Loneliness, Not Social Support, Is Associated with Cognitive Decline and Dementia Across Two Longitudinal Population-Based Cohorts
Abstract
Background:
Poor social health is likely associated with cognitive decline and risk of dementia; however, studies show inconsistent results. Additionally, few studies separate social health components or control for mental health.
Objective:
To investigate whether loneliness and social support are independently associated with cognitive decline and risk of dementia, and whether depressive symptoms confound the association.
Methods:
We included 4,514 participants from the population-based Rotterdam Study (RS; aged 71±7SD years) followed up to 14 years (median 10.8, interquartile range 7.4–11.6), and 2,112 participants from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K; aged 72±10SD years) followed up to 10 years (mean 5.9±1.6SD). At baseline, participants were free of major depression and scored on the Mini-Mental State Examination (MMSE) ≥26 for RS and ≥25 for SNAC-K. We investigated loneliness, perceived social support, and structural social support (specifically marital status and number of children). In both cohorts, dementia was diagnosed and cognitive function was repeatedly assessed with MMSE and a global cognitive factor (g-factor).
Results:
Loneliness was prospectively associated with a decline in the MMSE in both cohorts. Consistently, persons who were lonely had an increased risk of developing dementia (RS: HR 1.34, 95%CI 1.08–1.67; SNAC-K: HR 2.16, 95%CI 1.12–4.17). Adjustment for depressive symptoms and exclusion of the first 5 years of follow-up did not alter results. Neither perceived or structural social support was associated with cognitive decline or dementia risk.
Conclusion:
Loneliness, not social support, predicted cognitive decline and incident dementia independently of depressive symptoms.
INTRODUCTION
Dementia has a complex multifactorial etiology with risk factors ranging from genetic variants to lifestyle factors, and cardiovascular risk factors [1–3]. Recently, there has been increasing evidence that social health plays a role in the development of dementia [4–6]. Social health comprises the capacity to fulfil one’s potential and obligations, the ability to manage life with some degree of independence, and to participate in social activities [7]. An active and socially integrated lifestyle may protect against dementia [8]. The link between social health and dementia is underpinned by social network theory [9] and this link may be causative if evaluated using the contemporary causation conceptual framework [10]. The contemporary causation conceptual framework assesses the totality of evidence against a set of criteria to determine a causal link. As an example, Howick et al. [10] discuss how positive social relationships have been associated with longer life in animal studies, and a randomized controlled study in humans demonstrated that improved perceived social support can reduce depression. For the mechanistic criterion, Howick et al. [10] discussed Berkman et al. [9]’s social network theory, which draws upon the concepts of the stress buffering and main effects. Poor social health can overstimulate the body’s stress response through increased levels of the stress hormone cortisol, raise blood pressure and decrease blood flow to vital organs through higher tonic vascular resistance, impair the immune system’s ability to fight infections through lower production of white blood cells, and reduce sleep quality leading to less restorative sleep and daytime fatigue [11]. Positive social health, on the other hand, can reduce stress and anxiety, thus avoiding the negative effects on the biological pathways. Additionally, friends and family can provide direct support (emotional, instrumental, informational) and influence behaviour positively, hence, the main effect concept. Positive social health has been linked to a number of behavioral, anthropometric, and biomedical benefits, which are linked to decreased chronic disease including dementia [12].
Four systematic reviews have reported that better social health is associated with better cognitive well-being or lower risk of dementia. In 2016, Kuiper et al. [13]’s meta-analysis of 43 longitudinal cohort studies concluded that “Poor social relationships predicted cognitive decline”. In 2017, Kelly et al. [14] conclude in a systematic review that the 39 included studies (34 of which were observational) were consistent in demonstrating an association between more social activity, better social networks, and more social support with better cognitive function. In 2015, Kuiper et al. [5]’s meta-analysis of 19 longitudinal cohort studies showed that low social participation, less frequent social contact, and more loneliness were each associated with incident dementia. However, Kuiper et al. [5] reported inconsistent findings for social network size and no association between low satisfaction with social network and the risk of dementia. In 2018, Penninkilampi et al. [15]’s meta-analysis of 31 cohort and two case-control studies concluded that better social networks and social support were associated with a lower dementia risk. Notably, Kuiper et al. [5]’s and Penninkilampi et al. [15]’s systematic reviews are in conflict regarding loneliness’s influence on dementia risk. Additionally, structural aspects of social support play a role, as illustrated by a meta-analysis of 15 studies which concluded that marriage is protective against dementia [16].
Yet, studies show inconsistent results [5, 17, 18], and a few important issues have hardly been add-ressed. At present, the issue of reverse causality cannot be ruled out. Currently most studies of social health have a short follow-up duration [5, 13–15]. This hampers investigating reverse causality effects, which may be substantial as behavioral and cognitive changes can occur long before the diagnosis of dementia [19]. Penninkilampi et al. [15] attempted to address the issue of reverse causation by limiting a sub-analysis to long-term studies with a follow-up time of ≥10 years and reported that good social engagement was modestly protective of dementia risk. However, the issue with this approach is that long-term studies can yield relatively short median or mean participant observation times. For example, Saczynski et al. [20] followed participants for 32 years but only yielded a mean person observation time of 4.6 years. Of 91 studies identified across four systematic reviews plus three additional studies published last year, a quarter (n = 23) reported a follow-up duration of 10 years or more, however, only 10%(n = 9) had a mean person observation time of ≥10 years.
More recently the conversation regarding social health has turned to whether social support or lone-liness is more important for health, policy, and intervention purposes. Very few studies have assessed social support and loneliness simultaneously [5, 13–15]. Another limitation of current research assessing social health and cognition or dementia is adequate control for mental health, specifically depressive symptoms. Depression can be both an antecedent and consequence of poor social health. Consequently, loneliness and depressive symptoms may confound each other’s effects on health outcomes [21].
The aim of this study was to investigate the longitudinal associations of loneliness and social support with cognitive decline and incident dementia in the general population. Analyses were replicated across two countries, the Netherlands and Sweden, for validation and quality purposes.
METHODS
Study setting and inclusion population
The Rotterdam Study (RS) is an ongoing prospective population-based cohort, sampling inhabitants aged ≥45 of the suburban Ommoord district in Rotterdam [22]. The cohort was initiated in 1990; overall response rate was 72%. Examination rounds, including visits to the study center, were repeated every 4–5 years. For this study, we included 6,018 participants aged ≥55 years examined between 2002 and 2006. Of those, 5,917 participants had valid data on social health determinants investigated in this study (Table 1). We excluded participants with a Mini-Mental state Examination (MMSE) score < 25, dementia or major depression at baseline, leaving 4,514 participants available for analysis.
