Blood-Derived Progenitor Cells Are Depleted in Older Adults with Cognitive Impairment: A Role for Vascular Resilience?
Abstract
Background:
Depletion of blood-derived progenitor cells, including so called “early endothelial progenitor cells”, has been observed in individuals with early stage Alzheimer’s disease relative to matched older control subjects. These findings could implicate the loss of angiogenic support from hematopoietic progenitors or endothelial progenitors in cognitive dysfunction.
Objective:
To investigate links between progenitor cell proliferation and mild levels of cognitive dysfunction.
Methods:
We conducted in vitro studies of blood-derived progenitor cells using blood samples from sixty-five older adults who were free of stroke or dementia. Peripheral blood mononuclear cells from venous blood samples were cultured in CFU-Hill media and the number of colony forming units were counted after 5 days in vitro. Neuropsychological testing was administered to all participants.
Results:
Fewer colony forming units were observed in samples from older adults with a Clinical Dementia Rating global score of 0.5 versus 0. Older adults whose samples developed fewer colony forming units exhibited worse performance on neuropsychological measures of memory, executive functioning, and language ability.
Conclusion:
These data suggest blood progenitors may represent a vascular resilience marker related to cognitive dysfunction in older adults.
INTRODUCTION
Vascular risk factors increase risk of age-related cognitive decline and dementia [1]. Although the evidence of vascular risk factors contributing to the risk of cognitive dysfunction in older adults is robust, research on vascular resilience factors is sparce. One potential vascular resilience factor involves progenitor cells that originate from bone marrow and other tissue compartments and are present in adult peripheral blood [2]. Vascular protective cells, termed endothelial progenitor cells (EPCs), are crucial in blood vessel and capillary formation and healing, and also have been shown to mediate neuronal repair mechanisms following brain injury [3]. Two types of time dependent EPCs exist, named accordingly as early EPCs and late EPCs [also called endothelial colony forming cells (ECFCs)] based on the timing of their appearance in vitro [4]. Although some endothelial phenotypes are similar in both early EPCs and late EPCs, they differ in terms of morphology, proliferation rate, survival features, gene expression, and functional expression [5]. The differing functional roles found in early and late EPCs synergistically contribute to neovasculogenesisin vivo [4].
Early EPCs, a heterogeneous combination of monocytes/macrophages [6] and T-lymphocytes [7], are also called “circulating angiogenic cells”, “myeloid angiogenic cells”, “proangiogenic cells”, “hematopoietic endothelial progenitor cells”, “CFU-Hill cells”, and “small EPCs” [8–10]. Early EPCs are thought to support brain health by homing to sites of neural or cerebrovascular injury and secreting angiogenic growth factors and cytokines (e.g., vascular endothelial growth factor; VEGF), thereby activating adjacent endothelial cells and promoting angiogenesis by regenerating mature endothelial cells and neurogenesis by supporting neural cell growth [11, 12]. Early EPCs appear to be further protective against neurovascular injury by secreting a number of neurotropic factors in addition to VEGF [13], facilitating neuronal tissue repair [14–16], and contributing to the neurogenic [17], neurovascular [18], and oligovascular niche [17–20]. Putative early EPCs are cultured from peripheral blood mononuclear cells (PBMCs), historically using in vitro colony-forming assays measuring angiogenic activity [2]. These assays afford evaluation of colony-forming units (i.e., CFU-Hill colonies), which consist of hematopoietic progenitor cells and T lymphocytes [21], are indicative of EPC proliferative potential and are involved in stimulating, supporting, and regulating angiogenesis [4, 22–23]. The CFU-Hill assay is a well characterized assay for the early EPC phenotype, and putative early EPCs have the ability to form CFU-Hill colonies [24]. The colonies that form exhibit a highly specific pattern that includes a central core of round cells with elongated spindle-like cells that radiate from the core to the periphery. CFU-Hill colonies have been characterized to include myeloid progenitor cells, monocytes, T lymphocytes, and features of endothelial cells [25, 26]. Emergence of this type of colony therefore signifies the presence of such cell types. In contrast, late EPCs enhance vasculogenesis via their high proliferation potency upon stimulation by early EPCs [27]. Despite their unique roles, early and late EPCs play an interactive role in neuroprotection through their combined contributions to angiogenesis, neurogenesis, and neovasculogeneisis [4, 28, 29]. As such, these cells may help stave off the delirious effects of neurodegenerative processes and subsequently delay, or even prevent, clinical symptomatology in mild cognitive impairment (MCI) and dementia.
Evidence corroborating potentially protective effects of EPCs in brain injury and neurodegeneration comes from studies using cell surface EPCs markers (CD34+, CD133+, CD309+) and investigations into the EPC angiogenic secretome in individuals with MCI and/or Alzheimer’s disease (AD) [2]. Neurogenesis [30] and angiogenesis [31] are both impaired in AD, and circulating EPCs are accordingly depleted in AD [30, 31]. AD patients also present with reduced angiogenic activity of the EPC secretome [32]. More specifically, circulating CD34+ levels are reduced in early AD and related to AD neuropathology (i.e., amyloid-β levels) in the cerebrospinal fluid [33]. In vitro, patients with AD exhibit lower EPC levels and proliferation, where lower counts is related to worse cognitive functioning [31–35]. Case-control comparisons of progenitor levels in AD and cognitively normal older individuals have yielded mixed results [34, 36–39], warranting further studies to clarify if and how progenitor cells may confer resilience and buffer against cognitive decline in olderadults.
