Interaction of Alzheimer’s Disease-Associated Genetic Risk with Indicators of Socioeconomic Position on Mild Cognitive Impairment in the Heinz Nixdorf Recall Study
Abstract
Background:
The apolipoprotein E (APOE) ɛ4 allele is reported to be a strong genetic risk factor for mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Additional genetic loci have been detected that influence the risk for late-onset AD. As socioeconomic position (SEP) is also strongly related to cognitive decline, SEP has been suggested to be a possible modifier of the genetic effect on MCI.
Objective:
To investigate whether APOE ɛ4 and a genetic sum score of AD-associated risk alleles (GRSAD) interact with SEP indicators to affect MCI in a population-based cohort.
Methods:
Using data of 3,834 participants of the Heinz Nixdorf Recall Study, APOE ɛ4 and GRSAD by SEP interactions were assessed using logistic regression models, as well as SEP-stratified genetic association analysis. Interaction on additive scale was calculated using the relative excess risk due to interaction (RERI). All analysis were additionally stratified by sex.
Results:
Indication for interaction on the additive scale was found between APOE ɛ4 and low education on MCI (RERI: 0.52 [95% confidence interval (CI): 0.01; 1.03]). The strongest genetic effects of the APOE ɛ4 genotype on MCI were observed in groups of low education (Odds ratio (OR): 1.46 [95% CI: 0.79; 2.63] for≤10 years of education versus OR: 1.00 [95% CI: 0.43; 2.14] for≥18 years of education). Sex stratified results showed stronger effects in women. No indication for interaction between the GRSAD and SEP indicators on MCI was observed.
Conclusion:
Results indicate that low education may have an impact on APOE ɛ4 expression on MCI, especially among women.
INTRODUCTION
Alzheimer’s disease (AD) is a common age-related neurodegenerative disease. Mild cognitive impairment (MCI) represents an intermediate and potentially modifiable stage between normal cognition and dementia, including AD [1–3]. MCI involves minor problems of cognition, such as memory loss, problems with language, reasoning, attention, and visual depth perception [4]. As people with MCI can remain stable for many years or even return to a normal cognitive state over time, MCI is a promising target in the prevention of dementia [5, 6]. Studies have identified the apolipoprotein E (APOE) ɛ4 allele not only to be a strong genetic risk factor for AD [7–11], but also for MCI [1–3, 12–14]. Studies have further indicated that the effect of APOE ɛ4 on developing MCI is sex-dependent, with women being at greater risk than men [15, 16]. However, not all APOE ɛ4 carriers develop dementia, and about one-half of AD is not APOE ɛ4 associated [17], meaning that further genetic or non-genetic factors potentially may modify the expression of APOE ɛ4 risk. In accordance with the involvement of further genetic factors, genome-wide association studies (GWAS) have detected several genetic loci in addition to APOE that influence the risk for late-onset AD, together accounting for ∼31% of its genetic variance (25.2% of this variance was reported for APOE ɛ3 and ɛ4 alleles) [18]. In a population-based study, AD-related genetic variants detected by GWAS were also found to be associated with MCI status, even after excluding APOE ɛ4 from the score [19].
Research has shown that indicators of socioeconomic position (SEP), such as education and occupational status, are also strongly related to cognitive decline, following a social gradient [2, 20]. Compared to people from high socioeconomic groups, people with a lower SEP are more likely to be exposed to unfavorable health behaviors such as low physical activity and poor diet, which are linked to poor mental health, cognitive impairment, and dementia [21]. Studies suggested that interactions between the APOE ɛ4 genotype with such environmental factors (GxE) might modify the effect of APOE ɛ4 on cognitive impairment or dementia [7, 20, 22–25]. However, empirical studies examining gene-by-SEP interactions (GxSEP) on MCI are limited and the impact of sex on the genetic risk of MCI has not been sufficiently examined. The aim of this study therefore was to investigate whether APOE ɛ4 and a genetic sum score of AD-associated risk alleles (GRSAD) detected by GWAS interact with educational attainment and monthly household income as indicators of socioeconomic position (SEP) to affect MCI in a population-based cohort including sex-stratified analysis.
METHODS
Study population
Data of the baseline and the second examination of the prospective population-based Heinz Nixdorf Recall Study (Risk Factors, Evaluation of Coronary Calcium, and Lifestyle) were used. The rationale and the design of the study have been previously described [26]. Briefly, between 2000 and 2003 individuals were randomly selected from mandatory registries of the cities Bochum, Essen, and Mülheim/Ruhr. 4,814 study participants aged 45–75 years were enrolled for the baseline examination in 2001–2003 [27]. The study population derives from a population-based sample of participants without dementia, meaning that the rate of comorbidities represents the rate in the general population (e.g., mean BMI of 27.8 (standard deviation: 4.6), 472 (12.3%) participants had diabetes mellitus and 208 (5.4%) coronary heart disease at study baseline). After five years, 4,157 participants joined the second examination (n = 430 were lost to follow-up and n = 227 were deceased). The study was approved by the ethics committee of the University Hospital Essen and included extended quality management procedures and certification according to DIN ISO 9001 : 2000. Written informed consent was obtained from all participants.
Mild cognitive impairment
Standardized cognitive assessment procedures were introduced at the second examination. A detailed description of the assessment procedures has been reported earlier [28, 29]. Briefly, cognitive performance was conceptualized as a multidimensional test, using established measures of immediate and delayed verbal memory (eight word list, performance measured as number of words recalled in each trial), verbal fluency (semantic category “animals”, number of recalled words within one minute), speed of processing (labyrinth test, time in seconds needed to complete the task), and visuospatial ability (clock-drawing test, performance was rated from 1 (perfect clock) to 6 (poor performance)) [30–32]. The raw data for each subtest were z-transformed (mean = 0, standard deviation (SD)±1) according to three age groups (50–59 years, 60–69 years, and 70–80 years) and within every age group according to the following education groups (≤10 years, 11–13 years,≥14 years). Based on the age and education adjusted z scores, the performance was rated as impaired (> 1 SD below the mean). MCI was defined according to the then current International Working Group on MCI criteria [2]. In short, the MCI diagnosis required a cognitive complaint and therefore participants were asked if their cognitive performance had declined during the past two years. Furthermore, the diagnosis required a cognitive impairment that is insufficient to fulfill criteria for dementia (DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [33]) and reflects generally intact activities of daily living. A cognitive subtest was rated as impaired if the performance was more than 1 SD below the age and education-adjusted mean or a score of≥3 in the clock drawing test. Participants without MCI or dementia were categorized as no-MCI.
