Including General Audiences in a Virtual Scientific Dementia Conference: Will They Get Anything From It?
Abstract
Background:
Study participants, patients, and care partners are key stakeholders in research and have asked for greater inclusion in the dissemination of scientific learning. However, the participation of general audiences in scientific conferences dedicated to Alzheimer’s disease and Alzheimer’s disease related dementias (AD/ADRD) is not widely supported or studied.
Objective:
Our objectives were to evaluate the interest, level of engagement, and impact of including general audiences in a virtual dementia conference.
Methods:
A diverse group of lay participants, identified via community-based health advocacy groups and research centers, were invited to attend the 2021 Alzheimer’s Association International Conference (AAIC), with optional small-group discussions. Participants received complimentary access to all scientific sessions and were supported via navigation tips, recommended sessions, and a glossary of frequently used terms and acronyms.
Results:
Lay participants demonstrated a high level of engagement, even among those that were research-naïve, attending virtual sessions for an average of 11.7 hours across the five days and recommending a variety of sessions to each other on topics extending from prevention of dementia to new therapies and care. Most participants said they would attend the conference again and rated the quality of interaction as high, while requesting more opportunities to engage directly with researchers.
Conclusion:
General audiences, in particular research participants, are advocating for greater participation in scientific conferences. This program can serve as a model to accomplish inclusion; thereby acknowledging their invaluable contribution to science.
INTRODUCTION
Scientific conferences are widely recognized as a forum for research and collaboration. Research results are shared, the implications of recent advances are discussed, collaborations are built, and new directions for research are discussed. Often overlooked, however, is the importance of including patients and care partners in these forums. Patients and care partners are valuable members of the research enterprise, and their participation in scientific conferences not only provides them with the information they are seeking, but also benefits science by broadening the perspective of medical research and even diversifying the dissemination of new information [1–3]. The inclusion of patient and care partners in scientific conferences has been practiced in some disease areas, such as AIDS [4], rheumatology, and breast cancer, resulting in recommendations to achieve meaningful inclusion for in-person events [5, 6]. Despite this, the practice of inclusion is far from standard, and the participation of patients and care partners in virtual conferences dedicated to Alzheimer’s disease and Alzheimer’s disease related dementias (AD/ADRD) has not been studied in any meaningful way.
Current AD/ADRD research spans the disease continuum; therefore, the inclusion of general audiences must be broad and include individuals with elevated risk, defined by either genetic or biomarker status [7], those with a diagnosis of mild cognitive impairment (MCI) or dementia, and individuals providing care and support for people with dementia; who are critical partners in AD/ADRD research. Equally important is understanding how to accomplish meaningful inclusion of general audiences from diverse racial, ethnic, geographic, and socioeconomic backgrounds.
In 2018 and again in 2019 two research participants asked their local researcher to help them attend a scientific conference. They attended in person, checking in occasionally with the researcher. Both participants valued the experience and reported that attending the conference had increased their commitment to research. We expanded in 2020 to a small group of research participants who attended the Virtual Alzheimer’s Association International Conference (AAIC) and learned that participants valued unrestricted access to virtual scientific conferences, when supported with small group discussions [8]. Participants expressed satisfaction with the experience, learned information they viewed as helpful, and reported feeling “encouraged” and “hopeful” by being able to witness the depth and breadth of research work underway. The 2020 program did not adequately represent the diverse populations impacted by AD/ADRD, leading us to ask whether we could broaden inclusivity by expanding our recruitment approach, and whether individuals with no research experience would also find benefit in attending a scientific conference.
The focus of this study was to evaluate the feasibility of engaging a general audience in attending a scientific conference, with the goal of making the public feel more engaged and included in research. To support this engagement, individuals working in research or other health professionals (e.g., study coordinators, community outreach staff, or health advocacy staff) hereafter “professional participants” met with attendees and answered questions and guided discussion groups. These professional participants were comparable to people who would regularly attend AAIC.
Our objectives for lay participants were to 1) recruit racial/ethnically diverse individuals, including those naïve to research, 2) assess the level of engagement in the virtual conference, 3) identify topics of interest, and 4) evaluate impact of attending the virtual conference. The objective for professional participants was to assess whether participating in this program, particularly the small group discussions, would be viewed as beneficial in broadening their perspectives.
