Association Study and Meta-Analysis of Polymorphisms and Blood mRNA Expression of the ALDH2 Gene in Patients with Alzheimer’s Disease
Abstract
Background:
Late-onset Alzheimer’s disease (LOAD) is a complex disease in which neuroinflammation plays an important pathophysiological role, and exposure to neurotoxic substrates such as aldehydes may contribute. Blood mRNA expression levels of neuroinflammation-related genes appear to be potential biological markers of LOAD. A relationship between ALDH2 and LOAD has been suggested.
Objective:
Our objective was to examine blood ALDH2 expression in Japanese LOAD patients, conduct a genetic association study, and add new studies to an extended meta-analysis of the Asian population.
Methods:
A blood expression study (45 AD subjects, 54 controls) in which total RNA was isolated from whole peripheral blood samples and ALDH2 expression measured was conducted. In addition, a genetic association study (271 AD subjects, 492 controls) using genomic DNA from whole peripheral blood samples was conducted. Finally, a meta-analysis examined the relationship between ALDH2*2 frequency and the risk of LOAD.
Results:
ALDH2 mRNA expression was significantly higher in LOAD than in controls, and also higher in men with LOAD than in women with LOAD (p = 0.043). The genotypes in the two classified groups and the allele frequency were significantly different between AD and control subjects. The meta-analysis showed a significant difference in the ALDH2*2 allele, with an increased AD risk (OR = 1.38; 95% CI = 1.02–1.85; p = 0.0348, I2 = 81.1%).
Conclusion:
There was a significant increase in blood ALDH2 expression, and a genetic association with ALDH2*2 in LOAD. ALDH2 may have significant roles in the pathogenesis of LOAD in the Asian population.
INTRODUCTION
Late-onset Alzheimer’s disease (LOAD) is a complex disease affected by multiple genetic factors including APOE [1, 2], environmental factors such as lifestyle, and comorbidities [3]. Neuroinflammation is one of the important factors in the pathophysiology of LOAD [4], and exposure to neurotoxic substrates such as aldehydes may contribute to Alzheimer’s disease (AD)-associated pathologies [5]. Several studies have reported that blood mRNA expression levels of neuroinflammation-related genes were potential biological markers of LOAD [6–9].
Currently, extensive research has reported that mitochondrial dysfunction may be associated with various AD pathologies [10]. The aldehyde dehydrogenase (ALDH) superfamily plays a crucial role in several biological processes including development and detoxification pathways in the organism [11]. ALDH2, a gene that codes a protein localized in mitochondria, is crucial in the oxidative metabolism of toxic aldehydes in the brain, such as catecholaminergic metabolites 3,4-Dihydroxyphenylacetaldehyde (DOPAL) and 3,4-dihydroxyphenylglycolaldehyde (DOPEGAL) [12]. ALDH2 also protects against oxidative stress, such as that caused by the principal product of the lipid peroxidation process, 4-hydroxy-2-nonenal (4-HNE) [12]. Animal studies also suggested that ALDH2 was associated with age-dependent memory impairment and AD-like pathological changes [13, 14].
The rs671(A) allele of the ALDH2 gene causes the substitution of glutamate (Glu) to lysine (Lys) at codon 487 and inactivates ALDH2 enzyme [15]. The G allele and A allele are shown as ALDH2*1 and ALDH2*2, respectively. Thus, the genotype was shown as having three types, 1/1, 1/2, and 2/2. The A allele is very rare in Caucasians and widely prevalent in Asian populations [16]. ALHD2 is also a mitochondrial enzyme involved in the metabolism of acetaldehyde, a metabolite of ethanol. Deficiency of the ALDH2 enzyme due to ALDH2*2 has been reported to elevate ROS upon ethanol intake, increasing the risk of cytotoxicity and AD-related pathologies [5]. Kamino et al. showed that ALDH2*2 was associated with the development of LOAD in the Japanese population [17]. The recent meta-analysis including five studies (1,057 LOAD and 1,136 healthy controls from Asian populations) showed no significant association between ALDH2*2 and LOAD overall, but men with ALDH2*2 had a higher risk of LOAD [18]. Unfortunately, this meta-analysis is now outdated, and several large-scale genetic association studies of ALDH2*2 in the Asian population have been conducted since then.
Therefore, we examined blood ALDH2 expression in Japanese LOAD patients. Then, a genetic association study was conducted using our Japanese cohort, and new studies were added to the extended meta-analysis of the Asian population.
METHODS
Re-analysis of our transcriptome data using an AD mouse model (3xTg-AD)
We used our transcriptome data to reanalyze the Aldh2 mRNA levels in the AD mouse model (3xTg-AD) and control mice. Detailed methods were shown in our published paper [19]. In brief, mRNA was extracted from the blood and hippocampus of 3xTg-AD and control mice at different ages (at 12 and 52 weeks of age) and used for microarray analysis. The animal experiments were approved by the Animal Experiment Committee of Ehime University (#28–25) and were performed in accordance with the Guidelines for Animal Experiments at Ehime University. The microarray data were deposited in the GEO database (accession number GSE144459). From this database, we re-analyzed the Aldh2 mRNA levels.
Subjects for the blood expression study
Demographic data for each group of participants are shown in Table 1. A total of 45 AD subjects who visited Ehime University Hospital [mean age±standard deviation (SD) = 77.31±4.39 years] were diagnosed and classified as having probable AD dementia according to the criteria of the National Institute on Aging and the Alzheimer’s Association [20]. The control participants were 54 elderly individuals [mean age±SD = 77.1±5.57 years] without cognitive impairment, psychiatric signs, or a history of neuropsychiatric diseases as determined by at least two certified psychiatrists based on clinical interviews.