Socioeconomic position
Educational attainment and household income were used as indicators for SEP and assessed using a standardized computer-assisted face-to-face interview at study baseline. Education was defined by combining school and vocational training as total years of formal education according to the International Standard Classification of Education [34]. Duration of education was categorized into four groups as≤10 years, 11–13 years, 14–17 years, and≥18 years of education. The lowest educational group is equivalent to a minimum compulsory school attendance and no additional vocational degree, and the highest educational group is comparable to a vocational training including additional qualification or a university degree. Income was measured as the monthly household equivalent income (in Euros) calculated by weighting the participants’ total household net income by a factor for each household member [35]. Income was divided into four groups, using sex-specific quartiles.
For SEP stratified analyses, SEP variables categorized in the four groups were used. For all other analyses, income was included as a dichotomized variable with groups of low and high income using the sex-specific median as cut off and education was dichotomized in low education with < 14 years and high education with≥14 years. Education and income were analyzed separately to account for their different mechanisms in causing health inequalities [36, 37].
Genetic data
Lymphocyte DNA was isolated from EDTA anti-coagulated venous blood using the Chemagic Magnetic Separation Module I (Chemagen, Baesweiler, Germany). In order to discriminate between APOE alleles ɛ2, ɛ3, and ɛ4, the two single-nucleotide polymorphisms (SNPs) rs429358 and rs7412 were used, genotyped on the MetaboChip Illumina BeadArray. Study participants with at least one allele ɛ4 (2/4, 3/4, 4/4) were defined as APOE ɛ4 status positive, all others as APOE ɛ4 status negative.
Using the meta-analyses of genome-wide association studies by [38], 22 SNPs (Supplementary Table 1) representing independent genetic loci robustly associated with late-onset AD were identified on the global screening array 24v1.0 available for all study participants included in the analysis. Study participants were all of European ancestry. Neither SNPs rs62039712 at WWOX and rs3752246 at ABCA7 nor suitable proxy SNPs were found within genetic data of the study population and had to be excluded from analysis. An unweighted genetic risk score (GRSAD) was calculated by aggregating the total number of disease risk alleles for each individual across the selected 22 SNPs not including the APOE locus. In addition, a weighted GRSAD was calculated by summing up and weighting each of the risk alleles, by their previously published effect sizes [38]. The weighted GRS was then rescaled to reflect the number of risk alleles. As the main results using the weighted score did not differ to those resulting from the unweighted GRSAD, only the latter were reported.
Quality control was applied separately to each of the 22 SNPs prior to analysis and first performed on subject level including sex-, ethnicity-, and relatedness-checks, using Plink (v. 1.07). No deviation from Hardy-Weinberg equilibrium (HWE) (p≤0.001) was detected, and none of the SNPs had to be excluded with a missing genotype frequency > 5%. One individual was removed due to a low genotyping rate of > 5% missing genotypes.
Statistical analyses
Overall, 3,834 non-demented participants with complete information on the GRS, APOE ɛ4 status, and MCI were included in the analysis (Supplementary Figure 1). Participants with a physician’s diagnosis of dementia or AD, with intake of cholinesterase inhibitors (ATC, anatomic-therapeutic-chemical classification issued by the World Health Organization, code: N06DA) or other antidementia drugs (N06DX), or fulfilling the DSM-IV dementia diagnosis, were excluded from the analyses population (n = 22). Some participants had missing information on education (n = 5) or income (n = 225) and therefore populations differed in respective analyses.
Separate logistic regression models were fitted to estimate sex- and age-adjusted odds ratios (OR) and their corresponding 95% confidence intervals (95% CI) for the association of education, income, APOE ɛ4 status (carrier versus noncarrier), and the GRSAD with MCI (versus non-MCI). To assess interaction of APOE ɛ4 genotype/GRSAD by indicators of SEP on MCI and to give sufficient information on the magnitude of interaction, analyses were based on recommendations by Knol and van der Weele [39]. First, the genetic effects of APOE ɛ4 genotype/GRSAD on MCI were stratified by educational groups and income quartiles to see whether the genetic effect differs between SEP groups. Second, single reference joint effects of APOE ɛ4 genotype/GRSAD tertiles and SEP groups on MCI were calculated in separate logistic regression models for income quartiles and education categories, with the group of a negative APOE ɛ4 status/low GRSAD and a high socioeconomic position as reference category to check whether the genetic effect on MCI was modified by SEP. To assess APOExSEP and GRSADxSEP interaction on the multiplicative scale, APOE ɛ4 status, and GRSAD main effects and the corresponding interaction terms were included into age- and sex-adjusted regression models. To consider interaction on the additive scale, the relative excess risk due to interaction (RERI) was calculated. For this, the GRSAD was dichotomized using a median split. For interaction analyses, the SEP-variables income and education were dichotomized. Interaction analysis was also repeated for each AD-associated SNP and SEP indicator. Analyses were additionally stratified by sex.
Sensitivity analysis was performed using a more rigorously defined non-MCI group by including only those participants who did not report a cognitive complaint and did not show any impairment in the applied cognitive tests (n = 1380). In addition, sensitivity analysis including only participants with amnestic MCI (i.e., participants presenting with an objective impairment in memory (immediate and/or delayed verbal memory subtest) with or without impairment in any other cognitive tests applied; n = 293) was also performed to examine the robustness of study results.
The statistical computing software R v3.6.0 [40] was used for all analyses. For the calculation of the genetic risk score and for single SNP analyses Plink v1.07 software package for Windows was used [41].
RESULTS
In the study population, 560 participants were defined as having MCI (prevalence: 14.6%) (Table 1). Women had lower formal education and a lower median income than men. One fourth of the study population was positive for the APOE ɛ4 genotype. The number of genetic risk alleles ranged from 13 to 34 risk alleles with a mean of 24.1±2.9 in the whole study population. The mean number of AD associated risk alleles showed no differences between men (24.1±2.8) and women (24.2±2.8).
