Shortening of Saccades as a Possible Easy-to-Use Biomarker to Detect Risk of Alzheimer’s Disease
Abstract
Background:
Wide-ranging functional defects in eye movements have been reported in Alzheimer’s disease (AD) dementia. The detection of abnormal eye movements and reading problems may identify persons at risk of AD when clear clinical symptoms are lacking.
Objective:
To examine whether computer-based eye-tracking (ET) analysis of King-Devick (KD) test results differentiates cognitively healthy persons from persons with minor problems in cognitive testing or diagnosed mild AD.
Methods:
We recruited 78 participants (57 non-demented, 21 with mild AD) who underwent neurological examination, the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological test battery (CERAD-NB), and a Clinical Dementia Rating (CDR) interview. The non-demented participants were further divided into control (normal CERAD subtests, mean MMSE = 28) and objective mild cognitive impairment (MCI; decline in at least one CERAD memory score, mean MMSE = 27) groups. The KD reading test was performed using computer-based ET. The total time used for the reading test, errors made, fixation and saccade durations, and saccade amplitudes were analyzed.
Results:
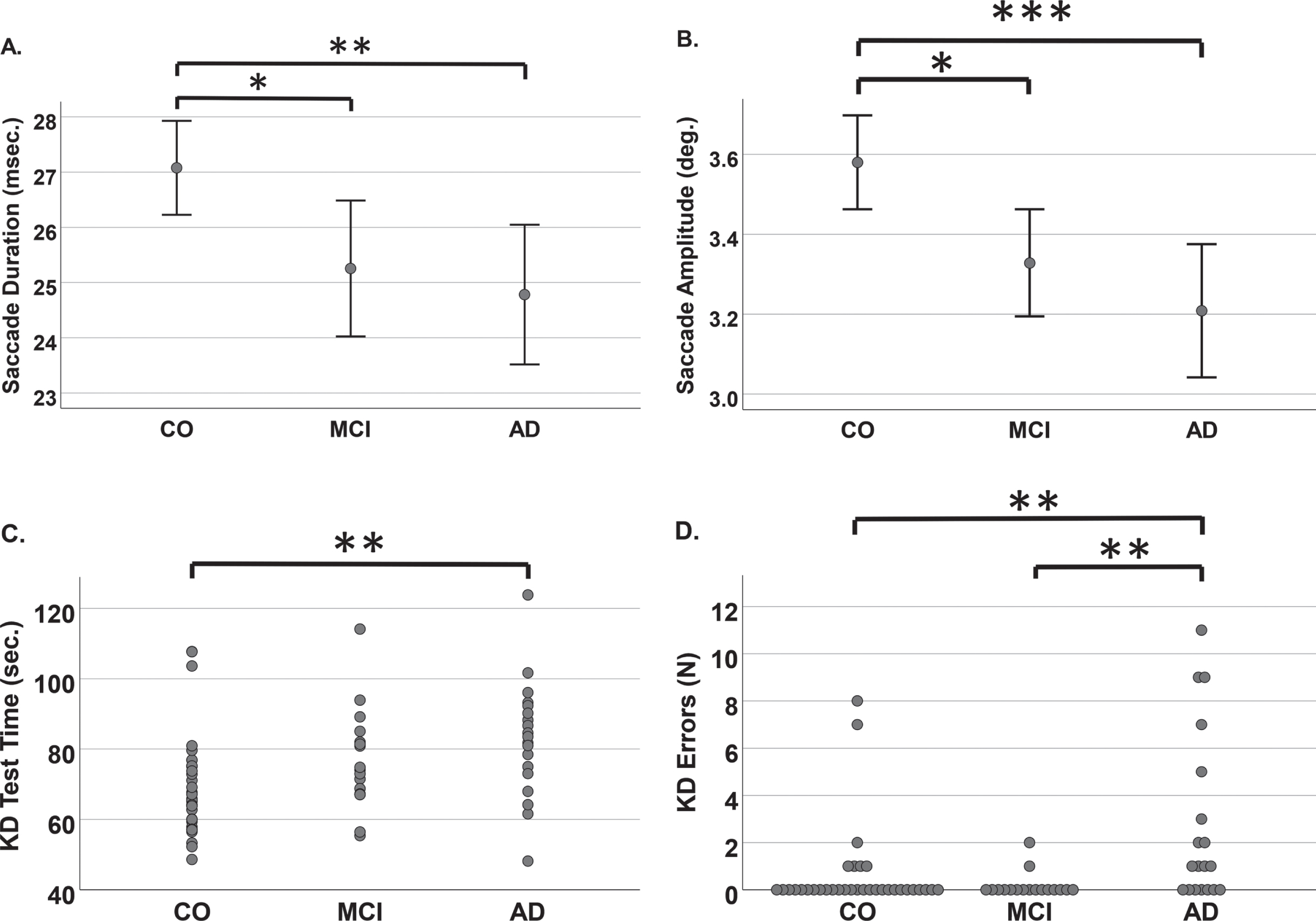
We found significant differences between the control, objective MCI, and AD groups in regard to the mean saccade amplitude (3.58, 3.33, and 3.21 ms, respectively, p < 0.03) and duration (27.1, 25.3, and 24.8 ms, respectively, p < 0.05). The KD error scores in the AD group differed significantly (p < 0.01) from the other groups.
Conclusion:
Computed ET analysis of the KD test may help detect persons with objective MCI early when clear clinical symptoms are lacking. The portable device for ET is easy to use in primary health care memory clinics.
