BIOFACE: A Prospective Study of Risk Factors, Cognition, and Biomarkers in a Cohort of Individuals with Early-Onset Mild Cognitive Impairment. Study Rationale and Research Protocols
Abstract
Background:
Mild cognitive impairment (MCI) due to Alzheimer’s disease (AD) diagnosis is based on cerebrospinal fluid (CSF) or neuroimaging biomarkers. Currently, non-invasive and inexpensive blood-based biomarkers are being investigated, such as neuronal-derived plasma exosomes (NPEs). Neuroinflammation and early vascular changes have been described in AD pathogenesis and can be traced in plasma and NPEs. However, they have not been studied in early onset MCI (EOMCI).
Objective:
To describe the rationale, design, and baseline characteristics of the participants from the BIOFACE cohort, a two-year observational study on EOMCI conducted at Fundació ACE. The study goal is to characterize the different phenotypes from a clinical, neuropsychological, and biomarker point of view and to investigate the CSF and plasma proteomics as well as the role of NPEs as early biomarkers of AD.
Methods:
Participants underwent extended neurological and neuropsychological batteries, multimodal biomarkers including brain MRI, blood, saliva, CSF, anthropometric, and neuro-ophthalmological examinations.
Results:
Ninety-seven patients with EOMCI were recruited. 59.8%were women. Mean age at symptom onset was 57 years; mean MMSE was 28. First degree and presenile family history of dementia was present in 60.8%and 15.5%, respectively. Depressive and anxiety disorders along with vascular risk factors were the most frequent comorbidities. 29%of participants were APOE ɛ4 carriers, and 67%showed a CSF normal ATN profile.
Conclusion:
BIOFACE is a two-year study of clinical, cognition, and biomarkers that will shed light on the physiopathology and the potential utility of plasma and NPEs as non-invasive early diagnostic and prognostic biomarkers in people younger than 65 years.
INTRODUCTION
Despite age being the main risk factor for the development of dementia, cognitive decline can also manifest at young ages. Traditionally, the arbitrary cut-off age of 65 years has been used to differentiate early onset dementia (EOD, ≤65 years old) from late onset dementia (LOD, > 65 years old). Epidemiological studies on EOD are scarce and show increasing incidence rate of dementia as age goes up (3.9, 22.9, and 67.7 cases per 100,000 inhabitants/year for the age group of 40–49 years, 50–59 years, and 60–64 years, respectively) [1].
EOD constitutes an important health and social problem as it affects people during a period of their life with work, economic, and family responsibilities. The underlying causes of cognitive decline at this age are very heterogeneous, ranging from neurodegenerative diseases such as Alzheimer’s disease (AD) or frontotemporal dementia (FTD), to non-degenerative pathologies such as psychiatric, cerebrovascular, inflammatory, traumatic, or toxic-metabolic disorders [2]. Given that some of these causes are potentially treatable, obtaining an adequate etiological diagnosis is crucial in these individuals. It is important to note that the most common cause of dementia, in both early and late onset groups, is AD [1]. Differences in the clinical presentation, cognitive and functional decline have been reported between early and late onset AD cases (EOAD and LOAD, respectively) [2], with non-amnestic presentations and a faster rate of disease progression being more frequent in EOAD than LOAD [3]. These characteristics may imply potential underlying differences in the neuroanatomical and biological pathways of the disease in this younger population.
Mild cognitive impairment (MCI) is defined as the prodromal stage before the onset of dementia during which cognitive deficits are observed but individuals do not experience any impairment in activities of daily living [4]. MCI can affect young people, and clinical and prognostic differences could also be found between early and late onset MCI (EOMCI and LOMCI, respectively) [5]. Nowadays, the diagnosis of MCI due to AD is based on clinical grounds and biomarkers of amyloidosis and neurodegeneration (amyloid-beta (Aβ) and tau proteins) in cerebrospinal fluid (CSF) and/or neuroimaging studies with amyloid and tau ligands [6]. However, these methods are invasive and expensive, respectively, and not widely available. As a result, plasma biomarkers are being investigated in terms to maximize cost effectiveness, as potential useful non-invasive diagnostic and prognostic instruments [7, 8].
A new plasma-derived biomarker under study is a class of extracellular vesicles called exosomes (endosome-derived membrane microvesicles). Exosomes are produced by most cells of the body, including neurons, and are transported to other neighboring cells and the circulatory system [9]. They contain proteins, lipids, RNA, and microRNAs involved in cellular signaling or protein degradation mechanisms [10]. Moreover, exosomes have specific surface markers that allow the identification of their cell of origin so they can be traced in different biological fluids (blood, CSF, saliva, or urine) [11]. In neurons, exosomes are involved in the processing of amyloid-β protein precursor (AβPP) and Aβ clearance, among other processes [12–14].
Neuronal-derived plasma exosomes (NPEs) studies in preclinical AD have shown that increased levels of Aβ1–42 and phosphorylated-tau at the 181 threonine (p181-tau) and 396 serine residues (pS396-tau), can predict 10 years in advance the onset of dementia in sporadic AD cases [11]. Likewise, in a clinical 36-months follow-up study, high levels of Aβ1–42, p181-tau, and pS396-tau, have also been detected in NPEs in MCI due to AD and AD dementia cases compared to controls and subjects with stable MCI [15]. These findings suggest NPEs could be a useful tool in the diagnosis process of AD.
In addition, vascular and oxidative stress biomarkers can be detected both in plasma and exosomes. Nowadays with proteomics, the analysis of proteins in different biologic tissues and fluids, we could study myriad pathways potentially involved in the pathophysiology of AD. The vascular hypothesis of AD considers that cerebral hypoperfusion is involved in the pathogenesis of the disease [16], while vascular pathophysiological changes have even been described preceding amyloidosis in LOAD [17]. In this sense, several vascular-related proteins have been postulated as early biomarkers in the pathogenesis of the disease: heart-type fatty acid binding protein (hFABP), Apolipoprotein A1 (APOA1), and Interferon-γ induced protein 10 (IP-10), which have been detected in high concentrations in CSF of patients with MCI at risk to develop AD [18–21]. Additionally, IP-10 and acrolein levels are also increased in plasma [17, 22]. However, these vascular-related changes and their pathophysiological relation with Aβ and tau proteins have not yet been well characterized in EOMCI.
Previous studies on the clinical features, risk factors, and biomarkers for conversion to dementia in EOMCI have been limited to patients with amnestic presentations [5] or MCI probably due to AD [23] and included a small number of participants [24]. Few large cohorts have been studied to characterize EOMCI from a biomarker-based point of view, assessing the different potential etiologies and clinical progression in longitudinal studies. Moreover, to our knowledge no clinical studies have evaluated the diagnostic and prognostic value of NPEs in EOMCI.
Here we present the BIOFACE study, a two year-term follow-up prospective observational study on EOMCI conducted at Fundació ACE in Barcelona, Spain. The study goal is to characterize from a clinical, neuropsychological and a multimodal biomarker-based perspective the different phenotypes of EOMCI. A special focus is given to compare the CSF and plasma biomarkers changes in order to elucidate the correlation of both physiological fluids as prognostic tools in EOMCI patients. Additionally, we aim to characterize NPEs derived biomarkers according to the CSF ATN classification [25], the burden of vascular pathology on brain MRI and the longitudinal cognitive decline (conversion to dementia) in EOMCI participants. The differentiation of a neurodegenerative disease from other causes of cognitive decline has significant social, work and family implications in young subjects, and it entails a substantial emotional burden. The protocol, design and baseline demographic and clinical characteristics of the BIOFACE study are described.
METHODS
Design
BIOFACE is single-center prospective observational study of participants with a diagnosis of EOMCI, with a two-year follow-up, conducted at Fundació ACE’s Memory Unit, Barcelona Alzheimer Research and Treatment Centre (Spain).
Objectives
The main objectives of the BIOFACE study are: 1) to characterize the signatures of a panel of neurobiological and inflammation proteomics in plasma, NPEs, and CSF according to the ATN classification [25] in EOMCI participants; 2) to correlate the vascular burden (as measured by brain MRI) with inflammation-related proteomic biomarkers measured in plasma, NPEs, and CSF; and 3) to evaluate the correlation of the same panel of neurobiological and inflammation proteomics in plasma, NPEs, and CSF samples of EOMCI participants. Secondary aims of this study are: 4) to analyze the demographic, neurological, and neuropsychological features associated with the different clinical phenotypes of EOMCI; 5) to characterize the biomarker profile (genetics, CSF, plasma, NPEs, and brain MRI) of the different clinical phenotypes of EOMCI; and 6) to analyze the natural history (progression to dementia versus stability versus reversion to normal cognition) of the different clinical phenotypes of EOMCI.
Subjects
BIOFACE included a total of 102 subjects with a diagnosis of EOMCI from Fundació ACE’s Memory Unit recruited and assessed from January 2018 to December 2019. Five subjects were later considered dropouts because of incomplete assessments at baseline. The majority of subjects (68%) were referred by their primary care physician (PCP) and less frequently (32%) were recruited from our Open-House Initiative (OHI). The OHI is a community-based engagement program that assesses cognitive status in individuals over 55 years for free and without the need of a physician’s referral. The OHI is part of the corporate social responsibility program of Fundació ACE [26].
