Physical Activity and Cognition in Sedentary Older Adults: A Systematic Review and Meta-Analysis
Abstract
Background:
Epidemiologic evidence suggests that physical activity benefits cognition, but results from randomized trials in sedentary individuals are limited and inconsistent.
Objective:
To evaluate the effects of physical activity on cognition among sedentary older adults.
Objective:
A systematic literature search for eligible studies published up to January 1, 2021, was performed on six international (PubMed, Cochrane Library, Web of Science, Sinomed, FMRS, and OVID) and three Chinese databases (Wanfang, China National Knowledge Infrastructure, and VIP). We estimated the effect of physical activity on the cognition of sedentary elderly by standardized mean differences (SMD) and 95% confidence intervals (CI) using a random-effects model. We evaluated publication bias using funnel plots and heterogeneity using I2 statistics. Subgroup analyses were conducted by baseline cognition, intervention duration, activity type, and country.
Results:
Seven randomized controlled trials (RCTs) comprising 321 (experimental group, 164; control group, 157) sedentary older adults were included in the meta-analysis. Physical activity significantly improved cognition in sedentary elderly adults compared with controls (SMD: 0.50, 95% CI:0.09–0.92). Subgroup analyses showed significant effects of baseline cognition impairment (SMD: 9.80, 95% CI: 5.81–13.80), intervention duration > 12 weeks (SMD: 2.85, 95% CI: 0.73–4.96), aerobic exercise (SMD: 0.74, CI: 0.19–1.29), and countries other than the United States (SMD: 10.50, 95% CI: 7.08–13.92).
Conclusion:
Physical activity might have a general positive effect on the cognition of sedentary older adults. Intervention > 12 weeks and aerobic exercise can effectively delay their cognitive decline; however, more rigorous RCTs are needed to support our findings.
INTRODUCTION
As of 2018, about 50 million people worldwide suffer from dementia, a number expected to increase to 152 million by 2050 [1]. Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 60% to 80% of all dementia cases [2]. However, its exact etiology and pathogenesis are still unclear. AD is the fourth largest cause of death in older adults after cancer, heart disease, and cerebrovascular disease [3, 4], whose single most important non-modifiable risk factor is age [5]. The increasing incidence of AD and the huge associated social and economic burden have stimulated research into multiple protective factors to prevent the occurrence and development of this neurodegenerative disease. Among them, reducing sedentary behavior or increasing physical activity (PA) is a low-cost and low-risk intervention proved to have a positive impact on the physical and mental health of patients with AD [6].
Sedentary behavior is defined as any behavior with an energy expenditure of≤1.5 metabolic equivalents and includes behaviors such as sitting, watching television, and lying down [7]. Sedentary behavior is associated with numerous health risks including type 2 diabetes, cardiovascular disease and all cause mortality [8–10]. Given the health risks of a sedentary lifestyle, recommendations for sedentary time suggest limiting discretionary sedentary time to < 2 h/day and accumulating > 2 h/day of light-intensity activity (i.e., standing and light walking) [11, 12]. For the elderly, watching TV or other visual content together with poor physical strength caused by disease or aging leads to the generation and/or maintenance of a sedentary lifestyle. The data show that the elderly have an average of 9.4 h of immobility per day, which is equivalent to 65% –80% of their waking time [13].
In observational studies, individuals who are physically active often show less cognitive decline and a lower risk of dementia than sedentary individuals [14]. A meta-analysis concluded that people who were not previously physically active start showing improved cognitive functioning after exercising for as little as four months [15]. Furthermore, exercise interventions may also reduce the rate of cognitive decline in people with cognitive impairment [16]. However, more recent studies are much less consistent. For example, a large randomized controlled trial (RCT) of sedentary adults showed no effect on cognition outcomes after 24 months of moderate intensity physical exercise [17], and no cognitive improvement in AD patients after 16 weeks of aerobic exercise training [18]. In addition, a recent meta-analysis of aerobic, resistance training and tai chi interventions in people older than 50 years showed little benefit of exercise on cognitive function [19]. In addition to these differences, it is still unclear how to combine the type, intensity, and time of exercise for a maximum benefit on the cognition of sedentary elderly people.
The present review and meta-analysis aimed to appropriately explore the effects of PA on the cognition of sedentary older adults. Considering the significant health and economic burden of dementia, the results of our study may serve as a basis for establishing guidelines and recommendations for future sedentary behavior interventions in the elderly and provide highly operational and popular non-pharmacological interventions for the prevention of cognitive decline.
METHODS
Literature search
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) reporting guidelines [20]. We did not publish or register a protocol for this study. A systematic literature search for eligible studies published before January 2021 was performed on six international databases (PubMed, The Cochrane Library, Web of Science, Sinomed, FMRS, and OVID) and three Chinese databases (Wanfang Data, VIP, and CKNI). The search items included a combination of Medical Subject Heading terms and free words. The following keywords were searched: “Exercise” OR “Physical Activity” OR “Activity, Physical” AND “sedentary behavior” OR “physical inactivity” OR “television time” OR “screen time” AND “cognition” OR “cognitive function” OR “brain function” were searched. In this search, we retrieved 20019 studies. Titles and abstracts from a final total of 1,464 studies were then reviewed for further inclusion. In addition, the reference lists of the retrieved original articles and relevant review articles were also comprehensively examined to identify further pertinent studies.
Inclusion and exclusion criteria
To be eligible for inclusion in this systematic review, studies had to satisfy the following criteria: 1) design: RCTs, had an exercise-only intervention in the experimental group and a non-exercise control group (i.e., subjects who maintain their current sedentary lifestyle); 2) eligibility of participants: subjects aged≥60 years with or without cognitive impairment and living a sedentary lifestyle; 3) Eligibility criteria: articles examining the association between physical activity and cognition in sedentary elderly adults; 4) sedentary behavior: Studies were included as long as they included older adults with sedentary lifestyles, regardless of the sedentary criteria; 5) Outcome measurements: involved global cognition or other specific domains such as memory and executive function; 6) only articles published in English or Chinese were included.
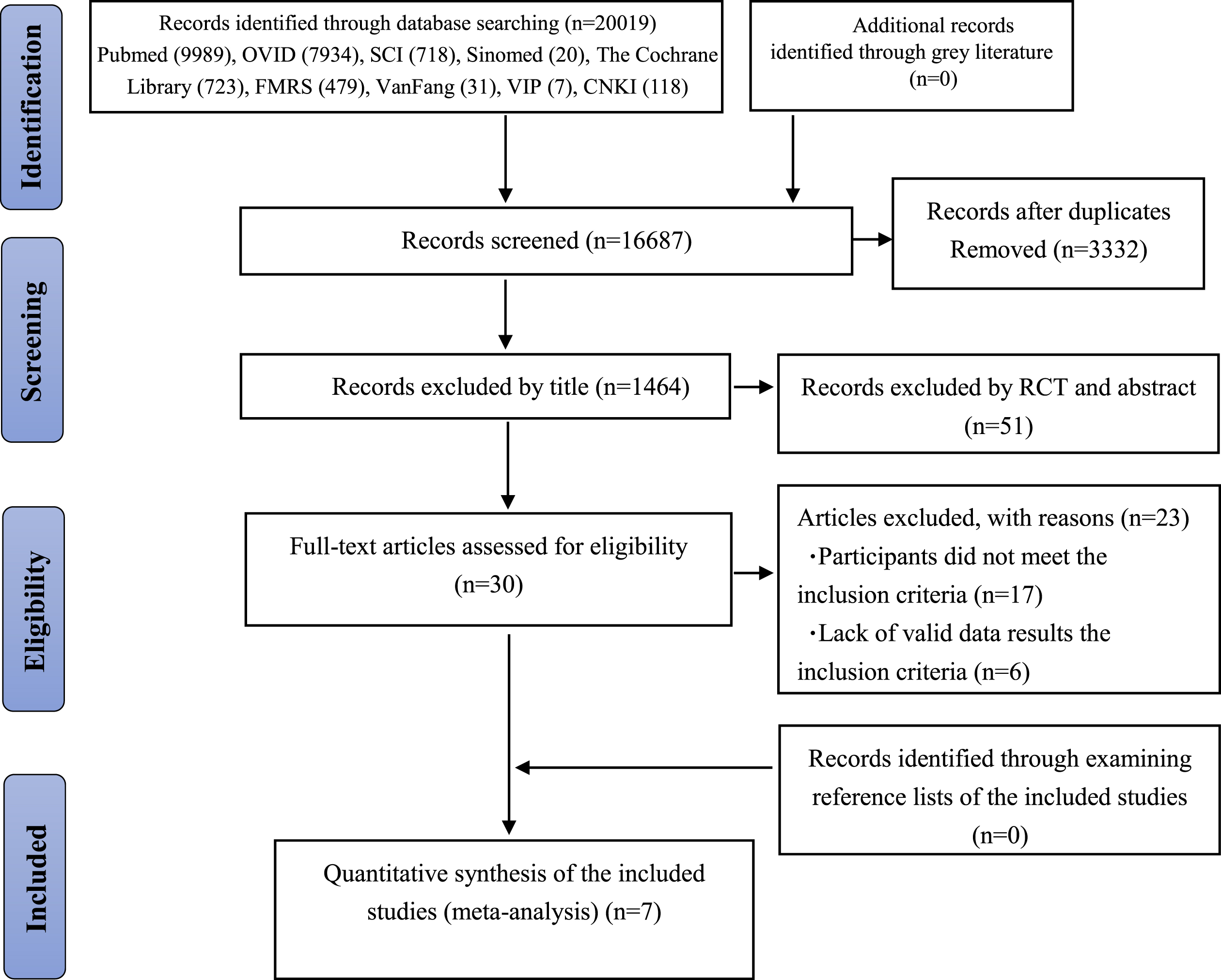
Studies that failed to meet the initial criteria were rejected on initial review. Reviews, conference papers, animal experiments and abstracts without available full text were also excluded. Qualitative studies or studies on the effects of exercise on cognitive function in combination with other interventions, such as cognition therapy or cognitive stimulation, were excluded. Two authors independently reviewed full texts of all articles that were considered relevant for inclusion in this review. A third author was consulted in case of disagreement. The study selection process is described in Fig. 1.