INTRODUCTION
Alzheimer’s disease (AD) pathology begins to develop in the brain up to 20 years before clinical symptoms [1]. AD has been shown to have a broad range of effects on eye functions and the oculomotor system. Visual field deficits, prolonged visual evoked potentials, abnormal eye movement recordings, and abnormal eye movements during reading have been reported to occur in AD dementia [2–4]. The severity of reading problems increases as AD progresses [5]. Recent studies have demonstrated disturbed eye movements (saccades and fixations) and reading problems in persons with mild cognitive impairment (MCI) [5–10].
If AD can be diagnosed in the prodromal stage, or a person at risk can be reliably detected by minor cognitive changes, available treatment can be initiated early to maintain cognition and daily functions longer [11]. Few biological biomarkers are currently available to help diagnose prodromal AD [12]. The main problems with these biomarkers are that none of them are sensitive enough or always available [13].
The detection of abnormal eye movements during a reading test may offer a new easy-to-use indication, or at least be a supportive biomarker candidate, for diagnosing persons with MCI who are at risk of AD. King-Devick (KD) is a short reading test originally developed to detect children with oculomotor and reading problems and, more recently, in contact sports to detect possible concussion, but it is a potential test for detecting cognitive impairments in other neurological conditions, such as Parkinson’s disease (PD) and AD. The KD test has also been piloted for distinguishing healthy controls from persons with AD even in the early stages of the disease [14].
Eye-tracking (ET) is a useful tool for detecting saccadic eye movements during the reading task. The control of saccades decreases with age, even in healthy persons [15]. ET recordings have also been used in research to find differences in oculomotor activity between healthy controls and persons with AD dementia [5, 7]. In addition to AD, ET technology has been used to screen cognitive dysfunction in other neurological disorders, such as PD, amyotrophic lateral sclerosis, multiple sclerosis, and epilepsy [16].
The Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological test battery (CERAD-NB) is a valid and widely used measure originally developed for screening cognitive impairment in the early stages of AD [17]. The CERAD-NB total score has been shown to be a more sensitive method of discriminating controls from MCI than the Mini-Mental State Examination (MMSE) [18].
Our aim in the present study was to examine whether computer-based ET analysis of the KD test differentiates cognitively healthy persons from persons with minor changes on cognitive tests, reflecting objective MCI and the risk of AD when clear clinical symptoms are still lacking.
METHODS
Ethics statement
The study adhered to the principles of the Declaration of Helsinki and has been evaluated and accepted by The Research Ethics Committee of the Northern Savo Hospital District (Dnro: 482/2017). All study participants were verbally informed, read the information letter, and signed the informed consent prior to participation. The proxy consent was signed for individuals diagnosed with dementia stage AD.
Study design, participants, and study protocol
A total of 78 volunteers > 60 years old were recruited at the Brain Research Unit of the University of Eastern Finland for this cross-sectional study. Among the participants, 57 were non-demented and 21 had mild AD dementia. The persons with AD were used as positive controls. The persons with AD had been examined by a neurologist with special competence in memory disorders prior to this study. The AD dementia diagnoses were made on the basis of the revised National Institute on Aging and Alzheimer’s Association (NIA/AA) criteria [19].
Brain MRI or CT had been performed for all participants with mild AD dementia, and they had all initiated suitable AD medication. All of the participants underwent cognitive evaluation with the CERAD-NB [17] and neurological examination. The interviews relating to Clinical Dementia Rating (CDR) were carried out with both the participants and family members [19] to obtain a broad spectrum of demographic information on medical history, medication, education level and length, daily function, and family risk for AD. The study participants were classified into three groups: cognitively healthy controls, objective MCI, and mild AD dementia (Fig. 1A). Based on CERAD test results, there were 12 amnestic MCI and 8 non-amnestic MCI participants. A non-demented person was considered to have normal cognition if they performed all of the CERAD subtests within normal variation and had no reported decline in daily function in the interviews (CDR 0). A participant was classified into the MCI group (CDR 0.5) if she or he failed at least one CERAD- memory subtest and were independent in daily activities. Final groups were confirmed by clinicians specialized in memory disorders (AK, MH, MR).
The exclusion criteria were diabetes, any signs of parkinsonism, upper motoneuron deficits, cerebellum disorders, dementia due to an etiology other than AD, moderate or severe AD (CDR 2 or 3). The exclusion criteria were also severe eye diseases affecting vision (e.g., macular disease, age-related macular degeneration, glaucoma, field of vision deficiency). To verify possible eye diseases, participants’ eyes were clinically examined by an ophthalmologist.
APOE genotyping
Genomic DNA was extracted from venous blood samples using the QIAamp DNA blood mini extraction kit (QIAGEN). APOE gene alleles were determined using TaqMan genotyping assays (App-lied Biosystems (ABI), Foster City, CA, USA) for two single nucleotide polymorphisms (rs429358 and rs7412) and an allelic discrimination method on the ABI 7000 platform [20].
Cognitive tests
The CERAD-NB [17] and MMSE were used to evaluate cognition. The Finnish version of the CERAD-NB includes all subtests from the original English test battery: The Boston Naming Test (15-item version; range 0–15), category fluency (animals; range from 0 to no limit), word list learning (range 0–30), word list recall (range 0–10), word list recognition (range 0–20), and constructional praxis (range 0–11). In addition, the clock drawing test (range 0–6) and constructional praxis delayed recall test (range 0–11) were added to the Finnish version [21]. The MMSE, wordlist learning, wordlist delayed recall %, wordlist recognition %, visuo-constructing copy, visuo-constructing recall %, and CERAD global memory score were used to distinguish study groups from each other based on their cognitive performance in verbal fluency and naming.