The inclusion criteria were as follows: a) age under 65 years old; b) Mini-Mental State Examination (MMSE) [27] score ≥24; c) Clinical Dementia Rating (CDR) [28] = 0.5; d) educational level of at least elementary school (≥6 years of formal education); e) capacity to provide written informed consent; f) fluent Spanish language skills. The exclusion criteria were: a) contraindication for brain MRI; b) presence of an underlying medical or neurological illness that could account for the cognitive impairment based on laboratory tests or brain imaging (including significant vascular burden such as large vessel stroke, Huntington’s disease or multiple sclerosis); c) current or previous major psychiatric disorder; d) active alcohol consumption or drug use; e) severe auditory/visual impairment.
As previously described in detail [29], a comprehensive cognitive, behavioral, and functional assessment battery was administrated to all participants. Briefly, they underwent a complete physical and neurological examination, as well as routine analyses of blood and structural brain neuroimaging. Information used to diagnose MCI came from both the patient and a family member and was gathered in two structured interviews conducted by a neurologist and a social worker, respectively. With all available information, a consensual diagnosis of MCI was achieved according to the criteria of Petersen [30] and López et al. [31] at a multidisciplinary team meeting [29]. Similar to López et al. [31], but extending it to the non-amnestic MCI, we added the additional qualifier of possible/probable MCI condition in function of the presence or absence, respectively, of psychiatric, neurological, or systemic illnesses that could otherwise explain their cognitive deficits or when there was insufficient information [32].
Visits and assessments scheduled
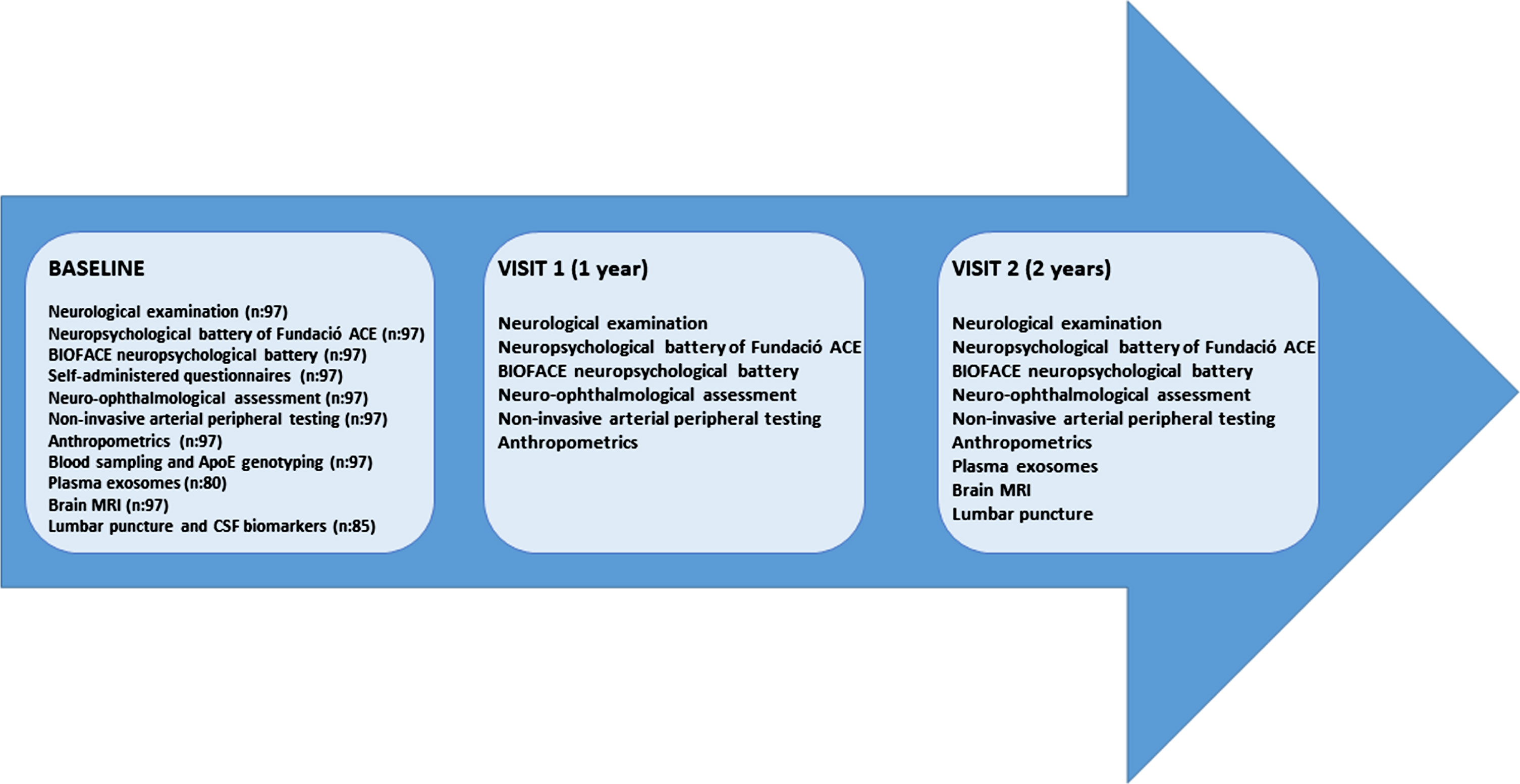
Extended neurological and neuropsychological batteries, anthropometrics and neuro-ophthalmological examinations, brain MRI, sampling of blood, saliva, and CSF were performed at baseline visit. All these procedures took place at the Memory Unit in Fundació ACE except the MRI acquisition that was performed at the Corachan Clinic, in Barcelona (Spain), our reference neuroimaging research center. All the procedures of each visit were done within a time window of three months. Detailed overviews of the assessments scheduled in the three consecutive annual visits of the study are shown in Fig. 1.
Fig. 1
Assessments scheduled in the three consecutive annual visits.
Neurological assessment
Clinical assessments at baseline and follow-up visits were performed by a neurologist specialized in cognitive disorders. Examinations included a structured personal medical, psychiatric, and social history, lifestyle habits, family history, current medication, and standardized full neurological and cognitive examinations. The scales used for assessment at baseline visits are outlined in Table 1. In the follow-up visits, MMSE [27], the Spanish version of the Neuropsychiatric Inventory Questionnaire (NPI-Q) [35], Motor assessment from the Unified Parkinson’s Disease Rating Scale (UPDRS-III) [36], CDR [28], and the Blessed scale [37] were administered.
Table 1
Scales used for neurological assessment at baseline
| Scales baseline | Function/topic assessed |
| Mini-Mental State Examination (MMSE) [27] | Global cognition |
| The memory subtest of the Spanish version of the 7 Minute Test [33] | Memory |
| Handedness Edinburgh Inventory [34] | Handedness |
| The Spanish version of the Neuropsychiatric Inventory Questionnaire (NPI-Q) [35] | Behavioral symptoms |
| Motor assessment from the Unified Parkinson’s Disease Rating Scale (UPDRS-III) [36] | Extrapyramidal signs |
| Clinical Dementia Rating (CDR) [28] and Blessed scales [37] | Functionality |
| Pittsburgh sleep quality index [38] | Sleep quality |
| International Physical Activity Questionnaire (IPAQ) (Spanish adaptation) [39] | Physical activity |
| Word accentuation test [40] | Premorbid intelligence quotient |
| Language subtests from the Barcelona’s Test [41] | Language |
| Order comprehension from the Boston Diagnostic Aphasia Examination [42] | Language (Comprehension) |
Neuropsychological assessment
At baseline and follow-up visits all patients participated in a two-day session comprehensive neuropsychological evaluation. In the first assessment the neuropsychological battery of Fundació ACE (NBACE) [43] and the Hospital Anxiety and Depression Scale (HADS) [44] were administered, according to our standardized protocol, to characterize the patient’s cognitive impairment. In a subsequent visit, an additional neuropsychological assessment consisting of classical neuropsychological tests, which are specific for the assessment of each cognitive domain, was administered to detect the prodromal stage and atypical forms of AD (see Table 2).
Table 2
Additional neuropsychological tests and the cognitive functions explored
| Neuropsychological instrument | Domain of cognition |
| FACEmemory® [45] (tablet format) | Associative memory |
| Free and Cued Selective Reminding Test [46] | Episodic memory |
| Rey figure [41] (copy and delayed recall) | Visuoconstructional and visual memory |
| Trail making test A and B [47] | Executive function |
| Stroop Test [48] | Executive function |
| Letter Fluency MRP [49] | Executive function |
| Boston Naming Test (60 items) [50] | Language |
| Pyramid and Palm Tree Test [51] | Language |
| Judgment of Line Orientation [52] | Visuospatial |
| Subtests of fragmented letters and dot counting from the Visual Object and Space Perception Battery [53] | Visuospatial/visuoperception |
| Ekman 60 Test of Facial Affect [54]. | Social cognition |
In addition, several self-administered questionnaires related to lifestyle and mood were applied at the baseline and final visits (see Table 3).