Table 1
Demographic data and clinical characteristics of control and LOAD subjects for the gene expression study
| Control (n = 54) | LOAD (n = 45) | p | |
| Male, n (%) | 18 (33.3) | 15 (33.3) | |
| Female, n (%) | 36 (66.7) | 30 (66.7) | 0.948 |
| Age, years | 77.1±5.57 | 77.3±4.39 | 0.848 |
| Diabetes mellitus (n) | – | 6 | |
| MMSE score | – | 19.0±3.90 | |
| NPI score | – | 12.31±18.3 | |
| ADAS score | – | 19.12±8.66 | |
| CDR score | – | 1.42±0.64 |
Sex; χ2 test, age; Student’s t test. Data are given as mean±SD unless indicated otherwise. There were no differences in the sex and age between control and LOAD subjects (sex 0.948 χ2 test; age 0.848 Student’s t-test).
Subjects for the genetic association study
Demographic data for each group of participants are shown in Table 2. A total of 271 AD subjects (74 males and 197 females, mean age±SD = 81.6±6.41 years) who visited Ehime University Hospital, Saiseikai Saijo Hospital, Kuroda Hospital, Maajiro Kujira Rehabilitation Hospital, Heisei Hospital, Japan Community Health Care Organization Uwajima Hospital, Matsukaze Hospital, Nomura Hospital, or Zaidan Niihama Hospital, Ehime, Japan, from December 2013 to May 2015 were enrolled. AD subjects were diagnosed and classified as having probable AD dementia according to the criteria of the National Institute on Aging and the Alzheimer’s Association [20]. The control participants were 492 elderly individuals (149 males and 343 females, mean age±SD = 80.8±5.84 years) without cognitive impairment, psychiatric signs, or a history of neuropsychiatric diseases as determined by at least two certified psychiatrists based on clinical interviews.
Table 2
Demographic data and clinical characteristics of control and LOAD subjects for genetic association study
| Control (n = 492) | LOAD (n = 271) | p | |
| Male, n (%) | 149 (30.3) | 74 (27.3) | |
| Female, n (%) | 343 (69.7) | 197 (72.7) | 0.387 |
| Age, y | 80.8±5.84 | 81.6±6.41 | 0.078 |
Sex; χ2 test, age; Student’s t test. Data are given as mean±SD unless indicated otherwise. There were no differences in sex and age between control and LOAD subjects (sex 0.387 χ2 test; age 0.078 Student’s t-test).
All participants were unrelated, of Japanese origin, and provided written, informed consent using forms that were approved by the institutional ethics committees of each hospital and of Ehime University Hospital (31-K8, 1901009, and 2109001).
Blood sample preparation for the expression study
Total RNA was isolated from whole peripheral blood samples using PaxGene Blood RNA Systems tubes (BD, Tokyo, Japan) according to the standard protocol. The RNA concentration and purity were measured with a NanoDrop-1000 (Thermo Fisher Scientific, Yokohama, Japan), and the 260/280 ratio was between 1.8 and 2.0. RNA (1.0μg) was used to synthesize cDNA with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time quantitative reverse transcription-PCR was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems). Specific TaqMan probes were Hs01007998_m1 (Applied Biosystems) for ALDH2 and Hs.PT.39a.22214836 (IDT) for GAPDH. GAPDH was used as a housekeeping gene because previous studies, including ours, consistently identified GAPDH as a suitable housekeeping gene for blood gene expression analysis using the Paxgene blood RNA system [21–24]. The final volume of reactions was 10μL with TaqMan Universal Master Mix (Applied Biosystems). The expression levels were examined in triplicate. The ΔΔCt method was used to determine relative expression levels using StepOne software (Applied Biosystems).
Blood sample preparation for the genetic association study
For genotyping, genomic DNA (gDNA) was obtained from whole peripheral blood samples collected in potassium EDTA tubes and extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Tokyo, Japan) according to the standard protocol. PCR was performed with 10.0 ng gDNA, 2.5μL of the 2×TaqMan genotyping Master Mix (Thermo Fisher Scientific), 0.125μL of the 20×TaqMan Assay mix, and 2.375μL of nuclease-free water. A specific TaqMan probe for the SNP analysis was selected for ALDH2 rs671, assay ID: C__11703892_10 (TaqMan Assays, Applied Biosystems). Cycling conditions were as follows: denaturation at 95°C for 10 min, followed by 50 cycles of 95°C for 15 s, and 60°C for 1 min.
Meta-analysis methods
Data were collected from PubMed, the Cochrane Library, ClinicalTrials.gov, and MEDLINE. The following keywords were used for the search: ‘aldehyde dehydrogenase 2’ and ‘Alzheimer’s disease’. Published studies to examine the association between ALDH2 and LOAD were carefully selected by two independent investigators (J.I. and M.U.). Inclusion criteria were as follows: 1) investigated the association of ALDH2*2 and LOAD risk; 2) case-control studies; 3) sufficient information for genotype counts for calculating the odds ratio (OR) and its corresponding 95% CI. Studies not meeting the above criteria were excluded.
Seventy potential studies were selected through searching and 67 studies were excluded due to not meeting the inclusion criteria. A total of seven potentially relevant case-control studies were identified, but one paper was excluded because the paper did not show the number of the ALDH2*2 allele [25]. Among six studies, five were identical to the preceding meta-analysis [18] and one was newly added [26]. Thus, six studies and the present study were included for meta-analysis (Fig. 1). The meta-analysis examined the relationship between the frequency of ALDH2*2 and the risk of LOAD. Odds ratios (ORs) and their 95% confidence intervals (95% CIs) were estimated for 1/1 versus the other carriers (1/2 and 2/2). To combine individual study results, the meta-analysis was conducted using EZR [27].
Fig. 1
The flow diagram of the literature search. Six studies and this study were included for meta-analysis.
Statistical analysis
Statistical analysis was performed using SPSS Statistics version 22.0 (IBM Corp., Tokyo, Japan). For analysis of the effects of ALDH2 alleles on each parameter, these alleles were divided into two groups (1/1 versus 1/2 and 2/2) [17]. ALDH2 mRNA expression levels and the participants’ age were compared between AD and controls using Student’s t-test or the Mann-Whitney U test after the Shapiro-Wilk test. Sex differences and distributions of ALDH2 alleles were compared with the χ2 test. Statistical significance was defined at the 95% level (p = 0.05). Meta-analysis and power calculations were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [27].
