Clinical and Economic Assessment in Early-Stage Dementia by Severity and Amyloid-β Status: A 5-Year Retrospective Claims Study of GERAS-US Patients
Abstract
Background:
The high burden of dementia and Alzheimer’s disease (AD) increases substantially as disease progresses. Characterizing early patterns of health care utilization among patients who develop cognitive impairment may deepen our understanding of early disease trajectory and potentially facilitate timely diagnosis and management.
Objective:
Describe clinical characteristics, healthcare utilization, and costs in early-stage dementia by disease severity and amyloid-β status before enrollment in an observational study (GERAS-US).
Methods:
Consented patients’ GERAS-US data were linked to available five-years of Medicare claims history before GERAS-US enrollment. Clinical characteristics, comorbidity, and pre-/post-diagnosis healthcare use and costs were assessed. Continuous and categorical variables were compared between severity and amyloid-status cohorts using t-test and Chi-square statistics; linear regression models were used to compare cost and utilization measures after adjusting for differences in patients’ observation time. Relative likelihood of observed diagnoses, comorbidity, and prescription drug use among cohorts were presented as OR and 90% confidence interval (CI).
Results:
Of 174 patients clinically diagnosed with early dementia (mild cognitive impairment (MCI): 101; mild dementia (MILD): 73), 55% were amyloid-positive. Memory loss was more likely in MILD versus MCI (OR:1.85, 90% CI 1.10–3.09) and in amyloid-positive versus amyloid-negative cohorts (OR:1.98, 90% CI 1.19–3.29). Mean annual healthcare costs after cognitive impairment/dementia diagnosis were significantly higher for MILD versus MCI ($1191 versus $712, p = 0.067) and amyloid-negative versus amyloid-positive ($1281 versus $701, p = 0.034). Diabetes was more prevalent in MILD and amyloid-negative cohorts.
Conclusion:
Comorbidity and economic burden increased in earliest stages of MCI and MILD and were higher in patients who were amyloid-negative.
INTRODUCTION
Alzheimer’s disease (AD) is a progressive, debilitating, neurodegenerative disease affecting the older adults [1]. It is the most common cause of dementia and was consistently the sixth leading cause of mortality in the United States (US) from 2015 to 2019 and was ranked seventh in 2020 after deaths due to Coronavirus disease 2019 [2, 3]. It is estimated that worldwide, around 55 million people are living with dementia; as per 2021 estimates, 6.2 million people in the US aged >65 years are living with AD dementia [1]. By 2050, the number of patients with clinical AD in the US is projected to double due to an aging population, and the total annual healthcare costs are projected to triple in the absence of effective treatments [1, 4]. In addition, the societal burden of AD in the US is significant, with unpaid informal care in 2020 amounting to 15.3 billion hours valued at $256.7 billion [1].
The disease advances over time from pre-clinical, asymptomatic to mild cognitive impairment (MCI), and mild, moderate, and severe dementia due to AD [1, 5]. Research has been conducted to identify risk factors and biomarkers that are associated with the prognosis of AD [6–9]. The neuropathological alterations and abnormal accumulation of amyloid and tau proteins are thought to begin 10–20 years before the onset of overt symptoms [10, 11]. Diagnosing dementia or AD early in the disease may facilitate disease management for both the patient and the caregiver [12]. However, because patients tend to be diagnosed later in the disease process, the patterns of comorbidities and health care utilization in the earliest stages have not been well characterized. AD is diagnosed by the presence of amyloid-β plaques or tau neurofibrillary tangles in the brain [13–15], and the degree of cognitive and functional impairment can be assessed by a variety of tools including Mini-Mental State Examination (MMSE) [16] and Functional Activities Questionnaire (FAQ) [17]. Together, these tools can characterize the early stages of AD.
GERAS-US, a three-year, prospective study, used cognitive tests to identify and characterize patients with early AD or dementia; amyloid status was determined using a Food and Drug Administration (FDA)-approved amyloid PET scan (florbetapir, flutemetamol, or florbetaben) or through assessment of cerebrospinal fluid [18–20]. In the GERAS-US study, 52% patients with amyloid-positive status [19], and patients clinically diagnosed with mild dementia (MILD) incurred greater societal costs than the patients with MCI [20]. The GERAS-US study also found that the MILD amyloid-negative cohort had greater comorbidity and economic burden than the MILD amyloid-positive cohort at baseline [19, 20]. Studying clinical characteristics, disease comorbidities, and healthcare utilization and costs of GERAS-US patients before their study enrollment will aid in characterizing the early disease state and may potentially help to identify specific patterns of comorbidities and health care utilization that could lead to earlier disease diagnosis. Designed to provide information regarding the burden of illness associated with dementia, this addendum study offers an efficient and effective method to examine outcomes by augmenting the clinical assessment data from the GERAS-US study with claims data related to all long-term healthcare resource utilization, treatment patterns, and costs over a longer course of treatment than would be feasible in clinical research alone. Through this study, a greater understanding of the disease state will be gained, as well as a greater understanding of the cost and health factors associated with the emergence of cognitive impairment.
The present study aimed to better understand the comorbidity profile and healthcare utilization and costs for a subset of patients from the GERAS-US study, who gave their consent for using up to five years of claims history before their baseline study visit. The study further described healthcare utilization and costs before and after a diagnosis of cognitive impairment, dementia, or AD was observed in the Medicare data and compared the patients according to the disease severity or amyloid-β status classifications made at entry in the GERAS-US study.
METHODS
Study design and population
A retrospective cohort study was conducted by linking GERAS-US (ClinicalTrials.gov: NCT02951598 [19]) patient data with Medicare claims data for up to five years (2013–2017) before entry in the GERAS-US study. The five years before the GERAS-US visit was the study period of this investigation. Detailed study design and initial cohort description of the GERAS-US study are described elsewhere [20]. Briefly, the GERAS-US study included patients aged 55 to 85 years with a diagnosis of dementia clinical syndrome; patients were classified according to disease severity and amyloid-β status [19]. This addendum study required Medicare eligibility to provide access to information in the Medicare claims prior to entry in GERAS-US. Medicare fee-for-service (FFS) Parts A and B (A/B) provide complete cost and utilization data supported by diagnosis and procedure codes. Medicare drug coverage (Medicare Part D) is optional, and individuals enrolled in Part D may not have Medicare FFS A/B eligibility. To evaluate differences in prescribed medications, we analyzed all individuals with Part D eligibility which provides data on prescribed medications. Of the 1,329 GERAS-US participants, 266 patients provided consent to use their Medicare data; among these, 241 patients had Medicare FFS A/B eligibility and/or Medicare Part D, as well as GERAS-US baseline data defining their severity cohort and amyloid status. Of these 241 patients, FFS A/B data were available for 174 patients, and Part D data were available for 190 patients.
Assessments
At entry in the GERAS-US, baseline assessments were used to classify patients by disease severity (MILD versus MCI) and amyloid-β status. Two outcome assessments, i.e., MMSE and FAQ tools, were used in the screening process to evaluate the degree of cognitive and functional impairment [20]. Amyloid status was established via a Food and Drug Administration (FDA)-approved amyloid PET scan (florbetapir, flutemetamol, or florbetaben) or a cerebrospinal fluid test either prior to or at the GERAS-US study enrolment. Patients who tested positive for amyloid biomarkers were characterised with AD and patients with amyloid-negative status were characterised as with clinical dementia syndrome [5].
These classifications were used to define MILD versus MCI cohorts and amyloid-positive versus amyloid-negative cohorts in this addendum study.
Demographics and comorbidities
Patient demographics, JEN Frailty Index (JFI), and Charlson comorbidity score visit at entry in GERAS-US were compared between cohorts for patients with FFS eligibility. Diagnoses observed in the Medicare data throughout the observation period were compared between cohorts at the level of International Classification of Diseases 9th Revision (ICD-9) diagnosis subcategories. The Center for Medicare and Medicaid Services (CMS) General Equivalence Mappings were used to map International Classification of Diseases 10th Revision (ICD-10) codes to ICD-9 codes [21]. Comorbidities present at entry in GERAS-US were defined using the diagnostic criteria specified in 27 chronic condition algorithms from the CMS Chronic Conditions Data Warehouse, which assess diagnoses observed over 1–3 years [22].