Fig. 1
Flowchart for searching and selection of the included studies.
Data extraction
The titles and abstracts of the studies identified in the initial search were imported into EndNote for initial filtering. Document screening and data extraction were independently performed by two researchers, with third-party arbitration for inconsistent results. Data extraction of the screened accepted studies, including author, publication year, journal, number of experimental and control groups, interventions and time, outcome indicator, and cognitive assessment method. The study characteristics are shown in Table 1.
Table 1
Baseline characteristics and intervention details of the included studies.
| Author | Year | Country | Age (y) Mean (SD) | Sample condition | Intervention type | Intervention (frequency, duration, intensity) | Outcome measures | |
| Experimental group | Control group | |||||||
| Matson et al. [13] | 2019 | USA | 60–89 (–) | 29 | 29 | work with health coaches, exercise | 2 in-person health coach visits and 4 biweekly calls; 12 weeks; moderate | Self-reported Health Outcomes |
| Nocera et al. [21] | 2015 | USA | 71.95 (5.24) | 10 | 8 | spin aerobic cycling | 3 times a week; 12 weeks; light | D-KEFS Verbal Fluency Test |
| Williamson et al. [22] | 2009 | USA | 77.44 (4.26) | 50 | 52 | multicomponent training | exercise sessions 40 –60 min per week for the first 2 months, 2 times per week in the next 4 months, and flexibility exercises 3 or more times per week; 12 months; moderate | DSST, RAVLT, 3MSE, Modified Stroop Test |
| Ansai et al. [23] | 2015 | Brazil | 82.4 (2.4) | 23 | 23 | multicomponent training | Three 1-h sessions per week on non-consecutive days; 16 weeks; moderate | MoCA, CDT, Verbal Fluency, Dual Task (TUGT-cognitive) |
| Venturelli et al. [24] | 2011 | Italy | 84 (5) | 12 | 12 | Exercise (walking) | a minimum of 30 min of moderate exercise (walking) 4 times a week; 24 weeks; moderate | MMSE, Clinical Dementia Rating Scale |
| Dillon et al. [25] | 2020 | Canada | 86.7 (5.3) | 14 | 11 | light walking | 338 min per week; 10 weeks; light and moderate | ADAS-Cog |
| Bouaziz et al. [26] | 2019 | France | 72.9 (2.5) | 27 | 29 | short-term interval aerobic training | 30-min twice a week; 9.5 weeks; moderate | TMT, PASAT, Verbal Fluency |
SD, standard deviation; MoCA, Montreal Cognitive Assessment; CDT, Clock Drawing Test; TUGT-cognitive, Timed Up and Go test associated with cognitive task; DSST, Digit Symbol Substitution Test; RAVLT, Rey Auditory Learning Test; 3MSE, Modified Mini-Mental State Examination; ADAS-Cog, The AD assessment scale-cognitive; SF-36, 36-Item Short-Form Health Survey; MMSE, Mini-Mental State Examination; TMT, Trail Making Test; PASAT, Paced Auditory Serial Addition Test; –, data not available.
Risk of bias
Studies meeting the inclusion criteria were individually scored by two authors independently according to the Cochrane risk of bias tool; the third author would be consulted in case of disagreement. Seven items regarding the risk of bias were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. The included RCTs were classified as being at low risk, high risk, or unclear risk in the above fields.
Statistical analysis
Analyses were conducted using Reviewer Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). For the meta-analysis, we synthesized continuous outcome data using the mean difference and standard deviation. When different measurements for the same outcome were performed in different studies, we used the standardized mean difference (SMD) instead to obtain a summary effect. We used a random-effects model with generic inverse-variance to pool the effect and its corresponding 95% CI. The I2 statistic was used to examine the heterogeneity of the included studies. If large, the source of heterogeneity was investigated through a sensitivity test and subgroup analysis. Subgroup analyses were conducted to explore potential sources of heterogeneity with the following subgroups: cognitive function at baseline, length of intervention duration, type of PA, and study countries. A two-sided p < 0.05 indicated significance. Publication bias was graphically illustrated using a funnel plot.
RESULTS
Identification of studies
The initial search identified a total of 20,019 articles (Ovid: 7,934 articles, PubMed: 9,989 articles, Sinomed: 20 articles, FMRS: 479 articles, SCI: 718 articles, The Cochrane Library: 723 articles, VIP: 7 articles, CKNI: 118 articles, Wanfang: 31 articles). We excluded 3,332 articles because of duplication. Upon application of the eligibility criteria, 1,464 studies were further excluded. A total of 51 full-text articles were then scrutinized, of which seven publications were eligible for this review [13, 21–26] . The whole screening process was completed independently by two reviewers. Figure 1 illustrates the study selection process.
Study characteristics
Table 1 presents the detailed characteristics of each study. The total number of participants in all seven included studies was 350. There were a total of 29 dropouts, leaving 321 subjects (experimental group 164, control group 157) with a mean baseline age > 60 years. Three studies were conducted in the United States [13, 21, 22]; the other four studies were conducted in Brazil [23], Italy [24], Canada [25], and France [26].
Measurement of sedentary behavior
Measurement of sedentary behavior varied considerably with a total of six different measures used across the seven studies. One study measured sedentary behavior via an objective method (accelerometry) [25]. Two studies measured exposure as sedentary time (i.e., time spent sitting, lying down, or sleeping) [21, 22]. Three studies examined sedentary behavior using a previously developed questionnaire [13, 23, 26]. Two studies did not exact explanation of how to assess sedentary behavior [23, 24], another study used the validated PA measures of the same psychosocial constructs, which includes sedentary habits (PACE and Self-Report Habit Index) [13], and the last study used an International Physical Activity Questionnaire [26].
