loss of appetite (χ2= 24.455, df = 12, p = 0.003), depressive mood (χ2= 21.397, df = 12, p = 0.045) and irritability significantly decreased in the course of the pandemic (χ2= 20.691, df = 12, p = 0.047).
In patients with dementia solely the presence of loneliness (χ2= 23.367, df = 12, p = 0.025) was associated with the beginning of the COVID-19 pandemic and showed a decrease in the course of time.
Emotional and behavioral symptoms of participants reported by relatives or caregivers
Results of caregiver perception of the occurrence or worsening of emotional or behavioral symptoms in participants since the beginning of the COVID-19 pandemic showed an increase in most assessed items. Caregivers living separate from the participant compared to those living with the participants reported more often the occurrence of three of four assessed neuropsychiatric symptoms: 1) sadness and loneliness, 2) anxiety and helplessness, and 3) insomnia. The 4th item dysphoria and irritability was reported equally often independently of caregivers’ living situation. The presence of neuropsychiatric symptoms rated with the NPI at the clinical visit was positively correlated with the occurrence of all emotional or behavioral symptoms reported by caregivers. Further, a higher GDS score was associated with caregiver reported occurrence of sadness and hopelessness, anxiety and helplessness and insomnia (Table 6).
Table 6
Emotional and behavioral symptoms of participants and burden and stressful factors reported by relatives or caregiver living with the participants versus living outside and their correlation with neuropsychiatric symptoms or MMSE score and depression score of participants
| group | Mann-Whitney | Spearman correlation | |||||||
| U-test | with total score | ||||||||
| Question: Occurrence of participants emotional or behavior symptoms since the beginning of the COVID-19 pandemic in March 2020 occurrence of.. | Mean±SD | NPI total | GDS | ||||||
| Range 1-3b | score | score | |||||||
| Total | same | lives | Test | p | ρ | p | ρ | p | |
| score | household | outside | statistica | ||||||
| N = 204 | N = 98 | N = 106 | |||||||
| sadness and hopelessness | 1.60±0.71 | 1.43±0.61 | 1.75±0.76 | Z = – 3.59 | < 0.001 | 0.337 | < 0.001 | 0.329 | < 0.001 |
| dysphoria and irritability | 1.70±0.75 | 1.68±0.71 | 1.73±0.78 | Z = – 0.47 | 0.636 | 0.350 | < 0.001 | 0.107 | 0.087 |
| anxiety and helplessness | 1.65±0.74 | 1.51±0.68 | 1.77±0.77 | Z = – 2.52 | 0.012 | 0.343 | < 0.001 | 0.282 | < 0.001 |
| Insomnia | 1.58±0.74 | 1.48±0.74 | 1.68±0.73 | Z = – 2.37 | 0.018 | – 0.308 | < 0.001 | 0.257 | < 0.001 |
| Question: Change of burden or stressful factors since the beginning of the COVID-19 pandemic in March 2020 Burden due to/because of ... | Mean±SD | MMSE | GDS | ||||||
| Range 1-3c | score | score | |||||||
| The COVID-19 pandemic and restrictions | 1.90±0.71 | 1.72±0.67 | 2.09±0.71 | Z = – 3.54 | < 0.001 | – 0.184 | 0.009 | 0.229 | 0.003 |
| Extra effort of care for the participant | 1.67±0.79 | 1.52±0.75 | 1.80±0.80 | Z = – 2.71 | 0.007 | – 0.191 | 0.007 | 0.088 | 0.263 |
| General concern for the participant | 1.97±0.77 | 1.78±0.72 | 2.16±0.77 | Z = – 3.23 | < 0.001 | – 0.125 | 0.080 | 0.223 | 0.004 |
| Concern to fall ill with the SARS-cov-2 virus | 1.43±0.58 | 1.50±0.57 | 1.36±0.59 | Z = – 1.88 | 0.061 | – 0.070 | 0.323 | 0.110 | 0.162 |
| Concern to die from the SARS-cov-2 virus | 1.10±0.31 | 1.14±0.38 | 1.07±0.25 | Z = – 1.59 | 0.111 | 0.059 | 0.405 | 0.077 | 0.327 |
| Concern that the participant fall ill with the SARS-cov-2 virus | 1.79±0.71 | 1.67±0.69 | 1.92±0.71 | Z = – 2.54 | 0.011 | – 0.005 | 0.094 | 0.167 | 0.033 |
| Concern that the participant die from the SARS-cov-2 virus | 1.51±0.67 | 1.36±0.61 | 1.65±0.70 | Z = – 3.18 | 0.001 | – 0.088 | 0.215 | 0.199 | 0.011 |
| Increased financial burden | 1.24±0.54 | 1.16±0.43 | 1.30±0.64 | Z = – 1.45 | 0.148 | – 0,084 | 0.499 | 0.105 | 0.185 |
aDue to deviations from a normal distribution the Mann-Whitney U-test was used. b1 = absent/2 = sometimes present/3 = frequently present, c1 = no or never/2 = slightly or occasionally/3 = significantly or frequently. SD, standard deviation.