METHODS
We began by identifying community-based health advocacy (including: Rememoirs, 2CEERIAS, Sanjeevani, Caregiver Action Network) and research organizations (Mt. Sinai Alzheimer’s Research Center and the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases at University of Texas Health San Antonio), selecting organizations with established relationships with individuals from groups not well-represented in AD/ADRD research. Lay participants were also invited from two advisory groups: the Advisory Group on Risk Evidence Education for Dementia (AGREED): Stakeholder Subcommittee [9] and the Alzheimer’s Clinical Trials Consortium (ACTC) Research Participant Advisory Board [10]. A few lay participants learned about the program through word-of-mouth from those who attended the prior year. Inclusion criteria were: Over 18 years of age, able to complete consent electronically, have an email address and internet access. The total number of individuals invited to participate were not tracked by the recruiting organizations.
Each organization was asked to identify a facilitator who would recruit local participants and host small-group discussions during the conference. The protocol, consent, and pre- and post-conference surveys were reviewed and approved by the University of Southern California Institutional Review Board (IRB). Where needed, partner organizations obtained IRB approval. The facilitators were trained on the program goals, given a quick-start guide and template email language to recruit participants, and a glossary of acronyms, abbreviations, and terms frequently used in AD/ADRD research (see Supplementary Table 1). On each day of the conference, a list of recommended sessions was provided to facilitators to share with the participants. Registration fees and technical support were provided by the Alzheimer’s Association and conference organizers. Each organization was asked to hold between one to five small-group discussions, during the same week as the live conference, with participants and share feedback with the program leads.
Data was collected by survey, emailed to participants both before and after the conference. Pre-conference surveys collected demographics, research experience, and topics of interest. Post-conference surveys collected estimates of time spent in conference and small group discussion and included open-ended questions for qualitative feedback. Surveys were reviewed with some participant feedback shared in our results. Where feasible and allowed by IRB, small group discussions were recorded and transcribed, allowing the authors to compile additional feedback from participants. Sessions recommended by participants were compiled from both post-conference surveys, notes from small group discussions, and emails from participants. Feedback was compiled and shared with the conference organizers to take into consideration for promoting inclusivity in future conferences and virtual programs.
The Research Attitude Questionnaire (RAQ) is a seven-question survey validated and used to assess community-dwelling volunteers’ attitudes toward research [11], to understand barriers to research participation in poor urban minority populations [12, 13], to estimate the likelihood of enrolling in a clinical trial [14], and to predict research drop-outs [15]. The RAQ utilizes a five-point Likert scale (strongly disagree to strongly agree) to collect responses (range: 7–35). Higher scores represent a more favorable attitude toward research.
The 2021 Alzheimer’s Association International Conference (AAIC) was held as a hybrid conference with on-demand and live sessions between July 26–30. Registration for participants and care partners was provided by the Alzheimer’s Association free of charge. Over 11,900 people from over 110 countries registered to attend AAIC 2021, either in person in Denver or remotely around the world. The conference included hybrid and virtual scientific sessions reporting the latest research discoveries, spanning basic science, biomarkers, clinical manifestations, drug development, public health, and dementia care. AAIC 2021 featured 300 + in-person posters and 2,500 + posters on the virtual platform. The virtual conference was smart-phone accessible and required the use of an internet browser to join; Chrome and Firefox were recommended. All sessions were recorded and available for an additional 30 days.
RESULTS
Participant characteristics
Thirty-one lay participants registered for the conference and completed a preconference survey, with one participant declining to provide demographics. The participants’ ages ranged from 44 to 77 (mean = 60.9, SD = 10.1) (Table 1). All participants had at least some college education, 80.6% were women, 54.8% self-identified their race as White, 22.5% as Asian, and 12.9% as Black. Among the lay participants, 12.9% self-identified as Latino/Hispanic ethnicity. Two participants (6.5%) identified as Lesbian, Gay, Bisexual, Transgender or Queer (LGBTQ), a majority (64.5%) had a parent or sibling diagnosed with early AD or dementia, 12.9% had been diagnosed with AD or dementia themselves, and 61.2% were current or former care partners for someone with AD or dementia. In total, 13 (41.9%) lay participants had taken part in a research study or clinical trial.
