Contribution of Memory Tests to Early Identification of Conversion from Amnestic Mild Cognitive Impairment to Dementia
Abstract
Background:
Memory tests using controlled encoding and cued recall paradigm (CECR) have been shown to identify prodromal Alzheimer’s disease (AD), but information about the effectiveness of CECR compared to other memory tests in predicting clinical progression is missing.
Objective:
The aim was to examine the predictive ability of a memory test based on the CECR paradigm in comparison to other memory/non-memory tests for conversion to dementia in patients with amnestic mild cognitive impairment (aMCI).
Methods:
270 aMCI patients from the clinical-based Czech Brain Aging Study underwent a comprehensive neuropsychological assessment including the Enhanced Cued Recall test (ECR), a memory test with CECR, two verbal memory tests without controlled encoding: the Auditory Verbal Learning Test (AVLT) and Logical memory test (LM), a visuospatial memory test: the Rey-Osterrieth Complex Figure test, and cognitive testing based on the Uniform Data Set battery. The patients were followed prospectively. Conversion to dementia as a function of cognitive performance was examined using Cox proportional hazard models.
Results:
144 (53%) patients converted to dementia. Most converters (89%) developed dementia due to AD or mixed (AD and vascular) dementia. Comparing the four memory tests, the delayed recall scores on AVLT and LM best predicted conversion to dementia. Adjusted hazard ratios (HR) of immediate recall scores on ECR, AVLT, and LM were similar to the HR of categorical verbal fluency.
Conclusion:
Using the CECR memory paradigm in assessment of aMCI patients has no superiority over verbal and non-verbal memory tests without cued recall in predicting conversion to dementia.
INTRODUCTION
Assessment of memory impairment is a key neuropsychological approach when predicting conversion to Alzheimer dementia (AD dementia) in patients with mild cognitive impairment (MCI), subjective cognitive decline, and in cognitively healthy older adults [1, 2]. Various neuropsychological tests are used in the diagnosis of AD, though practice differs across countries and within countries in relation to specialization of the clinical sites [3]. There is a need for consensus as some guidelines recommend the use of memory tests with a controlled encoding paradigm [4–6] whereas others do not [7, 8]. Controlled learning/encoding with semantic cues diminishes the interference of attention, strategy, and working memory during the encoding part of the test, based on the encoding specificity principle [9].
Patients with MCI are at increased risk of progression to dementia and those with memory impairment (amnestic MCI –aMCI) are at particularly high risk of converting to AD dementia [10]. It was suggested that memory recall deficit in memory tests with controlled encoding that is not normalized or significantly improved with cueing or recognition is specific for hippocampal impairment [11, 12]. Keeping with the current knowledge of localization of neuropathological changes in early AD, the so-called hippocampal type of memory impairment was postulated to be the core neuropsychological marker of prodromal AD [4, 13].
The most widely used test with the controlled encoding and cued recall (CECR) paradigm is the Free and Cued Selective Reminding Test (FCSRT), which includes free and total recall subtests [14]. The FCSRT uses category cues at both acquisition and retrieval in an attempt to ensure semantic encoding and enhance recall.
The hippocampal type of memory impairment was found to be highly specific and sensitive when predicting aMCI conversion to dementia in a longitudinal study with the FCSRT [15]. An additional study demonstrated the utility of FCSRT in a population-based cohort of older adults where free and total recall showed good specificity, sensitivity, and negative predictive value in predicting dementia; however, positive predictive values were low, and many subjects with poor free and total recall scores in the FCSRT remained free of dementia at 5 years [16]. In another longitudinal aging study, a decline in free recall was detected 7 years before the diagnosis of dementia [17].
One cross-sectional study brought indirect evidence for the superiority of FCSRT measures for discrimination between AD and non-AD etiology of MCI: both free and cued FCSRT recall were more closely related to a cerebrospinal fluid (CSF) biomarker signature indicative of AD in comparison to two free recall measures without a controlled encoding paradigm (Logical memory, CERAD test), though only delayed recalls were compared [18].
Apart from the FCSRT, other studies have been conducted demonstrating the relation of other memory tests with dementia conversion, though inclusion of the CECR paradigm and comparison of several memory tests in one battery is rare and consensus on which memory tests best predict conversion is unclear. One longitudinal study of patients with aMCI with 3 years follow-up found the TAVEC –Verbal Learning Test of the Complutense University (a test using free and cued recall –Spanish version of the Californian Verbal Learning Test) to be the best predictor of conversion to dementia in MCI patients compared to other tests in the battery; however, no other verbal memory test was used to allow direct comparison [19].
To the best of our knowledge, only one prior longitudinal study compared a memory test based on the CECR paradigm with a wordlist memory test based on uncontrolled learning and free recall. A short 6-item test was found to be superior to a 10-item version of the Auditory Verbal Learning Test (AVLT) in predicting conversion to AD dementia at 18 months follow-up [20]. Another longitudinal study used a modified 15-word version of the FCSRT, in which controlled encoding was not implemented, and found free recall in this test to be superior to a memory test using story recall [21].
Besides the memory tests using a list of words such as the AVLT or FCSRT, tests using story recall have been recommended in clinical practice and research to identify patients likely to convert to dementia. Among them, the Logical memory test (LM) in particular has been widely used in the United States, where it has been a part of the UDS [2, 22] and the PACC (preclinical AD cognitive composite) battery [23, 24]. This suggests the need of comparing the LM to tests using the CECR paradigm.
In summary, the clinical utility of memory tests with the free and cued paradigm has been demonstrated in numerous previous studies; however, there is no conclusive evidence of superiority of tests using CECR over other memory tests (without this paradigm) among older adults without dementia.
Recently, attention has been drawn to non-memory domains. In particular, semantic fluency has been identified as an independent predictor of the presence of AD pathology in cognitively normal older adults. Addition of category fluency to the PACC cognitive battery provided unique information about early cognitive decline not currently captured by the episodic memory, executive function, and global cognition components, and was suggested to improve detection of early Amyloid-beta-related cognitive decline [24, 25]. Moreover, recent work by the Czech Brain Aging Study has suggested that a dysnomic form of aMCI may exist, and that patients with dysnomic or severe multi-domain aMCI are more likely to progress to dementia [26].
In our previous cross-sectional study, we examined the potential of the Enhanced Cued Recall (ECR) test, which is an alternative version of the FCSRT based on the same paradigm, to reflect the hippocampal atrophy in nondemented older adults. We compared it with two other frequently used memory tests, the AVLT— a test with 15 words without this procedure, and the nonverbal Rey–Osterrieth complex figure test (ROCFT) and we found no superiority of the ECR test over the AVLT [27].
Building on previous research, the aim of this longitudinal clinical based study was to compare the potential of four memory tests (ECR, AVLT, ROCF, and LM) and other, non-memory tests to predict the conversion to dementia in aMCI patients. We expected that the ECR test using the CECR paradigm would be superior to other memory and non-memory tests to predict conversion to dementia.
METHODS
Participants
A total of 270 aMCI patients were recruited and followed prospectively with annual examinations at the Memory Clinic in Motol University Hospital in Prague, Czech Republic between 2005 and 2020 in the Czech Brain Aging Study (CBAS) [28].