Table 1
Characteristics of study population stratified by sex
| All | Men | Women | |
| (n = 3,834) | (n = 1,905) | (n = 1,929) | |
| Age (y) [0] mean±SD | 59.1±7.6 | 59.2±7.6 | 59.1±7.7 |
| MCI [0] n (%) | 560 (14.6%) | 275 (14.4%) | 285 (14.8%) |
| Amnestic MCI n (%) | 293 (7.6%) | 161 (8.5%) | 132 (6.8%) |
| APOE ɛ4 positive [0] n (%) | 981 (25.6%) | 479 (25.1%) | 502 (26.0%) |
| AD risk alleles (GRSAD) [0] mean±SD | 24.1±2.8 | 24.1±2.8 | 24.2±2.8 |
| Income (€ /mo) [225] median (IQR) | 2,833 (2,167–3,750) | 3,167 (2,250–4,074) | 2,750 (2,027–3,667) |
| Education (y) [5] n (%) | |||
| ≤10 | 378 (9.9%) | 76 (4.0%) | 302 (15.7%) |
| 11–13 | 2142 (55.9%) | 898 (47.2%) | 1244 (64.5%) |
| 14–17 | 871 (22.8%) | 653 (34.3%) | 218 (11.3%) |
| ≥18 | 438 (11.4%) | 274 (14.5%) | 164 (8.5%) |
[number of participants with missing values]; MCI, mild cognitive impairment; APOE, apolipoprotein E; AD, Alzheimer’s disease; SD, standard deviation; IQR, interquartile range (first quartile-third quartile).
A higher chance of having MCI was observed for participants with low income (OR: 1.38 [95% CI: 1.14; 1.68]) or < 14 years of formal education (OR: 1.37 [95% CI: 1.11, 1.69]) (Table 2). A positive APOE ɛ4 status was associated with a higher chance of MCI (OR: 1.27 [95% CI: 1.04; 1.55]), while no effect of the genetic risk score on MCI was observed. Sex-stratified analyses showed that low income had a greater effect on MCI in men (OR: 1.52 [95% CI: 1.15; 2.01]), while low education (OR: 1.58 [95% CI: 1.10; 2.33]) and a positive APOE ɛ4 status (OR: 1.40 [95% CI: 1.06; 1.84]) had a greater effect on MCI in women (Supplementary Table 2). The GRSAD showed no effect in both sexes.
Table 2
Sex- and age- adjusted odds ratios (OR) and corresponding 95% confidence intervals (95% CI) for the association of income, education, APOE ɛ4 genotype, and the Alzheimer’s disease-associated genetic risk score (GRSAD) with mild cognitive impairment calculated using separate logistic regression models
| n | OR (95% CI) | p | |
| Intercept | 3,609 | 0.02 (0.01; 0.04) | < 2*10–16 |
| Low income | 1.38 (1.14; 1.68) | 0.001 | |
| Sex | 1.04 (0.86; 1.25) | 0.68 | |
| Age | 1.03 (1.02; 1.05) | 4.0*10–8 | |
| Intercept | 3,829 | 0.02 (0.01; 0.04) | < 2*10–16 |
| Low education | 1.37 (1.11; 1.69) | 0.004 | |
| Sex | 0.94 (0.78; 1.14) | 0.52 | |
| Age | 1.04 (1.02; 1.05) | 7.4*10–9 | |
| Intercept | 3,834 | 0.01 (0.008; 0.03) | < 2*10–16 |
| APOE ɛ4 | 1.27 (1.04; 1.55) | 0.02 | |
| Sex | 1.03 (0.86; 1.23) | 0.79 | |
| Age | 1.04 (1.03; 1.05) | 7.1*10–10 | |
| Intercept | 3,834 | 0.02 (0.01; 0.05) | 1.5*10–13 |
| GRSAD | 0.99 (0.96; 1.03) | 0.94 | |
| Sex | 1.03 (0.86; 1.23) | 0.77 | |
| Age | 1.04 (1.03; 1.05) | 5.8*10–10 |
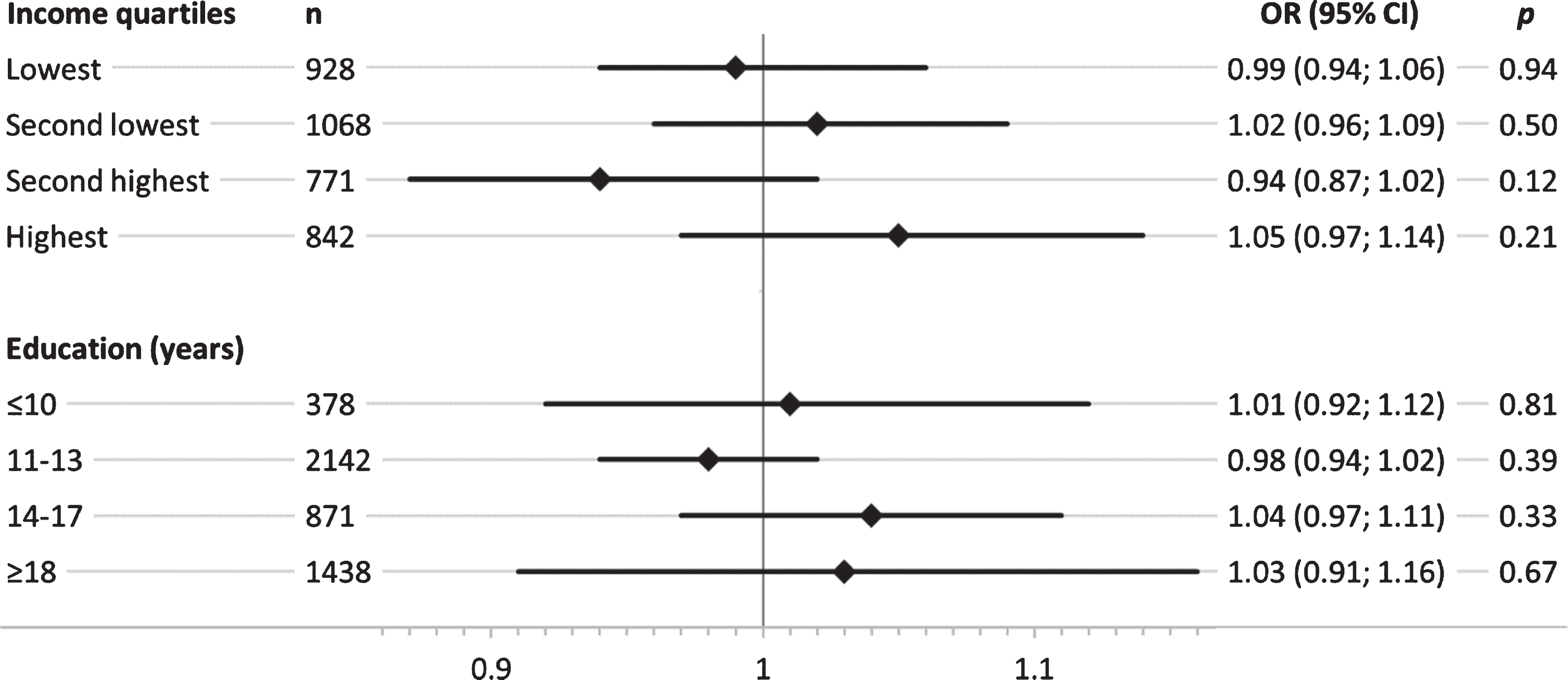
Overall, SEP-stratified analysis showed a stronger genetic effect of APOE ɛ4 in groups with low education (Fig. 2). This trend was not evident across income quartiles, where similar effect size estimates were observed in the lowest and the highest quartile. Results for the genetic effect on MCI across the two lower education groups revealed that participants with < 14 years of formal education showed a considerably higher genetic effect of APOE ɛ4 on MCI, while no effect was present in the two highest education groups ≥14 years (Fig. 1). Results of the SEP-stratified analysis for the GRSAD showed no trend of genetic effects on MCI across income quartiles or educational group (Fig. 2).
