Diagnosis and Care Use for People with Young-Onset Dementia in Primary Care in the Netherlands
Abstract
Background:
Timely diagnosis and adequate care is important for persons with young-onset dementia (YOD) and their caregivers, due to the high impact of the disease. Initiating care can be difficult for the general practitioner (GP) and other healthcare professionals.
Objective:
Provide insight in the care use of persons with YOD and identify factors influencing care use.
Methods:
A primary care register was used for this study. Information on the care use of persons with YOD was extracted from the GPs written notes. Information entailed time until start of care use, reasons and factors influencing the GP’s decision, and reasons and factors influencing actual care use were included. Analyses included quantitative explorative descriptive analyses, and qualitative manifest content analyses.
Results:
75 persons with YOD were included in this study. The main reason for GPs to refer for diagnosis was concerns of caregivers. After diagnosis, 72% of the persons were assigned a case manager, 42.7% received day care, and 44% were admitted to a long-term care facility. A higher percentage of persons without a case manager was admitted to a long-term care facility (64%) compared to the persons with a case manager (36%). Reasons for not initiating care were reluctancy of the persons with YOD or their caregivers, the person deceased, or because the GP did not refer for care.
Conclusion:
Care use differed between persons due to different needs and reasons. Although most persons with YOD receive care in the years after diagnosis, there are still factors that could be improved.
INTRODUCTION
Worldwide, around 3.9 million people have young-onset dementia (YOD) [1], defined as dementia with a symptom onset before the age of 65 years [2, 3]. The disease has a large impact on the lives of persons with YOD and their caregivers. Due to the heterogeneity in symptoms, the young age, and the relatively low prevalence of YOD, recognition of YOD is difficult, leading to a substantial diagnostic delay from three to over six years between first symptom onset and a diagnosis of YOD [4–6].
Persons with YOD face different challenges than persons with late-onset dementia (LOD) because they are in a different life phase. They may still be employed and have active social lives and family responsibilities. Changing roles due to dementia can be difficult to deal with and can cause financial problems, relationship problems, and a loss of sense of purpose for the person with YOD [7–9]. Caregiving can cause a substantial strain, leading to high levels of care burden, increased stress, and depression [7].
It is important for persons with YOD and their caregivers to receive adequate and timely healthcare and support, in order to help them cope with the impact and burden as much as possible. It seems evident that both persons with YOD and their caregivers require age-appropriate and tailored specialist support, treatment, and care to maintain a healthy balance between the needs of the person with YOD and of their caregivers [10]. Persons can have different needs regarding care services depending on their situation. For instance, different symptoms or subtypes of dementia require different treatment, or a person’s living situation requires different care services [11–13].
In the Netherlands, the general practitioner (GP) is responsible for referring persons for diagnostic follow-up, and one of the healthcare professionals who is responsible for arranging timely healthcare. Together with case managers, memory clinic specialists, and other healthcare professionals, the GP arranges day care, admission to long-term care facilities, and other dementia care. GPs furthermore function as a gatekeeper to hospital and specialized care. Case managers are nurses specialized in dementia care. They were introduced in the Netherlands to help GPs by coordinating and initiating care for all persons with dementia to facilitate timely care access, preferably in close contact with the GP [14].
Since GPs are the gatekeepers for specialized care, it is important to know which type of care they provide, and which factors influence care use. GP written notes reveal reasons and factors discussed with the patient about care use, and are therefore a valuable source of information to gain better insight in the care use of persons with YOD.
This study explores the initiation of diagnosis and post-diagnostic care use in YOD by using GPs written notes to describe a) the healthcare initiation and used care services, b) reasons and factors that influence the decisions of GPs for care use, and c) reasons and factors (such as subgroup differences) that influence the actual use of care services by persons with YOD.
METHODS
We conducted a register-based study using the Research Network Family Medicine (RNFM) database containing patient files from GPs in the south-eastern part of the Netherlands. The database is managed by the Department of Family Medicine at Maastricht University Medical Centre (MUMC+) and, for the present study, covers 28 practices with 150,000 current patients from 2014 onwards. Only anonymous data were used for this study.
Participants
The participants in this study were persons aged 70 years (to account for any diagnostic delay) or younger with a dementia diagnosis in the RNFM database. A dementia diagnosis was registered using the International Classification of Primary Care (ICPC) coding system [15]. A total of 103 persons with an ICPC P70 code of “dementia” between 2016 and 2020 were sampled from the RNFM database. Persons were included if they had a confirmed dementia diagnosis as described in the patient file and availability of data on the care trajectory after diagnosis. Out of 103 persons, 28 were excluded due to different exclusion criteria (see Table 1). Participants who moved or died a few months after diagnosis were excluded as well, since dementia care was not initiated for these participants. Persons without a clear dementia diagnosis were excluded when the ICPC P70 dementia code could not be confirmed by the GPs written notes of the follow-up data.
