Do They Align? Congruence Between Patient Preferences of People Living with Cognitive Impairments and Physicians’ Judgements for Person-Centered Care: An Analytic Hierarchy Process Study
Abstract
Background:
Person-centered care (PCC) requires knowledge about patient preferences. Among people living with cognitive impairments (PlwCI), evidence on quantitative, choice-based preferences, which allow to quantify, weigh, and rank care elements, is limited. Furthermore, data on the congruence of patient preferences with physicians’ judgements for PCC are missing. Such information is expected to support the implementation of PCC; state-of-the-art medical care aligned with patients’ preferences.
Objective:
To elicit patient preferences and physicians’ judgements for PCC and their congruence.
Methods:
Data from the mixed-methods PreDemCare study, including a cross-sectional, paper-and-pencil, interviewer-assisted analytic hierarchy process (AHP) survey conducted with n = 50 community-dwelling PlwCI and n = 25 physicians. Individual AHP weights (preferences/judgements) were calculated with the principal eigenvector method and aggregated per group by aggregation of individual priorities mode. Individual consistency ratios (CRs) were calculated and aggregated per group. Group differences in preferences/judgements were investigated descriptively by means and standard deviations (SDs) of AHP weights, resulting ranks, and boxplots. Additionally, differences between groups were investigated with independent paired t-test/Mann Whitney U-test. Sensitivity of AHP results was tested by inclusion/exclusion of inconsistent respondents, with an accepted threshold at CR≤0.3 for patients, and CR≤0.2 for physicians, due to better cognitive fitness of the latter group.
Results:
Patient preferences and physicians’ judgements did not differ significantly, except for the criterion Memory Exercises (AHP weights (mean (SD)): 0.135 (0.066) versus 0.099 (0.068), p = 0.01). We did not see rank-reversals of criteria after exclusion of inconsistent participants. Mean CR for patients at the criteria level was 0.261, and 0.181 for physicians.
Conclusion:
Physicians’ judgements in our setting aligned well with patients’ preferences. Our findings may be used to guide the implementation of preference-based PCC.
INTRODUCTION
Populations around the globe face demographic aging [1]. An increase in age-associated diseases, e.g., dementia diseases, is a challenge for health care systems worldwide [2]. Recent evidence from the Global Burden of Disease Study estimates the number of people living with a dementia disease to increase from 57.4 (95% uncertainty interval (UI) 50.4–65.1) million cases globally in 2019 to 152.8 (UI 130.8–175.9) million cases by 2050 [3]. In the development of dementia diseases, subjective and objective evidence of cognitive decline, e.g., mild cognitive impairment (MCI), have been found as transitional states, suggesting an increased risk for the development of a dementia disease [4, 5]. Currently, no curative disease-modifying treatment for people living with cognitive impairments (PlwCI) exists. PlwCI need a timely differential diagnosis [2, 6] and care, which ensures a high quality of life (QoL) [7].
According to the Alzheimer’s Association Dementia Care Practice Recommendations, a person-centered focus is the core of individualized and high-quality care across all care settings and throughout the disease course [7]. Over the years, person-centered care (PCC) has been included in many countries’ national guidelines and dementia plans [8–14], aimed at an improvement of QoL. PCC practices usually follow a non-pharmacological, sociopsychological treatment approach and are often delivered as multi-modal interventions [15], which have shown some success in delay of cognitive decline [3]. The PCC concept requires person customization of care [16], which in turn requires knowledge about the care recipient’s needs and preferences [17–20]. Among PlwCI and dementia, some evidence about preferences exists. However, evidence about preferences elicited through quantitative, in particular choice-based preference methods is limited [21, 22]. This includes a consideration of what can be defined as a “preference”. “Preference” or “prefer” stems from Latin “praeferre”, which means “place or set before” [23]. A preference can hence be defined as “1b: the power or opportunity of choosing” or “3: the act, fact or principle of giving advantages to some over others” [24], which may imply the necessity to make a choice to express a preference. Harrison Dening et al. [25] elicited preferences from dyads (people living with dementia and their family carers) during qualitative interviews. Van Haitsma et al. developed an extensive Preferences for Everyday Living Inventory (PELI) for elicitation of preferences in community-dwelling aged adults by inter alia (i.a.) Likert-type scales and open-ended questions [20]. These methods fall short to quantify, weigh, and rank patient-relevant elements of care, to measure their relative importance and identify most/least preferred choices, id est (i.e.) per definition “preferences”. Such information can be assessed with quantitative, choice-based preference measurement techniques from multi-criteria decision analysis (MCDA) [26]. MCDA techniques commonly used in health care research include i.a. discrete choice experiments (DCEs) [27], Best Worse Scaling (BWS) [28], and the Analytic Hierarchy Process (AHP) [29, 30]. For elicitation of quantitative, choice-based preferences among PlwCI, the AHP has been suggested suitable, due to the simple pairwise comparisons with only two individual aspects of a complex decision problem [31]. With the AHP, which supports systematic decision-making that takes multiple criteria into account, it may be possible to involve PlwCI in future care decisions (patient participation and shared decision-making) and ensure implementation of truly PCC for PlwCI.
To the best of our knowledge, the alignment of patient preferences with physicians’ judgments for PCC of PlwCI has not been investigated. Earlier studies of patient preferences versus physicians’ judgements in other indication areas found that experts’ judgements do not correlate well with subjective preferences of patients [32]. Knowledge about physicians’ judgments and their alignment with PlwCIs’ preferences is important, as physicians make decisions for their patients, are responsible for the diagnosis and monitoring of cognitive decline in their patients, and the provision of PCC, i.e., state of the art medical care aligned with patient’s preferences.
Hence, the aim of this study was to elicit patient preferences and physicians’ judgements for PCC of PlwCI, including an assessment of congruence of patient preferences and physicians’ judgements.
MATERIALS AND METHODS
Study design, sample size, study population, and setting
This report is based on data from the main study in the sequential mixed methods PreDemCare study [33], which followed the core components in the design of a patient preference study using methods of MCDA [34, 35]. The study team developed, pretested, and conducted a cross-sectional, (assisted) paper-and-pencil AHP survey. Detailed information about the complete course of the PreDemCare study can be found in the study protocol (see Mohr & Rädke et al. [33]).
Due to lack of an appropriate sample size calculation method for an AHP survey, we followed Ijzerman et al. [36], and applied the equation for sample size determination used in conjoint analysis ((NxTxA)/C≥500, where N = number of respondents, T = number of choice sets per respondent, A = number of scenarios per choice set, and C = maximum number of levels) [37, 38]. Thus, we needed to include a minimum of n = 24 participants per group. As we planned to conduct subgroup analyses including respective statistical analyses, we recruited n = 75 participants (n = 50 PlwCI and n = 25 physicians) [33]. Community-dwelling PlwCI for the patient survey were selected from clinical trials (ClinicalTrials.gov identifiers: NCT04741932, NCT01401582, NCT03359408, German Clinical Trials Register Reference No.: DRKS00025074) and the memory clinic at site of the DZNE Rostock/Greifswald, Mecklenburg Western-Pomerania, Germany. Eligibility criteria were:≥60 years, indication of MCI or early- to moderate-stage dementia by diagnosis or cognitive test-result (e.g., DemTect < 13 [39], Mini-Mental State Examination (MMSE) < 27 [40, 41]), capable to understand written and oral German, written consent provided by patient/legal guardian [33]. Study nurses identified eligible patients and functioned as gatekeepers to access the PlwCI for the AHP survey, as they are known and perceived as trustworthy by participants. The gatekeepers emphasized the independence of this study from the clinical trials. Informal caregivers (CGs) were invited to join as silent supporters. Additionally, the study nurses identified eligible physicians from their networks (with experience (past/current) in the treatment of dementia patients, from any setting in the federal state Mecklenburg-Western Pomerania, any age group, any specialty), who subsequently were invited via phone, e-mail, or ground mail to participate in the (non-assisted) AHP survey. The PreDemCare study [33] was evaluated and approved by the Ethics Committee at the University Medicine Greifswald (Ref.-No.: BB018-21).