Table 1
Inclusion of study populations from the Rotterdam Study and the Swedish National study on Aging and Care in Kungsholmen
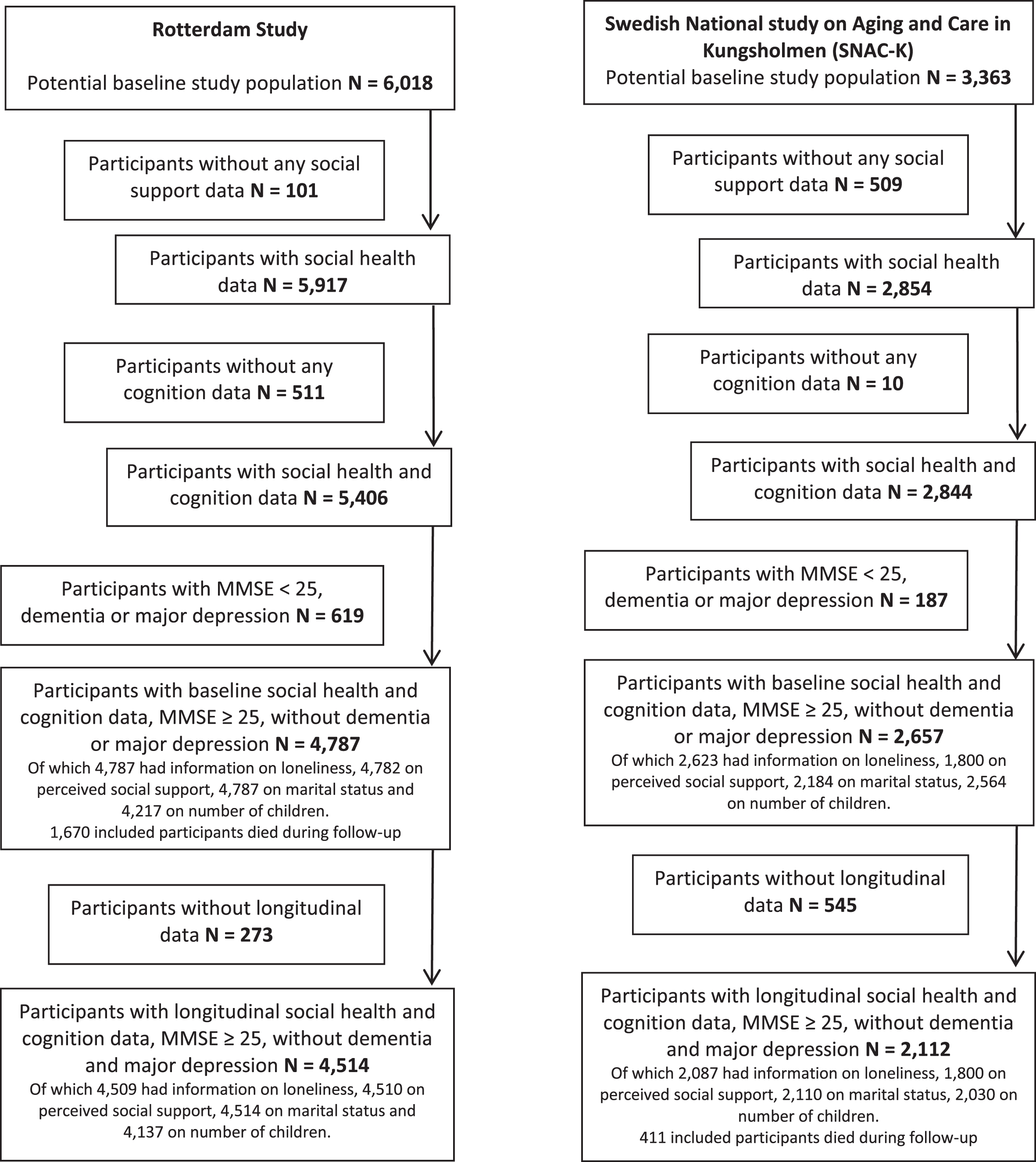 |
The Swedish National study on Aging and Care in Kungsholmen (SNAC-K) is an ongoing multidisciplinary study of aging and health [23]. Between 2001 and 2004, a random sample of inhabitants aged ≥60 years of the Kungsholmen area in central Stockholm were recruited. At baseline, 3,363 (response rate: 73%) persons were examined, of whom 2,854 had valid data on social health. Individuals aged > 78 years were re-examined every 3 years, younger age groups were re-examined every 6 years. Data for this study were obtained from the 3-, 6-, and 9-year follow-up examinations. We excluded participants with a MMSE score < 26, dementia, or major depression at baseline, leaving 2,112 participants available for analysis (Table 1).
The MMSE cut offs are predetermined for each cohort and both < 25 and < 26 cut offs are acceptable among older participants [24, 25]. As the SNAC-K study has a higher proportion of highly educated participants, they opted for the < 26 cut off [25].
For longitudinal analyses, we followed participants until the first of: Onset of dementia, loss to follow-up, study end (01-01-2016 for RS; 01-12-2010 for SNAC-K), or death. Participants were followed for 14 years in RS and 10 years in SNAC-K, yielding person observation times of median 10.8 (interquartile range 7.4 –11.6) and mean 5.9±1.6SD years respectively.
The excluded participants were more likely to be less healthy (p < 0.05 for a range of health indicators), more lonely (excluded: RS: 27%, SNAC-K: 39%; compared to included: 15%and 23%, respectively, p < 0.01), have less perceived social support (excluded: 56%, 25%; included: 69%, 56%SD, p < 0.01), not married (excluded: 48%, 26%; included: 69%, 56%, p < 0.01), and have less children (p < 0.05) than the included participants (Supplementary Table 1).
Approvals and consent
The Rotterdam Study has been approved by the institutional review board (Medical Ethics Committee) of the Erasmus Medical Center and by the review board of The Netherlands Ministry of Health, Welfare and Sports of the Netherlands (per the Population Study Act Rotterdam Study) [22]. As such, The Rotterdam Study has no “approval” number. The approval has been renewed every 5 years, as well as with the introduction of major new elements in the study (e.g., MRI investigations) [22]. Written informed consent was obtained from all participants [22].
All phases of SNAC-K and the use of inpatient registry data were approved by and the Regional Ethical Review Board in Stockholm (Dnrs: KI 01-114, 04-929/3, Ö26-2007, 2009/595-32, 2010/447-31/2, 2013/828-31/3 and 2016/730-31/1) [23]. Participants in the study completed a written informed consent form as stipulated by the ethics board. For participants with prevalent or incident cognitive impairment, consent was obtained from next of kin. For further information see: www.snac-k.se/about/ethics/.
Social health
Qualitative and structural aspects of social health were assessed during a personal interview by trained nurses. In RS, loneliness was assessed by one item on the Dutch version [26] of the Centre for Epidemiological Studies Depression Scale (CES-D) [27], with a Likert-score question: “Did you feel lonely last week?” [28]. Answers were dichotomized “never” or “rarely” versus “sometimes”, “regularly” or “usually”. In SNAC-K, feelings of loneliness were assessed with a single question answered “yes” or “no”. Perceived social support in RS was assessed with a modified 5-item questionnaire from the Health and Lifestyle Survey asking if the participant had people including family and friends who “do things that make me happy”, “I can count on”, “help if I need it”, “make me feel important in their lives”, “accept me as I am” with response options “never”, “sometimes” and “always”’, providing a score ranging from 0-10 dichotomized as optimal (10) or non-optimal (0-9). Social support in SNAC-K was addressed by five items: Satisfaction with social contacts, perceived material support, psychological support, sense of affinity with relatives and neighbors, and being part of a group of friends. Raw scores on social support items were standardized and averaged to create a social support index. In both studies, we assessed marital status (married, widowed or divorced, and single) and the number of children (no children, 1-2 children, ≥3 children) as measures of structural social support.