The present study aims to expand on prior work by investigating the link between in vitro CFU-Hill proliferation, and cognitive functioning, including memory, executive functioning, and language, in a cohort of independently living older adults who are free of stroke or dementia. We hypothesized that the degree of progenitor cell proliferation as derived from CFU-Hill colonies would be related to better neuropsychological functioning across cognitive domains.
METHODS
Following participant informed consent and approval by the University of Southern California (USC) Institutional Review Board (IRB), the study protocol was implemented at the Vascular Senescence and Cognition (VaSC) Laboratory in the USC Department of Psychology.
Participants
Sixty-five community dwelling older adults (Mage = 70.94; SDage = 7.39), free of dementia or clinical stroke, underwent venipuncture, neuropsychological testing, and brain MRI. Participants were recruited from the community via university platforms, including the USC School of Gerontology Healthy Minds Program and the USC Alzheimer’s Disease Research Center, as well as additional recruitment from the local community surround the USC campus via word-of-mouth, flyers, and community outreach events. Inclusion criteria for participants consisted of being a minimum age of 55 years and independently living. Exclusion criteria included history of dementia, clinical stroke, learning disability, traumatic brain injury, or other systemic or neurological illness or treatment that may affect central nervous system functioning. Vascular risk factors were determined by clinical interview and objective measures, including blood pressure, body mass index (weight in kg/ height in m2), dyslipidemia, (i.e., history of elevated total cholesterol, low density lipoprotein cholesterol or triglycerides, or reduced high density cholesterol), diabetes (history of type I or II), history of cardiovascular disease (angina, intermittent claudication, myocardial infarction, stent placement, coronary artery bypass graft), or history of transient ischemic attack. Blood pressure was measured twice from each arm on two separate days, and systolic and diastolic pressures were averaged across these time points. Pulse pressure was calculated as difference between systolic and diastolic pressure, and mean arterial pressure was calculated as the sum of diastolic blood pressure and one-third of the pulse pressure.
Procedures
Blood draw
Fasting venipuncture was performed prior to neuropsychological testing during each participant’s first visit, with approximately 25 mL of blood being drawn into EDTA coated tubes. Samples were centrifuged and PBMCs were isolated from the plasma before performing cellular assays as described below.
CFU-Hill in vitro culture
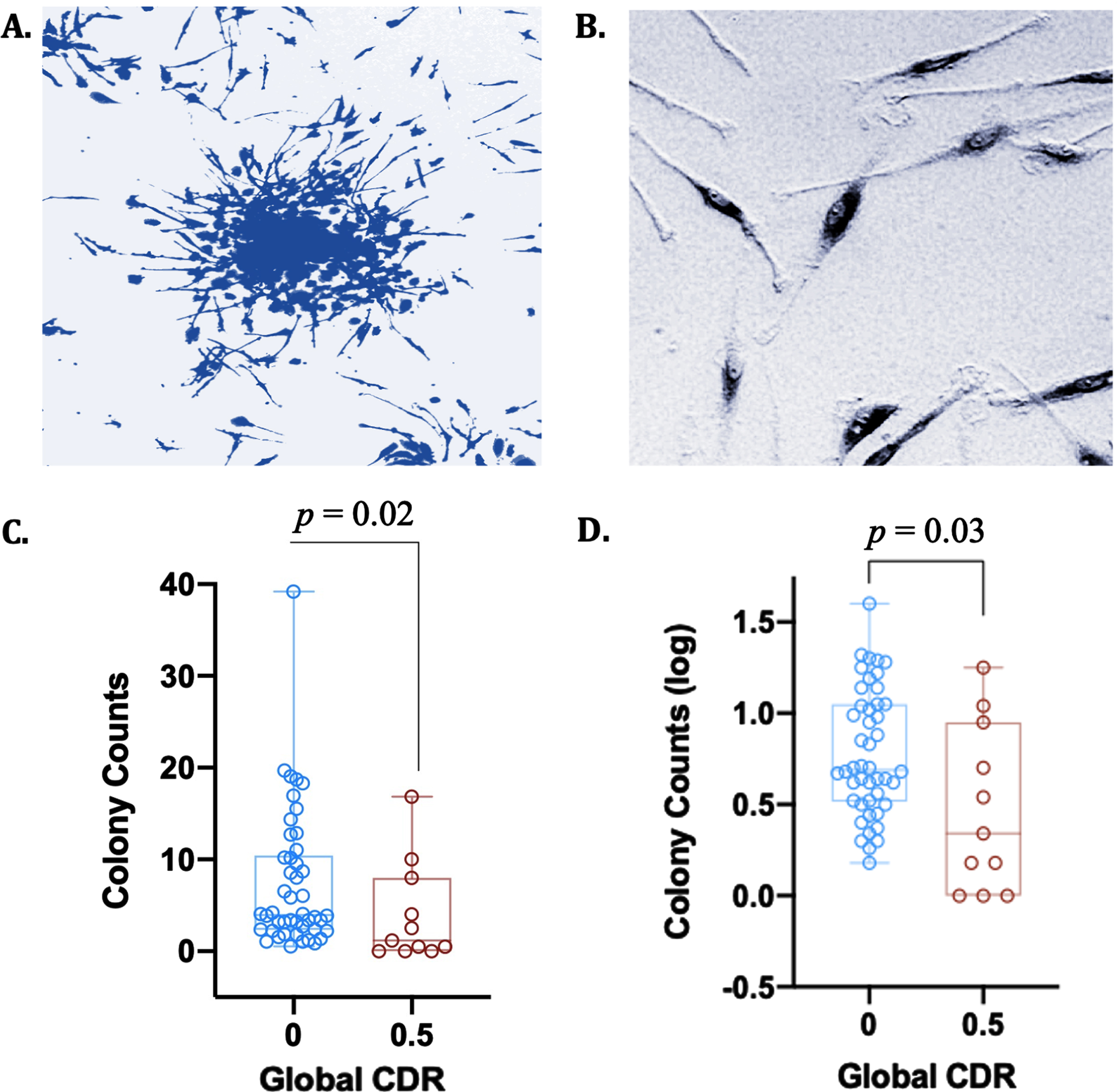
CFU-Hill colonies were cultured from PBMCs to isolate putative early EPCs. PBMCs were isolated from anticoagulated whole blood by density gradient centrifugation in Histopaque-1077 (Sigma Aldrich). Cells were first seeded on 12-well fibronectin-coated plates (Day 0) at 2.5×106 cells/well in CFU-Hill Liquid Medium (Stem Cell Tech). Non-adherent cells were then collected and replated onto 24-well fibronectin-coated plates at 1.0×106 cells/well (Day 2). CFU colonies were next scored as a central core of round cells surrounded by radiating spindle-shaped endothelial-like cells (Day 5) (Fig. 1A, B). Following day 5 in culture, CFU-Hill colonies were counted, and mean colony number calculated.