Additional comparison of these four neuropsychiatric symptoms between the three diagnostic groups using the Kruskal-Wallis-Test showed no significant differences. Further, correlation analysis of the MMSE score with results of these four items showed no significant association (detailed results not presented).
Burden and stressful factors for relatives or caregivers depending on their living situation
The assessment of 8 possibly burdensome and/or stressful factors due to the COVID-19 pandemic in caregivers living separate from the participants and those living with the participants indicated a slight or significant burden in all factors. Caregivers living separate from the participants reported a higher burden due to COVID-19 restrictions in general, general concerns for the participants, and in particular concerns that the participants may fall ill or die from theSARS-CoV-2 virus. Further, caregivers living separate from the participants reported more extra effort of care for the participants. Correlation analysis showed that a lower MMSE score was associated with a higher effort of care and more burden due to restrictions. A higher patient depression score at the time of assessment was associated with higher caregiver ratings in 4 out of 8 items (burden, extra effort of care, concerns to fall ill, or die from the SARS-CoV-2 virus (Table 6).
Additionally, we found significant differences between the three diagnostic groups regarding the participants/caregivers ratio (calculated as score of participants divided by the score of caregiver) on the degree of burden due to the COVID-19 pandemic and associated restrictions (Kruskal-Wallis-Test, df = 2, p = 0.003). While the ratio in the groups cognitively intact and MCI was positive (higher burden in participants rating compared to caregivers rating)— the ratio for participants with dementia was negative (lower burden in patient rating compared to caregiver rating). This result shows a higher subjective burden due to the COVID-19 pandemic in caregivers of patients with dementia compared to the burden perceived by the patients themselves (detailed results not presented).
DISCUSSION
In this prospective, observational, questionnaire-based study, we investigated the impact of the COVID-19 pandemic and its restrictions on emotion, cognitive function, social living, and areas of care in a memory clinic population. We assessed the perspective of participants and their caregivers during the first year of the pandemic in Austria. Further, we explored sources of information related to the COVID-19 pandemic and the use of digital communication technologies related to cognitive deficits and living situation of participants.
The clinical and demographic characteristics of our study population showed, as expected, in approximately 80% of patients mild to moderate cognitive deficits according to a diagnosis of dementia or MCI with the highest prevalence of AD and mixed AD. Although the gender distribution showed no significant differences between the diagnostic groups, the almost double proportion of women in the MCI and dementia group is in line with data from the literature [36, 37]. In accordance with a report of Livingston et al. (2020) [38] and others, the lowest level of education was found in the dementia group. The somehow surprising result that the NPI total score showed solely a trend toward higher sores in patients with dementia might be due to the mild to beginning moderate dementia stage of the included patients who completed the questionnaire. Taking analysis of non-responders into account, we assume that patients with moderate to severe dementia and/or a high rate of neuropsychiatric symptoms were not an appropriate study population for self-completed questionnaire studies. Based on clinical impressions within the first year of the COVID-19 pandemic, most of these patients were able to report their perception on the pandemic solely in a personal interview. Further, our results underpin the high importance of caregiver perceptions in the treatment of the elderly and in particular patients with dementia.
Participants perception of COVID-19 restrictions and information provided by the government
The evaluation regarding the subjective opinion on the necessity of COVID-19 restrictions showed no differences between participants with no, mild, or severe cognitive deficits. Most of the participants of the memory clinic rated the national COVID-19 restrictions as necessary and correct. On the other hand, about one third of patients with dementia rated the information regarding COVID-19 restrictions as not sufficient or understandable. These results suggest a disadvantage of people with cognitive deficits regarding the comprehensibility of COVID-19 restrictions and information [39].