Cognition
The MMSE and general cognition (g-factor) are both used extensively in clinical and research settings, however, measure slightly different aspects of cognition. MMSE is a measure of cognitive impairment, while the g-factor is a measure of cognitive function or cognitive intelligence. Hence, we will assess both as outcomes. In both RS and SNAC-K, participants underwent a neuropsychological test battery at each study visit. The five tests implemented in RS were the delayed 15-Word Verbal Learning Task, Stroop 3 test, Letter-Digit Substitution Task, Purdue Pegboard test, and Word-Fluency test [29]. In SNAC-K, similar tasks assessing perceptual speed (pattern comparison), episodic memory (free recall), semantic memory (vocabulary), letter fluency (letter F), and category fluency (animal fluency) [30]. To allow for direct comparability across tests, we generated z-scores for each cognitive test, except for MMSE because it is naturally skewed. A measure of general cognition (g-factor) was calculated per cohort as the first unrotated component obtained through a principal component analysis including abovementioned tests [29, 31]. The g-factor explained 51%(RS) to 53%(SNAC-K) of the variance in the cognitive tests. We calculated the g-factor after multiple imputation of missing cognitive test data for participants with at least two valid cognitive test measures in RS, and at least three in SNAC-K.
Assessment of dementia
In RS, participants were screened for all-cause dementia (hereafter: dementia) using the MMSE and the Geriatric Mental Schedule - organic level (GMS) at baseline and following visits [32]. Further investigation including the Cambridge Examination for Mental Disorders of the Elderly was performed when participants had a MMSE-score < 26 or GMS score > 0. In addition, all participants were continuously monitored for dementia through linkage of the study database with general practitioner medical records. A consensus panel, led by a consultant neurologist, determined the diagnosis according to the standard criteria for dementia (DSM-III-R). In SNAC-K, dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV), in which a three-step diagnostic procedure was followed [30]. Two physicians independently made a preliminary diagnosis, and, in case of disagreement, a third opinion was sought to reach a concordant diagnosis.
Covariates
Potential confounders were selected based on previous publications [33–35]. In both studies, education was categorized as primary, secondary/lower or intermediate vocational, and higher vocational/university. Smoking status was categorized as never, former, or current smoker. Problematic alcohol use for men was an intake of > 14 glasses of alcohol per week, for women an intake of > 7 glasses of alcohol per week. Presence of hypertension was defined as a resting blood pressure ≥160/100 mm Hg (as recommended at the time), or the use of blood pressure-lowering medication. Hypercholesterolemia was defined as a total cholesterol > 6.22 mmol/l or the use of lipid-lowering medication. Diabetes was defined in RS as a fasting serum glucose level ≥7.0 mmol/l or the use of anti-diabetic medication, and in SNAC-K as a HbA1c > 46 mmol/mol or the use of anti-diabetic medication. Activities of Daily Living (ADL) were obtained by a Dutch version of the Stanford Health Assessment Questionnaire (ADL disability index). Body mass index (BMI) was computed from measured height and weight (kg/m2). Prevalent coronary heart disease (CHD) was obtained from medical records.
In RS, depressive symptoms were assessed with the CES-D. Participants with a CES-D score ≥16 were interviewed by a clinician with the Dutch version of the Schedules for Clinical Assessment in Neuropsychiatry to diagnose major depressive disorder according to DSM-IV criteria, as described previously [36]. Analyses we adjusted for the CES-D score, without the loneliness item. In SNAC-K, depressive symptoms were assessed according to the Comprehensive Psychopathological Rating Scale (CPRS) [37, 38]. Major depression was diagnosed according to DSM-IV criteria.
Statistical analysis
We calculated Spearman’s rank correlations between loneliness and social support indicators. As main analysis, we investigated associations of loneliness and indicators of social support with 1) cognitive decline, using baseline and repeated measures of cognitive functioning analyzed with linear mixed models; 2) risk of dementia, analyzed with Cox regression models using time from baseline to censoring as the timescale. For cognitive decline, we focused on the interaction of the determinant with time (determinant*time), interpretable as the slope or the decline of cognitive functioning over time. The fixed effects were specified as: determinant, time from baseline, determinant*time, time*age, and other covariates as stated below. As random effects, we included only a random intercept. All analyses were adjusted for age, sex, and level of education (model 1), and additionally for smoking status, presence of problematic alcohol use, hypertension, hypercholesterolemia, diabetes mellitus, a disability index of impairments in activities of daily living, and body mass index (model 2).
We performed several sensitivity analyses. First, we adjusted for depressive symptoms in addition to excluding persons with depression. Considering poor social health can lead to depression [39], which may also impact the risk of cognitive decline or dementia, we also examined mediation by depressive symptoms. Thereby we assessed any indirect pathway from social health through depressive symptoms to cognitive decline or dementia using the Baron & Kenny [40] method. Of note, we could not assess temporality as social health and depressive symptoms were assessed at the same time point [41]. Second, to reduce the influence of reverse causality in the dementia analyses (i.e., preclinical dementia symptoms already affecting social support and loneliness before the diagnosis is made), we analyzed associations excluding persons censored in the first five years after baseline. Third, we explored independence of loneliness and indicators of social support by mutually adjusting each factor for each other. Finally, we included participants with major depressive disorder to facilitate comparison to studies that did not exclude these participants.
Statistical testing was performed two-sided at p < 0.05. We checked the proportional hazard assumptions using partial residual scatterplots over time and found no violations. In RS but not SNAC-K, the MMSE-score was inversed and log-transformed (ln(30.0001-MMSE)). In both studies, MMSE was standardized; g-factor was already expressed per standard deviation difference from the mean. Missing covariate data was imputed using 5 multiple imputations. Analyses were performed using SPSS version 24.0, and using the lme and survival package in R [42].
Data availability
Data cannot be shared for legal and ethical rea-sons. Data cannot be shared publicly as data are part of two large ongoing observational cohorts with a rigorous process to access data. Data are avail-able from Erasmus MC (contact via www.erasmusmc.nl/en/research/core-facilities/ergo-the-rotterdam-study) and Karolinska Institute (contact via www.snac-k.se/for-researchers/application-form/) for researchers who meet the criteria.
RESULTS
Age at baseline was 71.3±7.4SD years in RS and 71.6±9.9SD years in SNAC-K (Table 2). Compared to RS participants, SNAC-K participants were more likely male, single or widowed/divorced, and had a higher education. RS participants were less likely to report moderate to severe loneliness (15%RS, 23%SNAC-K) but similarly report optimal perceived social support (69%RS, 63%SNAC-K) compared to SNAC-K participants. Loneliness was moderately negatively correlated to marital status (Supplementary Table 2; rSpearman = –0.33 in RS; –0.30 in SNAC-K). Other correlations amongst social health factors were small (|rSpearman|≤0.20).