Fig. 1
CFU-Hill colony counts are attenuated with cognitive impairment on the CDR. A) Giemsa stain of a CFU-Hill colony cultured from blood in vitro; B) Endothelial-like cell, i.e., spindle-shaped cell (putative early EPC); C) CFU-Hill colony raw counts are depleted in individuals with greater cognitive dysfunction on CDR; D) CFU-Hill colony log counts are depleted in individuals with greater cognitive dysfunction on CDR.
APOE genotyping
APOE genotyping was performed using the blood cell pellet fraction. PureLink Genomic DNA Mini Kit (Thermo Fisher) isolated DNA. Isolated DNA was genotyped using the TaqMan SNP Genotyping Assay (Thermo Fisher) on an Applied Biosystems 7300 Real Time PCR System. ApoE gene SNPs were analyzed for dbSNP IDs rs429358 and rs7412. Allelic discrimination was determined using qPCR software. The APOE ɛ4 allele was labeled as rs429358-C + rs7412-C.
Cognitive testing
The cognitive test battery in the present study consisted of several neuropsychological tests in each cognitive domain of interest, in addition to a global measure of cognitive and functional performance related to the deficits in AD and other dementias. Cognitive domains for this study included memory [Verbal memory: Wechsler Memory Scale-Revised (WMS-R) Logical memory I and II; Visual memory: WMS-R Visual Reproduction I and II], executive functioning (Delis-Kaplan Executive Function System (DKEFS) Trail Making Test B, FAS Fluency, Golden Stroop), attention (DKEFS Trail Making Test A, Digit Span Forward), and language (Naming: Multilingual Naming Test [MiNT], Fluency: Animal and Fruits & Vegetables Naming.
The Clinical Dementia Rating Scale (CDR) was used as a measure of cognitive and functional impairment indicating MCI and/or very mild dementia. The CDR is a 5-point scale characterizing cognitive and functional domains applicable to AD and similar dementias. Functional domains were based on a participant and informant semi-structured interview, while cognitive domains were evaluated by administering a participant and informant semi-structured interview and brief cognitive tests. A global CDR score was calculated by scoring each domain and totaling the CDR Sum of Boxes based on criteria detailed elsewhere [40]. A global score of zero indicates no clinically meaningful cognitive or functional impairment, while a global score of 0.5 indicates MCI and/or very mild dementia. While different criteria are used to diagnose MCI, several previous studies have used a global CDR of 0.5 as a diagnostic criterion [41–44].
Statistical analyses
Data were first examined for outliers and deviations from normality using skewness and kurtosis measures. Log10-transformation was used to normalize the distribution of putative EPC proliferation and Trails B scores. While the CDR 0 and 0.5 groups were unequal in sample size, heteroscedasticity was not an issue between groups. Due to the relatively equal variances in both groups, the one-way ANOVA models are valid despite unequal sample sizes.
Participant groups (CDR 0 versus CDR 0.5) were compared across demographic characteristics and vascular risk factors using independent sample t-tests, chi-square tests, and one-way analysis of variance (ANOVA). Potential contributors to progenitor cell proliferation (i.e., age, sex, and vascular risk factors) were additionally analyzed. CFU-Hill colony counts were evaluated between groups using Univariate Analysis of Covariance (ANCOVA), controlling for age, sex, and education level. Hierarchical multiple linear regression analyses were run to determine if the addition of CFU-Hill counts improved the prediction of raw scores on neuropsychological tests above age, sex, and education level alone. Age, sex, and education level were thus included in the first model of each test, followed by CFU-Hill log counts in the second model. Nine subjects did not have CDR data and were thus excluded from these subsequent analyses.
RESULTS
Demographics and vascular risk factors
Participants with indications of mild cognitive and/or functional impairment as measured by the CDR (i.e., CDR of 0.5) were significantly older than participants who had a CDR of 0 (p < 0.001) but did not differ on other demographic or vascular risk factors (Table 1).