RESULTS
Gene expression study in AD mouse model (3xTg-AD)
Re-analysis of our transcriptome data using the AD mouse model (3xTg-AD) shows that Aldh2 mRNA expression in blood is increased with age in wild-type mice (ANOVA p < 0.01, Dunnett’s multiple comparison test: C12 versus C52 p < 0.01) and that in the hippocampus is significantly elevated in 3xTg-AD mice (ANOVA p < 0.001, Dunnett’s multiple comparison test: C12 versus AD12 p < 0.01 and C12 versus AD12 p < 0.0001) (Fig. 2).
Fig. 2
Re-analysis of our transcriptome data using the AD mouse model (3xTg-AD). Aldh2 mRNA expression in blood is increased with age in wild-type mice (ANOVA p < 0.01, Dunnett’s multiple comparison test: C12 versus C52 p < 0.01) and that in the hippocampus is significantly elevated in 3xTg-AD mice (ANOVA p < 0.001, Dunnett’s multiple comparison test: C12 versus AD12 p < 0.01 and C12 versus AD12 p < 0.0001). Lines are at means with 95% CI [19]. AD12, AD mouse at 12 weeks of age; AD52, AD mouse at 52 weeks of age; C12, control mouse at 12 weeks of age; C52, control mouse at 52 weeks of age.
![Re-analysis of our transcriptome data using the AD mouse model (3xTg-AD). Aldh2 mRNA expression in blood is increased with age in wild-type mice (ANOVA p < 0.01, Dunnett’s multiple comparison test: C12 versus C52 p < 0.01) and that in the hippocampus is significantly elevated in 3xTg-AD mice (ANOVA p < 0.001, Dunnett’s multiple comparison test: C12 versus AD12 p < 0.01 and C12 versus AD12 p < 0.0001). Lines are at means with 95% CI [19]. AD12, AD mouse at 12 weeks of age; AD52, AD mouse at 52 weeks of age; C12, control mouse at 12 weeks of age; C52, control mouse at 52 weeks of age.](https://content.iospress.com:443/media/jad/2022/87-2/jad-87-2-jad215627/jad-87-jad215627-g002.jpg)
Gene expression study
The ALDH2 mRNA expression level was significantly higher in subjects with LOAD than in controls (Fig. 2). ALDH2 mRNA expression levels were significantly higher in men with LOAD than in women with LOAD (men 1.51±0.43 versus women 1.28±0.31, Student’s t-test: p = 0.043). ALDH2 mRNA expression levels were not associated with the number of ALDH2*2 (r = 0.158 p = 0.307) or other clinical factors (presence of diabetes mellitus: r = –0.004 p = 0.987, Mini-Mental State Examination (MMSE): r = –0.11 p = 0.942, Neuropsychiatric inventory (NPI): r = –0.106 p = 0.497, Alzheimer’s Disease Assessment Scale (ADAS): r = 0.237 p = 0.184, Clinical Dementia Rating (CDR): r = –0.018 p = 0.911). The number of ALDH2*2 was not correlated with clinical factors (presence of diabetes mellitus: r = –0.224 p = 0.330, MMSE: r = –0.086 p = 0.578, NPI: r = –0.152 p = 0.337, ADAS: r = 0.076 p = 0.681, CDR: r = –0.030 p = 0.851).
Genetic association study
LOAD and control subjects did not differ in sex or age. The distributions of ALDH2 and rs671 are shown in Table 3. The genotypes in the two classified groups and the allele frequency in AD were significantly different from those of control subjects (genotypes, p = 0.015; allele frequencies, p = 0.044).
Table 3
Genotype and allele frequencies of the ALDH2 gene in patients with LOAD and control subjects
| Genotypes N (%) | 1/1 | 1/2 | 2/2 | p | Allele frequencies N (%) | 1 | 2 | p |
| LOAD (n = 271) | 142 (52.4) | 112 (41.3) | 17 (6.3) | 0.015 | LOAD | 396 (73.1) | 146 (26.9) | 0.044 |
| Control (n = 492) | 299 (60.8) | 175 (35.6) | 18 (3.7) | Control | 773 (78.6) | 211 (21.4) |
Differences in the ratio for each genotype and allele frequency between LOAD and control were significant according to the χ2 test.
Meta-analysis
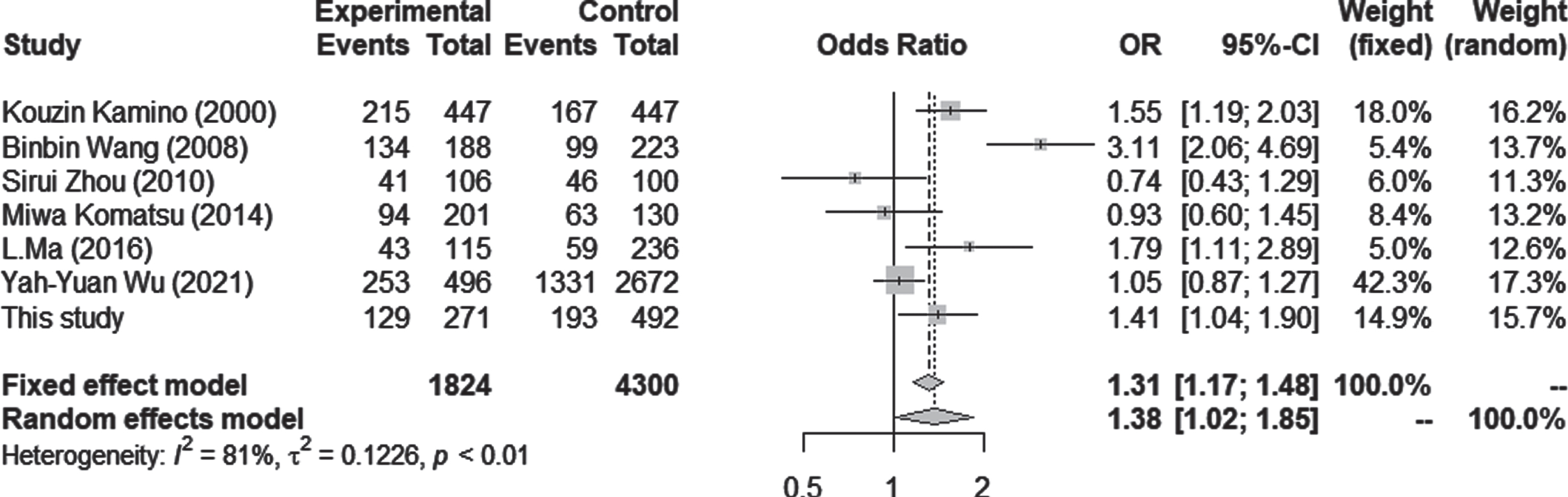
Seven case-control studies using Asian populations were included in the meta-analysis, which consisted of 1,824 cases and 4,300 controls. The association between the number of ALDH2*2 and AD was tested by estimating the ORs for 1/1 versus 1/2 + 2/2 carriers. There was a significant difference in the ALDH2*2 allele, with an increased AD risk under both random and fixed models (OR = 1.38; 95% CI = 1.02–1.85; p = 0.0348, I2 = 81.1%) (Fig. 3). Although statistical analysis for publication bias could not be done due to the small sample size, the funnel plots seemed not to have a significant bias (Supplementary Figure 1).