Table 2
Baseline characteristics of the study populations from the Rotterdam Study and the Swedish National study on Aging and Care in Kungsholmen
| Characteristic (unit) | Rotterdam Study | SNAC-K |
| N = 4,514 | N = 2,112 | |
| Social determinants | ||
| Presence of loneliness | 722 (15%) | 485 (23%) |
| Missing | 8 (0%) | 25 (1%) |
| Optimal perceived social support | 3,314 (69%) | 1,320 (63%) |
| Missing | 7 (0%) | 312 (15%) |
| Marital status | ||
| Single | 233 (5%) | 336 (16%) |
| Widowed/divorced | 1,228 (26%) | 745 (35%) |
| Married | 3,326 (69%) | 1,029 (49%) |
| Missing | – | 2 (0%) |
| Number of children | ||
| No children | 468 (10%) | 530 (25%) |
| One or two children | 2,227 (47%) | 1,090 (52%) |
| Three or more children | 1,442 (30%) | 410 (19%) |
| Missing | 650 (14%) | 82 (4%) |
| Covariates | ||
| Age at baseline (years) | 71.3±7.4 | 71.6±9.9 |
| Male | 2,048 (43%) | 1,340 (64%) |
| Education level | ||
| Lower education | 1,763 (37%) | 256 (12%) |
| Middle education | 2,296 (48%) | 1,046 (50%) |
| Higher education | 728 (15%) | 810 (38%) |
| Smoking status | ||
| Never smoked | 1393 (29%) | 985 (47%) |
| Past smoking | 670 (14%) | 850 (40%) |
| Current smoking | 2724 (57%) | 265 (13%) |
| Missing | – | 12 (1%) |
| Alcohol intake (glasses) | ||
| Men | ||
| > 14/week | 732 (35.8) | 89 (12%) |
| ≤14/week | 1,316 (64.2) | 679 (88%) |
| Missing (%) | – | 4 (1%) |
| Female | ||
| > 7/week | 829 (30%) | 291 (22%) |
| ≤7/week | 1,910 (70%) | 1,045 (78%) |
| Missing | – | 4 (0%) |
| Hypertension | 3,744 (78.2) | 1,560 (74%) |
| Missing | – | 3 (0%) |
| Hypercholesterolemia | 2,236 (46.7) | 1,106 (52%) |
| Missing | – | 47 (2%) |
| Diabetes mellitus | 695 (15%) | 168 (8%) |
| Activities of daily living (disability index) | 0.25 (0.13; 0.63) | 0 (0, 0) |
| Prevalent coronary heart disease | 274 (6%) | 320 (15%) |
| Body mass index (kg/m2) | 27 (25–30) | 25 (23–28) |
| Depressive symptoms score (CES-D or CPRS) | 3 (1–8) | 1 (0–3) |
| Cognitive functioning at baseline | ||
| Mini-mental state examination (score) | 28 (27–29) | 29 (28–30) |
| g-factor (score) | 0.43±0.89 | 0.12±0.69 |
| Missing | – | 108 |
Values are expressed as frequency (%) for categorical variables and mean±SD or median (IQR) for continuous variables. CES-D, Center for Epidemiological Studies –Depression Scale; N, sample size; SNAC-K, Swedish National study on Aging and Care in Kungsholmen.
Main analysis
Loneliness was associated with a decline in the MMSE-score even if adjusted for lifestyle factors (beta 0.01, 95%CI 0.00; 0.03, p = 0.04 in RS, beta –0.38, 95%CI –0.08; –0.68, p = 0.01 in SNAC-K; Table 3). Moreover, in SNAC-K, an association between loneliness and a decline in g-factor was observed (beta –0.05, 95%CI –0.09; –0.00, p = 0.03). In both studies, perceived social support, marital status and number of children were not associated with a subsequent change in MMSE. Likewise, we found no associations of any indicator of social support with g-factor, except for marital status in SNAC-K: Widowed/divorced persons experienced an increased decline in g-factor compared to married persons (beta –0.05, 95%CI –0.09; –0.01, p = 0.01; Table 3). For RS, when MMSE-scores were not inversed prior to log-transformation and standardization, the magnitude of associations remained comparable, however, were no longer statistically significant (beta –0.01, 95%CI –0.03; 0.00, p = 0.2; Supplementary Table 3).
Table 3
Associations of loneliness and social support with cognitive decline
| MMSE | ||||||
| Determinants | Rotterdam Study* | SNAC-K | ||||
| N; na | Beta (95%CI) | p | N; na | Beta (95%CI) | p | |
| Loneliness, yes | 4,509; 9,194 | 0.01 (0.00; 0.03) | 0.04 | 2,087; 8,208 | –0.38 (–0.08; –0.68) | 0.01 |
| Perceived social support, optimal | 4,510; 9,193 | –0.01 (–0.02; 0.00) | 0.20 | 1,800; 7,132 | –0.03 (–0.29; 0.24) | 0.85 |
| Marital status | 4,514; 9,201 | 2,110; 8,300 | ||||
| Married | 0 [Reference] | – | 0 [Reference] | – | ||
| Single | 0.02 (–0.00; 0.04) | 0.10 | 0.13 (–0.22; 0.48) | 0.45 | ||
| Widowed or divorced | 0.00 (–0.01; 0.01) | 0.76 | –0.22 (–0.52; 0.06) | 0.12 | ||
| No. children | 2,404; 4,959 | 2,030; 7,984 | ||||
| 0 children | 0.01 (–0.02; 0.04) | 0.57 | 0.19 (–0.12; 0.50) | 0.87 | ||
| 1–2 children | 0 [Reference] | – | 0 [Reference] | – | ||
| ≥3 children | 0.01 (–0.01; 0.02) | 0.79 | 0.07 (–0.26; 0.41) | 0.67 | ||
| g-factor | ||||||
| Determinants | Rotterdam Study | SNAC-K | ||||
| Loneliness, yes | 4,313; 8,572 | 0.00 (–0.00; 0.01) | 0.10 | 1,982; 7,284 | –0.05 (–0.09; –0.00) | 0.03 |
| Perceived social v | 4,314; 8,571 | –0.01 (–0.01; 0.00) | 0.30 | 1,905; 6,700 | –0.01 (–0.03, 0.05) | 0.67 |
| Marital status | 4,319; 8,581 | 2,002; 7,352 | ||||
| Married | 0 [Reference] | – | 0 [Reference] | – | ||
| Single | 0.00 (–0.01; 0.01) | 0.65 | 0.04 (–0.05; 0.05) | 0.88 | ||
| Widowed or divorced | 0.00 (–0.01; 0.00) | 0.78 | –0.05 (–0.09; –0.01) | 0.01 | ||
| No. children | 3,827; 7,579 | 1,926; 7,068 | ||||
| 0 children | 0.00 (–0.01; 0.00) | 0.20 | 0.02 (–0.03; 0.06) | 0.41 | ||
| 1–2 children | 0 [Reference] | – | 0 [Reference] | – | ||
| ≥3 children | 0.00 (–0.01; 0.00) | 0.17 | 0.01 (–0.04; 0.06) | 0.60 | ||
Estimates for determinant*time interaction term are provided, obtained with linear mixed models. This term is interpretable as the decline of cognition over time. We specified fixed effects as time from baseline, determinant, determinant*time, time*age, age, sex, education, smoking status, problematic alcohol use, presence of hypertension, presence of hypercholesterolemia, diabetes, activities of daily living disability index and body mass index. We specified only a random intercept. As outcomes were standardized, estimates indicate how categories of the determinants change the outcome in standard deviations per year. *Please note that due to inversing the MMSE score, positive coefficients indicate not better but worse MMSE scores c.q. cognitive decline for Rotterdam Study estimates. a Capital N is the number of unique participants, lowercase ‘n’ denotes total repeated assessments up to a maximum of 3 per person. MMSE, Mini Mental State Examination; CI, Confidence Interval; SNAC-K, Swedish National study on Aging and Care in Kungsholmen.
