Behavioral and Psychological Symptoms of Dementia in Different Dementia Disorders: A Large-Scale Study of 10,000 Individuals
Abstract
Background:
The majority of individuals with dementia will suffer from behavioral and psychological symptoms of dementia (BPSD). These symptoms contribute to functional impairment and caregiver burden.
Objective:
To characterize BPSD in Alzheimer’s disease (AD), vascular dementia (VaD), mixed (Mixed) dementia, Parkinson’s disease dementia (PDD), dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), and unspecified dementia in individuals residing in long-term care facilities.
Methods:
We included 10,405 individuals with dementia living in long-term care facilities from the Swedish registry for cognitive/dementia disorders (SveDem) and the Swedish BPSD registry. BPSD was assessed with the Neuropsychiatric Inventory - Nursing Home Version (NPI-NH). Multivariate logistic regression models were used to evaluate the associations between dementia diagnoses and different BPSDs.
Results:
The most common symptoms were aberrant motor behavior, agitation, and irritability. Compared to AD, we found a lower risk of delusions (in FTD, unspecified dementia), hallucinations (FTD), agitation (VaD, PDD, unspecified dementia), elation/euphoria (DLB), anxiety (Mixed, VaD, unspecified dementia), disinhibition (in PDD), irritability (in DLB, FTD, unspecified dementia), aberrant motor behavior (Mixed, VaD, unspecified dementia), and sleep and night-time behavior changes (unspecified dementia). Higher risk of delusions (DLB), hallucinations (DLB, PDD), apathy (VaD, FTD), disinhibition (FTD), and appetite and eating abnormalities (FTD) were also found in comparison to AD.
Conclusion:
Although individuals in our sample were diagnosed with different dementia disorders, they all exhibited aberrant motor behavior, agitation, and irritability. This suggests common underlying psychosocial or biological mechanisms. We recommend prioritizing these symptoms while planning interventions in long-term care facilities.
INTRODUCTION
Dementia is a complex syndrome associated with cognitive, behavioral, and psychological symptoms. The current diagnostic system emphasizes cognitive impairment and progressive cognitive decline as the key features of dementia [1]. However, cognitive impairment does not solely explain functional disability or poor quality of life in people with dementia. Instead, recent studies have shown that approximately 90% of individuals with dementia will suffer from additional psychological or behavioral symptoms and that these symptoms are important factors contributing to functional impairment and increased caregiver burden [2–5]. Behavioral and psychological symptoms of dementia (BPSD) (also referred to as neuropsychiatric symptoms of dementia) include changes in behavior, perception, content of thoughts as well as mood disorders [5]. These symptoms are commonly found across different dementia disorders such as Alzheimer’s disease (AD), vascular dementia (VaD), dementia with Lewy bodies (DLB), Parkinson’s disease dementia (PDD), or frontotemporal dementia (FTD). However, the frequency of particular symptoms tends to vary between diagnoses (e.g., [6–9]). Additionally, the place of residency seems to affect the manifestation of symptoms, with individuals residing in long-term care facilities presenting more symptoms from the hyperactivity spectrum (irritability, aberrant motor behavior, euphoria) and appetite disorders than those that are community dwelling [10]. The psychosocial/physical environment characteristics of long-term care facilities, such as interior design, lighting levels, and daily activities, seem to contribute to an increased risk of BPSD [11]. However, to our knowledge, studies conducted in long-term care facilities did not make a distinction between different types of dementia. From a clinical standpoint, it is important to determine whether residents in long-term care institutions with various dementia diagnoses present with different BPSD or if there is a more prevalent group of symptoms regardless of the type of dementia. A deeper insight into the profiles of BPSD in long-term care facilities may facilitate more cost-effective interventions. Therefore, the aim of this study was to provide a detailed and comprehensive description of presence of BPSD across different dementia diagnoses in individuals residing in long-term care facilities through a merge of two large Swedish quality registries.
MATERIALS AND METHODS
The Swedish registry for cognitive/dementia disorders (SveDem) was established in 2007 and focuses on improving the quality of care of persons with dementia. Individuals are registered at the time of the dementia diagnosis and during follow-up annually in specialist units, primary care centers or in long-term care facilities. Data on type of dementia, age, sex, and Mini-Mental State Examination (MMSE) scores at the time of the dementia diagnosis, medication, and living arrangements are collected. The types of dementia diagnosis registered are: AD, Mixed, VaD, DLB, PDD, FTD, unspecified dementia, and other dementia types (this category includes various dementia disorders, e.g., corticobasal degeneration and alcohol-related dementia). Clinicians are instructed to use the 10th revision of the International Classification of Diseases (ICD-10). Additionally, the McKeith criteria are used for DLB [12], the Lund-Manchester criteria are used for FTD [13], and the Movement Disorder Society Task Force criteria [14] are used for PDD. For a more detailed description of SveDem, please see Religa et al [15].
The Swedish BPSD registry was established in 2010 to improve the quality of care of persons with dementia with BPSD and to achieve a national standard of care throughout Sweden. It includes information about BPSD, current medical treatment and a checklist for possible causes of BPSD [17]. In the registry, assessment of BPSD is carried out using the Neuropsychiatric Inventory-Nursing Home Version (NPI-NH) [18], which includes twelve categories of neuropsychiatric symptoms: delusions, hallucinations, agitation/aggression, depression/depression, apathy, elation/euphoria, anxiety, disinhibition, irritability, aberrant motor behavior, sleep and night-time behavior changes, and appetite and eating abnormalities. The NPI-NH is based on the information provided by a caregiver familiar with the behavior of an individual. First, the frequency of symptoms is scored on a four-point scale from 1 to 4 (1: occasionally, 2: less than once a week, 3: very frequently, 4: more than once a day). Second, the severity of the behavior is rated on a three-point scale: mild, moderate, or severe. By multiplying the frequency and severity scores, the NPI-NH yields a rating for each category between 1 and 12. The total NPI score is calculated by adding up the scores across all categories, which can yield a maximum of 144 points. Category scores of 4 or more are treated as indicative of clinical significance [19]. Information about medical treatment is collected at the time of BPSD assessment. Specifically, the Anatomical Therapeutic Chemical (ATC) codes for the following medications were recorded: analgesics, antipsychotics (ATC code: N05A), anxiolytics (N05B), hypnotics (N05C), antidepressants (N06AA), acetylcholinesterase inhibitors (AChEI) (N06DA), N-Methyl-D-aspartate (NMDA) receptor antagonist (ATC codes: N06DX). Study has ethical approval (2015/2291-31/5).
Study sample size
In 2016, there were 44,482 persons registered in the BPSD registry and 68,515 in SveDem. After merging the two registries, 13,413 individuals with different dementia diagnosis and assessed for BPSD-symptoms were identified. Age was recorded at the time of dementia diagnosis and at the time of registration in the BPSD-registry. After excluding individuals with incomplete records (n = 256), duplicates (n = 111), diagnoses prior to 2007 (n = 81) and after 2017 (n = 1), there were 12,964 individuals. For the purposes of the current study, we additionally excluded persons with other types of dementia (n = 329), individuals who were included in the BPSD registry before being registered in SveDem (n = 498) and individuals living in community at the time of BPSD assessment (n = 1,298) or no information about their residency at the time of BPSD was available (n = 434). As a result, our final study sample consisted of 10,405 individuals (Supplementary Figure 1).