Data collection
Decision goal, (sub)criteria the AHP decision hierarchy, and survey
In line with recommendations [34, 35], the identification of the decision goal and sub(criteria) was initiated based on results from a previous literature study about key intervention categories to provide PCC in dementia [15]. Literature-derived conceptual (sub)criteria were reviewed by a small expert panel with n = 2 Dementia Care Managers (DCMs), i.e., dementia-specific qualified nurses [42–44] from site. Subsequently, individual interviews including a card game with n = 10 PlwCI and n = 3 informal/family CGs were conducted to identify patient-relevant (sub)criteria of PCC for inclusion in the AHP decision hierarchy. Detailed information about the qualitative interviews is reported in Mohr et al. [45]. The identified (sub)criteria were structured into an AHP decision hierarchy with 6×2 (sub)criteria to not cognitively overburden the decision-makers [46, 47]. A preliminary AHP survey for both patients and physicians was developed. Both survey versions were reviewed and pretested extensively during two clinical expert panels with n = 4 DCMs and n = 4 physicians to ensure content validity, and during individual pretests with n = 11 PlwCI as experts by experience and n = 3 family CGs to ensure face validity [48]. Subsequently, we finalized the AHP decision hierarchy and survey versions.
All n = 75 participants in this report completed the surveys individually. Among physicians, the paper-pencil questionnaire was distributed via e-mail or ground mail. Among patients, data were collected as interviewer-assisted paper-pencil questionnaires in their homes or daycare centers from October 2021 to January 2022. To ensure a comfortable and non-stressful survey situation, PlwCI could invite their informal CGs to support them during the survey. It was emphasized that informal CGs should not act as proxies and answer questions on behalf of the PlwCI. The AHP survey had to be interviewer-assisted, as many patients had visual impairments and needed help with reading. The interviewer conducted all surveys under strict adherence to a standardized interviewing procedure by Danner et al. [31]. The choice to have only one interviewer assist was based on observations from the previous interviews [45, 48], where attendance of two interviewers had resulted in nervousness among some PlwCI. All participants were informed about the purpose and content of the study, i.e., to elicit their care preferences and later on compare these to the physicians’ judgements. Participation required prior provided informed written consent, which the PlwCI or a legal guardian could provide.
The questionnaire for both groups was structured as follows: 1) a description of the study and an introduction to the criteria in lay language, including an example question with a pairwise comparison example to choose a side dish oriented in Danner et al. [31], 2) first part of the AHP survey (15 criteria pairwise comparisons), 3) introduction to sub-criteria, 4) second part of the AHP survey (6 sub-criteria pairwise comparisons), 5) a short self-developed sociodemographic questionnaire (different for both groups, see the Supplementary Material) including an evaluative question about survey difficulty. For a detailed description of included elements in the AHP survey, we refer to Mohr et al. [15, 45, 48]. We used the AHP judgement scale with verbal explanations of numeric values [49], including an adjustment of graphic design to meet the specific needs of this participant group [48]. Oriented in the standardized interview procedure [31], the assisting interviewer repeated after each pairwise comparison what the PlwCI said with her/his/their judgement, e.g.: “With your judgement you are saying that [X] is very much more important to you than [Y]; is this what you wanted to express?”, to make sure the tradeoffs presented were understood. Since the survey was administered as paper-pencil questionnaire for both groups, individual consistency could not be assessed immediately and participants could respectively not be asked to revise their judgements.
Sociodemographic and clinical factors
Participant characteristics in both groups, including age, gender, etc., were collected as categorical data to ensure anonymity. Subject to explicit informed written consent, PlwCI were asked whether data about 1) a diagnosis of MCI and/or dementia and 2) the most recent cognitive test result from the MMSE [41], could be obtained from the informal CGs, or from the study nurses at site. Furthermore, PlwCI were asked whether they could share a current medication plan. If not at hand, PlwCI were asked whether this information similarly could be obtained from the informal CGs or the study nurses at site. As part of the sociodemographic questionnaire and likewise subject to initial explicit informed written consent, PlwCI were asked to participate in a short cognitive test (DemTect [39]) to obtain a current cognitive test result.
Data analyses
Mathematical analyses: AHP
Importance weights for the (sub)criteria were calculated for each individual participant with the principal right eigenvector method [49–51]. The vector of weights (w) of the included (sub)criteria is represented by the principal right eigenvector [30, 31]. Multiplied by a matrix A, in case of a non-negative reciprocal matrix A, the principal right eigenvector is equal to the maximal eigenvalue, λmax, multiplied by w (A* w =λmax*w) of the matrix [31]. The principal right eigenvector can thus be calculated by matrix multiplication [31, 52]. To aggregate weights in both groups, individual weights were averaged arithmetically, i.e., by the aggregation of individual priorities (AIP) method. A detailed overview of both individual weights and aggregation calculations can be found in Danner et al. [31]. Local weights for (sub)criteria for each cluster summarize to one. Global weights for sub-criteria were calculated for each individual participant by multiplication of the local sub-criteria weights with the local weight of the respective criterion. Global sub-criteria weights were likewise aggregated by AIP method. At the criteria level, the consistency ratio (CR), as a measure of logical judgement performance in an AHP survey, was calculated [47]. The literature usually recommends a consistency threshold of 0.1–0.2 [53, 54]. However, particular circumstances, such as cognitive capacities of surveyed participants, can warrant the acceptance of a higher value at 0.3 [55, 56]. To achieve low inconsistency should not be the mere goal of the decision-making process; reasonable consistency is necessary, but does not suffice for calling a decision “a good decision” [57]. Reasons for observed inconsistency have been described in detail elsewhere [31, 57]. We used Expert Choice Comparion® [58] and the package ‘ahpsurvey’ [59] in RStudio to calculate weights and CRs. Likewise to Danner et al. [31], the sensitivity of AHP results was tested by inclusion and exclusion of inconsistent respondents in the analyses, as further sensitivity analyses were limited by lack of alternatives in the AHP hierarchy [29].
Statistical analyses: Participant characteristics, AHP rankings
Sociodemographic/clinical participant characteristics, including participants’ ratings of questionnaire difficulty, were analyzed by frequency counts (%) and means (standard deviations (SDs)). Due to the comparatively small sample sizes in both groups (patients/physicians), it was decided to recode/dichotomize the variables for patients’ age group (60–80 / 81 to > 90), living situation (own home / assisted living), family status (not alone / alone), education (10 years and below />10 years), self-rated health status (good / moderate / bad), and physicians’ age group (30–50 / 51 to > 70) as well as specialization (general practitioner (GP) / other specialists) for a more comprehensible reporting of participant characteristics. The original age groups as well as PlwCIs’ self-rated health status groups can be found in the Supplementary Material. AA, who has a pharmaceutical background, analyzed the medication plans oriented in Richling [60] in Microsoft®Excel. Based on the analyses, AA developed a continuous variable on sum of medications, which subsequently was used for report of patient characteristics (Supplementary Material). AA’s analyses were reviewed by NW, who is a medical doctor.
Group differences in rankings of (sub)criteria were initially investigated by descriptive statistics. This comprised calculation of means (by AIP method) and SDs of individual AHP weights per group, as well as the subsequent assignment of (sub)criteria rank from highest to lowest mean. If AHP elements ranks between groups reversed with two or more ranks, these were considered meaningful. As an additional graphical analysis, boxplots layered with means and SDs were developed. To further analyze differences in mean AHP weights for the six criteria (dependent variables) between groups, i.e., status (patient/ physician) and consistent/inconsistent participants (independent variables), we conducted univariable analyses with independent paired t-tests and Mann Whitney U-tests in case of violations of assumptions. All statistical analyses were conducted with R/RStudio.
RESULTS
Participant characteristics
Short versions of participant characteristics are depicted in Tables 1A and B. A comprehensive version can be viewed in the Supplementary Material.