Primary information sources and communication possibilities used within the first year of the COVID-19 pandemic
This study found that primary information sources about the COVID-19 pandemic varied between the diagnostic groups. While patients with dementia predominantly obtained their information from caregivers or television news, cognitively intact individuals and patients with MCI informed themselves through newspapers or the internet. Patients with dementia showed a very low rate of internet use and digital communication tools as an information source. Most importantly, only a small number of patients with MCI or dementia were able to start using digital communication after pandemic onset. Interestingly, the use of digital communication was higher in participants living in a partnership or the family across all diagnostic groups with highest rates in the groups cognitively intact individuals and MCI. Our results are in line with previous publications that highlight the important implication of digital communication in times of social isolation and lockdowns but also the low use and availability in the elderly population and especially dementia patients [40]. Already many years before the pandemic, it was reported that digital communication can improve well-being in elderly people [41]. Even after the beginning of the pandemic, studies showed that telemedicine and tele rehabilitation could provide treatment effects comparable with face– to-face interventions in dementia care [42] or other indications [43]. Nonetheless, the elderly population has always been considered to be “hard-to-reach” for digital technologies due to lack of interest, economic constraints, or cognitive deficits. Our findings indicate that, despite technological advances, the use of digital communication is still not very widespread, available, or frequently used by the elderly. Actually, one would assume that in particular people who live alone would have used digital communication more often to prevent social isolation and loneliness. This might not be true for the older population who seems to be dependent on help and support to use such new technologies. In support of this, a study by Bonsaksen et al. found a higher rate and psychological benefit of video-communication in people aged 60-69 but not in oldest age which is often associated with cognitive decline [44]. We conclude that digital communication offers a number of benefits for elderly people to prevent social isolation and promote social and cognitive activities. Notwithstanding, our results clearly show that there is a high need to bring digital communication technologies closer to old people and especially patients with dementia.
Regarding the use of information sources, our results are in agreement with other studies published within the first year of the pandemic that showed that television is an important and frequently used source of information in the elderly population [45, 46]. To the best of our knowledge, our study is the first that analyzed the use of type and number of information sources in cognitively intact elderly, patients with MCI, and dementia patients in the course of the first year of the COVID-19 pandemic. An important finding was that patients with MCI have expanded the number of information sources over time while patients with dementia have not. Providing reliable and understandable information during the pandemic must be considered as an essential aspect of the pandemic management. In our study, approximately 50% of dementia patients obtained information about the COVID-19 pandemic mainly from caregivers. In contrast to information from newspapers or television news, caregivers of patients with dementia could be individually addressed and supported in a clear and proper communication on the pandemic and associated restrictions. As reported in studies before and after the beginning of the pandemic, internet-based interventions and information platforms have become more important in dementia care within the last years [47–49]. However, our results show that the internet was only rarely used as a source of information on the COVID-19 pandemic in the elderly and especially in patients with dementia. Therefore, we propose that internet-based information has to be actively addressed to caregivers of patients with dementia. In order to reach people with dementia via the internet, the range of suitable trainings for them must be expanded.
Occurrence or worsening of physical and psychological symptoms within the first year of the COVID-19 pandemic
The results of this study indicate that the COVID-19 pandemic and associated restrictions led to an increase of number of psychological and physical symptoms in the elderly. We found that worsening or the occurrence of sleep disorders, motor disturbances, and vertigo were the most prevalent physical symptoms reported in our study cohort. In particular the increase of sleep disturbances may negatively influence cognitive function and behavior due to, e.g., disturbances of neuronal networks and neurotransmitter release [50]. Further, our results provide evidence of different prevalences of physical and psychological symptoms related to the particular diagnostic groups in the course of the first year of the pandemic. Cognitively intact participants and patients with MCI reported especially in the first months of the pandemic an increase of pain and a loss of appetite with a subsequent decrease until March 2021. These results are in line with previous findings from the beginning of the COVID-19 pandemic reporting a critical increase of depression, stress, and insomnia in the general population [51] and patients with dementia [39, 52]. Our findings on the time course of symptoms experienced support the hypothesis of Bakker et al. [53] that patients with MCI have the ability to adapt to the difficult circumstances of the pandemic and associated restrictions. Conversely, this means that patients with dementia and their caregivers need a higher level of attention and support to counteract negative consequences.