Statistical analyses
Continuous variables were reported as mean±standard deviation (SD), and categorical variables as counts and proportions. Baseline variables from SveDem and BPSD registries were compared across different dementias using Pearson chi-square for proportions and ANOVA for continuous variables. We had a small fraction of missing data ranging from 1.9 in the variable Delusions at the time of dementia diagnosis to 8.3 in MMSE at the time of dementia diagnosis (Supplementary Table 1).
Odds ratios (OR) and 95% Confidence Intervals (CIs) of having a clinically significant BPSD (NPI score > 3 in a given category) (versus no symptom) in each diagnosis comparing to AD were estimated with multivariate logistic regression. Then, the analyses were repeated with each diagnosis used as a reference group. All models were adjusted for age, sex, MMSE, and time between SveDem and BPSD registries.
Given that the main analyses were based on complete case data, we also performed a sensitivity analysis using imputed data to assess that the results were similar before and after imputation. Incomplete variables were imputed under fully conditional specification using the mice 3.0 package [20]. We created five multiple imputed datasets and performed multivariate logistic regression analysis in each of them. OR and 95% CIs of having a clinically significant BPSD (NPI score > 3 in a given category) for each of the 12 symptoms in each diagnosis comparing to AD were estimated in each imputed dataset separately and combined using Rubin’s rules. All analyses were undertaken in R version 3.6.0 (Foundation for Statistical Computing, Vienna, Austria) [21]
RESULTS
The characteristics of the sample are provided in Tables 1 and 2. Overall the most common diagnosis in the cohort was AD (34.1%), followed by VaD (16.4%), Mixed (15.6%), DLB (2.3%), PDD (1.7%), and FTD (1.9%). Further, there were 28.5% individuals with unspecified dementia. Mean age at the time of dementia diagnosis was 80.2±7.9 years and 64.8% were women. At the time of dementia diagnosis, 7.3% were being treated with antipsychotics, 30.5% with antidepressants, 18.9% with hypnotics, and 13.7% with anxiolytics. In AD, 69% individuals were treated with AChEI, 11.7% with a NMDA antagonist and 2.4% were receiving both NMDA and AChEI. On average, individuals were registered in the BPSD registry after 1013.5±660.2 days from registration in SveDem.
Table 1
Basic characteristics of the study population at the time of registration in SveDem
| Total | AD | VaD | Mixed | DLB | PDD | FTD | Unspecified | p | |
| 3,548 (34.1) | 1,708 (16.4) | 1,621 (15.6) | 236 (2.3) | 122 (1.7) | 200 (1.9) | 2,970 (28.5) | |||
| Female | 6,739 (64.8) | 2,443 (68.9) | 1,015 (59.4) | 1,066 (65.8) | 118 (50) | 47 (38.5) | 111 (55.5) | 1,939 (65.3) | < 0.001 |
| Age, y | 80.2 (7.9) | 78.3 (8.3) | 81.5 (7.2) | 81.3 (6.7) | 77.6 (6.9) | 75.9 (7) | 69.8 (9) | 82.2 (7.1) | < 0.001 |
| MMSE, score | 20.1 (5.1) | 20.4 (5.2) | 20.1 (4.9) | 20.1 (5) | 21.3 (4.9) | 20.8 (4.3) | 22.1 (6.1) | 19.4 (5.2) | < 0.001 |
| Time, days to registration in the BPSD-registry | 1,013.5 (660.2) | 1,169.6 (682.8) | 895.2 (622.4) | 997.3 (673.4) | 912.3 (611.8) | 951.2 (575) | 929.9 (585.9) | 920.2 (621.9) | < 0.001 |
| Antipsychotics | 717 (7.3) | 186 (5.5) | 114 (7) | 109 (6.9) | 30 (12.9) | 23 (20.2) | 25 (12.7) | 230 (8.6) | < 0.001 |
| Anxiolytics | 1,340 (13.7) | 349 (10.3) | 270 (16.6) | 162 (10.3) | 26 (11.2) | 16 (13.9) | 25 (12.7) | 492 (18.4) | < 0.001 |
| Hypnotics | 1,849 (18.9) | 546 (16.2) | 362 (22.3) | 284 (18.1) | 52 (22.3) | 20 (17.5) | 31 (15.7) | 554 (20.7) | < 0.001 |
| Antidepressants | 3,006 (30.5) | 1,001 (29.5) | 590 (36.1) | 414 (26.2) | 81 (34.8) | 45 (39.1) | 68 (34.2) | 807 (29.9) | < 0.001 |
| AChEI | 4,416 (44.3) | 2,380 (69) | 151 (9.2) | 789 (49.6) | 174 (74) | 65 (53.7) | 10 (5) | 847 (30.9) | < 0.001 |
| NMDA antagonist | 1,068 (10.8) | 397 (11.7) | 92 (5.7) | 321 (20.2) | 41 (17.7) | 24 (20.5) | 14 (7) | 179 (6.6) | < 0.001 |
| NMDA antagonist + AChEIs | 151 (1.5) | 81 (2.4) | 4 (0.2) | 20 (1.3) | 15 (6.5) | 9 (7.7) | 1 (0.5) | 21 (0.8) | < 0.001 |
AD, Alzheimer’s disease; Mixed, Mixed dementia; VaD, vascular dementia; DLB, dementia with Lewy bodies; PDD, Parkinson’s disease dementia; FTD, frontotemporal dementia; MMSE, Mini-Mental State Examination; Living arrangement, at the time of dementia diagnoses; NMDA, N-Methyl-D-aspartate, AChEIs, acetylcholineesterase inhibitors. Data are presented as mean (SD) or n (%).
Table 2
Basic characteristics of the study population at the time of registration in the BPSD registry
| Total | AD | VaD | Mixed | DLB | PDD | FTD | Unspecified | p | |
| Age | 82.4 (7.6) | 80.9 (8) | 83.4 (7) | 83.5 (6.4) | 79.6 (6.6) | 78 (6.9) | 71.8 (8.9) | 84.1 (7) | < 0.001 |
| Analgesics | 4,024 (38.7) | 1,297 (36.6) | 713 (41.7) | 636 (39.2) | 89 (37.7) | 47 (38.5) | 58 (29) | 1,184 (39.9) | 0.006 |
| Antipsychotics | 1,757 (16.9) | 615 (17.3) | 279 (16.3) | 242 (14.9) | 44 (18.6) | 33 (27) | 53 (26.5) | 491 (16.5) | < 0.001 |
| Anxiolytics | 2,049 (19.7) | 723 (20.4) | 336 (19.7) | 301 (18.6) | 37 (15.7) | 14 (11.5) | 30 (15) | 608 (20.5) | 0.031 |
| Hypnotics | 1,967 (18.9) | 672 (18.9) | 346 (20.3) | 337 (20.8) | 52 (22) | 18 (14.8) | 39 (19.5) | 503 (16.9) | 0.013 |
| Antidepressants | 4,635 (44.5) | 1,618 (45.6) | 808 (47.3) | 707 (43.6) | 103 (43.6) | 60 (49.2) | 78 (39) | 1,261 (42.5) | 0.012 |
| AChEI | 2,773 (26.7) | 1,361 (38.4) | 116 (6.8) | 519 (32) | 126 (53.4) | 44 (36.1) | 10 (5) | 597 (20.1) | < 0.001 |
| NMDA antagonist | 2,366 (22.7) | 1063 (30) | 186 (10.9) | 496 (30.6) | 92 (39) | 33 (27) | 22 (11) | 474 (16) | < 0.001 |
| NMDA+AChEI | 808 (7.8) | 444 (12.5) | 16 (0.937) | 150 (9.25) | 58 (24.6) | 15 (12.3) | 4 (2) | 121 (4.07) | < 0.001 |
| Total NPI | 23.2 (21.9) | 24.8 (22.5) | 22.5 (21.6) | 22.5 (22) | 24.9 (23.1) | 21.7 (16.8) | 26.3 (22.5) | 21.7 (21.2) | < 0.001 |
| Any symptom (NPI > 0) | 9,430 (92.3) | 3,228 (92.9) | 1,529 (91.1) | 1,466 (92.2) | 220 (94.4) | 115 (95) | 186 (94.9) | 2,686 (91.8) | 0.093 |
| Clinically significant symptom (NPI > 3) | 7,710 (75.5) | 2,630 (75.8) | 1,249 (74.4) | 1,183 (74.4) | 187 (80.6) | 105 (86.8) | 136 (69.4) | 2,220 (76) | 0.006 |
AD, Alzheimer’s disease; Mixed, Mixed dementia; VaD, vascular dementia; DLB, dementia with Lewy bodies; PDD, Parkinson’s disease dementia; FTD, frontotemporal dementia; NMDA, N-Methyl-D-aspartate; NPI, Neuropsychiatric Inventory. Data are presented as mean (SD) or n (%).