Table 1
Demographics of Participants
| Demographics | Lay Participants (proportion) | Professional Participants (proportion) |
| N = 41 | 31 (73.1%) | 10 (24.4%) |
| Mean Age (SD) | 60.9 (SD = 10.1) | 38.0 (SD = 14.1) |
| Women | 25 (80.6%) | 10 (100.0%) |
| College or advanced degree | 31 (100.0%) | 10 (100.0%) |
| Retired/not working | 16 (51.6%) | 2 (20.0%) |
| Self-identified Race | ||
| Asian | 7 (22.5%) | 3 (30.0%) |
| Black | 4 (12.9%) | 2 (20.0%) |
| White | 17 (54.8%) | 5 (50.0%) |
| Prefer not to answer | 2 (6.5%) | 0 (0.0%) |
| Self-identified Ethnicity | ||
| Latino/Hispanic | 4 (12.9%) | 1 (10.0%) |
| African | 3 (9.7%) | 1 (10.0%) |
| South Asian | 4 (12.9%) | 2 (20.0%) |
| East Asian | 2 (6.5%) | 1 (10.0%) |
| Caucasian | 14 (45.2%) | 4 (40.0%) |
| Prefer not to answer | 2 (6.5%) | 1 (10.0%) |
| Self-identify as Lesbian, Gay, Bisexual, Transgender or Queer (LGBTQ) | 2 (6.5%) | 0 (0.0%) |
| Other Characteristics | ||
| Family history of AD/dementia | 20 (64.5%) | 2 (20.0%) |
| Current/former dementia care partner | 19 (61.2%) | 1 (10.0%) |
| Dementia diagnosis | 4 (12.9%) | 0 (0.0%) |
| Previously taken part in research | 13 (45.1%) | 5 (50.0%) |
The average total RAQ for lay participants was 27.88, in groups defined by research experience, the average RAQ score was 29.71 for participants with research experience and 26.94 for research-naïve. Across all lay participants, the two items with the most neutral or negative responses (33%) were: “If I volunteer for medical research, I know my personal information will be kept private and confidential” and “Medical research will find cures for many major diseases during my lifetime.”
Professional participants that completed the pre-conference survey (N = 10) were all college educated women. Professional participants were on average younger than the lay participants (mean = 38.0, SD = 14.1) (Table 1). Professional participants self-reported their race as Asian (30.0%) Black/African American (20.0%), and White (50.0%). One professional participant (10.0%) self-reported ethnicity as Latino/Hispanic. 20.0% of professional participants had a family history of AD or dementia, with one either providing care for a person with dementia. All professional participants work in research except for one that is a clinical counselor and one working in health advocacy and outreach.
Level of engagement
Level of engagement was collected by the post-conference surveys, and from notes from small-group discussions. Fourteen lay participants attended day one, thirteen attended day two, thirteen attended day three, fourteen attended day four, and eight attended day five. Just under half of the participants attended virtual conference sessions after the end of the live event. One organization offered five small group discussions, and three organizations offered two small group discussions during the conference, with 29 participants attending at least one of the small-group discussions.
Lay participants spent an average of 11.7 hours on the virtual conference platform. Seven participants spent between 1–5 hours, three between 6–10 hours, five between 11–15 hours, and four spent between 20–40 hours. Participants with research experience spent a longer time on average (13.9 hours) compared to research naïve participants (9.1 hours). The participants appreciated full access to the conference program, being able to select topics of interest, without the burden of traveling or taking time off work, which was only possible with a hybrid or virtual conference. Many participants highlighted a key advantage of virtual conference— being able to pause, rewind, or watch a session for a second time. Despite the convenience of a virtual conference, eight lay participants stated they wished they had more time to attend the conference or small groups, but were unable to do so due to caregiving or work responsibilities. A few lay participants saw conference attendance as an important benefit to being a research participant and providing information they need to advocate participation to others: “As a clinical trial participant, I don’t expect to understand everything, but as an advocate who supports people often dealing with misconceptions about both cognitive impairments and the role of researchers, this is invaluable as a means to supporting them and recommending trust in ‘mainstream’ science.”
After the conference, 78% of lay participants said they would attend the conference again, with a higher rate of research naïve participants (70%) either disagreeing or neutral. When asked to explain, participants described dissatisfaction with the virtual platform, including searchability and other technical issues, with a few expressing dissatisfaction with the content and style of presentations.