Diagnoses of cognitive impairment, dementia, and AD
The ICD-9 diagnosis codes for identifying cognitive impairment, dementia, and AD in the Medicare data are presented in Supplementary Table 1 [23]. The proportion of patients observed with one of these diagnosis codes was calculated for each month leading up to entry in GERAS-US. While the AD diagnosis codes reported by health care providers in the Medicare data may not meet the current diagnostic criteria for AD, which require the presence of amyloid, the reporting of the AD diagnosis code as opposed to the codes for other cognitive impairment and dementia may identify patients with more severe cognitive decline; therefore, individuals observed with an AD diagnosis code were analyzed separately. At entry in the GERAS-US study, patients were clinically diagnosed with either MCI or MILD. For some patients this was their first diagnosis; others may have been diagnosed previously. Using the diagnosis codes observed in the Medicare data, time to diagnosis with any cognitive impairment, dementia, and AD was also estimated starting up to five years before the baseline visit. Relative likelihood of being diagnosed with dementia, AD (with or without cognitive impairment), only cognitive impairment and no AD/cognitive impairment before entry in GERAS-US were estimated between cohorts.
Medication usage
Patients with Part D Medicare (contains prescription information) eligibility were evaluated for pre-baseline medication usage and the relative likelihood for receiving specific drug groups were analyzed between cohorts. National drug codes were categorized by therapeutic class and drug groups following Medi-Span® database (Wolters Kluwer Health, Inc., Indianapolis, IN, USA) and Therapeutic Classification System© (Clinical Drug Information, LLC, Hudson, IL, USA).
Healthcare utilization and costs
All-cause healthcare utilization and costs were calculated before and after diagnosis of any cognitive impairment, adjusting for duration of FFS history in each phase. The pre-diagnosis phase consisted of time from the patient’s first month of FFS eligibility up to the month before their first observed diagnosis code with any cognitive impairment in the Medicare data. The post-diagnosis phase consisted of time from the first diagnosis of any cognitive impairment to the month before the baseline visit. Thus, not all patients may have claims information for both phases. Healthcare utilization and costs were reported as per patient per month (PPPM).
Statistical analyses
All analyses were conducted within the CMS Virtual Research Data Center using SAS© 7.15 (SAS Institute Inc., Cary, NC, USA). Presentation of findings on these cohorts is subject to the study’s data use agreement with CMS, which prevents the reporting of cell sizes under 11 [24]. Descriptive variables were summarized using number, proportion, mean, and standard deviation (SD). With the exception of the cost and utilization variables, continuous and categorical variables were compared using t-test and Chi-square statistics, respectively. Linear regression models were used to compare the cost and utilization measures after adjusting for the differences in person-months. Cox proportional hazards model with hazard ratio (HR) were used to compare time to any cognitive impairment diagnosis and the time to an AD diagnosis (as reported in the Medicare data) between cohorts. Relative likelihood of observed diagnoses, comorbidity, and prescription drug use among the cohorts were assessed with ORs. To identify characteristics associated with early cognitive decline and amyloid-β status that may warrant further investigation, the significance threshold was set at α= 0.1 or 90% CI to identify differences between cohorts; the underlying p-values are reported, where appropriate, noting differences significant at p < 0.10 and p < 0.05.
RESULTS
Patients’ profile
Of the 1,329 patients from the GERAS-US study, 266 patients provided consent to use their Medicare data; among these, 241 patients had Medicare FFS Parts A and B eligibility and/or Part D, as well as GERAS-US. Of these 241 patients, FFS A/B data were available for 174 patients and Part D data were available for 190 patients their entry in GERAS-US (Fig. 1).
Fig. 1
Patient selection and data attrition. Filled boxes in gray highlight the patient population studied for different variables. Patients with FFS eligibility were assessed for baseline characteristics, pre-baseline comorbidity, pre- and post-diagnosis healthcare use and costs, and regression modelling. Patients with Part D eligibility were assessed for pre-baseline prescription patterns. AD, Alzheimer’s disease; CI, cognitive impairment; FFS, fee-for-service; N, number of patients at each step.
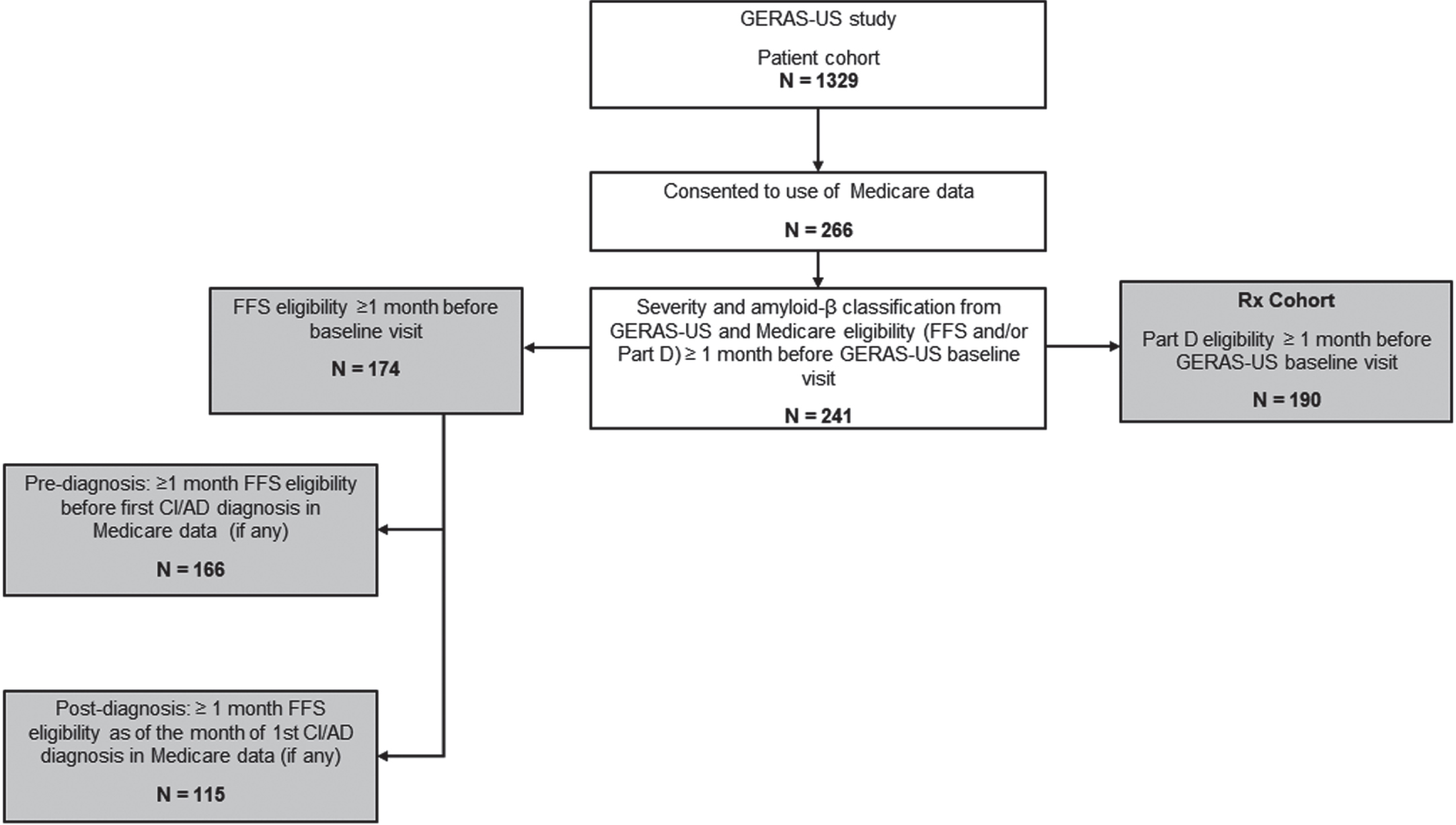
Of the 174 patients with FFS A/B eligibility, the MCI and MILD cohorts comprised 101 and 73 patients, respectively. The demographics were largely similar between the two cohorts, with mean (SD) age of 73.9±5.9 and 73.2±7.2 years and 49.5% and 49.3% female distribution in the MCI and MILD cohorts, respectively (Table 1). A significantly higher proportion of the MCI cohort versus the MILD cohort was white and from the South (p < 0.05). The MILD cohort had a significantly greater proportion of patients with a cognitive impairment diagnosis code in the Medicare data before the baseline GERAS-US visit, as well as higher Charlson comorbidity and JFI scores than the MCI cohort (p < 0.05; Table 1). A similar proportion of patients had amyloid-positive status in the MILD (56.2%) and MCI (54.5%) cohorts.