When registered in the BPSD registry, the mean age was 82.4±7.6 years, 16.9% of individuals were being treated with antipsychotics, 44.5% with antidepressants, 18.9% with hypnotics, and 19.7% with anxiolytics. In AD, 38.4% were treated with AChEI, 30% with NMDA antagonist and 12.5% were receiving both.
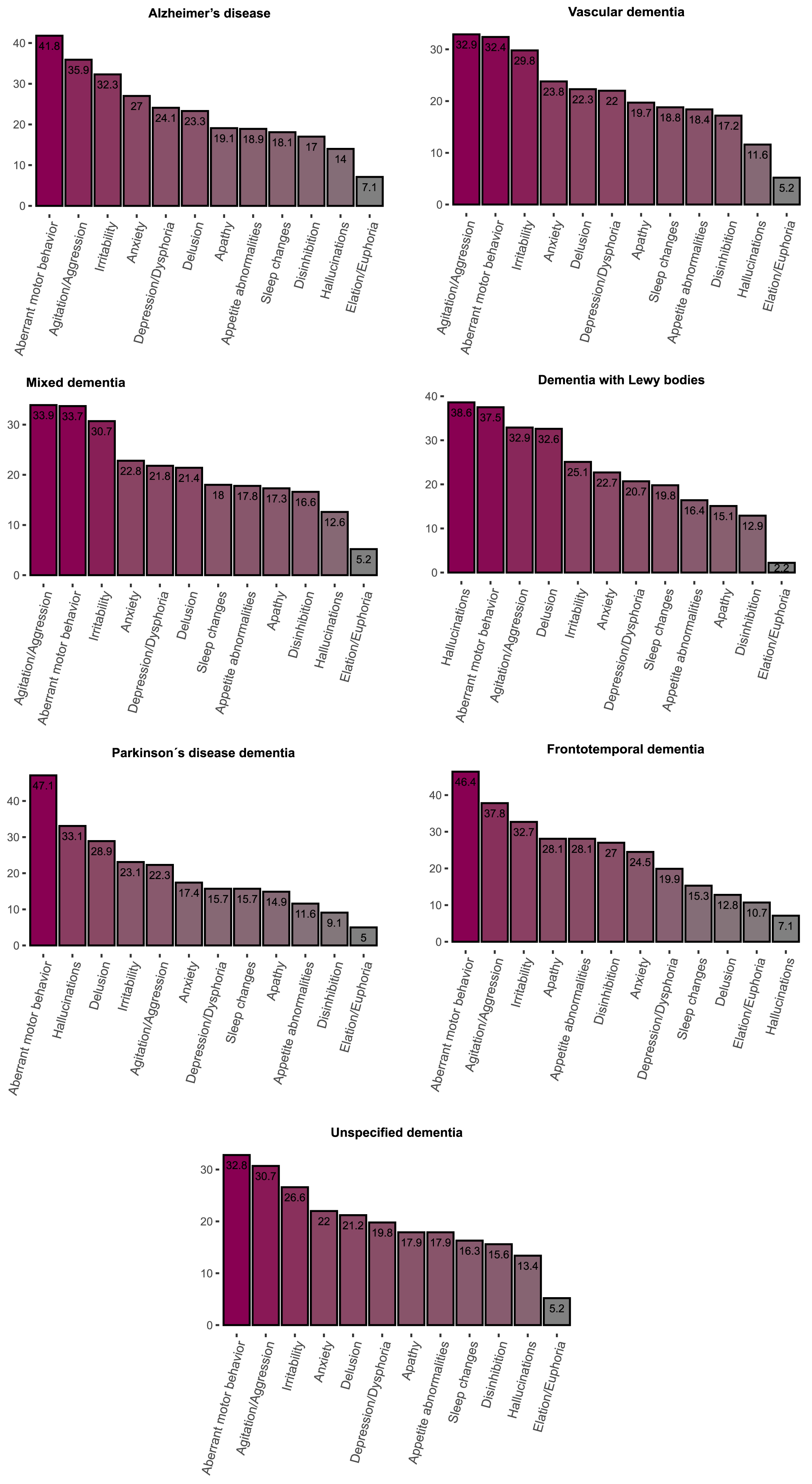
At least one clinically significant symptom (NPI score > 3 in a given category) was present in 75.5% of individuals and any symptom (NPI score > 0 in a given category) was present in 92.3% individuals. The most frequent clinically significant symptoms were aberrant motor behavior (38.4%), agitation (33.4%) and irritability (29.7%) (Table 3, Fig. 1). Mean of total NPI score was 23.2±21.9 points. The highest mean total NPI score was found in FTD (26.3±22.5), followed by DLB (24.9±23.1), AD (24.8±2 2.5), PDD (21.7±16.8), Mixed (22.5±22), VaD (22.5±22), and unspecified dementia (21.7±21.2).
