The Impact of Agitation in Dementia on Caregivers: A Real-World Survey
Abstract
Background:
Dementia patients frequently depend on caregivers. Agitation is a common behavioral dementia symptom particularly burdensome to patients and caregivers.
Objective:
To assess the association of agitation severity with non-professional caregiver hours, burden, health status, and productivity. Secondarily, to assess the association of agitation severity with these outcomes for patients receiving remote (not living with the patient) and proximate (living with the patient) caregiving.
Methods:
A retrospective analysis of physician and non-professional caregiver-reported data from a US point-in-time survey. Patients were aged ≥50 years, with early cognitive impairment or dementia. Regression analyses compared outcomes by agitation severity; covariates included age, sex, and clinical characteristics.
Results:
Data were included for 1,349 patients (non-agitated n = 656, agitated n = 693; no care n = 305, remote care n = 248, proximate care n = 691; unknown care n = 105). Greater agitation was significantly associated (p < 0.05) in all caregivers with increasing: Zarit Burden Interview (ZBI) Total Caregiver Burden, Personal Strain, Role Strain, and Guilt; Work Productivity and Activity Index (WPAI) presenteeism, overall work impairment, and total activity impairment. Higher ZBI Total Caregiver Burden, Personal Strain, and Role Strain were associated with greater agitation in proximate caregivers and higher ZBI Guilt associated with greater agitation in remote caregivers (p < 0.05). Higher WPAI presenteeism and total activity impairment were associated (p < 0.05) with greater agitation in proximate caregivers. Caregiving hours increased with increasing agitation for proximate caregiving (p = 0.001).
Conclusion:
Greater agitation severity was associated with higher caregiver burden and lower productivity, with higher indirect costs a likely outcome of agitation.
INTRODUCTION
In patients with dementia, cognitive, functional, and behavioral impairments lead to reduced patient autonomy. These symptoms often lead to dependence on non-professional or professional caregivers [1, 2]. According to analysis of data from the 2011 United States (US) National Health and Aging Trends Study, around 80% of patients with dementia required help of non-professional caregivers [3].
Agitation is a behavioral symptom of dementia. Behavioral or neuropsychiatric symptoms have been reported to be particularly burdensome to caregivers of dementia patients [4, 5], with disruptive behaviors such as agitation, aggression, and disinhibition presenting the greatest burden [6]. There is no widespread agreement on the elements involved in aggression, but the International Psychogeriatric Association (IPA) has published a provisional consensus on a definition of agitation in cognitive disorders, which includes a specification of ‘behaviors that cause excess disability’ [7]. Likely due at least in part to the uncertainty around what constitutes agitation, wide ranges in the prevalence of this symptom in dementia have been reported in the literature. A systematic review of studies of behavioral disturbances in dementia focused on agitation and included studies based on the IPA consensus definition; it included studies that did not explicitly use the term agitation but reported on behaviors included in the IPA definition [8]. This review reported the prevalence of agitation to range from 5% to 88% across all studies, with 21 (38%) studies reporting a prevalence of agitation of ≥50% [8].
The American Psychiatric Association Practice Guidelines advocate a personalized approach to the treatment of agitation in dementia, with antipsychotic drugs recommended when agitation symptoms are severe; however, there is no US Food and Drug Ad-ministration (FDA)-approved pharmacologic treatment for agitation in dementia [9].
The primary objective of this survey was to assess the association of agitation severity with the number of non-professional caregiver hours and non-professional caregiver burden, health status, and productivity for all patients, regardless of whether this caregiving was defined as proximate care (non-professional caregiver living with the patient) or remote care (non-professional caregiver not living with the patient). A secondary objective was to assess the association of agitation severity with these outcomes separately for patients receiving either proximate care or remote care.
MATERIALS AND METHODS
Study design
This was a retrospective analysis of physician and non-professional caregiver-reported data from a US point-in-time survey.
Data source
Data were drawn from the Adelphi Dementia Dis-ease Specific Programme (DSPtrademark) conducted in 2015/2016 in the US across the Midwest, North East, West, and South regions. DSPs are large, multinational point-in-time surveys of physicians and their consulting patients conducted in real-world clinical practice, which describe current disease management, disease-burden impact, and associated treatment effects (both clinical and physician-perceived). Full details of the DSP methodology [10], and methods specific to the Dementia DSP [11], have been published previously.
Study participants and eligibility criteria
A geographically representative sample of physicians from across the US were recruited to participate in the DSP by field-based interviewers. Physicians were eligible to participate in this survey if they reported that they were personally responsible for treatment decisions and management of patients with cognitive impairment (CI)/dementia.
Patients were eligible for inclusion if they were aged ≥50 years, had a physician-confirmed diagnosis of early CI, Alzheimer’s disease (AD), or mixed dementia (vascular/AD), and visited the physician between November 2015 and April 2016. Patients with dementia of purely vascular origin or due to environmental factors (e.g., traumatic head injury, alcoholism) were excluded.
Study procedures
Physicians were instructed to complete a patient record form for the next nine consecutive patients who visited the physician for routine care. Completion of the form was undertaken through consultation of existing patient clinical records, as well as the assessment of the patient at the time the form was completed.
Non-professional caregivers accompanying pati-ents were also invited to voluntarily complete a self-reported record form and provided their informed consent to participate.
It should be noted that the survey was designed to facilitate understanding of real-world clinical practice, and thus physicians could only report on data they had to hand at the time of the consultation. Therefore, the data represent the evidence they had when making any clinical treatment and other management decisions at that consultation. No tests, treatments, or investigations were performed as part of this survey. Physician participation was financially incentivized, with reimbursement upon survey completion. Patients and caregivers were not compensated for participation.
Measures
Physician- and caregiver-reported data
The physician-reported patient record form contained detailed questions on CI/dementia symptoms, presence of a professional or non-professional caregiver, professional caregiver hours/week, and relationship of non-professional caregiver to the patient. The caregiver-reported form recorded caregiver demographics, relationship to the patient being cared for, caregiver burden (via the Zarit Burden Interview [ZBI]), health status (via the EQ-5D-3L), and productivity (via the Work Productivity and Activity Impairment questionnaire [WPAI]).
Caregiver-reported questionnaires
The ZBI assesses caregiver perceptions of burden including the physical, psychological, social, and emotional impact their caring role had on their life and work [12, 13]. It comprises a 22-item subjectively worded self-report inventory, and non-professional caregivers were asked to respond to each item using a 5-point Likert scale ranging from 0 (never) to 4 (nearly always). The scores for the 22 items of the ZBI were summed to give a total score ranging from 0 to 88, representing Total Caregiver Burden, with higher scores indicating a higher level of perceived burden. Scores for two subscales (Personal Strain— reflecting psychological distress, and Role Strain— the general impact on the caregiver’s life) were calculated from ten and nine items of the ZBI, respectively, and a further factor (Guilt) from two items [13].
The EQ-5D-3L is a valid and reliable measure of caregiver health-related quality of life (HRQoL) [14, 15]. It assesses self-reported health status in five dimensions (Mobility, Self-care, Usual Activities, Pain/Discomfort, and Anxiety/Depression), via three response levels (no problems, some problems, extreme problems/unable to do). Application of country-specific scoring algorithms to the scores of the five domains results in a single health utility index, with a value of 1 indicating perfect health, a value of 0 indicating death, and a value of < 0 indicating that from a societal perspective being in this state is regarded as worse than death [16, 17]. The EQ-5D-3L also includes a 20 cm visual analog scale (VAS) on which the respondent rates their perceived health from 0 (the worst imaginable health) to 100 (the best imaginable health).
The WPAI questionnaire measured the impact of caregiving on non-professional caregivers’ productivity and activity during the past 7 days [18]. It includes six questions concerning employment status, hours worked, hours of work missed due to caregiving responsibilities, hours of work missed for other reasons, and the degree to which caregiving responsibilities affected productivity at work and regular daily activities (both on a scale of 0 = no effect to 10 = completely prevented). Absenteeism (calculated as: hours of work missed due to caregiving responsibilities/(hours worked + hours of work missed for any reason)×100)), presenteeism (calculated as: degree to which caregiving responsibilities affected productivity at work×10), overall work impairment (calculated as sum of absenteeism and presenteeism, and total activity impairment (calculated as: degree to which caregiving responsibilities affected regular daily activities×10) were each reported as percentage impairment.
Derivation of agitation score
We derived a means to calculate an agitation score that reflected elements of the IPA consensus on the definition of agitation. The IPA definition can be applied in epidemiologic, non-interventional clinical, pharmacologic, non-pharmacologic interventional, and neurobiological studies [7]. An overall agitation score was derived for each patient based on seven agitation-related physician-reported symptoms: aggression, disinhibition, wandering, agitation, irritability/lability, aberrant motor behavior, social interaction problems. Physicians rated each symptom as not present (0), mild (1), moderate (2), or severe (3), and scores were summed to provide a score between 0 (no symptoms) and 21 (all severe symptoms).