Table 1A
Patients’ (n = 50) characteristics *
| Characteristic | n (%) |
| Age groups (recoded) | |
| 60–80 | 22 (44.0) |
| 81 to > 90 | 28 (56.0) |
| Gender | |
| Female | 28 (56.0) |
| Male | 22 (44.0) |
| Living situation (recoded) | |
| Own home | 37 (74.0) |
| Assisted living | 12 (24.0) |
| Missing (Do not know) | 1 (2.0) |
| DemTect | 8.02 (3.49) a |
| MMSE | 23.5 (4.2) a |
| Diagnosis of MCI or dementia b | 40 (80.0) |
| Self-rated general health (recoded) | |
| Good | 18 (36.0) |
| Moderate | 25 (50.0) |
| Bad | 7 (14.0) |
| Self-rated assessment of survey difficulty | |
| Easy | 8 (16.0) |
| Rather easy | 16 (32.0) |
| Neutral | 17 (34.0) |
| Rather difficult | 9 (18.0) |
| Difficult | N/A |
*Complete characteristics of patients can be reviewed in Supplementary Table 1A. Original age groups: 60–70, 71–80, 81–90, >90; original living situation groups: own home, assisted living, community housing (e.g., with children), original self-rated general health groups: very good, good, satisfactory, less good, bad. aMean (SD); bICD-10: F00.1, F00.2, F00.9, F01.3, F01.9, F02.3, F03, F06.7, G30, U51.02, U51.11, U51.12.
Table 1B
Physicians’ (n = 25) characteristics *
| Characteristic | n (%) |
| Age groups (recoded) | |
| 30–50 | 13 (52.0) |
| 51 to > 70 | 12 (48.0) |
| Gender | |
| Female | 18 (72.0) |
| Male | 7 (28.0) |
| Field of specialty (recoded) | |
| Family medicine/ general practitioner | 16 (64.0) |
| Other specialist | 9 (36.0) |
| Self-rated assessment of survey difficulty a | |
| Easy | 6 (24.0) |
| Rather easy | 4 (16.0) |
| Neutral | 8 (32.0) |
| Rather difficult | 3 (12.0) |
| Difficult | 4 (16.0) |
| Missing | 1 (4.0) |
*Complete characteristics of physicians can be reviewed in Supplementary Table 1B. Original age groups: 30–40, 41–50, 51–60, 61–70, >70; original other specialist groups: psychiatry, neurology, and internal medicine. aOne participant chose both, hence percentage out of all 25 for both groups separately calculated.
56% of patients were 81 to > 90 years of age and indicated female gender. 80% had a diagnosis of MCI or dementia (Table 1A). No patient was per diagnosis and/or indicated by cognitive test results at an advanced stage of dementia (Table 1A and Supplementary Material). The majority (86%) rated their general health status as good or moderate. Among physicians, 52% were aged 30–50 years. The majority indicated female gender (72%) and worked as general practitioners (64%).
(Sub)criteria importance weights, rankings per group, and congruence between groups
Aggregated local AHP importance weights for each (sub)criterion per group for all patients (n = 50), consistent patients (CR n = 36), all physicians (n = 25), and consistent physicians (n = 21) are depicted in Table 2.
Table 2
AHP importance weights for (sub)criteria by patients and physicians
| Criteria and sub-criteria (rank-order) | Patients (n = 50), local weights, mean (SD) | Consistent patients (n = 36) c local weights, mean (SD) | Criteria and sub-criteria (rank-order) | Physicians (n = 25), local weights, mean (SD) | Consistent physicians (n = 21)d local weights, mean (SD) |
| Assistance with everyday activities | 0.206 (0.102) | 0.210 (0.112) | Assistance with everyday activities | 0.217 (0.087) | 0.212 (0.089) |
| – Informal/ family CG | 0.572 (0.263) | – N/A | – Informal/ family CG | 0.620 (0.218) | N/A |
| – Professional CG | 0.428 (0.263) | – N/A | – Professional CG | 0.380 (0.218) | N/A |
| Social exchange | 0.201 (0.008) | 0.199 (0.095) | Organization of health care | 0.192 (0.113) | 0.199 (0.107) |
| – Family and/or friends | 0.700 (0.184) | – N/A | – Communication | 0.658 (0.237) | N/A |
| – New contacts | 0.300 (0.184) | – N/A | – Integrated care structures | 0.342 (0.237) | N/A |
| Organization of health care | 0.173 (0.082) | 0.159 (0.080) | Social exchange | 0.183 (0.091) | 0.179 (0.095) |
| – Communication | 0.532 (0.235) | – N/A | – Family and/or friends | 0.735 (0.196) | N/A |
| – Integrated care structures | 0.468 (0.235) | – N/A | – New contacts | 0.265 (0.196) | N/A |
| Characteristics of professional CGs | 0.163 (0.079) | 0.152 (0.076) | Characteristics of professional CGs | 0.175 (0.072) | 0.174 (0.075) |
| – Empathy | 0.513 (0.193) | – N/A | – Empathy | 0.726 (0.161) | N/A |
| – Education and work experience | 0.487 (0.193) | – N/A | – Education and work experience | 0.274 (0.161) | N/A |
| Memory exercises | 0.135 (0.066) a | 0.147 (0.058) b | Physical activities | 0.134 (0.061) | 0.134 (0.052) |
| – Leisure activities | 0.653 (0.207) | – N/A | – How? (Format) | 0.584 (0.245) | N/A |
| – Learning something new | 0.347 (0.207) | – N/A | – Where? (Location) | 0.416 (0.245) | N/A |
| Physical activities | 0.121 (0.079) | 0.133 (0.079) | Memory exercises | 0.099 (0.068) a | 0.102 (0.072) |
| – Where? (Location) | 0.502 (0.253) | – N/A | – Leisure activities | 0.697 (0.225) | N/A |
| – How? (Format) | 0.498 (0.253) | – N/A | – Learning something new | 0.303 (0.225) | N/A |
As surveys were conducted individually and not as group decision, individual weights were calculated by the principal eigenvalue method [51] and aggregated by arithmetic mean similar to Danner et al. [31]. Sub-criteria weights were not calculated for consistent patients and physicians, as consistency ratio was calculated at level of criteria. For sub-criteria the CR = 0, as only two elements were compared. aNumbers in bold indicate significant differences (p < 0.05) between two independent groups (patients versus physicians) as calculated with Mann Whitney U test due to violation of assumptions. bNumbers in bold indicate significant differences (p < 0.05) between two independent groups (consistent versus inconsistent patients based on CR-threshold of CR≤0.3) as calculated with Mann Whitney U test due to violation of assumptions. cConsistency ration of≤0.3 [55, 56]. dConsistency ratio of CR≤0.2 [53, 54].
Both patients and physicians rated Assistance with Everyday Activities highest (mean AHP weights: 0.206 (SD: 0.102) versus 0.217 (SD: 0.087), p = 0.65). While patients viewed Social Exchange as the second most important criterion (mean: 0.201 (SD: 0.008), p = 0.43), physicians judged Organization of Health Care to be the second most important (mean: 0.192 (SD: 0.113), p = 0.43), and Social Exchange third most important (mean: 0.183 (SD: 0.091), p = 0.43). Characteristics of Professional CGs took the fourth place in both groups (mean: 0.163 (SD: 0.079) versus mean: 0.175 (SD: 0.072), p = 0.53). Memory Exercises was the only criterion, where we found a significant difference in AHP weights between groups (mean: 0.135 (SD: 0.066), fifth place for patients versus mean: 0.099 (SD: 0.068), sixth place for physicians, p = 0.01). A graphical display of patients’ preferences versus physicians’ judgements for criteria is depicted in Fig. 1.
Fig. 1
Box plots of AHP weights by patients and physicians for the criteria of PCC for PlwCI. The circles are outliers. The ends of each box represent the 25th and 75th percentiles, and the ends of each line show the 95% confidence interval. Lines within boxes represent medians (i.e., 50th percentiles). The red point shows the mean, the red lines the standard deviation (Table 2). AHP, Analytic Hierarchy Process; PlwCI, People living with Cognitive Impairments. Tests for differences between groups (patients/ physicians) in AHP-weights for Memory Exercises with Mann Whitney U test showed a slightly significant difference (p-value = 0.01). For remaining criteria, no significant differences in AHP-weights between groups was found.