Table 3
Frequency of BPSD across different dementia diagnoses
| Total | AD | VaD | Mixed | DLB | PDD | FTD | Unspecified | P | |
| Delusions | |||||||||
| No symptom (0) | 6,646 (65) | 2,262 (65.1) | 1,087 (64.7) | 1,059 (66.6) | 120 (51.5) | 62 (51.2) | 157 (80.1) | 1,899 (64.9) | < 0.001 |
| Mild symptoms (1–3) | 1,294 (12.7) | 404 (11.6) | 219 (13) | 191 (12) | 37 (15.9) | 24 (19.8) | 14 (7.1) | 405 (13.9) | |
| Clinically significant (> 3) | 2,279 (22.3) | 808 (23.3) | 374 (22.3) | 341 (21.4) | 76 (32.6) | 35 (28.9) | 25 (12.8) | 620 (21.2) | |
| Hallucinations | |||||||||
| No symptom (0) | 7,723 (75,6) | 2,637 (76) | 1,317 (78.4) | 1,235 (77.7) | 91 (39.1) | 51 (42.1) | 171 (87.2) | 2,221 (76) | < 0.001 |
| Mild symptoms (1–3) | 1,072 (10.5) | 347 (10) | 168 (10) | 155 (9.7) | 52 (22.3) | 30 (24.8) | 11 (5.6) | 309 (10.6) | |
| Clinically significant (> 3) | 1,419 (13.9) | 487 (14) | 195 (11.6) | 200 (12.6) | 90 (38.6) | 40 (33.1) | 14 (7.1) | 393 (13.4) | |
| Agitation/Aggression | |||||||||
| No symptom (0) | 4,238 (41,5) | 1,363 (39.3) | 740 (44.1) | 649 (40.8) | 103 (44.6) | 58 (47.9) | 73 (37.2) | 1,252 (42.8) | < 0.001 |
| Mild symptoms (1–3) | 2,561 (25.1) | 863 (24.9) | 386 (23) | 402 (25.3) | 52 (22.5) | 36 (29.8) | 49 (25) | 773 (26.4) | |
| Clinically significant (> 3) | 3,413 (33.4) | 1,246 (35.9) | 553 (32.9) | 539 (33.9) | 76 (32.9) | 27 (22.3) | 74 (37.8) | 898 (30.7) | |
| Depression/Dysphoria | |||||||||
| No symptom (0) | 5,264 (51.5) | 1,724 (49.7) | 864 (51.4) | 830 (52.2) | 101 (43.5) | 61 (50.4) | 119 (60.7) | 1,565 (53.6) | < 0.001 |
| Mild symptoms (1–3) | 2,713 (26.6) | 912 (26.3) | 446 (26.5) | 414 (26) | 83 (35.8) | 41 (33.9) | 38 (19.4) | 779 (26.7) | |
| Clinically significant (> 3) | 2,236 (21.9) | 836 (24.1) | 370 (22) | 346 (21.8) | 48 (20.7) | 19 (15.7) | 39 (19.9) | 578 (19.8) | |
| Anxiety | |||||||||
| No symptom (0) | 5,264 (51.5) | 1,900 (54.7) | 1,023 (60.9) | 960 (60.3) | 139 (59.7) | 71 (58.7) | 120 (61.2) | 1,796 (61.5) | < 0.001 |
| Mild symptoms (1–3) | 1,741 (17) | 635 (18.3) | 257 (15.3) | 268 (16.8) | 41 (17.6) | 29 (24) | 28 (14.3) | 483 (16.5) | |
| Clinically significant (> 3) | 2,462 (24.1) | 937 (27) | 399 (23.8) | 363 (22.8) | 53 (22.7) | 21 (17.4) | 48 (24.5) | 641 (22) | |
| Elation/Euphoria | |||||||||
| No symptom (0) | 8,861 (86.8) | 2,948 (84.9) | 1,492 (88.9) | 1,396 (87.9) | 212 (91.8) | 102 (84.3) | 152 (77.6) | 2,559 (87.6) | < 0.001 |
| Mild symptoms (1–3) | 743 (7.3) | 275 (7.9) | 99 (5.9) | 111 (7) | 14 (6.1) | 13 (10.7) | 23 (11.7) | 208 (7.1) | |
| Clinically significant (> 3) | 603 (5.9) | 248 (7.1) | 88 (5.2) | 82 (5.2) | 5 (2.2) | 6 (5) | 21 (10.7) | 153 (5.2) | |
| Apathy | |||||||||
| No symptom (0) | 7,092 (69.4) | 2,434 (70.1) | 1,157 (69) | 1,110 (69.8) | 165 (71.1) | 79 (65.3) | 119 (60.7) | 2,028 (69.4) | 0.001 |
| Mild symptoms (1–3) | 1,219 (11.9) | 374 (10.8) | 190 (11.3) | 205 (12.9) | 32 (13.8) | 24 (19.8) | 22 (11.2) | 372 (12.7) | |
| Clinically significant (> 3) | 1,901 (18.6) | 663 (19.1) | 331 (19.7) | 276 (17.3) | 35 (15.1) | 18 (14.9) | 55 (28.1) | 523 (17.9) | |
| Disinhibition | |||||||||
| No symptom (0) | 7,170 (70.2) | 2,406 (69.3) | 1,181 (70.3) | 1,145 (72) | 171 (73.7) | 94 (77.7) | 113 (57.7) | 2,060 (70.5) | < 0.001 |
| Mild symptoms (1–3) | 1,345 (13.2) | 474 (13.7) | 209 (12.4) | 181 (11.4) | 31 (13.4) | 16 (13.2) | 30 (15.3) | 404 (13.8) | |
| Clinically significant (> 3) | 1,694 (16.6) | 591 (17) | 289 (17.2) | 264 (16.6) | 30 (12.9) | 11 (9.1) | 53 (27) | 456 (15.6) | |
| Irritability | |||||||||
| No symptom (0) | 4,838 (47.4) | 1,584 (45.7) | 810 (48.3) | 730 (45.9) | 127 (55) | 57 (47.1) | 101 (51.5) | 1,429 (48.9) | < 0.001 |
| Mild symptoms (1–3) | 2,333 (22.9) | 766 (22.1) | 368 (21.9) | 372 (23.4) | 46 (19.9) | 36 (29.8) | 31 (15.8) | 714 (24.5) | |
| Clinically significant (> 3) | 3,034 (29.7) | 1,119 (32.3) | 500 (29.8) | 488 (30.7) | 58 (25.1) | 28 (23.1) | 64 (32.7) | 777 (26.6) | |
| Aberrant motor behavior | |||||||||
| No symptom (0) | 5,446 (53.3) | 1,671 (48.1) | 992 (59.1) | 883 (55.5) | 124 (53.4) | 49 (40.5) | 93 (47.4) | 1,634 (55.9) | < 0.001 |
| Mild symptoms (1–3) | 1,044 (10.2) | 352 (10.1) | 143 (8.5) | 171 (10.8) | 21 (9.1) | 15 (12.4) | 12 (6.1) | 330 (11.3) | |
| Clinically significant (> 3) | 3,721 (36.4) | 1,450 (41.8) | 543 (32.4) | 536 (33.7) | 87 (37.5) | 57 (47.1) | 91 (46.4) | 957 (32.8) | |
| Sleep and night-time behavior changes | |||||||||
| No symptom (0) | 6,585 (64.5) | 2,206 (63.6) | 1,050 (62.6) | 1,057 (66.6) | 141 (60.8) | 74 (61.2) | 126 (64.3) | 1,931 (66.2) | 0.0662 |
| Mild symptoms (1–3) | 1,817 (17.8) | 634 (18.3) | 312 (18.6) | 245 (15.4) | 45 (19.4) | 28 (23.1) | 40 (20.4) | 513 (17.6) | |
| Clinically significant (> 3) | 1,800 (17.6) | 629 (18.1) | 316 (18.8) | 285 (18) | 46 (19.8) | 19 (15.7) | 30 (15.3) | 475 (16.3) | |
| Appetite and eating abnormalities | |||||||||
| No symptom (0) | 7,434 (72.8) | 2,519 (72.6) | 1,214 (72.4) | 1,164 (73.3) | 172 (74.1) | 102 (84.3) | 122 (62.2) | 2,141 (73.3) | 0.02 |
| Mild symptoms (0–3) | 893 (8.7) | 295 (8.5) | 154 (9.2) | 142 (8.9) | 22 (9.5) | 5 (4.1) | 19 (9.7) | 256 (8.8) | |
| Clinically significant (> 3) | 1,880 (18.4) | 657 (18.9) | 309 (18.4) | 283 (17.8) | 38 (16.4) | 14 (11.6) | 55 (28.1) | 524 (17.9) |
AD, Alzheimer’s disease; Mixed, Mixed dementia; VaD, vascular dementia; DLB, dementia with Lewy bodies; PDD, Parkinson’s disease dementia; FTD, frontotemporal dementia. Data are presented as mean (SD) or n (%).
Fig. 1
Frequency of clinically significant BPSD across different dementia diagnoses.