Statistical analysis plan
Descriptive and bivariate analyses
Patient and non-professional caregiver demogra-phic data and clinical characteristics, and the relationship of caregivers to the patient they cared for (as reported by both physicians and caregivers) were assessed descriptively. For numeric variables, sample size, mean, and range were presented. For categorical variables, sample size and the number and percent in each category were presented. Variables were compared between agitated and non-agitated patients using t-tests for numerical variables and Fisher’s exact/chi-squared tests for categorical variables.
Multivariate analyses
Regression analysis was conducted to model the relationship of agitation score with each outcome (non-professional caregiver hours, burden, health status, and productivity), with data from all caregivers, regardless of whether caregiving was remote or proximate. Regression analyses were repeated a further two times, once including only data from caregivers providing remote care and once including only caregivers providing proximate care. Covariates adjusted for in all regression analyses were patient demographics (age and sex) and clinical characteristics (time since diagnosis, current Mini-Mental State Examination (MMSE) score).
Negative binomial regression was used to model the relationship of agitation score with outcomes measured as non-negative counts and all other regressions were linear. Details of the type of regression analysis used for different outcomes can be found in Supplementary Table 1. Standard errors in regressions were adjusted to allow for intragroup correlation within reporting physician. Results from negative binomial regressions used to analyze caregiving hours are presented as incidence rate ratio (95% confidence interval) (IRR [95% CI]). Negative binomial regression was considered appropriate for the analysis of caregiver hours as data cannot include a negative number. Pseudo r-squared values (typically McFadden’s r-square) were produced indicating how well the models fit. Other analyses focused on the differences between agitation severity levels for a number of caregiver-reported endpoints, which are considered continuous outcomes; results are therefore reported as regression coefficients (95% CI).
Linear regression coefficients significantly different from 0 indicate the predicted outcome is significantly different for different agitation scores. Coefficients are additive on the predicted outcome, so a coefficient greater than 0 implies a higher predicted outcome, and a coefficient less than 0 implies a lower predicted outcome. Negative binomial regression IRRs significantly different from 1 indicate the predicted number of events is significantly different for different agitation scores. IRRs are multiplicative on the number of events, so an IRR greater than 1 implies the more events, and an IRR less than 1 implies fewer events.
Missing data were not imputed; therefore, sample size could vary from variable to variable and is reported separately for each analysis. Data were considered missing if they were not available in the patient clinical records or responses were missing from caregivers.
All analyses were conducted in Stata v16.0 [19].
Ethics
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association (EphMRA) Code of Conduct [20]. Research relating to market or consumer behavior, involving healthcare professionals, patients, and/or caregivers does not require Clinical Research Ethics Committee or Independent Review Board approval. Using a check box, physicians and caregivers of patients provided informed consent for use of their anonymized and confidential data. Data were collected in such a way that patients, their caregivers and treating physicians could not be identified directly; all data were aggregated and de-identified before receipt. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 [21] and Health Information Technology for Economic and Clinical Health Act legislation [22].
RESULTS
Participants
The 2015/16 Adelphi Dementia DSP collected data from 150 physicians in the US; 59 (39.3%) were neurologists, 50 (33.3%) primary care physicians, 20 (13.3%) geriatricians, 20 (13.3%) psychiatrists, and 1 (0.7%) was a psycho-geriatrician.
Physicians provided data for 1,349 patients, ranging in age from 50 to 90 years, with a slightly higher proportion of females than males (Table 1). A total of 247 non-professional caregivers completed a self-reported record form.
Table 1
Patient demographics and clinical characteristics
| Total (n = 1,349) | Non-agitated (n = 656) | Agitated (n = 693) | p | Total (n = 1,244) | No Care (n = 305) | Remote Care (n = 248) | Proximate Care (n = 691) | p | |
| Age, y | |||||||||
| n | 1349 | 656 | 693 | 0.0307 | 1244 | 305 | 248 | 691 | < 0.0001 |
| Mean (SD) | 75.5 (9.0) | 75.0 (8.8) | 76.0 (9.1) | 75.6 (9.1) | 70.0 (8.6) | 79.3 (8.6) | 76.7 (8.4) | ||
| Range | 50–90 | 50–90 | 50–90 | 50–90 | 50–90 | 52–90 | 50–90 | ||
| Sex, n (%) | |||||||||
| n | 1346 | 655 | 691 | 0.3254 | 1241 | 305 | 247 | 689 | < 0.0001 |
| Male | 625 (46) | 295 (45) | 330 (48) | 566 (46) | 169 (55) | 90 (36) | 307 (45) | ||
| Female | 721 (54) | 360 (55) | 361 (52) | 675 (54) | 136 (45) | 157 (64) | 382 (55) | ||
| Ethnicity, n (%) | |||||||||
| n | 1347 | 654 | 693 | 0.0014 | 1243 | 305 | 247 | 691 | 0.1248 |
| White/Caucasian | 976 (73) | 500 (77) | 476 (69) | 906 (73) | 234 (77) | 182 (74) | 490 (71) | ||
| African American | 186 (14) | 70 (11) | 116 (17) | 173 (14) | 28 (9) | 35 (14) | 110 (16) | ||
| Hispanic/Latino | 99 (7) | 51 (8) | 48 (7) | 89 (7) | 24 (8) | 13 (5) | 52 (8) | ||
| Other | 86 (6) | 33 (5) | 53 (8) | 75 (6) | 19 (6) | 17 (7) | 39 (6) | ||
| Employment status, n (%) | |||||||||
| n | 1327 | 644 | 683 | 0.0007 | 1223 | 300 | 244 | 679 | < 0.0001 |
| Working full-time | 53 (4) | 34 (5) | 19 (3) | 51 (4) | 43 (14) | 0 (0) | 8 (1) | ||
| Working part-time | 46 (4) | 34 (5) | 12 (2) | 42 (3) | 30 (10) | 1 (< 1) | 11 (2) | ||
| Homemaker | 129 (10) | 61 (10) | 68 (10) | 116 (10) | 21 (7) | 21 (9) | 74 (11) | ||
| Student | 4 (0.3) | 3 (0.5) | 1 (0.1) | 4 (< 1) | 2 (1) | 1 (< 1) | 1 (< 1) | ||
| Retired | 1014 (76) | 480 (75) | 534 (78) | 934 (76) | 190 (63) | 199 (82) | 545 (80) | ||
| Unemployed | 81 (6) | 32 (5) | 49 (7) | 76 (6) | 14 (5) | 22 (9) | 40 (6) | ||
| Current hospital status, n (%) | |||||||||
| n | 1305 | 639 | 666 | 0.0007 | 1207 | 298 | 234 | 675 | < 0.0001 |
| Outpatient | 1226 (94) | 615 (96) | 611 (92) | 1133 (94) | 286 (96) | 195 (83) | 652 (97) | ||
| Inpatient | 79 (6) | 24 (4) | 55 (8) | 74 (6) | 12 (4) | 39 (17) | 23 (3) | ||
| Home circumstances, n (%) | |||||||||
| n | 1338 | 650 | 688 | < 0.0001 | 1236 | 302 | 247 | 687 | < 0.0001 |
| Lives with spouse/partner | 770 (58) | 410 (63) | 360 (52) | 713 (58) | 210 (70) | 37 (15) | 466 (68) | ||
| Lives with other family | 284 (21) | 115 (18) | 169 (25) | 254 (21) | 25 (8) | 23 (9) | 206 (30) | ||
| Lives alone | 102 (8) | 70 (11) | 32 (5) | 96 (8) | 59 (20) | 36 (15) | 1 (< 1) | ||
| Nursing home | 121 (9) | 30 (5) | 91 (13) | 117 (10) | 0 (0) | 116 (47) | 1 (< 1) | ||
| Sheltered housing/Assisted living | 31 (2) | 10 (2) | 21 (3) | 28 (2) | 5 (2) | 18 (7) | 5 (1) | ||
| Lives with friends | 9 (1) | 3 (0.5) | 6 (1) | 8 (1) | 2 (1) | 2 (1) | 4 (1) | ||
| Other | 21 (2) | 12 (2) | 9 (1) | 20 (2) | 1 (< 1) | 15 (6) | 4 (1) |
Supplementary Table 2 reports more details of patient demographics and clinical characteristics. SD, standard deviation.
Patient demographics and clinical characteristics for all patients
Physicians reported that 693 (48.6%) patients displayed ≥1 symptom of agitation, and 656 (51.4%) displayed no agitation symptoms.
Patients showing symptoms of agitation were slightly older than those not showing symptoms of agitation (p = 0.0307), but there was no difference in the proportion of male to female patients in the agitated and non-agitated groups (p = 0.3254) (Table 1). A lower proportion of agitated than non-agitated patients were White/Caucasian (p = 0.0014) and currently working (p = 0.0007) (Table 1). There was a difference between agitated and non-agitated patients’ home circumstances (p < 0.0001), with a lower proportion of agitated than non-agitated patients living with their spouse/partner or alone and a higher proportion of agitated than non-agitated patients living with other family members or in a nursing home (Table 1).