Table 1
In- and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| ICPC P70 code between 2016 – 2020 with confirming information in written notes GP | Dummy patients* (2) |
| Age<70 years at diagnosis | Not patient but spouse has dementia (2) |
| Data available on dementia care trajectory | Wrong ICPC code (2) |
| No data available (2) | |
| Down Syndrome (5) | |
| Patient moved within months after diagnosis, so no dementia care was started (3) | |
| Patient died within months after diagnosis, so no dementia care was started (4) | |
| No clear data on dementia diagnosis or trajectory after diagnosis (8) |
*Dummy patients were patient files created by GPs that did not belong to a single patient.
Data collection and analyses
When participants contacted or visited their GP, the GP entered reasons for the encounter, observations, and important information regarding healthcare use in the electronic patient file. Data used for this study were collected from the GPs written notes within the RNFM database. From the written notes, we extracted all data and information on the care use (from diagnostic referral to post-diagnosis) of the persons with YOD. All data available on healthcare use, reasons and factors influencing a GPs decision to initiate care, and reasons and factors for actual care use were extracted. For the diagnostic phase the following data were collected: the use of cognitive screening tests, having consultations with caregivers, referred specialist (e.g., neurologist, psychiatrist, geriatrician, etc.). For the diagnosis we extracted which subtype of dementia was diagnosed. For the post-diagnostic phase, we extracted care service use (anti-dementia drug prescription, case management, day care, and admission to long-term care facility). Furthermore, we extracted characteristics which could influence care use (dementia subtype, living arrangement) and reasons for initiating or not initiating care use.
Data were analyzed using explorative data analysis, often used for data description and vital for generating hypotheses [16]. This study aimed to summarize main characteristics and show subgroup differences. No predefined hypotheses were tested.
Quantitative data were used as categorical variables (referred specialist, dementia subtype, drug prescription (yes/no), assignment of case manager (yes/no), day care (yes/no), admission to long-term care facility (yes/no), living arrangement), which were analyzed using descriptive analyses to report percentages within different subgroups. Continuous variables (Mini-Mental State Examination (MMSE) test score, date of diagnosis, date of case management, date of day care, date of long-term care facility admission) were also analyzed using descriptive analyses, to report means (MMSE) or calculate the time from diagnosis until start of this type of care by subtracting the date of diagnosis from the start date of care use. Qualitative data entailed information on reasons for decisions made by the GP, or reasons for the actual use of care services. This information was analyzed using manifest content analyses. Here, the focus was on identifying and counting specific content [17], without specific in-depth analyses of the content. The specific content for this analysis included reasons or factors that influenced care use. Given the explorative design, there were no predefined aspects that were sought. All information on reasons or factors for care use were included. Analyses were conducted by one researcher (S.H.) and a subset was cross-checked by a second researcher (K.P.).