Associations between dementia type and BPSD
The most common clinically significant symptoms in all dementias were aberrant motor behavior, agitation/aggression and irritability, except for DLB and PDD. In DLB, hallucinations, aberrant motor behavior, and delusions were the most frequent symptoms, whereas in PDD aberrant motor behavior, hallucinations and delusions were the most frequent (Table 3). The multivariate logistic regression analyses showed that, compared to AD, individuals with VaD had higher risk of apathy but lower risk of agitation/aggression, anxiety, aberrant motor behavior; individuals with Mixed dementia had lower risk of clinically significant anxiety and aberrant motor behavior; individuals with DLB had higher risk of delusions and hallucinations and lower risk of elation/euphoria and irritability; individuals with PDD had higher risk of hallucinations but lower risk of agitation/ aggression and disinhibition, individuals with FTD had higher risk of apathy, disinhibition, appetite and eating abnormalities as well as lower risk delusion, hallucinations, depression/dysphoria; individuals with unspecified dementia had lower risk of delusion, agitation/aggression, depression/dysphoria, anxiety, irritability, aberrant motor behavior, sleep and night-time behavior changes (Table 4).
Table 4
Odds ratios (OR) and 95% confidence intervals (CI) of the association between dementia type and BPSD with the reference to AD in individuals residing in long-term care facility
| VaD | Mixed | DLB | PDD | FTD | Unspecified | |||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Delusions | 0.95 (0.82–1.11) | 0.542 | 0.91 (0.78–1.06) | 0.234 | 1.71 (1.25–2.33) | 0.001 | 1.35 (0.85–2.1) | 0.185 | 0.44 (0.27–0.68) | < 0.001 | 0.87 (0.77–1) | 0.044 |
| Hallucinations | 0.83 (0.69–1.01) | 0.066 | 0.94 (0.78–1.13) | 0.54 | 5.94 (4.31–8.2) | < 0.001 | 4.47 (2.86–6.95) | < 0.001 | 0.5 (0.27–0.85) | 0.016 | 1.01 (0.86–1.18) | 0.93 |
| Agitation/Aggression | 0.85 (0.73–0.98) | 0.029 | 1.01 (0.87–1.17) | 0.905 | 0.84 (0.61–1.16) | 0.291 | 0.46 (0.28–0.75) | 0.002 | 0.97 (0.67–1.41) | 0.871 | 0.85 (0.75–0.96) | 0.011 |
| Depression/Dysphoria | 0.94 (0.8–1.1) | 0.465 | 0.93 (0.79–1.09) | 0.361 | 1.01 (0.69–1.45) | 0.964 | 0.69 (0.39–1.16) | 0.181 | 0.57 (0.38–0.85) | 0.007 | 0.83 (0.72–0.95) | 0.007 |
| Elation/Euphoria | 0.9 (0.69–1.18) | 0.463 | 0.89 (0.68–1.16) | 0.395 | 0.32 (0.11–0.71) | 0.014 | 0.79 (0.3–1.69) | 0.58 | 1.25 (0.72–2.05) | 0.405 | 0.89 (0.7–1.11) | 0.307 |
| Anxiety | 0.85 (0.73–0.98) | 0.031 | 0.82 (0.71–0.96) | 0.012 | 0.83 (0.58–1.16) | 0.282 | 0.64 (0.37–1.06) | 0.097 | 0.73 (0.49–1.07) | 0.11 | 0.75 (0.66–0.85) | < 0.001 |
| Apathy | 1.21 (1.03–1.42) | 0.023 | 1.04 (0.88–1.22) | 0.684 | 0.87 (0.58–1.26) | 0.471 | 0.89 (0.5–1.49) | 0.678 | 1.54 (1.06–2.22) | 0.022 | 1.09 (0.94–1.25) | 0.256 |
| Disinhibition | 1.03 (0.87–1.22) | 0.714 | 1.02 (0.86–1.21) | 0.816 | 0.71 (0.46–1.05) | 0.097 | 0.4 (0.19–0.74) | 0.007 | 1.52 (1.03–2.2) | 0.03 | 0.97 (0.83–1.12) | 0.683 |
| Irritability | 0.94 (0.81–1.08) | 0.381 | 1.08 (0.93–1.24) | 0.322 | 0.67 (0.47–0.93) | 0.018 | 0.65 (0.4–1.04) | 0.081 | 0.69 (0.48–0.98) | 0.042 | 0.83 (0.73–0.95) | 0.005 |
| Aberrant motor behavior | 0.66 (0.58–0.76) | < 0.001 | 0.75 (0.66–0.86) | < 0.001 | 0.75 (0.55–1) | 0.053 | 1.12 (0.74–1.68) | 0.594 | 0.83 (0.59–1.15) | 0.257 | 0.73 (0.65–0.82) | < 0.001 |
| Sleep and night-time behavior changes | 0.92 (0.78–1.08) | 0.317 | 0.91 (0.77–1.07) | 0.267 | 0.98 (0.67–1.4) | 0.917 | 0.66 (0.37–1.13) | 0.148 | 0.82 (0.51–1.26) | 0.381 | 0.82 (0.71–0.95) | 0.008 |
| Appetite and eating abnormalities | 1.08 (0.92–1.27) | 0.33 | 1.02 (0.87–1.2) | 0.809 | 0.92 (0.62–1.32) | 0.649 | 0.63 (0.34–1.07) | 0.109 | 1.84 (1.27–2.63) | 0.001 | 1 (0.87–1.15) | 0.998 |
AD, Alzheimer’s disease; Mixed, Mixed dementia; VaD, vascular dementia; DLB, dementia with Lewy bodies; PDD, Parkinson’s disease dementia; FTD, frontotemporal dementia. Model adjusted for age, sex, MMSE, and time between registration to SveDem and BPSD registry.
The OR 95% (CI) and p-values for comparisons between other diagnoses are presented in Supplementary Tables 2A-F. The results obtained in imputed data sets were similar (Supplementary Table 3).
DISCUSSION
In this large-scale study of 10,000 individuals, we observed that BPSD was common across all dementia types, with 75.5% of all individuals exhibiting at least one clinically significant BPSD. Among all symptoms, aberrant motor behavior, agitation, and irritability were the most frequent, occurring commonly in all dementias. High levels of hyperactivity symptoms in long-term care facilities have been identified in previous studies [10]. However, to our knowledge our study is the first to show the high frequency of these symptoms regardless of dementia type.
Certain aspects of long-term care facilities’ environments appear to contribute to an increased risk of BPSD [11]. Dementia-related processes affect the perception of the environment and coping abilities [22, 23]. In this case, hyperactivity symptoms may be the result of increased vulnerability to stressors and higher susceptibility to over- or under- stimulation in individuals with dementia, which can lead to dysfunctional reactions, for example, agitation or irritability. Additionally, lack of stimulation may be compensated with behaviors that alleviate boredom, such as pacing or wandering. Another interpretation of the results is a potential overlap of pathophysiological mechanisms between different dementia subtypes. Agitation in AD is associated to damage to the insula, the anterior cingulate cortex, frontal cortex, and amygdala [24]. Medial frontal and frontoinsular degeneration is common in FTD, a diagnosis characterized by disinhibition and poor social functioning [25, 26]. Hence, there may be a pathophysiological link between behavioral abnormalities observed in FTD and presence of agitation in AD.
Associations between dementia type and BPSD
The main aim of this study was to compare BPSD in different dementia diagnoses. Due to their similar presentation of BPSD, AD, Mixed, and VaD are discussed as one group and DLB and PDD as another group.