Fig. 1
Sex- and age-adjusted odds ratios (OR) and corresponding 95% confidence intervals (95% CI) for the association of APOE ɛ4 genotype with mild cognitive impairment, stratified by income quartiles and education groups (years) in separate logistic regression models.
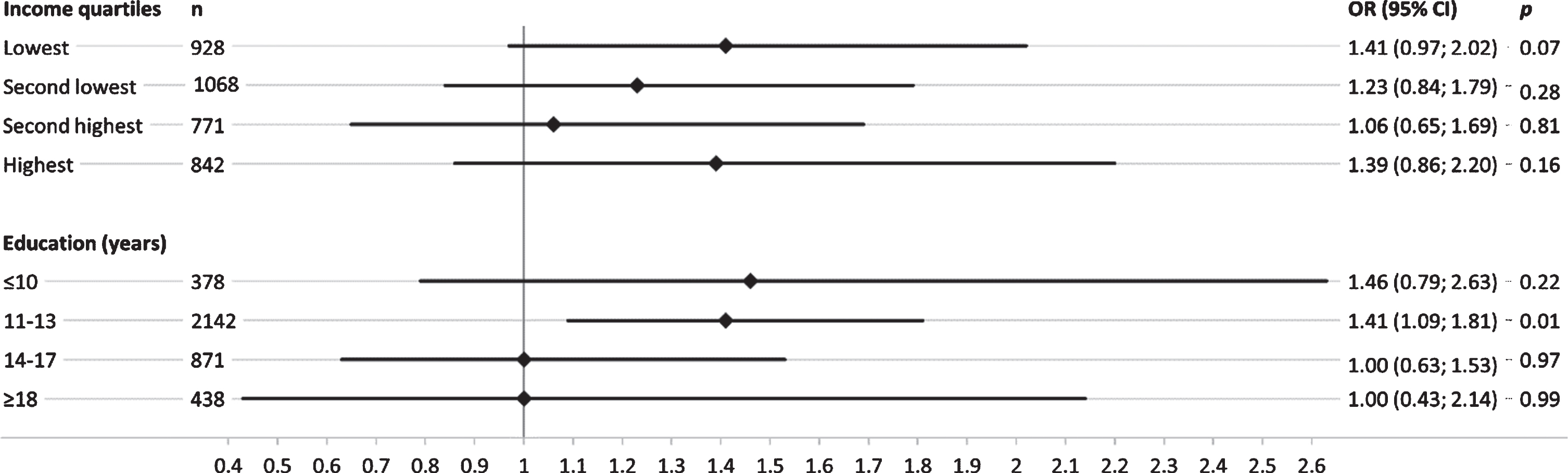
Fig. 2
Sex- and age-adjusted odds ratios (OR) and corresponding 95% confidence intervals (95% CI) for the association of the AD-associated genetic risk score (GRSAD) with mild cognitive impairment, stratified by income quartiles and education groups (years) in separate logistic regression models.
In the analysis of joint effects with a single reference group, ORs showed a trend within and between groups: with decreasing income and years of education and a positive APOE ɛ4 status compared to a negative APOE ɛ4 status, the joint effects were observed to be stronger. Compared to the reference group with the lowest SEP and a negative APOE ɛ4 status, the strongest joint effects were seen in groups with APOE ɛ4 genotype and lowest SEP (OR: 2.29 [95% CI: 1.53; 3.43] for income and OR: 2.29 [95% CI: 1.18; 4.33] for education) (Supplementary Figure 2). Less clear patterns were seen for the joint effects of SEP indicators and tertiles of the GRSAD on MCI (Supplementary Figure 3).
Some indication for positive interaction on the multiplicative scale was observed between APOE ɛ4 status and education (ORAPOE *Education_low: 1.42 [0.91; 2.24]). No indication for interaction on the multiplicative scale was found between APOE ɛ4 status and income, nor between the GRSAD with indicators of SEP (Table 3). Sex-stratified analyses revealed also some indication for APOE ɛ4 by education interaction in women (ORAPOE *Education_low: 2.17 [95% CI: 0.94; 5.48]), indicating that the observed APOE ɛ4 by education interaction was mainly present in women (Supplementary Table 3).