The majority of lay participants (66%) rated the quality of interaction with researchers as excellent or good. Comments from those rating quality of interaction as low included limitations of a virtual platform to support interactions, as well as not feeling comfortable speaking up in the scientific venue, and recommending researchers be invited to join small group discussions instead.
Topics of interest
Prior to the conference, lay participants expressed interest in sessions across a broad set of topics (see Fig. 1): 78.6% indicated an interest in prevention of dementia, 66.7% in clinical trials, 59% in caregiving, 54.8% in brain imaging, 52.4% in genetic risks, 50% in blood-based biomarkers, and 50% in underrepresented populations. Other topics of interest included Lewy body dementia, frontotemporal dementia, vascular dementia, medications being studied, markers of presymptomatic disease, and legal protections for the elderly. Sessions recommended by participants after attending the conference are listed in Table 2. Because participants were able to search the program for topics of interest, many of the sessions they attended were related to topics they expressed interest in prior to the conference. However, participants also recommended sessions for each other at the small group discussions where they found the content interesting and especially when the presenter was able to ‘tell the story’. Conference sessions that were recommended in small group discussions ranged from a study on dance therapy, to presentations of new amyloid-removing drugs, and the development of innovative cognitive tests that don’t require individuals to be literate to diagnosedementia.
Fig. 1
Lay Participants topics of interest prior to virtual conference.
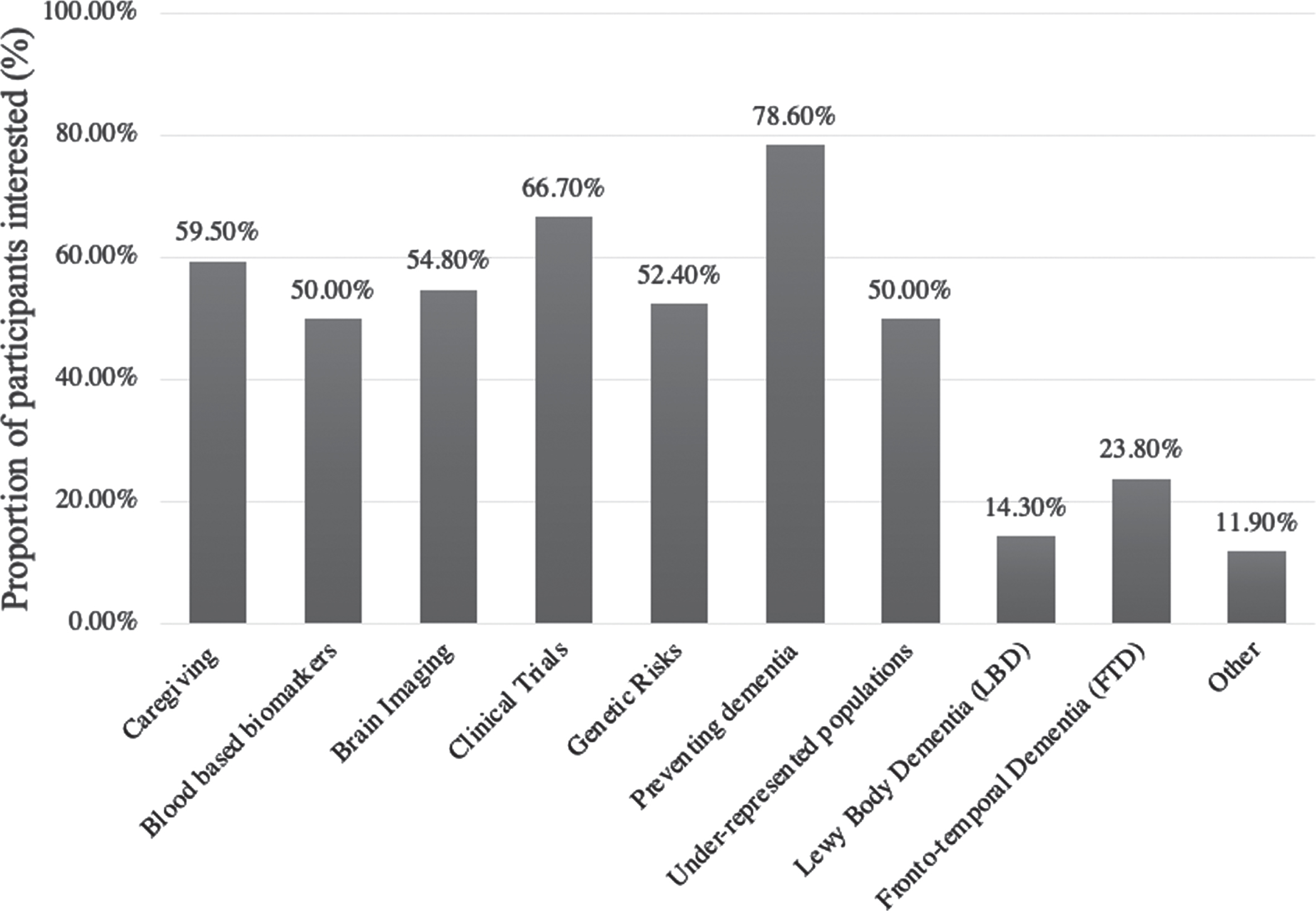
Table 2
Dementia conference sessions recommended by lay participants
| Aligning Towards Action in Social Determinants Research on Brain Health |
| Association between Birth in the Stroke Belt and Late-life Cognition with Assessment of Socioeconomic and Cardiovascular Risk Factors (START study) |
| Blood-based Biomarkers |
| Clinical Care Pathway for Alzheimer’s Disease |
| Clinical Trials (results and designs for new studies). Specifically, Aducanumab and Donanemab, BACE Inhibitors. |
| Cognitive Reserve (impact on lifespan, role in prevention) |
| COVID-19 and Dementia |
| Dancing to Slow Cognitive Decline |
| Decentralized Trials and Virtual Assessments |
| Dementia Care Research: Person-Centered Care and Characteristics Of Care |
| Dementia Care Practice (Supporting Communities) |
| Dementia Nomenclature Initiative (DeNoMi) |
| Diagnostic Criteria |
| Disclosure of AD Biomarker and Neuroimaging Results in Symptomatic Populations |
| Disparities in Dementia Care (globally, racial and ethnic excluded groups) |
| Drivers and Barriers to participation of Black and Hispanic groups in clinical trials |
| Early Diagnosis |
| Epidemiology: Cardiovascular Risk |