All individuals were referred to the clinic by general practitioners, neurologists, psychiatrists, or geriatricians based on memory complaints reported by themselves or their close informants. They underwent standard clinical and laboratory evaluations, brain MRI, and comprehensive neuropsychological examination at baseline and were followed prospectively with yearly clinical and neuropsychological evaluations, and interviews with informants in order to detect conversion to dementia. MRI was repeated every two years. Additional clinical visits were performed in case of unusual clinical worsening reported by the patient or his/her informant. The diagnosis was determined at the joint meetings of neuropsychologists with neurologists and was based on mutual agreement. When establishing the diagnosis, the clinicians used all available information including the results of previous tests and all other clinical information. In case of conversion, the patients underwent a new brain MRI used to confirm the final diagnosis. At baseline, all participants fulfilled Petersen’s criteria for aMCI including memory complaints, evidence of memory dysfunction on neuropsychological testing, generally intact activities of daily living, and absence of dementia [8]. The group included both single domain (isolated memory impairment) and multiple domain (memory impairment plus impairment of at least one other cognitive domain) aMCI participants. Memory impairment was established when the patient scored more than 1.5 standard deviations below the mean of age- and education-adjusted norms on any memory test
Individuals with a history of neurological or psychiatric disease potentially interfering with cognitive function (i.e., stroke, multiple sclerosis, Parkinson’s disease, major depressive symptomatology defined as >8 points on the 15-item Geriatric Depression Scale, psychosis, etc.), psychiatric medication usage excluding SSRI, or abnormal neurological examination including gait or movement difficulties were not included. In addition, we did not include patients with primary progressive aphasia. All participants in this study had signed written informed consent that was approved by a local ethics committee. The procedures were in accordance with the Helsinki Declaration of 1975 and later revision in 2000. The basic characteristics and results of neuropsychological assessment are summarized in Table 1.
Table 1
Demographic characteristics and cognitive performance
| Baseline Amnestic Mild Cognitive Impairment (n = 270) | Follow-Up | ||
| Non-Converters (n = 126) | Dementia Converters (n = 144) | ||
| Demographics | Mean±SD | Mean±SD | Mean±SD |
| Days of Follow-Up (y) | 2.80±2.02 | 3.20±2.32 | 2.44±1.65* |
| Gender (male/female) | 124/146 | 66/60 | 58/86 |
| Age | 71.71±8.45 | 69.15±8.36 | 73.97±7.89* |
| Education | 14.53±3.39 | 15.12±3.60 | 14.01±3.12* |
| GDS-15 | 4.12±3.25 | 4.52±3.34 | 3.76±3.13 |
| ∧APOE4 carriers (≥1 allele) | 45% (95) | 35% (33) | 53% (62)* |
| ∧APOE4/E4 carriers (2 alleles) | 6% (13) | 4% (4) | 8% (9) |
| Dementia Classification | % (n) | ||
| AD | – | – | 72% (104) |
| BV-FTD | – | – | 4% (6) |
| LBD | – | – | 5% (7) |
| Mixed Dementia | – | – | 17% (25) |
| VaD (without AD) | – | – | 1% (2) |
| Cognitive Performance (Baseline) | Mean±SD | Mean±SD | Mean±SD |
| MMSE | 26.30±2.68 | 27.49±1.94 | 25.24±2.80* |
| AVLT 1 | 3.88±1.48 | 4.37±1.44 | 3.42±1.36* |
| AVLT 5 | 7.88±2.44 | 8.91±2.30 | 6.87±2.14* |
| AVLT 1-5 | 31.34±8.46 | 35.22±7.61 | 27.55±7.49* |
| AVLT 30 | 3.43±3.03 | 4.92±2.81 | 1.95±2.48* |
| ECR-FR | 4.58±3.00 | 6.13±2.79 | 3.54±2.68* |
| ECR-TR | 13.23±3.24 | 14.50±2.09 | 12.38±3.58* |
| ROCF-R | 7.82±6.16 | 10.58±6.09 | 5.27±5.06* |
| ROCF-C | 27.07±6.33 | 27.92±5.42 | 26.29±6.99* |
| LOG-I | 9.36±4.25 | 11.27±4.04 | 7.35±3.49* |
| LOG-D | 5.74±5.08 | 8.27±4.93 | 3.08±3.69* |
| TMT A | 59.94±27.83 | 57.56±27.93 | 62.30±27.67 |
| TMT B | 173.97±80.76 | 145.91±71.02 | 202.28±80.38* |
| F-DigitSpan-NM | 5.68±1.23 | 5.85±1.34 | 5.54±1.11* |
| F-Digit Span-SC | 8.44±2.13 | 8.59±2.38 | 8.32±1.89 |
| B-DigitSpan-NM | 4.06±1.20 | 4.23±1.30 | 3.91±1.09* |
| B-DigitSpan-SC | 5.30±1.94 | 5.60±2.08 | 5.03±1.76* |
| Digit Symbol | 30.29±10.42 | 33.62±11.23 | 26.98±8.38* |
| BNT | 53.31±6.49 | 53.98±5.52 | 52.72±7.21 |
| P-VF | 33.82±11.73 | 34.36±11.88 | 33.35±11.63 |
| S-VF-A | 16.83±5.47 | 19.00±5.37 | 14.37±4.47* |
| S-VF-V | 8.91±2.84 | 9.61±2.98 | 8.12±2.47* |
*indicates statistical significance between non-converters and converters. Patients who did not convert with less than 360 days of follow-up were excluded (n = 12). ∧Sample size for apolipoprotein (APOE) was 213. AD, Alzheimer’s disease; BV-FTD, behavioral-variant frontotemporal dementia; LBD, Lewy body dementia; VaD, vascular dementia; MMSE, total score; AVLT 1, trial 1 recall; AVLT 5, trial 5 recall; AVLT 1-5, sum of trials 1 to 5; AVLT 30, recall after 30 min; ECR-FR, free recall; ECR-TR, total recall after cueing; ROCF-R, visual reproduction after 3 min; ROCF-C, copy score [44]; LOG-I, Logical Memory Immediate Recall from the Uniform Data Set; LOG-D, Logical Memory Delayed Recall from the Uniform Data Set; TMT A, given in seconds; TMT B, given in seconds; F-DigitSpan-NM, forward Digit Span –numbers; F-Digit Span-SC, forward Digit Span –score; B-DigitSpan-NM, backward Digit Span –numbers; B-DigitSpan-SC, backward Digit Span –score; Digit Symbol, Digit Symbol Score from the WAIS-R; BNT, Boston Naming Test; P-VF, Phonemic Verbal Fluency; S-VF-A, Semantic Verbal Fluency –Animals; S-VF-V, Semantic Verbal Fluency –Vegetables.
The conversion to dementia and its etiology was established during the regular consensual meetings of neurologists and neuropsychologists. The diagnosis was based on clinical history reported by the patient and the caregiver, neurological examination, neuropsychological assessment, and MRI. The main criterion to diagnose dementia was based on the impairment of activities of daily living reported by the patient’s informant [7]. Neuropsychological test results were used to assess the cognitive profile, which helped to specify the dementia’s etiology.
The diagnosis of different types of dementia was based on current criteria for probable AD [7], probable vascular dementia [30], probable dementia with Lewy bodies [31], or probable behavioral variant of frontotemporal dementia [32]. Patients labeled as mixed (AD+vascular) dementia were considered to have predominance of AD pathology accompanied by evidence of extensive vascular changes or vascular changes in areas important for cognition (hippocampus, thalamus). In the clinical phenotype, the patients manifested episodic memory impairment and impaired attention/working memory, executive function, and slow processing speed [33].