Only 10% of patients were considered by their physician to have severe dementia, with just over half considered to have mild cognitive impairment or mild dementia (Supplementary Table 2). Patients experiencing agitation tended to be at a more severe stage of dementia, as perceived by the physician, than those not experiencing agitation (p < 0.0001). The most recent MMSE score was lower for agitated than non-agitated patients (mean of 19.2 versus 22.0, respectively; p < 0.0001), also indicating more severe dementia in agitated than non-agitated patients (Supplementary Table 2).
Physicians reported on the type of caregiver for 1,244 patients, of whom 305 (24.5%) received no care, 248 (19.9%) remote care, and 691 (55.5%) proximate care. There was a significant difference in the age of patients based on type of care, with those receiving remote care the oldest and those receiving no care the youngest (p < 0.0001; Table 1). Patients’ home circumstances differed depending on type of care (p < 0.0001); a lower proportion of patients receiving remote care than patients receiving proximate or no care were living with their spouse/partner (15% versus 68% /70%) and a higher proportion of patients receiving remote care than patients receiving proximate or no care were living in a nursing home (47% vs 0% /0%) (Table 1). The proportion of female patients was highest in those receiving remote care and lowest in those receiving no care (p < 0.0001; Table 1). No difference was observed in ethnicity based on type of care (p = 0.1248), but the proportion of patients working was highest in those receiving no care and lowest in those receiving remote care (p < 0.0001) (Table 1)
Physician-perceived stage of dementia and most recent MMSE score both indicated that patients receiving remote care had the most severe dementia and those receiving no care the least severe dementia (both p < 0.0001; Supplementary Table 2). Agitation severity differed based on type of care, with patients with a remote caregiver having the highest mean agitation severity score and those with no caregiver the lowest score (p < 0.0001; Supplementary Table 2).
Caregiver demographics and relationship to patient
Of the 247 caregivers completing a self-reported record form, 144 (58%) reported that the patients they cared for showed symptoms of agitation, and 103 (42%) reported that the patients they cared for showed no symptoms of agitation. Of these 247 caregivers, 56 (22%) provided remote care, and 191 (77%) proximate care. No differences were seen in the demographics of non-professional caregivers between those providing care for agitated and non-agitated patients (Table 2). However, some differences were seen in the demographics of caregivers depending on remote/proximate caregiving, with those providing remote care being younger than those providing proximate care, and a higher proportion of those providing remote than proximate care working (both p < 0.0001; Table 2).
Table 2
Non-professional caregiver demographics
| Total (n = 247) | Non-agitated (n = 103) | Agitated (n = 144) | p | Remote Care (n = 56) | Proximate Care (n = 191) | p | |
| Age, y | |||||||
| n | 245 | 103 | 142 | 0.0740 | 55 | 190 | |
| Mean (SD) | 62.7 (13.8) | 64.6 (13.7) | 61.4 (13.8) | 52.6 (11.9) | 65.7 (13.0) | < 0.0001 | |
| Range | 30–90 | 30–90 | 31–89 | 31–89 | 30–90 | ||
| Sex, n (%) | |||||||
| n | 247 | 103 | 144 | 56 | 191 | ||
| Male | 62 (25) | 28 (27) | 34 (24) | 0.5536 | 10 (18) | 52 (27) | 0.1663 |
| Female | 185 (75) | 75 (73) | 110 (76) | 46 (82) | 139 (73) | ||
| Marital status, n (%) | |||||||
| n | 247 | 103 | 144 | 0.9692 | 56 | 191 | 0.4016 |
| Married/Cohabiting | 200 (81) | 85 (83) | 115 (80) | 42 (75) | 158 (83) | ||
| Single/Never married | 8 (3) | 3 (3) | 5 (4) | 1 (2) | 7 (4) | ||
| Divorced/Separated | 32 (13) | 12 (12) | 20 (14) | 10 (18) | 22 (12) | ||
| Widowed | 4 (2) | 2 (2) | 2 (1) | 2 (4) | 2 (1) | ||
| Other | 3 (1) | 1 (1) | 2 (1) | 1 (2) | 2 (1) | ||
| Employment status, n (%) | |||||||
| n | 247 | 103 | 144 | 0.1967 | 56 | 191 | < 0.0001 |
| Working full-time | 48 (19) | 14 (14) | 34 (24) | 29 (52) | 19 (10) | ||
| Working part-time | 40 (16) | 20 (19) | 20 (14) | 15 (27) | 25 (13) | ||
| Homemaker | 46 (19) | 17 (17) | 29 (20) | 8 (14) | 38 (20) | ||
| Student | 5 (2) | 3 (3) | 2 (1) | 0 (0) | 5 (3) | ||
| Retired | 95 (39) | 44 (43) | 51 (35) | 3 (5) | 92 (48) | ||
| Unemployed | 10 (4) | 5 (5) | 5 (4) | 0 (0) | 10 (5) | ||
| Other | 3 (1) | 0 (0) | 3 (2) | 1 (2) | 2 (1) |
Data are reported for caregivers who completed a record form. SD, standard deviation.
The most common relationship of caregivers to the patient was partner/spouse, with the proportion of patients cared for by their partner/spouse similar for agitated and non-agitated patients (Table 3). According to physicians, of patients receiving remote care, only 9% had care provided by their partners/spouse, while 69% of patients receiving proximate care were cared for by their partner/spouse (p < 0.0001). Daughters were the second most common caregivers overall at 24.3%. Physicians also reported that daughters were more commonly caregivers of agitated patients than non-agitated patients (p = 0.0023) and in providing proximate care compared to remote care (p < 0.0001) (Table 3). Sons were reported by physicians to more commonly provide proximate rather than remote care (p < 0.0001).
Table 3
Relationship of non-professional caregivers to patientsa
| Relationship | Total | Non-agitated | Agitated | p | Remote Care | Proximate Care | p |
| Physician-reportedb | |||||||
| n | 1,233 | 604 | 629 | 247 | 681 | ||
| Partner/Spouse, n (%) | 493 (40) | 235 (39) | 258 (41) | 0.4496 | 22 (9) | 471 (69) | < 0.0001 |
| Son, n (%) | 112 (9) | 45 (8) | 67 (11) | 0.0505 | 17 (7) | 95 (14) | < 0.0001 |
| Daughter, n (%) | 300 (24) | 124 (21) | 176 (28) | 0.0023 | 61 (25) | 239 (35) | < 0.0001 |
| Sibling, n (%) | 19 (2) | 4 (1) | 15 (2) | 0.0141 | 2 (1) | 17 (3) | 0.0026 |
| Other relative, n (%) | 50 (4) | 15 (3) | 35 (6) | 0.0061 | 10 (4) | 40 (6) | < 0.0001 |
| Friend/neighbor, n (%) | 19 (2) | 3 (1) | 16 (3) | 0.0034 | 7 (3) | 12 (2) | 0.0074 |
| Caregiver-reported | |||||||
| n | 247 | 103 | 144 | 56 | 191 | ||
| Partner/Spouse, n (%) | 119 (48) | 59 (57) | 60 (42) | 0.0155 | 1 (2) | 118 (62) | < 0.0001 |
| Son, n (%) | 20 (8) | 7 (7) | 13 (9) | 0.5261 | 10 (18) | 10 (5) | 0.0048 |
| Daughter, n (%) | 81 (33) | 30 (29) | 51 (35) | 0.2991 | 37 (66) | 44 (23) | < 0.0001 |
| Sibling, n (%) | 10 (4) | 2 (2) | 8 (6) | 0.2007 | 1 (2) | 9 (5) | 0.4633 |
| Other relative, n (%) | 14 (6) | 4 (4) | 10 (7) | 0.3050 | 6 (11) | 8 (4) | 0.0937 |
| Friend/neighbor, n (%) | 3 (1) | 1 (1) | 2 (1) | 1.0000 | 1 (2) | 2 (1) | 0.5393 |
aPhysicians reported on all types of caregivers, including professional caregivers. Sum of percentages are not equal to 100%; not all patients have a non-professional caregiver and some patients may have more than one caregiver. bPhysician-reported data for each patient with available data, which for some patients includes relationships to multiple caregivers.
Caregivers also reported partners/spouses to be the most common caregivers overall, with daughters the second most common (Table 3). Partners/spouses were more commonly caregivers for non-agitated than agitated patients (p = 0.0155) and in providing proximate versus remote care (p < 0.0001). The proportion of caregiver-reported daughters and sons providing care were higher in remote than proximate caregiving (p < 0.0001 and p = 0.0048, respectively).