Table 3
Sex- and age- adjusted odds ratios (OR) and corresponding 95% confidence intervals (95% CI) in logistic regression models including main effects and interaction terms for the interaction of APOE ɛ4 status and AD-associated genetic risk score (GRSAD) with education and income on mild cognitive impairment
| n | OR (95% CI) | p | |
| Intercept | 3,609 | 0.02 (0.01; 0.04) | < 2.0*10–16 |
| Income low | 1.35 (1.08; 1.71) | 0.01 | |
| APOE ɛ4 | 1.21 (0.87; 1.68) | 0.25 | |
| Sex | 1.04 (0.86; 1.25) | 0.70 | |
| Age | 1.04 (1.02; 1.05) | 6.0*10–8 | |
| APOE ɛ4* | 1.09 (0.71; 1.67) | 0.70 | |
| Income low | |||
| Intercept | 3,829 | 0.02 (0.01; 0.04) | < 2.0*10–16 |
| Education low | 1.24 (0.98; 1.59) | 0.08 | |
| APOE ɛ4 | 1.00 (0.67; 1.45) | 0.99 | |
| Sex | 0.94 (0.77; 1.13) | 0.50 | |
| Age | 1.03 (1.02; 1.05) | 1.0*10–8 | |
| APOE ɛ4* | 1.42 (0.91; 2.24) | 0.13 | |
| Education low | |||
| Intercept | 3,609 | 0.02 (0.004; 0.09) | 4.3*10–7 |
| Income low | 0.97 (0.19; 5.05) | 0.97 | |
| GRSAD | 0.99 (0.94; 1.05) | 0.81 | |
| Sex | 1.04 (0.86; 1.25) | 0.68 | |
| Age | 1.04 (1.02; 1.05) | 4.1*10–8 | |
| GRSAD* | 1.01 (0.95; 1.09) | 0.68 | |
| Income low | |||
| Intercept | 3,829 | 0.01 (0.002; 0.04) | 5.8*10–9 |
| Education low | 3.86 (0.70; 21.46) | 0.12 | |
| GRSAD | 1.03 (0.97; 1.10) | 0.32 | |
| Sex | 0.94 (0.77; 1.14) | 0.54 | |
| Age | 1.04 (1.02; 1.05) | 8.2*10–9 | |
| GRSAD* | 0.96 (0.89; 1.03) | 0.23 | |
| Education low |
On the additive scale, an indication for a positive interaction between APOE ɛ4 status and education was observed (RERI: 0.52 [95% CI: 0.01; 1.03]) (Table 4). Within sex-stratified analyses, an indication for a positive interaction between APOE ɛ4 status and education was only present among women (RERI: 1.00 [95% CI: 0.26; 1.75]) (Supplementary Table 4).
Table 4
Age- and sex-adjusted relative excess risk due to interaction (RERI) and corresponding 95% confidence intervals (95% CI) as a measure of interaction between APOE ɛ4xSEP and the GRSADxSEP on the additive scale
| n | RERI (95% CI) | p | |
| APOE ɛ4*Income | 3,609 | 0.22 (–0.35; 0.79) | > 0.05 |
| APOE ɛ4*Education | 3,829 | 0.52 (0.01; 1.03) | 0.02 |
| GRSAD*Income | 3,609 | –0.19 (–0.73; 0.35) | > 0.05 |
| GRSAD*Education | 3,829 | –0.35 (–0.94; 0.24) | > 0.05 |
The single SNP analysis of each of the 22 AD-associated SNPs revealed that only ∼50% of the AD risk alleles reported by [38] showed directionally consistent effect size estimates for the association with MCI within the study population (Supplementary Table 1). Overall, no strong indication was found for SNP by SEP interaction, except an indication for additive interaction at locus SPI1 with education (Supplementary Tables 5 and 6). Interaction effect size estimates of the single SNP interaction analysis differed strongly for both SEP indicators in the range of ORs of 0.82 to 1.53 and RERIs of –0.30 to 0.62 for interaction with income and in the range of ORs of 0.73 to 1.31 and RERIs of –0.40 to 0.27 for interaction with education. Results of sensitivity analysis revealed no substantial differences to the main study results (Supplementary Tables 7–12). While there was no indication for an association of the GRSAD with MCI, the GRSAD effect size estimate was at least directionally consistent when using the amnestic MCI phenotype (OR: 1.02 [95% CI: 0.97; 1.06]) (Supplementary Table 10).
DISCUSSION
The aim of the present study was to investigate whether APOE ɛ4 and a genetic sum score of AD-associated risk alleles interact with educational attainment and household income to affect MCI in a population-based cohort. Study results gave indication for positive interaction on the additive scale between APOE ɛ4 and low education, as the combination of the genetic effect and low education was more than the sum of their marginal effects. This was supported by results of stratified genetic association analysis, in which the strongest effects of the APOE ɛ4 genotype on MCI were seen in groups of low education. Also, single reference joint effects of all possible combinations of the APOE ɛ4 status and SEP groups showed the strongest effect on MCI in participants with a positive APOE ɛ4 status and low SEP. Sex stratified results showed stronger interaction effects in women. No indication for interaction was observed for the genetic sum score of AD-associated risk alleles with indicators of SEP.
To our knowledge, this is the first study to examine possible interactions between APOE ɛ4, an AD-associated genetic risk allele score and indicators of SEP on MCI within a population-based cohort including also sex-stratified analyses. The main finding that APOE ɛ4 and education interact on the additive scale to affect MCI is in line with results of a study by Arenaza-Urquijo et al. (2015), who have reported an interaction between APOE ɛ4 and education on frontal and temporal metabolism in a French study sample [25]. Likewise, another study has found an interaction between education and APOE ɛ4 on dementia in pooled data of three major population-based studies from Northern Europe [22]. Cook and Fletscher (2015) have reported an interaction between APOE ɛ4 genotype and years of schooling and also found that a higher educational attainment is adequate to cancel out the increased risk of the APOE ɛ4 genotype on late-life cognitive decline, using data of the Wisconsin Longitudinal Study [42]. Similar results were observed in the study presented here, which showed that no effect of APOE ɛ4 on MCI was detected in groups with≥14 years of formal education.
Sex-stratified analyses revealed that women had a much higher risk of MCI when carrying the APOE ɛ4 genotype compared to men. An indication for the APOE ɛ4 by education interaction was observed to be substantially stronger in women on both, multiplicative and additive scales. The direction of effects in men was consistent to those in women. A large number of epidemiological studies has supported sex differences in the association between a positive APOE ɛ4 genotype and cognitive deficits, with women being at higher risk of AD or cognitive decline [43–46]. A previous study in a longitudinal sample of 11,654 subjects recruited through the National Alzheimer’s Coordinating Center in the United States has demonstrated that the risk of clinical conversion conferred by the APOE ɛ4 allele and the transformation from healthy aging to MCI/AD and from MCI to AD is much greater in women than in men [15]. The authors have hypothesized that tau pathology could be a possible explanation for the observed sex differences. Female APOE ɛ4 carriers were found to show increased cerebrospinal fluid tau concentrations and tau/Aβ ratios, compared with male carriers.