Table 1
Demographics and clinical characteristics of patients clinically diagnosed with early dementia stratified by severity and amyloid-β status
| Cohorts for comparisonsa | Severity | Amyloid-β status | ||
| Description | MCI | MILD | Negative | Positive |
| N = 101 | N = 73 | N = 78 | N = 96 | |
| At index | ||||
| Age (y), mean (SD) | 73.87 (5.85) | 73.25 (7.17) | 72.74 (6.69) | 74.31 (6.15) |
| Sex: Female, n (%) | 50 (49.5) | 36 (49.3) | 39 (50.0) | 47 (49.0) |
| Race: White, % | >89b | 83.6b | >86 | >89 |
| Region: South, n (%) | 46 (45.5)b | 52 (71.2)b | 43 (55.1) | 55 (57.3) |
| Amyloid-β status, n (%) | ||||
| Negative | 46 (45.5) | 32 (43.8) | 78 (100) | – |
| Positive | 55 (54.5) | 41 (56.2) | – | 96 (100) |
| JEN Frailty Index, mean (SD)d | 5.19 (1.99)b | 5.95 (2.03)b | 5.69 (1.95) | 5.38 (2.10) |
| Charlson score, mean (SD)d | 0.92 (1.29)b | 1.43 (1.66)b | 1.36 (1.56) | 0.96 (1.39) |
| Comorbidities, % d | ||||
| Anemia | 25.6 | 27.7 | 28.4 | 25.0 |
| Chronic kidney disease | 22.1 | 29.2 | 26.9 | 23.8 |
| Depression | 31.4 | 35.4 | 37.3 | 29.8 |
| Diabetes | 24.4b | 38.5b | 40.3b | 22.6b |
| Hyperlipidemia | 69.8 | 58.5 | 62.7 | 66.7 |
| Hypertension | 70.9 | 73.8 | 71.6 | 72.6 |
| Ischemic heart disease | 26.7 | 35.4 | 28.4 | 32.1 |
| Prior to baseline study visit | ||||
| Diagnosis prior to visit, n (%) | ||||
| AD (with or without cognitive impairment) | 21 (20.8)b | 27 (37.0)b | 18 (23.1) | 30 (31.3) |
| Only cognitive impairment | 39 (38.6) | 28 (38.4) | 33 (42.3) | 34 (35.4) |
| No AD/cognitive impairment | 41 (40.6) | 18 (24.7) | 27 (34.6) | 32 (33.3) |
| Prior Part D history | ||||
| n (%) | 78 (77.2)c | 47 (64.4)c | 55 (70.5) | 70 (72.9) |
| Months, mean (SD) | 43.38 (17.62) | 42.60 (16.18) | 41.05 (17.84) | 44.69 (15.29) |
| Prior FFS history | ||||
| n (%) | 101 (100) | 73 (100) | 78 (100) | 96 (100) |
| Months, mean (SD) | 43.40 (17.62) | 41.19 (18.21) | 40.36 (19.39) | 44.19 (16.39) |
| Phase (months), mean (SD) | ||||
| Pre-diagnosise | 31.43 (19.24)b | 24.41 (16.79)b | 27.35 (19.10) | 29.37 (18.09) |
| Post-diagnosise | 22.77 (18.58) | 23.60 (16.46) | 22.04 (17.09) | 24.06 (17.95) |
aMCI versus MILD and amyloid-negative versus amyloid-positive p-values were estimated for continuous and categorical data using t-test or chi-square test, respectively. bp < 0.05. cp < 0.10. dRestricted to participants with FFS eligibility in the index month. eRefers to pre- and post-diagnosis of AD or cognitive impairment. AD, Alzheimer’s disease; FFS, fee-for-service; MCI, mild cognitive impairment; MILD, mild dementia; n, number of patients in each category; N, total number of patients in the cohort; SD, standard deviation. Baseline comorbidities were defined using chronic condition algorithms from the CMS Chronic Conditions Data Warehouse.
When stratified by amyloid-β status (n = 174), 78 patients were amyloid-negative, and 96 patients were amyloid-positive. Patient demographics and clinical characteristics were comparable between patients with amyloid-negative (mean±SD age: 72.7±6.7 years and 50.0% female) and amyloid-positive (mean±SD age: 74.3±6.1 years and 49.0% female) status (Table 1). Of the comorbid conditions present in all the cohorts, diabetes was significantly more prevalent in the MILD cohort versus the MCI cohort (38.5% versus 24.4%; p < 0.05) and in the amyloid-negative cohort versus the amyloid-positive cohort (40.3% versus 22.6%; p < 0.05 (Table 1).
Comorbidity
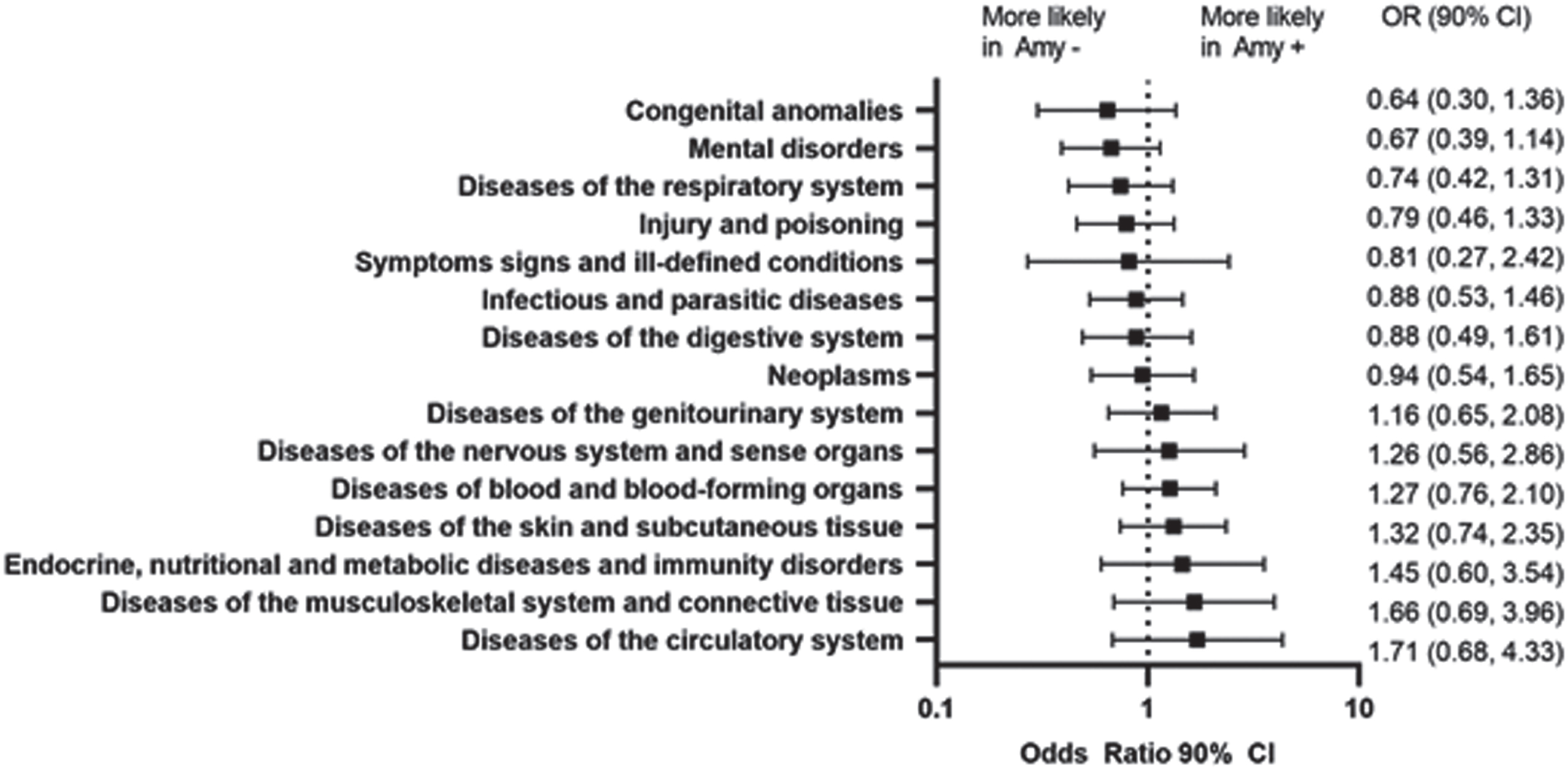
Prior to the baseline visit, the prevalence of major diagnostic categories was comparable between the amyloid-positive and amyloid-negative cohorts (Table 1; Fig. 2). Upon stratifying by both severity and amyloid-β status, the greatest differences in the comorbidities were observed in the MCI cohort, where most of the comorbidities were more likely observed in the amyloid-negative cohort. Only diseases of intestines and peritoneum and benign neoplasms were prevalent in MCI amyloid-positive cohort (Supplementary Figure 1).