| Evaluation of Dementia in Low Educated and Indigenous Populations |
| First Manifestations of AD Before MCI |
| Frontotemporal Dementia (all sessions) |
| Global Perspectives (risk factors, access to care) |
| Impact of COVID-19 Pandemic on Reserve and Resilience |
| Ketones and Improved Cognition in MCI |
| Links to ApoE and White Matter Energetics |
| Longitudinal Cascades of Alzheimer’s Biomarkers in Cognitively Normal Adults |
| Metabolic Syndrome in Relation to Cognitive Function and White Matter Integrity in Midlife |
| Neuroimaging: Imaging Linking with Genetics and Racial Differences |
| Nutrition For Dementia Prevention |
| Patient Engagement and Practice Considerations |
| Population Diversity in Clinical Trials |
| Prevention of Dementia (precision nutrition, physical activity, cognitive reserve, healthy lifestyle) |
| Psychosocial Factors &Environmental Design: environments, communities, &technology |
| Sex and Gender Differences |
| Tau Therapies and Tau in Blood, CSF as markers of disease |
| Therapeutic Approaches Targeting Innate Immunity in AD |
Impact of attending a virtual scientific conference
Attending this virtual scientific conference opened neurological research to communities and energized participants to share this information with others. One lay participant who cares for her mother with dementia and works as a health advocate with African American communities shared, “I didn’t know this side of Alzheimer’s and dementia world existed...Black and Brown communities that I engage with don’t know much about this either. This got me really excited to bring this into the masses! They could benefit from this information.” (Caregiver from Houston Texas, age 44).
Two clinical trial participants expressed that inclusion in the conference and small group discussions increased their satisfaction in research participation: “It was an honor to have researchers show interest in the opinions of trial participants. I came away feeling that my participation in a trial and my feedback were genuinely appreciated and valued.” (Clinical Trial Participant from Illinois –age 75) “As a participant in a trial, I appreciated the opportunity to hear the science behind trials and to hear the future for finding solutions to cognitive impairments . . . Being a cog in a wheel is one component, but understanding how the cog works and where it is going is enlightenment.” (Clinical Trial Participant from New England –age 69)
About half of the participants asked for more opportunities to interact directly with researchers, due to not feeling comfortable asking questions in the larger conference setting. “I did not participate in any interactions or group discussions with the researchers because I didn’t feel I was in the same academic language level.” (Caregiver from South Texas –age 65) “It would be nice to have more researchers in the small-group discussions to facilitate further connection with the research participants.” (Former research participant from Southern California –age 60).