King-Devick test
The KD test is a standardized, commercial reading test that has been used to evaluate concussions [14]. The test includes three, short, 1 to 2-minute number reading tasks, with a demonstration card preceding them. The participants read the numbers aloud from the cue cards as fast as possible. The lines of numbers on the cards become gradually more difficult to follow correctly. The scoring includes the total time used for the three cards combined and the total number of errors made.
Eye-tracking recordings and apparatus
ET recordings were carried out using a Windows PC with an USB web camera, microphone, and Tobii TX300 display with an integrated ET unit from Tobii Technology AB, Sweden [22]. The ET data were recorded in authentic hospital settings with fluorescent lighting and, often, some daylight in the room. All participants were 60 cm from the screen. The internal movement compensation by the Tobii TX300 unit was used to ensure data accuracy in the case of small head movements. The unit was calibrated for each participant using regular 9-point calibration with medium speed preset. In addition to ET data, audio and video of the participants were recorded using Tobii Studio 3.2.1 [23].
Data processing
Figure 1B visualizes the selection of participants for statistical analysis based on quality control of the ET and audio data. ET recordings for nine participants (4 control, 1 MCI, 4 AD), who did not have a sample success rate > 60% were disqualified from the ET data analysis. Most of the disqualified participants either wore strong multifocal glasses or had visibly drooping eyelids, which heavily degraded the quality of the ET data. Quality control and data export were conducted on the Tobii Studio 3.4.8 using the built-in I-VT filter with the recommended default values: 75 ms max gap length interpolation, 20 ms window length, 30 deg/s velocity threshold, merging adjacent fixations between 75 ms and 0.5 deg, and discarding fixations shorter than 60 ms [24].
Fig. 1
Division of participants into analysis groups. A) Non-demented participants underwent testing and were divided into control and mild cognitive impairment (MCI) groups. The Alzheimer’s disease (AD) group was confirmed similarly. B) Quality control of analysis groups. All eye-tracking (ET) data went through quality control and recordings with < 60% success rate were disqualified. All audio recordings went through quality control, with garbled or silent recordings being disqualified before the automated King-Devick (KD) results analysis. CERAD-NB, Consortium to Establish a Registry for Alzheimer’s Disease –neuropsychological test battery; CDR, clinical dementia rating; QC, quality control. *One participant from the KD MCI group was removed from the KD analysis due to an excessive number of errors (> 30) made in the last KD card, as they read the test in vertical columns instead of horizontal rows.
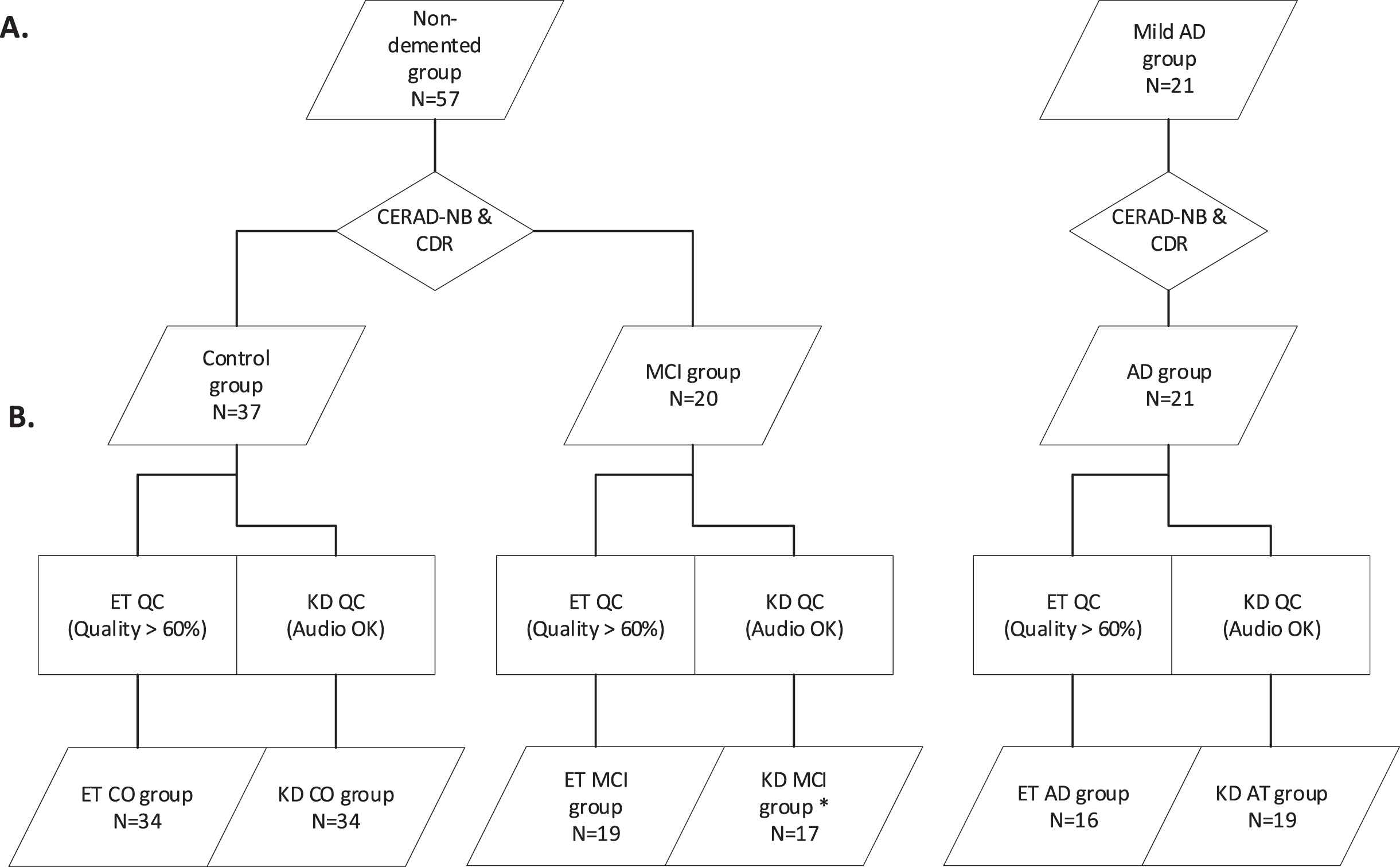
Participants’ audio and video were recorded simultaneously during the ET tests. In case of reliability issues with the setup, garbled or muted audio recordings were removed (4 control, 2 MCI, 1 AD). The successful audio recordings were normalized in Audacity 2.3.3 and all discussion or utterances that were not part of the test task output were cut out. The processed audio files were then timed towards a prepared text file, which contained the correct answers the participants were expected to say, using AaltoASR-align from the AaltoASR 1.1 module on CSC’s Taito supercluster [25]. The resulting TextGrid files with word timings were manually evaluated in ELAN 5.2 against the uncut audio files, required timing adjustments made, and the erroneous words tagged. At this stage, one MCI participant was removed from the analysis due to reading the last KD test card vertically in columns instead of horizontal rows, resulting in an excessive number of errors. The resulting final TextGrid audio transcription data were then joined with the ET metadata for more detailed analysis, including automated scoring of the KD test by counting the errors made.
