Relationship of Homocysteine Plasma Levels with Mild Cognitive Impairment, Alzheimer’s Disease, Vascular Dementia, Psychobehavioral, and Functional Complications
Abstract
Background:
Alzheimer’s disease (AD) may be a vascular disorder with neurodegenerative consequences opening possibility of preventing AD by targeting vascular risk factors including homocysteine.
Objective:
The study aims were to assess homocysteine distribution in different forms and severity of cognitive impairment (CogI) [mild cognitive impairment (MCI), probable AD (Prob-AD), possible AD (Poss-AD), and vascular dementia (VaD)] and in NoCogI, and to estimate possible association between hyperhomocysteinemia levels with functional deficit severity and psychobehavioral complications.
Methods:
In total, 929 (M = 366, F = 563; mean age of 72.55±6.24 years) patients were evaluated with cognitive, neuropsychiatric, affective, and functional assessment scales. Homocysteine serum was set on two levels: between 0 and 10μmol/L and > 10μmol/L. For each patient, blood concentration of folate, vitamin B12, hemoglobin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), cholesterol, triglycerides, and glycemia were measured.
Results:
CogI patients demonstrated significantly a higher frequency of homocysteine > 10 (p = 0.003), than NoCogI patients. Patients with moderate and severe dementia had a higher frequency of homocysteine > 10 (p < 0.0001), than MCI and mild dementia. Poss-AD and VaD had a higher frequency of homocysteine > 10 (p = 0.003), than Prob-AD patients. Homocysteine > 10 frequency is directly proportional to increased neuropsychiatric symptom severity (p < 0.0001), and functional impairment severity respectively for ADL (p < 0.0001) and IADL (p < 0.0001).
Conclusion:
Higher homocysteine level seems to be significantly related to cognitive impairment frequency and severity, possible AD and VaD, neuropsychiatric symptom severity, and functional impairment severity.
INTRODUCTION
Alzheimer’s disease (AD) is the most common type of dementia among western countries, corresponding to about 60–70% of cases [1], while vascular dementia (VaD) is the second, with about 5% to 10% of all cases [2]. AD is associated with tangles and plaques in the brain, loss of connections, inflammation, and death of brain cells; therefore, it is classified as a neurodegenerative disorder. AD usually progresses slowly and gradually gets worse as more brain cells die. An important projection indicated that the number of people affected by dementia is likely to reach 82 million in 2030 and 152 million in 2050 [1]. In AD, causes and progression are not well understood. Moreover, symptomatology, pathophysiology, and risk factors overlap with VaD. Some studies suggest that the combination of AD and cerebrovascular pathologies is possible and frequently causes the onset of a type of dementia called mixed dementia [3, 4]. It is now clear that pure cerebrovascular disease and pure AD represent two extremes of a spectrum [5, 6]. The concept that AD may be a vascular disorder with neurodegenerative consequences [7, 8] has offered an explanation into the nature of this disorder and opened the possibility of preventing AD by targeting vascular risk factors. The vascular risk factors known to date are many and act with different mechanisms of action. The wall of the vessels, in particular the vascular endothelium, is the site where the mechanisms of damage act. The endothelium of vessels is a single layer of dynamic cells which through a variety of stimuli produces vasoactive substances to maintain vascular tone, regulate blood flow, and affect cellular metabolism of tissues. Endothelial damage is a crucial aspect of atherosclerosis and precedes overt manifestation of disease. Endothelial dysfunction is involved in several diseases including hypertension, diabetes, atherosclerosis, renal and cardiac failure, and neurodegeneration [9–12]. On the basis of these findings, there has been a growing interest in the role played by the vascular system. Among all the vascular risk factors, much attention has recently been paid to homocysteine. Homocysteine is an amino acid containing a sulfhydryl group, and its levels are influenced by B vitamins cobalamin, vitamin B6, and folic acid that through their roles as cofactors for the enzymes involved in methionine metabolism [13]. Elevated plasma homocysteine is well recognized as a risk factor for cardiovascular [14, 15], renal [16], and cerebrovascular diseases [17]. Inflammatory processes in the vascular wall are crucial at the beginning and in the continuation of the pathology. The proinflammatory effect of homocysteine is related to reactive oxygen species (ROS) generation and involves the activation of nuclear transcription factor κB (NF-κB) which controls the genes for the expression of adhesion molecules, cytokines, and chemokines [18]. Elevated serum homocysteine levels have been found to be an independent risk factor for AD. Homocysteine levels are significantly higher among patients with dementia than among normal control subject and that the levels are inversely related to cognitive function in people with dementia [19–24]. It has even been shown that B vitamins taken at the earliest stages of cognitive impairment are able to slow down the rate of brain atrophy [25] as well as cognitive decline [26], and may reduce conversion rates into dementia [27]. The homocysteine neurotoxicity [28, 29] and its direct effect on brain atrophy has been established [30]. Furthermore, homocysteine has been shown to increase platelet aggregation which contributes to the occurrence of clinically apparent, silent brain infarcts [31, 32], white matter abnormalities [33], and for recurrent stroke [34]. In addition to stroke, increased levels have been shown to be related to increased cortical and hippocampal atrophy [35]. Homocysteine was associated with AD as diagnosed both clinical [36] and histopathological criteria [37] and recent meta-analysis demonstrates a causal link between plasma total homocysteine and the risk for AD and provides a new insight into the etiology and prevention of AD [38]. Currently, a study concluded that homocysteine-methionine cycle is a key metabolic sensor system mediating receptor-independent metabolism-associated danger signal recognition and modulating methylation in disease conditions such as metabolic disorders, autoimmune disease, and cardiovascular disease [39]. In light of these studies, our research hypothesis was that homocysteine plasma assay is related to the proper functioning of the methylation pathway.