One participant with early-stage AD recommended small groups be less than ten people, to ensure balance, or to hold dedicated small groups for individuals living with dementia. “I think I might have been the only person on the call living with dementia . . . It wasn’t a safe place (for me) to ask my questions. I left because the discussion made me feel worse about myself and my diagnosis.” (Research Participant from Upstate New York - age 60).
Having access to all sessions promoted lay participants to explore topics they are interested in and offered an opportunity to hear about progress in research less widely discussed outside of scientific arenas: “I learned new things every day of the conference. I am also encouraged by research on the social and lifestyle factors that influence AD outcomes. I feel that it is important to have interventions that are available to everyone and not just the privileged few.” (Clinical Trial Participant from New England –age 69) “I was unaware of all the studies and research that are being conducted around the world. Participating in this year’s conference was a great experience. I hope this becomes open to Caregivers to attend.” (Caregiver from South Texas –age 65).
Some lay participants expressed concern with the conference’s focus on AD: “It would be good to have assessment tools that are not so Alzheimer’s heavy so that FTD and Lewy body disease would not be screened out.” (Dementia advocate –age 24) “All in all, I was very impressed by the progress in the field of Alzheimer’s disease, and disappointed in the paucity of information on less common related dementias, such as Progressive Supranuclear Palsy, Corticobasal Degeneration, and Lewy body dementias.” (Research participant and Lewy body Dementia advocate from Minnesota - aged 63).
Prompted by the conference sessions, participants used the small group discussion to relate where their lived experience either countered or echoed the results of research. These experiences included challenges in obtaining a dementia diagnose, providing care, and experiences in learning risk information as part of a research study.
Impact on professional participants
Each of the six professional participants who responded to the post-conference survey shared that the small group discussions allowed them to learn about lay participant’s lived experiences. Having a better understanding of what information lay participants are seeking can help researchers develop better outreach programs that meet these interests: “I learned that participants are eager to know more about current science and research advancements in the areas of dementia and are eager to know specific research that applies to their own lives (i.e., caregiving, prevention, treatment).”
One researcher who works in drug studies was interested to learn that lay participants are also interested in other research: “Interest seemed to gravitate towards practical, lifestyle changes that could assist in preventing onset or worsening of cognitive decline.”
Another research assistant shared how she was impacted by participants sharing their lived experience: “What was most memorable for me was listening to stories about disclosure. When people were told about their amyloid burden or results/data about their brain health by their researchers/physicians, I did not realize how much of an emotional toll there would be for the participant because there is generally little conversation about this topic outside of this small community.”
One research professional, after hearing caregivers share their daily challenges, was able to better understand barriers people are dealing with that might prevent them taking part in research: “There is neither a cure nor enough financial help for patients who do not have the resources to even get through daily life, let alone sign up for clinical trials. Providing some sort of immediate support for these people may increase diversity of participation.”
DISCUSSION
We were able to identify and include in this virtual scientific conference a group of lay participants with some racial and ethnic diversity, as well as individuals from sexual and gender minoritized groups. A majority of the lay participants were naïve to research. The lay participants that took part represented the broad experience of disease, including care partners, those with elevated risk for disease, with two individuals living with a diagnosis of MCI or dementia taking part. However, men and individuals with secondary education were not adequately represented in our study. We do not have demographics or metrics of those invited to join versus those that agreed to join, this imbalance may be due to the makeup of the organizations used in recruitment. This limits our ability to translate results to all general audiences that may be interested in participating in virtualconferences.
One key metric demonstrating the levels of interest was the high rates (50% or greater) of participants who expressed interest in every topical area offered at the conference. We were unable to collect metrics on conference session attendance, so our learnings are limited to what was reported by participants. Based on these reports, roughly a third of participants logged on during each day of the five-day conference and attended a staggering variety of sessions. Eight participants expressed a desire to spend more time at the conference, which points to the high levels of interest, and flexibility offered with a virtual conference, especially for those that are working or providing care.