Statistical analyses
Demographic data and CERAD test results are shown as mean±standard deviation (SD) or as number of cases and proportions (%). For the comparison of means between groups, we used one-way analysis of variance (ANOVA) followed by the LSD post-hoc test. The group comparisons were made using Pearson’s chi-square. Statistical analyses were performed in SPSS 22 software (SPSS Inc, Chicago, IL, USA). p < 0.05 indicated a significant difference.
Data points for both the ET and audio-based analyses were collated and processed using custom-made Python 3.6.9 scripts. For each study participant, individual KD task-specific medians for fixation duration, saccade duration, and saccade amplitudes were calculated from the Tobii exported data, and the annotated audio transcripts were utilized for automated scoring of the KD test. Individual medians were preferred to raw data to compensate for the variability in ET data quality (i.e., sample success rate), as medians would not be affected by abnormal artificial outliers. The data were then imported into IBM SPSS Statistics 25 for statistical analysis. Data were evaluated by one-way ANOVA followed by a Bonferroni post-hoc test. ET recording and KD results are presented as means±standard deviation (SD).
RESULTS
The demographics of the study groups are presented in Table 1. Two copies of APOE ɛ4 alleles were not found in the control group, but 10% of the MCI group and 32% of the AD group had two copies of APOE ɛ4 alleles (p≤0.001; Table 1). Table 2 summarizes the results and significance of the group differences in the nine CERAD subtests. Six of the nine CERAD subtests showed significant differences between the control and MCI groups and between the MCI and AD groups in cognitive performance. Fifty-four (95%) of the non-demented participants (37 control, 17 MCI) achieved MMSE scores ≥25, indicating normal performance. In the demographic interview, 40% of participants in the MCI group reported some subjective cognitive problems, but only 2.7% of control participants reported the same.
Table 1
Demographic characteristics of the study participants
| Number of participants (N) | CO | MCI | AD | p |
| 37 | 20 | 21 | ||
| Gender, female (N) | 54.1% (20) | 45% (9) | 61.9% (13) | 0.555 |
| Age, y | 71 (5.1) | 72 (6.4) | 71 (6.8) | 0.961 |
| Education, y | 13 (4.3) | 11 (3.7) | 13 (4.3) | 0.278 |
| Family history | 51% | 45% | 57% | 0.739 |
| APOE4 carrier (N) | 37.8% (14) | 35% (7) | 78.9% (15)* | 0.006 |
| APOE4 two alleles (N) | 0% (0) | 10% (2) | 32% (6)* | 0.001 |
Values are presented as means (±SD) or percentages (N). CO, control; MCI, persons with mild cognitive impairment; AD, Alzheimer’s disease; APOE4, apolipoprotein E4; SD, standard deviation. *Total N = 19. Bolded p-values indicate significant differences between groups.
All nine CERAD subtests distinguished between the control and AD groups (p≤0.000 in seven tests, Table 2).
Table 2
Mean results for nine tests from the CERAD-NB test battery
| CERAD test battery | Max value | CO | MCI | AD | CO-MCI | MCI-AD | CO-AD |
| N = 37 | N = 20 | N = 21 | p | p | p | ||
| Verbal fluency | > 16 | 25.0 (7.3) | 19.5 (4.6) | 15.7 (6.4) | 0.003 | 0.08 | 0.000 |
| Naming | 15 | 13.4 (1.7) | 12.0 (3.2) | 11.2 (3.4) | 0.07 | 0.275 | 0.002 |
| MMSE score test | 30 | 28.4 (1.5) | 27.0 (2.0) | 23.9 (3.0) | 0.018 | 0.000 | 0.000 |
| Wordlist learning | 30 | 23.1 (30.7) | 17.8 (4.0) | 13.1 (2.1) | 0.000 | 0.000 | 0.000 |
| Wordlist delayed recall % | 100 | 95.1 (10.6) | 75.5 (48.3) | 38.0 (30.6) | 0.021 | 0.000 | 0.000 |
| Wordlist recognition % | 100 | 98.2 (3.6) | 85.3 (24.0) | 75.3 (11.6) | 0.001 | 0.017 | 0.000 |
| Visuo-construction | 11 | 10.5 (1.0) | 9.8 (1.6) | 9.4 (2.7) | 0.149 | 0.538 | 0.027 |
| Visuo-construction recall % | 100 | 95.1 (10.1) | 85.1 (19.8) | 58.9 (39.2) | 0.141 | 0.002 | 0.000 |
| CERAD global memory score | 30 | 27.8 (1.8) | 23.1 (2.9) | 17.0 (3.4) | 0.000 | 0.000 | 0.000 |
Values are presented as means (±SD). CO, control; MCI, mild cognitive impairment; AD, Alzheimer’s disease; CERAD, The Consortium to Establish a Registry for Alzheimer’s disease; MMSE, Mini-Mental State Examination; SD, standard deviation. Bolded p-values indicate significant differences between groups.