Consistent with findings for an increased decline in MMSE, participants who were lonely at baseline had a higher risk of developing dementia (HR 1.34, 95%CI 1.08–1.60, p = 0.01 in RS, HR 2.16, 95%CI 1.12–4.17, p = 0.02 in SNAC-K; Table 4). No other indicator of social support was associated with the risk of dementia.
Table 4
Associations of loneliness and social support with incident dementia, for full follow-up and after excluding the first 5 years of follow-up
| Determinants | Incident dementia | |||||||||
| Rotterdam Study | SNAC-K | |||||||||
| Events/person-years | Full follow-up | p | > 5 y follow-up | p | Events/person-years | Full follow-up | p | > 5 y follow-up | p | |
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |||||||
| Loneliness, yes | 504/44,698 | 1.34 (1.08; 1.67) | 0.01 | 1.37 (1.06; 1.77) | 0.02 | 292/12,399 | 2.16 (1.12; 4.17) | 0.02 | 2.10 (0.96; 4.57) | 0.06 |
| Perceived Social Support, optimal | 503/44,709 | 0.90 (0.75; 1.09) | 0.28 | 0.89 (0.71; 1.10) | 0.28 | 208/10,868 | 1.22 (0.59; 2.52) | 0.59 | 1.48 (0.61; 3.64) | 0.39 |
| Marital status | 504/44,753 | 296/12,527 | ||||||||
| Married | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] | ||||||
| Single | 0.69 (0.44; 1.07) | 0.10 | 0.85 (0.51; 1.40) | 0.52 | 0.57 (0.19; 1.78) | 0.34 | 0.59 (0.16; 2.19) | 0.43 | ||
| Widow/divorce | 0.96 (0.78; 1.19) | 0.72 | 1.00 (0.78; 1.29) | 0.96 | 1.03 (0.54; 1.99) | 0.92 | 1.17 (0.55; 2.48) | 0.68 | ||
| No. children | 504/39,529 | 289/12,048 | ||||||||
| 0 children | 0.82 (0.61; 1.11) | 0.19 | 0.97 (0.69; 1.35) | 0.84 | 0.41 (0.16; 1.02) | 0.06 | 0.61 (0.21; 1.77) | 0.37 | ||
| 1–2 children | 0 [Reference] | 0 [Reference] | 1 [Reference] | 1 [Reference] | ||||||
| ≥3 children | 1.19 (0.99; 1.44) | 0.07 | 1.22 (0.98; 1.53) | 0.08 | 1.35 (0.68; 2.69) | 0.39 | 2.00 (0.91; 4.38) | 0.09 | ||
Hazard ratio estimates were obtained with Cox regression models, analyzing association both in the full follow-up as well as after excluding the first 5 years of follow-up. Estimates were adjusted for age, sex, education, smoking status, problematic alcohol use, presence of hypertension, presence of hypercholesterolemia, diabetes, activities of daily living disability index and body mass index (model 2). CI, confidence interval; HR, hazard ratio; SNAC-K, Swedish National study on Aging and Care in Kungsholmen.
The above results were obtained from the fully adjusted models. Estimates for cognitive decline and for dementia adjusted only for age, sex and education were highly similar to fully adjusted results (Supplementary Table 4). When loneliness and indicators of social support were mutually adjusted for each other, findings remained unchanged (findings not reported).
Sensitivity analysis
When additionally adjusting for depressive symptoms, the relation of loneliness with decline in MMSE remained (beta 0.01, 95%CI 0.00; 0.03 in RS; beta –0.37, 95%CI –0.67; –0.07 in SNAC-K). Similarly, correcting for depressive symptoms did not attenuate the association of loneliness with dementia risk (HR 1.31, 95%CI 1.03–1.66 in RS; HR 2.09, 95%CI 1.10–3.95 in SNAC-K).
Through mediation analysis, we observed no indirect mediation pathways from social health through depressive symptoms to cognitive decline or dementia in RS or SNAC-K (Supplementary Table 5). Additionally, for example in RS, social health measures were associated with depressive symptoms (loneliness β/HR 7.2 per CES-D unit, p < 0.001; social support –2.2, p < 0.001; widowed/divorced versus married 2.02, p < 0.001; > 3 versus 1–2 children –0.50, p = 0.02), but depressive symptoms was not associated with a decline in MMSE (β 0.00, p = 0.27), decline in g-factor (β 0.00, p = 0.09), or incident dementia (HR 1.01, p = 0.13).
Excluding the first five years of follow-up time only slightly reduced effect sizes for associations of loneliness with dementia risk in RS (HR 1.37, 95%CI 1.06–1.77) and SNAC-K (HR 2.10, 95%CI 0.96–4.57; Table 4). Results for SNAC-K no longer reached statistical significance (Table 4).
Additionally adjusting the associations with loneliness for the other social support indicators did not meaningfully change results for cognitive decline (beta 0.01, 95%CI 0.00; 0.03 in RS; beta 0.15, 95%CI –0.31; 0.60 in SNAC-K) or risk of dementia (HR 1.37, 95%CI 1.08–1.74 in RS; HR 3.49, 95%CI 1.31–9.28 in SNAC-K).
There was no meaningful change in associations of loneliness with dementia when we included persons with major depressive disorder at baseline, although the statistical significance in SNAC-K was attenuated (Table 5).
Table 5
Associations of loneliness and social support with incident dementia, in full follow-up and after excluding the first 5 years of follow-up, including participants with major depressive disorder
| Determinants | Incident dementia | ||||
| Rotterdam Study | |||||
| Events/person-years | Full follow-up | p | > 5 y follow-up | p | |
| HR (95%CI) | HR (95%CI) | ||||
| Loneliness, yes | 521/45,191 | 1.40 (1.13; 1.72) | < 0.001 | 1.36 (1.06; 1.75) | 0.02 |
| Perceived Social Support, optimal | 520/45,182 | 0.88 (0.74; 1.06) | 0.18 | 0.89 (0.72; 1.10) | 0.28 |
| Marital status | 521/45,235 | ||||
| Married | 1 [Reference] | – | 1 [Reference] | – | |
| Single | 0.71 (0.46; 0.09) | 0.12 | 0.84 (0.51; 1.39) | 0.49 | |
| Widow/divorce | 0.98 (0.80; 1.21) | 0.88 | 1.02 (0.80; 1.31) | 0.86 | |
| No. children | 521/39,977 | ||||
| 0 children | 0.86 (0.64; 1.15) | 0.30 | 0.97 (0.70; 1.36) | 0.87 | |
| 1–2 children | 1 [Reference] | – | 1 [Reference] | – | |
| ≥3 children | 1.19 (0.99; 1.43) | 0.07 | 1.23 (0.99; 1.53) | 0.07 | |
| SNAC-K | |||||
| Loneliness, yes | 301/12,479 | 2.15 (1.11; 4.16) | 0.02 | 2.09 (0.96; 4.56) | 0.06 |
| Perceived Social Support, optimal | 209/10,877 | 1.22 (0.59; 2.52) | 0.59 | 1.49 (0.61; 3.64) | 0.39 |
| Marital status | 306/12,625 | ||||
| Married | 1 [Reference] | 1 [Reference] | |||
| Single | 0.57 (0.19; 1.78) | 0.34 | 0.59 (0.16; 2.20) | 0.43 | |
| Widow/divorce | 1.03 (0.54; 1.98) | 0.93 | 1.17 (0.55; 2.48) | 0.68 | |
| No. children | 298/12,124 | ||||
| 0 children | 0.41 (0.16; 1.02) | 0.06 | 0.61 (0.21; 1.78) | 0.37 | |
| 1–2 children | 1 [Reference] | 1 [Reference] | |||
| ≥3 children | 1.35 (0.68; 2.69) | 0.39 | 2.00 (0.91; 4.38) | 0.08 | |
DISCUSSION
The results of this study based on data from two longitudinal population-based studies show that loneliness, but not social support, was associated with cognitive decline and an increased risk of dementia. These associations remained when adjusted for depressive symptoms and after excluding the first five years of follow-up time. For social support, neither perceived nor structural aspects were associated with cognitive decline or dementia. Notably, the two cohorts provided consistent findings despite different measurements of loneliness and social support (independent variables) and diagnostic criteria for dementia (dependent variables).