Table 1
Demographics and clinical characteristics for the CDR 0 and 0.5 group
| CDR 0 | CDR 0.5 | ||
| Demographics | p | ||
| n | 45 | 11 | |
| Age | 69.5 (6.4) | 76.6 (5.8) | 0.001 |
| Sex, % male | 15 (33.3%) | 7 (63.6%) | 0.09 |
| APOE4 carriers | 8 (21.6%) | 2 (20.0%) | 1.00 |
| Education | 16.5 (2.2) | 16.5 (1.6) | 0.96 |
| Race, % Non-Hispanic White | 9 (64.4%) | 6 (54.5%) | 0.46 |
| % Hispanic | 3 (6.4%) | 0 (0%) | 0.50 |
| % Black | 9 (20%) | 2 (18.2%) | 0.49 |
| % Asian | 4 (8.9%) | 1 (9.1%) | 0.40 |
| % Other | 0 (0%) | 2 (3.6%) | 0.07 |
| Vascular Risk Factors | |||
| Hypertension | 19 (42.2%) | 5 (45.5%) | 1.00 |
| Diabetes | 6 (13.3%) | 2 (18.2%) | 0.65 |
| Smoking (Current) % | 5 (11.1%) | 1 (9.1%) | 1.00 |
| Smoking (History) % | 20 (44.4%) | 7 (63.6%) | 0.32 |
| Dyslipidemia % | 25 (55.6%) | 5 (45.5%) | 0.74 |
| Transient Ischemic Attack % | 1 (2.3%) | 1 (9.1%) | 0.36 |
| Body Fat % | 36.3 (7.0) | 32.7 (7.3) | 0.17 |
| Systolic Blood Pressure (mmHg) | 131.3 (13.2) | 133.4 (8.9) | 0.64 |
| Diastolic Blood Pressure (mmHg) | 78.0 (9.2) | 78.5 (9.1) | 0.86 |
| Mean Arterial Pressure (mmHg) | 113.5 (10.9) | 115.1 (8.0) | 0.68 |
| Pulse Pressure (mmHg) | 53.4 (10.4) | 54.9 (6.3) | 0.67 |
CFU-Hill colony proliferation and cognitive dysfunction
CFU-Hill colony log counts were significantly depleted in participants with a CDR of 0.5 (M = 0.47, SD = 0.46) compared to participants with a CDR of 0 (M = 0.78, SD = 0.35); t(55) = 2.52, p = 0.02. After correcting for age, sex, and education, CFU-Hill colony log counts remained significantly depleted in participants with a CDR of 0.5 compared to those with a CDR of 0, F(1, 51) = 5.84, p = 0.03 (Fig. 1C, D). Per a post hoc power analysis using G*Power [45], the ANCOVA analysis examining a group difference between CDR 0 and CDR 0.5 was of sufficient sample size (N = 56) to detect a medium effect size and significant difference between the two groups at >80% power, with the inclusion of 3 covariates (age, education level, sex). Levene’s test indicated equal variances between groups for both the CFU-Hill raw colony counts (F = 0.82, p = 0.37) and log-transformed counts (F = 2.38, p = 0.13), indicating that heteroscedasticity was not an issue in this analysis.
Lower CFU-Hill colony counts predicted worse performances on tests of executive functioning, verbal memory, and language after controlling for age, sex, and education [(1) Animals: F(4, 60) = 4.10, p = 0.005; R2 = 0.16; β= 0.41; ΔF = 12.51, p = 0.001; (2) Fruits and Vegetables: F(4, 60) = 4.00, p = 0.006; R2 = 0.21; β= 0.35; ΔF = 9.01, p = 0.004; (3) FAS: F(4, 60) = 7.81, p < 0.001; R2 = 0.09; β= 0.30; ΔF = 7.04, p = 0.007; (4) Trails B: F(4, 60) = 5.13; p = 0.001; β= 0.25; R2 = 0.26; ΔF = 5.07, p = 0.03; (5) WMS-IV Logical Memory I: F(4, 60) = 9.17; p < 0.001; β= 0.28; R2 = 0.38; ΔF = 7.92, p = 0.02] (Table 2). CFU-Hill colony counts did not significantly predict other cognitive tests in our study after controlling for age, sex, and education (p > 0.5; Table 2).
Table 2
CFU-Hill colony counts predict short-term verbal memory, executive functioning, and language
| Cognitive domain | Cognitive test/subtest | β | R2 | ΔR2 | p |
| Memory | WMS-IV Logical Memory I | 0.28 | 0.32 | 0.07 | 0.012* |
| WMS-IV Logical Memory II | 0.17 | 0.28 | 0.03 | 0.13 | |
| WMS-IV Visual Reproduction I | 0.19 | 0.11 | 0.03 | 0.13 | |
| WMS-IV Visual Reproduction II | 0.18 | 0.09 | 0.03 | 0.15 | |
| Executive function | Trails B (time) | –0.27 | 0.4 | 0.07 | 0.009** |
| Phonological fluency (FAS) | 0.29 | 0.34 | 0.08 | 0.008** | |
| Golden Stroop | –0.03 | 0.44 | 0 | 0.77 | |
| Digit span backward | 0.14 | 0.12 | 0.02 | 0.25 | |
| Attention | Trails A | 0.21 | 0.1 | 0.04 | 0.09 |
| Digit span forward | –0.07 | 0.16 | 0 | 0.59 | |
| Language | MiNT visual naming test | 0.17 | 0.11 | 0.02 | 0.46 |
| Semantic fluency (Animals) | 0.39 | 0.27 | 0.15 | 0.001** | |
| Semantic fluency (Fruits/vegetables) | 0.35 | 0.21 | 0.12 | 0.004** |
N = 65. All values are based on statistics from model 2 of the hierarchical multiple regression model (where model 1 predictors included age, sex, education, and model 2 predictors included age, sex, education, and CFU-Hill log counts). *p < 0.05; **p < 0.01; ***p < 0.001.