Alzheimer’s disease, mixed dementia, and vascular dementia
In the present study, the most common clinically significant symptoms in AD, Mixed, and VaD were aberrant motor behavior, agitation/aggression, and irritability. We found that AD individuals had a higher risk of anxiety, aberrant motor behavior compared with Mixed and VaD as well as agitation in comparison with VaD. Individuals with VaD had higher risk of apathy in comparison to AD. The higher risk of agitation/aggression in Mixed compared to VaD was the only difference found between these two diagnoses. Although previous studies explored the differences between AD, Mixed, and VaD [6, 9, 27–33], their results were not conclusive. Additionally, to our knowledge only three of the previous studies used analyses adjusted for potential confounders [6, 30, 32] of which two used NPI to assess the symptoms [6, 32]. Our study is in line with the work by Caputo et al. (2008) [32] regarding a higher risk of anxiety, agitation and aberrant motor behavior in AD than in VaD. In the study by Fuh et al. (2005) [6] the mean composite scores in sleep disturbance domains in individuals with cortical VaD were higher than those in individuals with AD. Further, there was a trend for patients with cortical VaD and subcortical VaD to have higher scores than AD in apathy. However, in our study it was not possible to differentiate between different subtypes of VaD, which could explain why we did not find similar results to previous studies as our VaD patients included individuals with both cortical and subcortical pathology.
Dementia with Lewy bodies and Parkinson’s disease dementia
DLB and PDD are clinically similar disorders that are defined by the order in which dementia and parkinsonism manifest (the 1-year rule) [12, 14]. Although DLB and PDD share the core characteristics (hallucinations, cognitive fluctuations, dementia, and parkinsonism) [34], previous studies observed a higher mean total NPI score as well as higher frequencies of delusions, hallucinations, agitation, anxiety, irritability, aberrant motor behavior in DLB compared to PDD [7, 35, 36]. Differentiating between PDD and DLB in clinical practice can be difficult, Our and previous studies suggest that observing neuropsychiatric symptoms, especially the frequency of psychotic and hyperactivity symptoms, could speak in favor DLB diagnosis.
In line with previous reports, a higher risk of hallucinations and delusions in DLB compared to other diagnoses was also found in our study [8, 32, 37]. On the other hand, we found that individuals with DLB had a lower risk of elation/euphoria relative to all other types of dementia, except for PDD. Individuals with PDD had a lower risk of anxiety and other symptoms from the hyperactivity spectrum (agitation/aggression, disinhibition, and irritability). Although this is consistent with previous reports [7, 38, 39], future studies are needed to assess the nature of the mechanism behind lower risk of hyperactivity symptoms in PDD. Individuals with PDD had a higher risk of hallucinations than other types of dementia (except DLB), and a higher risk of aberrant motor behavior than VaD and unspecified dementia. Aberrant motor behavior was the most common symptom in PDD, affecting nearly 50% of individuals. This is a broad category encompassing different behaviors, such as pacing or repetitive behavior and therefore may not be specific enough to capture qualitative differences between diagnoses. It has been shown that stereotypical motor behavior (e.g., punding) is frequent in PD and can be a result of dopaminergic therapy [40]. Therefore, scores in aberrant motor behavior in PDD may be elevated by the stereotypical motor behavior emerging as a complication due to the treatment. However, the exact reason why the difference was found only in reference to VaD and unspecified dementia cannot be explained in this study and needs further investigation.
Frontotemporal dementia
The most frequent symptoms in FTD were aberrant motor behavior, agitation/aggression, irritability, and apathy. Moreover, in FTD, behavioral changes are one of the core symptoms of the disease, which explains the highest total NPI score found in our study in this group. Furthermore, compared with the other dementia diagnoses, we found a higher risk of apathy (except for VaD, PDD and unspecified dementia), disinhibition (except for VaD), appetite and eating abnormalities in addition to a lower risk of delusions, hallucinations (except for VaD) and depression (except for PDD and unspecified dementia). We also found a higher risk of elation/euphoria in FTD in comparison to DLB. Our results agree with the findings reported by previous studies [9, 37, 41–44]. There is consistent evidence showing that in individuals with FTD, apathy, disinhibition and appetite and eating abnormalities are frequent symptoms, with abnormal behavior being the core diagnostic feature of FTD [13]. In line with our results, several previous studies showed that the risk of psychotic symptoms in FTD is generally low [45].
Unspecified dementia
Aberrant motor behavior, agitation/aggression and irritability were the most common clinically significant symptoms in unspecified dementia. Additionally, the lowest total NPI score was found in this group. This was in line with the lower risk of several symptoms in unspecified dementia compared with other types of dementia observed in our cohort. On the other hand, individuals with unspecified dementia had higher risk of elation/euphoria than DLB and disinhibition than PDD. However, these differences were likely driven by the generally low risk of elation/euphoria and disinhibition in these diagnoses. The diagnosis of unspecified dementia is often set in a primary care setting without access to more extensive investigations needed to reveal the etiology of dementia [46]. The unspecified dementia group may therefore include people with various pathologies and thus lack any specific patterns of BPSD.
General discussion and limitations
Our study was based on the merging of two large quality registries, which enabled a comprehensive large-scale comparison of BPSD between all major subtypes of dementia in a single study. Although BPSD has been studied before, previous work typically involved smaller sample sizes [9, 30] or focused on specific diagnoses (e.g., AD and VaD [6], PD, PDD, and DLB [7], AD and DLB [8]) hampering the full understanding of the behaviors and symptoms expressed by these disorders. To our knowledge, our study is the most comprehensive investigation performed on BPSD so far, which increased substantially our statistical power for detecting clinically meaningful aspects and well as reduced the probability of finding false positives or that the results are driven by particular small groups of individuals. At the time of the BPSD assessment, only 1,298 individuals were still living in the community. Hence, the sample size was too small to compare symptoms between different types of dementia in individuals living in community. However, there is an increasing number of individuals being constantly included in the registry and it will be interesting to compare symptoms between individuals living in community and long-term care facilities. Similar profiles of BPSD in AD, Mixed, VaD, DLB, and PDD could be explained by shared pathophysiologic mechanisms [47–49]. Moreover, the presence of symptoms in VaD may vary due to the variability of the location and the extension of ischemic damage [50]. The VaD cohort in our study was heterogenous and included cases with multi-infarct dementia, strategic infarct dementia as well as subcortical ischemic dementia. There is also a frequent coexistence of AD pathology with VaD, DLB, and PDD [47–49]. Additionally, potential diagnostic accuracy problems could contribute to these results. For example, some DLB individuals may be misdiagnosed as AD and vice-versa. Moreover, information about the severity of dementia at the time of assessment of BPSD was not available and therefore we used the measurement at the time of dementia diagnosis. The cross-sectional design with a single BPSD measure may obscure differences between different dementia diagnoses. Therefore, a longitudinal design with repeated BPSD assessments should complement the present study. The different sample sizes and their characteristics, study designs and methods to estimate BPSD used in previous studies render the comparisons between our results and previous findings difficult. For instance, in contrast to most previous studies, the majority of individuals in our cohort was older. Additionally, comorbidities are not registered in the registries we used and therefore could not be adjusted for in the analyses. Furthermore, we cannot rule out that the differences between our and previous studies are due to cultural variation in BPSD [51]. Moreover, we had a fraction of missing data, which was dealt with using the multiple imputation method.