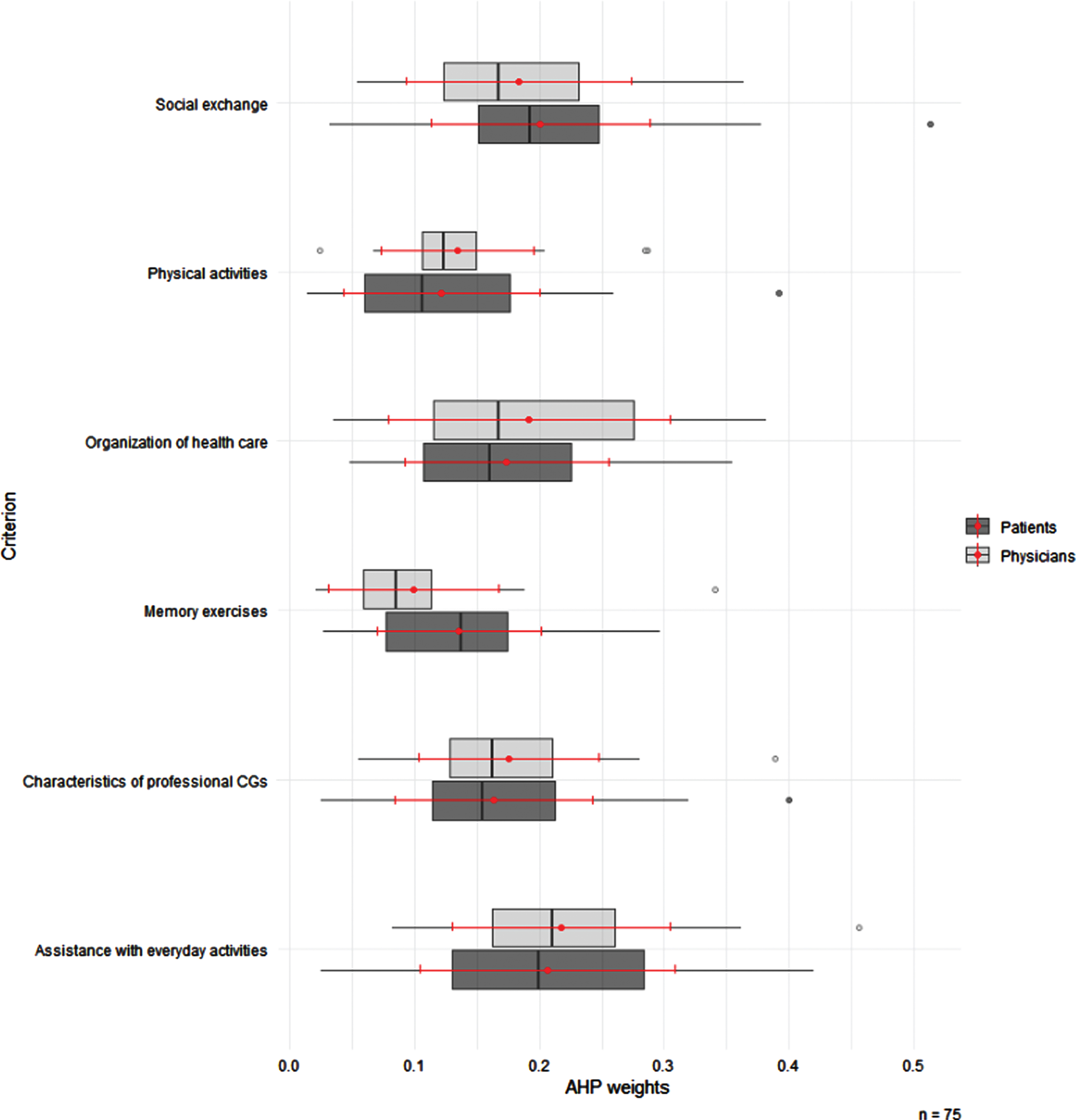
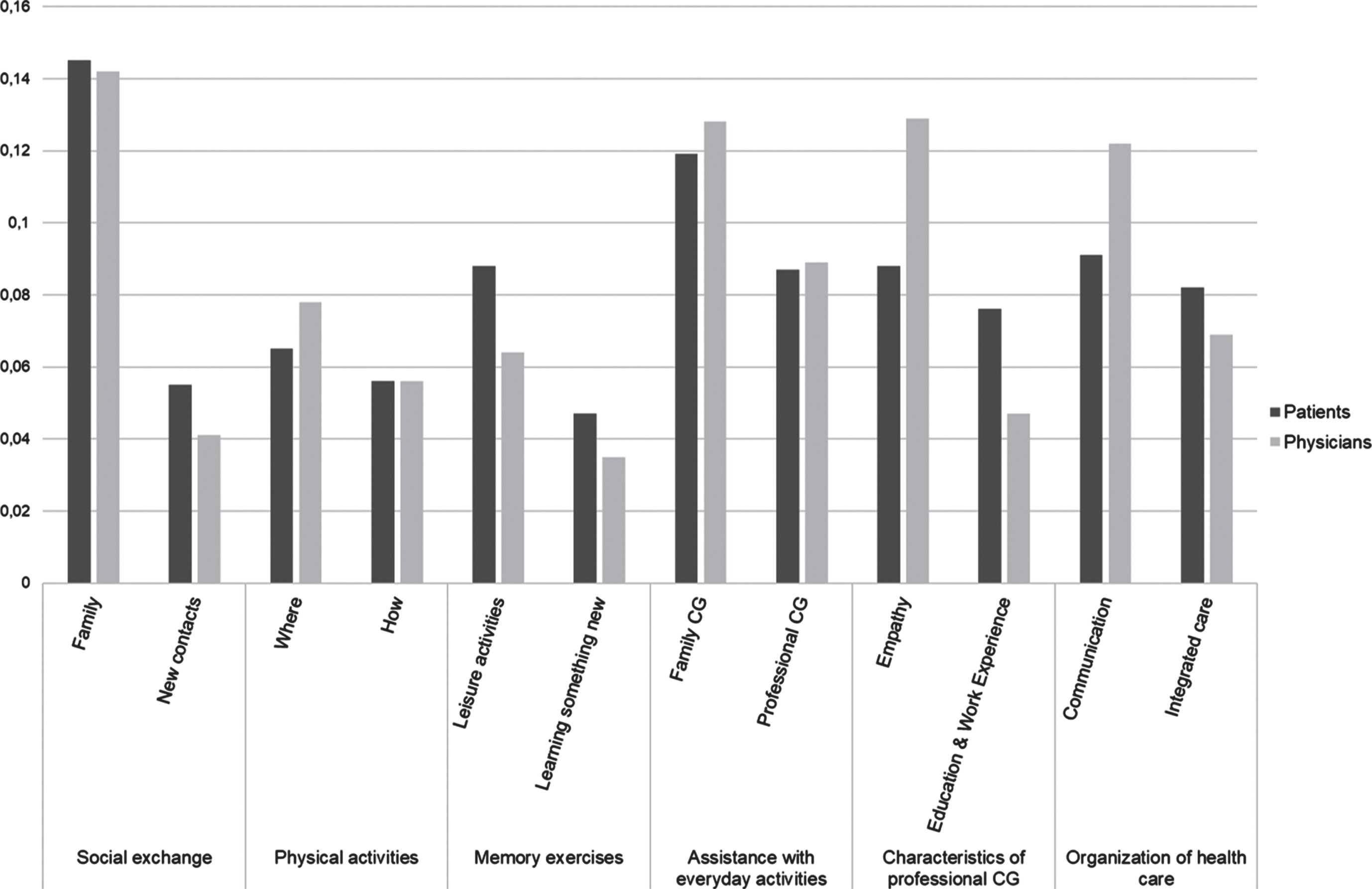
Figure 2 shows aggregated global weights per group for sub-criteria, sorted from highest to lowest mean-value per cluster. Social Exchange with Family and/or friends was prioritized highest among both patients and physicians, whilst Social Exchange with New Contacts and Memory Exercises by Learning something new was prioritized lowest in both groups. Global weights differed in particular for Empathy versus Education and work experience as Characteristics of Professional CGs and Communication versus Integrated care structures for Organization of Health Care. Physicians gave greater importance to Empathy and Communication than the patients (Fig. 2).
Fig. 2
Global weights (aggregated, mean), for sub-criteria among patients (n = 50) and physicians (n = 25). Global weights are the local weights of sub-criteria multiplied by the local weights of the respective parent criterion per person. CG, Caregiver.