DISCUSSION
The present study findings indicate attenuated proliferation of blood-derived progenitors in vitro is related to cognitive dysfunction in older adults. Specifically, older adults with cognitive impairment (CDR 0.5 versus 0) show lower levels of progenitor proliferation in vitro than those who are cognitively normal. Additional findings indicate decreased progenitor proliferation correlates with worse performance on neuropsychological tests of memory, executive function, and language ability. Together these findings could suggest blood-derived progenitors as a blood-based marker of vascular resilience in cognitive dysfunction in older adults.
Our observations of decreased in vitro progenitor proliferation in older adults with cognitive dysfunction are consistent with prior studies linking circulating progenitor cell levels to dementia and stroke and extend the association to more mild levels of cognitive dysfunction. Prior studies have utilized flow cytometry to identify circulating progenitors by various cell surface markers (e.g., CD34, CD133, CD309) and have observed depletion of circulating progenitor cell levels in normal aging [46] and in association with cognitive function [47], MCI [48], cognitive impairment due to coronary artery disease [49], vascular cognitive impairment [39], and AD dementia [32–34, 36]. Far fewer studies have examined in vitro markers of progenitor cell proliferation, but one study found decreased progenitor cell proliferation in AD dementia relative to normal aging using the CFU-Hill assay [35]. Although it is challenging to conduct in vitro proliferative assays on a large number of participants, these studies are uniquely valuable in providing a window into the function of progenitor cells in older adults with cognitive impairment. Flow cytometry studies are also of great value to track progenitor cell depletion, but the number of circulating progenitor cells does not necessarily correspond to cell function, and their remains a lack of consensus regarding the optimal combination of markers that identify progenitor cells. The present study corroborates and extends these findings from flow cytometry and in vitro culture assay studies by demonstrating clear links between decreased progenitor cell proliferation in vitro and mild levels of cognitive dysfunction. These findings could suggest that progenitor cells represent a resilience factor that may be depleted during the early stages of cognitive decline in older adults; however, this hypothesis must be evaluated by future longitudinal studies.
The cognitive domains related to progenitor cell proliferation in the present study are immediate memory, executive dysfunction and verbal fluency, all of which are implicated in aging and vascular disease [50] and have also been linked to MCI and AD dementia [51, 52]. Memory, executive and fluency deficits are commonly observed in the early stages of MCI and can be part of the dementia prodrome [52], suggesting attenuated progenitor proliferation may be related to dementia risk at an early stage. Importantly, our findings reveal a relationship between progenitor proliferation and cognition that is detectible even in patients with no history of stroke. These findings warrant further investigation into progenitor cell functioning as an early-stage vascular process related to cognitive decline. The present study also did not assess for AD or cerebrovascular pathological markers, thereby limiting conclusions regarding the role of progenitor cell proliferation in a specific pathophysiological process. It is possible that attenuation of progenitor proliferation reflects a nonspecific decrease in progenitor vitality, possibly indicating or even contributing to susceptibility to neurocognitive dysfunction.
While the mechanism contributing to the association between progenitor cell proliferation and age-related cognitive dysfunction is unknown, there are several potential explanations. One mechanism may involve cellular senescence in the aging brain, which has been shown to contribute to cognitive impairment and decline [53]. Endothelial cell senescence has recently emerged as a possible mechanism underlying cerebral microvascular pathologies. Specifically, single-cell RNA sequencing indicated that approximately 10% of cerebral microvascular endothelial cells were senescent in the aging mouse brain [54]. Applying single-cell RNA sequence technology to progenitor cells derived from human whole blood may help elucidate the degree to which senescent endothelial progenitor cells in particular are associated with cognitive dysfunction. Additionally, recent work suggests hematopoietic stem/progenitor cells (HSPCs) show diminished vascular reparative capacity and impaired mobilization following ischemic injury in aging mice. These deficits in HSPC function are reversible following the activation of factors that stimulate vascular repair (i.e., angiotensin-converting enzyme-2 and the Mas Receptor) [55]. These findings underscore the hypothesis that reduced vascular repair capacity of progenitor cells occurs in aging, which in turn may increase susceptibility to vascular injury and subsequent clinical consequences, including cognitive impairment. In addition to the cellular senescence occurring in the aging vasculature, several other cerebral metabolic changes are also co-occurring in the vasculature as a result of aging that may also lead to progenitor cell exhaustion, including alterations in pro-geronic factors (e.g., serum response factor, insulin-like growth factor-1 and VEGF-A), increased oxidative stress, changes in DNA metabolism, and lifestyle factors such as nutrition and dietary habits [56–58]. Future studies on progenitor cells and aging would benefit from including these cellular and lifestyle factors to delineate to what extent different cell types, factors in the vasculature, and lifestyle choices are associated with age-related cognitive dysfunction.