Table 3
Self-administered questionnaires of BIOFACE
| Self -administered questionnaire | Lifestyle and mood domain explored |
| Starkstein Apathy Scale [55] | Apathy |
| Clinically Useful Depression Outcome Scale (CUDOS) [56] | Depressive symptoms |
| Fear of Alzheimer’s Disease Scale (FADS) [57] | Fear of suffering Alzheimer’s disease |
| Hamilton Anxiety Rating Scale (HARS) [58] | Anxiety symptoms |
| General Health Goldberg’s Questionnaire (GHQ28) [59] | Health |
| Toronto Alexithymia scale (TAS-20) [60] | Alexithymia |
| Zuckerman-Kuhlman Personality Questionnaire (ZKPQ) [61] | Personality |
Acquisition and processing protocol of brain MRI images
All BIOFACE’s cohort participants underwent a brain MRI with a Siemens VIDA 3T at Corachan Clinic’s Radiology Department (Barcelona, Spain) at the baseline visit. The anatomical images for the volumetric study were acquired using a T1-weighted 3D gradient echo sequence (T1 MPRAGE isotropic) with a slice thickness of 1.2 mm, FOV 270 mm, 243×256×95 matrix, voxel measurement of 1.1×1.1×1.2 mm, TI of 968 ms, TR 2,200 ms, and TE 2.23 ms. Diffusion tensor imaging (DTI) study was acquired by diffusion-enhanced ecoplanar sequence (EPI), with 64 directions and b1 values of 0 and b2 of 1000 s/mm2. To complete the acquisition, a T2-weighted axial sequence, an isotropic 3D FLAIR, and a T2-weighted gradient recalled echo* axial sequence were performed to detect ischemic damage and microbleeds.
Another brain MRI will be acquired at the follow-up visit 2, with the same characteristics and conditions described for the baseline visit, and before the performance of the lumbar puncture.
MRI studies were examined by a group of experienced neuroradiologists and reported according to standard practice. Regional atrophy was measured with the following visual rating scales: global cortical atrophy (GCA) [62], medial temporal atrophy (MTA) [63], parietal atrophy (PA) [64], and several variables to measure vascular disease such as the Fazekas scale [65]. In the visual rating scale, a score of zero indicates that there is no atrophy, while scores of one to three (PA and GCA) or four (MTA) indicate an increasing degree of atrophy. The Fazekas scale, taking into account deep and periventricular white matter hyperintensities (DWMH and PVWMH, respectively) was scored according to the same patterns; a score of zero indicates a none or a single punctate WMH lesion, while scores of one to three indicate multiple, beginning confluency and large confluent lesions, respectively.
The images will be processed at Fundació ACE Neuroimaging Laboratory. All images, stored in a PACS system, will undergo an automated de-identification process. Initially the structural MRI images, enhanced in T1, will be processed with Freesurfer 6.0.1 (https://surfer.nmr.mgh.harvard.edu/). Cortical and subcortical segmentation of structural images will be performed. This procedure allows segmenting the GM, WM, and subcortical structures, after normalizing the intensity and correcting topological defects, giving robust measurements of the hippocampal volume, mean cortical thickness and the volume of WM hyperintensities (WMH). The DTI is preprocessed with Functional MRI of the Brain (FMRIB)’s Diffusion Toolbox), implemented in the FSL 5.0 software package (FMRIB Software Library; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Initially, images are corrected to eliminate distortions and being normalized to the same anatomical space with the T1. Lately, they are fitted to a voxel-to-voxel diffusion tensor model to obtain fractional anisotropy, mean axial and radial diffusion images, according to the Johns Hopkins University atlas.
Lumbar puncture protocol
Lumbar punctures (LP) were performed at baseline visit at Fundació ACE by an experienced neurologist under fasting conditions. CSF was collected passively in two 10 ml polypropylene tubes (Sarstedt Ref 62.610.018) centrifuged (2000×g 10 min at 4°C), aliquoted and stored in polypropylene tubes (Sarstedt Ref 72.694.007) at –80°C until its use. Time delay between CSF collection and storage was less than 2 h. The collection protocol follows the recommendations of the Alzheimer’s Biomarkers Standardization Initiative [66]. The day of the analysis, one aliquot of 0.5 mL was thawed and used for the determination of Aβ1–40, Aβ1–42, Total Tau (T-tau), and p181-tau. Amyloid and tau proteins were quantified by chemiluminescence enzyme immunoassay (CLEIA) using the Lumipulse G 600 II automatic platform (Fujirebio Inc) [67]. Another LP will be performed at follow-up visit 2, in the same conditions stated, for CSF biomarkers and proteomics analysis.
CSF biomarkers. Participants were classified into three categories according to the ATN scheme [25]: Normal AD biomarkers (A-T-N-), Alzheimer’s disease continuum (including A+T-N-, A+T+N-, A+T+N+ and A+T-N+), and non-AD pathologic changes (including A-T+N-, A-T-N+ and A-T+N+), where A refers to aggregated Aβ, T to aggregated tau, and N to neurodegeneration or neuronal injury. Cut-offs from the FACEBREP cohort were used to dichotomize each CSF biomarker into +/–as follows: ratio Aβ1–42/Aβ1–40 < 0.063 for A; p181-tau > 54 pg/ml for T; and T-tau > 412 pg/ml for N) (Orellana et al, submitted for publication).
Acquisition, processing, and analysis of plasma exosomes
Consecutively to CSF extraction, matched samples of plasma and serum were obtained from each patient at baseline visit and will be obtained at follow-up visit 2. Samples were collected in BD Vacutainer tubes. Three of them (1× SSTII Advance and 2× K2-EDTA) were centrifuged (2000×g, 10 min at 4°C) to obtain serum and plasma, respectively. Samples were aliquoted and stored at –80°C until their use.
The exosomes were isolated and purified from plasma samples based on Thery’s protocol with small modifications [68]. First, 3.5 mL of plasma samples were centrifuged (10,000 g, 30 min, 4°C) to remove cellular debris and the supernatant was then ultracentrifuged (100,000 g, 60 min, 4°C) to pellet the exosomes. All centrifugations were done with Sorvall Discovery M150SE (Thermo Scientific) ultracentrifuge using S100AT6 rotor. The isolation of NPEs is performed by immunomagnetic separation of L1CAM positive exosomes. Briefly, the pellet is resuspended in 500μL buffer TRIS HCl pH 7.4 (previously filtered and autoclaved) and incubated with L1CAM antibody-modified magnetic particles (anti L1CAM-MPs) for 60 min at RT with slight rotation. To remove the supernatant, anti L1CAM-MPs with the exosomes attached were attracted with an external magnet. Finally, anti L1CAM-MPs are washed 3× with 500μL of washing buffer (TRIS HCl pH 7.4). Characterization of the exosomes by their size is established by Nanoparticle Tracking Analysis (NTA) with a Nanosight LM10 microscope (Malvern Technologies).
Neurology and inflammation biomarkers quantification in plasma, NPEs, and CSF samples
Total protein concentration of obtained NPEs was first measured by Pierce BCA Protein Assay Kit (ThermoFisher). Specific protein concentration of plasma, NPEs and CSF samples were quantified using the validated, highly sensitive, and specific ProSeek® multiplex immunoassay, developed by Olink Proteomics (Uppsala, Sweden). Two commercially available ProSeek® Multiplex panels (Inflammation & Neurology) were used to measure the concentrations of 184 proteins.
To prepare the NPEs samples for Pierce BCA Protein Assay and Olink platform, the L1CAM + exosomes were lysed with 50μL lysis buffer (50 mM TRIS pH7.4, 150 mM NaCl, 1 mM EDTA pH8, 1%Triton x100, 0.01%sodium deoxycholate).
APOE genotyping
Study of genomic DNA was carried out after the extraction of 12 ml of blood in polypropylene tubes with EDTA (Vacutainer, violet stopper). DNA extraction was carried out with the Chemagen (Perkin Elmer) DNA purification system that allows the processing of 96 samples at a time. Subsequently, we performed APOE genotyping using TaqMan probe analysis of SNP ID RS429358 and SNP ID RS7412 using a Real-Time PCR Quant Studio 3 system (Thermofisher).
Neuro-ophthalmology examination
Participants underwent a neuro-ophthalmology exam performed by an optometrist at all study visits. The assessment included: review of past ophthalmological history and treatments, visual acuity (using the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart [69]), intraocular pressure measurement (by Icare tonometry [70]) and optical coherence tomography (OCT). Retinal structural and angiography studies were performed using the DRI OCT Triton - Swept Source (SS) OCT, software v.1.22.1 (Topcon Co. Tokyo, Japan). The availability of high-resolution B-scan mode made pupil dilatation unnecessary. The same optometrist screened all images for possible abnormalities after each OCT imaging session. All data were reviewed by an ophthalmologist to rule out ophthalmologic pathologies and check retinal images.
Anthropometrics and non-invasive arterial peripheral testing
In all the study visits, body composition and arterial peripheral testing were obtained at Fundació ACE’s nursing station. Using the Tanita® device, a trained nurse measured height, weight, body mass index, abdominal perimeter, %of muscle, fat, water, and bone. Additionally, using the Vicorder® device, blood pressure, heart frequency, arterial rigidity index, and ankle-arm index were determined.
Statistical analysis
In this paper we present the baseline sociodemographic and clinical characteristics of the sample, using descriptive statistics. Categorical variables are presented as percentages and continuous variables as means and standard deviations, using SPSS version 20.0 software (SPSS Inc Chicago, IL).
Ethical approval
BIOFACE study protocol was approved by the Ethics Committee of Hospital’s Clinic (Barcelona, Spain). Written informed consent was obtained from all participants after the aims and procedures of the study were fully explained by the neurologist in charge of the study, according to Spanish biomedical laws and to the principles of the Declaration of Helsinki.