RESULTS
Baseline characteristics
The total sample available for analysis in this study was 75 participants. The mean age at diagnosis was 63.2 years and 56.6% of the participants were female (Table 2). 62.7% of the participants were living with their spouses. 22.7% lived alone, of which about a third were widowed. Others were living in a healthcare facility or with other family members (Table 2).
Table 2
Baseline characteristics of the study sample (n = 75)
| Age, (mean, range) | 63.2 (32–69) |
| Female (n, %) | 43 (57.3%) |
| Follow-up time after diagnosis (number of persons) | |
| <1 y | 75 |
| 1 – 2 y | 59 |
| 2 – 3 y | 33 |
| 3 – 4 y | 19 |
| 4 – 5 y | 6 |
| 5 – 6 y | 1 |
| Living arrangement (n, %) | |
| Living with a spouse | 47 (62.7%) |
| Living alone | 17 (22.7%) |
| Living in healthcare facility* | 7 (9.3%) |
| Living with daughter | 1(1.3%) |
| Unclear | 3 (4%) |
*Assisted living or care facilities due to diseases other than dementia.
Diagnostic phase
Considerations for referral
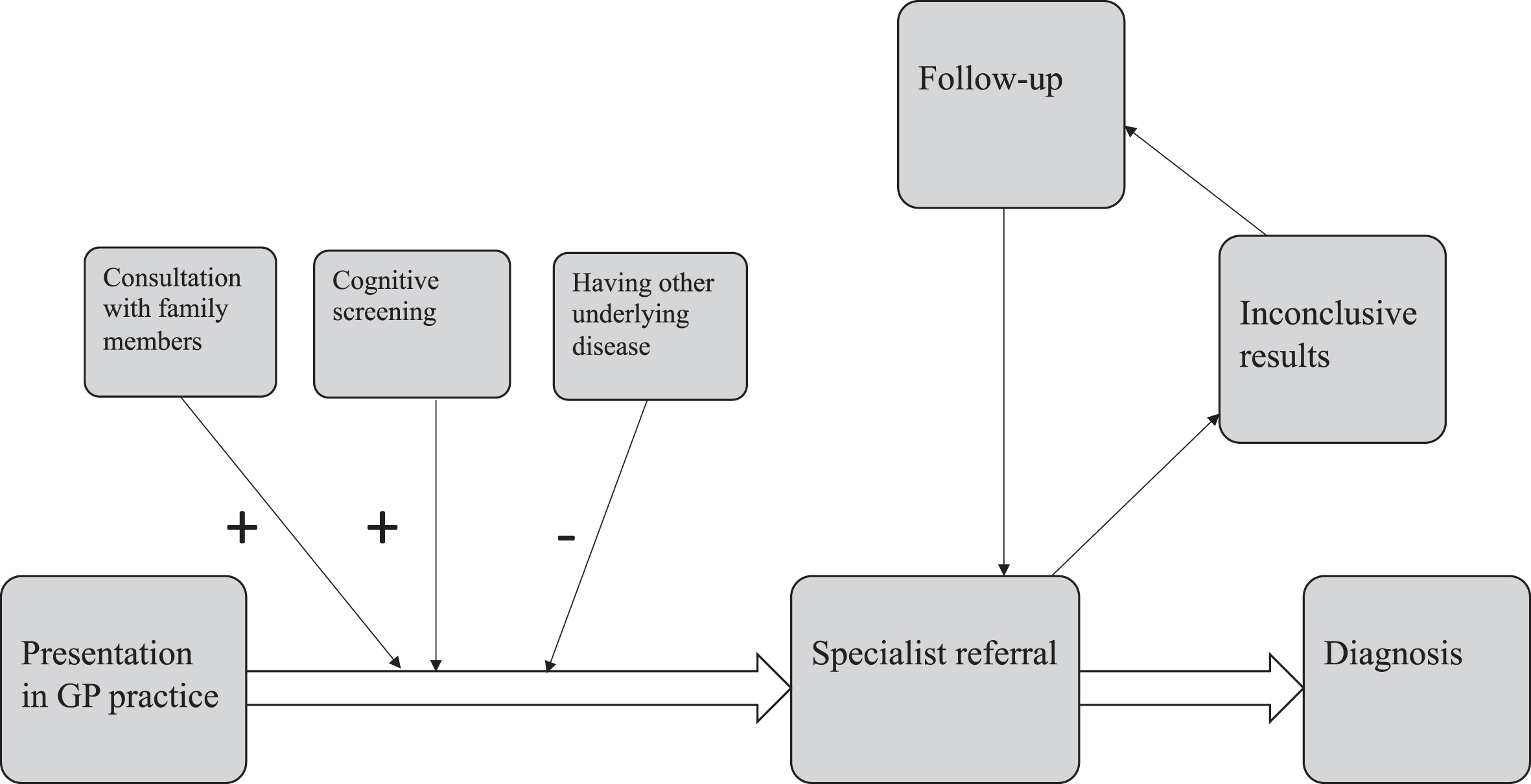
There were several factors that influenced the decision of a GP to refer a person for diagnostic follow-up (Fig. 1). In 65.3% of the persons, the GP had consultations with spouses, children, or other caregivers to get a better understanding of the home situation and elucidate current concerns from caregivers. These consultations usually led the GP to refer a person to a specialist. In 39.5% of the cases, GPs performed a cognitive screening test before referral, using the MMSE (mean score 22.2, standard deviation 5.4). Already having a diagnosis of a neurological disease such as Parkinson’s disease led to a delay in recognition of dementia and consequently a delay in referral for diagnostic follow-up.
Fig. 1
Diagnostic pathway and factors influencing diagnostic referral.
Diagnosis
Nearly all persons (96%) were referred to a specialist for the dementia diagnosis (see Table 3 and Fig. 1). When specialists were not able to diagnose dementia during the first visit, persons were followed up by the specialist until a diagnosis was made. GP files did not report whether specialists referred to other healthcare professionals to aid in the diagnosis. It is therefore unclear whether more than one specialist was involved in the diagnosis. The GP patient files described the dementia subtype in only 55% of the cases (Table 3). Alzheimer’s disease was reported most (25%), followed by vascular dementia (12%).
Table 3
Overview diagnosing specialists and diagnosed subtypes of dementia
| Healthcare specialist that made the diagnosis | Number of persons |
| General Practitioner | 3 (4%) |
| Neurologist | 16 (21.3%) |
| Psychiatrist | 2 (2.7%) |
| Geriatrician | 19 (25.3%) |
| Unclear but in a memory clinic setting | 15 (20%) |
| Unclear | 20 (26.7%) |
| Subtype of dementia* | |
| Alzheimer’s disease | 19 (25.3%) |
| Vascular dementia | 9 (12%) |
| Frontotemporal dementia/Primary progressive aphasia | 5 (6.7%) |
| Parkinson’s dementia/Lewy body dementia | 4 (5.3%) |
| Other** | 3 (4%) |
| Mixed dementia | 2 (2.7%) |
*Not all subtypes of diagnoses were recorded; **Other refers to dementias due to amyotrophic lateral sclerosis, Huntington’s disease or brain tumor.