Measurement of cognition
Table 1 describes the measures of cognitive function used. Sixteen different measures of cognitive function were used across the seven studies. The first study in Table 1 used the Self-reported Health Outcomes [13]. The second study used the Verbal Fluency Test of the Delis-Kaplan Executive Function System [21]. The third study was based on the LIFE-P (Lifestyle Interventions and Independence for Elders pilot) study and assessed CF using a battery adapted from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. The cognitive battery consisted of four primary components: Digit Symbol Substitution Test (DSST) as a measure of psychomotor speed and working memory. The modified Stroop test was used as a measure of processing speed, cognitive flexibility, and inhibition or disinhibition. The modified Mini-Mental State Examination (3MSE) is a widely used measure of global cognitive functioning. The Rey Auditory Verbal Learning Test (RAVLT) is a test of short- and long-term verbal memory that assesses the ability to learn a list of 15 common words [22]. The fourth study used the Montreal Cognitive Assessment (MoCA; 0–30), Clock Drawing Test (CDT; 0–10), verbal fluency and dual task to assess cognitive function [23]. The fifth study used the Mini-Mental State Exam (MMSE) and the Clinical Dementia Rating Scale. A psychologist specialized in neuropsychology assessed the global cognitive functions of the participants through the MMSE [24]. The sixth study used the AD assessment scale–cognitive subscale (ADAS-Cog) [25]. As shown in Table 1, that study, used verbal fluency tasks, the Trail Making Test, and the Paced Auditory Serial Addition Test (PASAT). Lexical-semantic memory was measured via the Verbal Fluency. Executive function was measured via the Trail Making Test [26].
The studies examined the following areas of cognition: 1) two studies measured memory [22, 26]; 2) two measured executive function [21, 26]; 3) one measured processing speed [22]; and 4) three studies created scores for overall cognitive function [22, 24, 26].
Quality assessment
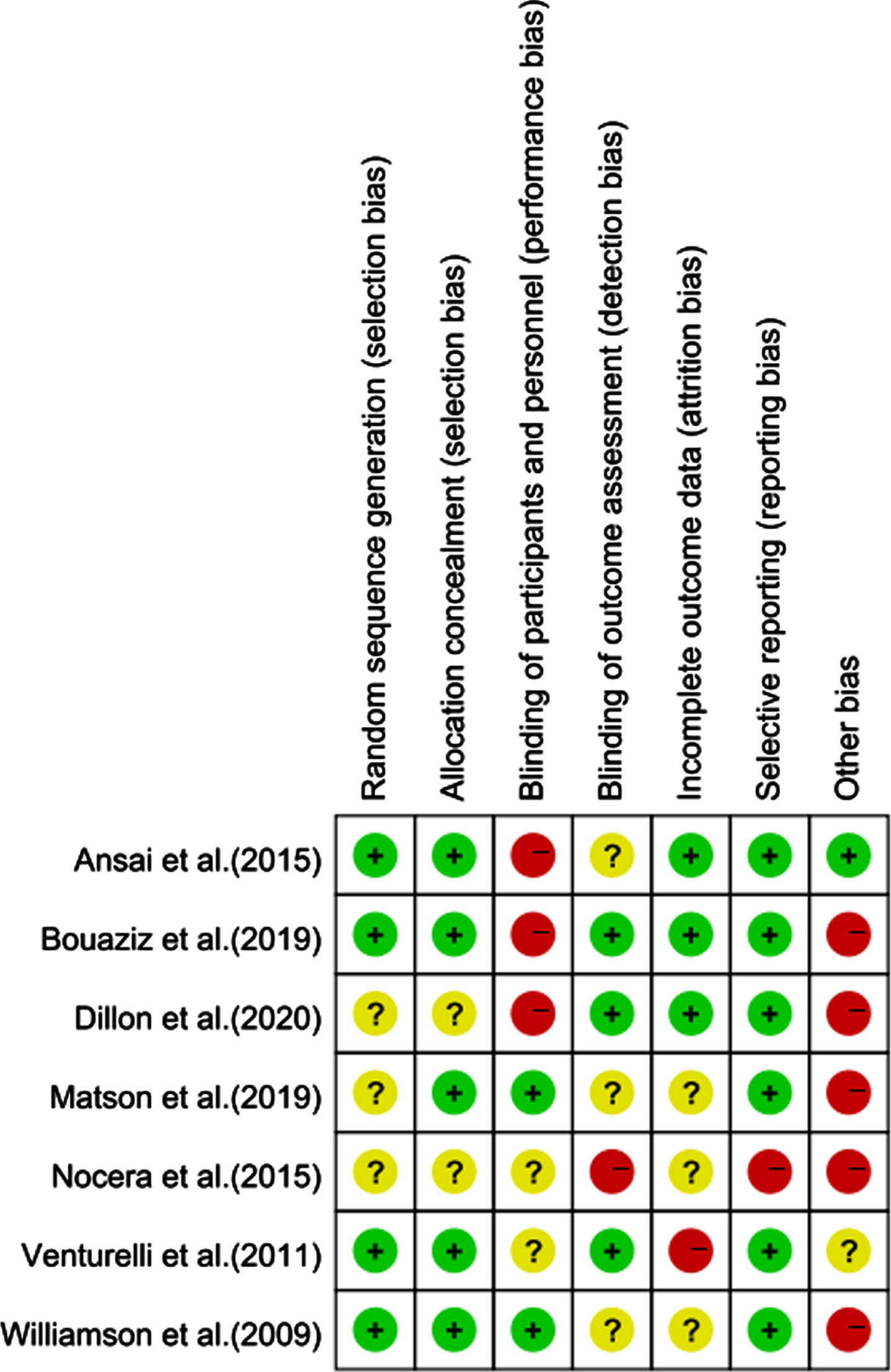
The seven studies included were published be-tween 2009 and 2020. Of them, four reported random assignment procedures and three reported a blinding procedure. All described movement patterns and cognitive measures in the experimental group. The general quality of the articles was high. Figures 2 and 3 show the quality evaluations for allstudies.
Fig. 2
Risk of bias in trials of each item.
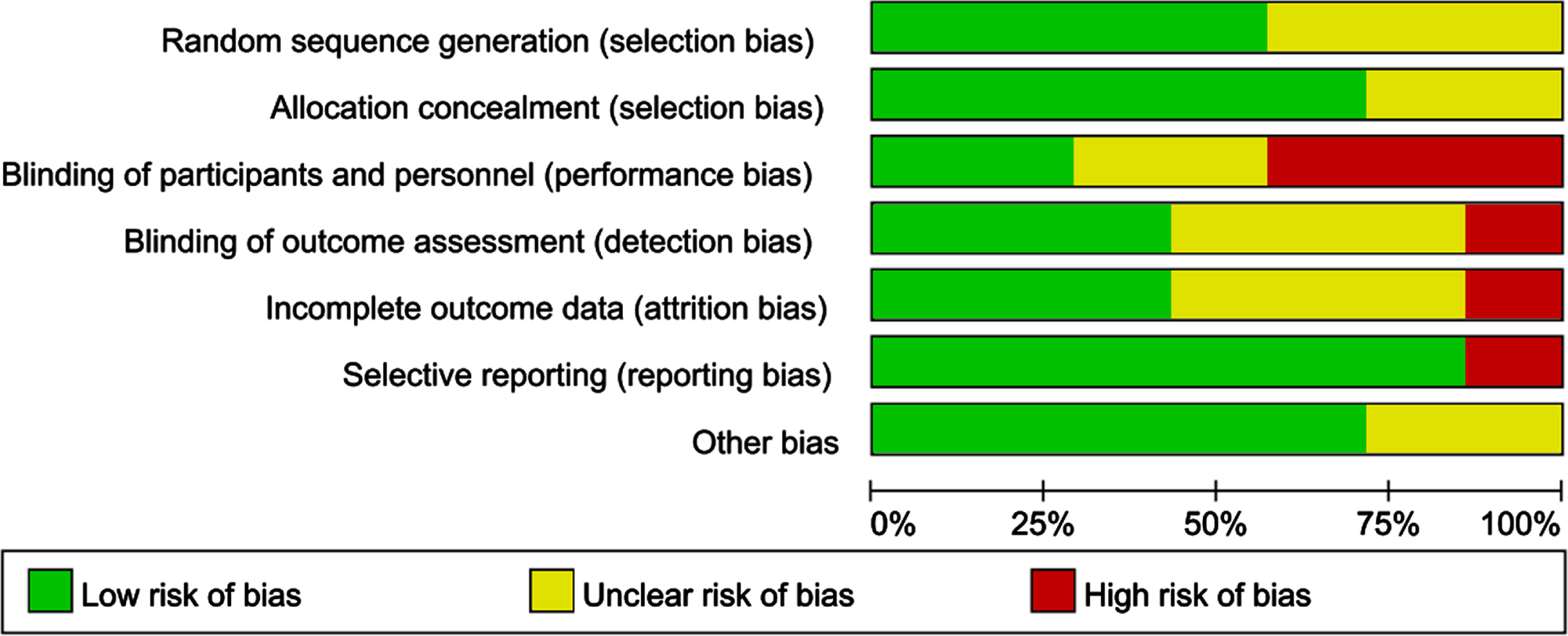
Fig. 3
Assessment for the risk of bias in the included studies. Green circle, the risk of bias was low; Red circle, the risk of bias was high; Yellow circle, the risk of bias was unclear.