Emotional and behavioral symptoms
We found significant differences in caregiver perception of the occurrence of emotional and behavioral symptoms in outpatients of our memory clinic within the first year of the COVID-19 pandemic (Table 6). Caregivers not living with the participants reported a higher rate of sadness and loneliness, anxiety and helplessness, and insomnia since the beginning of the pandemic compared to caregivers living with the participants. Correlation analysis with the GDS score and results from NPI during the outpatient visit correlated with the caregivers’ perception independently of the caregivers’ living situation. Our results are in line with results of Wurm et al. in an Austrian memory clinic population who found an increase of neuropsychiatric symptoms associated with lockdown restrictions at the beginning of the pandemic [54]. Numerous reports published within the first year of the pandemic also reported a critical increase of neuropsychiatric symptoms directly related with a SARS-CoV-2 infection or in the general population [16]. In elderly individuals, factors such as social distancing and cancelation of cognitive stimulation programs might have led to loneliness and in further consequence to the occurrence or worsening of behavioral symptoms such as apathy, anxiety, and agitation [55]. In contrast to others, our study takes into account the influence of the caregivers’ living situation on the occurrence and perception of emotional and behavioral symptoms in elderly participants with different degrees of cognitive impairment. The higher rates of emotional and behavioral symptoms of participants reported by caregivers living separated from the participants may have different causes. Firstly, the forced separation due to restrictions may have interfered with caregiver support and have led to poorer health monitoring and omission of professional care. Secondly, the loss or decrease of interpersonal interaction may have increased the risk of insufficient nutrition, dehydration, or lack of medication intake. Finally, the general increase of insecurity and anxiety may have sensitized caregivers to symptoms in the vulnerable population of elderly persons, especially with cognitive deficits.
Burden and stressful factors for relatives or caregivers
The evaluation of stressful factors for caregivers in connection with the COVID-19 pandemic showed a significant influence of caregivers’ living situation on burden and concern for the participants. Further, depressive mood and more severe cognitive deficits were associated with higher caregiver burden due to COVID-19 restrictions. Concerns that the participant dies or falls ill with the SARS-CoV-2 virus and extra efforts of care were significantly more prevalent in caregivers who do not live in the same household as the participants. We presume that factors such as social distancing and reduction of personal contacts may have reinforced these stressful factors in caregivers. In support of this a study by Ronan et al., conducted independently of the COVID-19 pandemic, reported a high caregiver burden in independently living adult children caring for dementia patients [56].
Limitations
This study is limited by its retrospective design in which participants and caregivers were asked to recall facts or symptoms from the past. Especially in a study population with a high percentage of participants with memory deficits, the validity of collected data might be limited. To address this bias, we excluded patients in moderate or severe dementia stages and those with a high percentage of missing items. Further, the clinical and neuropsychodiagnostic evaluation of participants close in time to the completion of the questionnaire allowed a good estimate of the validity of data and given answers. Another limitation of this study is the single center design and the inclusion of a very selected memory clinic population in one region in Austria. Consequently, our results cannot be generalized to the general elderly population or people of other countries who had more strict or other COVID-19 restrictions. Further, our results of the negative effects of the COVID-19 pandemic on especially emotional and behavioral symptoms cannot be put into a causal relationship due to a missing control group.
Conclusion
The current COVID-19 pandemic has a significant impact on many areas of daily life. This interruption of routine activities is particularly stressful for cognitively impaired elderly and their caregivers due to their sensitivity to environmental changes. In summary, our results showed that the COVID-19 pandemic and its restrictions had a number of negative consequences on social, emotional and cognitive factors in elderly people with cognitive impairment. Despite the importance of digital communication in times of social distancing and lockdown, elderly people with cognitive decline oftentimes are not able to use these tools without the support of caregivers. In the future, it is important to promote digital media in order to prevent negative consequences of the pandemic on the vulnerable group of elderly people with cognitive decline and their caregivers.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and its supplementary material.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220887.