Neuropsychological assessment
All individuals were interviewed using the following questionnaires: Clinical Dementia Rating [34], Functional Activities Questionnaire to assess activities of daily living [35], Hachinski Ischemic Scale, and 15-item Geriatric Depression Scale (GDS-15) [36]. The neuropsychological battery included the Mini-Mental State Examination (MMSE), Digit Span forward and backward tests, Digit Symbol, Trail Making Tests (TMT) A and B, Boston Naming Test (30 odd-items version), Semantic Verbal Fluency (Animals, Vegetables), Phonemic Verbal Fluency (Czech version, letters N, K, P) [37, 38] and visuospatial tests (The Rey–Osterrieth complex figure test (ROCFT) –copy condition) [39].
Four memory tests were used:
1) Memory test with controlled encoding and free and cued recall –A modified version of the FCSRT called Enhanced Cued Recall (ECR test in Czech validated version) [40, 41]. The test uses category cues at both acquisition and retrieval to ensure semantic encoding and to enhance recall. The subject is asked to search through a card containing line drawings of four objects and to identify the one that belongs to a category named by the examiner, such as fruit. Each of the 16 items to be learned appears on one of four cards that are used. After each item is correctly identified on the first card, the card is removed and immediate recall of the four items is tested by cueing with the category prompt. Errors are corrected. The other 12 items are presented four at a time in the same manner. A learning phase and subsequent interfering task (Clock Drawing Test) were followed by one free recall and subsequent cued recall for items not freely recalled. Free recall (ECR-FR) and total recall (ECR-TR = free + cued recall) were evaluated.
2) Verbal memory test with uncontrolled encoding and delayed recall –Auditory verbal learning test (AVLT) [42, 43]. The examiner reads a list of 15 words from List A at the rate of one word per 1.5 s after instructing the participant to listen and remember them. The examiner writes down the words recalled, then rereads the test for trials II to V with immediate recall recorded after every trial. After the fifth trial, the words from List B are read and recalled. Following the List B trial, the examiner asks the patient to recall as many words from List A as possible (trial VI). A 30-min delayed recall trial is administered to measure retention. In our study, word span under overload conditions (trial I: AVLT 1), final acquisition (trial V: AVLT 5), total acquisition (Σ I-V: AVLT 1–5), and delayed recall after 30 min (trial VII- AVLT 30) were analyzed.
3) Story learning memory test –Logical memory test (LM) [38]. The examiner reads a story and the subject is asked to recall it immediately and after a 20-min delay. The number of correctly recalled items was analyzed.
4) Visuospatial memory test –Rey-Osterrieth Complex Figure Test (ROCFT) [44]. Participants were asked to copy and, after a 3-min delay, recall a line drawing of a figure. The subject had not been previously instructed to memorize the figure. The copy and the reproduction of the drawing were scored by an experienced rater (neuropsychologist) using the 36-points Meyers system. Both the copy and reproduction were used in the final analysis.
To allow the direct comparison of the memory tests, several verbal tests were used in the neuropsychological battery, potentially leading to the memory interference effect. To minimize this effect, we ensured that the administration of memory tests did not overlap in the battery (i.e., the learning phase of the new test started after the delayed recall of the previous one).
Results of the neuropsychological battery including memory tests are summarized in Table 1.
Statistical analysis
Initially, demographic characteristics and baseline cognitive scores were compared for individuals who did versus did not convert using t-tests for differences in means and chi-square tests for differences in frequencies. Subsequently, cognitive test scores were converted to z-scores for ease of comparison across individual cognitive tests. To allow further direct comparison, the z-scores for TMT A and B were reversed. In the main analyses, conversion to dementia as a function of cognitive performance was examined using Cox proportional hazard models in R3.6.1 [45], which yields hazard ratios (HRs). HRs greater than 1.00 indicate increased risk, those lower than 1.00 indicate decreased risk. 95% confidence intervals are also reported. When the entire confidence interval for one cognitive test falls outside the confidence interval for another test, we can infer that the difference in the magnitude of the effect is statistically significant for the two tests.
First, age, sex, and education were included as covariates in the models and the corresponding HRs and confidence intervals were extracted. Next, global cognition as measured by the MMSE was added as a covariate to all models. We opted to control for MMSE to better understand whether certain neuropsychological tests are less predictive of progression from aMCI to dementia than a simple measure of global functioning MMSE, and conversely to highlight tests that are robust even after controlling for global cognition. In a third step, we included the GDS-15 and APOE ɛ4 variant as covariates. If a HR from a non-memory test was statistically significant in the first step (controlling for age, sex, education), it was further analyzed after controlling for delayed LM, which was identified as the best predictor of conversion in our analyses with respect to effect magnitude. Multiple logistic regression was conducted to extract the Area Under the Curve (AUC) with DeLong 95% confidence intervals from the first set of models (controlling for age, sex, and education). Values of 0.80 suggest excellent discriminatory ability for a given neuropsychological test after adjustment for age, sex, and education. In order to visualize the time to conversion, a Kaplan-Meier curve with 95% confidence intervals was provided with the cumulative number of incident dementia cases per year.
RESULTS
A total of 270 patients with aMCI who reached out to the memory clinic were recruited at baseline. During the follow-up, 144 (53%) individuals converted to dementia. The majority of converters developed AD dementia or mixed (AD+vascular) dementia (72% and 17%, respectively). The mean and median follow-up times were 2.80 (SD = 2.02 years, range 0.23–13.85) and 2.19 respectively for the full sample. The mean time to conversion was 2.44 years (SD = 1.65 years, range 0.23–10.41) and the median time was 2.12 years. The mean follow-up time of non-converters was 3.20 years (SD = 2.32, range 0.99–13.85) and the median follow-up time was 2.45 years. The Kaplan-Meier curve analyzing time to conversion is provided in Fig. 1.
Fig. 1
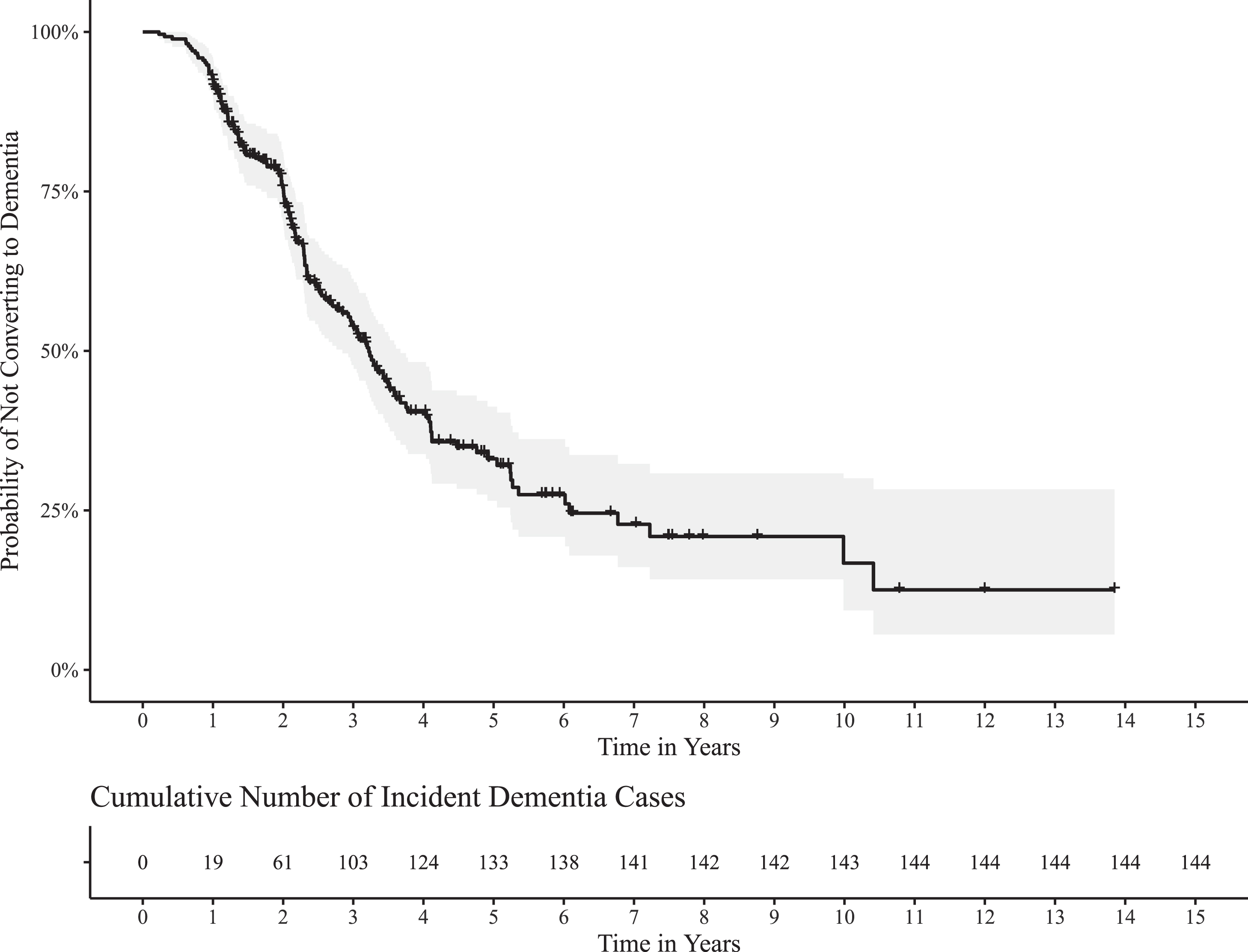
Basic demographic characteristics of the group and comparison of converters versus non-converters are in the Table 1. The HRs linked to different neuropsychological scores are listed in Table 2.