Inconsistency in judgements and sensitivity of results
Mean CR for patients at the criteria level was 0.261 for patients, and 0.181 for physicians, with model inconsistency for both groups just below the defined threshold (patients CR≤0.3, physicians CR≤0.2). At the level of sub-criteria, the CR was 0, as this results when only two elements are compared. Among patients, 14 (28%) provided judgments with a CR of > 0.3, among physicians, 4 (16%) provided judgements with a CR of > 0.2.
For both patients and physicians, we could not see rank reversals of criteria when inconsistent respondents were excluded (Table 2). We found a significant difference between consistent versus inconsistent patients for the AHP weights of Memory Exercises (mean: 0.147 (SD: 0.058) versus mean: 0.105 (SD: 0.077), p = 0.02), but not for any other criterion. No significant differences in AHP weights of criteria between consistent versus inconsistent physicians could be identified.
Rating of questionnaire difficulty
Among patients, the majority of respondents (66%) rated the survey as rather easy or neutral. No patient rated the survey as difficult. 12/25 physicians rated the survey as easy/neutral, and 4/25 as rather easy/difficult respectively.
DISCUSSION
The aim of this study was to elicit patient preferences and physicians’ judgements for PCC of PlwCI, including an assessment of congruence. For both groups “Assistance with everyday activities” was the most important criterion. “Physical activities” and “Memory exercises” were least important in both groups. Overall, patient preferences and physicians’ judgements in terms of AHP elements’ ranking aligned well. We did not see rank reversals of criteria after exclusion of inconsistent respondents in either group. Significant differences in weights per group were found for Memory Exercises, both between patients versus physicians and consistent versus inconsistent patients. Model inconsistency in both groups was below the defined threshold, which may contribute to confidence in our results. The majority of patients rated the survey as rather easy or neutral.
Some (sub)criteria in our study are similar to important elements of care in other studies. Chester et al. [61] likewise identified “social and recreational activities” as an attribute of importance. However, the authors used a DCE with dyads. The use of a DCE for our study had been discussed among the authors, but the method was deemed too cognitively demanding for PlwCI. Carpenter et al. [62] conducted a concept mapping to identify domains for psychosocial preference measurements. The authors did not focus on PlwCI, but generally included aged adults. Still, the identified domains are similar to our (sub)criteria: social contact, growth activities, leisure activities, self-dominion, support aids, CGs, and care. Another instrument for preference elicitation among aged adults is the PELI by van Haitsma et al. [20], which similar to our instrument focuses on preferences for psychosocial activities. However, preferences in the PELI are reported to be assessed by Likert-type scales and open-ended questions. Hence, PELI differs from a choice-based preference elicitation instrument such as ours, as it not explicitly may require a choice, i.e., to express a “preference” per definition [23, 24]. The scale used in our instrument explicitly demands a choice, by asking which of two elements is more important and by how much [49, 63]. Another recent study [64] reports the understanding of patient preferences among individuals with Lewy bodies dementia, however focused on clinical care elements. The study was conducted as interview study without a choice-based preference elicitation instrument and included dyads. The authors identified “communication” and “finding local resources” as elements of importance, probably related to our criteria 1) Social Exchange and 6) Organization of health care. To summarize, despite the methodological and sample differences of earlier research, similar care elements of importance as in our study were identified, which contributes to confidence in our results. The application of a choice-based preference elicitation instrument assures that we elicited actual preferences per definition [23, 24].
Our study investigates the congruence between patient preferences and physicians’ judgements for PCC of PlwCI. Mühlbacher & Juhnke [32] reviewed studies that examined this relationship across different indications and methodologies, and found that patient preferences and physicians’ judgements often differed. In our study, patient’s and physician’s rank order of criteria did not show any meaningful differences. We saw some differences in global AHP weights for sub-criteria, where, interestingly, physicians gave greater importance to Empathy as Characteristic of Professional CGs and Communication in Organization of Health Care than the patients (Fig. 2). At the level of criteria, significant differences in AHP weights could be identified for Memory Exercises. Two rank reversals of criteria could be identified between groups. However, in both cases the criteria switched only one place in the ranking and did not jump considerably. Casparie & van der Waal [65] reported considerable jumps of elements important in the care of people living with diabetes, however, still rated patients’ and diabetologists’ preferences to show a rather high degree of agreement. In this regard, the authors emphasized that both patients and diabetologists’ ranked the same criterion highest, a phenomenon we also saw in our study for the criterion Assistance with everyday activities. Pfisterer et al. [66], on the other hand, found the level of agreement between patients and potential proxies (other than spouses) to be at best slight to fair. However, the authors looked at treatment options for urinary incontinence, which is difficult to compare to criteria of PCC for PlwCI as in our study. Overall, our study did not identify meaningful or significant differences in patient preferences and physicians’ judgements, which is a promising result for the implementation of PCC in our setting. Physicians in our study setting may know well what matters in PCC of PlwCI, which could enhance shared decision-making and hence improve the quality of care for PlwCI. Future research may consider to use a different method to elicit preferences versus judgements for PCC of PlwCI, e.g., BWS or group decision-based AHP, to check the reliability of the obtained results. However, as concluded by Mühlbacher & Juhnke [32], one method or technique will not always result in a disagreement while another method will.
With regard to the physicians’ judgements, an expert opinion, one might, however, have expected a different ranking of criteria. A recent systematic review by Bahar-Fuchs et al. [67] found cognitive training for people with mild to moderate dementia probably be associated with small to moderate positive effects on global cognition. Another systematic review by Blondell et al. [68] found an association between higher levels of physical activity and a reduced risk of cognitive decline and dementia. Based on the findings from these reviews, one might have expected the physicians as clinical experts to express greater importance for those criteria focused on individual health status, such as Memory Exercises and Physical activities. Despite this expectation, physicians ranked these lowest –similar to the patients. This may, however, be explained by the remaining included criteria. In particular, when confronted with the highest ranked criterion, Assistance with everyday activities, some patients emphasized verbally that none of the other criteria could be considered without Assistance with everyday activities being provided. Under consideration of the surveyed patient group, aged PlwCI, this may be expected. It may be that the criteria such as Assistance with everyday activities, Characteristics of professional CGs, and Organization of health care were perceived as minimum requirements for PCC by all participants, and this priority could explain why the criteria focused on improvement of individuals’ health states were given lesser importance. A deeper understanding of why PlwCI assigned Physical Activities and Memory Exercises the lowest importance, might also be of interest for future qualitative research, to improve uptake of such health-promoting activities for healthy ageing and potentially improve adherence to respective components in large multi-modal prevention trials such as the Age.Well study [69]. In a recent debate article, Montero-Odasso, Ismail & Livingston [70] discussed the conclusion from the Lancet Commission on Dementia Prevention that up to 35% of dementia cases could be prevented by modifying nine risk factors. Per the Lancet Commission’s 2020 report, 2% reduction in dementia prevalence could be achieved if physical inactivity in later life as modifiable risk factor would be eliminated [71]. However, Montero-Odasso et al. highlighted that large randomized controlled trials (>250 participants per arm, minimum of 6 months follow-up), primarily set to prevent dementia using lifestyle interventions, had merely shown modest or even negative results [70]. One may question whether these findings may have been impacted by low adherence due to low preferences for such interventions among the study participants. Cardona et al. [72] recently presented baseline analyses from the Age.Well study, which i.a. includes physical activity as one intervention component. The authors included 1,030 participants in their analyses. Approximately half (51.8%) engaged in physical activity≥2 times per week for at least 30 min at baseline. Self-efficacy, i.e., the belief in one’s ability to succeed in a given task [73], was an important predictor of physical activity participation among persons at risk of dementia and multi-morbidity (p < 0.001) in Cardona et al.’s analyses [72]. Previous research found that self-efficacy can drive individuals to prefer more challenging tasks and also persist more in the face of challenges encountered [74]. Hence, one could discuss whether better self-efficacy could influence individuals to express greater preference for challenging tasks, such as physical activity, and hence show better adherence to such interventions. However, results from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study found high self-reported adherence to the intervention component of physical activity (90%). Still, the overall effect from the multi-domain intervention of diet, exercise, cognitive training, and vascular risk monitoring on cognitive improvements in comparison to the control group was small [75]. Future clinical trials on the effect of non-pharmacological, preventive treatment approaches in dementia may include an assessment of preferences to study the relationship with treatment adherence in more detail. Finally, the low importance assigned to Memory Exercises and Physical Activities among physicians in our study, may be an expression of the fact that health-promoting/disease preventive approaches still are not well established in German primary care [76]. Whether importance differs according to the specialty of physicians may be of interest for future research.