A study by Müller-Gerards et al. (2019) have observed an indication for a positive interaction between subjective cognitive decline and APOE ɛ4 genotype on incident MCI during 5 years of follow-up in women. The authors have mentioned the role of sex hormones, such as the loss of estrogen through menopause, as a possible explanation for the observed sex differences [47]. Several studies have found that estrogen use in elderly women has a favorable effect on cognition and prevention of dementia, independent of age, education, ethnicity, and APOE genotype [48–50], while other studies have not found an association [51]. Beyond that, another study has found an interaction between estrogen use and the presence of the APOE ɛ4 genotype, whereas current estrogen use reduced the risk of cognitive impairment compared with never users in APOE ɛ4 negative women and not in APOE ɛ4 positive women [52].
The present study did not find an association between a sum score of 22 AD-associated risk alleles on MCI, which contrasts results of previous studies that found associations between genetic sum scores of AD-associated risk alleles and MCI in the population-based Rotterdam Study [19, 53]. This may be in part explained by differences in the SNPs used for calculating the genetic sum scores, because compared to previous studies, the GRS used here was based on a more recent genome-wide meta-analysis including more individuals [38]. However, in the sensitive analysis including only cases of amnestic MCI, the GRSAD effect size estimate was at least directionally consistent. This seems plausible, as amnestic MCI usually shows a higher progression to AD compared to non-amnestic MCI.
The effect of the APOE ɛ4 genotype on non-amnesic MCIs that are not prodromal to AD may be explained by the effect of APOE on the lipid and lipoprotein metabolism which affects brain function in various ways [54]. The fact that no indication for interaction between the AD-associated risk alleles and SEP was observed in the present study is in line with a study using data of the UK Biobank where an increased dementia risk was reported within groups of high genetic risk for AD, while no interaction was reported with SEP-related lifestyle risk score including smoking, physical activity, diet, and alcohol consumption [55].
The present study adds further knowledge to the results of previous studies with the investigation of interactions between SEP and the APOE ɛ4 genotype and additional AD-associated variants on MCI in sex-stratified analyses. Strengths of the present study include a population-based study sample and the use of two different individual SEP indicators in the analyses. Further, evidence for interaction was not only based on testing APOExSEP and GRS-ADxSEP interaction terms in regression models, but also on stratified analyses, single references joint effects and calculating the RERI. Despite these strengths, the following limitations need to be acknowledged: The power of the study was not sufficient for detection of small interaction effect size estimates. Especially for robustly analyzing single SNP by SEP interactions, larger samples are required. Further, the neuropsychological tests used in this study were rather limited. Nevertheless, the cognitive assessment was validated in 656 participants against an MCI diagnosis that was based on a detailed neuropsychological examination conducted by a neuropsychologist and a neurological examination by a senior neurologist and found a good accuracy in identifying participants with MCI (Area under the curve (AUC) = 0.82 (0.78–0.85)) [28].
In conclusion, the results of the present study provide an indication of an interaction of APOE ɛ4 with education on MCI in a population-based study sample, showing stronger genetic effects in groups of low education, especially among women. This gives support to the hypothesis that SEP influences the expression of genetic risk related to MCI. Higher educated groups seem to be better equipped to reduce their genetic susceptibility for MCI. However, it has to be assumed that SEP does not directly affect MCI and SEP-related factors not considered in the present analysis may be responsible for the observed differences in the genetic risk for MCI. Literature suggests that cognitive leisure activities and diet as modifiable SEP-related risk factors play a role in the development of MCI [21, 56]. However, further studies are needed to investigate the risk factors involved.
ACKNOWLEDGMENTS
We are indebted to all study participants and to both the dedicated personnel of the study center of the Heinz Nixdorf Recall study and to the investigative group, in particular to U. Slomiany, E.M. Beck, A. Öffner, S. Münkel, R. Peter, and H. Hirche. Advisory Board: Meinertz T., Hamburg, Germany (Chair); Bode C., Freiburg, Germany; deFeyter P.J., Rotterdam, Netherlands; Güntert B, Halli, Austria; Gutzwiller F., Bern, Switzerland; Heinen H., Bonn, Germany; Hess O., Bern, Switzerland; Klein B., Essen, Germany; Löwel H., Neuherberg, Germany; Reiser M., Munich, Germany; Schmidt G., Essen, Germany; Schwaiger M., Munich, Germany; Steinmüller C., Bonn, Germany; Theorell T., Stockholm, Sweden; Willich S.N., Berlin, Germany.
The authors thank the Heinz Nixdorf Foundation [Chairman: Martin Nixdorf; Past Chairman: Dr jur. Gerhard Schmidt (†)], for their generous support of this study. Parts of the study were also supported by the German Research Council (DFG) [DFG project: EI 969/2-3, ER 155/6-1;6-2, HO 3314/2-1;2-2;2-3;4-3, INST 58219/32-1, JO 170/8-1, KN 885/3-1, PE 2309/2-1, SI 236/8-1;9-1;10-1,], the German Ministry of Education and Science [BMBF project: 01EG0401, 01GI0856, 01GI0860, 01GS0820_WB2-C, 01ER1001D, 01GI0205], the Ministry of Innovation, Science, Research and Technology, North Rhine-Westphalia (MIWFT-NRW), the Else Kröner-Fresenius-Stiftung [project: 2015_A119] and the German Social Accident Insurance [DGUV project: FF-FP295]. Furthermore the study was supported by the Competence Network for HIV/AIDS, the deanship of the University Hospital and IFORES of the University Duisburg-Essen, the European Union, the German Competence Network Heart Failure, Kulturstiftung Essen, the Protein Research Unit within Europe (PURE), the Dr. Werner-Jackstädt Stiftung and the following companies: Celgene GmbH München, Imatron/GE-Imatron, Janssen, Merck KG, Philips, ResMed Foundation, Roche Diagnostics, Sarstedt AG&Co, Siemens HealthCare Diagnostics, Volkswagen Foundation.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0244r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-210244.