Meta-analysis results
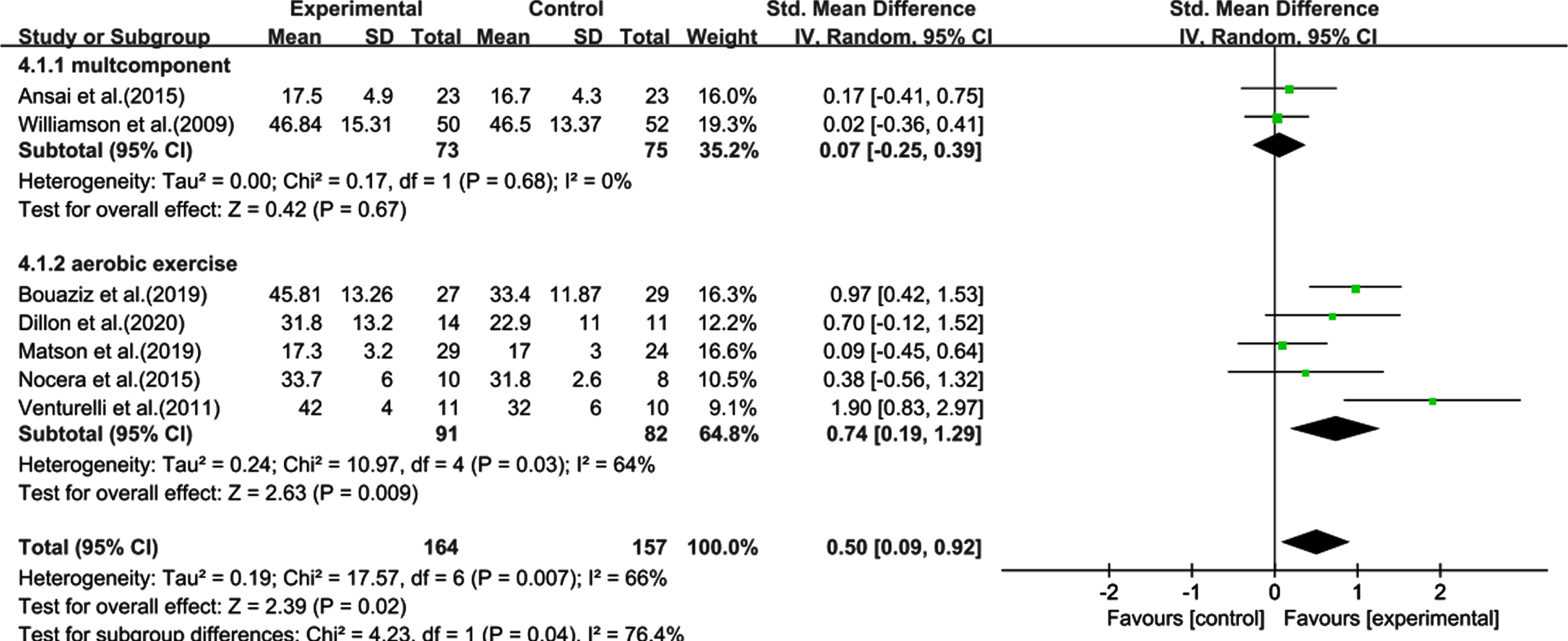
Primary outcome
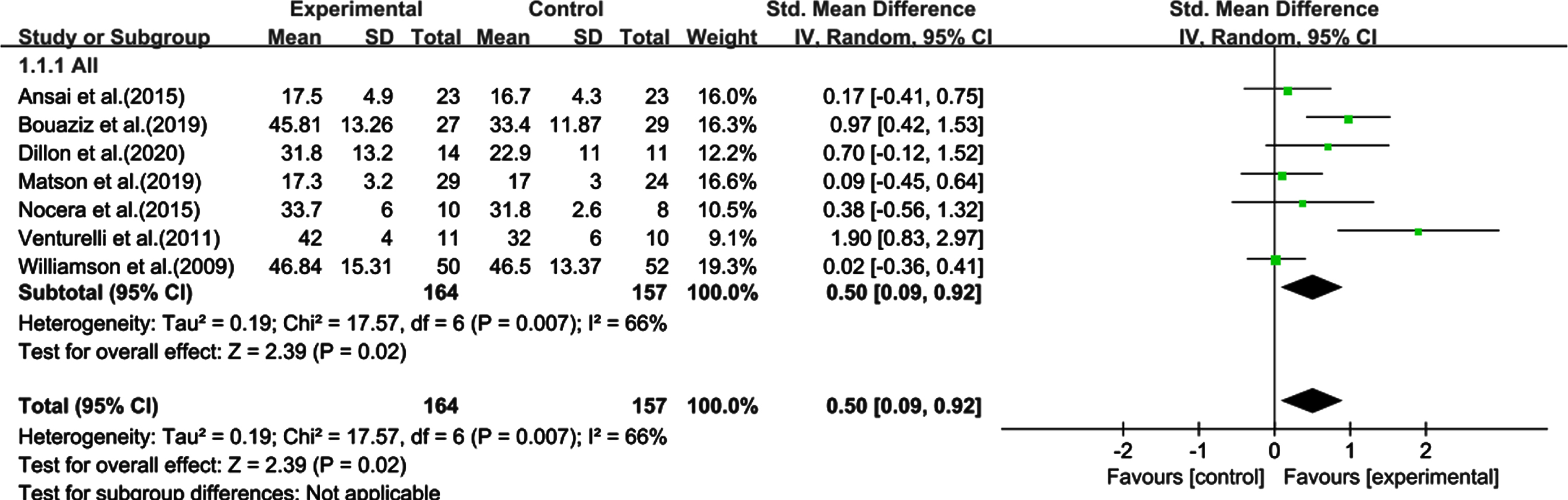
The statistical analysis showed the comprehensive effect of PA on cognition during the whole intervention period. The heterogeneity test results showed a moderate degree of statistical heterogeneity among the studies (χ2 = 17.57, I2 = 66%, p = 0.007), therefore, the random effects model was adopted for analysis. The meta-analysis results showed a significant combined effect size of SMD: 0.50, 95% CI [0.09–0.92], p = 0.02, indicating that physical exercise can improve cognitive function in sedentary elderly individuals (Fig. 4).
Fig. 4
Effect of physical activity on the cognitive function of sedentary elderly individuals. CI, confidence interval; SD, standard deviation.
Results of the subgroup analysis
Subgroup analysis by baseline cognition
The heterogeneity test of the baseline normal cognition group was significant (χ2 = 12.38, I2 = 68%, p = 0.01), but the combined effect size was not (SMD: 1.02, 95% CI: –0.25–2.30, p = 0.12). However, the heterogeneity test was significant in the impairment cognition group (χ2 = 29.25, I2 = 79%, p < 0.0001), as was the combined effect size (SMD: 1.84, 95% CI: 0.62–3.06, p = 0.003) (Fig. 5).
Fig. 5
Subgroup analysis categorized by baseline cognitive function. CI, confidence interval; SD, standard deviation.