Impact of agitation for all patients
Caregiver hours
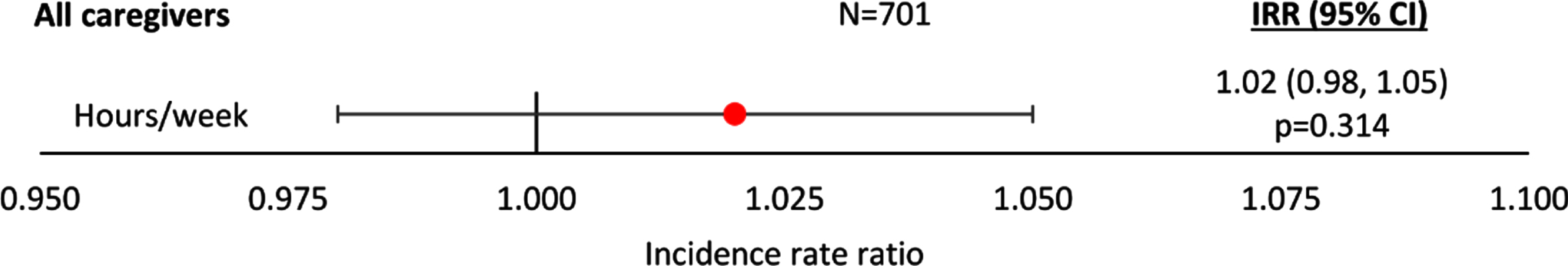
Regression analysis showed that the relationship between the number of hours per week of non-professional caregiving and increasing agitation severity score was positive, but non-significant, when all patients were included in the analysis (p = 0.314; Fig. 1). Regression results including IRR and 95% CI can be found in Supplementary Figure 1.
Fig. 1
Negative binomial regression analysis of caregiver hours by agitation score –all patients. An IRR significantly different from 1 indicates caregiver hours differ significantly for different agitation scores; an IRR > 1 implies greater hours with higher agitation scores, an IRR < 1 implies fewer hours with higher agitation scores. All regressions were controlled for patient demographics (age and sex) and clinical characteristics (time since diagnosis, current MMSE score). Coef, coefficient; CI, confidence interval; IRR, incidence rate ratio; MMSE, Mini-Mental State Examination.
Caregiver burden
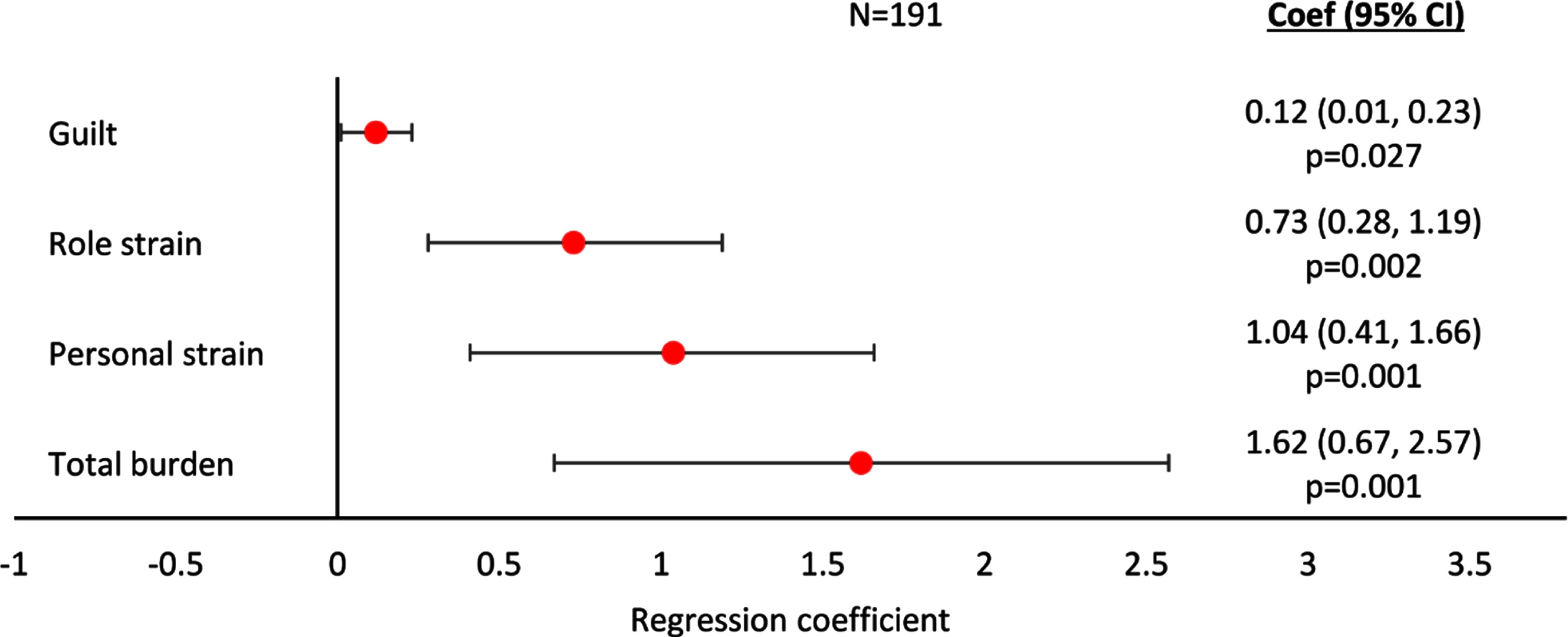
Regression analyses showed ZBI-assessed Total Caregiver Burden, Personal Strain, Role Strain, and Guilt all increased with increasing agitation severity score when the analyses considered all patients, regardless of whether receiving remote or proximate caregiving (all p < 0.01, except Guilt p < 0.05; Fig. 2). The association with agitation severity was most marked for Total Caregiver Burden, and Personal Strain. Regression results including coefficients and 95% CI can be found in Supplementary Figure 2.
Fig. 2
Linear regression analysis of caregiver burden assessed with ZBI by agitation score –all patients. A regression coefficient significantly different from 0 indicates the predicted outcomes differs significantly for different agitation scores; a coef > 0 implies a worse outcome with higher agitation scores, a coef < 0 implies a better outcome with higher agitation scores. All regressions were controlled for patient demographics (age and sex) and clinical characteristics (time since diagnosis, current MMSE score). Coef, coefficient; CI, confidence interval; MMSE, Mini-Mental State Examination; ZBI, Zarit Burden Interview.
Caregiver health status
Caregivers’ EQ-5D-3L health utility index and EQ-5D-3L VAS were negatively (indicating poorer health status), but non-significantly, associated with increasing agitation severity (EQ-5D-3L health utility index regression coefficient [95% CI]: –0.003 [–0.009, 0.003]; p = 0.294; EQ-5D-3L VAS score regression coefficient [95% CI]: –0.036 [–1.09, 0.27]; p = 0.259). The regression plots can be found in Supplementary Figure 3.
Caregiver productivity
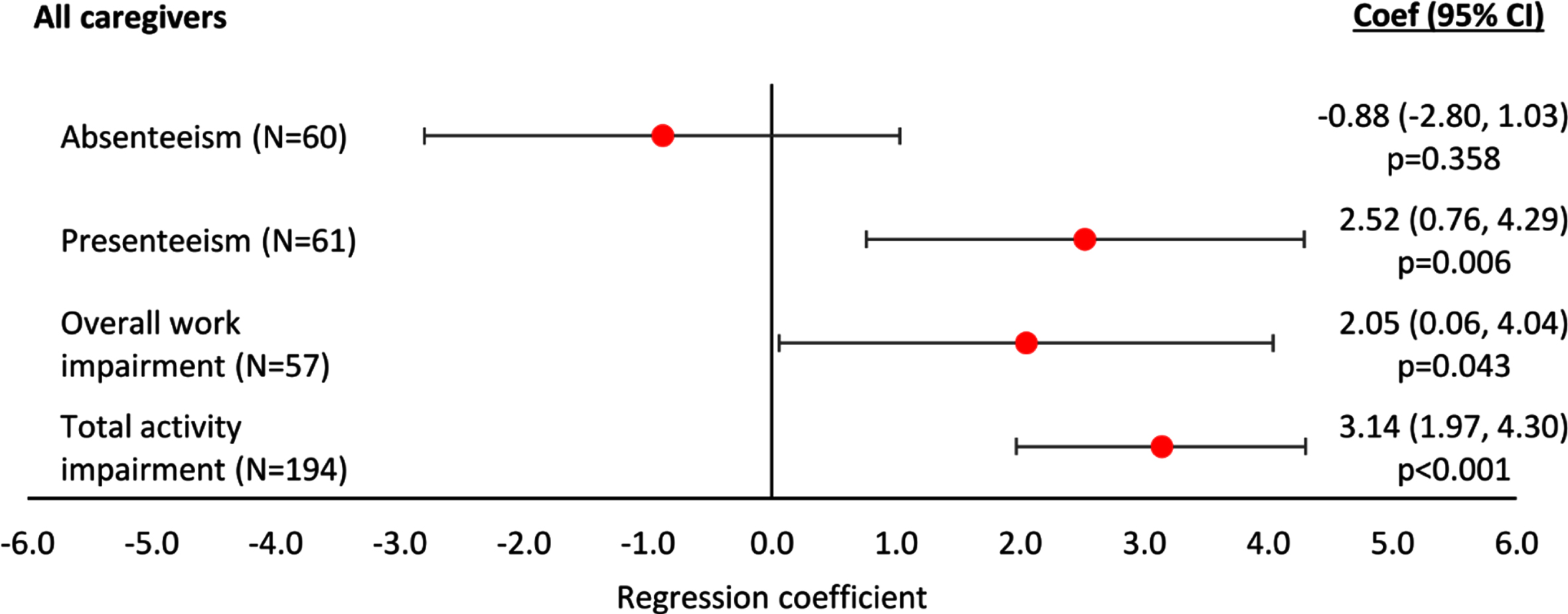
WPAI-assessed presenteeism, overall work im-pairment and total activity impairment all increased with increasing agitation severity score based on regression analyses (p < 0.01, p < 0.05 and p < 0.001, respectively; Fig. 3). Absenteeism was negatively, but non-significantly, associated with increasing agitation severity (Fig. 3). The regression plots can be found in Supplementary Figure 4.