Fig. 3
Relative ALDH2 mRNA expression levels in AD and control subjects. The mean expression level is significantly higher in AD subjects (1.35±0.36) than in control subjects (1.19±0.38) (Student’s t-test, p = 0.030). Lines are at means with 95% CI.
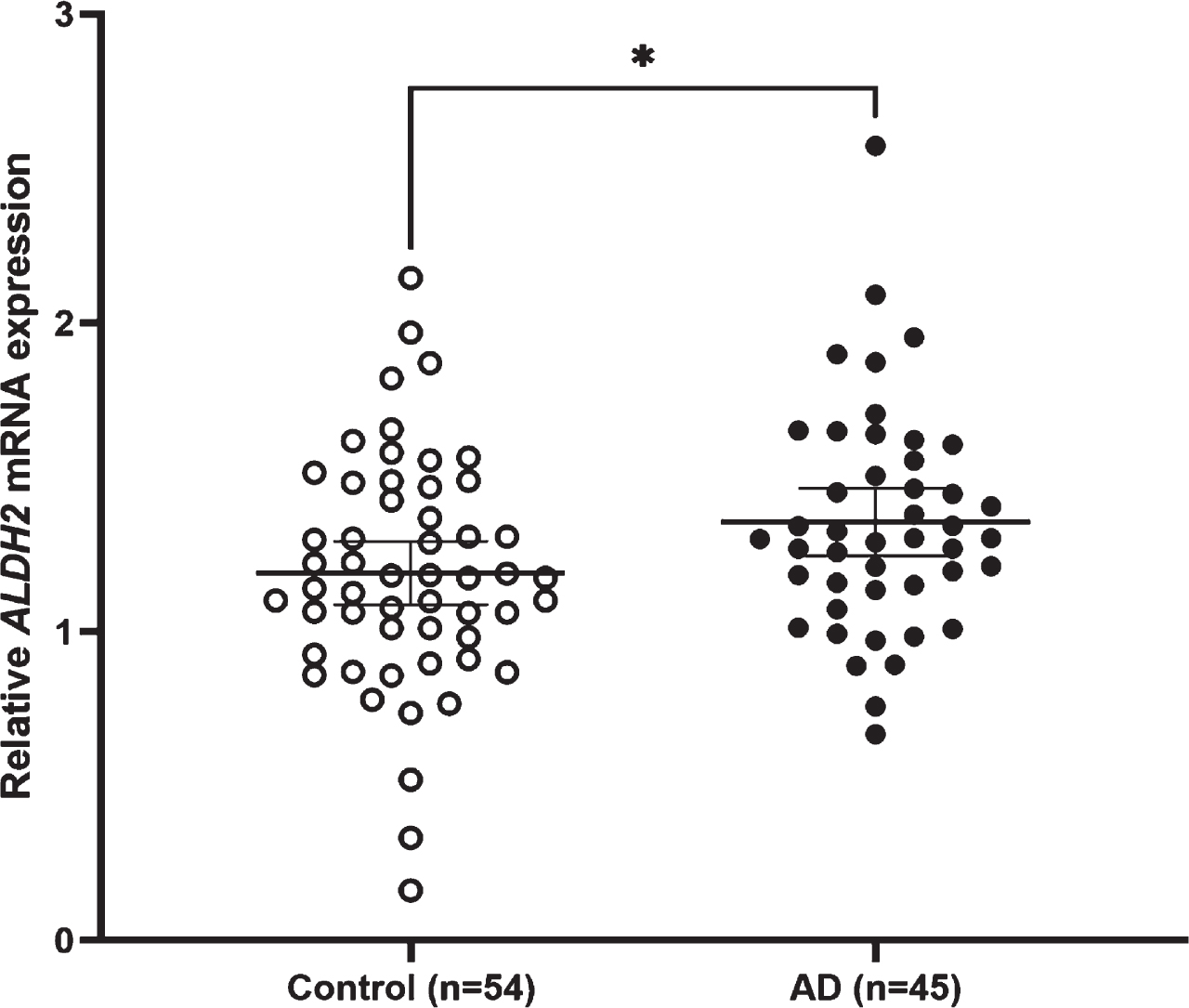
Fig. 4
Relative ALDH2 mRNA expression levels in male and female LOAD. The mean expression level is significantly higher in male LOAD subjects (1.51±0.43) than in female LOAD subjects (1.28±0.31) (Student’s t-test: p = 0.043). Lines are at means with 95% CI.