Overall model consistency in both groups was below the defined threshold per group. Similar to Danner et al. [31], we observed those respondents with a lot of high judgements (judgements≥5) to have higher internal inconsistency. Likewise, respondents with many equal judgements (judgements = 1) showed lower observed inconsistency. A deeper statistical analysis of how ranking behavior among respondents may have affected their individual CRs lies outside the scope of this paper. Still, it may be of interest for future research. Contrary to Danner et al. [31], the exclusion of inconsistent participants did not result in rank reversals of criteria in our sample, however a significant difference in weights for Memory Exercises among patients. The majority of PlwCI rated the AHP survey to be rather easy or neutral, which may be an indicator of good acceptability and feasibility with this patient group.
Limitations
Our study has several limitations. Our AHP decision hierarchy included qualitative (sub)criteria, which may yield room for interpretation and hence influence the assigned importance. However, we adjusted for interpretation by inclusion of patient-understandable descriptions and definitions for each (sub)criterion [45, 48]. Kuruoglu et al. [77] similarly incorporated qualitative criteria in their AHP decision hierarchy for choice of a family physician. The possibility to cover qualitative aspects, can also be viewed as a strength of the AHP method, which allows for the inclusion of potentially important qualitative (sub)criteria. Our AHP decision hierarchy had been developed with a variety of (participatory) research methods (literature review, qualitative interviews, pretests, expert panels) including both PlwCI as experts by experience and clinical experts. The variety of (participatory) research approaches contribute to confidence in the content- and face validity of our AHP decision hierarchy and the included (sub)criteria. An average of 60–90 min survey time in the patient group is rather long and may have resulted in fatigued and hence less concentrated respondents, which in turn may have led to greater inconsistency, as was observed previously [31]. We did, however, consider this problem by adaptation of the survey outline; first the most challenging pairwise comparisons of criteria and last sociodemographic questions and rating of survey difficulty. The inclusion of PlwCI based on i.a. MMSE results may raise the question about the specificity and sensitivity of the MMSE as a cognitive screening instrument amongst MCI and early-stage dementia. The DemTect has been found more sensitive to detect cognitive impairments in early stages [39, 78]. We considered this problem early on by inclusion of the DemTect in our survey, to obtain a recent cognitive test result. The physician’s sample might suffer from a selection bias, i.e., the included physicians might be more engaged than the average with regard to the care of their patients. This might be reflected in our findings, which did not show meaningful differences in AHP element rankings. It could be that the included physicians due to their general higher engagement align better in their judgements with patient preferences than less engaged physicians. Here, subgroup analyses to consider heterogeneities in the sample, e.g., general practitioners versus other specialists or female versus male physicians, may yield some information on the extent of a potential selection bias. Heterogeneities by an analysis of weights and respective ranks in different subgroups were only briefly addressed in this report (patients versus physicians). However, extensive subgroup analyses lie outside the scope of this paper, which primary focus was to assess the congruence of patient preferences and physicians’ judgements. One may question whether the AIP method for aggregation was appropriate for our study. As our study similarly to Danner et al. [31] was conducted with individual representatives of two populations and not in group settings, the AIP method for aggregation was deemed more appropriate than the aggregation of individual judgements method. The latter is commonly applied when the AHP is used as a group decision making instrument, as only this aggregation method by application of the geometric mean on individual judgements can assure the reciprocal axiom of the AHP for the combined judgements matrix [79]. Generalizability of our results, similar to other patient preference studies, is limited due to choice of setting [31]. This study was conducted in one federal state of Germany. Nevertheless, we surveyed community-dwelling PlwCI and physicians from different geographical areas in the state, which may contribute to diversity and generalizability of our results across this state, and possibly beyond. We did not exclude inconsistent respondents from our analyses, which may have prevented loss of external validity, as discussed by Mühlbacher et al. [80].
Conclusion
This study provides data about patient preferences and physicians’ judgements for PCC of PlwCI, assessed with a quantitative, choice-based preference instrument. Our findings show that physicians judgements in the selected study setting aligned well with what their patients want and prefer in terms of care. Respectively, outpatient care for PlwCI may prioritize interventions focused on assistance with everyday activities, social exchange, and an organization of health care that includes shared decision-making and integrated care structures. Our findings may form a basis for the implementation of truly PCC for PlwCI, i.e., state-of-the-art medical care aligned with patients’ preferences.
ACKNOWLEDGMENTS
We would like to thank all participants for their collaboration and contribution to this study. We would like to thank Ulrike Kempe, Sebastian Lange, Mandy Freimark, and study personnel colleagues at the German Institute for Neurodegenerative Disease e.V. (DZNE) Site Rostock/Greifswald for their support with participant recruitment. The first author, WM, would like to thank Lena Hofbauer, MSc., German Institute for Neurodegenerative Disease e.V. (DZNE) Site Rostock/Greifswald, for her continuous support in the application of R and RStudio. Additionally, WM would like to thank Michelle Pfaff, German Institute for Neurodegenerative Disease e.V. (DZNE) Site Rostock/Greifswald, for her support with development of sub-criteria graphs. Furthermore, the first author would like to thank the Hans & Ilse Breuer Foundation for their support. WM is funded by the Hans & Ilse Breuer Foundation under the Alzheimer Doctoral Scholarship. This research was completed independently from the funding agency, which had no role in determining the study design, analysis, results, or discussion.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0753r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220753.