Otherwise to measure the homocysteine levels in order to verify that our results were in line with the ones of studies performed in other populations, we focused to homocysteine distribution in the different forms and severity of cognitive impairment. Finally, we looked for the possible association between levels of hyperhomocysteinemia with the severity of the functional deficit and psychobehavioral complications.
MATERIALS AND METHODS
The present study was conducted according to the Declaration of Helsinki, the Guidelines for Good Clinical Practice and the guidelines for Strengthening the Reporting of Observational Studies in Epidemiology, and it was approved by the local ethics committee for human experimentation (Prot. N. 3877/DS). The study was an observational study, in which the assignment of an intervention to the participants, its effect assessment, and health-related biomedical or behavioral outcomes are not considered. In the present study, healthy participants were recruited as control subjects.
Study sample
From January 2012 to July 2019, we screened older subjects who had consecutively attended the Cognitive Impairment Evaluation Unit of the Complex Unit of Geriatrics of Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) “Casa Sollievo della Sofferenza” for possible study enrollment. The control group patients were recruited in the Geriatric Unit and were categorized as no cognitive impairment (NoCogI) patients by cognitive, neuropsychiatric, and functional assessments. We obtained written informed consent for research from each patient, or from relatives or a legal guardian. All subjects were Caucasian, not including people of Jewish, Eastern European, or North African descent; with most individuals having Southern Italian ancestry, living in Southern Italy for at least three generations.
Inclusion criteria were: 1) age ≥60 years; 2) diagnosis of mild cognitive impairment (MCI) according to the National Institute on Aging-Alzheimer’s Association (NIAAA) [40] criteria; 3) diagnosis of Probable/Possible AD (Prob-AD/Poss-AD) according to the NINCDS-ADRDA [41] and NIAAA [42] criteria; 4) diagnosis of vascular dementia (VaD) according to the criteria of the National Institute of Neurological Disorders and Stroke - Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) Work Group [43]; 5) ability to provide an informed consent or availability of a relatives or a legal guardian in the case of severe demented patients. Exclusion criteria were: 1) presence of serious comorbidity, tumors, other diseases or physiological status (ascertained blood infections, disorders of the thyroid, kidneys, or liver), that could be causally related to cognitive impairment; 2) history of alcohol or drug abuse, head trauma, and other causes that can cause memory impairment.
As control group, we included older patients consecutively evaluated in the same center who had no cognitive impairment and neuropsychiatric symptoms.
Clinical, cognitive, neuropsychiatric, and functional assessment
The medical status of the patients was collected by a structured interview, a clinical evaluation, and a review of records from the patients’ general practitioners.
In all patients, cognitive status was defined with the Mini-Mental State Examination (MMSE) [44], Frontal Assessment Battery (FAB) [45], and Clock Drawing Test (CDT) [46], after a brief interview with the caregiver. Diagnosis of dementia was always supported by neuroimaging evidence (computed tomography scan and/or nuclear magnetic resonance). Differential diagnosis between AD and VaD was also based on the Hachinski Ischemic Score (HIS) [47] to address unclear AD/VaD diagnoses.