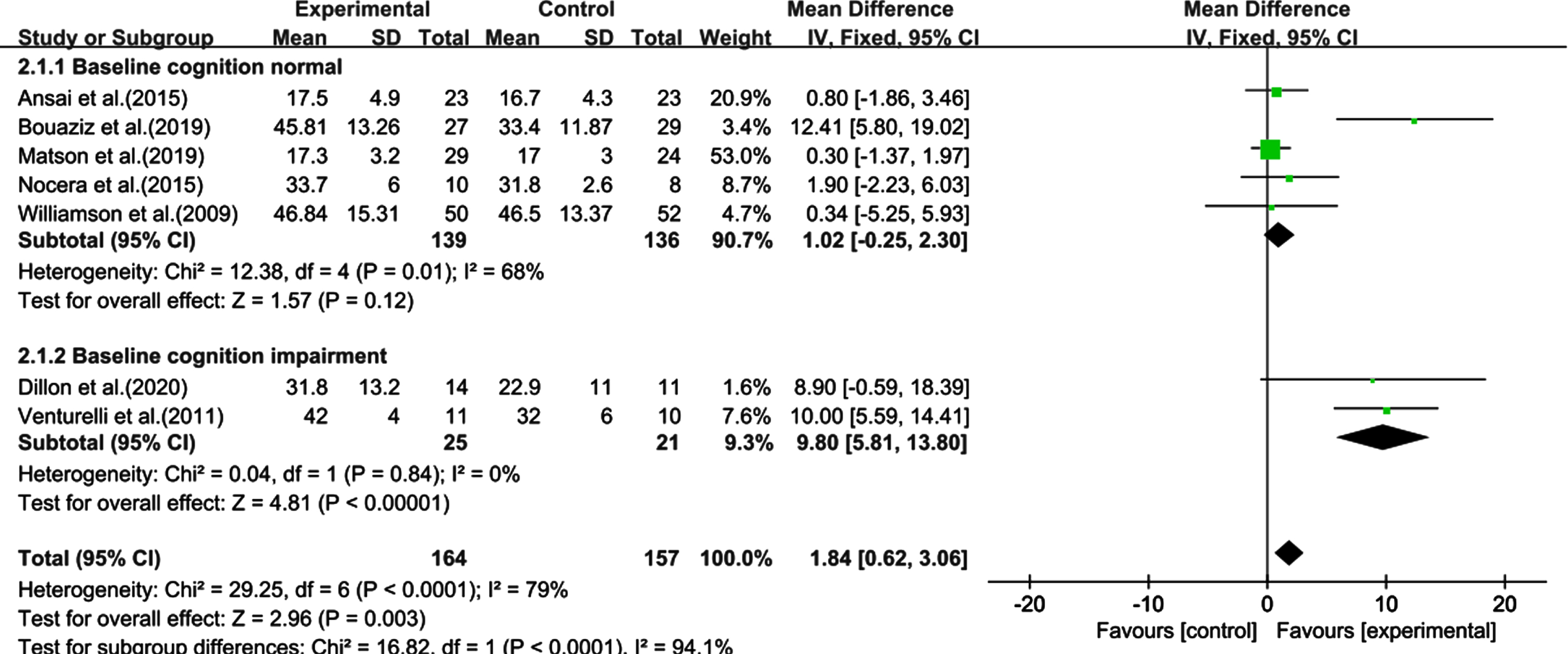
Subgroup analysis by intervention duration
The studies with an intervention duration of less than 12 weeks were compared with those with a duration more than 12 weeks. The heterogeneity test within 12 weeks was significant (χ2 = 14.77, I2 = 80%, p = 0.002), but the combined effect size was not (SMD: 1.34, 95% CI: 0.15–2.83, p = 0.08). The heterogeneity test for intervention duration more than 12 weeks was also significant (χ2 = 13.16, I2 = 85%, p = 0.001), as was the combined effect size (SMD: 2.85, 95% CI: 0.73–4.96, p = 0.008). Therefore, an intervention duration more than 12 weeks led to significant cognitive improvement in the sedentary elderly (Fig. 6).
Fig. 6
Subgroup analysis categorized by the length of intervention duration. CI, confidence interval; SD, standard deviation.
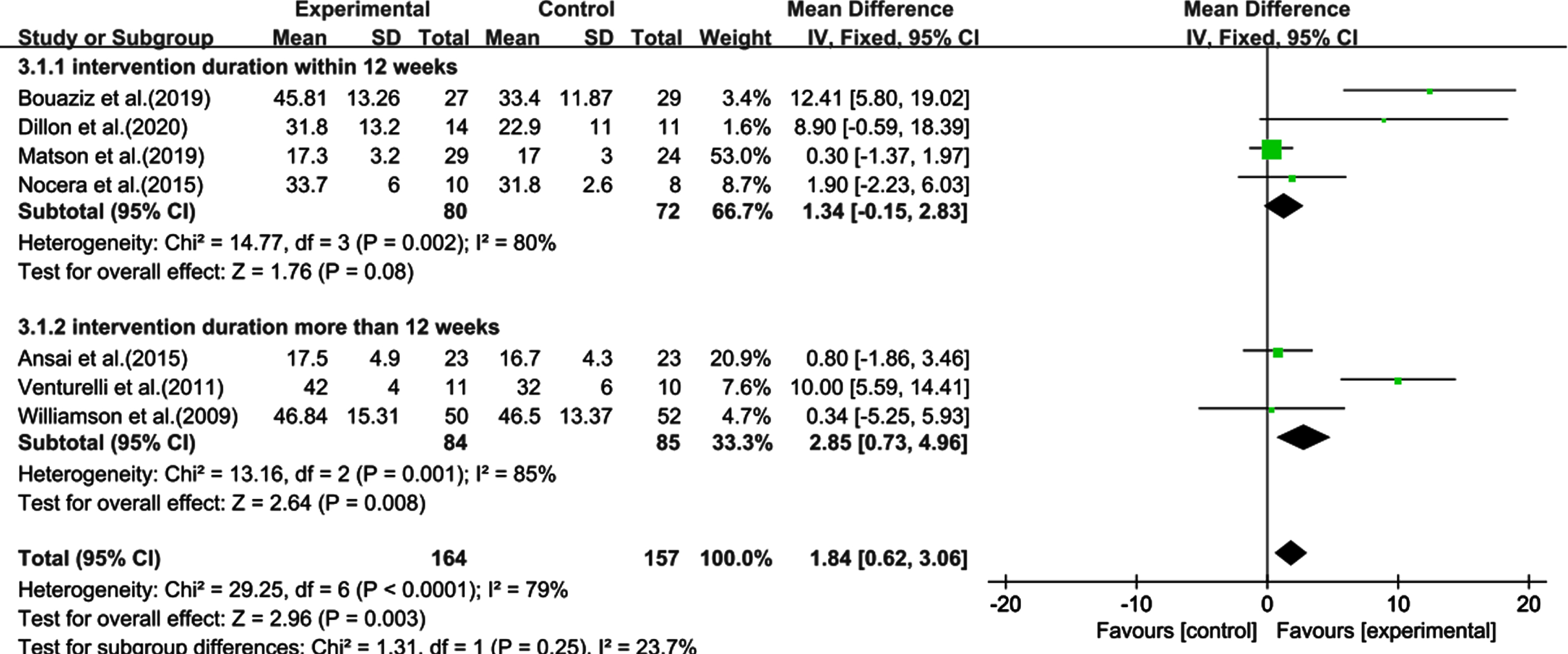
Subgroup analysis by PA forms
Multi-component exercise intervention and aerobic exercise studies were compared. Heterogeneity test of multi-groups showed a non-significant (χ2 = 0.17, I2 = 0%, p = 0.68) or combined effect size (SMD: 0.07, 95% CI: 0.25–0.39, p = 0.67). However, for the aerobic exercise group, the result was significant (χ2 = 10.97, I2 = 64%, p = 0.03), as was the combined effect size (SMD: 0.74, 95% CI: 0.19–1.29, p = 0.009). These results showed that aerobic exercise had a significant effect on the cognitive improvement of the sedentary elderly (Fig. 7).
Fig. 7
Subgroup analysis categorized by forms of physical activity. CI, confidence interval; SD, standard deviation.