Conclusions and Implications
In this study, BPSD were frequent in all major types of dementias. The most frequent BPSD were agitation/aggression, aberrant motor behavior, and irritability. A high frequency of these symptoms has already been demonstrated in several previous studies. Importantly, although individuals in our sample were diagnosed with different dementia disorders, we found similarities in BPSD between the different diagnoses. This might have practical implication in terms of planning cost-effective interventions. Our results suggest that, if limited resources are available, the strategies implemented to reduce BPSD should focus first on these symptoms, regardless of the primary diagnosis. Due to their intrinsic complexity, the treatment of BPSD is challenging. Antipsychotic treatment is commonly used to alleviate some of the symptoms [5, 52]. However, it is associated with only modest improvement and serious side effects [53]. There are several non-pharmacological strategies targeting physical environment that have been proven to be effective in alleviating hyperactivity symptoms in long-term care settings. These strategies involve designing home-like environments, configuration of spatial layout and orientation cues, optimizing the level of lightning and noise [11]. Additionally, outdoor activities, music therapy and interventions improving communication with individuals with dementia have shown positive effects in reducing hyperactivity [11, 54].
ETHICS STATEMENT
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the regional ethical review board in Stockholm, Sweden with ethical number: 2015/2291-31/5. All patients receive information about the use of their information and have the right to refuse participation or withdraw their data from the registry at any time.
ACKNOWLEDGMENTS
SveDem is supported financially by the Swedish Associations of Local Authorities and Re- gions, Gun och Bertil Stohnes Stiftelse, CIMED grant, Alzheimerfonden, Swedish Brain Foundation, and Margaretha af Ugglas’ foundation and Swedish Research Council (Drn 2012-2291 and Drn 2016-02317), and by grants provided by the Stockholm County Council (ALF project). BPSD registry is supported financially by the Swedish Associations of Local Authorities.
The authors are grateful to the SveDem and BPSD registry. We thank all patients, caregivers, reporting units and coordinators as well as registries’ steering committee.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5198r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215198.
REFERENCES
[1] | American Psychiatric Associa ((2013) ) American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), Arlington, VA. |
[2] | Okabe K , Nagata T , Shinagawa S , Inamura K , Tagai K , Nukariya K , Shigeta M ((2020) ) Effects of neuropsychiatric symptoms of dementia on reductions in activities of daily living in patients with Alzheimer’s disease. Geriatr Gerontol Int 20: , 584–588. |
[3] | Moheb N , Mendez MF , Kremen SA , Teng E ((2017) ) Executive dysfunction and behavioral symptoms are associated with deficits in instrumental activities of daily living in frontotemporal dementia. Dement Geriatr Cogn Disord 43: , 89–99. |
[4] | Baharudin AD , Din NC , Subramaniam P , Razali R ((2019) ) The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health 19: , 447. |
[5] | Cerejeira J , Lagarto L , Mukaetova-Ladinska EB ((2012) ) Behavioral and psychological symptoms of dementia. Front Neurol 3: , 73. |
[6] | Fuh J-L ((2005) ) Neuropsychiatric profiles in patients with Alzheimer’s disease and vascular dementia. J Neurol Neurosurg Psychiatry 76: , 1337–1341. |
[7] | Aarsland D , Ballard C , Larsen JP , McKeith I ((2001) ) A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry 16: , 528–536. |
[8] | Hashimoto M , Yatabe Y , Ishikawa T , Fukuhara R , Kaneda K , Honda K , Yuki S , Ogawa Y , Imamura T , Kazui H , Kamimura N , Shinagawa S , Mizukami K , Mori E , Ikeda M ((2015) ) Relationship between dementia severity and behavioral and psychological symptoms of dementia in dementia with Lewy bodies and Alzheimer’s disease patients. Dement Geriatr Cogn Disord Extra 5: , 244–252. |
[9] | Srikanth S , Nagaraja AV , Ratnavalli E ((2005) ) Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci 236: , 43–48. |
[10] | Zhao Q-F , Tan L , Wang H-F , Jiang T , Tan M-S , Tan L , Xu W , Li J-Q , Wang J , Lai T-J , Yu J-T ((2016) ) The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord 190: , 264–271. |
[11] | Chaudhury H , Cooke HA , Cowie H , Razaghi L ((2018) ) The influence of the physical environment on residents with dementia in long-term care settings: A review of the empirical literature. Gerontologist 58: , e325–e337. |
[12] | McKeith IG , Boeve BF , Dickson DW , Halliday G , Taylor J-P , Weintraub D , Aarsland D , Galvin J , Attems J , Ballard CG , Bayston A , Beach TG , Blanc F , Bohnen N , Bonanni L , Bras J , Brundin P , Burn D , Chen-Plotkin A , Duda JE , El-Agnaf O , Feldman H , Ferman TJ , ffytche D , Fujishiro H , Galasko D , Goldman JG , Gomperts SN , Graff-Radford NR , Honig LS , Iranzo A , Kantarci K , Kaufer D , Kukull W , Lee VMY , Leverenz JB , Lewis S , Lippa C , Lunde A , Masellis M , Masliah E , McLean P , Mollenhauer B , Montine TJ , Moreno E , Mori E , Murray M , O’Brien JT , Orimo S , Postuma RB , Ramaswamy S , Ross OA , Salmon DP , Singleton A , Taylor A , Thomas A , Tiraboschi P , Toledo JB , Trojanowski JQ , Tsuang D , Walker Z , Yamada M , Kosaka K ((2017) ) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89: , 88–100. |
[13] | ((1994) ) Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 57: , 416–418. |
[14] | Emre M , Aarsland D , Brown R , Dubois B ((2007) ) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22: , 1689–1707. |
[15] | Religa D , Fereshtehnejad S-M , Cermakova P , Edlund A-K , Garcia-Ptacek S , Granqvist N , Hallbäck A , Kåwe K , Farahmand B , Kilander L , Mattsson U-B , Nägga K , Nordström P , Wijk H , Wimo A , Winblad B , Eriksdotter M ((2015) ) SveDem, the Swedish Dementia Registry – a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PLoS One 10: , e0116538. |
[16] | Cermakova P , Fereshtehnejad S-M , Johnell K , Winblad B , Eriksdotter M , Religa D ((2014) ) Cardiovascular medication burden in dementia disorders: A nationwide study of 19,743 dementia patients in the Swedish Dementia Registry. Alzheimers Res Ther 6: , 34. |
[17] | Bränsvik V , Granvik E , Minthon L , Nordström P , Nägga K ((2021) ) Mortality in patients with behavioural and psychological symptoms of dementia: A registry-based study. Aging Ment Health 25: , 1101–1109. |
[18] | Wood S , Cummings JL , Hsu M-A , Barclay T , Wheatley MV , Yarema KT , Schnelle JF ((2000) ) The use of the Neuropsychiatric Inventory in nursing home residents: Characterization and measurement. Am J Geriatr Psychiatry 8: , 75–83. |
[19] | Lyketsos CG , Lopez O , Jones B , Fitzpatrick AL , Breitner J , DeKosky S ((2002) ) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. JAMA 288: , 1475. |