Table 2
Cox proportional hazard models with neuropsychological tests predicting conversion from amnestic mild cognitive impairment to all-cause dementia
| Adjusted Hazard Ratio(Age, Sex,Education) | p | AUC [DeLong 95% CI] | Adjusted Hazard Ratio + MMSE (Age, Sex, Education, MMSE) | p | Adjusted Hazard Ratio +GDS-15 + APOE ɛ4(Age, Sex, Education,GDS-15, APOE ɛ4) | p | |
| Neuropsychological Tests | |||||||
| MMSE* | 1.82 [1.54, 2.14] | <0.001 | 0.798 [0.745, 0.852] | – | – | 1.71 [1.42, 2.06] | <0.001 |
| AVLT 1 | 1.58 [1.30, 1.92] | <0.001 | 0.764 [0.704, 0.823] | 1.42 [1.15, 1.75] | 0.001 | 1.31 [1.04, 1.66] | 0.02 |
| AVLT 5 | 1.60 [1.32, 1.93] | <0.001 | 0.793 [0.736, 0.849] | 1.36 [1.11, 1.66] | 0.003 | 1.45 [1.15, 1.83] | 0.002 |
| AVLT 1-5 | 1.73 [1.43, 2.09] | <0.001 | 0.803 [0.749, 0.858]∧ | 1.46 [1.19, 1.80] | <0.001 | 1.53 [1.22, 1.93] | <0.001 |
| AVLT 30 | 2.25 [1.75, 2.90] | <0.001 | 0.826 [0.772, 0.879]∧ | 1.93 [1.48, 2.52] | <0.001 | 1.81 [1.34, 2.45] | <0.001 |
| ECR-FR | 1.78 [1.43, 2.21] | <0.001 | 0.794 [s0.731, 0.856] | 1.46 [1.16, 1.84] | 0.001 | 1.62 [1.26, 2.09] | <0.001 |
| ECR-TR | 1.44 [1.23, 1.69] | <0.001 | 0.761 [0.695, 0.828] | 1.26 [1.06, 1.50] | 0.009 | 1.31 [1.08, 1.58] | 0.005 |
| ROCF-R | 2.10 [1.62, 2.72] | <0.001 | 0.801 [0.745, 0.858]∧ | 1.76 [1.34, 2.32] | <0.001 | 1.74 [1.30, 2.33] | <0.001 |
| ROCF-C | 1.36 [1.14, 1.62] | <0.001 | 0.727 [0.664, 0.791>] | 1.13 [0.94, 1.37] | 0.19 | 1.33 [1.09, 1.62] | 0.005 |
| Log-I* | 1.68 [1.35, 2.08] | <0.001 | 0.819 [0.762, 0.876]∧ | 1.35 [1.06, 1.72] | 0.01 | 1.50 [1.17, 1.91] | 0.001 |
| Log-D* | 2.43 [1.83, 3.22] | <0.001 | 0.839 [0.786, 0.892]∧ | 2.05 [1.51, 2.78] | <0.001 | 2.52 [1.76, 3.60] | <0.001 |
| TMT A* | 1.19 [0.98, 1.44] | 0.08 | 0.722 [0.649, 0.795] | 1.08 [0.87, 1.33] | 0.48 | 1.17 [0.94, 1.45] | 0.16 |
| TMT B* | 1.70 [1.39, 2.07] | <0.001 | 0.744 [0.679, 0.808] | 1.32 [1.04, 1.67] | 0.02 | 1.60 [1.27, 2.01] | <0.001 |
| F-DigitSpan-NM* | 1.13 [0.95, 1.35] | 0.17 | 0.716 [0.654, 0.778] | 1.08 [0.90, 1.31] | 0.40 | 1.15 [0.93, 1.42] | 0.19 |
| F-Digit Span-SC* | 1.12 [0.93, 1.35] | 0.22 | 0.709 [0.647, 0.772] | 1.04 [0.86, 1.26] | 0.69 | 1.13 [0.90, 1.41] | 0.29 |
| B-DigitSpan-NM* | 1.15 [0.94, 1.40] | 0.17 | 0.713 [0.651, 0.775] | 0.94 [0.77, 1.15] | 0.55 | 1.12 [0.90, 1.39] | 0.32 |
| B-DigitSpan-SC* | 1.16 [0.95, 1.42] | 0.15 | 0.713 [0.651, 0.775] | 0.90 [.73, 1.12] | 0.35 | 1.14 [0.91, 1.43] | 0.25 |
| Digit Symbol* | 1.51 [1.24, 1.84] | <0.001 | 0.754 [0.692, 0.817] | 1.30 [1.05, 1.62] | 0.02 | 1.97 [1.44, 2.70] | <0.001 |
| BNT* | 1.33 [1.10, 1.63] | 0.004 | 0.728 [0.661, 0.796] | 1.23 [0.99, 1.51] | 0.06 | 1.20 [0.96, 1.49] | 0.11 |
| P-VF | 1.35 [1.09, 1.67] | 0.007 | 0.730 [0.662, 0.798] | 1.21 [0.97, 1.51] | 0.08 | 1.45 [1.14, 1.84] | 0.002 |
| S-VF-A* | 1.68 [1.29, 2.20] | <0.001 | 0.811 [0.745, 0.877]∧ | 1.40 [1.06, 1.85] | 0.02 | 1.87 [1.39, 2.53] | <0.001 |
| S-VF-V* | 1.78 [1.34, 2.38] | <0.001 | 0.780 [0.709, 0.851] | 1.49 [1.11, 2.00] | 0.008 | 1.75 [1.29, 2.39] | <0.001 |
*indicates that this test is part of the Uniform Data Set (UDS). ∧indicates that this test has an Area Under the Curve (AUC) above 0.80, suggesting excellent discrimination ability. AUC was extracted from multiple logistic regression with each cognitive test as the main predictor variable and age, sex, and education as covariates. For Hazard ratios 95% confidence intervals are reported in the brackets. MMSE, total score; AVLT 1, trial 1 recall; AVLT 5, trial 5 recall; AVLT 1-5, sum of trials 1 to 5; AVLT 30, recall after 30 min; ECR-FR, free recall; ECR-TR, total recall after cueing; ROCF-R, visual reproduction after 3 min; ROCF-C, copy score [44]; LOG-I, Logical Memory Immediate Recall from the Uniform Data Set; LOG-D, Logical Memory Delayed Recall from the Uniform Data Set; TMT A, given in seconds; TMT B, given in seconds; F-DigitSpan-NM, forward Digit Span –numbers; F-Digit Span-SC, forward Digit Span –score; B-DigitSpan-NM, backward Digit Span –numbers; B-DigitSpan-SC, backward Digit Span –score; Digit Symbol, Digit Symbol Score from the WAIS-III; BNT, Boston Naming Test; P-VF, Phonemic Verbal Fluency; S-VF-A, Semantic Verbal Fluency –Animals; S-VF-V, Semantic Verbal Fluency –Vegetables.