ET recordings
Median performance values of ET recordings were calculated for individual participants on the basis of their performances for each card. Then, those individual card-specific medians were used in the statistical analyses. The saccades were longer in controls (mean 27.1±4.3 ms) than in the MCI group (mean 25.3±4.6 ms) or AD group (mean 24.8±4.4 ms). The differences were significant between both the control and MCI groups and between the control and AD groups (Fig. 2A). Comparable differences between the study groups were also detected in saccadic amplitudes. Saccadic amplitudes were decreased in the MCI group (mean 3.33±0.51 deg) and further decreased in the AD group (mean 3.21±0.57 deg) compared to healthy controls (3.58±0.60 deg). Differences were significant between the control and MCI groups (p = 0.024) and between the control and AD groups (p < 0.001; Fig. 2B). The AUC values of ROC analysis for classifying controls from MCI and AD groups were 0.636 for saccade amplitude and 0.641 for saccade duration.
Fig. 2
The total time, saccade duration and amplitude during the King-Devick (KD) test were calculated from eye-tracker recordings. Number of errors were calculated from the audio recordings. Saccade data are shown as means with 95% confidence intervals. A) The mean duration and B) the mean amplitude of saccades were both significantly decreased in the mild cognitive impairment (MCI) and Alzheimer’s disease (AD) groups compared to the control group. C) Scatter plot of the King-Devick (KD) test time (as total time used in seconds). On average, the MCI group was slower than control group, and the AD group was the slowest. D) Stacking scatter plot of the number of errors made during the KD test. In the AD group, more than half of the participants made errors, whereas other groups were mostly error-free. Outliers in the control group read some part of the test out of order. One MCI participant read the last slide of the KD test out of order, making more than 30 errors, and was removed from analysis. * p≤0.05, **p≤0.01, ***p≤0.001.
The duration of fixation was only slightly decreased in the MCI group, and more so in the AD group than in the control group (Table 3).
Table 3
King-Devick (KD) reading test scores and related eye-tracking (ET) recordings
| King -Devick analysis | CO | MCI | AD | CO-MCI | MCI-AD | CO-AD |
| N = 34 | N = 17 | N = 19 | p | p | p | |
| Total time, s | 69.3 (14.2) | 76.6 (14.2) | 82.7 (16.5) | 0.319 | 0.665 | 0.008 |
| Total number of errors | 0.62 (1.81) | 0.18 (0.53) | 2.74 (3.62) | 1.000 | 0.004 | 0.005 |
| Eye-tracking analysis | N = 34 | N = 19 | N = 16 | |||
| Fixation duration, ms | 249 (47) | 247 (42) | 241 (45) | 0.965 | 1.000 | 1.000 |
| Saccade duration, ms | 27.1 (4.3) | 25.3 (4.6) | 24.8 (4.4) | 0.040 | 1.000 | 0.010 |
| Saccade amplitude, deg | 3.58 (0.60) | 3.33 (0.51) | 3.21 (0.57) | 0.024 | 0.849 | < 0.001 |
Values are presented as means (±SD). CO, control; MCI, mild cognitive impairment; AD, Alzheimer’s disease. Bolded p-values indicate significant differences between groups.
King-Devick test
Table 3 summarizes the results of the KD test and ET recordings. As expected, the control group was the fastest and AD group the slowest. The reading speed was slower in the MCI group (mean 76.6±14.2 ms) than in the control group (mean 69.3±14.2 ms) but faster than in the AD group (mean 82.7±16.5 ms). The difference in reading speed between the control and AD groups was significant (p = 0.008; Fig. 2C). In the AD group, most participants made at least one error, and the AD group differed significantly from both the control (p = 0.005) and MCI (p = 0.004) groups, in which most participants did not make any errors during the KD test (Fig. 2D). The AUC values for classifying controls from MCI and AD groups were 0.727 for KD total time and 0.745 for errors made in the KD test.
DISCUSSION
In the present study, computer-based ET analysis of the KD test was able to detect changes in eye movements in persons with cognitive problems earlier than comparable previous studies. The durations and amplitudes of eye saccades in persons with minor cognitive problems (i.e., objective MCI group) changed towards the values detected in study participants with mild AD dementia. We found out that both the duration and amplitude of saccades were significantly decreased in the MCI and AD groups compared to the control group. As expected, the controls were significantly faster on the KD test than the AD patients. The AD group had the highest number of reading errors, and less than half (38.9%) of the AD group managed to complete the KD test without making errors. In contrast, most participants in the control and MCI groups (79% and 88.2%, respectively) completed the KD test error-free. The higher number of errors in the control group compared to the objective MCI group was due to two persons in the control group who made multiple errors during the test (Fig. 2B).