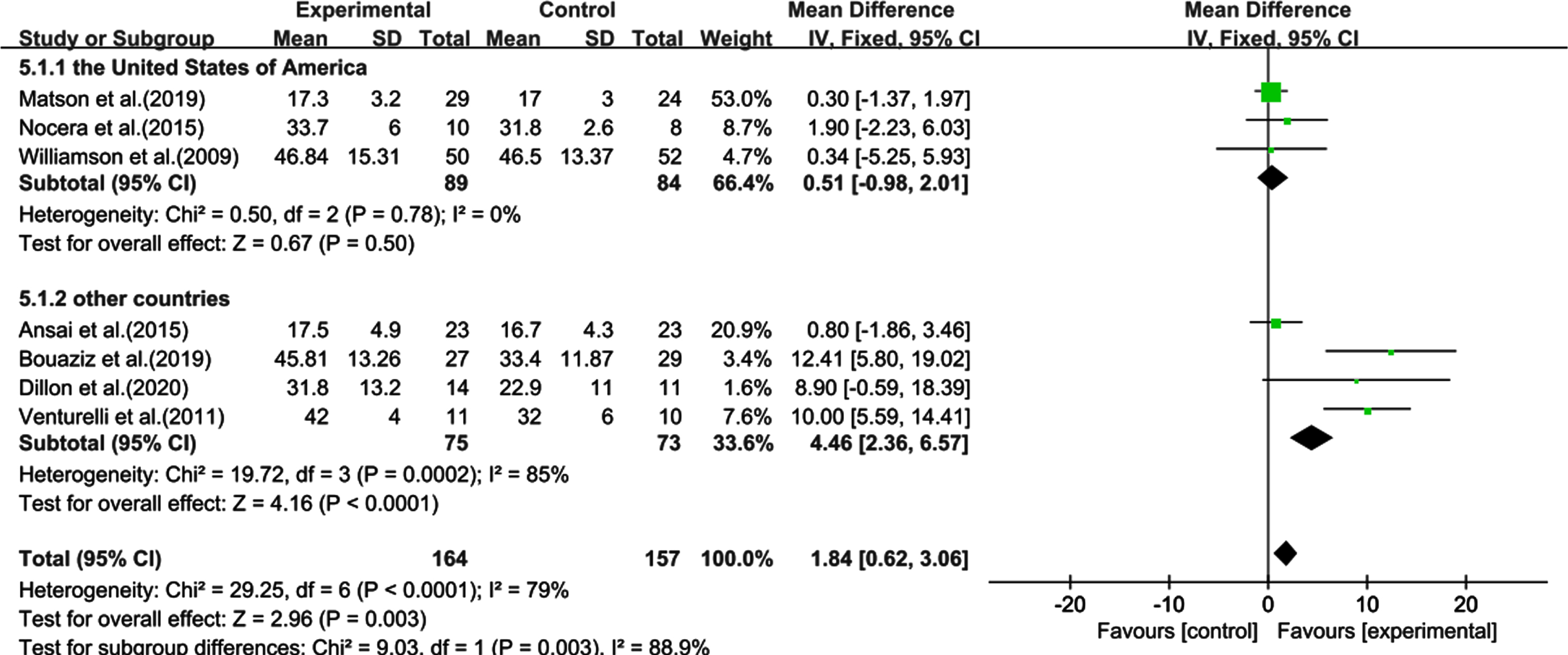
Subgroup analysis by country
The heterogeneity test of the US showed non-significant results (χ2 = 0.50, I2 = 0%, p = 0.78) and combined effect size (SMD: 0.51, 95% CI: 0.98–2.01, p = 0.50). In contrast, the heterogeneity test of the other countries showed significant effects (χ2 = 19.72, I2 = 85%, p = 0.0002) and a significant combined effect size (SMD: 4.46, 95% CI: 2.36–6.57, p < 0.0001), indicating that the cognitive improvement of sedentary elderly people in countries other than the US was statistically significant (Fig. 8).
Fig. 8
Subgroup analysis categorized by country. CI, confidence interval; SD, standard deviation.
Sensitivity and publication bias
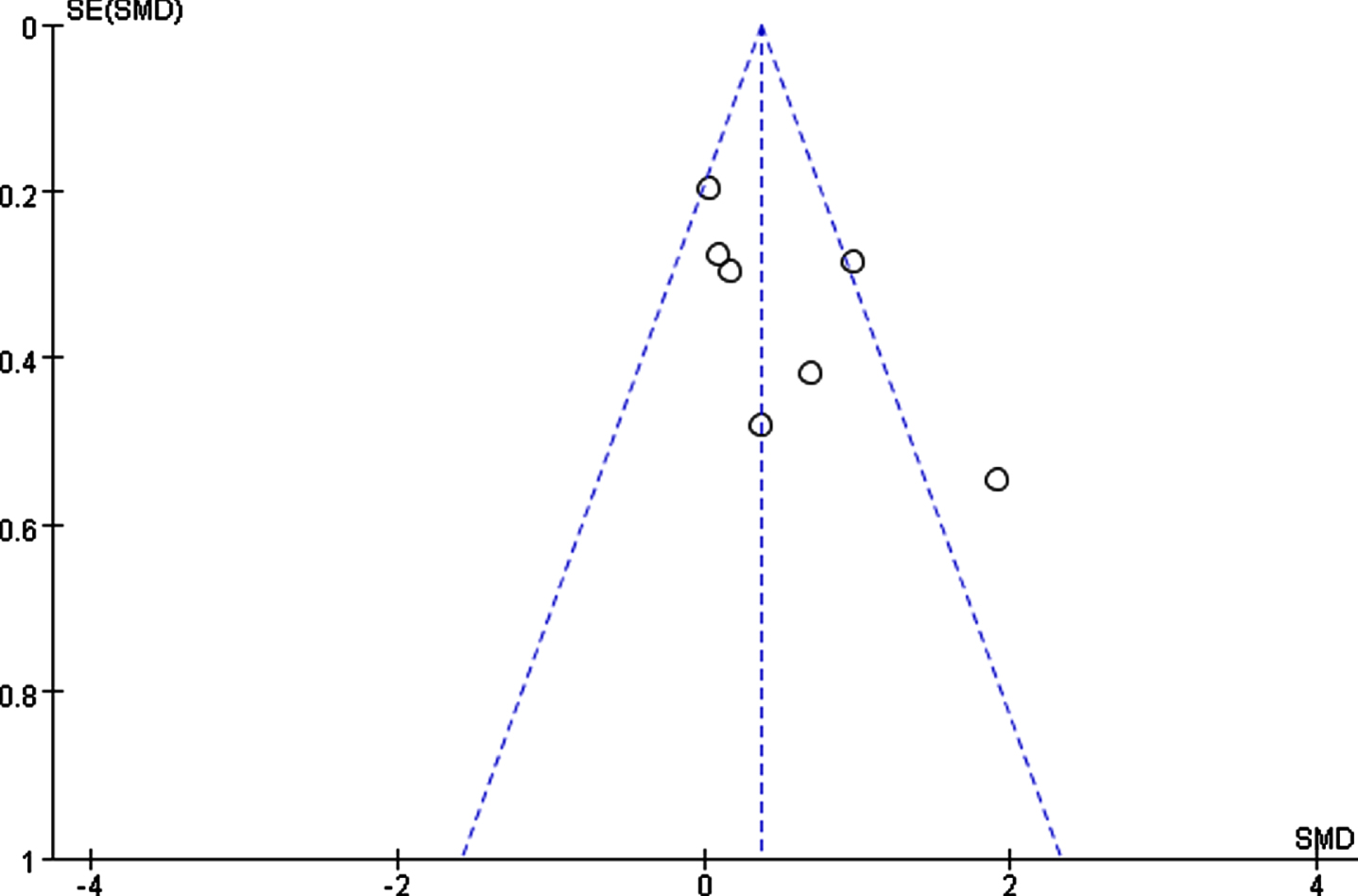
We conducted sensitivity analyses to identify potential sources of heterogeneity in the association between PA and cognition among sedentary older adults. After eliminating one study, we obtained a range of combined effect size of SMD from 0.35 to 0.62 and an I2 from 46% to 72% (p < 0.05). The processing results indicate low data sensitivity. None of the studies interfered much with the results of this meta-analysis, indicating its good stability and reliability. The results of the funnel plot test (Fig. 9) showed that one study fell outside the dotted line and two intersected with it, indicating heterogeneity. However, the distribution was roughly symmetric, suggesting no significant publication bias.
Fig. 9
Funnel plot for studies of physical activity and cognitive function in sedentary elderly individuals. SE, standard error; SMD, standardized mean differences.
DISCUSSION
To the best of our knowledge, this is the first meta-analysis to evaluate the effects of PA on the cognition of sedentary older adults. Our results showed that after a period over 12 weeks of intervention, the cognitive function scores of the sedentary elderly in the experimental group were significantly improved compared with the control group. Furthermore, PA had a positive effect on delaying the cognition decline of sedentary older adults. Sensitivity analysis showed that the study had good reliability and stability. In addition, we performed four subgroup analyses to explain the sources of heterogeneity across the seven studies. We found that baseline cognition, intervention duration, PA forms, and geographic location differently affect the degree of the delay in cognition decline in sedentary older adults.