The neuropsychiatric symptoms were assessed using the Neuropsychiatric Inventory (NPI) [48]: it consists of 12 neuropsychiatric domains (delusions, hallucinations, depression, anxiety, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy, aberrant motor activity, sleep disturbance, and eating disorder). Caregiver distress due to the behavioral problems of the patient was also assessed by the NPI-Distress (NPI-D) [48, 49], a subscale of the NPI which evaluates stress caused by each behavioral disturbance of the patient.
In all patients, functional status was assessed by activities of daily living (ADL) [50] and instrumental activities of daily living (IADL) [51] scales.
Value quantification of homocysteine and other biochemical concentrations
The blood samples (3–5 mL of blood) were collected intravenously from all patients’ upper limb in the morning. Then blood samples were stored in Vacutainer tubes containing citrate; within not more than 30 min, the samples were transferred to the department of biochemistry and analyzed in full autoanalyzer.
The serum homocysteine was set on two levels: between 0 and 10μmol/L and > 10μmol/L.
The blood concentration of folate, vitamin B12, hemoglobin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), cholesterol, triglycerides, and glycemia were measured in all patients.
Statistical analyses
For dichotomous variables, hypotheses regarding differences between the groups were tested using chi-square test. This analysis was made using the 2-Way Contingency Table Analysis. For continuous variables, normal distribution was verified by the Shapiro-Wilk normality test and the one-sample Kolgomorov-Smirnov test. For normally-distributed variables, hypotheses regarding differences among the groups were compared by means of the Welch two sample t-test or by means of the analysis of variance (ANOVA) under general linear model. For non-normally distributed variables, hypotheses regarding differences among the groups were compared by means of the Wilcoxon rank sum test with continuity correction or by means of the Kruskal-Wallis rank sum test. Risks (adjusted by age) will be reported as odds ratios (OR) along with their 95% confidence interval (CI). All the statistical analyses were made with the R Ver. 2.8.1 statistical software package (The R Project for Statistical Computing; available at URL http://www.r-project.org/). Tests in which the p value was smaller than the type I error rate α= 0.05 were declared significant.
RESULTS
During the enrolment period, 1,103 older patients were screened for the inclusion in the study. Of these, 3 patients were excluded because they were younger than 60 years, 9 patients had an incomplete examination, and 162 patients were without no cognitive impairment and with neuropsychiatric symptoms. Thus, the final population included 929 patients, 366 men (39.40%) and 563 women (60.6%) with a mean age of 72.55±6.24 years and a range from 60 to 93 years. Therefore, 126 patients (M: 70, F: 56; mean age of 77.31±7.26 years, range 62–92 years) were included in the NoCogI group, and 803 patients (M: 296, F: 507; mean age of 71.81±5.72 years, range 60–93 years) were included in cognitive impairment (CogI) group (of these 197 were MCI, 267 were AD, 198 were VaD, and 141 were Poss-AD).
As explained in Table 1, the CogI patients were allocated in four groups according to cognitive impairment severity: 1) 197 patients with MCI, 2) 241 patients with mild dementia, 3) 282 patients with moderate dementia, and 4) 83 patients with severe dementia.
Table 1