Post-diagnostic phase
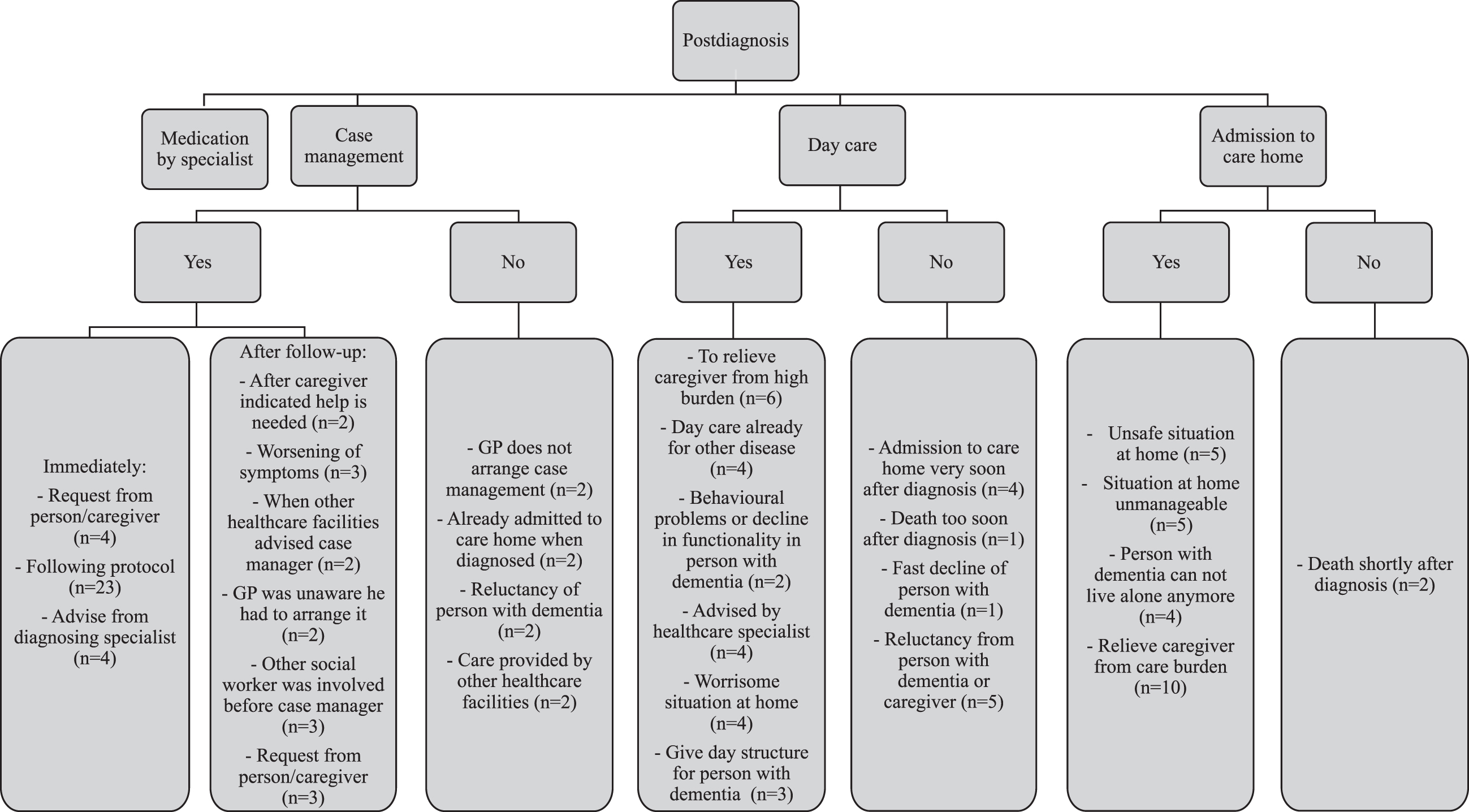
In 20% of the cases, the GP reported disagreements with persons with YOD or their caregivers regarding the decision to initiate specific types of care or support. Main reasons for these disagreements were that either the person or the caregiver did not want day care or admission to a long-term care facility. Figure 2 shows the different care services persons with YOD used, and the reasons for use, delay or not using these facilities.
Fig. 2
Reasons for initiating or not initiating specific types of care.
Medication
Pharmacotherapy aimed at delaying dementia progression was always initiated by a specialist. In total, 30.6% (n = 23) of the participants received such medication, with 54.6% receiving galantamine, 36.4% receiving rivastigmine and 9% receiving memantine. Of the persons receiving medication, 13 (70%) were diagnosed with Alzheimer’s disease dementia, two (2.7%) with Parkinson’s dementia, and one (1.3%) each with Lewy body dementia, primary progressive aphasia, or mixed dementia. For five medication users, no subtype was recorded.
Dementia case management
72% (n = 53) of the participants were assigned a case manager, 22% were not, and for 6% it was not reported. Most participants received case management within one year after diagnosis (Table 4). Reasons for assigning a case manager (if reasons were reported) are listed in Fig. 2.
Table 4
Time from diagnosis until the start of care
| Time since diagnosis | Dementia case manager (n = 53) | Day care (n = 32) | Admission to a long-term care facility (n = 33) |
| Before diagnosis | 5/75* (6.7%) | 5/75 (6.7%) | 3/75 (4%) |
| 0–1 year after diagnosis | 40/70 (57.1%) | 12/70 (1.7%) | 11/72 (15.3%) |
| 1–2 years after diagnosis | 6/25 (24%) | 7/47 (14.9%) | 10/52 (19.2%) |
| 2–3 years after diagnosis | 1/13 (4%) | 2/23 (8.7%) | 4/29 (13.8%) |
| 3–4 years after diagnosis | 1/8 (7.7%) | 1/10 (10%) | 4/17 (23.5%) |
| 4–5 years after diagnosis | 0/1 (0%) | 0/4 (0%) | 1/4 (25%) |
| Unknown | 0 | 5 | 0 |
*Nominator is number of persons getting the care service in that year of follow-up, denominator is number of persons who did not receive that care service yet and were not lost to follow up yet (persons ‘at risk’ for receiving that care service).