Fig. 3
Linear regression analysis of WPAI-assessed productivity and activity by agitation score –all patients. A regression coefficient significantly different from 0 indicates the predicted outcomes differs significantly for different agitation scores; a coef > 0 implies a worse outcome with higher agitation scores, a coef < 0 implies a better outcome with higher agitation scores. All regressions were controlled for patient demographics (age and sex) and clinical characteristics (time since diagnosis, current MMSE score). Coef, coefficient; CI, confidence interval; MMSE, Mini-Mental State Examination; WPAI, Work Productivity and Activity Index.
Impact of agitation dependent on type of care
Caregiver hours
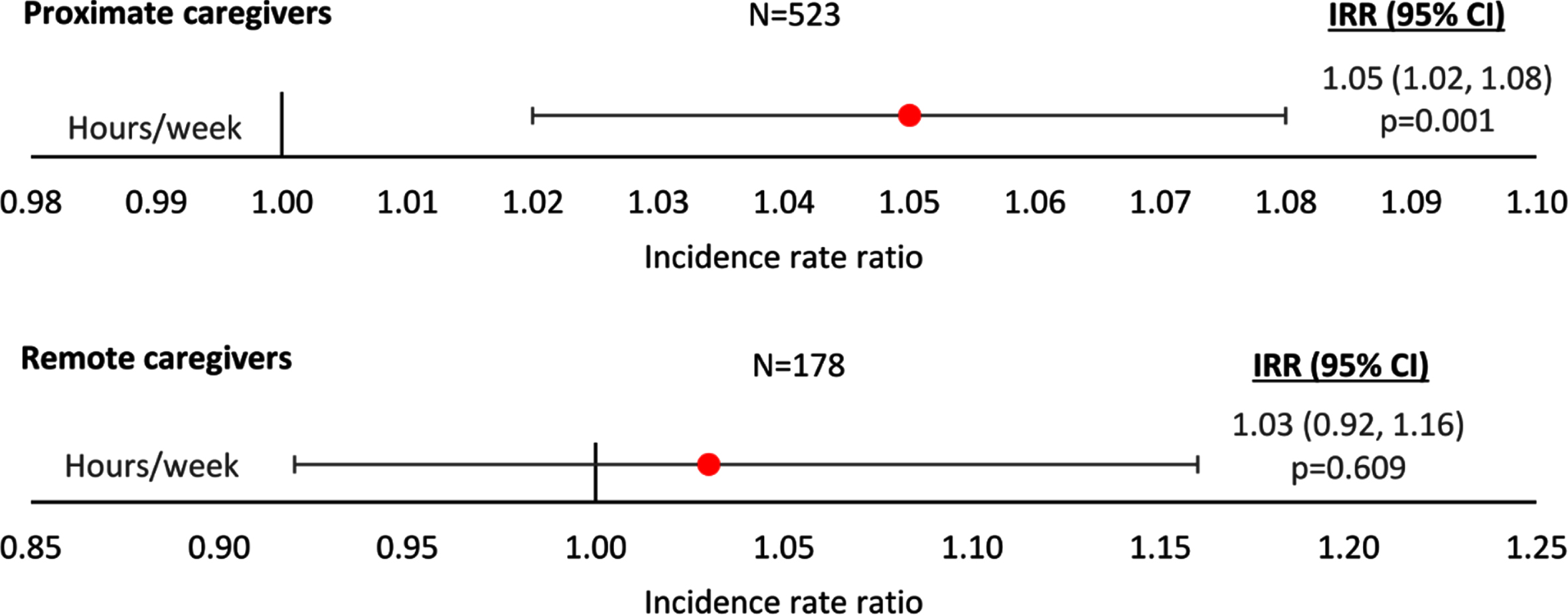
The number of non-professional caregiver hours/week was higher for patients receiving proximate care than remote care, at all agitation severity score levels. Regression analysis showed a significant increase in the number of hours per week of caregiving with increasing agitation severity score for patients receiving proximate care (p = 0.001; Fig. 4), but not for those receiving remote care (IRR [95% CI]: 1.03 [0.92, 1.16]; p = 0.609). The regression plots can be found in Supplementary Figure 5.
Fig. 4
Linear regression analysis of caregiver hours by agitation score and type of care. Proximate Care, caregiver living with patient; Remote Care, caregiver not living with patient. An IRR significantly different from 1 indicates caregiver hours differ significantly for different agitation scores; an IRR > 1 implies greater hours with higher agitation scores, an IRR < 1 implies fewer hours with higher agitation scores. All regressions were controlled for patient demographics (age and sex) and clinical characteristics (time since diagnosis, current MMSE score). Coef, coefficient; CI, confidence interval; IRR, incidence rate ratio; MMSE, Mini-Mental State Examination.
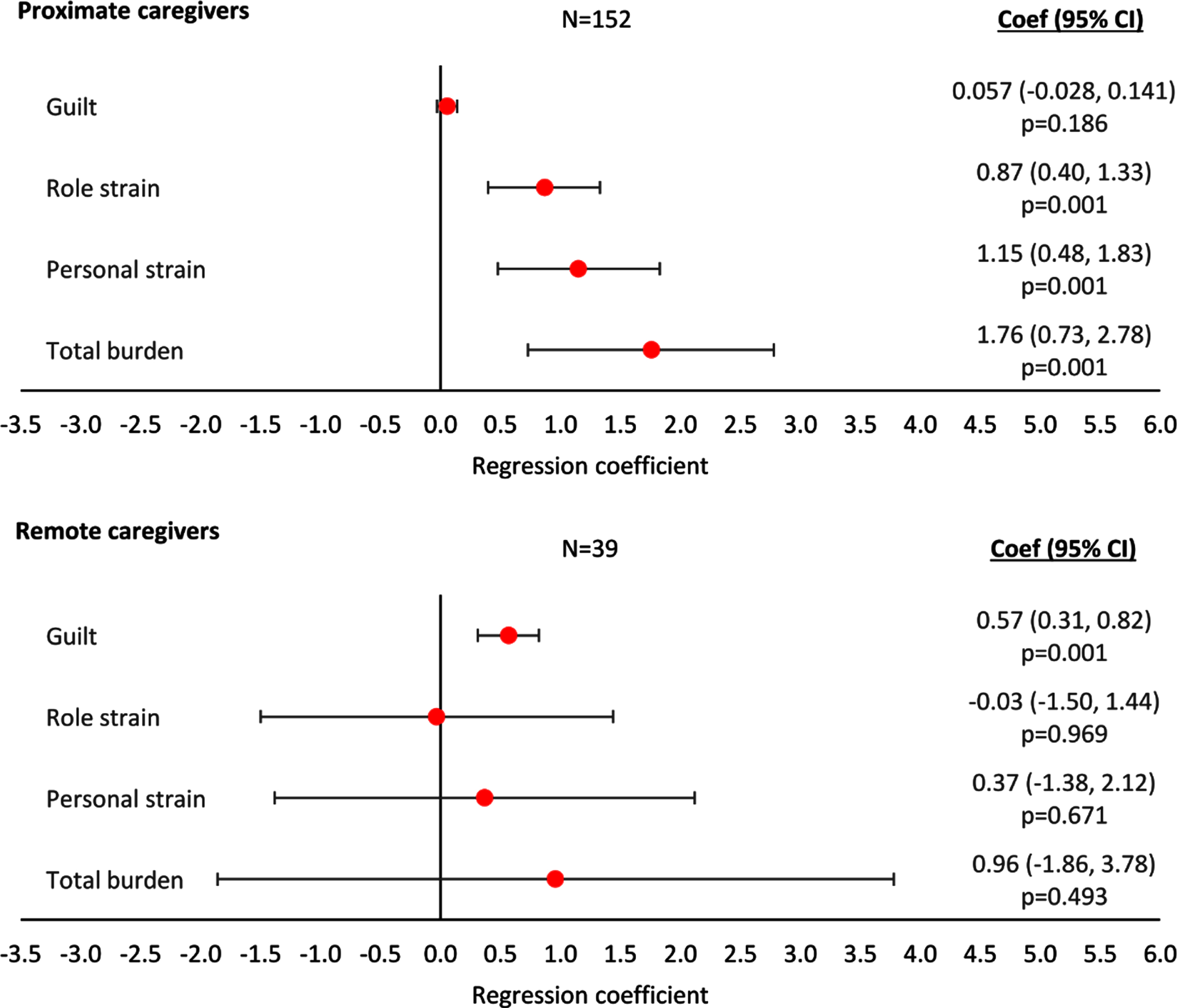
Caregiver burden
Based on the findings from regression analyses, ZBI Total Caregiver Burden, Personal Strain, and Role Strain all increased with increasing agitation severity score for caregivers providing proximate care (p < 0.01, p < 0.01, and p < 0.001, respectively; Fig. 5) but not for caregivers providing remote care (all p > 0.05; Fig. 5). Regression analyses showed an association of increasing ZBI Guilt with increasing agitation severity score for caregivers providing remote care (p < 0.001; Fig. 5), but not for caregivers providing proximate care (p > 0.05; Fig. 5). The regression plots can be found in Supplementary Figure 6.