REFERENCES
[1] | World Health Organization (2022) Ageing and health., Last updated October 1, 2022, Accessed October 14 2022 https://www.who.int/news-room/fact-sheets/detail/ageing-and-health |
[2] | Prince M , Comas-Herrera A , Knapp M , Guerchet M , Karagiannidou M (2016) World Alzheimer Report 2016. Improving healthcare for people living with dementia: Cov-erage, quality and costs now and in the future. Alzheimer’s Disease International, London. |
[3] | Nichols E , Steinmetz JD , Vollset SE , Fukutaki K , Chalek J , Abd-Allah F , Abdoli A , Abualhasan A , Abu-Gharbieh E , Akram TT , et al. ((2022) ) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7: , e105–e125. |
[4] | Libon DJ , Delano-Wood L , Bondi MW , Au R ((2014) ) Mild cognitive impairment. In Encyclopedia of the Neurological Sciences (Second Edition), Aminoff MJ, Daroff RB, eds. Academic Press, Oxford, pp. 72–75. |
[5] | Bruscoli M , Lovestone S ((2004) ) Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr 16: , 129–140. |
[6] | Prince M , Bryce R , Ferri C 2011 World Alzheimer Report 2011. The benefits of early diagnosis and intervention. Alzheimer’s Disease International, London. |
[7] | Alzheimer’s Association 2022 Dementia Care Practice Recommendations. https://www.alz.org/professionals/professional-providers/dementiacarepracticerecommendations Accessed October 14 2022 |
[8] | National Institute for Health and Care Excellence(2018)Dementia: Assessment, management and support for peo-ple living with dementia and their carers (NG97). National Institute for Health and Care Excellence, United Kingdom. |
[9] | Danish Health Authority (2019) National klinisk ret-ningslinje for forebyggelse og behandling af adfærdsmæs-sige og psykiske symptomer hos personer med demens (National clinical guideline for prevention and treatment of behavioral and mental symptoms among people with dementia). Danish Health Authority, Copenhagen, Den-mark. |
[10] | The National Board of Health and Welfare (2017) Nationella riktlinjer för vård och omsorg vid demenssjuk-dom (National guidelines for care of dementia diseases). Socialstyrelsen, Stockholm, Sweden. |
[11] | Norwegian Ministry of Health and Care Services (2015) Dementia Plan 2020 - A More Dementia-friendly Society. Norwegian Ministry of Health and Care Services. |
[12] | Dely H , Verschraegen J , Setyaert J (2018) You and me, together we are human - A reference framework for quality of life, housing and care for people with dementia. Flanders Centre of Expertise on Dementia, Antwerpen, Belgium. |
[13] | NHMRCPartnership Centre for Dealing with Cognitive and Related Functional Decline in Older People (2016) Clinical Practice Guidelines and Principles of Care for People with Dementia. NHMRC Partnership Centre for Dealing with Cognitive and Related Functional Decline in Older People, Sydney, Australia. |
[14] | Savaskan E , Bopp-Kistler I , Buerge M , Fischlin R , Georgescu D , Giardini U , Hatzinger M , Hemmeter U , Justiniano I , Kressig RW , Monsch A , Mosimann UP , Mueri R , Munk A , Popp J , Schmid R , Wollmer MA (2014) Empfehlungen zur Diagnostik und Therapie der Behavioralen und Psychologischen Symptome der Demenz (BPSD) (Recommendations for diagnosis and treatment of behavioral and mental symptoms in dementia). Verlag H, Switzerland.. |
[15] | Mohr W , Rädke A , Afi A , Edvardsson D , Mühlichen F , Platen M , Roes M , Michalowsky B , Hoffmann W ((2021) ) Key intervention categories to provide person-centered dementia care: A systematic review of person-centered interventions. J Alzheimers Dis 84: , 343–366. |
[16] | Morgan S , Yoder L ((2012) ) A concept analysis of person-centered care. J Holist Nurs 30: , 6–15. |
[17] | Kitwood T , Bredin K ((1992) ) Towards a theory of dementia care: Personhood and well-being. Ageing Soc 12: , 269–287. |
[18] | Kitwood TM , Kitwood T (1997) Dementia reconsidered: The person comes first, Open university press Buckingham. |
[19] | Edvardsson D , Varrailhon P , Edvardsson K ((2014) ) Promoting person-centeredness in long-term care: An exploratory study. J Gerontol Nurs 40: , 46–53. |
[20] | Van Haitsma K , Curyto K , Spector A , Towsley G , Kleban M , Carpenter B , Ruckdeschel K , Feldman PH , Koren MJ ((2012) ) The preferences for everyday living inventory: Scale development and description of psychosocial preferences responses in community-dwelling elders. Gerontologist 53: , 582–595. |
[21] | Wehrmann H , Michalowsky B , Lepper S , Mohr W , Raedke A , Hoffmann W ((2021) ) Priorities and preferences of people living with dementia or cognitive impairment–a systematic review. Patient Prefer Adherence 15: , 2793. |
[22] | Lepper S , Rädke A , Wehrmann H , Michalowsky B , Hoffmann W ((2020) ) Preferences of cognitively impaired patients and patients living with dementia: A systematic review of quantitative patient preference studies. J Alzheimers Dis 77: , 885–901. |
[23] | Online Etymology Dictionary (2021-2022) prefer (v.). https://www.etymonline.com/word/prefer?ref=etymonline_crossreferencecrossreference, Accessed April 13 2022 |
[24] | Webster Dictionary Preference., https://www.merriam-webster.com/dictionary/preference Accessed April 13 2022 . |
[25] | Harrison Dening K , King M , Jones L , Vickerstaff V , Sampson EL ((2016) ) Correction: Advance care planning in dementia: Do family carers know the treatment preferences of people with early dementia? PLoS One 11: , e0161142. |
[26] | Mühlbacher A (2017) Ohne Patientenpräxferenzen kein sinnvoller Wettbewerb (Without patient preferences no meaningful competition). Deutsches Ärzteblatt, (35– 36): A 1584-1590. |
[27] | Lancsar E , Louviere J ((2008) ) Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics 26: , 661–677. |
[28] | Mühlbacher AC , Kaczynski A , Zweifel P , Johnson FR ((2016) ) Experimental measurement of preferences in health and healthcare using best-worst scaling: An overview. Health Econ Rev 6: , 1–14. |
[29] | Schmidt K , Aumann I , Hollander I , Damm K , von der Schulenburg J-MG ((2015) ) Applying the Analytic Hierarchy Process in healthcare research: A systematic literature review and evaluation of reporting. BMC Med Inform Decis Mak 15: , 112. |
[30] | Mühlbacher AC , Kaczynski A ((2013) ) Der Analytic Hierarchy Process (AHP): Eine Methode zur Entscheidungsunterstützung im Gesundheitswesen (The Analytic Hierarchy Process (AHP): A method for decision support in health care). PharmacoEconomics German Research Articles 11: , 119–132. |
[31] | Danner M , Vennedey V , Hiligsmann M , Fauser S , Gross C , Stock S ((2016) ) How well can analytic hierarchy process be used to elicit individual preferences? Insights from a survey in patients suffering from age-related macular degeneration. Patient 9: , 481–492. |
[32] | Mühlbacher AC , Juhnke C ((2013) ) Patient preferences versus physicians’ judgement: Does it make a difference in healthcare decision making? Appl Health Econ Health Policy 11: , 163–180. |
[33] | Mohr W , Rädke A , Michalowsky B , Hoffmann W ((2022) ) Elicitation of quantitative, choice-based preferences for person-centered care among people living with dementia in comparison to physicians’ judgements in Germany: Study protocol for the mixed-methods PreDemCare-study. BMC Geriatr 22: , 567. |
[34] | Thokala P , Devlin N , Marsh K , Baltussen R , Boysen M , Kalo Z , Longrenn T , Mussen F , Peacock S , Watkins J ((2016) ) Multiple criteria decision analysis for health care decision making—an introduction: Report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health 19: , 1–13. |
[35] | Marsh K , IJzerman M , Thokala P , Baltussen R , Boysen M , Kaló Z , Lönngren T , Mussen F , Peacock S , Watkins J ((2016) ) Multiple criteria decision analysis for health care decision making—emerging good practices: Report 2 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health 19: , 125–137. |
[36] | Ijzerman MJ , van Til JA , Snoek GJ ((2008) ) Comparison of two multi-criteria decision techniques for eliciting treatment preferences in people with neurological disorders. Patient 1: , 265–272 |
[37] | Johnson RM , Orme BK (1996) HowMany Questions Should You Ask in Choice-Based Conjoint Studies?, Sawtooth Soft-ware, Inc., Sequim, WA. |
[38] | Orme BK ((2010) ) Getting Started with Conjoint Analy-sis: Strategies for Product Design and Pricing Research, Research Publishers LLC, Madison, USA. |
[39] | Kalbe E , Kessler J , Calabrese P , Smith R , Passmore A , Brand Ma , Bullock R ((2004) ) DemTect: A new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry 19: , 136–143. |
[40] | Kessler J , Denzler P , Markowitsch H (1990) Mini-Mental-Status-Test (MMST). Deutsche Fassung. (Mini-Mental-State-Examination (MMSE). German Version.), Hogrefe Testzentrale Göttingen. |