Cognitive tests predicting conversion to dementia
At baseline, the converters were significantly older and less educated, and they differed in the majority of neuropsychological tests, but not in the number of depressive symptoms on the GDS-15. In Cox proportional hazard models adjusted for age, sex, and education, the risk of conversion was best predicted by the delayed recall in three memory tests (Delayed LM, AVLT 30, and ROCFT –reproduction), followed by MMSE, immediate recall scores and tests of other cognitive domains. Among the memory tests, the HR for delayed (HR = 2.43) and AVLT delayed recall after 30 min (HR = 2.25) reflected the relatively greatest effect, followed closely by the ROCFT reproduction (HR = 2.10), all conferring more than two times greater risk of conversion per one standard deviation decrease in the scores. The immediate recalls in other memory tests (AVLT 1-5, LM) and ECR-free recall score had HRs between 1.68–1.78 which was similar to semantic verbal fluency animals and vegetables (HR = 1.68 and 1.78 respectively) and TMT B (HR = 1.70). All digit span tests and the TMT-A did not reach statistical significance. The results are summarized in Table 2.
Six neuropsychological tests reported excellent discriminatory ability as determined by the AUC (≥0.80). These tests were: delayed LM (AUC = 0.839), AVLT 30 (AUC = 0.826), immediate LM (AUC = 0.819), semantic verbal fluency –animals (AUC = 0.811), AVLT 1-5 (AUC = 0.803), and ROCFT –reproduction (AUC = 0.801). In addition, the MMSE (AUC = 0.798), ECR-free recall (AUC = 0.794), and the AVLT 5 (AUC = 0.793) also reported near-excellent discriminatory ability.
3.2Controlling for global cognition and memory performance
After controlling for MMSE, ROCFT–Copy, Boston Naming Test, and phonemic verbal fluency were no longer statistically significant predictors of conversion to dementia (Table 2). Controlling for MMSE modestly reduced the HRs of all memory tests, though Delayed LM, AVLT 30, and ROCFT–reproduction remained most strongly associated with conversion. Among the non-memory tests that were statistically significant after controlling for MMSE (TMT B, Digit Symbol Test, and semantic fluency animals and vegetables), only semantic fluency vegetables (HR = 1.49) and the Digit Symbol Test (HR = 1.38) remained significant predictors of conversion when controlling for delayed LM (Table 3).
Table 3
Effect of non-memory tests on conversion after controlling for memory performance
| Adjusted Hazard Ratio + MMSE + Memory Performance (Age, Sex, Education, MMSE, Log-D) | p | |
| Neuropsychological Tests | ||
| TMT B* | 1.23 [0.90, 1.68] | 0.19 |
| Digit Symbol* | 1.38 [1.05, 1.81] | 0.02 |
| S-VF-A* | 1.36 [1.00, 1.86] | 0.05 |
| S-VF-V* | 1.49 [1.10, 2.02] | 0.01 |
For Hazard ratios 95% confidence intervals are reported in the brackets. *indicates that this test is part of the Uniform Data Set (UDS). TMT B, given in seconds; Digit Symbol, Digit Symbol Score from the WAIS-III; S-VF-A, Semantic Verbal Fluency –Animals, S-VF-V, Semantic Verbal Fluency –Vegetables.
4DISCUSSION
Analyzing a neuropsychological battery which comprised one non-verbal and three widely used verbal memory tests, we found three of them to be better predictors of conversion to dementia than the tests representing other cognitive domains. Comparing the memory tests, we found the delayed recall in LM, AVLT, and ROCFT to be the best predictors of conversion to dementia in aMCI. Thus, our results do not support the superiority of the ECR a memory test with 16 items using controlled encoding and cued recall, to the memory tests without this paradigm (AVLT, LM) or a nonverbal memory test ROCFT. The predictive power of the immediate recall memory scores was similar to semantic verbal fluency. In addition, semantic verbal fluency vegetables was predictive of conversion to dementia beyond delayed memory performance, global cognition, and relevant demographics.
The predictive power of neuropsychological tests in non-demented older adults has been a topic of several longitudinal studies. Previous research has consistently shown the superiority of memory tests over the tests of other cognitive domains in predicting future dementia in non-demented older adults [15, 16, 46]. These results are in general agreement with our study demonstrating better predictive power of three of four memory tests (AVLT, LM, and ROCFT reproduction) over the tests of executive functions (TMT B), attention and working memory (digit span forward and backward), language (Boston Naming Test), and visuoconstruction (ROCFT copy condition), as well as general cognition measured by MMSE.
The superiority of delayed recall over other memory scores is not surprising as delayed memory represents the most sensitive measure of the memory deficit and its decline precedes the decline in immediate scores by several years [47, 48].
In our study we found that future dementia was better predicted by free rather than total recall in ECR, although the latter score has been considered specific for hippocampal dysfunction. This paradox can be explained by the ceiling effect. In a recently published longitudinal study, the total recall in the FCSRT began to decline no sooner than 2 years before dementia onset and its impairment remained rather mild until the onset of dementia, contrasting with free recall which began to decline 7 years before dementia onset [49]
To the best of our knowledge, only one longitudinal study used several memory tests simultaneously with different encoding and recall paradigms, and compared a memory test with controlled encoding and cued recall to a memory test without this paradigm [20]. The authors tested 40 MCI patients with a neuropsychological battery comprising two memory tests: MIS (Memory Impairment Screen) plus –a memory tests with 6 words using controlled encoding and cued recall, and a 10-item version of AVLT. They found cued recall in the MIS plus to be a better predictor of conversion at 18 months than delayed free recall in a 10-item version of AVLT. The authors indicated that a score of 0 or 1 out of 6 on the MIS plus may be a good indicator of future (within 18 months) conversion to AD dementia among MCI patients. The low initial performance among future converters suggested a rather substantial memory impairment at baseline; however, even the non-converters in this study performed relatively poorly, and it is possible that the advantage of the MIS over the short version of AVLT was caused by the presence of floor effect in the AVLT test in both clinical groups compared to the considerably less difficult MIS test. As the authors stated, the other weakness of their study was a very short period of follow-up raising questions about the conversion in following years in the rest of the group. Thus, the application of these results to non-demented older adults in general seems problematic.