Visual field deficits, prolonged visual evoked potentials, and abnormal eye movements have been reported in AD dementia [2, 26]. There have also been reports of disturbed saccades and fixations and reading problems in persons with MCI [5, 7–10]. Thus, our study results are in line with those of previous studies. Our participants with MCI had higher MMSE scores, for a normal performance on average, than persons with MCI in previous studies, indicating that the described eye problems occur very early in the course of the disease [32, 33].
The KD test is a standardized, commercially available reading test [14]. We consider it to be a potential method of detecting cognitive impairment in neurodegenerative disorders, such as AD. The KD test has also been piloted to distinguish healthy controls from persons with MCI with higher sensitivity than behavioral reading test results alone [14, 27]. ET recordings have previously been reported in research to detect differences in oculomotor activity between healthy controls and persons with AD dementia [5, 7]. Our findings support ET recording being useful for adding value to the KD test, as we were able to show significant group-wise differences between the cognitively healthy control group and persons with minor cognitive problems and mild AD dementia in regard to saccadic eye movements. Considering the AUC results and large in-group variance, neither saccadic duration nor saccadic amplitude alone are reliable classification tools on an individual level; they need to be utilized in conjunction with other results.
We used CERAD-NB as a tool to divide non-demented participants into two study groups: cognitively healthy controls and persons with minor cognitive problems. Furthermore, we tested that the AD group differed from the two other groups on the basis of the CERAD tests. Verbal fluency [28–30], praxis [28, 30], recognition memory [30], and the Boston Naming Test [29] have been reported to be the best individual subtests of the CERAD-NB for distinguishing AD stages. Notably, 60% of the MCI participants had not noticed any cognitive problems themselves, even if the CERAD-NB test indicated minor cognitive difficulties. Based on the MMSE alone, almost all of the non-demented participants would have been considered to be “cognitively normal”. Our findings support previous reports of the usability and sensitivity of the CERAD-NB compared to the MMSE [18] in detecting persons with MCI-AD (prodromal AD) [31]. In our experiment, the MCI group represents persons with even milder cognitive problems (mean MMSE 27) than in previous similar studies with MCI and a mean MMSE score of 25.7 [32] or 25.62 [33].
As expected, an APOE ɛ4 allele (at least one copy) was 2-fold more common in the AD group than in the other study groups. None of the participants in the control group had two copies of the APOE ɛ4 alleles. APOE ɛ4 is a well-known genetic risk factor for late-onset familiar and sporadic AD [34, 35], and the risk of AD increases if a person has two APOE ɛ4 alleles [36]. These findings support the hypothesis of successful grouping using the above-mentioned clinical methods and the differences between the study groups.
Strengths and limitations
The number of participants in each group was limited, but this study was designed to be a pilot study to determine whether the KD test complemented by ET can detect differences between control, MCI, and AD groups. Due to the limited number of cases in this study, the early diagnostic value will be confirmed in larger study cohorts. However, significant group differences were noted even though the number of participants was limited. The number of errors in the reading test varied between individuals within the study groups, but was highest in the AD group, in which most participants had at least one error, whereas the majority in other groups were error-free. We speculate that the high variability inside the mild AD group may be due to the progressive nature of AD and large range in the CDR global 1 score (persons with CDR-SOB scores 4.5–9 indicate mild AD); therefore, test results may vary greatly.
The analysis of ET recordings requires technology and expertise, but the recordings can be made in the primary health care setting. The recording device is portable, and a trained nurse could carry out the tests and ET. The health care professional who carries out the CERAD-NB test battery must be trained and obtain the expertise to analyze the results. However, primary health care nurses can also be trained to carry out the test battery and physicians to analyze the results, as has been done in Finland. By using the CERAD-NB test battery instead of the MMSE alone, we were able to detect very early cognitive deficits, and the results of ET analyses supported clinical findings. Thus, our findings support both the usage of CERAD-NB as a screening tool in both research and the clinic to detect persons with MCI and the KD test with ET analysis to support early diagnosis of AD.
Significant differences between the study groups show that the selected tests and methods are valid and encourage us to continue to develop screening for early detection of detection of neurocognitive disorders.
Implications
Theoretically, it is possible to find new early signs of AD and develop preclinical biomarkers due to the pathology of the disease beginning years before the onset of cognitive symptoms [37]. New, objective methods that are easy to carry out to detect early signs of AD are important to identify because only early AD diagnosis and treatment initiation may delay progression of the disease and postpone the dementia stage and need for institutional care [38].
We already have some biological biomarkers in research and clinical use to support early diagnosis of AD in the prodromal stage. To diagnose preclinical AD, biomarkers indicating the underlying disease pathology are also warranted and being intensively researched (e.g., plasma tests) [39]. Cerebrospinal fluid (CSF) contains typical AD proteins (Aβ peptides, tau peptides) that can be isolated by lumbar puncture [40]. However, lumbar puncture is invasive and needs to be carried out at a specialized medical unit. Other currently used biomarkers of AD, such as FDG or amyloid PET, are expensive [37, 39, 40] and cannot be used in primary health care. Use of the CERAD-NB test battery requires training but is sensitive in detecting early AD-related cognitive changes [31]. We report that saccadic eye movement alterations occur very early in the disease course, even earlier than the other reports have indicated [5, 7, 9]. These findings support the idea that eyes and eye functions are interesting research targets for new AD biomarkers.