REFERENCES
[1] | Wilson N , Kvalsvig A , Barnard LT , Baker MG ((2020) ) Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis 26: , 1339–1441. |
[2] | Sun H , Ning R , Tao Y , Yu C , Deng X , Zhao C , Meng S , Tang F , Xu D ((2020) ) Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: A retrospective study. J Am Geriatr Soc 68: , E19–E23. |
[3] | Dowd JB , Andriano L , Brazel DM , Rotondi V , Block P , Ding X , Liu Y , Mills MC ((2020) ) Demographic science aids in understanding the spread and fatality rates of COVID-19. Proc Natl Acad Sci U S A 117: , 9696–9698. |
[4] | Kuo CL , Pilling LC , Atkins JL , Masoli JAH , Delgado J , Kuchel GA , Melzer D ((2020) ) ApoE e4e4 genotype and mortality with COVID-19 in UK Biobank. J Gerontol A Biol Sci Med Sci 75,: , 1801–1803. |
[5] | Naughton SX , Raval U , Pasinetti GM ((2020) ) Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J Alzheimers Dis 76: , 21–25. |
[6] | Yang Y , Li W , Zhang Q , Zhang L , Cheung T , Xiang YT ((2020) ) Mental health services for older adults in China during the COVID-19 outbreak. Lancet Psychiatry 7: , e19. |
[7] | Perry G ((2020) ) Alzheimer’s disease patients in the crosshairs of COVID-19. J Alzheimers Dis 76: , 1. |
[8] | Greenberg NE , Wallick A , Brown LM ((2020) ) Impact of COVID-19 pandemic restrictions on community-dwelling caregivers and persons with dementia, Psychol Trauma 12: , S220–S221. |
[9] | Canevelli M , Valletta M , Toccaceli Blasi M , Remoli G , Sarti G , Nuti F , Sciancalepore F , Ruberti E , Cesari M , Bruno G ((2020) ) Facing dementia during the COVID-19 outbreak. J Am Geriatr Soc 68: , 1673–1676. |
[10] | Capozzo R , Zoccolella S , Frisullo ME , Barone R , Dell’Abate MT , Barulli MR , Musio M , Accogli M , Logroscino G ((2020) ) Telemedicine for delivery of care in frontotemporal lobar degeneration during COVID-19 pandemic: Results from Southern Italy. J Alzheimers Dis 76: , 481–489. |
[11] | Lara B , Carnes A , Dakterzada F , Benitez I , Pinol-Ripoll G ((2020) ) Neuropsychiatric symptoms and quality of life in Spanish patients with Alzheimer’s disease during the COVID-19 lockdown. Eur J Neurol 9: , 1744–1747. |
[12] | Defrancesco M , Bancher C , Dal-Bianco P , Hinterhuber H , Schmidt R , Struhal W , Ransmayr G , Stogmann E , Marksteiner J ((2021) ) [Positionpaper of the Austrian Alzheimer Association (Österreichische Alzheimer Gesellschaft, ÖAG): Effects of the COVID-19 pandemic in Austria on people with dementia and their care environment-problem areas, recommendations, and strategies]. Neuropsychiatr 35: , 35–47. |
[13] | Iodice F , Cassano V , Rossini PM ((2021) ) Direct and indirect neurological, cognitive, and behavioral effects of COVID-19 on the healthy elderly, mild-cognitive-impairment, and Alzheimer’s disease populations. Neurol Sci 42: , 455–465. |
[14] | Hwang JM , Kim JH , Park JS , Chang MC , Park D ((2020) ) Neurological diseases as mortality predictive factors for patients with COVID-19: A retrospective cohort study. Neurol Sci 41: , 2317–2324. |
[15] | Mahalakshmi AM , Ray B , Tuladhar S , Bhat A , Paneyala S , Patteswari D , Sakharkar MK , Hamdan H , Ojcius DM , Bolla SR , Essa MM , Chidambaram SB , Qoronfleh MW ((2021) ) Does COVID-19 contribute to development of neurological disease? Immun Inflamm Dis 9: , 48–58. |
[16] | Alonso-Lana S , Marquie M , Ruiz A , Boada M ((2020) ) Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front Aging Neurosci 12: , 588872. |
[17] | Manca R , De Marco M , Venneri A ((2020) ) The impact of COVID-19 infection and enforced prolonged social isolation on neuropsychiatric symptoms in older adults with and without dementia: A review. Front Psychiatry 11: , 585540. |
[18] | Zhuang YP , Zhong HJ ((2021) ) Impact of COVID-19 on the clinical status of patients with Wilson disease. World J Gastroenterol 27: , 4248–4251. |