One more longitudinal study compared a memory test using free and cued recall with other verbal memory test [21]. However, according to its description published elsewhere [50], it seems that the version of Free and Cued Recall Test (FCRT) used in that study did not include controlled encoding procedures, and the paradigm of this test was much closer to the California verbal learning test than to the original FCSRT.
In our study, we found delayed recall in LM and AVLT to be the best predictors of conversion to dementia in patients with aMCI. For several decades, delayed free recall has been considered to be the episodic memory measure with the greatest sensitivity for early detection of AD [51]. Still, its specificity was judged to be problematic because other cognitive deficits beyond pure memory impairment (attentional difficulties and strategy problems) may interfere with poor performance. This was one of the reasons why the tests with controlled encoding and cued recall were developed. Although the effectiveness of memory tests based on CECR paradigm in predicting dementia was demonstrated [15, 16], there is no longitudinal evidence showing their superiority over tests without this paradigm.
According to our results, it is possible that memory tests with the CECR paradigm predict dementia with less accuracy compared to standard memory tests challenging also attention and strategy to encode the to-be-learned material. The reason may be the ceiling effect, caused by easier learning and recall in less impaired patients in the predementia stage— the CECR paradigm probably increases specificity for hippocampal impairment, but on the other hand, it can diminish sensitivity [27].
Previously, several attempts were made to overcome this issue, including the 48-item version of FCSRT which was developed but has not been used probably because of its extensive time requirement and difficulty for even mildly impaired patients. Another solution which combines the CECR paradigm with a novel memory binding paradigm has been proposed in early AD diagnostics, and newly developed tests were introduced [52], such as the Face-Name Associative Memory Exam or Memory Binding Test (MBT). There is growing evidence showing performance in these tests to be associated with biomarkers indicative of AD very early during the disease trajectory. In one longitudinal study, the MBT was shown to outperform conventional memory and non-memory tests, including the FCSRT, in prediction of incident dementia [53]; however, further studies are needed to support its clinical usefulness.
Among non-memory tests, the deficit in semantic verbal fluency conferred the same risk of conversion to dementia as the immediate scores in memory tests and, contrary to phonemic verbal fluency, predicted the conversion even when the analysis was controlled for MMSE score and delayed LM. This is analogous to the previous results showing semantic fluency to predict incident dementia even when controlling for memory test scores [53] and brings other arguments that deficits in semantic fluency may constitute a dysnomic aMCI phenotype that progresses to dementia more quickly than memory impairment alone [26]. It has been previously shown that semantic fluency is greatly reduced in early stages of AD [24, 25, 54–56], qualitatively impaired already in patients with subjective cognitive decline [57], and the predictive power to predict future conversion to dementia in MCI patients was only slightly inferior to memory tests [58]. At the functional level, the impairment of semantic fluency in AD is probably caused mainly by the degradation of semantic knowledge and impairment of associations between concepts in semantic knowledge manifesting as reduced cluster size [59]. We believe that analysis of advanced verbal fluency measures such as clustering and switching strategies could reveal an even greater potential of semantic fluency test in predicting dementia. Moreover, semantic fluency impairment in AD is more pronounced compared to phonemic fluency [60]. The reason for this differential impairment could be the dependence of semantic verbal fluency on temporal lobes demonstrated previously on fMRIs [61, 62]. As the majority of convertors in our study progressed to AD dementia, which affects temporal lobes early in the disease course, our results are in line with previous evidence.
The major strength of our study is the use of an extensive neuropsychological battery, including the UDS and complemented by several widely used memory tests. To the best of our knowledge, we are the first study comparing head-to-head four widely used memory tests in a longitudinal design in order to compare their power to predict future dementia. In addition, using longitudinal data from 270 aMCI patients, this is the largest longitudinal study analyzing the predictive power of several memory tests in this clinical population (compared to 30 patients in [20], 105 patients in [19], 38 patients in [46], and 251 patients in [15]).
Our study also has several limitations. We used two memory tests with almost the same number of words (15 in AVLT×16 in ECR), the tests differed in the encoding paradigm (controlled encoding in ECR to strengthen acquisition versus uncontrolled encoding but five consecutive trials in AVLT to strengthen acquisition) and recall conditions (free and cued recall in ECR×free recall in AVLT), and differed in other characteristics: number of learning trials (5 trials in AVLT×1 trial in ECR) and time between learning and recall (10 min in ECR×30 min in AVLT), making the interpretation of the results complex and the generalization difficult.
We used Peterson’s criteria as they are most widely used in clinical praxis and our paper was intended mainly for clinical use. We are aware that compared to other criteria [63], this approach can cause overdiagnosing of MCI, leading to more patients classified as MCI at baseline remaining stable or reverting back to normal during the follow-ups. In terms of our study, this could underestimate the predictive power of the examined neuropsychological tests. As the date of death was not recorded in the Czech Brain Aging Study dataset, we were unable to conduct Fine-Gray competing risks models to control for the competing risk of death.
Another source of bias may be our long inclusion timeline (i.e., including participants from 2005–2020). However, this methodology is a necessary byproduct of recruitment in prospective cohort studies. It should be noted that the conversion rate in our sample was higher than expected in the typical community-dwelling population, which is common in memory clinic samples. Some HR confidence intervals overlapped, suggesting that tests may not truly be statistically different from each other when predicting conversion to dementia. This may be caused by the real absence of difference but can also indicate that although we assume to be the largest study comparing memory tests as predictors of the conversion to dementia in MCI, still our sample size and follow-up time did not allow to draw clear differences among tests. Future work with larger sample sizes and longer follow-up periods may reduce this problem and therefore provide more definitive conclusions on test superiority or ranking. However, we also acknowledge that very large sample sizes may reveal clinically irrelevant results. To this end, we hope that future work will focus on effect sizes and the width of confidence intervals rather than conventional p-values.
In conclusion, we found that delayed scores in three memory tests (AVLT, LM, and ROCFT) had the highest power to predict conversion to dementia in aMCI patients. Thus, superiority of the ECR, a test employing the CECR paradigm previously proposed to be specific for a true memory impairment, to AVLT and LM, tests previously shown to be more susceptible to non-memory interference effects, was not supported by the results of this study. This could be at least partially due to a ceiling effect of the ECR in the mildly impaired cohort of aMCI patients. Further studies comparing the potential of uncontrolled learning and free recall and CECR paradigm to better predict conversion to dementia are needed to unravel the issue. Novel challenging tests combining the memory binding process with the CECR paradigm might be a promising direction. Memory tests were not the only predictors of incident dementia. The predictive power of semantic verbal fluency was comparable to the power of immediate recall memory scores. Semantic verbal fluency continues to relate significantly to conversion after adjustment for delayed memory, which supports its clinical usefulness for the cognitive deficit progression monitoring.
ACKNOWLEDGMENTS
The research leading to these results has received funding from the EEA/ Norway Grants 2014–2021 and the Technology Agency of the Czech Republic –project number TO01000215. This work was also supported by the European Regional Development Fund –Project ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868), Czech ministry of health –grant NV19-08-00472 and MH CZ –DRO, University Hospital Motol, Prague, Czech Republic No. 00064203 and by funds from the University of South Florida Nexus Initiative (UNI) Award and IPE2 2. LF UK Grant No. 6980382.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5364r2).