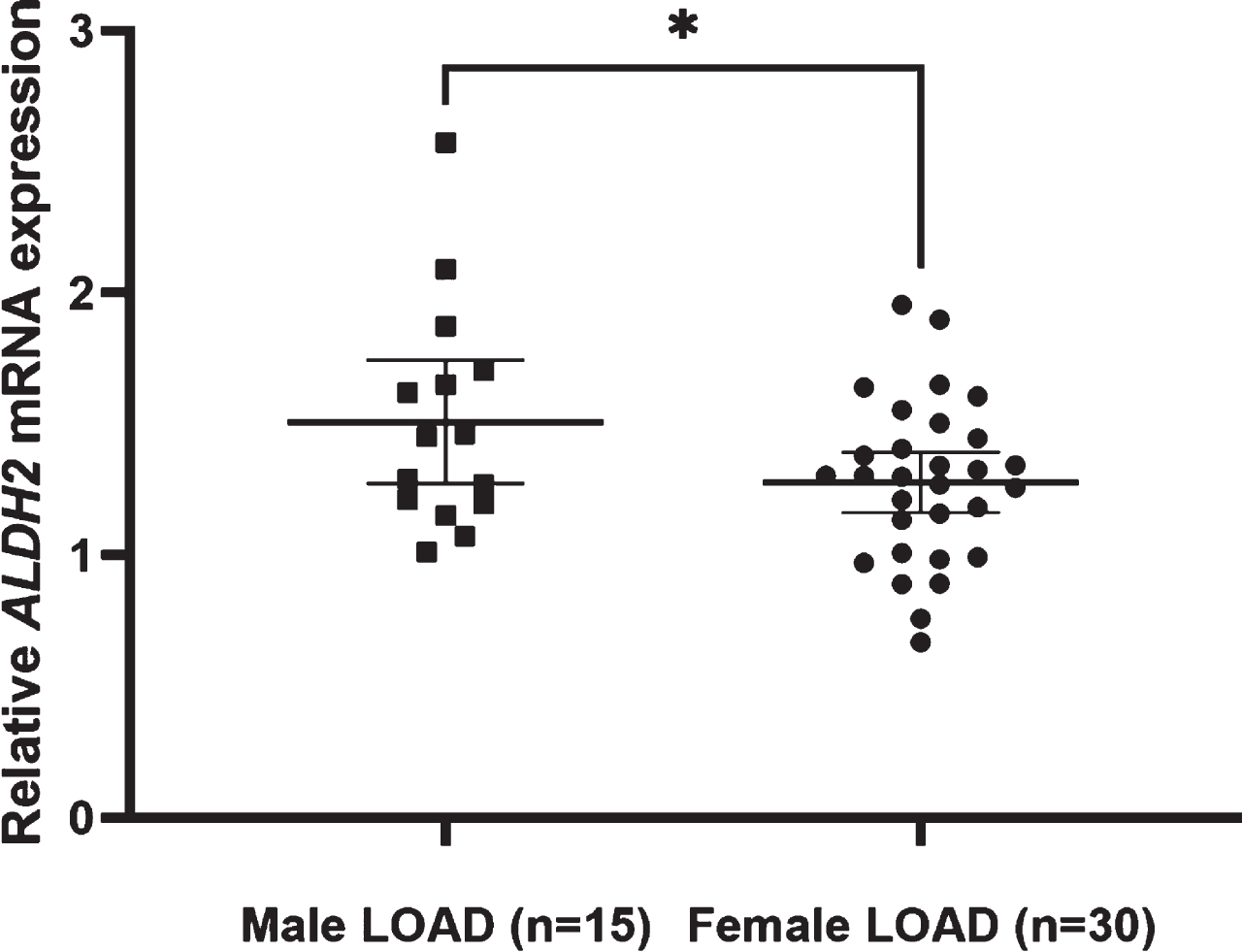
Fig. 5
Forest plots for the meta-analysis in the Asian population. Pooled ORs and 95% CIs were examined for 1/1 versus 1/2 + 2/2. Both fixed effect and random effects models showed significantly higher odds ratios for ALDH2*2 in LOAD.
DISCUSSION
There were three major findings in this study. First, this was the first study to show elevated blood ALDH2 mRNA expression in LOAD. This result was consistent with the re-analysis of our transcriptome study using an AD mouse model (3xTg-AD) showing that ALDH2 mRNA expression in blood was increased with age in WT mice, and that in the hippocampus, it was significantly elevated in both young and old 3xTg-AD mice (Fig. 2) [19]. ALDH2*2 genotypes did not alter the ALDH2 mRNA expression in our study, which is consistent with the previous studies showing that this SNP did not change relative ALDH mRNA levels in genetically engineered knock-in mice that express the human ALDH2*1 (wild-type allele) or ALDH2*2 gene (mutant allele) but reduced the ALDH enzyme activity [28]. ALDH2 is a nuclear gene, but it is transported and functions in the mitochondrial matrix [29]. Mitochondria are well known to be impaired from the early stage of LOAD [30–34]. ALDH2 is involved in metabolizing aldehydes [35, 36] such as acetaldehyde, 4-HNE, 3,4-dihydroxyphenylacetaldehyde (DOPAL, MAO product of dopamine), and 3,4-dihydroxyphenylglycoaldehyde (PDPEGAL, MAO product of norepinephrine), which accumulates in AD patients’ brains from the early stage [37–39]. Because ALDH2 activity is known to protect neurons against neurotoxicity induced by toxic aldehydes during oxidative stress and plays a role in neurodegenerative conditions such as AD [40, 41], elevated blood ALDH2 expression may be associated with a protective response to toxic aldehydes in mitochondria. We found that ALDH2 mRNA expression levels were significantly higher in men with LOAD than in women with LOAD. This may be related to the sex differences in alcohol consumption because it is reported that drinking behavior in Japanese elderly men was significantly higher than that in women and ALDH2*2 was a major factor associated with drinking behavior [42]. Moreover, regardless of ALDH2*2 genotype, ALDH2 mRNA in peripheral blood of healthy young Japanese volunteers increased following ethanol ingestion [43]. These studies indicate that alcohol consumption should be included as a covariate in a future study on ALDH2 gene expression and association.