Despite some lay participants expressing initial concerns about their ability to understand the content of the conference, participants expressed feeling “encouraged” after attending the conference, emphasized the progress being made, the quantity of work being done, and new learnings in social and prevention of disease. A common recommendation was for small group discussions to include a researcher, to allow for questions to be asked in a safe space. The small group discussions were successful in enhancing the conference experience for both lay and professional participants, providing a platform to triangulate, clarify individual understandings and gain deeper knowledge. Lay participants naïve to research expressed less satisfaction with the conference than those that had previously taken part in research. However, those same research naïve participants gave positive feedback on the small groups and hearing directly from research participants and research professionals. Training research participants as ‘ambassadors’ to aid in the dissemination of new scientific learnings and build trust in under-represented communities should be explored in future studies.
Feedback from the professional participants highlight how these exchanges can help researchers understand the lived experiences of people with a diagnosis and care partners. However, more work is needed to understand whether greater understanding will lead to improvement in outreach approaches or study design.
One key limitation for virtual conferences is that in order to attend, participants had to have free time to attend the sessions, excluding those who are working or providing care. Digital conferences also require access to the internet, with high bandwidth requirements for video participation; although the percentage of older adults in the United States who access the internet is increasing, internet access can still be a substantial barrier [16]. Finally, while some aspects of this design could translate to supporting general audiences for an in-person conference, there would be a distinct set of accessibility and inclusion issues, like financial or physical barriers to travel, and navigating the physical setting of a scientific conference (meeting rooms, audio and visual accommodations). General audiences that work and provide care will also have challenges in attending in person conferences, which should be considered.
Conclusion
This program has demonstrated that virtual conferences offer unique opportunities for inclusion of general audiences, when supported by small group discussions. General audiences expressed an eagerness to learn the latest science, and to hear directly from the scientists doing the work, even when information is not being communicated in a ‘lay friendly’ manner. Lay participants utilized the small group discussions to share recommended sessions with each other, to discuss their learnings with each other, and connect with other individuals also interested in research. Learning was enhanced when researchers were available to answer questions, allowing for deeper understanding of new concepts or information. In the small groups, participants naïve to research embraced the opportunity to hear from people like them about what it’s like to take part in a study. Research professionals also benefited from the exchanges, learning more about lived experience of people that take part in research as well as those living with a diagnosis and providing care and support. General audiences, in particular research participants, are advocating for greater participation in scientific conferences. This program can serve as a model to accomplish inclusion; thereby acknowledging their invaluable contribution to science.
ACKNOWLEDGMENTS
Ms. Walter, Ms. Shaffer, and Ms. Ziolkowski receive support from the Alzheimer’s Clinical Trials Consortium (ACTC), which is funded by a cooperative grant from the National Institute on Aging (NIA), National Institutes of Health (NIH) (U24AG057437). Dr. Aggarwal receives support from the following NIA grants (P30AG010161 (Bennett), P30AG072975 (Bennett).
We would like to express our gratitude to all ACTC Research Participant Advisory Board members and AGREED Leadership and members of the AGREEDementia Stakeholder Subcommittee for their committed involvement, guidance, and expertise throughout each phase of this project. We also thank the staff and leadership of the organizations that helped identify participants: the Glenn Biggs Institute at UT Health San Antonio, Rush Alzheimer’s Disease Center/Rush University Medical Center, Mount Sinai ADRC, 2CEERIAS, Caregivers Action Network and Sanjeevani.
We are grateful for ACTC and the Alzheimer’s Association leadership for allowing us to partner with them to develop equitable and innovative ways to engage our communities in AD/ADRD research to improve the lives of persons living with dementia, their families, and the communities in which they live. USC’s instance of REDCap was utilized for survey collection (UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5681r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215681.