RESULTS
Participant recruitment began in January 2018 and continued until December 2019. All participants performed the Memory Unit procedure described in Fig. 1. One hundred and two patients were recruited, five subjects were lately considered dropouts because they did not complete assessments at baseline and, as a result, ninety-seven patients were included in this study. They were mainly referred by their PCP (68%) (see Table 4). Eleven individuals withdrew informed consent to perform the lumbar puncture, while this procedure could not be performed in one additional subject. Finally, 85 subjects completed all the procedures of the study baseline visit, although in 5 of them not enough blood to perform exosomes studies could be obtained. Twelve patients did not undergo the lumbar puncture, but they completed the rest of the study tests.
Table 4
Baseline socio-demographic characteristics of the sample
| Demographic characteristics | Mean (±SD)/n (%) |
| Age at baseline visit (y) | 60.51 (±4.63) |
| Gender (women) | 58 (59.8%) |
| Ethnicity (Caucasian) | 93 (96%) |
| Education (y) | 12.13 (±5.08) |
| Bilingualism | 68 (70.1%) |
| Laterality | |
| Right-handed | 85 (87.6%) |
| Left-handed | 4 (4.2%) |
| “Converted” left-handed | 8 (8.2%) |
| Labor status | |
| Active | 37 (38.1%) |
| Housewife | 6 (6.2%) |
| Retired | 18 (18.6%) |
| Unemployed | 11 (11.3%) |
| Sick leave | 11 (11.3%) |
| Disability | 14 (14.4%) |
| Marital status | |
| Married | 67 (69.1%) |
| Single | 7 (7.2%) |
| Divorced | 16 (16.5%) |
| Widow/widower | 7 (7.2%) |
| Recruitment source | |
| Primary physician (PCP) | 66 (68%) |
| Open House Initiative | 31 (32%) |
Sociodemographic characteristics
Baseline sociodemographic data of the sample are detailed in Table 4. Most of participants were women (59.8%) and Caucasian (96%), and they had a mean of 12.13 (±5.07) years of formal education. The mean age at symptom onset was 57 (±4.14) years, while the mean age for seeking a neurologic evaluation was 60.51 (±4.63) years. All participants were fluent in Spanish and a high percentage (70.1%) was bilingual (Spanish and another language, mainly Catalan). History of dementia in first-degree relatives was present in 60.8%of participants, 15.5%of whom were diagnosed before 65 years of age (Table 5).
Table 5
Baseline clinical characteristics of the sample
| Clinical characteristics | n (%)/ mean (±SD) |
| Age of symptoms onset | 57.19 (±4.14) |
| Syndromic diagnosis | |
| Possible amnestic MCI | 32 (33%) |
| Possible non-amnestic MCI | 33 (34%) |
| Probable amnestic MCI | 16 (16.5%) |
| Probable non-amnestic MCI | 16 (16.5%) |
| Family history of dementia | |
| First degree | 59 (60.8%) |
| Presenile dementia | 15 (15.5%) |
| Past medical history | |
| Hypertension | 29 (29.9%) |
| Diabetes | 10 (10.3%) |
| Dyslipidemia | 33 (33.4%) |
| Current smoking habit | 23 (24%) |
| Obstructive Sleep Apnea- | 14 (14.4%) |
| Hypopnea Syndrome (OSAHS) | |
| Persistent depressive disorder | 54 (57.7%) |
| Generalized anxiety disorder | 45 (46.4%) |
| Fibromyalgia | 20 (20.6%) |
| Chronic fatigue syndrome | 13 (13.4%) |
| Medications | |
| Antiplatelets | 17 (17.5%) |
| Antihypertensives | 31 (32%) |
| Antidiabetics | 8 (8.2%) |
| Hypolipidemic drugs | 26 (26.8%) |
| Antidepressants | 51 (52.6%) |
| Anxiolytics | 25 (25.8%) |
| Antiepileptics | 13 (13.4%) |
| Neuroleptics | 1 (1%) |
| Anti-inflammatory | 1 (1%) |
| Analgesics | 3 (3.1%) |
| Opiates | 8 (8.2%) |
| Thyroid hormones | 12 (12.4%) |
| Hypnotics | 17 (17.5%) |
| B12 vitamin | 2 (2.1%) |
| Antihistamines | 2 (2.1%) |
| Symptoms at onset | |
| Memory | 83 (85.6%) |
| Language | 56 (57.7%) |
| Orientation | 4 (4.1%) |
| Executive dysfunction | 41 (42.3%) |
| Behavior | 2 (2.1%) |
| Depression | 41 (42.3%) |
| Others | 4 (4.1%) |
| MMSE | 28 (±1.70) |
MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Clinical characteristics
Cardiovascular risk factors were the most prevalent risk factors in the study cohort (Table 5). The most frequent was dyslipidemia (33.4%) followed by hypertension (29.9%) and smoking habit (24%), while only 10.3%of the subjects were diabetic. Past medical history of psychological symptoms was also rather frequent (57.7%presented depression and 46.4%anxiety) along with rheumatological diseases (20.6%had been diagnosed with fibromyalgia and 13.4%with chronic fatigue). The most commonly prescribed pharmacological treatments before inclusion in the study were antidepressants (52.60%), antihypertensives (32%), lipid-lowering medication (26.8%), and anxiolytics (25.8%).
Cognitive characteristics
The most frequent symptoms at onset were memory problems (85.6%) followed by language (57.7%) and executive dysfunction (42.3%). The syndromic cognitive diagnoses after the baseline assessment were as follows: possible amnestic MCI (33%), possible non-amnestic MCI (34%), probable amnestic MCI (16.5%), and probable non-amnestic MCI (16.5%) (see Table 5).
Biomarkers
Regarding the APOE genotype, 29%of the participants (22/75) were APOE ɛ4 carriers, while the frequency of APOE ɛ2 was 9.3%(7/75) (see Table 6). In relation with AD CSF biomarkers and according to the ATN classification [25], three groups of categories were stablished: Alzheimer’s disease continuum (including A+T-N-, A+T+N-, A+T+N+ and A+T-N+) was present in 15 participants (17.6%), non-AD pathologic changes (including A-T+N-, A-T-N+ and A-T+N+) in 13 participants (15.3%), and finally, normal AD biomarkers (A-T-N-) in 57 subjects (67%) (Table 6). Lastly, preliminary data based on MRI visual rating scales are shown in Table 6. 92.7%of the participants had an MTA score < 2, while 7.2%showed pathological scores (≥2) in the right hemisphere, with 94.8%versus 5.2%in the left hemisphere, and similar percentages in GCA and PA scores (94.8%versus 5.2%, respectively). WMH load measured with the Fazekas scale showed 95.87%of participants with a score 0–1, and 4.12%with a score≥2. Other quantitative measures will be performed using the Freesurfer program and will be presented in future articles derived from this study.
Table 6
Biomarkers at baseline visit
| BIOMARKERS | ||
| MRI (n = 97) | ||
| GCA | ||
| 0/1/2/3 | 42/50/5/0 | |
| MTA | R | L |
| 0/1/2/3/4 | 75/15/6/1/0 | 76/16/3/2/0 |
| PA | R | L |
| 0/1/2/3 | 54/39/3/1 | 45/47/3/2 |
| Fazekas DWMH | R | L |
| 0/1/2/3 | 44/49/3/1 | 40/53/3/1 |
| Fazekas PVWMH | ||
| 0/1/2/3 | 92/2/3/0 | |
| CSF (n = 85) | ||
| AD continuum | 15 | |
| Non-AD pathologic change | 13 | |
| Normal AD biomarkers | 57 | |
| APOE status (n = 75) | ||
| ɛ4 (at least 1 allele) | 22 | |
| ɛ2 (at least 1 allele) | 7 | |
| NPEs (n = 80) | ||
| Serum/ Plasma (n = 97) | ||
| OCT (= 97) | ||
| Vicorder©, Tanita© (n = 97) | ||
GCA, Global cortical atrophy; MTA, medial temporal atrophy; PA, posterior atrophy; DWMH, deep white matter hyperintensities; PVWMH, periventricular white matter hyperintensities; R, right; L, left; APOE, Apolipoprotein E; NPEs, neuronal-derived plasma exosomes; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; OCT, optical coherence tomography. Data are shown as n of participants.
DISCUSSION
Longitudinal studies with multimodal biomarkers are needed to characterize EOMCI patients in order to deepen the knowledge of the different underlying biological processes involved in this condition and to determine which biomarker(s) are more accurate and cost-effective to diagnose and predict future conversion to dementia, which is especially important in this age group. In this regard, the BIOFACE study aims to characterize the different phenotypes of EOMCI from a clinical, neuropsychological, and biomarker-based perspective, with a special focus on investigating the potential role of plasma and NPEs proteomics for an accurate and early diagnosis of AD.
In our sample, the socio-demographic characteristics confirm previous findings from other groups showing that individuals more likely to volunteer for research studies are mostly women and tend to have a higher level of education [71]. One of the characteristics of our cohort is that participants were recruited using two different methods: the majority (68%) were referred by their PCP to our Memory Clinic, which is the standard patient referral; while 32%were recruited from the OHI [26]. This could reflect the difficulty that young patients sometimes experience in order to be referred to a specialized memory clinic [72], having to look for other alternatives to obtain a cognitive assessment [73–75].