Demographic, cognitive, clinical, and biochemical characteristics of older patients according to cognitive impairment severity
| MCI | Mild dementia | Moderate dementia | Severe dementia | p | |
| N = 197 | n = 241 | n = 282 | n = 83 | ||
| Sex | |||||
| Males/Females | 77/120 | 86/155 | 102/180 | 31/52 | 0.890 |
| Males (%) | 39.10 | 35.70 | 36.20 | 37.30 | |
| Age (y) | |||||
| Mean±SD | 75.58±6.79 | 69.95±4.57 | 71.23±4.66 | 70.17±5.06 | < 0.0001 |
| Range | 60.31 –93.49 | 60.01 –83.68 | 60.22 –82.34 | 61.31 –85.23 | |
| MMSE | |||||
| Mean±SD | 25.30±0.99 | 20.98±1.68 | 14.44±2.29 | 5.95±3.57 | < 0.0001 |
| Range | 24.00 –27.00 | 18.20 –23.80 | 10.20 –18.00 | 0 –10.00 | |
| FAB | |||||
| Mean±SD | 15.18±1.84 | 10.76±4.34 | 5.56±4.06 | 1.12±2.15 | < 0.0001 |
| Range | 13.00 –18.00 | 0 –18.00 | 0 –16.00 | 0 –12.00 | |
| CDT | |||||
| Mean±SD | 1.00±0 | 4.07±1.56 | 5.46±0.98 | 5.98±0.15 | < 0.0001 |
| Range | 1.00 –1.00 | 1.00 –6.00 | 1.00 –6.00 | 5.00 –6.00 | |
| ADL | |||||
| Mean±SD | 6.00±0 | 4.75±1.37 | 3.62±1.44 | 1.87±0.82 | < 0.0001 |
| Range | 6.00 –6.00 | 2.00 –6.00 | 2.00 –6.00 | 1.00 –5.00 | |
| IADL | |||||
| Mean±SD | 8.00±0 | 3.48±2.65 | 1.21±1.60 | 0 | < 0.0001 |
| Range | 8.00 –8.00 | 0 –7.00 | 0 –7.00 | 0 | |
| NPI Total score | |||||
| Mean±SD | 0 | 23.81±19.12 | 33.32±20.17 | 44.44±20.60 | < 0.0001 |
| Range | 0 | 0 –87.00 | 0 –90.00 | 0 –87.00 | |
| NPI Distress Total score | |||||
| Mean±SD | 0 | 9.02±6.19 | 12.38±6.51 | 15.18±6.48 | < 0.0001 |
| Range | 0 | 0 –32.00 | 0 –33.00 | 0 –30.00 | |
| NPI-Delusion | |||||
| Mean±SD | 0 | 0.56±2.22 | 1.41±3.31 | 2.66±4.03 | < 0.0001 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Hallucination | |||||
| Mean±SD | 0 | 0.50±2.11 | 1.26±2.93 | 3.13±4.39 | < 0.0001 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Agitation/Aggression | |||||
| Mean±SD | 0 | 2.88±3.64 | 4.16±3.82 | 5.85±4.29 | < 0.0001 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Depression | |||||
| Mean±SD | 0 | 4.53±4.37 | 5.22±4.48 | 5.27±4.98 | 0.174 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Anxiety | |||||
| Mean±SD | 0 | 3.36±4.35 | 3.07±4.37 | 3.55±4.85 | 0.606 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Euphoria | |||||
| Mean±SD | 0 | 0.06±0.64 | 0.03±0.34 | 0.01±0.11 | 0.663 |
| Range | 0 | 0 –9.00 | 0 –4.00 | 0 –1.00 | |
| NPI-Apathy/Indifference | |||||
| Mean±SD | 0 | 4.57±4.49 | 7.50±4.39 | 8.87±4.22 | < 0.0001 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Disinhibition | |||||
| Mean±SD | 0 | 0.06±0.54 | 0.05±0.60 | 0.16±1.08 | 0.445 |
| Range | 0 | 0 –6.00 | 0 –9.00 | 0 –9.00 | |
| NPI-Irritability/Lability | |||||
| Mean±SD | 0 | 2.49±3.63 | 4.05±4.13 | 5.34±4.64 | < 0.0001 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Aberrant Motor Behavior | |||||
| Mean±SD | 0 | 0.07±0.82 | 0.13±1.03 | 1.20±3.10 | < 0.0001 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Sleep/night time behavior | |||||
| Mean±SD | 0 | 3.26±3.96 | 4.33±4.38 | 5.71±4.78 | < 0.0001 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Appetite/eating change | |||||
| Mean±SD | 0 | 1.47±3.01 | 2.10±3.36 | 2.70±4.08 | 0.008 |
| Range | 0 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| Homocysteine, μmol/L | |||||
| Mean±SD | 9.46±4.53 | 11.81±7.31 | 13.07±8.89 | 12.96±7.82 | < 0.0001 |
| Range | 2.80 –40.50 | 4.70 –90.00 | 3.70 –78.90 | 4.00 –49.90 | |
| Vitamin B12, ng/mL | |||||
| Mean±SD | 357.01±282.01 | 326.42±231.54 | 339.29±413.95 | 289.69±209.21 | 0.442 |
| Range | 3.00 –2395.00 | 4.00 –2000.00 | 4.00 –6000.00 | 4.00 –1521.00 | |
| Folate, pg/mL | |||||