The cross-sectional design of this study represents a study limitation that precludes any interpretations regarding causality. Age is the greatest risk factor of MCI, vascular cognitive impairment and dementia, and AD, reflected in our samples as the CDR 0.5 group which was significantly older than the CDR 0 group. However, age was not related to progenitor cell proliferation, suggesting that depletion of progenitor cell colony counts in the CDR 0.5 group cannot be explained by solely demographic factors. With regards to other demographic factors, although the relative diversity of our sample with respect to African American representation is a study strength, the complexity of the sample racial makeup together with the relatively small sample size precluded inclusion of racial subgroups in most of the statistical analyses. However, there were no significant differences in racial makeup between cognitively unimpaired and impaired participants. Additional study limitations include the small sample size of the CDR 0.5 group, although it is noted that significant relationships between colony counts and neuropsychological measures were observed in the total sample. Longitudinal studies evaluating progenitor cell proliferation as a predictor of cognitive decline will provide crucial information regarding the potential value of progenitor cells as early indicators of cognitive decline. Lastly, the well-established CFU-Hill assay used in the present study does not definitively characterize EPC phenotype. True EPCs are challenging to isolate and thus define due to the lack of a specific molecular determinant or cell surface marker that is uniquely associated with this cell population. Thus, while CFU-Hill colonies do not specifically isolate EPCs, these colonies express several cell antigens consistent with an EPC phenotype (i.e., CD31, CD105, CD144, CD146, von Willebrand factor, and KDR/VEGF-R2) [59, 60]. Further studies of the interplay among progenitors, cognitive dysfunction, structural brain integrity, and disease-specific biomarkers are warranted to evaluate the potential role of progenitor cell proliferation as a resilience indicator with implications for dementia risk assessment and prevention. We did not include imaging markers in the present study as we did not have sufficient brain MRI data available for analysis; however, future studies should incorporate brain MRI to examine associations between progenitor cells, cognition, and brain integrity.
ACKNOWLEDGMENTS
We thank the study participants and staff at the University of Southern California and the University of California, Irvine for making this study possible.
FUNDING
This work was supported by National Institutes of Health grants (R01AG064228, R01AG060049, R01AG082073, P30AG066519, P01AG052350) and the Alzheimer’s Association (AARG-17-532905).
CONFLICT OF INTEREST
Daniel Nation is an Editorial Board Member of this journal but was not involved in the peer-review process. All other authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
REFERENCES
[1] | Sahathevan R , Brodtmann A , Donnan GA ((2012) ) Dementia, stroke, and vascular risk factors; a review. Int J Stroke 7: , 61–73. |
[2] | Asahara T , Kawamoto A , Masuda H ((2011) ) Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 29: , 1650–1655. |
[3] | Herrmann M , Binder A , Menzel U , Zeiter S , Alini M , Verrier S ((2014) ) CD34/CD133 enriched bone marrow progenitor cells promote neovascularization of tissue engineered constructs in vivo. Stem Cell Res 13: , 465–477. |
[4] | Hur J , Yoon CH , Kim HS , Choi JH , Kang HJ , Hwang KK , Oh BH , Lee MM , Park YB ((2004) ) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24: , 288–293. |
[5] | Hristov M , Weber C ((2004) ) Endothelial progenitor cells: Characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med 8: , 498–508. |
[6] | Rehman J , Li J , Orschell CM , March KL ((2003) ) Peripheral blood ‘endothelial progenitor cells’ are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: , 1164–1169. |
[7] | Asakage M , Tsuno NH , Kitayama J , Kawai K , Okaji Y , Yazawa K , Kaisaki S , Osada T , Watanabe T , Takahashi K , Nagawa H ((2006) ) Early-outgrowth of endothelial progenitor cells can function as antigen-presenting cells. Cancer Immunol Immunother 55: , 708–716. |
[8] | Medina RJ , Barber CL , Sabatier F , Dignat-George F , Melero-Martin JM , Khosrotehrani K , Ohneda O , Randi AM , Chan JK , Yamaguchi T , Van Hinsbergh VW ((2017) ) Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Transl Med 6: , 1316–1320. |
[9] | Timmermans F , Plum J , Yöder MC , Ingram DA , Vandekerckhove B , Case J ((2009) ) Endothelial progenitor cells: Identity defined? J Cell Mol Med 13: , 87–102. |
[10] | Yoder MC ((2018) ) Endothelial stem and progenitor cells (Stem cells): (2017 Grover Conference Series). Pulm Circ 8: , 1. |
[11] | Imitola J , Raddassi K , Park KI , Mueller FJ , Nieto M , Teng YD , Frenkel D , Li J , Sidman RL , Walsh CA , Snyder EY ((2004) ) Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 101: , 18117–18122. |