Fig. 2
Prevalence of Non-Cognitive impairment/dementia diagnoses prior to study entry and the comparative likelihood by amyloid status. Amy-, amyloid-negative; Amy+, amyloid-positive; CI, confidence interval; OR, odds ratio.
Diagnosis of cognitive impairment or AD in the Medicare data
Prior to the baseline visit, patients in the MILD cohort were more likely than patients in the MCI cohort to be diagnosed with any cognitive impairment (HR 1.53, 90% CI 1.13–2.08) (Fig. 3A–C). Up to the month before the baseline visit, the proportion of patients receiving an AD diagnosis were significantly higher in the MILD cohort compared with the MCI cohort (37.0% versus 20.8%, p = 0.015) (Table 1 and Supplementary Figure 2A).
Fig. 3
Graphical representation of proportion of patients observed with cognitive impairment diagnoses in the Medicare data each month starting from five years up to the month before GERAS-US baseline visit for (A) MCI, (B) MILD, (D) amyloid-negative, and (E) amyloid-positive cohorts. The HR (90% CI) for diagnosis of any cognitive impairment or dementia and the diagnosis of AD are shown by (C) severity and (F) amyloid-β status. AD, Alzheimer’s disease; Amy-, amyloid-negative; Amy+, amyloid-positive; CI, confidence interval; Dx, diagnosis; MCI, mild cognitive impairment; MILD, mild dementia.
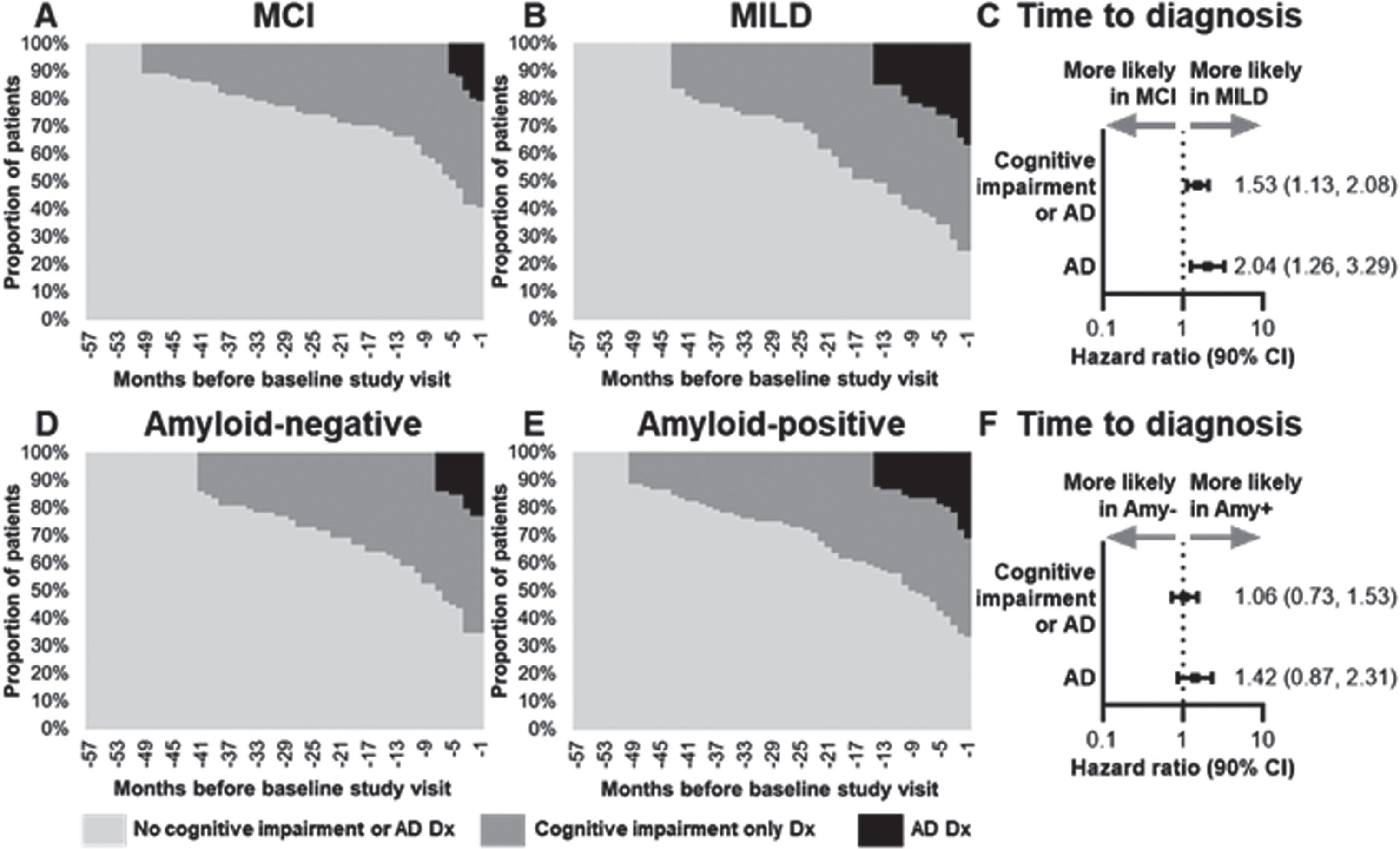
Prior to the baseline visit, patients with amyloid-positive versus amyloid-negative status were equally likely to be diagnosed with any cognitive impairment (HR 1.06, 90% CI 0.73–1.53). Compared with patients in the MCI cohort, patients in the MILD cohort were more likely to be diagnosed with specific components of cognitive impairment such as unspecified dementia (OR 1.86, CI 1.01–3.45), memory loss (OR 1.85, CI 1.10–3.09), and other alteration of consciousness (OR 2.98, CI 1.05–8.46) (Supplementary Figure 2A). The likelihood of diagnosis with neurodegenerative diseases or specific components of cognitive impairment was similar in patients with amyloid-positive and amyloid-negative status except for memory loss, which was more likely in the amyloid-positive cohort (OR 1.98, 90% CI 1.19–3.29) prior to the baseline visit (Supplementary Figure 2B).
Healthcare utilization and costs
In the pre- and post-diagnosis phase, patients in the MILD cohort had higher PPPM healthcare utilization (e.g., hospitalization, emergency department visits, and outpatient visits) than patients in the MCI cohort, with similar findings in the amyloid-negative versus amyloid-positive cohort (Supplementary Tables 2 and 3). For amyloid-negative versus amyloid-positive cohorts, only mean outpatient specialists’ visits were significantly higher for the amyloid-negative than amyloid-positive cohort (0.99 versus 0.71, p = 0.036) in the pre-diagnosis phase.