REFERENCES
[1] | Gainotti G , Quaranta D , Vita MG , Marra C ((2014) ) Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis 38: , 481–495. |
[2] | Vyhnálek M , Marková H , Laczó J , De Beni R , Di Nuovo S ((2019) ) Assessment of memory impairment in early diagnosis of Alzheimer’s disease. Curr Alzheimer Res 16: , 975–985. |
[3] | Maruta C , Guerreiro M , de Mendonça A , Hort J , Scheltens P ((2011) ) The use of neuropsychological tests across Europe: The need for a consensus in the use of assessment tools for dementia. Eur J Neurol 18: , 279–285. |
[4] | Dubois B , Feldman HH , Jacova C , Dekosky ST , Barberger-Gateau P , Cummings J , Delacourte A , Galasko D , Gauthier S , Jicha G , Meguro K , O’brien J , Pasquier F , Robert P , Rossor M , Salloway S , Stern Y , Visser PJ , Scheltens P ((2007) ) Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol 6: , 734–746. |
[5] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , Souza de LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[6] | Hort J , O’Brien JT , Gainotti G , Pirttila T , Popescu BO , Rektorova I , Sorbi S , Scheltens P ; EFNS Scientist Panel on Dementia ((2010) ) EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol 17: , 1236–1248. |
[7] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[8] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: , 183–194. |
[9] | Tulving E , Thomson DM ((1973) ) Encoding specificity and retrieval processes in episodic memory. Psychol Rev 80: , 352–373. |
[10] | Petersen RC ((2011) ) Clinical practice. Mild cognitive impairment. N Eng J Med 364: , 2227–2234. |
[11] | Dubois B , Albert ML ((2004) ) Amnestic MCI or prodromal Alzheimer’s disease? . Lancet Neurol 3: , 246–248. |
[12] | Tounsi H , Deweer B , Ergis AM , Van der Linden M , Pillon B , Michon A , Dubois B ((1999) ) Sensitivity to semantic cuing: An index of episodic memory dysfunction in early Alzheimer disease. Alzheimer Dis Assoc Disord 13: , 38–46. |
[13] | Buschke H , Sliwinski MJ , Kuslansky G , Lipton RB ((1997) ) Diagnosis of early dementia by the Double Memory Test: Encoding specificity improves diagnostic sensitivity and specificity. Neurology 48: , 989–997. |
[14] | Buschke H ((1984) ) Cued recall in amnesia. J Clin Neuropsychol 6: , 433–440. |
[15] | Sarazin M , Berr C , De Rotrou J , Fabrigoule C , Pasquier F , Legrain S , Michel B , Puel M , Volteau M , Touchon J , Verny M , Dubois B ((2007) ) Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology 69: , 1859–1867. |
[16] | Auriacombe S , Helmer C , Amieva H , Berr C , Dubois B , Dartigues JF ((2010) ) Validity of the free and cued selective reminding test in predicting dementia: The 3C study. Neurology 74: , 1760–1767. |
[17] | Grober E , Hall CB , Lipton RB , Zonderman AB , Resnick SM , Kawas C ((2008) ) Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc 14: , 266–278. |
[18] | Wagner M , Wolf S , Reischies FM , Daerr M , Wolfsgruber S , Jessen F , Popp J , Maier W , Hüll M , Frölich L , Hampel H , Perneczky R , Peters O , Jahn H , Luckhaus C , Gertz H-J , Schröder J , Pantel J , Lewczuk P , Kornhuber J , Wiltfang J ((2012) ) Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology 78: , 379–386. |
[19] | García-Herranz S , Díaz-Mardomingo MC , Peraita H ((2016) ) Neuropsychological predictors of conversion to probable Alzheimer disease in elderly with mild cognitive impairment. J Neuropsychol 10: , 239–255. |
[20] | Dierckx E , Engelborghs S , Raedt RD , Buggenhout MV , Deyn PPD , Verté D , Ponjaert-Kristoffersen I ((2009) ) Verbal cued recall as a predictor of conversion to Alzheimer’s disease in Mild Cognitive Impairment. Int J Geriatr Psychiatry 24: , 1094–1100. |
[21] | Belleville S , Gauthier S , Lepage E , Kergoat MJ , Gilbert B ((2014) ) Predicting decline in mild cognitive impairment: A prospective cognitive study. Neuropsychology 28: , 643–652. |
[22] | Beekly DL , Ramos EM , Lee WW , Deitrich WD , Jacka ME , Wu J , Hubbard JL , Koepsell TD , Morris JC , Kukull WA , NIA Alzheimer’s Disease Centers ((2007) ) The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 21: , 249–258. |
[23] | Donohue MC , Sperling RA , Salmon DP , Rentz DM , Raman R , Thomas RG , Weiner M , Aisen PS ; Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Cooperative Study ((2014) ) The preclinical Alzheimer cognitive composite: Measuring amyloid-related decline. JAMA Neurol 71: , 961–970. |
[24] | Papp KV , Rentz DM , Orlovsky I , Sperling RA , Mormino EC ((2017) ) Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: The PACC5. Alzheimers Dement (N Y) 3: , 668–677. |
[25] | Jutten RJ , Sikkes SAM , Amariglio RE , Buckley RF , Properzi MJ , Marshall GA , Rentz DM , Johnson KA , Teunissen CE , Van Berckel BNM , Van der Flier WM , Scheltens P , Sperling RA , Papp KV ; Alzheimer Disease Neuroimaging Initiative; National Alzheimer’s Coordinating Center, the Harvard Aging Brain Study and the Alzheimer Dementia Cohort ((2020) ) Identifying sensitive measures of cognitive decline at different clinical stages of Alzheimer’s disease. J Int Neuropsychol Soc 27: , 426–438. |
[26] | Jester DJ , Andel R , Cechová K , Laczó J , Lerch O , Marková H , Nikolai T , Vyhnálek M , Hort J ((2021) ) Cognitive phenotypes of older adults with subjective cognitive decline and amnestic mild cognitive impairment: The Czech Brain Aging Study. J Int Neuropsychol Soc 27: , 329–342. |
[27] | Vyhnalek M , Nikolai T , Andel R , Nedelska Z , Rubínová E , Marková H , Laczó J , Bezdicek O , Sheardova K , Hort J ((2014) ) Neuropsychological correlates of hippocampal atrophy in memory testing in nondemented older adults. J Alzheimers Dis 42: (Suppl 3), S81–S90. |
[28] | Sheardova K , Vyhnalek M , Nedelska Z , Laczo J , Andel R , Marciniak R , Cerman J , Lerch O , Hort J ((2019) ) Czech Brain Aging Study (CBAS): Prospective multicentre cohort study on risk and protective factors for dementia in the Czech Republic. . BMJ Open 9: , e030379. |
[29] | Laczó J , Andel R , Vlček K , Macoška V , Vyhnálek M , Tolar M , Bojar M , Hort J ((2011) ) Spatial navigation and APOE in amnestic mild cognitive impairment. Neurodegener Dis 8: , 169–177. |
[30] | Román GC , Tatemichi TK , Erkinjuntti T , Cummings JL , Masdeu JC , Garcia JH , Amaducci L , Orgogozo JM , Brun A , Hofman A , Moody DM , O’Brien MD , Yamaguchi T , Grafman J , Drayer BP , Bennett DA , Fisher M , Ogata J , Kokmen E , Bermejo F , Wolf PA , Gorelick PB , Bick KL , Pajeau AK , Bell MA , DeCarli C , Culebras A , Korczyn AD , Bogousslavsky J , Hartmann A , Scheinberg P ((1993) ) Vascular dementia: Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop*. Neurology 43: , 250–260. |