Our cohort presents a high frequency (60.8%) of family history of dementia, supporting the importance of genetic factors in the pathogenesis of the disease and the fact that the burden of inherited dementia is higher in these young patients [2]. Although compared with elderly individuals, younger patients tend to have fewer comorbidities such as renal and heart disease, and lower medication use [2], it should be pointed out that the most common comorbidities in our sample are persistent depressive disorder (57.7%) and generalized anxiety disorder (46.4%), which implies a more frequent use of antidepressants (52.6%), anxiolytics (25.8%), and hypnotics (17.5%), medications that have been related to cognitive impairment [2]. Co-existent cerebrovascular disease is also less common in younger patients [2], but in our sample cardiovascular risk factors are quite represented (the most frequent is dyslipidemia, in 33.4%, followed by hypertension in 29.9%and smoking habit in 24%).
Regarding the cognitive characteristics of our cohort, we postulate that the high prevalence of psychiatric disorders could be the reason for having a higher prevalence of “possible” compared to “probable” MCI syndromic categories (67%versus 33%, respectively). Besides, it is important to note that the study includes an extensive neuropsychological assessment, in order to characterize the different clinical patterns of EOMCI and to detect atypical forms of presentation. The differential diagnosis of MCI is often broad and, although the most common underlying causes of cognitive impairment are neurodegenerative conditions, their clinical features in patients younger than 65 years old can differ from those seen at older ages [2]. Despite presenting similar histopathological characteristics [76], differences in clinical and neuropsychological profiles have been observed between EOMCI and LOMCI patients [3], as well as a faster progression of cognitive and functional decline in younger individuals [5]. EOMCI is associated with a higher prevalence of atypical symptoms [3, 77], compared to the typical amnestic disorder of LOMCI cases [78]. Several studies have found that subjects with EOMCI show lower performance on praxis, visuospatial skills, language, and executive functions tests compared with subjects with LOMCI. In contrast, subjects with EOMCI tend to get better scores in memory tests [79, 80]. Due to the atypical symptoms and non-amnestic presentations in EOMCI, AD is often not properly diagnosed in this younger population [72]. The fact that younger individuals have generally a higher educational level and thus better cognitive reserve also contributes to this delayed diagnosis [81]. As a result, in order to detect a broad spectrum of neurodegenerative disorders, the BIOFACE study included EOMCI patients with diverse neuropsychological profiles without restrictions, in order to compare their different phenotypes and biological characteristics, and to assess etiologies and prognosis. In this sense, it is very important to extend the neuropsychological tests included in the study, otherwise the clinical phenotype is not going to be well defined and getting an accurate etiological diagnosis (mainly based on clinical and neuropsychological grounds, with biomarker support) would not be achieved.
Additionally, the study also comprises several questionnaires to assess neuropsychiatric symptoms (NPS). A significant number of individuals with MCI who are later diagnosed with a neurodegenerative disorder have initially received a psychiatric diagnosis, being depression the most common [82]. Previous studies found higher prevalence of NPS in EOAD [83] and also, in MCI (NPS have been reported in 35%to 85%of adults with MCI [84]). In addition, NPS may be the first manifestation of an underlying cognitive disorder and their occurrence for the first time in later life should increase suspicion for a neurodegenerative condition [85]. Moreover, the co-occurrence of NPS with MCI has been associated with a more rapid cognitive decline [85]. As a result, due to the high prevalence of NPS in early clinical stages, they can serve as diagnostic and prognostic indicators [85].
We also analyze the APOE genotype, the main genetic risk factor for AD, because of the potential contribution that APOE ɛ4 allele might have in the clinical presentation and the disease course. The presence of APOE ɛ4 allele has been associated with an earlier age of onset and with a typical amnestic phenotype in AD, while patients with atypical non-amnestic early-onset disease seldom carry the APOE ɛ4 allele [86]. In our preliminary data of the sample, there is a 29%of APOE ɛ4 carriers, slightly higher with respect to previous studies in general population [86] but lower than in other prospective cohorts including MCI [87, 88].
Availability of CSF biomarkers highlights the importance of the biological characterization in order to achieve a more precise etiological diagnosis. Most of the participants in the BIOFACE cohort (67%) are ATN negative, while 18%are under the “AD continuum” and 15%show non-AD pathologic changes. Again, the high prevalence of psychiatric symptoms and other comorbidities like fibromyalgia or cerebral small vessel disease in the study cohort, could be the reason of having such high percentage of normal AD biomarkers (A-T-N-).
Regarding the structural MRI data presented, most of the participants showed low scores on visual rating scales (Table 6). In this sense, studies regarding MRI data in patients with early-onset cognitive impairment have shown that none of the existing visual rating scales met the requirements for being a good biomarker (because of their low sensitivity and specificity), so these results should be interpreted with caution [89]. Moreover, we should consider the fact that our sample is remarkably young and on an early stage of the cognitive impairment spectrum, where subtle structural changes might be present but can’t be observed in visual scales yet. Thus, long-term monitoring is necessary in order to obtain more relevant quantitative MRI data.
The BIOFACE’s protocol also includes other biomarkers that will allow to measure longitudinally different pathophysiological processes: in addition to structural MRI, DTI will be explored as an early prognostic and diagnostic marker, as studies have shown that in preclinical AD, microstructural measures of the rate of water diffusion are altered even before volumetric changes occur [90]. Further, the need of non-invasive and cost-effective tools for early diagnosis entails consideration of others novel biomarkers that are also included in this study, such as OCT of the retina, which includes structural and vascular measurements. OCT is an inexpensive, widely available, quick, and innocuous procedure, and several studies have shown thinning of several retinal layers in AD and MCI patients compared to healthy controls [91].
In addition, we should consider that the scientific interest in biomarkers has recently moved to those based on plasma measurements, due to the invasive, expensive, and not widely available nature of current CSF and neuroimaging ones. Plasma is a biological fluid easy to obtain, even in a primary care setting. In this sense, exosomes, which can be isolated from plasma samples, are interesting targets for the early diagnosis of neurodegenerative diseases, such as AD. The function of exosomes remains unknown, but different roles have been suggested: they were originally thought to be a mechanism for cells to discard unwanted proteins and other molecules and, more recently, they appear to have a function in intercellular communication [10]. Indeed, exosomes may participate in the propagation of misfolded proteins as a number of proteins associated with neurodegenerative diseases have been shown to be released by cells in association with exosomes [92]. Furthermore, it has been shown that Aβ peptides are released into the extracellular environment in association with neuronal exosomes [14], as well as AβPP and carboxyterminal fragments (proteolytically cleaved products of AβPP) [13], and could be involved in Aβ plaque formation in AD [14]. Additionally, α- and β-secretases have been identified in exosomes, indicating that cleavage of AβPP can occur within them [13]. Taking this into consideration, NPEs are potential diagnostic biomarkers for AD, because they contain both hallmark AD pathogenic proteins, not only amyloid peptides but also tau species, which are secreted by neurons in association with exosomes [93], so it has been suggested exosomes can propagate tau aggregation [15]. Interestingly, in mice models, tau pathology was induced in brains of normal mice after injection of NPEs derived from AD patients [15]. In addition, clinical studies have shown increased levels of p-tau and Aβ1–42 in NPEs from AD patients (including MCI due to AD) compared to cognitively normal subjects [15]. These findings were observed also in preclinical AD (i.e., cognitively unimpaired individuals with abnormal biomarkers [94]) [11]. All in all, we can state that NPEs likely reflect cerebral pathology, serving as a “liquid biopsy”, and also, they may have prognostic and disease monitoring potential.
Furthermore, it is noteworthy that not only amyloid and tau are involved in the pathogenesis of AD. Recent evidence provided by in vivo studies, neuroimaging or human genetics have highlighted the involvement of other molecular mechanisms in AD pathophysiology: blood-brain barrier disruption, inflammation, oxidative stress, or vascular damage [95]. In this regard, neuroinflammation has recently been suggested as a direct contributor to the progression of the disease and in the promotion of AD pathology [96] and inflammatory-related proteins can be traced in plasma and exosomes (like tumor necrosis factor-α, interleukin-1-β) [97], as well as other proteins related with cellular metabolic processes: proteins involved in insulin dysregulation (insulin receptor substrate 1), synaptic proteins, low-density lipoprotein receptor-related protein 6, and heat shock proteins [98].
In this sense, proteomics studies in CSF and plasma of patients across the AD continuum have identified altered levels of some proteins involved in vascular, inflammatory, and other pathways, which could distinguish AD dementia and MCI due to AD from cognitively normal individuals, suggesting the potential contribution of these other biomarkers for early detection of neurodegeneration [95]. Some of these novels altered biomarkers were: chitinase-1 (a marker of microglial activation), matrix metalloproteinase 10 (related to immunity), and SPARC-related modular calcium-binding protein 2 (SMOC2) (related to vascular dysfunction), which were increased in CSF; interleukin-8, hydroxyacylglutathione hydrolase (HAGH) and caspase 8 (involved in synaptic plasticity and regulation of microglial pro-inflammatory activation), which were increased in plasma; while several CSF proteins correlated with their analogues in plasma (e.g., HAGH, SMOC2) [95]. However, plasma analysis presents some limitations, such the difficulty of measuring, standardizing thresholds, and replication of obtained results [99]. In the BIOFACE study, panels of neurobiology and inflammatory proteins involved in different pathways will be studied in CSF, plasma, and NPEs. The identification of clinically relevant protein signatures may help to a better understanding and diagnosis of neurodegenerative diseases. If enough accuracy is shown, NPEs could be considered as reliable and early AD biomarkers, with additional benefits of being non-invasive and available in different clinical settings, as only a sample of blood is required for their analysis.