[20] | Van BuurenS, Groothuis-Oudshoorn K mice: Multivariate imputation by chained equations in R. J Stat Softw 45: , 1–67. |
[21] | R Core Team ((2013) ) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. |
[22] | Smith M , Gerdner LA , Hall GR , Buckwalter KC ((2004) ) History, development, and future of the progressively lowered stress threshold: A conceptual model for dementia care: PLST REVIEW. J Am Geriatr Soc 52: , 1755–1760. |
[23] | Kales HC , Gitlin LN , Lyketsos CG ((2015) ) Assessment and management of behavioral and psychological symptoms of dementia. BMJ 350: , h369. |
[24] | Rosenberg PB , Nowrangi MA , Lyketsos CG ((2015) ) Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol Aspects Med 43–44: , 25–37. |
[25] | Brambati SM , Rankin KP , Narvid J , Seeley WW , Dean D , Rosen HJ , Miller BL , Ashburner J , Gorno-Tempini ML ((2009) ) Atrophy progression in semantic dementia with asymmetric temporal involvement: A tensor-based morphometry study. Neurobiol Aging 30: , 103–111. |
[26] | Rabinovici GD , Seeley WW , Kim EJ , Gorno-Tempini ML , Rascovsky K , Pagliaro TA , Allison SC , Halabi C , Kramer JH , Johnson JK , Weiner MW , Forman MS , Trojanowski JQ , DeArmond SJ , Miller BL , Rosen HJ ((2008) ) Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Dementiasr 22: , 474–488. |
[27] | Anor CJ , O’Connor S , Saund A , Tang-Wai DF , Keren R , Tartaglia MC ((2017) ) Neuropsychiatric symptoms in Alzheimer disease, vascular dementia, and mixed dementia. Neurodegener Dis 17: , 127–134. |
[28] | D’Onofrio G , Sancarlo D , Panza F , Copetti M , Cascavilla L , Paris F , Seripa D , G. Matera M , Solfrizzi V , Pellegrini F , Pilotto A ((2012) ) Neuropsychiatric symptoms and functional status in Alzheimer’s disease and vascular dementia patients. Curr Alzheimer Res 9: , 759–771. |
[29] | Ballard C , Neill D , O’Brien J , McKeith IG , Ince P , Perry R ((2000) ) Anxiety, depression and psychosis in vascular dementia: Prevalence and associations. J Affect Disord 59: , 97–106. |
[30] | Chiu M-J , Chen T-F , Yip P-K , Hua M-S , Tang L-Y ((2006) ) Behavioral and psychologic symptoms in different types of dementia. J Formos Med Assoc Taiwan Yi Zhi 105: , 556–562. |
[31] | Lyketsos CG ((2000) ) Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am J Psychiatry 157: , 708–714. |
[32] | Caputo M , Monastero R , Mariani E , Santucci A , Mangialasche F , Camarda R , Senin U , Mecocci P ((2008) ) Neuropsychiatric symptoms in 921 elderly subjects with dementia: A comparison between vascular and neurodegenerative types. Acta Psychiatr Scand 117: , 455–464. |
[33] | Fernandez-Martinez M , Castro J , Molano A , Zarranz J , Rodrigo R , Ortega R ((2008) ) Prevalence of neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. Curr Alzheimer Res 5: , 61–69. |
[34] | Walker L , Stefanis L , Attems J ((2019) ) Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies – current issues and future directions. J Neurochem 150: , 467–474. |
[35] | Chiu P-Y , Tsai C-T , Chen P-K , Chen W-J , Lai T-J ((2016) ) Neuropsychiatric symptoms in Parkinson’s disease dementia are more similar to Alzheimer’s disease than dementia with Lewy bodies: A case-control study. PLoS One 11: , e0153989. |
[36] | Bougea A , Stefanis L , Paraskevas GP , Emmanouilidou E , Efthymiopoulou E , Vekrelis K , Kapaki E ((2018) ) Neuropsychiatric symptoms and α-Synuclein profile of patients with Parkinson’s disease dementia, dementia with Lewy bodies and Alzheimer’s disease. J Neurol 265: , 2295–2301. |
[37] | Perri R , Monaco M , Fadda L , Caltagirone C , Carlesimo GA ((2014) ) Neuropsychological correlates of behavioral symptoms in Alzheimer’s disease, frontal variant of frontotemporal, subcortical vascular, and Lewy body dementias: A comparative study. J Alzheimers Dis 39: , 669–677. |
[38] | Lee W-J , Tsai C-F , Gauthier S , Wang S-J , Fuh J-L ((2012) ) The association between cognitive impairment and neuropsychiatric symptoms in patients with Parkinson’s disease dementia. Int Psychogeriatr 24: , 1980–1987. |
[39] | Starkstein SE , Sabe L , Petracca G , Chemerinski E , Kuzis G , Merello M , Leiguarda R ((1996) ) Neuropsychological and psychiatric differences between Alzheimer’s disease and Parkinson’s disease with dementia. J Neurol Neurosurg Psychiatry 61: , 381–387. |
[40] | Miwa H ((2007) ) Stereotyped behavior or punding in Parkinson’s disease. J Neurol 254: , 61–67. |
[41] | Levy ML ((1996) ) Alzheimer disease and frontotemporal dementias: Behavioral distinctions. Arch Neurol 53: , 687. |
[42] | Rozzini L , Lussignoli G , Padovani A , Bianchetti A , Trabucchi M ((1997) ) Alzheimer disease and frontotemporal dementia. Arch Neurol 54: , 350–350. |
[43] | Bozeat S ((2000) ) Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry 69: , 178–186. |
[44] | Mendez MF , Perryman KM , Miller BL , Cummings JL ((1998) ) Behavioral differences between frontotemporal dementia and Alzheimer’s disease: A comparison on the BEHAVE-AD Rating Scale. Int Psychogeriatr 10: , 155–162. |
[45] | Bathgate D , Snowden JS , Varma A , Blackshaw A , Neary D ((2001) ) Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand 103: , 367–378. |
[46] | Cermakova P , Johnell K , Fastbom J , Garcia-Ptacek S , Lund LH , Winblad B , Eriksdotter M , Religa D ((2015) ) Cardiovascular diseases in ∼30,000 patients in the Swedish Dementia Registry. J Alzheimers Dis 48: , 949–958. |
[47] | Kapasi A , DeCarli C , Schneider JA ((2017) ) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol (Berl) 134: , 171–186. |
[48] | Merdes AR , Hansen LA , Jeste DV , Galasko D , Hofstetter CR , Ho GJ , Thal LJ , Corey-Bloom J ((2003) ) Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60: , 1586–1590. |
[49] | Apaydin H , Ahlskog JE , Parisi JE , Boeve BF , Dickson DW ((2002) ) Parkinson disease neuropathology: Later-developing dementia and loss of the levodopa response. Arch Neurol 59: , 102–112. |
[50] | Moretti R , Torre P , Antonello RM , Cazzato G ((2006) ) Behavioral alterations and vascular dementia. Neurologist 12: , 43–47. |
[51] | Shah A , Dalvi M , Thompson T ((2005) ) Behavioural and psychological signs and symptoms of dementia across cultures: Current status and the future. Int J Geriatr Psychiatry 20: , 1187–1195. |
[52] | Magierski R , Sobow T , Schwertner E , Religa D ((2020) ) Pharmacotherapy of behavioral and psychological symptoms of dementia: State of the art and future progress. Front Pharmacol 11: , 1168. |
[53] | Schwertner E , Secnik J , Garcia-Ptacek S , Johansson B , Nagga K , Eriksdotter M , Winblad B , Religa D ((2019) ) Antipsychotic treatment associated with increased mortality risk in patients with dementia. A registry-based observational cohort study. J Am Med Dir Assoc 20: , 323–329.e2. |
[54] | Livingston G , Kelly L , Lewis-Holmes E , Baio G , Morris S , Patel N , Omar RZ , Katona C , Cooper C ((2014) ) Non-pharmacological interventions for agitation in dementia: Systematic review of randomised controlled trials. Br J Psychiatry 205: , 436–442. |