The question arises as to how sedentary behavior accelerates cognition decline. Previous data suggested that sedentary time may cause impairment of glucose and lipid metabolism [27, 28], which is considered a risk factor for cognitive function decline and all-cause dementia [29]. In addition, sedentary behavior may, in turn, induce or aggravate individual inflammation, which has also been identified as a potential risk factor for dementia [30, 31]. At present, the specific effect mechanism of physical exercise on the cognition of sedentary elderly individuals is not clear. Research studies have shown that cerebral perfusion is an important mechanism for maintaining cognition [32, 33]. PA can not only increase blood flow in the brain and improve cardiovascular function, but also affect the entire metabolic system. In addition, as physical exercise involves cognitive and social activities, it may enhance overall brain function. In the elderly, cardiovascular and metabolic efficiency are reduced, PA might effectively compensate for these issues, delaying the negative effects of sedentarism in the elderly.
In some of the included studies, PA was found to affect not only overall cognitive function, but also some specific effects cognitive domains, in sedentary elderly subjects. For example, Nocera et al. [21] found that PA can affect the verbal fluency of the elderly, usually considered as a component of executive function. In the study by Ansai and Rebelatto, the naming and attention domains improved significantly as well [23]. It was found that 9.5 weeks of interval aerobic training programs helped improve a number of cognitive functions, such as attention, mental flexibility, and working memory [26]. This suggested that the improvements in cognition could be directly attributed to the improvement in mood and quality of life caused by PA. This can be explained by the positive effects of PA over psychological stress and depression. In animal models, PA decreases amyloid load [34], positively affects hippocampal neuronal function [35] as well as hippocampal and parietal cortical cholinergic function and spatial learning [36], increases brain-derived neurotrophic factor levels, and may prevent the formation of oxidative stress associated with other forms of neuronal damage [37]. All these findings suggest that PA is an effective intervention for delaying cognitive decline. However, as the included studies had scattered evaluations of cognitive domains, we could not perform a subgroup analysis, which would require the inclusion of more relevant RCTs.
Considering that a substantial heterogeneity was observed in the included studies, we further performed a subgroup analysis to determine the potential sources of heterogeneity. Subgroup analysis showed that cognition of participants with baseline cognitive impairment was significantly more severe than that of normal cognition, which was consistent with previously reported results showing that intervention, such as physical activities including aerobic exercises, can improve cognitive function among older adults with MCI [38, 39]. In addition, the subgroup analysis of the duration revealed significant differences between the intervention group and the control group for different intervention durations (≤12w, > 12w). After > 12 weeks of intervention, the cognition of sedentary older adults was better than shorter interventions. However, the effect of PA intervention duration is still controversial. Rao et al. [40] conducted a meta-analysis that indicated that longer sessions were not associated with better prognosis, similar to the Rolland and Vellas’s study [41]. Although no clear relationship was found between intervention duration and effect size, the longest intervention in the study did not produce a large effect; therefore, longer interventions should be performed with caution. Previous studies also showed no difference between the experimental and control groups in overall cognition and other areas after a 3-month intervention [42]. Conversely, other trials with longer duration or follow-up found some degree of cognitive improvement [43, 44]. However, this difference may also be due to compliance issues. If we can raise the awareness of the importance of PA and regular voluntary exercise as well as the harm of sedentary behavior in the elderly, this conclusion might change. Therefore, it is important to improve compliance of the sedentary elderly, and an intervention more than 12 weeks is preferred to modify their sedentary lifestyle and protect their cognition. However, future studies are needed to verify thispossibility.
Interestingly, the results of the subgroup analysis of PA forms showed that the studies using aerobic exercise showed more efficiency than those using multicomponent exercise, indicating that aerobic exercise, rather than multicomponent exercise could considerably improve the cognition of sedentary elderly individuals. Nevertheless, the multicomponent group should not be considered ineffective in delaying cognitive decline, because a large number of studies have confirmed that both aerobic and multicomponent exercises have a positive effect on cognition. Moreover, only two of the studies included in this meta-analysis used multicomponent training, and it is likely that the result of the subgroup analysis of different intervention types is less reliable due to the small sample size. More clinical studies on the use of multicomponent exercise to improve cognition are needed to provide stronger evidence in the future. We also performed a subgroup analysis by country where the study was conducted; there were subtle differences between the US and other countries, but the reasons underlying this finding remain to be investigated. Therefore, more RCTs involving people from different backgrounds are needed to explore whether any of these cofactors influence theresults.
Clinical implications
This study shows that PA is clinically correlated with cognitive protection in the sedentary elderly. For this population, a PA intervention > 12 weeks can effectively postpone cognitive decline; aerobic exercise also has a positive effect on cognitive function. This study provides certain theoretical support for practical training strategies. Further, it points toward the importance of further research on the type, frequency, and intensity of PA that can better diminish the impact of sedentarism on cognitive function in older adults.
Strengths and limitations
There are some potential limitations of our study. First, the number of articles included was small (only seven studies); and the total sample size was small as well. Second, we limited our search to articles written in English and Chinese, which may lead to some limitations. Third, inconsistent assessment tools for cognitive outcome indicators may lead to bias in actual size estimates, but this is unavoidable due to the lack of literature on the cognitive state of sedentary older adults. Fourth, as the included studies focused on different cognitive domains, it was not possible to determine how PA might affect specific cognitive domains in sedentary older adults. This could be direction for future research. Last, we did not publish or register a study protocol for this systematic review and meta-analysis.
Our study has the following strengths: 1) we conducted a comprehensive literature search using nine medical databases, and the study selection and quality assessments were performed independently by two researchers, which ensured strict quality control during the study process, including data collection; 2) all studies in our analysis were RCTs, which is the most common type of interventional study and has certain advantages over other types of studies [45]; 3) we measured the subjects’ general characteristics at baseline, and the included population was homogenous. Through subgroup analysis, the baseline status of each group was relatively consistent, the comparability was good, bias was low, and the external implementation of the results was strong [46]; 4) despite the heterogeneity observed in the study, the consistent results of the sensitivity analysis indicate that our findings are reliable and robust.
Conclusion
Taken together, the current evidence suggests that PA has positive effects on cognitive function in sedentary older adults, especially in those who have impairment cognition. An intervention more than 12 weeks and aerobic exercise can effectively postpone cognitive decline in the sedentary elderly. However, it is still unclear which PA intensity and frequency are more effective in improving cognition in the sedentary elderly. Therefore, future multicenter, large sample, high-quality RCTs should be conducted to determine the best protocol and exercises for preventing cognitive decline in the elderly. Based on the above, we suggest that older adults should reduce sedentary time as much as possible, and exercise regularly to delay the appearance of cognitive impairment, thus improving their quality of life, reducing the burden on families, and achieving the corresponding public health benefits.
ACKNOWLEDGMENTS
We thank all staff members involved in this study. This work was supported by the Natural Science Foundation of Hebei Province (grant nos. C2020206044 and H2018206361) and the National Natural Science Foundation of China (grant no. 82171582).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0073r1).