All in all, this broad and accurate characterization is particularly important in this group of young patients, in which a precise diagnosis is very relevant due to work-related, family, and social responsibilities. 38%of BIOFACE participants are employed, therefore, we should consider the implications their cognitive impairment may entail for their daily job, with concerns about their ability to keep on working. Besides, legal and financial planning are important aspects to be considered as soon as possible in patients with MCI, especially in those due to a neurodegenerative disorder likely to progress to dementia over time [100, 101]. As a result, the diagnosis of a neurodegenerative underlying condition could not be done lightly and a comprehensive evaluation including neuropsychological testing and biomarkers is critical.
Our study has some limitations to be noted. First of all, not all recruited participants gave their consent for a CSF sample (n = 85, from a total of 97 patients). Lumbar puncture is an invasive process, not completely innocuous, that can cause pain or discomfort to the patient and, as a result, some of them were reluctant to perform it. However, we decided to follow-up these patients clinically and with the rest of procedures stated in the protocol. Secondly, the small sample size may prevent us from making robust conclusions. In spite of that, we should consider that this is a single-center study and patients with an early-onset cognitive impairment are less frequently derived to a Memory Clinic. Finally, NPEs are difficult to isolate and process and it requires a specialized laboratory with characterization equipment, such transmission electron microscope and immunoprecipitation techniques. As a result, in case NPEs shed light on biomarker field for clinical and preclinical AD, it would be necessary to create laboratory networks for their analysis.
In conclusion, BIOFACE is a longitudinal study designed to delve into the knowledge of the pathophysiology of EOMCI and to investigate the correlation between CSF and plasma proteomics in EOMCI patients, as well as the role of NPEs as early biomarkers of AD. This project will shed light on the research of non-invasive and cost-effective methods to early and precisely diagnose younger-onset cognitive impairment, sometimes even before overt clinical symptoms, with the ultimate aim of develop prevention strategies and identify patients that could benefit of future disease-modifying therapies.
ACKNOWLEDGMENTS
The authors are grateful to the study participants and their families, who generously participated in the study. We are also grateful to all the Fundació ACE’s professionals for their continuous support and, especially, to the BIOFACE study group, for making this work possible. This work will be included in the doctoral thesis of EEA from the University International of Catalonia.
This study has been funded by the Instituto de Salud Carlos III (ISCIII) Acción Estratégica en Salud, integrated in the Spanish National RCDCI Plan and financed by ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER-Una manera de hacer Europa) grant PI17/01474 awarded to MB (Mercè Boada) and SG. MM received funding from the Instituto de Salud Carlos III (ISCIII) Acción Estratégica en Salud, integrated in the Spanish National RCDCI Plan and financed by ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER-Una manera de hacer Europa) grant PI19/00335. MB (Mireia Bernuz) received funding from the Spanish Ministry of Science and Innovation project PID2019 106625RB I00. AC received funding from the Spanish Ministry of Science, Innovation and Universities under the grant Juan de la Cierva (FJC2018-036012-I).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0254r2).
REFERENCES
[1] | Vieira RT , Caixeta L , Machado S , Silva AC , Nardi AE , Arias-Carrión O , Carta MG ((2013) ) Epidemiology of early-onset dementia: A review of the literature. Clin Pract Epidemiol Ment Health 9: , 88–95. |
[2] | Rossor MN , Fox NC , Mummery CJ , Schott JM , Warren JD ((2010) ) The diagnosis of young-onset dementia. Lancet Neurol 9: , 793–806. |
[3] | Mendez MF ((2012) ) Early-onset Alzheimer’s disease: Non-amnestic subtypes and type 2 AD. Arch Med Res 43: , 677–685. |
[4] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: , 183–194. |
[5] | Ye BS , Seo SW , Lee Y , Kim SY , Choi SH , Lee YM , Kim DH , Han HJ , Na DL , Kim EJ ((2012) ) Neuropsychological performance and conversion to Alzheimer’s disease in early-compared to late-onset amnestic mild cognitive impairment: CREDOS study. Dement Geriatr Cogn Disord 34: , 156–166. |
[6] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman H , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[7] | Schindler SE , Bollinger JG , Ovod V , Mawuenyega KG , Li Y , Gordon BA , Holtzman DM , Morris JC , Benzinger T , Xiong C , Fagan AM , Bateman RJ ((2019) ) High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93: , e1647–e1659. |
[8] | Thijssen EH , La Joie R , Wolf A , Strom A , Wang P , Iaccarino L , Bourakova V , Cobigo Y , Heuer H , Spina S , VandeVrede L , Chai X , Proctor NK , Airey DC , Shcherbinin S , Duggan Evans C , Sims JR , Zetterberg H , Blennow K , Karydas AM , Teunissen CE , Kramer JH , Grinberg LT , Seeley WW , Rosen H , Boeve BF , Miller BL , Rabinovici GD , Dage JL , Rojas JC , Boxer AL ; Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators ((2020) ) Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 26: , 387–397. |
[9] | Vingtdeux V , Sergeant N , Buée L ((2012) ) Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front Physiol 3: , 229. |
[10] | Coleman BM , Hill AF ((2015) ) Extracellular vesicles - their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev Biol 40: , 89–96. |
[11] | Fiandaca MS , Kapogiannis D , Mapstone M , Boxer A , Eitan E , Schwartz JB , Abner EL , Petersen RC , Federoff HJ , Miller BL , Goetzl EJ ((2016) ) Identification of pre-clinical Alzheimer’s disease by a profile of pathogenic proteins in neurally-derived blood exosomes: A case-control study. Alzheimers Dement 11: , 600–607.e1. |
[12] | Yuyama K , Sun H , Usuki S , Sakai S , Hanamatsu H , Mioka T , Kimura N , Okada M , Tahara H , Furuwaka J , Fujitani N , Shinohara Y , Igarashi Y ((2015) ) A potential function for neuronal exosomes: Sequestering intracerebral amyloid-β peptide. FEBS Lett 589: , 84–88. |
[13] | Sharples RA , Vella LJ , Nisbet RM , Naylor R , Perez K , Barnham KJ , Masters CL , Hill AF ((2008) ) Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J 22: , 1469–1478. |
[14] | Rajendran L , Honsho M , Zahn TR , Keller P , Geiger KD , Verkade P , Simons K ((2006) ) Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103: , 11172–11177. |
[15] | Winston CN , Goetzl EJ , Akers JC , Carter BS , Rockenstein EM , Galasko D , Masliah E , Rissman RA ((2016) ) Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 3: , 63–72. |
[16] | de la Torre J ((2018) ) The vascular hypothesis of Alzheimer’s disease: A key to preclinical prediction of dementia using neuroimaging. J Alzheimers Dis 63: , 35–52. |
[17] | Iturria-Medina Y , Sotero RC , Toussaint PJ , Mateos-Pérez JM , Evans AC ; Alzheimer’s Disease Neuroimaging Initiative ((2016) ) Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 7: , 11934. |
[18] | Desikan RS , Thompson WK , Holland D , Hess CP , Brewer JB , Zetterberg H , Blennow K , Andreassen OA , McEvoy LK , Hyman BT , Dale AM ; Alzheimer’s Disease Neuroimaging Initiative ((2013) ) Heart fatty acid binding protein and Aβ-associated Alzheimer’s neurodegeneration. Mol Neurodegener 8: , 39. |
[19] | Chiasserini D , Parnetti L , Andreasson U , Zetterberg H , Giannandrea D , Calabresi P , Blennow K ((2010) ) CSF levels of heart fatty acid binding protein are altered during early phases of Alzheimer’s disease. J Alzheimers Dis 22: , 1281–1288. |
[20] | Slot RE , Van Harten AC , Kester MI , Jongbloed W , Bouwman FH , Teunissen CE , Scheltens P , Veerhuis R , van der Flier WM ((2017) ) Apolipoprotein A1 in cerebrospinal fluid and plasma and progression to Alzheimer’s disease in non-demented elderly. J Alzheimers Dis 56: , 687–697. |
[21] | Galimberti D , Schoonenboom N , Scheltens P , Fenoglio C , Bouwman F , Venturelli E , Guidi I , Blankenstein MA , Bresolin N , Scarpini E ((2006) ) Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol 63: , 538–543. |
[22] | Igarashi K , Yoshida M , Waragai M , Kashiwagi K ((2015) ) Evaluation of dementia by acrolein, amyloid-β and creatinine. Clin Chim Acta 450: , 56–63. |