[31] | McKeith IG , Boeve BF , Dickson DW , Halliday G , Taylor J-P , Weintraub D , Aarsland D , Galvin J , Attems J , Ballard CG , Bayston A , Beach TG , Blanc F , Bohnen N , Bonanni L , Bras J , Brundin P , Burn D , Chen-Plotkin A , Duda JE , El-Agnaf O , Feldman H , Ferman TJ , Ffytche D , Fujishiro H , Galasko D , Goldman JG , Gomperts SN , Graff-Radford NR , Honig LS , Iranzo A , Kantarci K , Kaufer D , Kukull W , Lee VMY , Leverenz JB , Lewis S , Lippa C , Lunde A , Masellis M , Masliah E , McLean P , Mollenhauer B , Montine TJ , Moreno E , Mori E , Murray M , O’Brien JT , Orimo S , Postuma RB , Ramaswamy S , Ross OA , Salmon DP , Singleton A , Taylor A , Thomas A , Tiraboschi P , Toledo JB , Trojanowski JQ , Tsuang D , Walker Z , Yamada M , Kosaka K ((2017) ) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89: , 88–100. |
[32] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EGP , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini M-L , Rosen H , Prioleau-Latham CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: , 2456–2477. |
[33] | Custodio N , Montesinos R , Lira D , Herrera-Pérez E , Bardales Y , Valeriano-Lorenzo L ((2017) ) Mixed dementia: A review of the evidence. Dement Neuropsychol 11: , 364–370. |
[34] | Morris JC ((1997) ) Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9: (Suppl 1), 173–176;discussion 177-178. |
[35] | Pfeffer RI , Kurosaki TT , Harrah CH Jr , Chance JM , Filos S ((1982) ) Measurement of functional activities in older adults in the community. J Gerontol 37: , 323–329. |
[36] | Sheikh JI , Yesavage JA ((1986) ) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol 5: , 165–173. |
[37] | Nikolai T , Štěpánková H , Michalec J , Bezdicek O , Horáková K , Marková H , Růžička E , Kopeč cek M ((2015) ) Testy verbální fluence, česká normativní studie pro osoby vyššího věku. Cesk Slov Neurol N 78: , 292–229. |
[38] | Nikolai T , Stepankova H , Kopecek M , Sulc Z , Vyhnalek M , Bezdicek O ((2018) ) The uniform data set, Czech version: Normative data in older adults from an international perspective. J Alzheimers Dis 61: , 1233–1240. |
[39] | Drozdová K , Štěpánková H , Lukavský J , Bezdícek O , Kopecek M ((2015) ) Normativní studie testu Reyovy-Osterriethovy komplexnífigury v populaci českých seniorů. Cesk Slov Neurol N 78: , 542–549. |
[40] | Grober E , Buschke H , Crystal H , Bang S , Dresner R ((1988) ) Screening for dementia by memory testing. Neurology 38: , 900–903. |
[41] | Topinková E , Jirák R , Kozený J ((2002) ) Krátká neurokognitivní baterie pro screening demence v klinické praxi: Sedmiminutový screeningový test. Neurologie Pro Praxi 3: , 323–328. |
[42] | Bezdicek O , Stepankova H , Moták L , Axelrod BN , Woodard JL , Preiss M , Nikolai T , Ružička E , Poreh A ((2014) ) Czech version of Rey Auditory Verbal Learning test: Normative data. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 21: , 693–721. |
[43] | Rey A ((1958) ) L’examen clinique en psychologie. [The psychological examination]. Presses Universitaires de France, Paris. |
[44] | Meyers JE , Meyers KR ((1995) ) Rey complex figure test under four different administration procedures. Clin Neuropsychol 9: , 63–67. |
[45] | R Core Team (2013) R: A language and environment for statistical computing. Retrieved from: https://www.rproject.org. |
[46] | Rabin LA , Paré N , Saykin AJ , Brown MJ , Wishart HA , Flashman LA , Santulli RB ((2009) ) Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 16: , 357–376. |
[47] | Larrabee GJ , Youngjohn JR , Sudilovsky A , Crook TH ((1993) ) Accelerated forgetting in Alzheimer-type dementia. J Clin Exp Neuropsychol 15: , 701–712. |
[48] | Reed BR , Paller KA , Mungas D ((1998) ) Impaired acquisition and rapid forgetting of patterned visual stimuli in Alzheimer’s disease. J Clin Exp Neuropsychol 20: , 738–749. |
[49] | Grober E , Veroff AE , Lipton RB ((2018) ) Temporal unfolding of declining episodic memory on the Free and Cued Selective Reminding Test in the predementia phase of Alzheimer’s disease: Implications for clinical trials. Alzheimers Dement (Amst) 10: , 161–171. |
[50] | Mottron L , Morasse K , Belleville S ((2001) ) A study of memory functioning in individuals with Autism. J Child Psychol Psychiatry 42: , 253–260. |
[51] | Welsh K , Butters N , Hughes J , Mohs R , Heyman A ((1991) ) Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol 48: , 278–281. |
[52] | Rentz DM , Parra Rodriguez MA , Amariglio R , Stern Y , Sperling R , Ferris S ((2013) ) Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: A selective review. Alzheimers Res Ther 5: , 58. |
[53] | Mowrey WB , Lipton RB , Katz MJ , Ramratan WS , Loewenstein DA , Zimmerman ME , Buschke H ((2018) ) Memory binding test predicts incident dementia: Results from the Einstein Aging Study. J Alzheimers Dis 62: , 293–304. |
[54] | Bayles KA , Salmon DP , Tomoeda CK , Jacobs D , Caffrey JT , Kaszniak AW , Tröster AI ((1989) ) Semantic and letter category naming in Alzheimer’s patients: A predictable difference. Dev Neuropsychol 5: , 335–347. |
[55] | Binetti G , Magni E , Cappa SF , Padovani A , Bianchetti A , Trabucchi M ((1995) ) Semantic memory in Alzheimer’s disease: An analysis of category fluency. J Clin Exp Neuropsychol 17: , 82–89. |
[56] | Salmon DP , Heindel WC , Lange KL ((1999) ) Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: Implications for the integrity of semantic memory. J Int Neuropsychol Soc 5: , 692–703. |
[57] | Nikolai T , Bezdicek O , Markova H , Stepankova H , Michalec J , Kopecek M , Dokoupilova M , Hort J , Vyhnalek M ((2018) ) Semantic verbal fluency impairment is detectable in patients with subjective cognitive decline. Appl Neuropsychol Adult 25: , 448–457. |
[58] | Palmer K , Bäckman L , Winblad B , Fratiglioni L ((2003) ) Detection of Alzheimer’s disease and dementia in the preclinical phase: Population based cohort study. BMJ 326: , 245. |
[59] | Carew TG , Lamar M , Cloud BS , Grossman M , Libon DJ ((1997) ) Impairment in category fluency in ischemic vascular dementia. Neuropsychology 11: , 400–412. |
[60] | Monsch AU , Bondi MW , Butters N , Salmon DP , Katzman R , Thal LJ ((1992) ) Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol 49: , 1253–1258. |
[61] | Baldo JV , Schwartz S , Wilkins D , Dronkers NF ((2006) ) Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc 12: , 896–900. |
[62] | Birn RM , Kenworthy L , Case L , Caravella R , Jones TB , Bandettini PA , Martin A ((2010) ) Neural systems supporting lexical search guided by letter and semantic category cues: A self-paced overt response fMRI study of verbal fluency. Neuroimage 49: , 1099–1107. |
[63] | Jak AJ , Bondi MW , Delano-Wood L , Wierenga C , Corey-Bloom J , Salmon DP , Delis DC ((2009) ) Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 17: , 368–375. |