REFERENCES
[1] | Boyle PA , Buchman AS , Wilson RS , Kelly JF , Bennett DA ((2010) ) The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology 34: , 43–49. |
[2] | Winblad B , Palmer K , Kivipelto M , Jelic V , Fratiglioni L , Wahlund L-O , Nordberg A , Bäckman L , Albert M , Almkvist O , Arai H , Basun H , Blennow K , Leon M de , DeCarli C , Erkinjuntti T , Giacobini E , Graff C , Hardy J , Jack C , Jorm A , Ritchie K , van Duijn C , Visser P , Petersen RC ((2004) ) Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256: , 240–246. |
[3] | Kryscio RJ , Schmitt FA , Salazar JC , Mendiondo MS , Markesbery WR ((2006) ) Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology 66: , 828–832. |
[4] | Alzheimer’s Society ((2019) ) What is mild cognitive impairment (MCI)? Factsheet 470LP, https://www.alzheimers.org.uk/sites/default/files/2019-09/470lp-what-is-mild-cognitive-impairment-mci-190521.pdf. |
[5] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[6] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: , 183–194. |
[7] | Farrer LA , Cupples LA , Haines JL , Hyman B , Kukull WA , Mayeux R , Myers RH , Pericak-Vance MA , Risch N , van Duijn CM ((1997) ) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278: , 1349–1356. |
[8] | Corder EH , Saunders AM , Strittmatter WJ , Schmechel DE , Gaskell PC , Small GW , Roses AD , Haines JL , Pericak-Vance MA ((1993) ) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261: , 921–923. |
[9] | Saunders AM , Strittmatter WJ , Schmechel D , George-Hyslop PH , Pericak-Vance MA , Joo SH , Rosi BL , Gusella JF , Crapper-MacLachlan DR , Alberts MJ ((1993) ) Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43: , 1467–1472. |
[10] | Ashford JW ((2004) ) APOE genotype effects on Alzheimer’s disease onset and epidemiology. JMN 23: , 157–166. |
[11] | Bennett DA , Wilson RS , Schneider JA , Evans DA , Aggarwal NT , Arnold SE , Cochran EJ , Berry-Kravis E , Bienias JL ((2003) ) Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology 60: , 246–252. |
[12] | Lopez OL , Jagust WJ , Dulberg C , Becker JT , DeKosky ST , Fitzpatrick A , Breitner J , Lyketsos C , Jones B , Kawas C , Carlson M , Kuller LH ((2003) ) Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 2. Arch Neurol 60: , 1394–1399. |
[13] | Alzheimer’s Association ((2019) ) Alzheimer’s disease facts and figures. Alzheimers Dement 15: , 321–387. |
[14] | Tervo S , Kivipelto M , Hänninen T , Vanhanen M , Hallikainen M , Mannermaa A , Soininen H ((2004) ) Incidence and risk factors for mild cognitive impairment: A population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord 17: , 196–203. |
[15] | Altmann A , Tian L , Henderson VW , Greicius MD ((2014) ) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 75: , 563–573. |
[16] | Ungar L , Altmann A , Greicius MD ((2014) ) Apolipoprotein E, gender, and Alzheimer’s disease: An overlooked, but potent and promising interaction. Brain Imaging Behav 8: , 262–273. |
[17] | Myers RH , Schaefer EJ , Wilson PW , D’Agostino R , Ordovas JM , Espino A , Au R , White RF , Knoefel JE , Cobb JL , McNulty KA , Beiser A , Wolf PA ((1996) ) Apolipoprotein E epsilon4 association with dementia in a population-based study: The Framingham study. Neurology 46: , 673–677. |
[18] | Ridge PG , Hoyt KB , Boehme K , Mukherjee S , Crane PK , Haines JL , Mayeux R , Farrer LA , Pericak-Vance MA , Schellenberg GD , Kauwe JSK ((2016) ) Assessment of the genetic variance of late-onset Alzheimer’s disease. Neurobiol Aging 41: , 200.e13–200.e20. |
[19] | Adams HHH , Bruijn RFAG de , Hofman A , Uitterlinden AG , van Duijn CM , Vernooij MW , Koudstaal PJ , Ikram MA ((2015) ) Genetic risk of neurodegenerative diseases is associated with mild cognitive impairment and conversion to dementia. Alzheimers Dement 11: , 1277–1285. |
[20] | Sattler C , Toro P , Schönknecht P , Schröder J ((2012) ) Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res 196: , 90–95. |
[21] | UCL Institute of Health Equity ((2016) ) Inequalities in Mental Health, Cognitive Impairment and Dementia Among Older People, http://www.instituteofhealthequity.org/resources-reports/inequalities-in-mental-health-cognitive-impairment-and-dementia-among-older-people/inequalities-in-mental-health-cognitive-impairement-and-dementia-among-older-people.pdf. |
[22] | Wang H-X , Gustafson DR , Kivipelto M , Pedersen NL , Skoog I , Windblad B , Fratiglioni L ((2012) ) Education halves the risk of dementia due to apolipoprotein ɛ4 allele: A collaborative study from the Swedish brain power initiative. Neurobiol Aging 33: , 1007.e1–7. |
[23] | Ferrari C , Xu W-L , Wang H-X , Winblad B , Sorbi S , Qiu C , Fratiglioni L ((2013) ) How can elderly apolipoprotein E ɛ4 carriers remain free from dementia? Neurobiol Aging 34: , 13–21. |
[24] | Meng X , D’Arcy C ((2013) ) Apolipoprotein E gene, environmental risk factors, and their interactions in dementia among seniors. Int J Geriatr Psychiatry 28: , 1005–1014. |
[25] | Arenaza-Urquijo EM , Gonneaud J , Fouquet M , Perrotin A , Mézenge F , Landeau B , Egret S , La Sayette V de , Desgranges B , Chételat G ((2015) ) Interaction between years of education and APOE ɛ4 status on frontal and temporal metabolism. Neurology 85: , 1392–1399. |
[26] | Schmermund A , Möhlenkamp S , Stang A , Grönemeyer D , Seibel R , Hirche H , Mann K , Siffert W , Lauterbach K , Siegrist J , Jöckel K-H , Erbel R ((2002) ) Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: Rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J 144: , 212–218. |
[27] | Stang A , Moebus S , Dragano N , Beck EM , Möhlenkamp S , Schmermund A , Siegrist J , Erbel R , Jöckel KH ((2005) ) Baseline recruitment and analyses of nonresponse of the Heinz Nixdorf Recall Study: Identifiability of phone numbers as the major determinant of response. Eur J Epidemiol 20: , 489–496. |