[23] | Tábuas-Pereira M , Baldeiras I , Duro D , Santiago B , Ribeiro MH , Leitão MJ , Oliveira C , Santana I ((2016) ) Prognosis of early-onset vs. late-onset mild cognitive impairment: Comparison of conversion rates and its predictors. Geriatrics (Basel) 1: , 11. |
[24] | Kim SH , Seo SW , Yoon DS , Chin J , Lee BH , Cheong HK , Han SH , Na DL ((2010) ) Comparison of neuropsychological and FDG-PET findings between early- versus late-onset mild cognitive impairment: A five-year longitudinal study. Dement Geriatr Cogn Disord 29: , 213–223. |
[25] | Jack CR Jr , Bennet DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R ; Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[26] | Rodríguez-Gómez O , Abdelnour C , Jessen F , Valero S , Boada M ((2015) ) Influence of sampling and recruitment methods in studies of subjective cognitive decline. J Alzheimers Dis 48 Suppl 1: , S99–S107. |
[27] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini- Mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[28] | Hughes CP , Berg L , Danziger WL , Coben LA , Martin RL ((1982) ) A new clinical scale for the staging of dementia. Br J Psychiatry 140: , 566–572. |
[29] | Boada M , Tárraga L , Hernández I , Valero S , Alegret M , Ruiz A , López OL , Becker JT , Fundació ACE Alzheimer Research Center and Memory Clinic ((2014) ) Design of a comprehensive Alzheimer’s Disease Clinic and Research Center in Spain to meet critical patient and family needs. Alzheimers Dement 10: , 409–415. |
[30] | Petersen RC , Morris JC ((2005) ) Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62: , 1160–1163. |
[31] | Lopez OL , Kuller LH , Becker JT , Dulberg C , Sweet RA , Gach HM , DeKosky ST ((2007) ) Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol 64: , 416–420. |
[32] | Espinosa A , Alegret M , Valero S , Vinyes-Junqué G , Hernández I , Mauleón A , Rosende-Roca M , Ruiz A , López O , Tárraga L , Boada M ((2013) ) A longitudinal follow-up of 550 mild cognitive impairment patients: Evidence for large conversion to dementia rates and detection of major risk factors involved. J Alzheimers Dis 34: , 769–780. |
[33] | Pérez-Martinez DA , Baztán JJ , González-Becerra M , Socorro A ((2005) ) Evaluation of the diagnostic value of a Spanish adaptation of the Buschke Memory Impairment Screen in the detection of dementia and cognitive impairment. Rev Neurol 40: , 644–648. |
[34] | Oldfield RC ((1971) ) The assessment and analysis of handedness: The Edinburg inventory. Neuropsychologia 9: , 97–113. |
[35] | Boada M , Cejudo JC , Tárraga L , López OL , Kaufer D ((2002) ) Neuropsychiatric inventory questionnaire (NPI-Q): Spanish validation of an abridged form of the Neuropsychiatric Inventory (NPI). Neurologia 17: , 317–323. |
[36] | Fahn S , Elton R ; UPDRS Development Committee ((1987) ) Unified Parkinson’s Disease Rating Scale. In Recent developments in Parkinson’s disease, Vol. 2, Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Macmillan Health Care Information, Florham Park, NJ, pp. 153–163. |
[37] | Blessed G , Tomlinson BE , Roth M ((1968) ) The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 114: , 797–811. |
[38] | Buysse DJ , Reynolds CF , 3rd Monk TH , Berman SR , Kupfer DJ ((1989) ) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: , 193–213. |
[39] | Roman-Viñas B , Serra-Majem L , Hagströmer M , Ribas-Barba L , Sjöström M , Segura-Cardona R ((2010) ) International physical activity questionnaire: Reliability and validity in a Spanish population. Eur J Sport Sci 10: , 297–304. |
[40] | Del Ser T , González-Montalvo JI , Martínez-Espinosa S , Delgado-Villapalos C , Bermejo F ((1997) ) Estimation of premorbid intelligence in Spanish people with the word accentuation test and its application to the diagnosis of dementia. Brain Cogn 33: , 343–356. |
[41] | Peña-Casanova J , Gramunt-Fombuena N , Quiñones-Úbeda S , Sánchez-Benavides G , Aguilar M , Badenes D , Molinuevo JL , Robles A , Barquero MS , Payno M , Antúnez C , Martínez-Parra C , Frank-García A , Fernández M , Alfonso V , Sol JM , Blesa R ; NEURONORMA Study Team ((2009) ) Spanish multicenter normative studies (NEURONORMA project): Norms for the rey-osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch Clin Neuropsychol 24: , 371–393. |
[42] | Goodglass H , Kaplan E , Barresi B ((2001) ) The assessment of aphasia and related disorders (3rd ed.). Lippincott Williams & Wilkins, Philadelphia, PA. |
[43] | Alegret M , Espinosa A , Valero S , Vinyes-Junqué G , Ruiz A , Hernández I , Rosende-Roca M , Mauleón A , Becker JT , Tárraga L , Boada M ((2013) ) Cut-off scores of a brief neuropsychological battery (NBACE) for Spanish individual adults older than 44 years old. PLoS One 8: , e76436. |
[44] | Zigmond AS , Snaith RP ((1983) ) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: , 361–370. |
[45] | Alegret M , Muñoz N , Roberto N , Rentz DM , Valero S , Gil S , Marquié M , Hernández I , Riveros C , Sanabria A , Pérez-Cordón A , Espinosa A , Ortega G , Mauleón A , Abdelnour C , Rosende-Roca M , Papp KV , Orellana A , Benaque A , Tarraga L , Ruiz A , Boada M ((2020) ) A computerized version of the short form of the Face-Name Associative Memory Exam (FACEmemory®) for the early detection of Alzheimer’s disease. Alzheimers Res Ther 12: , 25. |
[46] | Grober E , Ocepek-Welikson K , Teresi J ((2009) ) The free and cued selective reminding test: Evidence of psychometric adequacy. Psychol Sci Q 51: , 266–282. |
[47] | Reitan RM , Wolfson D ((1985) ) The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Neuropsychology Press, Tucson, AZ. |
[48] | Stroop JR ((1935) ) Studies of interference in serial verbal reactions. J Exp Psychol 18: , 643–662. |
[49] | Artiola L , Hermosillo D , Heaton R , Pardee RE ((1999) ) Manual de Normas y Procedimientos Para la bateria Neuropsicológica En Espa&ol. M Press, Tucson, AZ. |
[50] | Kaplan E , Goodglass H , Weintraub S ((2001) ) Boston Naming Test. Lea and Febiger, Philadelphia, PA. |
[51] | Howard D , Patterson K ((1992) ) Pyramids and palm trees: A test of semantic access from pictures and words. Thames Valley Test, Windsor, UK. |
[52] | Benton AL , Varney NR , Hamsher KS ((1978) ) Visuospatial judgment. A clinical test. Arch Neurol 35: , 364–367. |
[53] | Warrington EK , James M ((1991) ) The visual object and space perception battery. Thames Valley Company, Bury St Edmunds. |
[54] | Ekman P , Friesen WV ((1976) ) Pictures of facial affect. Consulting Psychologists Press, Palo Alto, CA. |
[55] | Marin RS , Biedrzycki RC , Firinciogullari S ((1991) ) Reliability and validity of the apathy evaluation scale. Psychiatry Res 38: , 143–162. |
[56] | Zimmerman M , Chelminski I , McGlinchey JB , Posternak MA ((2008) ) A clinically useful depression outcome scale. Compr Psychiatry 49: , 131–140. |
[57] | French SL , Floyd M , Wilkins S , Osato S ((2012) ) The Fear of Alzheimer’s Disease Scale: A new measure designed to assess anticipatory dementia in older adults. Int J Geriatr Psychiatry 27: , 521–528. |
[58] | Hamilton M ((1959) ) The assessment of anxiety states by rating. Br J Med Psychol 32: , 50–55. |
[59] | Goldberg DP , Hillier VF ((1979) ) A scaled version of the General Health Questionnaire. Psychol Med 9: , 139–145. |
[60] | Bagby RM , Taylor GJ , Parker JDA ((1994) ) The twenty-item Toronto Alexithymia scale-II. Convergent, discriminant, and concurrent validity. J Psychosom Res 38: , 33–40. |
[61] | Gomá-i-Freixanet M S , Puntí VJ , Zuckerman M ((2004) ) Psychometric properties of the Zuckerman-Kuhlman Personality Questionnaire in a Spanish sample. Eur J Psychol Assess 20: , 134–146. |
[62] | Scheltens P , Pasquier F , Weerts JGE , Barkhof F , Leys D ((1997) ) Qualitative assessment of cerebral atrophy on MRI: Inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol 37: , 95–99. |
[63] | Scheltens P , Leys D , Barkhof F , Huglo D , Weinstein HC , Vermersch P , Kuiper M , Steinling M , Wolters EC , Valk J ((1992) ) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55: , 967–972. |
[64] | Koedam EL , Lehmann M , van der Flier WM , Scheltens P , Pijnenburg YA , Fox N , Barkhof F , Wattjes MP ((2011) ) Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol 21: , 2618–2625. |
[65] | Fazekas F , Chawluk JB , Alavi A , Hurtig HI , Zimmerman RA ((1987) ) MR signal abnormalities at 1.5T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149: , 351–356. |
[66] | Vanderstichele H , Bibl M , Engelborghs S , Le Bastard N , Lewczuk P , Molinuevo JL , Parnetti L , Perret-Liaudet , Shaw LM , Teunissen C , Wouters D , Blennow K ((2012) ) Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 8: , 65–73. |