[19] | Nguyen M , Pachana NA , Beattie E , Fielding E , Ramis MA ((2015) ) Effectiveness of interventions to improve family-staff relationships in the care of people with dementia in residential aged care: A systematic review protocol. JBI Database System Rev Implement Rep 13: , 52–63. |
[20] | Bremer D , Inhestern L , von dem Knesebeck O ((2017) ) Social relationships and physician utilization among older adults-A systematic review, PLoS One 12: , e0185672. |
[21] | Arai A , Khaltar A , Ozaki T , Katsumata Y ((2021) ) Influence of social interaction on behavioral and psychological symptoms of dementia over 1 year among long-term care facility residents. Geriatr Nurs 42: , 509–516. |
[22] | Shen C , Rolls E , Cheng W , Kang J , Dong G , Xie C , Zhao XM , Sahakian B , Feng J ((2022) ) Associations of social isolation and loneliness with later dementia. Neurology 99: , e164–e175. |
[23] | Miyashita S , Yamada T , Mikami T , Miyashita H , Chopra N , Rizk D ((2020) ) Impact of dementia on clinical outcomes in elderly patients with coronavirus 2019 (COVID-19): An experience in New York. Geriatr Gerontol Int 20: , 732–734. |
[24] | Kremers EM , Janssen JHM , Nieuwboer MS , Olde Rikkert MGM , Peeters G ((2022) ) The psychosocial adaptability of independently living older adults to COVID-19 related social isolation in the Netherlands: A qualitative study. Health Soc Care Community 30: , e67–e74. |
[25] | Hackett RA , Steptoe A , Cadar D , Fancourt D ((2019) ) Social engagement before and after dementia diagnosis in the English Longitudinal Study of Ageing. PLoS One 14: , e0220195. |
[26] | Kotwal AA , Holt-Lunstad J , Newmark RL , Cenzer I , Smith AK , Covinsky KE , Escueta DP , Lee JM , Perissinotto CM ((2021) ) Social isolation and loneliness among San Francisco Bay Area older adults during the COVID-19 shelter-in-place orders. J Am Geriatr Soc 69: , 20–29. |
[27] | Cotterell N , Buffel T , Phillipson C ((2018) ) Preventing social isolation in older people. Maturitas 113: , 80–84. |
[28] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412–2414. |
[29] | Petersen RC , Doody R , Kurz A , Mohs RC , Morris JC , Rabins PV , Ritchie K , Rossor M , Thal L , Winblad B ((2001) ) Current concepts in mild cognitive impairment. Arch Neurol 58: , 1985–1992. |
[30] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr. , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[31] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[32] | Rosen WG , Mohs RC , Davis KL ((1984) ) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141: , 1356–1364. |
[33] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[34] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: , S10–16. |
[35] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1982) ) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: , 37–49. |
[36] | Chua XY , Ha NHL , Cheong CY , Wee SL , Yap PLK ((2019) ) The changing profile of patients in a geriatric medicine led memory clinic over 12 years. J Nutr Health Aging 23: , 310–315. |
[37] | Ruitenberg A , Ott A , van Swieten JC , Hofman A , Breteler MM ((2001) ) Incidence of dementia: Does gender make a difference? Neurobiol Aging 22: , 575–580. |
[38] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimaki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbaek G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[39] | Ryoo N , Pyun JM , Baek MJ , Suh J , Kang MJ , Wang MJ , Youn YC , Yang DW , Kim SY , Park YH , Kim S ((2020) ) Coping with dementia in the middle of the COVID-19 pandemic. J Korean Med Sci 35: , e383. |
[40] | Smith AC , Thomas E , Snoswell CL , Haydon H , Mehrotra A , Clemensen J , Caffery LJ ((2020) ) Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare 26: , 309–313. |
[41] | Ihm J , Hsieh YP ((2015) ) The implications of information and communication technology use for the social well-being of older adults. Inf Commun Soc 18: , 1123–1138. |