REFERENCES
[1] | Duffett L ((2017) ) Patient engagement: What partnering with patient in research is all about. Thromb Res 150: , 113–120. |
[2] | de Wit MP , Abma TA , Koelewijn-van Loon MS , Collins S , Kirwan J ((2014) ) What has been the effect on trial outcome assessments of a decade of patient participation in OMERACT? J Rheumatol 41: , 177–184. |
[3] | Utengen A , Rouholiman D , Gamble JG , Grajales FJ , III , Pradhan N , Staley AC , Bernstein L , Young SD , Clauson KA , Chu LF ((2017) ) Patient participation at health care conferences: engaged patients increase information flow, expand propagation, and deepen engagement in the conversation of tweets compared to physicians or researchers. J Med Internet Res 19: , e280. |
[4] | Rogstad KE , James NJ , Bowman CA ((1994) ) Physicians’ perceived value of international AIDS conferences and attitudes towards patient attendance. Genitourin Med 70: , 336–338. |
[5] | Chu LF , Utengen A , Kadry B , Kucharski SE , Campos H , Crockett J , Dawson N , Clauson KA ((2016) ) “Nothing about us without us”-patient partnership in medical conferences. BMJ 354: , i3883. |
[6] | Frank L , Shubeck E , Schicker M , Webb T , Maslow K , Gitlin L , Hummel CH , Kaplan EK , LeBlanc B , Marquez M , Nicholson B , O’Brien G , Phillips L , Van Buren B , Epstein-Lubow G ((2020) ) Contributions ofpersons living with dementia to scientific research meetings. Results from the National Research Summit on Care, Services, and Supports for Persons With Dementia and Their Caregivers. Am J Geriatr Psychiatry 28: , 421–430. |
[7] | Sperling RA , Jack CR Jr., , Aisen PS ((2011) ) Testing the right target and right drug at the right stage. Sci Transl Med 3: , 111cm133. |
[8] | Walter S , Wheaton B , Huling Hummel C , Tyrone J , Ziolkowski J , Shaffer E , Aggarwal NT ((2021) ) Can virtual scientific conferences facilitate two-way learning between dementia researchers and participants? . J Prev Alzheimers Dis 8: , 387–388. |
[9] | Rosen AC , Alber J , Al-Janabi OM , Arias JJ , Bardach SH , Blacker D , Denny SS , Dorociak K , Edwards DF , Erickson CM , Fargo K , Frank L , Gleason CE , Goldman J , Green RC , Grill JD , Heidebrink JL , Henderson VW , Hummel CH , Jwa AS , Karlawish J , Lah JJ , Langbaum JB , Langston AH , Largent EA , Lee AKW , Lingler J , Milne R , Moore RC , Mozersky J , Nosheny RL , Parker MW , Roberts JS , Rogalski EJ , Rumbaugh M , Saykin AJ , Shapiro R , Stites SD , Tyrone J , Vogel B , Walter S , Wang LS , Wijsman E , Aggarwal NT ((2020) ) The formation of the advisory group on risk evaluation education for dementia. Alzheimers Dement 16: (Supp 10), e045562. |
[10] | Walter S , Shaffer E , Ziolkowski J , Chan N , Hummel RCH , Heyde R , Meserve N , Childs N , Aisen P , ((2020) ) Preferences for Disclosure of Biomarker and Genetic Results in Alzheimer’s Research: Feedback from a Participant Advisory Board. J Prev Alzheimers Dis 7: , S55–S119. |
[11] | Rubright JD , Cary MS , Karlawish JH , Kim SY ((2011) ) Measuring howpeople view biomedical research: Reliability and validity analysisof the Research Attitudes Questionnaire. J Empir Res HumRes Ethics 6: , 63–68. |
[12] | Neugroschl J , Sewell M , De La Fuente A , Umpierre M , Luo X , Sano M ((2016) ) Attitudes and perceptions of research in aging and dementiain an urban minority population. J Alzheimers Dis 53: , 69–72. |
[13] | Zhou Y , Elashoff D , Kremen S , Teng E , Karlawish J , Grill JD ((2017) ) African Americans are less likely to enroll in preclinical Alzheimer’s disease clinical trials. Alzheimers Dement (N Y) 3: , 57–64. |
[14] | Jefferson AL , Lambe S , Chaisson C , Palmisano J , Horvath KJ , Karlawish J ((2011) ) Clinical research participation among aging adults enrolled in an Alzheimer’s Disease Center research registry. J Alzheimers Dis 23: , 443–452. |
[15] | Stites SD , Turner RS , Gill J , Gurian A , Karlawish J , Grill JD , Alzheimer’s Disease Cooperative Study ((2021) ) Research Attitudes Questionnaire scores predict Alzheimer’s disease clinical trial dropout. Clin Trials 18: , 237–244. |
[16] | Anderson M , Mobile technology and home broadband 2019. Pew Research Study 2019. https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/, Accessed May 14, 2020. |