In some cases (n = 4), the specialists recommended a case manager to the GP. Written notes showed three GPs were not familiar with the regulations regarding the initiation of dementia care, which delayed the arrangement of a case manager. Of those persons with YOD who did not receive case management (n = 22), four persons had advanced dementia at the time of diagnosis and were either already living in a long-term care facility or moved there within a few months. Two persons already received healthcare for treatment of the primary cause of dementia (one with a brain tumor, one with Parkinson’s disease).
Of the persons with a case manager, 43.4% (n = 23) received day care and 35.8% (n = 19) was admitted to a long-term care facility. Of the persons without a case manager, 40.9% (n = 9) received day care and 63.6% (n = 14) were admitted to a long-term care facility.
Day care
Table 4 shows how many persons received day care and the time in their trajectory at which the day care started. 42.7% (n = 32) of the persons received day care within the duration of this study. For most persons, day care started within two years after diagnosis. 6.7% (n = 5) of the persons received day care already before diagnosis due to other underlying neurodegenerative causes such as Parkinson’s disease, or brain tumor. For 26.7% (n = 20) of the persons, day care was not reported within the duration of this study. The other 30.6% (n = 23) did not receive day care. Figure 2 shows reasons that were reported for initiating or not initiating day care. The most important reasons were to relieve the caregivers’ burden (n = 6), give structure for the person with dementia (n = 3), or worrisome situation at home (n = 4). Reasons not to start day care were mostly death (n = 1), admission to a long-term care facility (n = 4), or unwillingness from the person with YOD or their caregiver (n = 5).
Admission to a long-term care facility
44% of the persons (n = 33) were admitted to a long-term care facility within the duration of this study (Table 4 and Fig. 2). 5.3% (n = 4) were not admitted to a long-term care facility because they died before institutionalization (due to other illness or euthanasia). Of the persons living alone, 58.9% (n = 10) were admitted to a long-term care facility, compared to 41.4% (n = 24) of the persons not living alone. The main reason for institutionalization was an unmanageable (n = 5) or unsafe (n = 5) situation at home, or because the caregiver was not able to provide the necessary care anymore (n = 10). Delay of admission occurred when the person with dementia or their caregiver refused admission.
Caregivers
Primary caregivers of the persons with YOD were mostly family members. For 64% of the persons, the spouse was the primary caregiver, sometimes supported by other relatives such as children or cousins. Children were the primary caregiver in 17.3% of the persons with YOD. Other primary caregivers were siblings (n = 2), a parent (n = 1), care counselors (n = 3), a friend (n = 1), or former spouse (n = 1). 5.3% of the persons did not have a primary caregiver. The GP discussed caregiving issues with the caregivers in only in a third of the participants. Most of these discussions were positive and involved talking about the burden the caregiver felt (n = 8), recommending specific caregiver support groups (n = 6), planning separate GP consultations with the caregiver (n = 2), or initiating day care to relieve the caregiver (n = 2). Sometimes the case manager was the one to monitor caregiver burden (n = 4). In two cases, the GP reported that the caregiver indicated the burden was too high, but the GP thought differently, therefore not referring them for further help.
DISCUSSION
This study provided insight in care use of persons with YOD and factors influencing this care trajectory as described in GP records. We found that consultations with family members were the main trigger for GPs to refer to a specialist during the diagnostic stage, while having another known underlying neurological disease delayed the referral for diagnostic follow-up for dementia. In the post-diagnostic stage, most persons with YOD received a case manager already in the first year of diagnosis. If no case manager was assigned the main reasons were the involvement of other healthcare facilities such as mental health facilities, or the person with dementia did not want support from a case manager. Furthermore, 32 persons received day care, and 33 persons were admitted to a long-term care facility. Main reasons for these were to relieve some burden of the caregiver, behavioral problems, decline in functionality in the person with dementia, or an unmanageable situation at home. Persons without a case manager were more often admitted to a long-term care facility (63.3%) than persons with a case manager (35.8%).
Recognizing YOD was difficult for the GP. This was illustrated by the result that in 65.3% of the cases the GP needed consultations with caregivers to better understand the situation and refer for diagnosis. Especially other underlying neurological causes made understanding and relating the symptoms to dementia difficult. This delay in recognition contributes to the long time to diagnosis in persons with YOD [4–6]. Cognitive screening tests were used in 39.5% of the participants, but in some persons MMSE tests could not be performed due to illiteracy, lack of understanding or refusal from the person with YOD. When the results of the MMSE indicated no cognitive impairment, caregivers expressed that the test did not reflect the current situation. Indeed, MMSE may underestimate cognitive impairment in young persons or dementia subtypes other than Alzheimer’s disease [18]. This may contribute to the delay in recognition of YOD. Without reliable screening tests GPs may delay referring to specialists for further diagnosis. Therefore, consultations with caregivers are very important. They can provide additional information for a GP to make an informed decision for referral. Indeed, we found in 65% of the cases consultations with caregivers were reported, and these were the main trigger for the GP to refer persons to a specialist. This is also reflected in studies investigating caregivers’ perspectives on the diagnostic trajectory [19–22]. Here, caregivers mentioned the decision of a GP to refer to a specialist was usually contingent on additional caregiver information. In a study among healthcare professionals, they emphasized the need for more awareness of YOD, so persons and their caregivers consult the GP earlier, and GPs refer to the right service earlier [23].