[12] | Schänzer A , Wachs FP , Wilhelm D , Acker T , Cooper-Kuhn C , Beck H , Winkler J , Aigner L , Plate KH , Kuhn HG ((2004) ) Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol 14: , 237–248. |
[13] | He T , Smith LA , Harrington S , Nath KA , Caplice NM , Katusic ZS ((2004) ) Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke 35: , 2378–2384. |
[14] | Yuan F , Chang S , Luo L , Li Y , Wang L , Song Y , Qu M , Zhang Z , Yang GY , Wang Y ((2018) ) Cxcl12 gene engineered endothelial progenitor cells further improve the functions of oligodendrocyte precursor cells. Exp Cell Res 367: , 222–231. |
[15] | Park KJ , Park E , Liu E , Baker AJ ((2014) ) Bone marrow-derived endothelial progenitor cells protect postischemic axons after traumatic brain injury. J Cereb Blood Flow Metab 34: , 357–366. |
[16] | Wang T , Fang X , Yin ZS ((2018) ) Endothelial progenitor cell-conditioned medium promotes angiogenesis and is neuroprotective after spinal cord injury. Neural Regen Res 13: , 887. |
[17] | Shen Q , Goderie SK , Jin L , Karanth N , Sun Y , Abramova N , Vincent P , Pumiglia K , Temple S ((2004) ) Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304: , 1338–1340. |
[18] | Ma F , Morancho A , Montaner J , Rosell A ((2015) ) Endothelial progenitor cells and revascularization following stroke. Brain Res 1623: , 150–159. |
[19] | Arai K , Lo EH ((2009) ) An oligovascular niche: Cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci 29: , 4351–4355. |
[20] | Pham LDD , Hayakawa K , Seo JH , Nguyen MN , Som AT , Lee BJ , Guo S , Kim KW , Lo EH , Arai K ((2012) ) Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia 60: , 875–881. |
[21] | Yoder MC , Mead LE , Prater D , Krier TR , Mroueh KN , Li F , Krasich R , Temm CJ , Prchal JT , Ingram DA ((2007) ) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: , 1801–1809. |
[22] | Asosingh K , Aldred MA , Vasanji A , Drazba J , Sharp J , Farver C , Comhair SA , Xu W , Licina L , Huang L , Anand-Apte B ((2008) ) Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 172: , 615–627. |
[23] | Li Y , Chang S , Li W , Tang G , Ma Y , Liu Y , Yuan F , Zhang Z , Yang GY , Wang Y ((2018) ) Cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res Ther 9: , 139. |
[24] | Ahrens I , Domeij H , Topcic D , Haviv I , Merivirta RM , Agrotis A , Leitner E , Jowett JB , Bode C , Lappas M , Peter K ((2011) ) Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS One 6: , e23210. |
[25] | Hirschi KK , Ingram DA , Yoder MC ((2008) ) Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: , 1584–1595. |
[26] | Basile DP , Yoder MC ((2014) ) Circulating and tissue resident endothelial progenitor cells. J Cell Physiol 229: , 10–16. |
[27] | Smadja DM , Bieche I , Uzan G , Bompais H , Muller L , Boisson-Vidal C , Vidaud M , Aiach M , Gaussem P ((2005) ) PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler Thromb Vasc Biol 25: , 2321–2327. |
[28] | Simard T , Jung RG , Motazedian P , Di Santo P , Ramirez FD , Russo JJ , Labinaz A , Yousef A , Anantharam B , Pourdjabbar A , Hibbert B ((2017) ) Progenitor cells for arterial repair: Incremental advancements towards therapeutic reality. Stem Cells Int 2017: , 8270498. |
[29] | Szmitko PE , Fedak PWM , Weisel RD , Stewart DJ , Kutryk MJB , Verma S ((2003) ) Endothelial progenitor cells: New hope for a broken heart. Circulation 107: , 3093–3100. |
[30] | Moreno-Jiménez EP , Flor-García M , Terreros-Roncal J , Rábano A , Cafini F , Pallas-Bazarra N , Ávila J , Llorens-Martín M ((2019) ) Adult hippocampal neurogenesis isabundant in neurologically healthy subjects and drops sharply inpatients with Alzheimer’s disease. Nat Med 25: , 554–560. |
[31] | Vagnucci Jr AH , Li WW ((2003) ) Alzheimer’s disease and angiogenesis. Lancet 361: , 605–608. |
[32] | Lee ST , Chu K , Jung KH , Jeon D , Bahn JJ , Kim JH , Lee K , Kim S , Roh JK ((2010) ) Dysfunctional characteristics of circulating angiogenic cells in Alzheimer’s disease. J Alzheimers Dis 19: , 1231–1240. |
[33] | Maler JM , Spitzer P , Lewczuk P , Kornhuber J , Herrmann M , Wiltfang J ((2006) ) Decreased circulating CD34+ stem cells in early Alzheimer’s disease: Evidence for a deficient hematopoietic brain support? Mol Psychiatry 11: , 1113–1115. |
[34] | Kong XD , Zhang Y , Liu L , Sun N , Zhang MY , Zhang JN ((2011) ) Endothelial progenitor cells with Alzheimer’s disease. Chin Med J (Engl) 124: , 901–906. |
[35] | Lee ST , Chu K , Jung KH , Park HK , Kim DH , Bahn JJ , Kim JH , Oh MJ , Lee SK , Kim M , Roh JK ((2009) ) Reduced circulating angiogenic cells in Alzheimer disease. Neurology 72: , 1858–1863. |
[36] | Bigalke B , Schreitmüller B , Sopova K , Paul A , Stransky E , Gawaz M , Stellos K , Laske C ((2011) ) Adipocytokines and CD34+ progenitor cells in Alzheimer’s disease. PloS one, 2011. Adipocytokines and CD34+ progenitor cells in Alzheimer’s disease. PloS One 6: , p.e20286. |
[37] | Breining A , Silvestre JS , Dieudonné B , Vilar J , Faucounau V , Verny M , Néri C , Boulanger CM , Boddaert J ((2016) ) Biomarkers ofvascular dysfunction and cognitive decline in patients withAlzheimer’s disease: No evidence for association in elderlysubjects. Aging Clin Exp Res 28: , 1133–1141. |