Our findings contradict the prior systematic reviews [5, 13–15], which reported that aspects of social support were protective of cognitive decline or dementia risk. We hypothesize that the length of the follow-up time may be limiting current research. A previous study of Fratiglioni et al. [18] in SNAC-K indicated that a large social network can help delay the clinical symptoms of age- and disease-related brain changes over an average 3 years of follow-up. However, we now show that with longer follow-up that loneliness, not social support, was associated with cognitive decline.
Our findings are consistent with Kuiper et al. [5]’s finding that more loneliness was associated with increased risk of dementia. However, our findings contradict both Penninkilampi et al. [15]’s systematic review reporting that loneliness was not associated with dementia risk and a sub-analysis by Kuiper et al. [13] reporting no association between being lonely and cognitive decline. Notably, Penninkilampi et al. [15] outlined their limitation of only obtaining four papers published between 2012 and 2017 which assessed loneliness as a risk factor for dementia. We have identified a number of individual studies that are aligned with our findings [43–50], including three that were published last year. Notably one also assessed reverse causality by censoring the first 5 years of follow-up and results were consistent that loneliness was associated with increased risk for all-cause dementia among 1,905 Swedish aged 60 or more years over a 20 year follow-up time (mean 11.1 years) [49].
We found no consistent associations of marital status and number of children with cognitive decline and risk for dementia. A systematic review and meta-analysis by Sommerlad et al. showed that older widowed women were at a greater risk of dementia than other age-gender categories [16]. This association disappeared when education and physical health were adjusted for, in line with our findings.
Pre-existing depressive symptoms could confound the association of social health with cognitive decline and dementia, yet adjustment for depressive symptoms did not meaningfully change our findings. Furthermore, mediation analyses suggested no indirect pathways from social health through depressive symptoms to cognitive decline or dementia. Van Winkel et al. described the complex relation between depression and loneliness as a self-reinforcing loop between loneliness, negative appraisals of social company, being alone and the development of major depressive disorder. Poor control for mental health, such as depressive symptoms, may obscure the interpretation of the association between the different social health factors and the risk of dementia [5]. Furthermore, depression has typically been associated with dementia in short term follow-up studies [51], whereas the strength of the association between loneliness and cognitive functioning did not weaken over at least five years. While loneliness can lead to depression [39], loneliness is distinctly different from depression [52] and they are much less correlated that what people may think (e.g. low correlation at age 18 r = 0.38 [53]). Hence, we hypothesize that the pathway from loneliness to cognitive decline and dementia is independent of depression.
There was considerable consistency in results for the MMSE-score and g-factor. In both studies, we found an association between the MMSE-score and loneliness and for the g-factor, an association was found in SNAC-K and in RS a non-significant trend could be demonstrated. The g-factor is more age-sensitive and partly reflects executive functioning; the MMSE is the most common screening instrument for dementia and often used to detect early symptoms reliably. This suggests that our results are robust and do not depend on whether the assessment examines basic cognitive functions such as orientation and attention or higher cognitive functions such as vocabulary and episodic memory.
There are several possible pathways for how social health impacts cognition and dementia [5, 13–15]. Elevated cortisol levels, increased markers of immune activation, and expression of inflammatory genes have been described in lonely individuals, which are associated with cognitive decline and dementia [54, 55]. As loneliness is believed to be a cognitive discrepancy between one’s actual relationships and one’s social desires, distress and low mood associated with loneliness could reduce social interaction. Interacting with people provides cognitive stimulation and builds cognitive reserve, which optimizes cognitive performance to compensate for cognitive difficulties related to pathology. While we found no confounding or mediation by depression or depressive symptoms, we cannot exclude that other emotional causes or consequences of loneliness play an important role.
Limitations and strengths
This study was performed in the Netherlands and Sweden, two countries with well-developed social welfare systems and relatively low social inequity [56]. These countries could be considered homogenous, particularly in terms of socioeconomic position. Greater population variability may provide greater opportunity for confounding. Additionally, many participants reported optimal social support. These factors may explain why we, in contrast to others [5, 13–15], found no association between perceived or structural social support and cognitive decline. In these two countries, family and close friends may play a less prominent role in providing welfare and care, and results may not be generalizable to countries with less developed social welfare systems or greater social inequality.
Notably, the magnitude of association differed between RS and SNAC-K (30%and > 110%in-creased risk of dementia, respectively) which could be due to 1) differences between samples, including being from different countries; 2) RS possibly having a slightly healthier cohort due to social support and loneliness being collected at the second or third wave (which is the baseline for this manuscript); 3) possible selective drop-out in SNAC-K as participants self-identifying as lonely may be less able to attend follow-up during which dementia diagnosis was determined; and 4) the follow-up time and mean person observation time differing.
The inconsistency in social interaction and loneliness measures is a common limitation of this research area [57, 58]. While social support measures incorporated number of friends as characteristic of the social network, there are more extensive measures of social support available. However, the use of a single-item measure of loneliness is not necessarily a limitation; a single-item is commonly used, has been acknowledged as valid and is likely more appropriate for an older age group [59]. A common limitation of longitudinal assessment is the generalizability of findings to contemporary cohorts. However, there is evidence that the prevalence of loneliness has not changed over generations of older adults [60]. Another common limitation for longitudinal studies is the healthy cohort effect, where participants who enter and remain in the study are general healthier than people who do not participate and participants who drop-out. Therefore, our findings may be conservative as excluded participants were more likely to be less healthy and have poorer social health, hence, our magnitude of findings would likely be stronger if these participants remained in the study. Lastly, we could not assess the temporal associations of social health and depressive symptoms on incident cognition or dementia. While poor social health can lead to depression [39], the reverse is also true [61], and future research may consider further assessing temporal associations and their impact on cognitive decline and dementia.
Study strengths include the application of the same methodology in two independent cohorts which yielded consistent results. Further we simultaneously investigated two measures of cognitive decline and dementia risk over considerable follow-up periods, with adequate power (a high number of dementia events), analyzed several social health factors, and carefully adjusted for potential important confounders including depressive symptoms. Loneliness was measured using one question, which is commonly used, preferable for older people and has good face and predictive validity [28, 59]. A prominent strength of the RS was the longer median person observation time (10.8 years), which allowed us to demonstrate that findings did not alter when we reduced the influence of reverse causality by censoring the first five years from baseline.
Implications
Loneliness is a serious societal problem across all ages. The prospective associations in this study implicate loneliness in the etiology of cognitive decline and dementia. Our findings highlight the importance of developing successful preventive measures for loneliness per se, which has been claimed as a 21st century epidemic [62, 63].