We found that 72% of the persons with YOD were assigned a case manager, most within one year following diagnosis. This is higher compared to the Dutch average of 60% of the persons with a dementia diagnosis at the GP and living at home [24]. Previous research has shown that the use of a case manager has a positive impact on the use of existing services and the management of problems experienced by both persons with YOD and their caregivers [25]. Our results showed that there was minimal difference in the use of day care between persons with and without a case manager, but a higher percentage of persons without a case manager were admitted to a long-term care facility. This may have several explanations. Some persons were admitted to a long-term care facility already a few months after diagnosis, making a case manager unnecessary. For others, it might be that the case manager prolonged the time until admission to a long-term care facility, because a case manager could help arrange care needed to facilitate a person with YOD living at home as long as possible. A previous systematic review has shown that case management reduced the caregiver burden [26], which may lead to a prolonged time between dementia diagnosis and admission to a long-term care facility.
Case managers also support and advise GPs with the coordination of healthcare use, since they have a more in-depth knowledge on the available care services [27]. In the free text, we found that some GPs were not aware of their responsibilities and roles when arranging dementia care (Fig. 2). Fostering communication on healthcare facilities between the GP, the diagnosing specialist and the case manager could therefore improve knowledge and timely arrangement of healthcare [28], especially since GPs may not be aware of specific YOD services, due to the low prevalence of YOD in the general practice.
Within the duration of this study, 32 persons (42.7%) received day care and 34 persons (45%) were admitted to a long-term care facility. However, follow-up time for most persons in this study was less than five years, which might give an underestimation of the number of persons eventually using day care or being admitted to a long-term care facility since these might be initiated in a later phase of the disease trajectory. In a previous study, the amount of care given by a caregiver delayed the time until day care or admission to a long-term care facility [12]. Additionally, one study found that the time from symptom onset to admission to a long-term care facility in persons with YOD was 9 years [29]. This might also explain the large number of persons not being admitted to a long-term care facility in our study yet.
We also found that in 20% of the cases GPs reported disagreements between the GP and persons with YOD or their caregivers about the use of dementia care, mostly regarding day care or admission to a long-term care facility. Persons with dementia but also their caregivers were reluctant to start day care or admission to a long-term care facility, which delayed the start of these care services. Previous research has shown this reluctance is also common in persons with LOD and is caused by the feeling of loss of independence [30]. Reluctance of caregivers may be due to the needs paradox, they only retrospectively realize care should have been initiated sooner, but a lack of acceptancy in the earlier stages of the care process led to a delay in accepting care [31]. A GP could therefore help in engaging in communication about the reasons for the reluctance of care use and emphasize meaningful dementia healthcare. The needs paradox for instance could be addressed by offering interventions that increase the knowledge on changing roles and focus on the enhancement of the positive experiences, thereby strengthening the relationship and reducing negative consequences [31].
Especially in YOD, the caregiver burden is high [32], with caregivers having to fulfill different roles. This is an important factor for GPs to consider and monitor, but we found that in only a third of the cases the GP reported discussing caregiver issues and in even fewer cases reported on providing help. However, this information might also be reported in the patient files of the caregiver, which makes underestimation in our study likely. It is essential for GPs to help caregivers of persons with YOD by advising on support groups or arranging timely dementia care to relieve caregiver burden or arrange for the case manager to help with the support of caregivers.
Strengths and limitations
This study has several strengths. To the best of our knowledge, this is the first study obtaining insight in the care trajectory of persons with YOD from GPs written notes. We were therefore able to identify factors that influenced the decisions GPs made regarding different aspects of the care trajectory. With the use of the patient files, there was minimal recall bias. Also, all persons with YOD in the GP register were sampled, leading to minimal selection bias.
However, several limitations exist within this study. The written notes from the GPs used for this study are primarily used as a memory aid for GPs during consultation and not for research purposes. Therefore, not all potentially relevant information for this study might have been documented, and it is likely that some information bias is present. There is a high heterogeneity between GPs in what they report in their notes, since there is no standard on what should be included in the written notes. Not all care is arranged by the GP, and although the GP should be notified on care use by other healthcare professionals, this may not always be the case in practice and/or this might not always be reported by the GP, leading to an underestimation of the total use of care. Also, the information extracted on the reasons for initiating or not initiating care us (Fig. 2) were mostly only briefly addressed, and underlying causes for these reasons may not have been mentioned. This might have led to misinterpretation of these reasons by the researchers.