Fig. 5
Linear regression analysis of caregiver burden assessed with ZBI by agitation score and type of care. Proximate Care, caregiver living with patient; Remote Care, caregiver not living with patient. A regression coefficient significantly different from 0 indicates the predicted outcomes differs significantly for different agitation scores; a coef > 0 implies a worse outcome with higher agitation scores, a coef < 0 implies a better outcome with higher agitation scores. All regressions were controlled for patient demographics (age and sex) and clinical characteristics (time since diagnosis, current MMSE score). Coef, coefficient; CI, confidence interval; MMSE, Mini Mental State Examination; ZBI, Zarit Burden Interview.
Caregiver health status
Regression analyses did not show a statistical association of caregivers’ EQ-5D-3L health utility index or VAS score with agitation severity score for caregivers providing either remote or proximate care; the regression plots can be found in Supplementary Figure 7. However, mean scores for EQ-5D-3L health utility index were negatively associated (indicating poorer health status), but not significantly, with increasing agitation severity in both proximate caregiving (regression coefficient [95% CI]: –0.004 [–0.012, 0.003]; p = 0.262) and the remote caregiving (regression coefficient [95% CI]: –0.006 [–0.017, 0.004]; p = 0.232). We also observed a similar relationship (not significant) between mean scores of EQ–5D–3L VAS and increasing agitation severity in proximate caregiving (regression coefficient [95% CI]: –0.550 [–1.354, 0.254]; p = 0.178). This relationship was far weaker for remote caregiving (regression coefficient [95% CI]: –0.101 [–1.212, 1.416]; p = 0.876).
Caregiver productivity
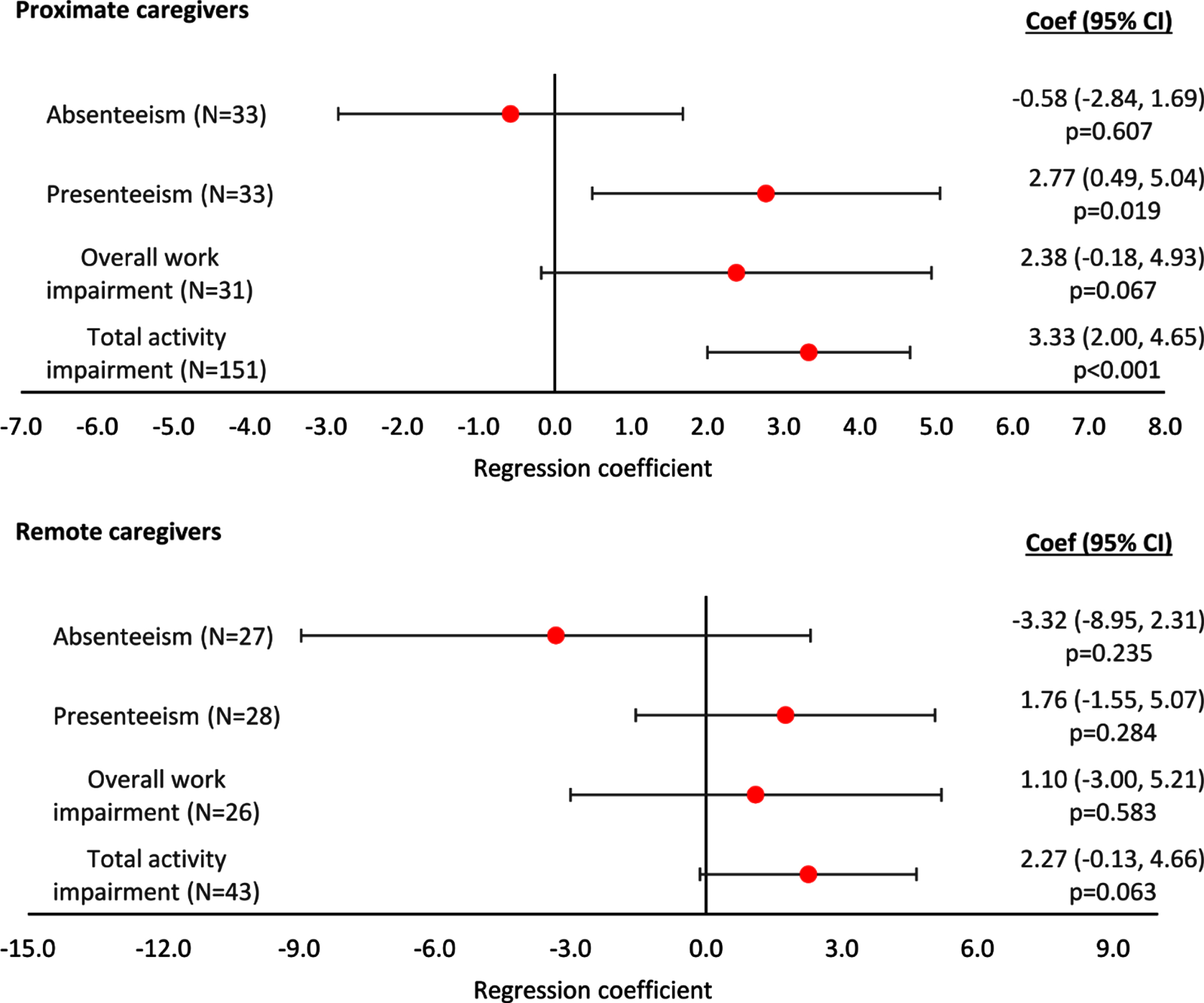
WPAI-assessed caregiver presenteeism, overall work impairment, and total activity impairment were positively, but non-significantly, associated with increasing agitation severity score in both remote and proximate caregiving, while significant relationships in regression analyses were observed only in presenteeism and total activity impairment for proximate caregivers (p = 0.019 and p < 0.001, respectively; Fig. 6). A non-significant association for caregiver absenteeism to decrease with increasing agitation severity was observed in both the remote and proximate caregiving was observed (Fig. 6). The regression plots can be found in Supplementary Figure 8.
Fig. 6
Linear regression analysis of WPAI-assessed productivity and activity by agitation score and type of care. Proximate Care, caregiver living with patient; Remote Care, caregiver not living with patient. A regression coefficient significantly different from 0 indicates the predicted outcomes differs significantly for different agitation scores; a coef > 0 implies a worse outcome with higher agitation scores, a coef < 0 implies a better outcome with higher agitation scores. All regressions were controlled for patient demographics (age and sex) and clinical characteristics (time since diagnosis, current MMSE score). Coef, coefficient; CI, confidence interval; MMSE, Mini Mental State Examination; WPAI, Work Productivity and Activity Index.
Summary of findings
A summary of key statistics for all regression analyses showing a statistically significant association of agitation severity with an outcome can be found in Table 4.
Table 4
Summary of statistically significant findings
| Outcome | Assessed by | Regression analysis of agitation severity score versus outcome | |
| All caregivers | |||
| Burden | Zarit Burden Interview | Guilt: | n = 191; Coef (95% CI) = 0.12 (0.01, 0.23); p = 0.027 |
| Role strain: | n = 191; Coef (95% CI) = 0.73 (0.28, 1.19); p = 0.002 | ||
| Personal strain: | n = 191; Coef (95% CI) = 1.04 (0.41, 1.66); p = 0.001 | ||
| Total burden: | n = 191; Coef (95% CI) = 1.62 (0.67, 2.56); p = 0.001 | ||
| Productivity | Work Productivity and Activity Impairment questionnaire | Presenteeism: | n = 61; Coef (95% CI) = 2.52 (0.76, 4.29); p = 0.006 |
| Overall work impairment: | n = 57; Coef (95% CI) = 2.05 (0.06, 4.04); p = 0.043 | ||
| Total activity impairment: | n = 194; Coef (95% CI) = 3.14 (1.97, 4.30); p < 0.001 | ||
| Proximate caregivers | |||
| Hours/week | Physician-reported caregiver time | n = 523; | IRR (95% CI) = 1.05 (1.02, 1.08); p = 0.001 |
| Burden | Zarit Burden Interview | Role strain: | n = 152; Coef (95% CI) = 0.87 (0.40, 1.33); p < 0.001 |
| Personal strain: | n = 152; Coef (95% CI) = 1.15 (0.48, 1.83); p = 0.001 | ||
| Total burden: | n = 152; Coef (95% CI) = 1.76 (0.73, 2.78); p = 0.001 | ||
| Productivity | Work Productivity and Activity Impairment questionnaire | Presenteeism: | n = 33; Coef (95% CI) = 2.77 (0.49, 5.04); p = 0.019 |
| Total activity impairment: | n = 151; Coef (95% CI) = 3.33 (2.00, 4.65); p < 0.001 | ||
| Remote caregivers | |||
| Burden | Zarit Burden Interview | Guilt: | n = 39; Coef (95% CI) = 0.57 (0.31, 0.82); p = 0.001 |
All regressions were controlled for patient demographics (age and sex) and clinical characteristics (time since diagnosis, current MMSE score). Coef, coefficient; CI, confidence interval; IRR, incidence rate ratio; MMSE, Mini-Mental State Examination; ZBI, Zarit Burden Interview.