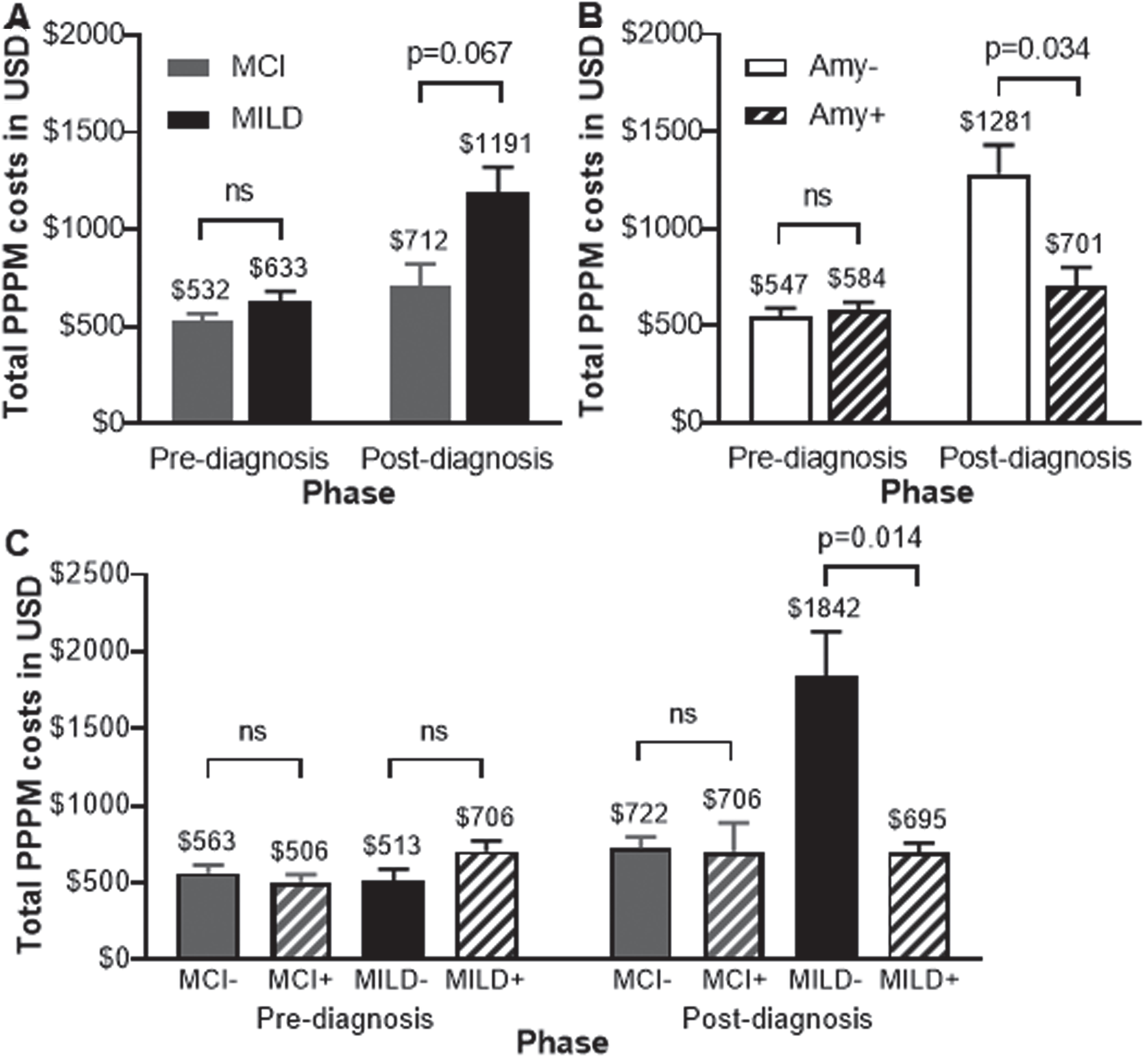
Healthcare costs before diagnosis, were not significantly different between MCI and MILD cohorts (Supplementary Table 2) or between amyloid-negative and amyloid-positive cohorts (Supplementary Table 3). Total PPPM all-cause healthcare costs were evaluated pre- and post-diagnosis between study cohorts to understand the change in healthcare utilization and claims before and after diagnosis. The negative report higher comorbidities demonstrated no differences in total PPPM all-cause healthcare costs before diagnosis between MCI and MILD, or between amyloid-negative and amyloid-positive cohorts (Fig. 4A, B). After diagnosis, total all-cause healthcare costs incurred were higher in the MILD versus MCI cohort ($1,191 versus $712, p = 0.067), and higher in the amyloid-negative versus amyloid-positive cohort ($1,281 versus $701, p = 0.034), respectively (Fig. 4A, B). The MILD amyloid-negative cohort incurred the highest cost, followed by the MILD amyloid-positive cohort (Fig. 4C). Home health cost was a major driver for cost differences observed in the severity and amyloid-β status cohorts in the post-diagnosis phase. Post-diagnosis home health costs were higher in the MILD versus MCI cohort and amyloid-negative versus amyloid-positive cohort.
Fig. 4
Data are represented as mean and standard error of the mean for costs incurred per patient per month in USD. Differences in total all-cause healthcare costs were assessed using linear regression models adjusting for patient observation months in each phase based on (A) severity, (B) amyloid-β status separately, and (C) both parameters considered together. Amy-, amyloid-negative; Amy+, amyloid-positive; MCI, mild cognitive impairment; MCI-, MCI and amyloid-negative; MCI+, MCI and amyloid-positive; MILD, mild dementia; MILD-, MILD and amyloid-negative; MILD+, MILD and amyloid-positive; ns, not significant; USD, United States Dollar.
Medication usage
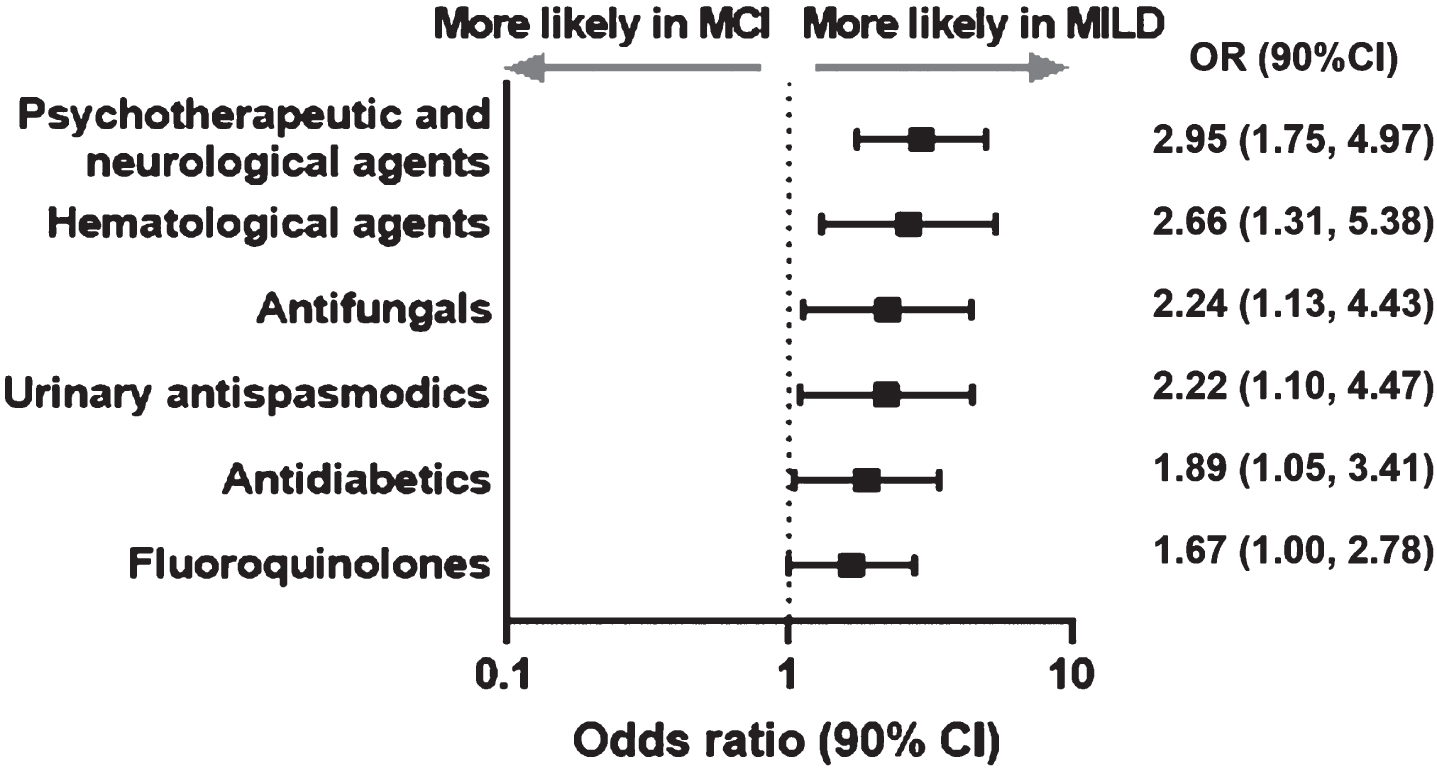
The likelihood of use of all the drugs classes before the baseline visit was greater in the MILD cohort versus the MCI cohort (Fig. 5). Among all the medications used psychotherapeutic and neurological agents (OR 2.95, 90% CI 1.75–4.97), were more likely to be used in the MILD cohort versus the MCI cohort (Fig. 5).