ACKNOWLEDGMENTS
The contributions of participants of the Rotterdam Study and general practitioners and pharmacists of Ommoord, Rotterdam are gratefully acknowledged. Likewise, we thank all participants and staff involved in data collection and management in the Swedish National Study on Aging and Care in Kungsholmen.
This project is part of the CoSTREAM consortium (www.costream.eu) and received funding from the European Union’s Horizon 2020 research and innovation programme (grant no. 667375).
The Rotterdam Study is supported by the Erasmus MC University Medical Center the Netherlands Organisation for Scientific Research (NWO), The Netherlands Organisation for Health Research and Development (ZonMW), the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. This work was partly supported by the JPND project Social Health And Reserve in the Dementia patient journey (SHARED) and financed through projects funded by the Netherlands Organisation for Health Research and Development (grant numbers 733051082 and 733050831). Henning Tiemeier received support from a ZonMW VICI grant (016.VICI.170.200). Rosanne Freak-Poli is supported by a Heart Foundation of Australia post-doctoral fellowship (101927).
SNAC-K (http://www.snac.org) is financially supported by the Swedish Ministry of Health and Social Affairs; the participating county councils and municipalities; the Swedish Research Council; and the Swedish Research Council for Health, Working Life and Welfare. Weili Xu received grants from the Swedish Research Council (No 2017-00981), the Konung Gustaf V:soch Drottning Victorias Frimurare Foundation (No. 2018-2019), Demensfonden (2018) and Alzheimerfonden (2018–2019).
None of the funding organizations or sponsors were involved in the design and conduct of the study; collection, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0330r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-210330.
REFERENCES
[1] | Jack CR Jr. , Knopman DS , Jagust WJ , Shaw LM , Aisen PS , Weiner MW , Petersen RC , Trojanowski JQ ((2010) ) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: , 119–128. |
[2] | Kivipelto M , Rovio S , Ngandu T , Kareholt I , Eskelinen M , Winblad B , Hachinski V , Cedazo-Minguez A , Soininen H , Tuomilehto J , Nissinen A ((2008) ) Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: A population-based study. J Cell Mol Med 12: , 2762–2771. |
[3] | Kivipelto M , Ngandu T , Fratiglioni L , Viitanen M , Kareholt I , Winblad B , Helkala EL , Tuomilehto J , Soininen H , Nissinen A ((2005) ) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62: , 1556–1560. |
[4] | Karp A , Paillard-Borg S , Wang HX , Silverstein M , Winblad B , Fratiglioni L ((2006) ) Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord 21: , 65–73. |
[5] | Kuiper JS , Zuidersma M , Oude Voshaar RC , Zuidema SU , van den Heuvel ER , Stolk RP , Smidt N ((2015) ) Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 22: , 39–57. |
[6] | Bennett DA , Schneider JA , Tang Y , Arnold SE , Wilson RS ((2006) ) The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol 5: , 406–412. |
[7] | Huber M , Knottnerus JA , Green L , van der Horst H , Jadad AR , Kromhout D , Leonard B , Lorig K , Loureiro MI , van der Meer JW , Schnabel P , Smith R , van Weel C , Smid H ((2011) ) How should we define health? BMJ 343: , d4163. |
[8] | Fratiglioni L , Paillard-Borg S , Winblad B ((2004) ) An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 3: , 343–353. |
[9] | Berkman LF , Glass T , Brissette I , Seeman TE ((2000) ) From social integration to health: Durkheim in the new millennium. Soc Sci Med 51: , 843–857. |
[10] | Howick J , Kelly P , Kelly M ((2019) ) Establishing a causal link between social relationships and health using the Bradford Hill Guidelines. SSM Popul Health 8: , 100402. |
[11] | Cacioppo S , Capitanio JP , Cacioppo JT ((2014) ) Toward a neurology of loneliness. Psychol Bull 140: , 1464–1504. |
[12] | Hu J , Fitzgerald SM , Owen AJ , Ryan J , Joyce J , Chowdhury E , Reid CM , Britt C , Woods RL , McNeil JJ , Freak-Poli R ((2021) ) Social isolation, social support, loneliness and cardiovascular disease risk factors: A cross-sectional study among older adults. Int J Geriatr Psychiatry 36: , 1795–1809. |
[13] | Kuiper JS , Zuidersma M , Zuidema SU , Burgerhof JG , Stolk RP , Oude Voshaar RC , Smidt N ((2016) ) Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. Int J Epidemiol 45: , 1169–1206. |
[14] | Kelly ME , Duff H , Kelly S , McHugh Power JE , Brennan S , Lawlor BA , Loughrey DG ((2017) ) The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Syst Rev 6: , 259. |
[15] | Penninkilampi R , Casey AN , Singh MF , Brodaty H ((2018) ) The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. J Alzheimers Dis 66: , 1619–1633. |
[16] | Sommerlad A , Ruegger J , Singh-Manoux A , Lewis G , Livingston G ((2018) ) Marriage and risk of dementia: Systematic review and meta-analysis of observational studies. J Neurol Neurosurg Psychiatry 89: , 231–238. |
[17] | Amieva H , Stoykova R , Matharan F , Helmer C , Antonucci TC , Dartigues JF ((2010) ) What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med 72: , 905–911. |
[18] | Fratiglioni L , Wang HX , Ericsson K , Maytan M , Winblad B ((2000) ) Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet 355: , 1315–1319. |
[19] | Verlinden VJ , van der Geest JN , de Bruijn RF , Hofman A , Koudstaal PJ , Ikram MA ((2016) ) Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement 12: , 144–153. |
[20] | Saczynski JS , Pfeifer LA , Masaki K , Korf ES , Laurin D , White L , Launer LJ ((2006) ) The effect of social engagement on incident dementia: The Honolulu-Asia Aging Study. Am J Epidemiol 163: , 433–440. |
[21] | Cacioppo JT , Hughes ME , Waite LJ , Hawkley LC , Thisted RA ((2006) ) Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychol Aging 21: , 140–151. |
[22] | Ikram MA , Brusselle GGO , Murad SD , van Duijn CM , Franco OH , Goedegebure A , Klaver CCW , Nijsten TEC , Peeters RP , Stricker BH , Tiemeier H , Uitterlinden AG , Vernooij MW , Hofman A ((2017) ) The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 32: , 807–850. |
[23] | Lagergren M , Fratiglioni L , Hallberg IR , Berglund J , Elmstahl S , Hagberg B , Holst G , Rennemark M , Sjolund BM , Thorslund M , Wiberg I , Winblad B , Wimo A ((2004) ) A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res 16: , 158–168. |
[24] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[25] | Crum RM , Anthony JC , Bassett SS , Folstein MF ((1993) ) Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 269: , 2386–2391. |
[26] | Beekman AT , Deeg DJ , Van Limbeek J , Braam AW , De Vries MZ , Van Tilburg W ((1997) ) Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): Results from a community-based sample of older subjects in The Netherlands. Psychol Med 27: , 231–235. |