REFERENCES
[1] | Alzheimer’s Disease International, Guerchet M , Prince M , Prina M (2020) Numbers of people with dementia world wide: An update to the estimates in the World Alzheimer Report 2015.https://www.alzint.org/resource/numbers-of-people-with-dementia-worldwide |
[2] | GBD 2016 Dementia Collaborators ((2019) ) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: , 88–106. |
[3] | Chen X , Huang D , Lin A ((2009) ) Causes and countermeasures study on psychiatric disabled adults in Guangdong province. Chin J Rehabilitation Med 10: , 24. |
[4] | Xue J , Li J , Liang J , Chen S ((2018) ) The prevalence of mild cognitive impairment in China: A systematic review. Aging Dis 9: , 706–715. |
[5] | Thapa N , Kim B , Yang JG , Park HJ , Jang M , Son HE , Kim GM , Park H ((2020) ) The relationship between chronotype, physical activity and the estimated risk of dementia in community-dwelling older adults. Int J Environ Res Public Health 17: , 3701. |
[6] | Rolland Y , Abellan van Kan G , Vellas B ((2008) ) Physical activity and Alzheimer’s disease: From prevention to therapeutic perspectives. J Am Med Dir Assoc 9: , 390–405. |
[7] | Pate RR , O’Neill JR , Lobelo F ((2008) ) The evolving definition of “sedentary". Exerc Sport Sci Rev 36: , 173–178. |
[8] | Owen N , Healy GN , Matthews CE , Dunstan DW ((2010) ) Too much sitting: The population health science of sedentary behavior. Exerc Sport Sci Rev 38: , 105–113. |
[9] | Ford ES , Caspersen CJ ((2012) ) Sedentary behaviour and cardiovascular disease: A review of prospective studies. Int J Epidemiol 41: , 1338–1353. |
[10] | Falck RS , Davis JC , Liu-Ambrose T ((2017) ) What is the association between sedentary behaviour and cognitive function? A systematic review. Br J Sports Med 51: , 800–811. |
[11] | Owen N , Sugiyama T , Eakin EE , Gardiner PA , Tremblay MS , Sallis JF ((2011) ) Adults’ sedentary behavior determinants and interventions. Am J Prev Med 41: , 189–196. |
[12] | Buckley JP , Hedge A , Yates T , Copeland RJ , Loosemore M , Hamer M , Bradley G , Dunstan DW ((2015) ) The sedentary office: An expert statement on the growing case for change towards better health and productivity. Br J Sports Med 49: , 1357–1362. |
[13] | Matson TE , Anderson ML , Renz AD , Greenwood-Hickman MA , McClure JB , Rosenberg DE ((2019) ) Changes in self-reported health and psychosocial outcomes in older adults enrolled in sedentary behavior intervention study. Am J Health Promot 33: , 1053–1057. |
[14] | Rockwood K , Middleton L ((2007) ) Physical activity and the maintenance of cognitive function. Alzheimers Dement 3: , S38–S44. |
[15] | Angevaren M , Aufdemkampe G , Verhaar HJ , Aleman A , Vanhees L ((2008) ) Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 16: , CD005381. |
[16] | Lautenschlager NT , Cox KL , Flicker L , Foster JK , van Bockxmeer FM , Xiao J , Greenop KR , Almeida OP ((2008) ) Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 300: , 1027–1037. |
[17] | Conradsson D , Löfgren N , Nero H , Hagströmer M , St°ahle A , Lökkåhle J , Franzén E ((2015) ) The effects of highly challenging balance training in elderly with Parkinson’s disease: A randomized controlled trial. Neurorehabil Neural Repair 29: , 827–836. |
[18] | Cott CA , Dawson P , Sidani S , Wells D ((2002) ) The effects of a walking/talking program on communication, ambulation, and functional status in residents with Alzheimer disease. Alzheimer Dis Assoc Disord 16: , 81–87. |
[19] | Kelly ME , Loughrey D , Lawlor BA , Robertson IH , Walsh C , Brennan S ((2014) ) The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res Rev 16: , 12–31. |
[20] | Moher D , Liberati A , Tetzlaff J , Altman DG ; PRISMA Group ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: , e1000097. |
[21] | Nocera JR , McGregor KM , Hass CJ , Crosson B ((2015) ) Spin exercise improves semantic fluency in previously sedentary older adults. J Aging Phys Act 23: , 90–94. |
[22] | Williamson JD , Espeland M , Kritchevsky SB , Newman AB , King AC , Pahor M , Guralnik JM , Pruitt LA , Miller ME ; LIFE Study Investigators ((2009) ) Changes in cognitive function in a randomized trial of physical activity: Results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci 64: , 688–694. |
[23] | Ansai JH , Rebelatto JR ((2015) ) Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: A randomized controlled trial. Geriatr Gerontol Int 15: , 1127–1134. |
[24] | Venturelli M , Scarsini R , Schena F ((2011) ) Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen 26: , 381–388. |
[25] | Dillon K , Prapavessis H ((2020) ) REducing SEDENTary behavior among mild to moderate cognitively impaired assisted living residents: A pilot randomized controlled trial (RESEDENT Study). J Aging Phys Act 29: , 27–35. |
[26] | Bouaziz W , Schmitt E , Vogel T , Lefebvre F , Leprêtre PM , Kaltenbach G , Geny B , Lang PO ((2019) ) Effects of a short-term Interval Aerobic Training Programme with active Recovery bouts (IATP-R) on cognitive and mental health, functional performance and quality of life: A randomised controlled trial in sedentary seniors. Int J Clin Pract 73: , e13219. |
[27] | Tremblay MS , Colley RC , Saunders TJ , Healy GN , Owen N ((2010) ) Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab 35: , 725–740. |
[28] | Duvivier BM , Schaper NC , Bremers MA , van Crombrugge G , Menheere PP , Kars M , Savelberg HH ((2013) ) Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS One 8: , e55542. |
[29] | Panza F , D’Introno A , Colacicco AM , Capurso C , Pichichero G , Capurso SA , Capurso A , Solfrizzi V ((2006) ) Lipid metabolism in cognitive decline and dementia. Brain Res Rev 51: , 275–292. |
[30] | Alves BC , Silva TR , Spritzer PM ((2016) ) Sedentary lifestyle and high-carbohydrate intake are associated with low-grade chronic inflammation in post-menopause: A cross-sectional study. Rev Bras Ginecol Obstet 38: , 317–324. |
[31] | Dregan A , Chowienczyk P , Gulliford MC ((2015) ) Are inflammation and related therapy associated with all-cause dementia in a primary care population? J Alzheimers Dis 46: , 1039–1047. |
[32] | Leeuwis AE , Benedictus MR , Kuijer JPA , Binnewijzend MAA , Hooghiemstra AM , Verfaillie SCJ , Koene T , Scheltens P , Barkhof F , Prins ND , van der Flier WM ((2017) ) Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimers Dement 13: , 531–540. |
[33] | Wolters FJ , de Bruijn RF , Hofman A , Koudstaal PJ , Ikram MA ; Heart Brain Connection Collaborative Research Group ((2016) ) Cerebral vasoreactivity, apolipoprotein E, and the risk of dementia: A population-based study. Arterioscler Thromb Vasc Biol 36: , 204–210. |
[34] | Adlard PA , Perreau VM , Pop V , Cotman CW ((2005) ) Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci 25: , 4217–4221. |
[35] | Shen H , Tong L , Balazs R , Cotman CW ((2001) ) Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience 107: , 219–229. |
[36] | Fordyce DE , Farrar RP ((1991) ) Physical activity effects on hippocampal and parietal cortical cholinergic function and spatial learning in F344 rats. Behav Brain Res 43: , 115–123. |
[37] | Smith AD , Zigmond MJ ((2003) ) Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol 184: , 31–39. |
[38] | Zheng G , Xia R , Zhou W , Tao J , Chen L ((2016) ) Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 50: , 1443–1450. |
[39] | Song D , Yu DSF , Li PWC , Lei Y ((2018) ) The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int J Nurs Stud 79: , 155–164. |
[40] | Rao AK , Chou A , Bursley B , Smulofsky J , Jezequel J ((2014) ) Systematic review of the effects of exercise on activities of daily living in people with Alzheimer’s disease. Am J Occup Ther 68: , 50–56. |
[41] | Rolland Y , Pillard F , Klapouszczak A , Reynish E , Thomas D , Andrieu S , Rivière D , Vellas B ((2007) ) Exercise program for nursing home residents with Alzheimer’s disease: A 1-year randomized, controlled trial. J Am Geriatr Soc 55: , 158–165. |
[42] | Barnes DE , Santos-Modesitt W , Poelke G , Kramer AF , Castro C , Middleton LE , Yaffe K ((2013) ) The Mental Activity and eXercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med 173: , 797–804. |
[43] | , Doi T , Verghese J , Makizako H , Tsutsumimoto K , Hotta R , Nakakubo S , Suzuki T , Shimada H ((2017) ) Effects of cognitive leisure activity on cognition in mild cognitive impairment: Results of a randomized controlled trial. J Am Med Dir Assoc 18: , 686–691. |
[44] | Zhu Y , Wu H , Qi M , Wang S , Zhang Q , Zhou L , Wang S , Wang W , Wu T , Xiao M , Yang S , Chen H , Zhang L , Zhang KC , Ma J , Wang T ((2018) ) Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin Interv Aging 13: , 1691–1700. |
[45] | Hong H , Wang C , Rosner GL ((2021) ) Meta-analysis of rare adverse events in randomized clinical trials: Bayesian and frequentist methods. Clin Trials 18: , 3–16. |
[46] | Smith PJ , Blumenthal JA , Hoffman BM , Cooper H , Strauman TA , Welsh-Bohmer K , Browndyke JN , Sherwood A ((2010) ) Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med 72: , 239–252. |