[28] | Wege N , Dlugaj M , Siegrist J , Dragano N , Erbel R , Jöckel K-H , Moebus S , Weimar C ((2011) ) Population-based distribution and psychometric properties of a short cognitive performance measure in the population-based Heinz Nixdorf Recall Study. Neuroepidemiology 37: , 13–20. |
[29] | Dlugaj M , Weimar C , Wege N , Verde PE , Gerwig M , Dragano N , Moebus S , Jöckel K-H , Erbel R , Siegrist J ((2010) ) Prevalence of mild cognitive impairment and its subtypes in the Heinz Nixdorf Recall study cohort. Dement Geriatr Cogn Disord 30: , 362–373. |
[30] | Fleischmann OW ((1994) ) Nürnberger Alters-Inventar (NAI), Hogrefe Verlag für Psychologie, Göttingen. |
[31] | Aschenbrenner S , Tucha O , Lange K ((2000) ) Regensburger Wortflüssigkeitstest (RWT), Hogrefe Verlag für Psychologie, Göttingen. |
[32] | Shulman KI ((2000) ) Clock-drawing: Is it the ideal cognitive screening test? Int J Geriatr Psychiatry 15: , 548–561. |
[33] | American Psychiatric Association ((1994) ) Diagnostic criteria from DSM-IV. American Psychiatric Association, Washington, DC. |
[34] | UNESCO ((2006) ) International standard classification of education ISCED 1997, English edition. - Re-edition, UNESCO-UIS, Montreal. |
[35] | Hagenaars A , Vos K de , Zaidi MA ((1994) ) Poverty statistics in the late 1980s: Research based on micro-data, Eurostat, Office for Official Publications of the European Communities, Luxembourg. |
[36] | Galobardes B , Shaw M , Lawlor DA , Lynch JW , Davey Smith G ((2006) ) Indicators of socioeconomic position (part 1). J Epidemiol Community Health 60: , 7–12. |
[37] | Geyer S , Hemström O , Peter R , Vågerö D ((2006) ) Education, income, and occupational class cannot be used interchangeably in social epidemiology. Empirical evidence against a common practice. J Epidemiol Community Health 60: , 804–810. |
[38] | Kunkle BW , Grenier-Boley B , Sims R , Bis JC , Damotte V , Naj AC , Boland A , Vronskaya M , van der Lee SJ , Amlie-Wolf A , et al. ((2019) ) Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 51: , 414–430. |
[39] | Knol MJ , VanderWeele TJ ((2012) ) Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 41: , 514–520. |
[40] | The R Core Team (1999-2012) R: A Language and Environment for Statistical Computing. Reference Index, https://cran.r-project.org/doc/manuals/fullrefman.pdf. |
[41] | Purcell S , Neale B , Todd-Brown K , Thomas L , Ferreira MAR , Bender D , Maller J , Sklar P , Bakker PIW de , Daly MJ , Sham PC ((2007) ) PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: , 559–575. |
[42] | Cook CJ , Fletcher JM ((2015) ) Can education rescue genetic liability for cognitive decline? Soc Sci Med 127: , 159–170. |
[43] | Molero AE , Pino-Ramírez G , Maestre GE ((2001) ) Modulation by age and gender of risk for Alzheimer’s disease and vascular dementia associated with the apolipoprotein E-ɛ4 allele in Latin Americans: Findings from the Maracaibo Aging Study. Neurosci Lett 307: , 5–8. |
[44] | Payami H , Grimslid H , Oken B , Camicioli R , Sexton G , Dame A , Howieson D , Kaye J ((1997) ) A prospective study of cognitive health in the elderly (Oregon Brain Aging Study): Effects of family history and apolipoprotein E genotype. Am J Hum Genet 60: , 948–956. |
[45] | Mortensen EL , Høgh P ((2001) ) A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology 57: , 89–95. |
[46] | Neu SC , Pa J , Kukull W , Beekly D , Kuzma A , Gangadharan P , Wang L-S , Romero K , Arneric SP , Redolfi A , Orlandi D , Frisoni GB , Au R , Devine S , Auerbach S , Espinosa A , Boada M , Ruiz A , Johnson SC , Koscik R , Wang J-J , Hsu W-C , Chen Y-L , Toga AW ((2017) ) Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol 74: , 1178–1189. |
[47] | Müller-Gerards D , Weimar C , Abramowski J , Tebrügge S , Jokisch M , Dragano N , Erbel R , Jöckel K-H , Moebus S , Winkler A ((2019) ) Subjective cognitive decline, APOE ɛ4, and incident mild cognitive impairment in men and women. Alzheimers Dement (Amst) 11: , 221–230. |
[48] | Jacobs DM , Tang MX , Stern Y , Sano M , Marder K , Bell KL , Schofield P , Dooneief G , Gurland B , Mayeux R ((1998) ) Cognitive function in nondemented older women who took estrogen after menopause. Neurology 50: , 368–373. |
[49] | Paganini-Hill A , Henderson VW ((1994) ) Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol 140: , 256–261. |
[50] | Tang M-X , Jacobs D , Stern Y , Marder K , Schofield P , Gurland B , Andrews H , Mayeux R ((1996) ) Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 348: , 429–432. |
[51] | Barrett-Connor E ((1993) ) Estrogen replacement therapy and cognitive function in older women. JAMA 269: , 2637. |
[52] | Yaffe K , Haan M , Byers A , Tangen C , Kuller L ((2000) ) Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology 54: , 1949–1954. |
[53] | Ahmad S , Bannister C , van der Lee SJ , Vojinovic D , Adams HHH , Ramirez A , Escott-Price V , Sims R , Baker E , Williams J , Holmans P , Vernooij MW , Ikram MA , Amin N , van Duijn CM ((2018) ) Disentangling the biological pathways involved in early features of Alzheimer’s disease in the Rotterdam Study. Alzheimers Dement 14: , 848–857. |
[54] | Marais AD ((2019) ) Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology 51: , 165–176. |
[55] | Lourida I , Hannon E , Littlejohns TJ , Langa KM , Hyppönen E , Kuzma E , Llewellyn DJ ((2019) ) Association of lifestyle and genetic risk with incidence of dementia. JAMA 322: , 430–437. |
[56] | Wirth M , Villeneuve S , La Joie R , Marks SM , Jagust WJ ((2014) ) Gene-environment interactions: Lifetime cognitive activity, APOE genotype, and β-amyloid burden. J Neurosci 34: , 8612–8617. |