Second, there was a significant association between ALDH2*2 and the development of LOAD in the present Japanese cohort. The frequencies of ALDH2*2 were significantly increased in subjects with LOAD compared to those of control subjects. The odds ratio was 1.41, which is consistent with the preceding study using another Japanese cohort [17]. The variant from G to A in exon12 of ALDH2 (ALDH2*2) causes very low levels of mitochondrial aldehyde dehydrogenase activity [44]. ALDH2-KO mice showed decreased cognitive function [13], and Alda-1, which activates the ALDH2 enzyme, improved the pathological and clinical outcome in the AD mouse model [45].
Third, the present comprehensive meta-analysis combining seven genetic studies also showed a significant association between ALDH2*2 and the development of LOAD in the Asian population. This is the first meta-analysis to establish a significant association between ALDH2*2 and LOAD in the Asian population. Although none of the recent genome wide association studies using Caucasian samples [46] or Asian samples [47, 48] detected genome-wide significance of the ALDH2 gene, this analysis suggested that ALDH2*2 has some role in the pathogenesis of LOAD in the Asian population. Interestingly, a recent genome-wide associated study in a Han Chinese population showed that ALDH2*2 was associated with serum uric acid by affecting alcohol consumption [49]. Similarly, the role of ALDH2*2 in the pathogenesis of LOAD may be affected by alcohol consumption and sex effects, which may have made it difficult for past genome-wide association studies to confirm the significance of the ALDH2 gene.
There are some limitations in this study. The power analysis using EZR revealed the minimum number of AD and controls required for our gene expression analysis were 77 and 92, respectively. Thus, our results for gene expression analysis may not have adequate power. Due to the low power, the negative findings in this study cannot be interpreted as showing no associations. Elevated blood ALDH2 mRNA expression in LOAD subjects may arise from other factors such as glucose [50, 51]. Although the presence of diabetes mellitus did not affect ALDH2 mRNA expression in the present study, the effects of other comorbidities need to be examined. A receiver operating characteristic curve using ALDH2 mRNA levels for diagnosis of LOAD revealed that the area under the curve was 0.623. This was relatively small, so this biomarker alone was insufficient for proper diagnosis. Combination with other biomarkers may increase the sensitivity and specificity [19, 22]. In addition, the effect of alcohol consumption, which may affect the gene expression of ALDH2 and the development of LOAD, was not examined. This study included only studies from East Asia, because ALDH2*2 is very rare in other populations. Therefore, in the future, larger studies from various regions in the world are needed. Furthermore, other approaches, such as epigenetic analysis and functional analysis of mitochondria, may be useful for demonstration of the involvement of ALDH2 in the pathophysiology of LOAD. ALDH2 protein levels and enzyme activity should be measured in a future study because increased mRNA levels are not necessarily indicative of increased protein levels [52].
Conclusion
There was a significant increase in blood ALDH2 expression and a genetic association with ALDH2*2 in LOAD. ALDH2 may have significant roles in the pathogenesis of LOAD in the Asian population.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Chiemi Onishi for technical assistance. This study was supported by the Japan Agency for Medical Research and Development (JP20dk0207025 and JP20dk0307076) and JSPS KAKENHI Grant Numbers 18H02752, 18K07564, 20K07971, and 20K16628.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5627r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215627.
REFERENCES
[1] | Liu H , Lutz M , Luo S , Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Association between polygenic risk score and the progression from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis 84: , 1323–1335. |
[2] | Wightman DP , Jansen IE , Savage JE , Shadrin AA , Bahrami S , Holland D , Rongve A , Børte S , Winsvold BS , Drange OK , Martinsen AE , Skogholt AH , Willer C , Bråthen G , Bosnes I , Nielsen JB , Fritsche LG , Thomas LF , Pedersen LM , Gabrielsen ME , Johnsen MB , Meisingset TW , Zhou W , Proitsi P , Hodges A , Dobson R , Velayudhan L , Heilbron K , Auton A ; 23andMe Research Team, Sealock JM , Davis LK , Pedersen NL , Reynolds CA , Karlsson IK , Magnusson S , Stefansson H , Thordardottir S , Jonsson PV , Snaedal J , Zettergren A , Skoog I , Kern S , Waern M , Zetterberg H , Blennow K , Stordal E , Hveem K , Zwart JA , Athanasiu L , Selnes P , Saltvedt I , Sando SB , Ulstein I , Djurovic S , Fladby T , Aarsland D , Selbæk G , Ripke S , Stefansson K , Andreassen OA , Posthuma D ((2021) ) A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat Genet 53: , 1276–1282. |
[3] | Yu JT , Xu W , Tan CC , Andrieu S , Suckling J , Evangelou E , Pan A , Zhang C , Jia J , Feng L , Kua EH , Wang YJ , Wang HF , Tan MS , Li JQ , Hou XH , Wan Y , Tan L , Mok V , Tan L , Dong Q , Touchon J , Gauthier S , Aisen PS , Vellas B ((2020) ) Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry 91: , 1201–1209. |