Conclusion
We found significant group differences between controls and persons with MCI or AD dementia in regard to saccadic duration and amplitude using ET analysis of the KD test. Cognitive disturbances were milder in the MCI group than in previous comparable studies. These findings suggest that ET analysis of the KD test may help detect AD very early. Thus, this method is a promising supportive, or even indicative, biomarker candidate for further studies when the goal is to find easy-to-use tools for distinguishing persons with AD risk. The ET analysis of the KD test could be used to screen participants for clinical trials aiming to develop care for persons at risk or in the early stages of AD. Furthermore, the portable device used to detect eye movements can be used in primary health care units.
ACKNOWLEDGMENTS
We are deeply grateful to all of the study participants. We also thank BEGAD research project study nurses Ulla Vanhanen, Kati Mönttinen, and Kristiina Holopainen for recruiting the study participants. We thank biostatistician Tuomas Selander for help with the statistical analysis. This work was supported by Juho and Lempi Pitkänen Foundation.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5551r2).
REFERENCES
[1] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[2] | Lueck KL , Mendez MF , Perryman KM ((2000) ) Eye movement abnormalities during reading in patients with Alzheimer disease.. Neuropsychiatry Neuropsychol Behav Neurol 13: , 77–82. |
[3] | Cummings JL , Houlihan JP , Hill MA ((1986) ) The pattern of reading deterioration in dementia of the Alzheimer type: Observations and implications.. Brain Lang 29: , 315–323. |
[4] | Sadun AA , Borchert M , DeVita E , Hinton DR , Bassi CJ ((1987) ) Assessment of visual impairment in patients with Alzheimer’s disease.. Am J Ophthalmol 104: , 113–120. |
[5] | Fernandez G , Mandolesi P , Rotstein NP , Colombo O , Agamennoni O , Politi LE ((2013) ) Eye movement alterations during reading in patients with early Alzheimer disease.. Invest Ophthalmol Vis Sci 54: , 8345–8352. |
[6] | Holden JG , Cosnard A , Laurens B , Asselineau J , Biotti D , Cubizolle S , Dupouy S , Formaglio M , Koric L , Seassau M , Tilikete C , Vighetto A , Tison F ((2018) ) Prodromal Alzheimer’s disease demonstrates increased errors at a simple and automated anti-saccade task.. J Alzheimers Dis 65: , 1209–1223. |
[7] | Fernandez G , Manes F , Politi LE , Orozco D , Schumacher M , Castro L , Agamennoni O , Rotstein NP ((2016) ) Patients with mild Alzheimer’s disease fail when using their working memory: Evidence from the eye tracking technique.. J Alzheimers Dis 50: , 827–838. |
[8] | Fernandez G , Manes F , Rotstein NP , Colombo O , Mandolesi P , Politi LE , Agamennoni O ((2014) ) Lack of contextual-word predictability during reading in patients with mild Alzheimer disease.. Neuropsychologia 62: , 143–151. |
[9] | Wilcockson TDW , Mardanbegi D , Xia B , Taylor S , Sawyer P , Gellersen HW , Leroi I , Killick R , Crawford TJ ((2019) ) Abnormalities of saccadic eye movements in dementia due to Alzheimer’s disease and mild cognitive impairment.. Aging (Albany NY) 11: , 5389–5398. |
[10] | Pereira MLGF , Camargo MVZA , Bellan AFR , Tahira AC , Dos Santos B , Dos Santos J , Machado-Lima A , Nunes FLS , Forlenza OV ((2020) ) Visual search efficiency in mild cognitive impairment and Alzheimer’s disease: An eye movement study.. J Alzheimers Dis 75: , 261–275. |
[11] | FlemingR, ZeiselJ, BennettK (2020) World Alzheimer Report 2020. Design, Dignity, Dementia: Dementia-related design and the built environment. Alzheimer’s Disease International, London, UK. |
[12] | Scheltens P , De Strooper B , Kivipelto M , Holstege H , Chetelat G , Teunissen CE , Cummings J , van der Flier WM ((2021) ) Alzheimer’s disease. Lancet 397: , 1577–1590. |
[13] | Cohen AD , Landau SM , Snitz BE , Klunk WE , Blennow K , Zetterberg H ((2019) ) Fluid and PET biomarkers for amyloid pathology in Alzheimer’s disease.. Mol Cell Neurosci 97: , 3–17. |
[14] | Galetta KM , Chapman KR , Essis MD , Alosco ML , Gillard D , Steinberg E , Dixon D , Martin B , Chaisson CE , Kowall NW , Tripodis Y , Balcer LJ , Stern RA ((2017) ) Screening utility of the King-Devick test in mild cognitive impairment and Alzheimer disease dementia.. Alzheimer Dis Assoc Disord 31: , 152–158. |
[15] | Peltsch A , Hemraj A , Garcia A , Munoz DP ((2011) ) Age-related trends in saccade characteristics among the elderly. Neurobiol Aging 32: , 669–679. |
[16] | Tao L , Wang Q , Liu D , Wang J , Zhu Z , Feng L ((2020) ) Eye tracking metrics to screen and assess cognitive impairment in patients with neurological disorders. Neurol Sci 41: , 1697–1704. |
[17] | Welsh KA , Butters N , Mohs RC , Beekly D , Edland S , Fillenbaum G , Heyman A ((1994) ) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44: , 609–614. |