[67] | Leitao MJ , Silva-Spínola A , Santana I , Olmedo V , Nadal A , Le Bastard N , Baldeiras I ((2019) ) Clinical validation of the Lumipulse G cerebrospinal fluid assays for routine diagnosis of Alzheimer’s disease. Alzheimer Res Ther 11: , 91. |
[68] | Théry C , Amigorena S , Raposo G , Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3: Unit 3.22. |
[69] | Bokinni Y , Shah N , Maguire O , Laidlaw DA ((2015) ) Performance of a computerised visual acuity measurement device in subjects with age-related macular degeneration: Comparison with gold standard ETDRS chart measurements. Eye (Lond) 29: , 1085–1091. |
[70] | Pakrou N , Gray T , Mills R , Landers J , Craig J ((2008) ) Clinical Comparison of the Icare tonometer and Goldmann applanation tonometry. J Glaucoma 17: , 43–47. |
[71] | Rosenthal R , Rosnow RL ((1975) ) The Volunteer Subject, John Wiley, New York. |
[72] | Balasa M , Gelpi E , Antonell A , Rey MJ , Sánchez-Valle R , Molinuevo JL , Lladó A ; Neurological Tissue Bank/University of Barcelona/Hospital Clínic NTB/UB/HC Collaborative Group ((2011) ) Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology 76: , 1720–1725. |
[73] | Rodríguez-Gómez O , Sanabria A , Pérez-Cordón A , Sánchez-Ruiz D , Abdelnour C , Valero S , Hernandez I , Rosende-Roca M , Mauleon A , Vargas L , Alegret M , Espinosa A , Ortega G , Guitart M , Gailhajanet A , Sotolongo-Grau O , Moreno-Grau S , Ruiz S , Tarragona M , Serra J , Martin E , Peleja E , Lomeña F , Campos F , Vivas A , Gomez-Chiari M , Tejero MA , Giménez J , Pesini P , Sarasa M , Martinez G , Ruiz A , Tarraga L , Boada M ((2017) ) FACEHBI: A prospective study of risk factors, biomarkers and cognition in a cohort of individuals with subjective cognitive decline. Study rationale and research protocols. J Prev Alzheimers Dis 4: , 100–108. |
[74] | Boada M , Santos-Santos MA , Rodríguez-Gómez O , Alegret M , Cañabate P , Lafuente A , Abdelnour C , Buendía M , de Dios MJ , Morera A , Sanabria Á , Campo L , Ruiz A , Tárraga L ((2018) ) Patient engagement: The Fundació ACE framework for improving recruitment and retention in Alzheimer’s disease research. J Alzheimers Dis 62: , 1079–1090. |
[75] | Abdelnour C , Rodríguez-Gómez O , Alegret M , Valero S , Moreno-Grau S , Sanabria A , Hernández I , Rosende-Roca M , Vargas L , Mauleon A , Sánchez D , Espinosa A , Ortega G , Pérez-Cordón A , Diego S , Gailhajanet A , Guitart M , Sotolongo-Grau O , Ruiz A , Tárraga L , Boada M ((2017) ) Impact of recruitment methods in subjective cognitive decline. J Alzheimers Dis 57: , 625–632. |
[76] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34: , 939–944. |
[77] | Palasí A , Gutiérrez-Iglesias B , Alegret M , Pujadas F , Olabarrieta M , Liébana D , Quintana M , Álvarez-Sabin J , Boada M ((2015) ) Differentiated clinical presentation of early and late-onset Alzheimer’s disease: Is 65 years of age providing a reliable threshold? J Neurol 262: , 1238–1246. |
[78] | Binetti G , Magni E , Padovani A , Cappa SF , Bianchetti A , Trabucchi M ((1993) ) Neuropsychological heterogeneity in mild Alzheimer’s disease. Dementia 4: , 321–326. |
[79] | Imamura T , Takatsuki Y , Fujimori M , Hirono N , Ikejiri Y , Shimomura T , Hashimoto M , Yamashita H , Mori E ((1998) ) Age at onset and language disturbances in Alzheimer’s disease. Neuropsychologia 36: , 945–949. |
[80] | Joubert S , Gour N , Guedj E , Didic M , Guériot C , Koric L , Ranjeva JP , Felician O , Guye M , Ceccaldi M ((2016) ) Early-onset and late-onset Alzheimer’s disease are associated with distinct patterns of memory impairment. Cortex 74: , 217–232. |
[81] | Bennett DA , Wilson RS , Schneider JA , Evans DA , Mendes de Leon CF , Arnold SE , Barnes L , Bienias JL ((2003) ) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60: , 1909–1915. |
[82] | Woolley JD , Khan BK , Murthy NK , Miller BL , Rankin KP ((2011) ) The diagnostic challenge of psychiatric symptoms in neurodegenerative disease. J Clin Psychiatry 72: , 126–133. |
[83] | Zhao QF , Tan L , Wang HF , Jiang T , Tan MS , Tan L , Xu W , Li J , Wang J , Lai TJ , Yu JT ((2016) ) The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord 190: , 264–271. |
[84] | Monastero R , Mangialasche F , Camarda C , Ercolani S , Camarda R ((2009) ) A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis 18: , 11–30. |
[85] | Gallagher D , Fischer CE , Iaboni A ((2017) ) Neuropsychiatric symptoms in mild cognitive impairment: An update on prevalence, mechanisms, and clinical significance. Can J Psychiatry 62: , 161–169. |
[86] | van der Flier WM , Pijnenburg YA , Fox NC , Scheltens P ((2011) ) Early-onset versus late-onset Alzheimer’s disease: The case of the missing APOE ɛ4 allele. Lancet Neurol 10: , 280–288. |
[87] | Ellis KA , Bush AI , Darby D , De Fazio D , Foster J , Hudson P , Lautenschlagher NT , Lenzo N , Martins RN , Maruff P , Masters C , Milner A , Pike K , Rowe C , Savage G , Szoeke C , Taddei K , Villemagne V , Woodward M , Ames D ; AIBL Research Group ((2009) ) The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr 21: , 672–687. |
[88] | Risacher SL , Shen L , West JD , Kim S , McDonald DC , Beckett LA , Harvey DJ , Jack CR Jr , Weiner MW , Saykin AJ , Alzheimer’s Disease Neuroimaging Initiative (ADNI) ((2010) ) Longitudinal MRI atrophy biomarkers: Relationship to conversion in ADNI cohort. Neurobiol Aging 31: , 1401–1418. |
[89] | Falgás N , Balasa M , Bargalló N , Borrego-Écija S , Ramos-Campoy O , Fernández-Villullas G , Bosch B , Olives J , Tort-Merino A , Antonell A , Castellví M , Allen IE , Sánchez-Valle R , Lladó A ((2020) ) Diagnostic accuracy of MRI visual rating scales in the diagnosis of early onset cognitive impairment. J Alzheimers Dis 73: , 1575–1583. |
[90] | Molinuevo JL , Ripolles P , Simó M , Lladó A , Olives J , Balasa M , Antonell A , Rodríguez-Fornells A , Rami L ((2014) ) White matter changes in preclinical Alzheimer’s disease: A magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid β protein 42 levels. Neurobiol Aging 35: , 2671–2680. |
[91] | London A , Benhar I , Schwartz M ((2013) ) The retina as a window to the brain - from eye research to CNS disorders. Nat Rev Neurol 9: , 44–53. |
[92] | Bellingham SA , Guo BB , Coleman BM , Hill AF ((2012) ) Exosomes: Vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 3: , 124. |
[93] | Saman S , Kim WH , Raya M , Visnick Y , Miro S , Saman S , Jackson B , McKee AC , Alvarez VE , Lee NCY , Hall GF ((2012) ) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287: , 3842–3849. |
[94] | Jessen F , Amariglio RE , Van Boxtel M , Breteler M , Ceccaldi M , Chételat G , Dubois B , Dufouil C , Ellis KA , van der Flier WM , Glodzik L , van Harten AC , de Leon MJ , McHugh P , Mielke MM , Molinuevo JL , Mosconi L , Osorio RS , Perrotin A , Petersen RC , Rabin LA , Rami L , Reisberg B , Rentz DM , Sachdev PS , de la Sayette V , Saykin AJ , Scheltens P , Shulman MB , Slavin MJ , Sperling RA , Stewart R , Uspenskaya O , Vellas B , Visser PJ , Wagner M ; Subjective Cognitive Decline Inititaive (SCD-I) Working Group ((2014) ) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10: , 844–852. |
[95] | Whelan CD , Mattsson N , Nagle MW , Vijayaraghavan S , Hyde C , Janelidze S , Stomrud E , Lee J , Fitz L , Samad TA , Ramaswamy G , Margolin RA , Malarstig A , Hansson O ((2019) ) Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer’s disease. Acta Neuropathol Commun 7: , 169. |
[96] | Webers A , Heneka MT , Gleeson PA ((2020) ) The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol Cell Biol 98: , 28–41. |
[97] | Counil H , Krantic S ((2020) ) Synaptic activity and (neuro)inflammation in Alzheimer’s disease: Could exosomes be an additional link? J Alzheimers Dis 74: , 1029–1043. |
[98] | Watson L , Hamlett ED , Stone TD , Sims-Robinson C ((2019) ) Neuronally derived extracellular vesicles: An emerging tool for understanding Alzheimer’s disease. Mol Neurodegener 14: , 22. |
[99] | Altuna-Azkargorta M , Mendioroz-Iriarte M (2018) Biomarcadores sanguíneos en la enfermedad de Alzheimer. Neurología, doi:10.1016/j.nrl.2018.03.006. |
[100] | Budson A , Solomon P ((2016) ) Memory Loss, Alzheimer’s disease and Dementia: A practical guide for clinicians. Elsevier, Inc. |
[101] | Martin RC , Gerstenecker A , Triebel KL , Falola M , McPherson T , Cutter G , Marson DC ((2018) ) Declining financial capacity in mild cognitive impairment: A six-year longitudinal study. Arch Clin Neuropsychol 34: , 152–161. |