[4] | Maccioni RB , Navarrete LP , Gonzalez A , Gonzalez-Canacer A , Guzman-Martinez L , Cortes N ((2020) ) Inflammation: A major target for compounds to control Alzheimer’s disease. J Alzheimers Dis 76: , 1199–1213. |
[5] | Joshi AU , Van Wassenhove LD , Logas KR , Minhas PS , Andreasson KI , Weinberg KI , Chen CH , Mochly-Rosen D ((2019) ) Aldehyde dehydrogenase 2 activity and aldehydic load contribute to neuroinflammation and Alzheimer’s disease related pathology. Acta Neuropathol Commun 7: , 190. |
[6] | Sao T , Yoshino Y , Yamazaki K , Ozaki Y , Mori Y , Ochi S , Yoshida T , Mori T , Iga JI , Ueno SI ((2018) ) MEF2C mRNA expression and cognitive function in Japanese patients with Alzheimer’s disease. Psychiatry Clin Neurosci 72: , 160–167. |
[7] | Sao T , Yoshino Y , Yamazaki K , Ozaki Y , Mori Y , Ochi S , Yoshida T , Mori T , Iga JI , Ueno SI ((2018) ) TREM1 mRNA expression in leukocytes and cognitive function in Japanese patients with Alzheimer’s disease. J Alzheimers Dis 64: , 1275–1284. |
[8] | Lee T , Lee H ((2020) ) Prediction of Alzheimer’s disease using blood gene expression data. Sci Rep 10: , 3485. |
[9] | Kumon H , Yoshino Y , Funahashi Y , Mori H , Ueno M , Ozaki Y , Yamazaki K , Ochi S , Mori T , Iga JI , Nagai M , Nomoto M , Ueno SI ((2021) ) PICALM mRNA expression in the blood of patients with neurodegenerative diseases and geriatric depression. J Alzheimers Dis 79: , 1055–1062. |
[10] | Swerdlow RH ((2018) ) Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimers Dis 62: , 1403–1416. |
[11] | Vasiliou V , Pappa A , Petersen DR ((2000) ) Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact 129: , 1–19. |
[12] | Deza-Ponzio R , Herrera ML , Bellini MJ , Virgolini MB , Herenu CB ((2018) ) Aldehyde dehydrogenase 2 in the spotlight: The link between mitochondria and neurodegeneration. Neurotoxicology 68: , 19–24. |
[13] | D’Souza Y , Elharram A , Soon-Shiong R , Andrew RD , Bennett BM ((2015) ) Characterization of Aldh2 (-/-) mice as an age-related model of cognitive impairment and Alzheimer’s disease. Mol Brain 8: , 27. |
[14] | Ohsawa I , Nishimaki K , Murakami Y , Suzuki Y , Ishikawa M , Ohta S ((2008) ) Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci 28: , 6239–6249. |
[15] | Yoshida A , Huang IY , Ikawa M ((1984) ) Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci U S A 81: , 258–261. |
[16] | Goedde HW , Agarwal DP , Harada S , Rothhammer F , Whittaker JO , Lisker R ((1986) ) Aldehyde dehydrogenase polymorphism in North American, South American, and Mexican Indian populations. Am J Hum Genet 38: , 395–399. |
[17] | Kamino K , Nagasaka K , Imagawa M , Yamamoto H , Yoneda H , Ueki A , Kitamura S , Namekata K , Miki T , Ohta S ((2000) ) Deficiency in mitochondrial aldehyde dehydrogenase increases the risk for late-onset Alzheimer’s disease in the Japanese population. Biochem Biophys Res Commun 273: , 192–196. |
[18] | Liu H , Ge W , Chen W , Kong X , Jian W , Wang A ((2020) ) Association between ALDH2 gene polymorphism and late-onset Alzheimer disease: An up-to-date meta-analysis. Curr Alzheimer Res 17: , 105–111. |
[19] | Ochi S , Iga JI , Funahashi Y , Yoshino Y , Yamazaki K , Kumon H , Mori H , Ozaki Y , Mori T , Ueno SI ((2020) ) Identifying blood transcriptome biomarkers of Alzheimer’s disease using transgenic mice. Mol Neurobiol 57: , 4941–4951. |
[20] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr. , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[21] | Livak KJ , Schmittgen TD ((2001) ) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: , 402–408. |
[22] | Watanabe SY , Iga J , Ishii K , Numata S , Shimodera S , Fujita H , Ohmori T ((2015) ) Biological tests for major depressive disorder that involve leukocyte gene expression assays. J Psychiatr Res 66-67: , 1–6. |
[23] | Watanabe S , Iga J , Nishi A , Numata S , Kinoshita M , Kikuchi K , Nakataki M , Ohmori T ((2014) ) Microarray analysis of global gene expression in leukocytes following lithium treatment. Hum Psychopharmacol 29: , 190–198. |
[24] | Hu N , Tan MS , Yu JT , Sun L , Tan L , Wang YL , Jiang T , Tan L ((2014) ) Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. J Alzheimers Dis 38: , 497–501. |
[25] | Kim JM , Stewart R , Shin IS , Jung JS , Yoon JS ((2004) ) Assessment of association between mitochondrial aldehyde dehydrogenase polymorphism and Alzheimer’s disease in an older Korean population. Neurobiol Aging 25: , 295–301. |
[26] | Wu YY , Lee YS , Liu YL , Hsu WC , Ho WM , Huang YH , Tsai SJ , Kuo PH , Chen YC ((2021) ) Association study of alcohol dehydrogenase and aldehyde dehydrogenase polymorphism with Alzheimer disease in the Taiwanese population. Front Neurosci 15: , 625885. |
[27] | Kanda Y ((2013) ) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: , 452–458. |
[28] | Hirohashi K , Ohashi S , Amanuma Y , Nakai Y , Ida T , Baba K , Mitani Y , Mizumoto A , Yamamoto Y , Kikuchi O , Matsubara J , Yamada A , Miyamoto S , Seno H , Matsuda T , Muto M ((2020) ) Protective effects of Alda-1, an ALDH2 activator, on alcohol-derived DNA damage in the esophagus of human ALDH2*2 (Glu504Lys) knock-in mice. Carcinogenesis 41: , 194–202. |
[29] | Chen CH , Ferreira JC , Gross ER , Mochly-Rosen D ((2014) ) Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol Rev 94: , 1–34. |