[18] | Paajanen T , Hanninen T , Tunnard C , Mecocci P , Sobow T , Tsolaki M , Vellas B , Lovestone S , Soininen H , Addneuromed C ((2010) ) CERAD neuropsychological battery total score in multinational mild cognitive impairment and control populations: The AddNeuroMed study. J Alzheimers Dis 22: , 1089–1097. |
[19] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[20] | De la Vega FM , Lazaruk KD , Rhodes MD , Wenz MH ((2005) ) Assessment of two flexible and compatible SNP genotyping platforms: TaqMan SNP Genotyping Assays and the SNPlex Genotyping System. Mutat Res 573: , 111–135. |
[21] | Hänninen T , Pulliainen V , Salo J , Hokkanen L , Erkinjuntti T , Koivisto K , Viramo P , Hilkka Soininen, the Expert Group of the Finnish Memory Research Units ((1999) ) Suomen muistitutkimusyksiköiden asiantuntijaryhmä. Kognitiiviset testit muistihäiriöiden ja alkavan dementian varhaisdiagnostiikassa: CERAD-tehtäväsarja. Suom Lääkäril 54: , 1967–1975. [in Finnish] |
[22] | Tobii Technology (2010) Tobii TX300 Eye Tracker, https://www.tobiipro.com/siteassets/tobii-pro/brochures/tobii-pro-tx300-brochure.pdf. |
[23] | Tobii Technology (2016) Tobii Studio User’s Manual, https://www.tobiipro.com/siteassets/tobii-pro/user-manuals/tobii-pro-studio-user-manual.pdf. |
[24] | Olsen A , Matos R (2012) Identifying parameter values for an I-VT fixation filter suitable for handling data sampled with various sampling frequencies. ETRA’12: Proceedings of the Symposium on Eye Tracking Research and Applications, pp. 317–320. |
[25] | Kurimo M (2016) AaltoASR. https://github.com/aalto-speech/AaltoASR, April 5, 2017, Accessed on October 5, 2021. |
[26] | Mendez MF , Tomsak RL , Remler B ((1990) ) Disorders of the visual system in Alzheimer’s disease. J Clin Neuroophthalmol 10: , 62–69. |
[27] | King D , Hume P , Gissane C , Clark T ((2015) ) Use of the King-Devick test for sideline concussion screening in junior rugby league. J Neurol Sci 357: , 75–79. |
[28] | Barth S , Schonknecht P , Pantel J , Schroder J ((2005) ) Mild cognitive impairment and Alzheimer’s disease: An investigation of the CERAD-NP test battery. Fortschr Neurol Psychiatr 73: , 568–576. |
[29] | Bertolucci PH , Okamoto IH , Brucki SM , Siviero MO , Toniolo Neto J , Ramos LR ((2001) ) Applicability of the CERAD neuropsychological battery to Brazilian elderly. Arq Neuropsiquiatr 59: , 532–536. |
[30] | Welsh K , Butters N , Hughes J , Mohs R , Heyman A ((1991) ) Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol 48: , 278–281. |
[31] | Paajanen T , Hanninen T , Tunnard C , Hallikainen M , Mecocci P , Sobow T , Tsolaki M , Vellas B , Lovestone S , Soininen H ((2014) ) CERAD neuropsychological compound scores are accurate in detecting prodromal Alzheimer’s disease: A prospective AddNeuroMed study. J Alzheimers Dis 39: , 679–690. |
[32] | Oyama A , Takeda S , Ito Y , Nakajima T , Takami Y , Takeya Y , Yamamoto K , Sugimoto K , Shimizu H , Shimamura M , Katayama T , Rakugi H , Morishita R ((2019) ) Novel method for rapid assessment of cognitive impairment using high-performance eye-tracking technology. Sci Rep 9: , 12932. |
[33] | Chehrehnegar N , Nejati V , Shati M , Esmaeili M , Rezvani Z , Haghi M , Foroughan M ((2019) ) Behavioral and cognitive markers of mild cognitive impairment: Diagnostic value of saccadic eye movements and Simon task. Aging Clin Exp Res 31: , 1591–1600. |
[34] | Munoz SS , Garner B , Ooi L ((2019) ) Understanding the role of ApoE fragments in Alzheimer’s disease. Neurochem Res 44: , 1297–1305. |
[35] | Corder EH , Saunders AM , Strittmatter WJ , Schmechel DE , Gaskell PC , Small GW , Roses AD , Haines JL , Pericak-Vance MA ((1993) ) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261: , 921–923. |
[36] | Corbo RM , Scacchi R ((1999) ) Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet 63: , 301–310. |
[37] | Kaipainen A , Jaaskelainen O , Liu Y , Haapalinna F , Nykanen N , Vanninen R , Koivisto AM , Julkunen V , Remes AM , Herukka SK ((2020) ) Cerebrospinal fluid and MRI biomarkers in neurodegenerative diseases: A retrospective memory clinic-based study. J Alzheimers Dis 75: , 751–765. |
[38] | Hongisto K , Vaatainen S , Martikainen J , Hallikainen I , Valimaki T , Hartikainen S , Suhonen J , Koivisto AM ((2015) ) Self-Rated and caregiver-rated quality of life in Alzheimer disease with a focus on evolving patient ability to respond to questionnaires: 5-year prospective ALSOVA cohort study. Am J Geriatr Psychiatry 23: , 1280–1289. |
[39] | Karikari TK , Benedet AL , Ashton NJ , Lantero Rodriguez J , Snellman A , Suarez-Calvet M , Saha-Chaudhuri P , Lussier F , Kvartsberg H , Rial AM , Pascoal TA , Andreasson U , Scholl M , Weiner MW , Rosa-Neto P , Trojanowski JQ , Shaw LM , Blennow K , Zetterberg H , Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry 26: , 429–442. |
[40] | Seppala TT , Nerg O , Koivisto AM , Rummukainen J , Puli L , Zetterberg H , Pyykko OT , Helisalmi S , Alafuzoff I , Hiltunen M , Jaaskelainen JE , Rinne J , Soininen H , Leinonen V , Herukka SK ((2012) ) CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78: , 1568–1575. |