Fig. 5
Significant differences in pre-baseline prescription drug groups used in MILD versus MCI cohort. CI, confidence interval; MCI, mild cognitive impairment; MILD, mild dementia; OR, odds ratio.
On stratifying by both disease severity and amyloid-β status, antidepressants (OR 0.41, 90% CI 0.17–0.99) were 40% more likely prescribed in patients with amyloid-negative status than in patients with amyloid-positive status within the MILD cohort. In the MCI cohort, anticonvulsants (OR 0.39, 90% CI 0.20–0.74) were 40% more likely to be prescribed in patients with amyloid-negative than amyloid-positive status. Only genitourinary agents (OR 2.95, 90% CI 1.34–6.50) and ophthalmic agents (OR 2.57, 90% CI 1.38–4.78) were more likely to be prescribed in patients with amyloid-positive than amyloid-negative status within the MCI cohort.
DISCUSSION
The current addendum study is an expansion of the GERAS-US study and focused on describing up to 5 years of patients’ medical history and healthcare costs before enrollment in the GERAS-US study. At entry in the GERAS-US study, addendum patients were clinically diagnosed with early and were categorized as having MILD (41.95%) or MCI (58.04%); the percentage of patients with amyloid-positive status was comparable between the MILD (56.2%) and MCI (54.5%) cohorts. The finding that nearly half of the patients with a diagnosis of MCI or MILD at their baseline visit were amyloid-negative provides a unique opportunity to compare early patterns of comorbidities, healthcare utilization, and costs between the patients with amyloid-negative and amyloid-positive status. Assessing clinical characteristics, differences in patterns of disease comorbidities, healthcare utilization, and costs for up to 5 years before entry into the GERAS US study aid in characterizing the early disease state; in addition, identifying patterns of comorbidities provide useful information regarding the burden of illness associated with AD and dementia and the difficulty in arriving at a definitive diagnosis without the benefit of biomarker confirmation.
The results of the current study are aligned with the parent GERAS-US study, wherein 52% patients were classified as amyloid-positive irrespective of the level of cognitive and functional impairment [19], versus 55.17% in the current study. Results from the Imaging Dementia –Evidence for Amyloid Scanning (IDEAS) study showed amyloid positivity using PET scans in 55% patients diagnosed with MCI and 70% of patients diagnosed with MILD [25]. The percentage of patients diagnosed with MCI were comparable with the findings of the current study. In the current study, the MILD cohort received a diagnosis earlier in the study period compared to the MCI cohort, supporting their baseline severity classification. There are many factors that may influence if or when an individual is diagnosed with cognitive impairment or dementia. While evaluating these factors was beyond the scope of the current study, confusion and memory issues are early symptoms of MCI and AD related disease [1]. Memory loss was an important feature that was more likely observed in the MILD cohort than in the MCI cohort and in the amyloid-positive cohort than in the amyloid-negative cohort. This is in line with a previous report showing greater cognitive and memory decline in the amyloid-positive cohort compared with the amyloid-negative cohort [26]. These findings reinforce the importance of close monitoring of patients presenting with memory loss or changes in cognition to aid in earlier diagnosis and support earlier disease management and treatment.
Diseases of the circulatory system and diseases of the musculoskeletal system and connective tissue were more prevalent in the patients from the amyloid-positive cohort, suggesting a possible association between amyloid (or amyloid deposition) and other organ systems. Emerging data suggest an association between amyloid deposits and inflammatory cutaneous diseases, retinal depositions, and the effect of circulating blood-derived amyloid-β in amyloid-β related pathologies [27–29]. The correlation between different organ systems and amyloid-β depositions is not well explored and warrants further study.
In the addendum study, the patients with clinical dementia syndrome, specifically in the MCI amyloid-negative cohort were more likely to have pre-baseline comorbidities compared with MCI amyloid-positive cohort based on claims diagnosis. Diagnosis of mental disorders, unspecified dementia, and dementia without behavioral disorders were also more common among amyloid-negative cohort. This is consistent with the GERAS-US study where the amyloid-negative cohort was clinically more complex in both MCI and MILD with higher rates of depression and sleep disorder than the amyloid-positive cohort at baseline [19]. The higher likelihood of receiving a prescription for psychotherapeutics and neurological agents in MILD versus MCI cohort is consistent with increased cognitive and functional impairment with greater disease severity. Predictive risk factors such as use of antipsychotics and antihypertensives, history of hyperlipidemia or diabetes may align with factors associated but not specific with later coding of AD related dementia or other forms of dementia [30]. These findings are consistent with the increased cognitive and functional impairment as well as specific comorbidities seen in the patients in MILD cohort.
Higher use of antidepressants is also consistent with the greater prevalence of depression and other mental health diagnoses in the amyloid negative cohort. These results are in line with a recent study, which demonstrated a lack of relationship between amyloid and depressive symptoms [31]. Recently, more frequent use of anti-psychotics and anti-depressants were reported in dementia with Lewy bodies mildly impaired patients compared with late-onset AD related dementia and cognitively healthy people [32]. In addition, there are several studies that have failed to find a correlation between depression and amyloid-β pathology [33–35]. They concluded that the association of depressive symptoms with dementia and cognitive impairment appears to be independent of amyloid plaques and neurofibrillary pathology [36].
Healthcare use and costs were largely similar before diagnosis in MILD and MCI cohorts and increased after diagnosis of any cognitive impairment irrespective of severity or amyloid-β status. After diagnosis, total costs were higher for both MILD versus MCI and amyloid-negative versus amyloid-positive cohorts. Findings suggest that even in the early stages of symptomatic disease, economic burden increases with greater cognitive and functional impairment of the patients. The economic burden of patients classified as having dementia clinical syndrome was similar to that of amyloid positive patients indicating they may in fact be on a neurodegenerative pathway. Encouraging patients’ engagement with the healthcare system early in the course of their symptoms, may help to identify patterns that facilitate timely disease diagnosis and management. The increased costs in the MILD amyloid negative versus positive patients post-diagnosis are consistent with the findings suggesting that amyloid-negative patients had more comorbidities than the MILD amyloid-positive patients [19, 20]. Notable drivers in post-diagnosis cost differences by amyloid-β status were durable medical equipment, home health services and skilled nursing facility use, suggesting an overall decline in the patient health with increased need for supportive care.