Furthermore, follow-up time in this study was relatively short, leading to missing information of dementia care use in a large part of the sample. This refrained us from analyzing the average time between diagnosis and the start of care use such as day care or admission to a long-term care facility, since those persons with a long time between diagnosis and the start of care use would not be included in our analyses. This could have given a distorted representation of the actual time to start of care use. Results may also only be applicable in the Netherlands since other countries make use of different care systems. Furthermore, in this study it was unclear whether the availability of tailored care may influence the decision to start using this care, since it meets the needs of young persons better than regular dementia care. Moreover, we were unable to relate the start of care use to the disease stage. It is possible that persons in our study were still in an early stage, where dementia care is not yet necessary whereas others were already in a more advanced stage, where some dementia care was not feasible anymore (i.e., a person had to be admitted to a long-term care facility because day care was not feasible to start due to the severe cognitive decline).
Implications and conclusion
This study was a first exploration into the care use and factors influencing this care use in YOD. This has led to some implications for future research. We found several aspects of YOD care might differ from LOD care (e.g., the high number of persons with YOD receiving case management compared to the national average). Future research should therefore aim to investigate differences and similarities in the care use between persons with YOD and LOD. This could optimize dementia care for both groups.
Furthermore, this study aimed to describe and explore care use, rather than test hypothesis of differences between groups. Future research could use the results of this study to investigate differences in care use between different subgroups of people, for instance differences between persons living alone and persons living together.
This study was aimed to investigate the care use as reported by the GP. However, since the GP is not the only healthcare specialist arranging care, future research should aim to address the care use as reported by other healthcare professionals such as case managers or specialists. The combination of information from different resources may give the most elaborate overview of factors influencing care use. We found case managers helped the GP by taking over care planning, but more collaboration between healthcare professionals may benefit dementia care even more.
Lastly, there are many more dementia care services (home care, psychosocial care, other drug use, etc.), which are not explored in this study, but might be of interest for future studies.
Understanding factors that influence care use is important for improvement of care use. This study showed that most persons with YOD already receive care in the first years after diagnosis, but there are factors that could be improved. Especially reluctance of persons with YOD or their caregivers to start certain care services is something the GP should address, so they could try to understand reasons behind it and help finding solutions to access the most optimal care at the right time.
ACKNOWLEDGMENTS
This research was funded by Gieskes-Strijbis fonds, Alzheimer Netherlands, and the Dutch Knowledge Centre on Young-Onset Dementia.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0713r1).
REFERENCES
[1] | Hendriks S , Peetoom K , Bakker C , Van Der Flier WM , Papma JM , Koopmans R , Verhey FRJ , De Vugt M , Köhler S , Withall A ((2021) ) Global prevalence of young-onset dementia: A systematic review and meta-analysis. JAMA Neurol 78: , 1080–1090. |
[2] | van de Veen D , Bakker C , Peetoom K , Pijnenburg Y , Papma J , group Ps , de Vugt M , Koopmans R ((2022) ) Provisional consensus on the nomenclature and operational definition of dementia at a young age, a Delphi study. Int J Geriatr Psychiatry 37: , 10.1002/gps.5691. |
[3] | van de Veen D , Bakker C , Peetoom K , Pijnenburg Y , Papma JM , de Vugt M , Koopmans R , Group PS ((2021) ) An integrative literature review on the nomenclature and definition of dementia at a young age. J Alzheimers Dis 83: , 1891–1916. |
[4] | Draper B , Cations M , White F , Trollor J , Loy C , Brodaty H , Sachdev P , Gonski P , Demirkol A , Cumming RG ((2016) ) Time to diagnosis in young-onset dementia and its determinants: The INSPIRED study. J Geriatr Psychiatry 31: , 1217–1224. |
[5] | Kvello-Alme M , Bråthen G , White LR , Sando SB ((2021) ) Time to diagnosis in young onset Alzheimer’s disease: A population-based study from Central Norway. J Alzheimers Dis 82: , 965–974. |
[6] | Van Vliet D , De Vugt ME , Bakker C , Pijnenburg YAL , Vernooij-Dassen M , Koopmans R , Verhey FRJ ((2013) ) Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med 43: , 423–432. |
[7] | Van Vliet D , de Vugt ME , Bakker C , Koopmans RTCM , Verhey FRJ ((2010) ) Impact of early onset dementia on caregivers: A review. Int J Geriatr Psychiatry 25: , 1091–1100. |