[27] | Radloff LS ((1977) ) The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measurement 1: , 385. |
[28] | Nicolaisen M , Thorsen K ((2014) ) Loneliness among men and women–a five-year follow-up study. Aging Ment Health 18: , 194–206. |
[29] | Hoogendam YY , Hofman A , van der Geest JN , van der Lugt A , Ikram MA ((2014) ) Patterns of cognitive function in aging: The Rotterdam Study. Eur J Epidemiol 29: , 133–140. |
[30] | Marseglia A , Fratiglioni L , Laukka EJ , Santoni G , Pedersen NL , Backman L , Xu W ((2016) ) Early cognitive deficits in type 2 diabetes: A population-based study. J Alzheimers Dis 53: , 1069–1078. |
[31] | Deary IJ ((2012) ) Intelligence. Annu Rev Psychol 63: , 453–482. |
[32] | de Bruijn RF , Bos MJ , Portegies ML , Hofman A , Franco OH , Koudstaal PJ , Ikram MA ((2015) ) The potential for prevention of dementia across two decades: The prospective, population-based Rotterdam Study. BMC Med 13: , 132. |
[33] | Wilson RS , Krueger KR , Arnold SE , Schneider JA , Kelly JF , Barnes LL , Tang Y , Bennett DA ((2007) ) Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry 64: , 234–240. |
[34] | Holwerda TJ , Deeg DJ , Beekman AT , van Tilburg TG , Stek ML , Jonker C , Schoevers RA ((2014) ) Feelings of loneliness, but not social isolation, predict dementia onset: Results from the Amsterdam Study of the Elderly (AMSTEL). J Neurol Neurosurg Psychiatry 85: , 135–142. |
[35] | Crooks VC , Lubben J , Petitti DB , Little D , Chiu V ((2008) ) Social network, cognitive function, and dementia incidence among elderly women. Am J Public Health 98: , 1221–1227. |
[36] | Luijendijk HJ , van den Berg JF , Dekker MJ , van Tuijl HR , Otte W , Smit F , Hofman A , Stricker BH , Tiemeier H ((2008) ) Incidence and recurrence of late-life depression. Arch Gen Psychiatry 65: , 1394–1401. |
[37] | Asberg M , Montgomery SA , Perris C , Schalling D , Sedvall G (1978)Acomprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl, pp. 5-27. |
[38] | Karlsson B , Johnell K , Sigstrom R , Sjoberg L , Fratiglioni L ((2016) ) Depression and depression treatment in a population-based study of individuals over 60 years old without dementia. Am J Geriatr Psychiatry 24: , 615–623. |
[39] | Cacioppo JT , Hawkley LC , Thisted RA ((2010) ) Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging 25: , 453–463. |
[40] | Baron RM , Kenny DA ((1986) ) The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51: , 1173–1182. |
[41] | Gelfand LA , Mensinger JL , Tenhave T ((2009) ) Mediation analysis: A retrospective snapshot of practice and more recent directions. J Gen Psychol 136: , 153–176. |
[42] | R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, https://www.R-project.org/. |
[43] | Lobo A , Lopez-Anton R , de-la-Camara C , Quintanilla MA , Campayo A , Saz P ((2008) ) Non-cognitive psychopathological symptoms associated with incident mild cognitive impairment and dementia, Alzheimer’s type. Neurotox Res 14: , 263–272. |
[44] | Tilvis RS , Pitkala KH , Jolkkonen J , Strandberg TE ((2000) ) Social networks and dementia. Lancet 356: , 77–78. |
[45] | Tilvis RS , Kahonen-Vare MH , Jolkkonen J , Valvanne J , Pitkala KH , Strandberg TE ((2004) ) Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci 59: , 268–274. |
[46] | Shankar A , Hamer M , McMunn A , Steptoe A ((2013) ) Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosom Med 75: , 161–170. |
[47] | Donovan NJ , Wu Q , Rentz DM , Sperling RA , Marshall GA , Glymour MM ((2017) ) Loneliness, depression and cognitive function in older U.S. adults. Int J Geriatr Psychiatry 32: , 564–573. |
[48] | Lara E , Caballero FF , Rico-Uribe LA , Olaya B , Haro JM , Ayuso-Mateos JL , Miret M ((2019) ) Are loneliness and social isolation associated with cognitive decline? Int J Geriatr Psychiatry 34: , 1613–1622. |
[49] | Sundstrom A , Nordin Adolfsson A , Nordin M , Adolfsson R ((2020) ) Loneliness increases the risk of all-cause dementia and Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci 75: , 919–926. |
[50] | Kuiper JS , Smidt N , Zuidema SU , Comijs HC , Oude Voshaar RC , Zuidersma M ((2020) ) A longitudinal study of the impact of social network size and loneliness on cognitive performance in depressed older adults. Aging Ment Health 24: , 889–897. |
[51] | Mirza SS , de Bruijn RF , Direk N , Hofman A , Koudstaal PJ , Ikram MA , Tiemeier H ((2014) ) Depressive symptoms predict incident dementia during short- but not long-term follow-up period. Alzheimers Dement 10: , S323–S329 e321. |
[52] | Spithoven AWM , Cacioppo S , Goossens L , Cacioppo JT ((2019) ) Genetic contributions to loneliness and their relevance to the evolutionary theory of loneliness. Perspect Psychol Sci 14: , 376–396. |
[53] | Matthews T , Danese A , Wertz J , Odgers CL , Ambler A , Moffitt TE , Arseneault L ((2016) ) Social isolation, loneliness and depression in young adulthood: A behavioural genetic analysis. Soc Psychiatry Psychiatr Epidemiol 51: , 339–348. |
[54] | Shen XN , Niu LD , Wang YJ , Cao XP , Liu Q , Tan L , Zhang C , Yu JT ((2019) ) Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry 90: , 590–598. |
[55] | Hawkley LC , Cacioppo JT ((2010) ) Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann Behav Med 40: , 218–227. |
[56] | Organization for Economic Cooperation and Development (OECD) (2011) Divided We Stand: Why Inequality Keeps Rising. OECD Publishing. https://www.oecd.org/els/soc/49170768.pdf, Accessed September 21, 2021. |
[57] | Courtin E , Knapp M ((2017) ) Social isolation, loneliness and health in old age: A scoping review. Health Soc Care Community 25: , 799–812. |
[58] | Valtorta NK , Kanaan M , Gilbody S , Hanratty B ((2016) ) Loneliness, social isolation and social relationships: What are we measuring? A novel framework for classifying and comparing tools. BMJ Open 6: , e010799. |
[59] | Campaign to End Loneliness, Measuring Your Impact on Loneliness in Later Life, http://www.campaigntoendloneliness.org/wp-content/uploads/Loneliness-Measurement-Guidance1.pdf, Accessed October 24, 2019. |
[60] | Hawkley LC , Wroblewski K , Kaiser T , Luhmann M , Schumm LP ((2019) ) Are U.S. older adults getting lonelier? Age, period, and cohort differences. Psychol Aging 34: , 1144–1157. |
[61] | McHugh Power J , Hannigan C , Hyland P , Brennan S , Kee F , Lawlor BA ((2020) ) Depressive symptoms predict increased social and emotional loneliness in older adults. Aging Ment Health 24: , 110–118. |
[62] | Cacioppo JT , Cacioppo S ((2018) ) The growing problem of loneliness. Lancet 391: , 426. |
[63] | Kar-Purkayastha I ((2010) ) An epidemic of loneliness. Lancet 376: , P2114–2115. |
[64] | Columbia University Irving Medical Center, Population Health: Methods: Causal Mediation, Columbia University, www.publichealth.columbia.edu/research/population-health-methods/causal-mediation, Accessed October 6, 2021. |