DISCUSSION
In this large, point-in-time survey of physicians and non-professional caregivers, we observed that the burden of caregiving for non-professional caregivers increased with increasing severity of agitation in patients with dementia. Furthermore, caregiver presenteeism, overall work impairment and total activity impairment were greater with more severe agitation. We also explored how these outcomes were associated with agitation considering only remote, and only proximate, caregiving.
The profile of patients experiencing agitation indicated that they were generally older and at a more advanced, severe stage of their disease than those without symptoms of agitation. Other US observational studies have also reported increasing agitation to be associated with increasing dementia severity [23, 24]. Similarly, patients receiving remote care were older and had more advanced, severe dementia, and more severe agitation symptoms than those receiving no care or proximate care. The relationship between agitation and remote/proximate caregiving may be confounded by the care setting, as more patients receiving remote care than those receiving proximate care lived in a nursing home. The observation that greater dementia severity was associated with a higher proportion of remote caregiving might be expected, as the care needs of advanced dementia can make home care extremely challenging [25, 26].
We observed partners/spouses to be the most common caregivers in both agitated and non-agitated patients. The high proportion of partners/spouses providing care that we observed has been reported in a systematic review of studies of non-professional caregivers for dementia [27]. However, we found that, according to physicians, although the majority of patients receiving proximate care were cared for by their partner/spouse, fewer than 10% of patients received remote care from partners/spouses. Only one caregiver (2%) of those completing record forms and providing remote care was the partner/spouse of the patient.
In all analyses, there was a trend for the time spent caregiving to increase with increasing agitation severity, although this was significant only when considering proximate caregiving (p = 0.001). More caregiving time was reported for proximate than remote caregiving giving at all levels of agitation. The mean hours of caregiving reported for patients with no agitation symptoms and those with an agitation severity score of 6 were 52.9 h and 58.6 h, respectively, for all caregivers, 12.3 h and 14.7 h, respectively, for remote caregivers, and 60.9 h and 81.5 h, respectively, for proximate caregivers. The association of caregiving time with agitation severity and remote/proximate caregiving must be interpreted with care as this will be affected by the patient’s living situation, with a lower likelihood of patients with more severe dementia and agitation symptoms living at home. To the authors’ knowledge, this is the first published analysis exploring the association of agitation symptoms and remote/proximate caregiving on caregiving time for dementia patients.
When considering all patients, all elements of caregiver burden increased with increasing agitation score. A search of the literature failed to identify other US point-in-time studies of the relationship between caregiver burden and patient agitation in dementia; however, a three-year Australian longitudinal study reported higher caregiver burden (measured via the ZBI) for patients with symptoms of agitation than those without [28], and a three-year longitudinal study in Spain found increased ZBI-assessed caregiver burden to be associated with the patient experiencing more severe neuropsychiatric symptoms [29].
Regression analyses of data related to all patients showed that as agitation severity increased, caregiver presenteeism, overall work impairment and total activity impairment worsened. To our knowledge, this is a novel finding, as a literature search identified no studies showing links between agitation severity in dementia patients and caregiver productivity. Further research is needed to confirm this finding and better understand the economic impact of agitation in dementia on caregivers’ work environments.
Analysis of caregiver burden in remote versus proximate caregiving revealed interesting findings, with ZBI Total Caregiver Burden, Personal Strain, and Role Strain all increasing with increasing agitation severity for proximate caregivers and ZBI Guilt increasing with increasing agitation severity for remote caregivers. As many patients receiving remote care were in nursing homes, results suggest that guilt associated with placing a dementia patient in the nursing home setting may be a source of stress for remote caregivers. These results are consistent with published evidence. For example, a Spanish observational study reported increased ZBI Total Caregiver Burden to be associated with the caregiver living with the patient [29], but not remote, caregivers. Furthermore, in a recent study, caregiver burden was found to be higher in caretakers of younger patients, male patients, or parents, which was reflected in our finding that burden was higher in proximate care and patients in proximate care tended to be younger, were more likely to be cared for by their children, and that a lower number of female patients were in proximate care. Lower guilt, however, was associated with older patients who are more likely to be in remote care potentially indicating that the guilt of placing a patient in remote care plays a larger role in caretaker burden than age [5]. Presenteeism and total activity impairment increased with increasing patient agitation severity for proximate caregivers, but not remote caregivers. This finding may also be related to the higher proportion of remote care patients residing in nursing homes, as work-related impairment may have been reduced for caregivers who had the additional support of nursing home staff.
Despite finding that both increased agitation and proximal care setting significantly increased caregiver burden we were unable to find any impact on caregiver HRQoL as measured by the EQ-5D-3L and EQ-VAS. This was perhaps unsurprising as it has been shown that EQ-5D and ZBI scores correlate poorly. EQ-5D showed little difference in scores as disease severity increased and low sensitivity to changes in caregiver circumstance [30]. Our finding that HRQoL did not correlate with increase burden does not necessarily indicate that increased burden does not affect HRQoL, it may be that a generic HRQoL measure such as the EQ-5D fails to capture the unique challenges to HRQoL faced by those caring for AD patients.
A number of limitations should be considered in the evaluation of our findings. Some limitations reflect the survey methodology and are commonly seen with this methodological approach. This was not a true random sample; physicians were asked to provide data for a consecutive series of patients to avoid selection bias, but patients may be those who visit their physician more frequently than the general dementia population. This criterion might result in selecting patients with more severe symptoms, but this bias would be offset to some degree as physicians were allowed to select target patients. Identification of the target patient group was based on the judgement of the respondent physician, and physicians may have been less likely to select severely ill patients. In addition, the survey excluded physicians based in residential care facilities, such as nursing homes; thus, more severe dementia cases might not be fully represented in this sample. It is also worth noting that the caregiver report form was entirely voluntary, this could risk selecting for caregivers with the lowest burden as they have more time to fill out the form, or possibly those with the highest burden as they are more motivated to report on their burden
Recall bias might have affected physician and caregiver responses; however, data were collected at the time of the patient’s appointment, reducing the likelihood of recall bias. The point-in-time design of the survey prevents conclusions about causal relationships, although identification of significant associations is possible. Our methods ensured physicians were unaware of responses on caregiver-reported forms, but it was not possible to confirm no information exchange between physicians and caregivers. Despite data collection taking place across 2015/2016, little has changed in terms of management and impact of disease, therefore, the data are likely suggestive of contemporaneous burden of agitation.
Other limitations are more specific to this analysis. We were not able to evaluate differences in our results attributable to physician specialty, although we recognize that the level of knowledge and management strategies for dementia might differ between primary care physicians and specialists. There is no firm consensus on the definition of agitation; in this survey, the presence of one or more of seven agitation-related physician-reported symptoms was sufficient to consider a patient to be agitated. We acknowledge that other definitions of agitation (such as that used in the NPI-Q) would define agitation differently than the definition used in this study. The definition of agitation used was constructed to best utilize the data available through the DSP and reflects the IPA consensus definition of agitation [7]. In addition, the IPA definition was defined more recently than NPI-Q and reflects a more current understanding of the wide range of agitation symptoms. The method employed in calculating agitation severity scores gave equal and weighting to all symptoms and assumed a linear relationship between mild, moderate, and severe symptom severity. Care must be taken in interpreting the associations observed between agitation severity, remote/proximate caregiving, and hours of caregiving. Given that a higher proportion of patients reside in a nursing home as dementia advances and agitation worsens, remote and proximate care experiences may be qualitatively different for caregivers. Additionally, we chose to run separate analyses on care setting and agitation rather than a covariant analysis to increase data flexibility and ease of interpretation, this did, however, lower the power of our analyses potentially meaning some significant associations appeared not to be significant.
Despite such limitations, real world studies play an important part in highlighting areas of concern that are not addressed in clinical trials. Moreover, there is a paucity of published data on the association of agitation with caregiver burden and productivity in dementia, hence the importance of our analysis.