Finally, several comorbidities were significantly associated with the MILD versus MCI and the amyloid-negative versus amyloid-positive cohorts, with diabetes being more strongly associated with the MILD and amyloid-negative cohorts. The prevalence of diabetes in these cohorts is in line with several studies that have reported diabetes as a risk factor for neurodegenerative diseases, vascular dementia, and cognitive decline [27, 37–41]. Patients with Type II diabetes often have other cardiovascular risk factors and conditions such as high blood pressure, dyslipidemia, cardiovascular disease, and cerebrovascular disease that may contribute to cognitive impairment independent of amyloid plaques. This medical complexity may in part explain the higher costs and higher incidence of diabetes among the amyloid negative patients [42]. Exploring this association of high prevalence of diabetes in amyloid negative patients may lead healthcare professionals towards a new diagnostic pathway for early profiling of neurodegenerative diseases.
Strengths and limitations
This study focused on a cohort of patients clinically diagnosed with early dementia where detailed information about their diagnostic, treatment, and healthcare history prior to cohort entry were compared based on disease severity and amyloid-β status. This study provides the advantage of both prospective and retrospective study designs by linking clinical data from the prospective observational GERAS-US study with patients’ Medicare claims prior to their enrollment in GERAS-US. This study provides a unique opportunity to answer questions related to early dementia clinical syndrome in a cohort where amyloid status is known. The study also had several limitations. Medicare claims provide a rich source of real-world data containing detailed information on the cost and use of healthcare resources, as well as diagnosis and procedure codes. However, the diagnosis and procedure codes present in claims data are used primarily for reimbursement purposes; as such they do not necessarily reflect the definitive picture of a patient’s clinical condition, especially with regards to disease severity. The addendum study used the clinical classification of cognitive impairment or dementia clinical syndrome made at entry in the GERAS-US study and accessed claims data to provide supplemental information to identify factors associated with the emergence of cognitive impairment in these patients. Only 241 of the 1,329 GERAS-US study consented and contributed to this addendum study. In comparison to published statistics on the GERAS-US patients that were classified according to their severity and amyloid-β status (n = 1,198), the addendum cohort did not differ from the GERAS-US cohort in terms of gender, race, or the prevalence of hypertension and diabetes at baseline (data not shown) [19, 20]. However, the mean age was higher in addendum cohort in the GERAS-US cohort (73.6 versus 70.4, p < 0.0001). Over 25% of the GERAS-US cohort was less than 65 years of age. Medicare eligibility, which is available to most older adults at age 65, was required for the addendum study and it is likely driving this age difference. As such, the addendum study findings cannot be generalized and extrapolated beyond Medicare beneficiaries. Additionally, more addendum patients completed some college (68.5% versus 50.9%, p < 0.0001) and fewer addendum patients had depression at baseline (28.7% versus 39.0%, p < 0.05). Despite the difference in the prevalence of depression and the inability of this study to fully stratify by severity and amyloid-β status, the results described above did corroborate findings on the higher rate of depression among amyloid-negative patients, the higher rate of prescriptions for psychotherapeutic agents among MILD patients, and the rate of amyloid positivity in the GERAS-US study [19, 25]. Additionally, the sample size for comorbidities (diabetes, chronic kidney disease, ischemic heart disease, and anemia) and prescription medication usage was small, which can lead to more variable ORs. In addition, some of the associations between variables may be exaggerated, requiring a larger sample size for more precise estimates. Prescription claims were used as a proxy for medication use; whether patients took the medications as prescribed was not confirmed through chart review or during patient contact. Since not all patients were diagnosed with cognitive impairment or AD before entry in the GERAS-US study and were not part of both the pre- and post-diagnosis phases, the addendum study did not include a longitudinal assessment of cost and use patterns.
Conclusions
In conclusion, the study confirmed that comorbidity and economic burden increased with greater disease severity in patients with early symptomatic disease. In addition, the comorbidity and economic burden was also greater in the amyloid-negative cohort than in the amyloid-positive cohort. The patients with dementia clinical syndrome are on the path of cognitive and functional impairment and developing neurodegenerative disorders. Higher incidence of comorbidities in the amyloid-negative group increases the economic burden by increased engagement with the healthcare system. The differences observed between the amyloid-positive and amyloid-negative cohorts provides additional information for healthcare providers to consider in making a definitive diagnosis of the disease among patients with dementia clinical syndrome. The findings also highlight the unmet need and disease burden in patients with mild dementia, irrespective of amyloid status.
ACKNOWLEDGMENTS
This study was sponsored by Eli Lilly and Company. The authors would like to thank Jennifer Zimmer, employee or Eli Lilly and Company for her contributions toward the clinical insights of the study results and for her review of the manuscript. The authors would like to thank Emily Kubisiak for her contributions to the management of the study consents, data analyses, and the initial summary of the study’s findings. Medical writing services were provided by Minal Jaggar and Era Seth, employees of Eli Lilly Services India Pvt. Ltd.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0415r3).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220415.
REFERENCES
[1] | Alzheimer’s Association ((2021) ) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement, 17: , 327–406. |
[2] | Kochanek KD , Xu J , Arias E ((2020) ) Mortality in the United States, 2019. NCHS Data Brief 1–8. |
[3] | Ahmad FB , Anderson RN ((2021) ) The leading causes of death in the US for 2020. JAMA, 325: , 1829–1830. |
[4] | Rajan KB , Weuve J , Barnes LL , McAninch EA , Wilson RS , Evans DA ((2021) ) Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement, 17: , 1966–1975. |
[5] | Jack CR Jr, Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R , Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement, 14: , 535–562. |
[6] | Vos SJ , Verhey F , Frolich L , Kornhuber J , Wiltfang J , Maier W , Peters O , Ruther E , Nobili F , Morbelli S , Frisoni GB , Drzezga A , Didic M , van Berckel BN , Simmons A , Soininen H , Kloszewska I , Mecocci P , Tsolaki M , Vellas B , Lovestone S , Muscio C , Herukka SK , Salmon E , Bastin C , Wallin A , Nordlund A , de Mendonca A , Silva D , Santana I , Lemos R , Engelborghs S , Van der Mussele S , Alzheimer’s Disease Neuroimaging Initiative, Freund-Levi Y , Wallin AK , Hampel H , van der Flier W , Scheltens P , Visser PJ ((2015) ) Prevalence and prognosis of Alzheimer’s disease at themild cognitive impairment stage. Brain, 138: , 1327–1338. |
[7] | DeTure MA , Dickson DW ((2019) ) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener, 14: , 1–18. |
[8] | Wang Y , Xu C , Park J-H , Lee S , Stern Y , Yoo S , Kim JH , Kim HS , Cha J ; Alzheimer’s Disease Neuroimaging Initiative ((2019) ) Diagnosis and prognosis of Alzheimer’s disease using brain morphometry and white matter connectomes. Neuroimage Clin, 23: , 101859. |