[42] | Yi JS , Pittman CA , Price CL , Nieman CL , Oh ES ((2021) ) Telemedicine and dementia care: A systematic review of barriers and facilitators. J Am Med Dir Assoc 22: , 1396–1402e1318. |
[43] | Cassarino L , Santoro F , Gelardi D , Panerai S , Papotto M , Tripodi M , Cosentino FII , Neri V , Ferri R , Ferlito S , Modica D , Fisicaro F , Pennisi M , Bella R , Lanza G ((2022) ) Post-stroke aphasia at the time of COVID-19 pandemic: A telerehabilitation perspective. J Integr Neurosci 21: , 8. |
[44] | Bonsaksen T , Thygesen H , Leung J , Ruffolo M , Schoultz M , Price D , Ostertun Geirdal A ((2021) ) Video-based communication and its association with loneliness, mental health and quality of life among older people during the COVID-19 outbreak. Int J Environ Res Public Health 18: , 6284. |
[45] | Dura-Perez E , Goodman-Casanova JM , Vega-Nunez A , Guerrero-Pertinez G , Varela-Moreno E , Garolera M , Quintana M , Cuesta-Vargas AI , Barnestein-Fonseca P , Gomez Sanchez-Lafuente C , Mayoral-Cleries F , Guzman-Parra J ((2022) ) The impact of COVID-19 confinement on cognition and mental health and technology use among socially vulnerable older people: Retrospective cohort study. J Med Internet Res 24: , e30598. |
[46] | Goodman-Casanova JM , Dura-Perez E , Guzman-Parra J , Cuesta-Vargas A , Mayoral-Cleries F ((2020) ) Telehealth home support during COVID-19 confinement: Survey study among community-dwelling older adults with mild cognitive impairment or mild dementia. J Med Internet Res 22: , e19434. |
[47] | Leng M , Zhao Y , Xiao H , Li C , Wang Z ((2020) ) Internet-based supportive interventions for family caregivers of people with dementia: Systematic review and meta-analysis. J Med Internet Res 22: , e19468. |
[48] | Boots LM , de Vugt ME , van Knippenberg RJ , Kempen GI , Verhey FR ((2014) ) A systematic review of Internet-based supportive interventions for caregivers of patients with dementia. Int J Geriatr Psychiatry 29: , 331–344. |
[49] | Nakanishi M , Yamasaki S , Endo K , Niimura J , Ziylan C , Bakker T , Granvik E , Nagga K , Nishida A ((2021) ) e-Learning and web-based tools for psychosocial interventions addressing neuropsychiatric symptoms of dementia during the COVID-19 pandemic in Tokyo, Japan: Quasi-experimental study. JMIR Med Educ 7: , e30652. |
[50] | Lanza G , DelRosso LM , Ferri R ((2022) ) Sleep and homeostatic control of plasticity. Handb Clin Neurol 184: , 53–72. |
[51] | Rossi R , Socci V , Talevi D , Mensi S , Niolu C , Pacitti F , Di Marco A , Rossi A , Siracusano A , Di Lorenzo G ((2020) ) COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatry 11: , 790. |
[52] | Cagnin A , Di Lorenzo R , Marra C , Bonanni L , Cupidi C , Laganà V , Rubino E , Vacca A , Provero P , Isella V , et al. ((2020) ) Behavioral andpsychological effects of coronavirus disease-19 quarantine inpatients with dementia. Front Psychiatry 11: , 578015. |
[53] | Bakker ED , van Maurik IS , Mank A , Zwan MD , Waterink L , van den Buuse S , van den Broeke JR , Gillissen F , van de Beek M , Lemstra E , van den Bosch KA , van Leeuwenstijn M , Bouwman FH , Scheltens P , van der Flier WM ((2022) ) Psychosocial effects of COVID-19 measures on (pre-)dementia patients during second lockdown. J Alzheimers Dis 86: , 931–939. |
[54] | Wurm R , Parvizi T , Silvaeih S , Berger-Sieczkowski E , Goeschl S , Konig T , Lehrner J , Stogmann E ((2022) ) Reduction of physical activity during the COVID-19 pandemic is related to increased neuropsychiatric symptoms in memory clinic patients. Clin Med (Lond) 22: , 177–180. |
[55] | Simonetti A , Pais C , Jones M , Cipriani MC , Janiri D , Monti L , Landi F , Bernabei R , Liperoti R , Sani G ((2020) ) Neuropsychiatric symptoms in elderly with dementia during COVID-19 pandemic: Definition, treatment, and future directions. Front Psychiatry 11: , 579842. |
[56] | O’Caoimh R , Calnan M , Dhar A , Molloy DW ((2021) ) Prevalence and predictors of caregiver burden in a memory clinic population. J Alzheimers Dis Rep 5: , 739–747. |