We have reported separately that increased healthcare resource utilization and direct healthcare costs were associated with increased severity of agitation symptoms in patients with dementia, based on analysis of the data source utilized for the current analysis [31]. The focus of the current survey was on the humanistic impact of agitation in dementia on caregivers, but our findings highlight further potential cost implications of agitation in dementia. The increase in caregiving time and lost productivity of caregivers caring for patients with agitation will certainly translate into higher indirect cost. Lost caregiver productivity needs further study, as an addition to the considerable cost burden of dementia, which is already well documented [32, 33].
In conclusion, our data demonstrated a strong relationship of agitation with burden and productivity impairment for non-professional caregivers. We observed agitation severity to be associated with higher burden and reduced productivity in non-professional caregivers, particularly those providing proximate caregiving.
ACKNOWLEDGMENTS
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Dementia Disease Specific Programme (DSP)trademark. Otsuka Pharmaceutical Development & Commercialization, Inc., and Lundbeck, LLC, did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here used data from the Adelphi Dementia DSP. The DSP is a wholly owned Adelphi product. Otsuka Pharmaceutical Development & Commercialization, Inc., and Lundbeck, LLC, are two of multiple subscribers to the DSP.
Medical writing support under the guidance of the authors was provided by Carole Evans, PhD, on behalf of Adelphi Real world, in accordance with Good Publication Practice (GPP3) guidelines (Annals of Internal Medicine. 2015;163(6):461-4).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5670r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215670.
REFERENCES
[1] | Jennings LA , Reuben DB , Evertson LC , Serrano KS , Ercoli L , Grill J , Chodosh J , Tan Z , Wenger NS ((2015) ) Unmet needs of caregivers of individuals referred to a dementia care program. J Am Geriatr Soc 63: , 282–289. |
[2] | McLaughlin T , Feldman H , Fillit H , Sano M , Schmitt F , Aisen P , Leibman C , Mucha L , Ryan JM , Sullivan SD , Spackman DE , Neumann PJ , Cohen J , Stern Y ((2010) ) Dependence as a unifying construct in defining Alzheimer’s disease severity. Alzheimers Dement 6: , 482–493. |
[3] | Kasper JD , Freedman VA , Spillman BC , Wolff JL ((2015) ) The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health Aff (Millwood) 34: , 1642–1649. |
[4] | Allegri RF , Sarasola D , Serrano CM , Taragano FE , Arizaga RL , Butman J , Loñ L ((2006) ) Neuropsychiatric symptoms as a predictor of caregiver burden in Alzheimer’s disease. Neuropsychiatr Dis Treat 2: , 105–110. |
[5] | Cheng ST ((2017) ) Dementia caregiver burden: A research update and critical analysis. Curr Psychiatry Rep 19: , 64. |
[6] | Lucijanić J , Baždarić K , Librenjak D , Lucijanić M , Hanževački M , Jureša V ((2020) ) A validation of the Croatian version of Zarit Burden Interview and clinical predictors of caregiver burden in informal caregivers of patients with dementia: A cross-sectional study. Croat Med J 61: , 527–537. |
[7] | Cummings J , Mintzer J , Brodaty H , Sano M , Banerjee S , Devanand DP , Gauthier S , Howard R , Lanctôt K , Lyketsos CG , Peskind E , Porsteinsson AP , Reich E , Sampaio C , Steffens D , Wortmann M , Zhong K ; International Psychogeriatric Association ((2015) ) Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr 27: , 7–17. |
[8] | Anatchkova M , Brooks A , Swett L , Hartry A , Duffy RA , Baker RA , Hammer-Helmich L , Sanon Aigbogun M ((2019) ) Agitation in patients with dementia: A systematic review of epidemiology and association with severity and course. Int Psychogeriatr 11: , 1–14. |
[9] | Reus VI , Fochtmann LJ , Eyler AE , Hilty DM , Horvitz-Lennon M , Jibson MD , Lopez OL , Mahoney J , Pasic J , Tan ZS , Wills CD , Rhoads R , Yager J ((2016) ) The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry 173: , 543–546. |
[10] | Anderson P , Benford M , Harris N , Karavali M , Piercy J ((2008) ) Real world physician and patient behaviour across countries: Disease-Specific Programmes - a means to understand. Curr Med Res Opin 24: , 3063–3072. |
[11] | Ritchie CW , Black CM , Khandker RK , Wood R , Jones E , Hu X , Ambegaonkar BM ((2018) ) Quantifying the diagnostic pathway for patients with cognitive impairment: Real world data from seven European and North American countries. J Alzheimers Dis 62: , 457–466. |
[12] | Zarit SH , Reever KE , Bach-Peterson J ((1980) ) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20: , 649–655. |
[13] | Siegert RJ , Jackson DM , Tennant A , Turner-Stokes L ((2010) ) Factor analysis and Rasch analysis of the Zarit Burden Interview for acquired brain injury carer research. J Rehabil Med 42: , 302–309. |
[14] | Rabin R , de Charro F ((2001) ) EQ-5D: A measure of health status from the EuroQol Group. Ann Med 33: , 337–343. |
[15] | Wang VW , Kandiah N , Lin X , Wee HL ((2016) ) Comparison of caregiver-rated 3-level EuroQol-5D (EQ-5D-3L) and Health Utilities Index Mark 3 (HUI3) in patients with dementia in Singapore. Value Health 19: , A810. |
[16] | Hurst NP , Kind P , Ruta D , Hunter M , Stubbings A ((1997) ) Measuring health-related quality of life in rheumatoid arthritis: Validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 36: , 551–559. |
[17] | Szende A , Oppe M , Devlin N (2007) EQ-5D Value Sets: Inventory, Comparative Review and User Guide. Springer, Netherlands. |
[18] | Reilly MC , Zbrozek AS , Dukes EM ((1993) ) The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 4: , 353–365. |
[19] | StataCorp (2019) Stata Statistical Software: Release 16. StataCorp LLC, College Station, TX. |
[20] | EphMRA Code of Conduct (2020) https://www.ephmra.org/4857/ephmra-2020-code-of-conduct-doc-f.pdf. Accessed on 12 March 2021. |
[21] | US Health Insurance Portability and Accountability Act 1996 - US Department of Health and Human Services. https://www.hhs.gov/hipaa/for-professionals/index.html. Accessed on 12 March 2021. |
[22] | Health Information Technology for Economic and Clinical Health (HITECH) Act 2009. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Accessed on 12 March 2021. |
[23] | Brodaty H , Connors MH , Xu J , Woodward M , Ames D; PRIME study group ((2015) ) The course of neuropsychiatric symptoms in dementia: A 3-year longitudinal study. J Am Med Dir Assoc 16: , 380–387. |
[24] | Zahodne LB , Ornstein K , Cosentino S , Devanand DP , Stern Y ((2015) ) Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry 23: , 130–140. |
[25] | Alzheimer’s Association (2020) Late-Stage Caring. https://www.alz.org/help-support/caregiving/stages-behaviors/late-stage. Accessed on 16 October 2020. |
[26] | Dementia Care Central (2020) Stages of Alzheimer’s & Dementia: Durations & Scales Used to Measure Progression (GDS, FAST & CDR). https://www.dementiacarecentral.com/aboutdementia/facts/stages/#stages. Accessed on 16 October 2020. |
[27] | Farina N , Page TE , Daley S , Brown A , Bowling A , Basset T , Livingston G , Knapp M , Murray J , Banerjee S ((2017) ) Factors associated with the quality of life of family carers of people with dementia: A systematic review. Alzheimers Dement 13: , 572–581. |
[28] | Connors MH , Seeher K , Teixeira-Pinto A , Woodward M , Ames D , Brodaty H ((2020) ) Dementia and caregiver burden: A three-year longitudinal study. Int J Geriatr Psychiatry 35: , 250–258. |
[29] | Conde-Sala JL , Turró-Garriga O , Calvó-Perxas L , Vilalta-Franch J , Lopez-Pousa S , Garre-Olmo J ((2014) ) Three-year trajectories of caregiver burden in Alzheimer’s disease. J Alzheimers Dis 42: , 623–633. |
[30] | Reed C , Barrett A , Lebrec J , Dodel R , Jones RW , Vellas B , Wimo A , Argimon JM , Bruno G , Haro JM ((2017) ) How useful is the EQ-5D in assessing the impact of caring for people with Alzheimer’s disease? Health Qual Life Outcomes 15: , 16. |
[31] | Jones E , Aigbogun MS , Pike J , Berry M , Houle CR , Husbands J ((2021) ) Agitation in dementia: Real-world impact and burden on patients and the healthcare system. J Alzheimers Dis 83: , 89–101. |
[32] | Cantarero-Prieto D , Leon PL , Blazquez-Fernandez C , Juan PS , Cobo CS ((2020) ) The economic cost of dementia: A systematic review. Dementia (London) 19: , 2637–2657. |
[33] | Fishman P , Coe NB , White L , Crane PK , Park S , Ingraham B , Larson EB ((2019) ) Cost of dementia in Medicare managed care: A systematic literature review. Am J Manag Care 1: , e247–e253. |