[41] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. Apractical method for grading the cognitive state of patients for theclinician. J Psychiatr Res 12: , 189–198. |
[42] | Kleinke F , Michalowsky B , Rädke A , Platen M , Mühlichen F , Scharf A , Mohr W , Penndorf P , Bahls T , van den Berg N , Hoffmann W ((2022) ) Advanced nursing practice and interprofessional dementia care (InDePendent): Study protocol for a multi-center, cluster-randomized, controlled, interventional trial. Trials 23: , 290. |
[43] | Eichler T , Thyrian JR , Dreier A , Wucherer D , Köhler L , Fiß T , Böwing G , Michalowsky B , Hoffmann W ((2014) ) Dementia care management: Going new ways in ambulant dementia care within a GP-based randomized controlled intervention trial. Int Psychogeriatr 26: , 247–256. |
[44] | van den Berg N , Heymann R , Meinke C , Baumeister SE , Fleßa S , Hoffmann W ((2012) ) Effect of the delegation of GP-home visits on the development of the number of patients in an ambulatory healthcare centre in Germany. BMC Health Serv Res 12: , 355. |
[45] | Mohr W , Rädke A , Afi A , Mühlichen F , Platen M , Michalowsky B , Hoffmann W ((2022) ) Development of a quantitative instrument to elicit patient preferences for person-centered dementia care stage 1: A formative qualitative study to identify patient relevant criteria for experimental design of an analytic hierarchy process. Int J Environ Res Public Health 19: , 7629. |
[46] | Saaty TL , Ozdemir MS ((2003) ) Why the magic number seven plus or minus two. Math Comput Model 38: , 233–244. |
[47] | Ozdemir MS ((2005) ) Validity and inconsistency in the analytic hierarchy process. Appl Math Comput 161: , 707–720. |
[48] | Mohr W , Rädke A , Afi A , Mühlichen F , Platen M , Scharf A , Michalowsky B , Hoffmann W ((2022) ) Development of a quantitativepreference instrument for person-centered dementia care - Stage 2:Insights from a formative qualitative study to design and pretest adementia-friendly analytic hierarchy process survey. Int JEnviron Res Public Health 19: , 8554. |
[49] | Saaty TL ((1977) ) A scaling method for priorities in hierarchical structures. J Math Psychol 15: , 234–281. |
[50] | Saaty TL (1980) The analytic hierarchy process: Planning, priority setting, resource allocation. McGraw-Hill, NewYork, pp. S. XIII, pp. 287. |
[51] | Saaty TL ((2003) ) Decision-making with the AHP: Why is the principal eigenvector necessary. Eur J Oper Res 145: , 85–91. |
[52] | Dolan JG , Isselhardt BJ , Cappuccio JD ((1989) ) The analytic hierarchy process in medical decision making: A tutorial. Med Decis Mak 9: , 40–50. |
[53] | Saaty TL ((1994) ) Highlights and critical points in the theory and application of the analytic hierarchy process. Eur J Oper Res 74: , 426–447. |
[54] | Hummel JM , Bridges JFP , Ijzerman MJ ((2014) ) Group decision making with the analytic hierarchy process in benefit-risk assessment: A tutorial. Patient 7: , 129–140. |
[55] | Goepel KD (2013) Implementing the analytic hierarchy process as a standard method for multi-criteria decision making in corporate enterprises–a new AHP excel template with multiple inputs. In Proceedings of the International Symposium on the Analytic Hierarchy Process, Vol. 10, Creative Decisions Foundation Kuala Lumpur, pp. 1-10. |
[56] | Hummel JM , Steuten LG , Groothuis-Oudshoorn C , Mulder N , IJzerman MJ ((2013) ) Preferences for colorectal cancer screening techniques and intention to attend: A multi-criteria decision analysis. Appl Health Econ Health Policy 11: , 499–507. |
[57] | Expert Choice (2021) Expert Choice Comparion Help Center - Inconsistency Ratio.https://comparion.knowledgeowl.com/help/inconsistency-ratio Accessed January 18, 2022. |
[58] | Expert Choice (2022) Expert Choice Comparion® AHP Software for Collaborative Decision Making Solution. Arlington, VA. URL: https://www.expertchoice.com/comparion |
[59] | Cho F (2019) Analytic Hierarchy Process for Survey Data in R. https://cran.r-project.org/web/packages/ahpsurvey/vignettes/my-vignette.htmlLast updated November 1039 24, 2019, Accessed August 02, 2021. |
[60] | Richling I (2017) Medikationsanalyse - Grundlagen und Fallbeispiele für das Medikationsmanagement (Medicationanalysis - basics and case examples for medication manage-ment), Deutscher Apotheker Verlag, Stuttgart, Germany. |
[61] | Chester H , Clarkson P , Davies L , Sutcliffe C , Davies S , Feast A , Hughes J , Challis D , Group. H-DPM ((2018) ) People with dementia and carer preferences for home support services in early-stage dementia. Aging Mental Health 22: , 270–279. |
[62] | Carpenter BD , Van Haitsma K , Ruckdeschel K , Lawton MP ((2000) ) The psychosocial preferences of older adults: A pilot examination of content and structure. Gerontologist 40: , 335–348. |
[63] | Saaty TL ((2008) ) Decision making with the analytic hierarchy process. Int J Serv Sci 1: , 83–98. |
[64] | Armstrong MJ , Gamez N , Alliance S , Majid T , Taylor AS , Kurasz AM , Patel B , Smith GE ((2021) ) Clinical care and unmet needs of individuals with dementia with Lewy bodies and caregivers: An interview study. Alzheimer Dis Assoc Disord 35: , 327–334. |
[65] | Casparie AF , van der Waal MA ((1995) ) Differences in preferences between diabetic patients and diabetologists regarding quality of care: A matter of continuity and efficiency of care? Diabet Med 12: , 828–832. |
[66] | Pfisterer MHD , Johnson TM , Jenetzky E , Hauer K , Oster P ((2007) ) Geriatric patients’ preferences for treatment of urinary incontinence: A study of hospitalized, cognitively competent adults aged 80 and older. J Am Geriatr Socy 55: , 2016–2022. |
[67] | Bahar-Fuchs A , Martyr A , Goh AM , Sabates J , Clare L ((2019) ) Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev 3: , CD013069. |
[68] | Blondell SJ , Hammersley-Mather R , Veerman JL ((2014) ) Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 14: , 510. |
[69] | Zülke A , Luck T , Pabst A , Hoffmann W , Thyrian JR , Gensichen J , Kaduszkiewicz H , König H-H , Haefeli WE , Czock D , Wiese B , Frese T , Röhr S , Riedel-Heller SG ((2019) ) AgeWell.de –study protocol of a pragmatic multi-center cluster-randomized controlled prevention trial against cognitive decline in older primary care patients. BMC Geriatr 19: , 203. |
[70] | Montero-Odasso M , Ismail Z , Livingston G ((2020) ) One third of dementia cases can be prevented within the next 25-years by tackling risk factors. The case “for” and “against”. Alzheimers Res Ther 12: , 81. |
[71] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[72] | Cardona MI , Weißenborn M , Zöllinger I , Kroeber ES , Bauer A , Luppa M , Pabst A , Czock D , König HH , Wiese B , Gensichen J , Frese T , Kaduszkiewicz H , Hoffmann W , Riedel-Heller SG , ThyrianJR ((2022) ) Physical activity determinants in older German adults at increased dementia risk with multimorbidity: Baseline results of the AgeWell.de Study. Int J Environ Res Public Health 19: , 3164. |
[73] | Bandura A (1997) Self-efficacy: The exercise of control, W. H. Freeman and Company, New York. |
[74] | Bandura A , Locke EA ((2003) ) Negative self-efficacy and goal effects revisited. J Appl Psychol 88: , 87–99. |
[75] | Ngandu T , Lehtisalo J , Solomon A , Levälahti E , Ahtiluoto S , Antikainen R , Bäckman L , Hänninen T , Jula A , Laatikainen T , Lindström J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial, Lancet 385: , 2255–2263. |
[76] | Holmberg C , Sarganas G , Mittring N , Braun V , Dini L , Heintze C , Rieckmann N , Muckelbauer R , Müller-Nordhorn J ((2014) ) Primary prevention in general practice - views of German general practitioners: A mixed-methods study. BMC Fam Pract 15: , 103. |
[77] | Kuruoglu E , Guldal D , Mevsim V , Gunvar T ((2015) ) Which family physician should I choose? The analytichierarchy process approach for ranking of criteria in the selection of a family physician. BMC Med Inform Decis Mak 15: , 1–8. |
[78] | Kohn N , Kalbe E , Georg H , Kessler J ((2007) ) Vergleich MMST und DemTect: Spezifität und Sensitivität bei primär kognitiven Störungen (Comparison MMSE and DemTect: Specificity and Sensitivity in primary cognitive disorders). Aktuelle Neurol 34: , P672. |
[79] | Forman E , Peniwati K ((1998) ) Aggregating individual judgments and priorities with the analytic hierarchy process Eur J Operational Res 108: , 165–169. |
[80] | Mühlbacher A , Bethge S , Kaczynski A , Juhnke C ((2015) ) Objective criteria in the medicinal therapy for type II diabetes: An analysis of the patients’ perspective with analytic hierarchy process and best-worst scaling Gesundheitswesen 78: , 326–336. |