[38] | Stellos K , Panagiota V , Sachsenmaier S , Trunk T , Straten G , Leyhe T , Seizer P , Geisler T , Gawaz M , Laske C ((2010) ) Increased circulating progenitor cells in Alzheimer’s disease patients with moderate to severe dementia: Evidence for vascular repair and tissue regeneration? J Alzheimers Dis 19: , 591–600. |
[39] | Taguchi A , Matsuyama T , Nakagomi T , Shimizu Y , Fukunaga R , Tatsumi Y , Yoshikawa H , Kikuchi-Taura A , Soma T , Moriwaki H , Nagatsuka K ((2008) ) Circulating CD34-positive cells provide a marker of vascular risk associated with cognitive impairment. J Cereb Blood Flow Metab 28: , 445–449. |
[40] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR). Neurology 43: , 2412. |
[41] | Meguro K , Ishii H , Yamaguchi S , Ishizaki J , Sato M , Hashimoto R , Meguro M , Lee E , Tanaka Y , Kasuya M , Sekita Y ((2004) ) Prevalence and cognitive performances of clinical dementia rating 0.5 and mild cognitive impairment in Japan: The Tajiri Project. Alzheimer Dis Assoc Disord 18: , 3–10. |
[42] | O’Bryant SE , Waring SC , Cullum CM , Hall J , Lacritz L , Massman PJ , Lupo PJ , Reisch JS , Doody R , Texas Alzheimer’s Research Consortium ((2008) ) Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: A Texas Alzheimer’s research consortium study. Arch Neurol 65: , 1091–1095. |
[43] | Chang YL , Bondi MW , McEvoy LK , Fennema-Notestine C , Salmon DP , Galasko D , Hagler DJ , Dale AM , Alzheimer’s Disease Neuroimaging Initiative ((2011) ) Global clinical dementia rating of 0.5 in MCI masks variability related to level of function. Neurology 76: , 652–659. |
[44] | Aretouli E , Okonkwo OC , Samek J , Brandt J ((2011) ) The fate of the 0.5s: Predictors of 2-year outcome in mild cognitive impairment. J Int Neuropsychol Soc 17: , 277–288. |
[45] | Faul F , Erdfelder E , Buchner A , Lang A-G ((2009) ) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 41: , 1149–1160. |
[46] | Heiss C , Keymel S , Niesler U , Ziemann J , Kelm M , Kalka C ((2005) ) Impaired progenitor cell activity in age-related endothelialdysfunction. J Am Coll Cardiol 45: , 1441–1448. |
[47] | Hajjar I , Goldstein FC , Waller EK , Moss LD , Quyyumi A ((2016) ) Circulating progenitor cells is linked to cognitive decline in healthy adults. Am J Med Sci 351: , 147–152. |
[48] | Nation DA , Tan A , Dutt S , McIntosh EC , Yew B , Ho JK , Blanken AE , Jang JY , Rodgers KE , Gaubert A ((2018) ) Circulating progenitor cells correlate with memory, posterior cortical thickness, and hippocampal perfusion. J Alzheimers Dis 61: , 91–101. |
[49] | Moazzami K , Wittbrodt MT , Lima BB , Kim JH , Hammadah M , Ko YA , Obideen M , Abdelhadi N , Kaseer B , Gafeer MM , Nye JA ((2020) ) Circulating progenitor cells and cognitive impairment in men and women with coronary artery disease. J Alzheimers Dis 74: , 659–668. |
[50] | Charlton RA , Morris RG , Nitkunan A , Markus HS ((2006) ) The cognitive profiles of CADASIL and sporadic small vessel disease. Neurology 66: , 1523–1526. |
[51] | Traykov L , Raoux N , Latour F , Gallo L , Hanon O , Baudic S , Bayle C , Wenisch E , Remy P , Rigaud AS ((2007) ) Executive functions deficit in mild cognitive impairment. Cogn Behav Neurol 20: , 219–224. |
[52] | Bondi MW , Jak AJ , Delano-Wood L , Jacobson MW , Delis DC , Salmon DP ((2008) ) Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychol Rev 18: , 73–90. |
[53] | Sikora E , Bielak-Zmijewska A , Dudkowska M , Krzystyniak A , Mosieniak G , Wesierska M , Wlodarczyk J ((2021) ) Cellular senescence in brain aging. Front Aging Neurosci 13: , 646924. |
[54] | Kiss T , Nyúl-Tóth Á , Balasubramanian P , Tarantini S , Ahire C , DelFavero J , Yabluchanskiy A , Csipo T , Farkas E , Wiley G , Garman L , Csiszar A , Ungvari Z ((2020) ) Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. GeroScience 42: , 429–444. |
[55] | Joshi S , Chittimalli K , Jahan J , Vasam G , Jarajapu YP ((2021) ) ACE2/ACE imbalance and impaired vasoreparative functions of stem/progenitor cells in aging. GeroScience 43: , 1423–1436. |
[56] | Balasubramanian P , DelFavero J , Ungvari A , Papp M , Tarantini A , Price N , de Cabo R , Tarantini S ((2020) ) Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res Rev 64: , 101189. |
[57] | Sanchez-Roman I , Ferrando B , Holst CM , Mengel-From J , Rasmussen SH , Thinggaard M , Bohr VA , Christensen K , Stevnsner T ((2022) ) Molecular markers of DNA repair and brain metabolism correlate with cognition in centenarians. GeroScience 44: , 103–125. |
[58] | Kiss T , Nyúl-Tóth Á , Gulej R , Tarantini S , Csipo T , Mukli P , Ungvari A , Balasubramanian P , Yabluchanskiy A , Benyo Z , Conley SM ((2022) ) Old blood from heterochronic parabionts accelerates vascular aging in young mice: Transcriptomic signature of pathologic smooth muscle remodeling. GeroScience 44: , 953–981. |
[59] | Mead LE , Prater D , Yoder MC , Ingram DA ((2008) ) Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol Chapter 2: , Unit 2C.1. |
[60] | Bouvard C , Gafsou B , Dizier B , Galy-Fauroux I , Lokajczyk A , Boisson-Vidal, C , Fischer AM , Helley, D ((2010) ) α6-Integrin subunit plays a major role in the proangiogenic properties of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 30: , 1569–1575. |