[9] | Tosun D , Demir Z , Veitch DP , Weintraub D , Aisen P , Jack CR Jr , Jagust WJ , Petersen RC , Saykin AJ , Shaw LM , Trojanowski JQ , Weiner MW ; Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Contribution of Alzheimer’s biomarkers and risk factors to cognitive impairment and decline across the Alzheimer’s disease continuum. Alzheimers Dement, 18: , 1370–1382. |
[10] | Perl DP ((2010) ) Neuropathology of Alzheimer’s disease. Mt Sinai J Med, 77: , 32–42. |
[11] | Bateman RJ , Xiong C , Benzinger TL , Fagan AM , Goate A , Fox NC , Marcus DS , Cairns NJ , Xie X , Blazey TM , Holtzman DM , Santacruz A , Buckles V , Oliver A , Moulder K , Aisen PS , Ghetti B , Klunk WE , McDade E , Martins RN , Masters CL , Mayeux R , Ringman JM , Rossor MN , Schofield PR , Sperling RA , Salloway S , Morris JC , Dominantly Inherited Alzheimer Network ((2012) ) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med, 367: , 795–804. |
[12] | Rasmussen J , Langerman H ((2019) ) Alzheimer’s disease - why we need early diagnosis. Degener Neurol Neuromuscul Dis, 9: , 123–130. |
[13] | Nelson PT , Alafuzoff I , Bigio EH , Bouras C , Braak H , Cairns NJ , Castellani RJ , Crain BJ , Davies P , Del Tredici K , Duyckaerts C , Frosch MP , Haroutunian V , Hof PR , Hulette CM , Hyman BT , Iwatsubo T , Jellinger KA , Jicha GA , Kovari E , Kukull WA , Leverenz JB , Love S , Mackenzie IR , Mann DM , Masliah E , McKee AC , Montine TJ , Morris JC , Schneider JA , Sonnen JA , Thal DR , Trojanowski JQ , Troncoso JC , Wisniewski T , Woltjer RL , Beach TG ((2012) ) Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol, 71: , 362–381. |
[14] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7: , 270–279. |
[15] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr. , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7: , 263–269. |
[16] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. Apractical method for grading the cognitive state of patients for theclinician. J Psychiatr Res, 12: , 189–198. |
[17] | Teng E , Becker BW , Woo E , Knopman DS , Cummings JL , Lu PH ((2010) ) Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord, 24: , 348–353. |
[18] | Clark CM , Pontecorvo MJ , Beach TG , Bedell BJ , Coleman RE , Doraiswamy PM , Fleisher AS , Reiman EM , Sabbagh MN , Sadowsky CH , Schneider JA , Arora A , Carpenter AP , Flitter ML , Joshi AD , Krautkramer MJ , Lu M , Mintun MA , Skovronsky DM ; AV-45-A16 Study Group ((2012) ) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: A prospective cohort study. Lancet Neurol, 11: , 669–678. |
[19] | Robinson RL , Rentz DM , Bruemmer V , Scott Andrews J , Zagar A , Kim Y , Schwartz RL , Ye W , Fillit HM ((2019) ) Observation of patient and caregiver burden associated with early Alzheimer’s disease in the United States: Design and baseline findings of the GERAS-US Cohort Study. J Alzheimers Dis, 72: , 279–292. |
[20] | Robinson RL , Rentz DM , Andrews JS , Zagar A , Kim Y , Bruemmer V , Schwartz RL , Ye W , Fillit HM ((2020) ) Costs of early stage Alzheimer’s disease in the United States: Cross-sectional analysis of a prospective cohort study (GERAS-US). J Alzheimers Dis, 75: , 437–450. |
[21] | Centers for Medicare & Medicaid Services, 2017 ICD-10- CM and GEMs, https://www.cms.gov/Medicare/Coding/ICD10/2017-ICD-10-CM-and-GEMs. |
[22] | Centers for Medicare & Medicaid Services, Chronic Conditions Data Warehouse, https://www2.ccwdata.org/web/guest/condition-categories. |
[23] | Gilden DM , Kubisiak JM , Sarsour K , Hunter CA ((2015) ) Diagnostic pathways to Alzheimer disease: Costs incurred in a Medicare population. Alzheimer Dis Assoc Disord, 29: , 330–337. |
[24] | Centers for Medicare & Medicaid Services, CMS Cell Size Suppression Policy, https://resdac.org/articles/cms-cell-size-suppression-policy. |
[25] | Rabinovici GD , Gatsonis C , Apgar C , Chaudhary K , Gareen I , Hanna L , Hendrix J , Hillner BE , Olson C , Lesman-Segev OH , Romanoff J , Siegel BA , Whitmer RA , Carrillo MC ((2019) ) Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA, 321: , 1286–1294. |
[26] | Roberts RO , Aakre JA , Kremers WK , Vassilaki M , Knopman DS , Mielke MM , Alhurani R , Geda YE , Machulda MM , Coloma P , Schauble B , Lowe VJ , Jack CR Jr, Petersen RC ((2018) ) Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol, 75: , 970–979. |
[27] | Awal G , Kaur S ((2018) ) Association of cutaneous amyloidosis with neurodegenerative amyloidosis: Correlation or coincidence? J Clin Aesthet Dermatol, 11: , 25–27. |
[28] | Frost S , Kanagasingam Y , Macaulay L , Koronyo-Hamaoui M , Koronyo Y , Biggs D , Verdooner S , Black K , Taddei K , Shah T , Rainey-Smith S , Bourgeat P , Salvado O , Doecke J , Wilson B , Villemagne V , Rowe CC , Martins R , AIBL Research Group ((2014) ) O3-13-01: Retinal amyloid fluorescence imaging predicts cerebral amyloid burden and Alzheimer’s disease. Alzheimers Dement, 10: , P234–P235. |
[29] | Bu XL , Xiang Y , Jin WS , Wang J , Shen LL , Huang ZL , Zhang K , Liu YH , Zeng F , Liu JH , Sun HL , Zhuang ZQ , Chen SH , Yao XQ , Giunta B , Shan YC , Tan J , Chen XW , Dong ZF , Zhou HD , Zhou XF , Song W , Wang YJ ((2018) ) Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol Psychiatry, 23: , 1948–1956. |
[30] | Potashman M , Parcher B , Zhou J , Hou Q , Stefanacci R ((2022) ) Identification of cognitively impaired patients at risk for development of Alzheimer’s disease dementia: An analysis of US Medicare claims data. Expert Rev Pharmacoecon Outcomes Res, 22: , 773–786. |
[31] | Babulal GM , Roe CM , Stout SH , Rajasekar G , Wisch JK , Benzinger TLS , Morris JC , Ances BM ((2020) ) Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. J Alzheimers Dis, 74: , 1045–1055. |
[32] | de Oliveira FF , Miraldo MC , de Castro-Neto EF , de Almeida SS , Matas SLA , Bertolucci PHF , Naffah-Mazzacoratti MDG ((2021) ) Associations of neuropsychiatric features with cerebrospinal fluid biomarkers of amyloidogenesis and neurodegeneration in dementia with Lewy bodies compared with Alzheimer’s disease and cognitively healthy people. J Alzheimers Dis, 81: , 1295–1309. |
[33] | Wilson RS , Schneider JA , Bienias JL , Arnold SE , Evans DA , Bennett DA ((2003) ) Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology, 61: , 1102–1107. |
[34] | Madsen K , Hasselbalch BJ , Frederiksen KS , Haahr ME , Gade A , Law I , Price JC , Knudsen GM , Kessing LV , Hasselbalch SG ((2012) ) Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol Aging, 33: , 2334–2342. |
[35] | Taylor WD ((2017) ) Lack of a role for Alzheimer’s disease pathology in late-life depression, or just no relationship with amyloid? Am J Psychiatry, 174: , 197–198. |
[36] | Saldanha NM , Suemoto CK , Rodriguez RD , Leite REP , Nascimento C , Ferreti-Rebustini R , da Silva MM , Pasqualucci CA , Nitrini R , Jacob-Filho W ((2021) ) β-amyloid pathology is not associated with depression in a large community sample autopsy study. J Affect Disord, 278: , 372–381. |
[37] | Samaras K , Makkar S , Crawford JD , Kochan NA , Wen W , Draper B , Trollor JN , Brodaty H , Sachdev PS ((2020) ) Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: The Sydney Memory and Ageing Study. Diabetes Care, 43: , 2691–2701. |
[38] | Ganguli M , Beer JC , Zmuda JM , Ryan CM , Sullivan KJ , Chang CH , Rao RH ((2020) ) Aging, diabetes, obesity, and cognitive decline: A population-based study. J Am Geriatr Soc, 68: , 991–998. |
[39] | Moran C , Beare R , Wang W , Callisaya M , Srikanth V , Alzheimer’s Disease Neuroimaging I ((2019) ) Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology, 92: , e823–e830. |
[40] | Marseglia A , Fratiglioni L , Kalpouzos G , Wang R , Backman L , Xu W ((2019) ) Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: A population-based cohort study. Alzheimers Dement, 15: , 25–33. |
[41] | Chornenkyy Y , Wang WX , Wei A , Nelson PT ((2019) ) Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol, 29: , 3–17. |
[42] | Sastre AA , Vernooij RW , Harmand MGC , Martínez G ((2017) ) Effectof the treatment of type 2 diabetes mellitus on the development ofcognitive impairment and dementia. Cochrane Database Syst Rev, 6: CD003804. |