[8] | Bayly M , O’Connell ME , Kortzman A , Peacock S , Morgan DG , Kirk A ((2021) ) Family carers’ narratives of the financial consequences of young onset dementia. Dementia 20: , 2708–2724. |
[9] | Busted LM , Nielsen DS , Birkelund R ((2020) ) “Sometimes it feels like thinking in syrup”– the experience of losing sense of self in those with young onset dementia. Int J Qual Stud Health Well-being 15: , 1734277. |
[10] | Chirico I , Ottoboni G , Linarello S , Ferriani E , Marrocco E , ChattatR ((2022) ) Family experience of young-onset dementia: The perspectives of spouses and children. Aging Mental Health 26: , 2243–2251. |
[11] | Millenaar JK , Bakker C , Koopmans RTCM , Verhey FRJ , Kurz A , de Vugt ME ((2016) ) The care needs and experiences with the use of services of people with young-onset dementia and their caregivers: A systematic review. Int J Geriatr Psychiatry 31: , 1261–1276. |
[12] | Janssen O , Vos SJB , Handels R , Vermunt L , Verheij R , Verhey FRJ , van Hout H , Visser PJ , Joling KJ ((2020) ) Duration of care trajectories in persons with dementia differs according to demographic and clinical characteristics. J Am Med Dir Assoc 21: , 1102–1107. |
[13] | Buckley JS , Salpeter SR ((2015) ) A risk-benefit assessment of dementia medications: Systematic review of the evidence. Drugs Aging 32: , 453–467. |
[14] | Netherlands/Vilans A (2013) Zorgstandaard dementie [The national standard for dementia care] (in Dutch). |
[15] | Bentsen BG ((1986) ) International classification of primary care. Scand J Prim Health Care 4: , 43–50. |
[16] | Chatfield C ((1986) ) Exploratory data analysis. Eur J Oper Res 23: , 5–13. |
[17] | Hsieh H-F , Shannon SE ((2005) ) Three approaches to qualitative content analysis. Qual Health Res 15: , 1277–1288. |
[18] | Tombaugh TN , McIntyre NJ ((1992) ) The Mini-Mental State Examination: A comprehensive review. J Am Geriatr Soc 40: , 922–935. |
[19] | Bruinsma J , Peetoom K , Bakker C , Boots L , Verhey F , de Vugt M ((2022) ) ‘They simply do not understand’: A focus group study exploring the lived experiences of family caregivers of people with frontotemporal dementia. Aging Mental Health 26: , 277–285. |
[20] | Olivieri P , Hamelin L , Lagarde J , Hahn V , Guichart-Gomez E , Roué-Jagot C , Sarazin M ((2021) ) Characterization of the initial complaint and care pathways prior to diagnosis in very young sporadic Alzheimer’s disease. Alzheimers Res Therapy 13: , 90. |
[21] | Van Vliet D , de Vugt ME , Bakker C , Koopmans RTCM , Pijnenburg YAL , Vernooij-Dassen MJFJ , Verhey FRJ ((2011) ) Caregivers’ perspectives on the pre-diagnostic period in early onset dementia: A long and winding road. Int Psychogeriatr 23: , 1393–1404. |
[22] | O’Malley M , Carter J , Stamou V , LaFontaine J , Oyebode J , Parkes J ((2021) ) Receiving a diagnosis of young onset dementia: A scoping review of lived experiences. Aging Mental Health 25: , 1–12. |
[23] | Spreadbury JH , Kipps CM ((2018) ) Understanding important issues in young-onset dementia care: The perspective of healthcare professionals. Neurodegener Dis Manag 8: , 37–47. |
[24] | Ministry of Health, Welfare and Sports (October 2020). National Dementia Strategy 2021-2030. www.government.nl. |
[25] | Khanassov V , Vedel I ((2016) ) Family physician– case manager collaboration and needs of patients with dementia and their caregivers: A systematic mixed studies review. Ann Fam Med 14: , 166–177. |
[26] | Corvol A , Dreier A , Prudhomm J , Thyrian JR , Hoffmann W , Somme D ((2017) ) Consequences of clinical case management for caregivers: A systematic review. Int J Geriatr Psychiatry 32: , 473–483. |
[27] | Giebel C , Robertson S , Beaulen A , Zwakhalen S , Allen D , Verbeek H ((2021) ) “Nobody seems to know where to even turn to”: Barriers in accessing and utilising dementia care services in England and The Netherlands. Int J Environ Res Public Health 18: , 12233. |
[28] | Prince M , Comas-Herrera A , Knapp M , Guerchet M , Karagiannidou M ((2016) ) World Alzheimer Report 2016. Improving healthcare for people living with dementia: Coverage, quality and costs now and in the future. Alzheimer’s Disease International, London. |
[29] | Bakker C , de Vugt ME , van Vliet D , Verhey FRJ , Pijnenburg YA , Vernooij-Dassen MJFJ , Koopmans RTCM ((2013) ) Predictors of the time to institutionalization in young-versus late-onset dementia: Results from the Needs in Young Onset Dementia (NeedYD) study. J Am Med Dir Assoc 14: , 248–253. |
[30] | Stephan A , Bieber A , Hopper L , Joyce R , Irving K , Zanetti O , Portolani E , Kerpershoek L , Verhey F , De Vugt M ((2018) ) Barriers and facilitators to the access to and use of formal dementia care: Findings of a focus group study with people with dementia, informal carers and health and social care professionals in eight European countries. BMC Geriatr 18: , 131. |
[31] | Boots LMM , Wolfs CAG , Verhey FRJ , Kempen GIJM , de Vugt ME ((2015) ) Qualitative study on needs and wishes of early-stage dementia caregivers: The paradox between needing and accepting help. Int Psychogeriatr 27: , 927–936. |
[32] | Lim L , Zhang A , Lim L , Choong T-M , Silva E , Ng A , Kandiah N ((2018) ) High caregiver burden in young onset dementia: What factors need attention? J Alzheimers Dis 61: , 537–543. |