[30] | Mise A , Yoshino Y , Yamazaki K , Ozaki Y , Sao T , Yoshida T , Mori T , Mori Y , Ochi S , Iga JI , Ueno SI ((2017) ) TOMM40 and APOE gene expression and cognitive decline in Japanese Alzheimer’s disease subjects. J Alzheimers Dis 60: , 1107–1117. |
[31] | Spuch C , Ortolano S , Navarro C ((2012) ) New insights in the amyloid-beta interaction with mitochondria. J Aging Res 2012: , 324968. |
[32] | Hirai K , Aliev G , Nunomura A , Fujioka H , Russell RL , Atwood CS , Johnson AB , Kress Y , Vinters HV , Tabaton M , Shimohama S , Cash AD , Siedlak SL , Harris PL , Jones PK , Petersen RB , Perry G , Smith MA ((2001) ) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21: , 3017–3023. |
[33] | Du H , Guo L , Fang F , Chen D , Sosunov AA , McKhann GM , Yan Y , Wang C , Zhang H , Molkentin JD , Gunn-Moore FJ , Vonsattel JP , Arancio O , Chen JX , Yan SD ((2008) ) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 14: , 1097–1105. |
[34] | Gan X , Huang S , Wu L , Wang Y , Hu G , Li G , Zhang H , Yu H , Swerdlow RH , Chen JX , Yan SS ((2014) ) Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochim Biophys Acta 1842: , 220–231. |
[35] | Guo JM , Liu AJ , Zang P , Dong WZ , Ying L , Wang W , Xu P , Song XR , Cai J , Zhang SQ , Duan JL , Mehta JL , Su DF ((2013) ) ALDH2 protects against stroke by clearing 4-HNE. Cell Res 23: , 915–930. |
[36] | Kimura M , Yokoyama A , Higuchi S ((2019) ) Aldehyde dehydrogenase-2 as a therapeutic target. Expert Opin Ther Targets 23: , 955–966. |
[37] | Fukuda M , Kanou F , Shimada N , Sawabe M , Saito Y , Murayama S , Hashimoto M , Maruyama N , Ishigami A ((2009) ) Elevated levels of 4-hydroxynonenal-histidine Michael adduct in the hippocampi of patients with Alzheimer’s disease. Biomed Res 30: , 227–233. |
[38] | Markesbery WR , Lovell MA ((1998) ) Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging 19: , 33–36. |
[39] | Lovell MA , Ehmann WD , Mattson MP , Markesbery WR ((1997) ) Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging 18: , 457–461. |
[40] | Ohsawa I , Kamino K , Nagasaka K , Ando F , Niino N , Shimokata H , Ohta S ((2003) ) Genetic deficiency of a mitochondrial aldehyde dehydrogenase increases serum lipid peroxides in community-dwelling females. J Hum Genet 48: , 404–409. |
[41] | Degl’Innocenti D , Ramazzotti M , Sarchielli E , Monti D , Chevanne M , Vannelli GB , Barletta E ((2019) ) Oxadiazon affects the expression and activity of aldehyde dehydrogenase and acylphosphatase in human striatal precursor cells: A possible role in neurotoxicity. Toxicology 411: , 110–121. |
[42] | Sasaki T , Nishimoto Y , Hirata T , Abe Y , Takebayashi T , Arai Y ((2021) ) ALDH2 p.E504K variation and sex are major factors associated with current and quitting alcohol drinking in Japanese oldest old. Genes (Basel) 12: , 799. |
[43] | Kimura Y , Nishimura FT , Abe S , Fukunaga T , Tanii H , Saijoh K ((2009) ) A promoter polymorphism in the ALDH2 gene affects its basal and acetaldehyde/ethanol-induced gene expression in human peripheral blood leukocytes and HepG2 cells. Alcohol Alcohol 44: , 261–266. |
[44] | Farres J , Wang X , Takahashi K , Cunningham SJ , Wang TT , Weiner H ((1994) ) Effects of changing glutamate 487 to lysine in rat and human liver mitochondrial aldehyde dehydrogenase. A model to study human (Oriental type) class 2 aldehyde dehydrogenase. J Biol Chem 269: , 13854–13860. |
[45] | Yang Y , Chen W , Wang X , Ge W ((2021) ) Impact of mitochondrial aldehyde dehydrogenase 2 on cognitive impairment in the AD model mouse. Acta Biochim Biophys Sin (Shanghai) 53: , 837–847. |
[46] | Kunkle BW , Grenier-Boley B , Sims R , Bis JC , Damotte V , Naj AC , Boland A , Vronskaya M , van der Lee SJ , Amlie-Wolf A , et al. ((2019) ) Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet 51: , 414–430. |
[47] | Kang S , Gim J , Lee J , Gunasekaran TI , Choi KY , Lee JJ , Seo EH , Ko PW , Chung JY , Choi SM , Lee YM , Jeong JH , Park KW , Song MK , Lee HW , Kim KW , Choi SH , Lee DY , Kim SY , Kim H , Kim BC , Ikeuchi T , Lee KH ((2021) ) Potential novel genes for late-onset Alzheimer’s disease in East-Asian descent identified by APOE-stratified genome-wide association study. J Alzheimers Dis 82: , 1451–1460. |
[48] | Shigemizu D , Mitsumori R , Akiyama S , Miyashita A , Morizono T , Higaki S , Asanomi Y , Hara N , Tamiya G , Kinoshita K , Ikeuchi T , Niida S , Ozaki K ((2021) ) Ethnic and trans-ethnic genome-wide association studies identify new loci influencing Japanese Alzheimer’s disease risk. Transl Psychiatry 11: , 151. |
[49] | Zhang D , Yang M , Zhou D , Li Z , Cai L , Bao Y , Li H , Shan Z , Liu J , Lv D , Liu Y , Xu C , Ling J , Xu Y , Zhang S , Huang Q , Shi Y , Zhu Y , Lai M ((2018) ) The polymorphism rs671 at ALDH2 associated with serum uric acid levels in Chinese Han males: A genome-wide association study. Gene 651: , 62–69. |
[50] | Wang HJ , Kang PF , Wu WJ , Tang Y , Pan QQ , Ye HW , Tang B , Li ZH , Gao Q ((2013) ) Changes in cardiac mitochondrial aldehyde dehydrogenase 2 activity in relation to oxidative stress and inflammatory injury in diabetic rats. Mol Med Rep 8: , 686–690. |
[51] | Fang T , Cao R , Wang W , Ye H , Shen L , Li Z , Hu J , Gao Q ((2018) ) Alterations in necroptosis during ALDH2-mediated protection against high glucoseinduced H9c2 cardiac cell injury. Mol Med Rep 18: , 2807–2815. |
[52] | Boyum A ((1964) ) Separation of white blood cells. Nature 204: , 793–794. |