| Mean±SD | 11.04±31.25 | 10.77±21.89 | 10.70±31.34 | 8.18±8.79 | 0.878 |
| Range | 2.00 –429.00 | 2.00 –252.00 | 2.00 –443.00 | 2.00 –57.00 | |
| Haemoglobin, g/dL | |||||
| Mean±SD | 13.76±1.52 | 13.50±1.61 | 13.43±1.62 | 13.29±1.70 | 0.077 |
| Range | 10.00 –20.00 | 9.00 –19.00 | 8.00 –18.00 | 9.00 –17.00 | |
| CRP, mg/dL | |||||
| Mean±SD | 0.47±2.59 | 0.05±0.25 | 0.04±0.12 | 0.20±0.87 | 0.004 |
| Range | 0 –34.00 | 0 –3.30 | 0 –0.90 | 0 –6.90 | |
| ESR, mm/min | |||||
| Mean±SD | 24.85±14.74 | 27.63±16.81 | 29.44±19.77 | 28.47±20.39 | 0.056 |
| Range | 2.00 –70.00 | 2.00 –78.00 | 2.00 –174.00 | 2.00 –120.00 | |
| Cholesterol, mg/dL | |||||
| Mean±SD | 187.70±41.45 | 185.69±41.37 | 186.30±42.24 | 180.60±41.14 | 0.634 |
| Range | 82.00 –321.00 | 97.00 –305.00 | 54.00 –338.00 | 98.00 –320.00 | |
| Triglycerides, mg/dL | |||||
| Mean±SD | 106.15±43.77 | 111.11±48.22 | 115.43±66.65 | 117.07±50.59 | 0.261 |
| Range | 40.00 –328.00 | 33.00 –383.00 | 36.00 –721.00 | 52.00 –331.00 | |
| Glycemia, mg/dL | |||||
| Mean±SD | 100.37±32.36 | 102.95±37.33 | 102.88±36.01 | 102.16±39.88 | 0.875 |
| Range | 68.00 –340.00 | 64.00 –371.00 | 51.00 –292.00 | 65.00 –270.00 | |
| Hypertension | |||||
| Yes –N (%) | 156 (79.2) | 194 (80.5) | 225 (79.8) | 67 (80.7) | 0.923 |
| No –N (%) | 41 (20.8) | 47 (19.5) | 57 (20.2) | 16 (19.3) | |
| Alcohol consumption | |||||
| Yes –N (%) | 8 (4.1) | 12 (5.0) | 16 (5.7) | 4 (4.8) | |
| No –N (%) | 189 (95.9) | 229 (95.0) | 266 (94.3) | 79 (95.2) | 0.887 |
| Tobacco use | |||||
| Yes –N (%) | 27 (13.3) | 32 (13.3) | 37 (13.1) | 11 (13.3) | 0.998 |
| No –N (%) | 170 (86.3) | 209 (86.7) | 245 (86.9) | 72 (86.7) |
MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; FAB, Frontal Assessment Battery; CDT, Clock Drawing Test; ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; NPI, Neuropsychiatric Inventory; CRP, C-Reactive Protein; ESR, Erythrocyte Sedimentation Rate.
Clearly, the patients with severe dementia were more cognitively impaired in MMSE (p < 0.001), in FAB (p < 0.001), and in CDT (p < 0.001), showed obviously a major impairment in ADL (p < 0.001) and IADL (p < 0.001), and a major neuropsychiatric implication in NPI Total score (p < 0.001), NPI Distress Total score (p < 0.001), NPI-Delusion (p < 0.001), NPI-Hallucination (p < 0.001), NPI-Agitation/Aggression (p < 0.001), NPI-Apathy/Indifference (p < 0.001), NPI-Irritability/Lability (p < 0.001), NPI-Aberrant Motor Behavior (p < 0.001), NPI-Sleep/night time behavior (p < 0.001), and NPI-Appetite/eating change (p = 0.008), than other groups of patients.
The serum homocysteine concentration is higher in moderate and severe dementia patients (p < 0.001), than MCI and mild dementia. Finally, MCI patients are older (p < 0.001) and had a higher level of CRP (p = 0.004).
The groups did not differ in sex distribution (p = 0.890), NPI-Depression (p = 0.174), NPI-Anxiety (p = 0.606), NPI-Euphoria (p = 0.663), NPI-Disinhibition (p = 0.445), vitamin B12 (p = 0.442), folate (p = 0.878), hemoglobin (p = 0.077), ESR (p = 0.056), cholesterol (p = 0.634), triglycerides (p = 0.261), glycemia (p = 0.875), alcohol consumption (p = 0.887), and tobacco use (p = 0.998).
As shown in Table 2, all patients with dementia (N = 606; M: 219, F: 387; mean age of 70.57±4.71 years, range 60–85 years) were allocated in three groups according to dementia type: 1) 267 patients with Prob-AD, 2) 198 patients with VaD, and 3) 141 patients with Poss-AD.
Table 2
Demographic, cognitive, clinical, and biochemical characteristics of older patients according to dementia type
| ALL | Prob-AD | VaD | Poss-D | p | |
| N = 606 | n = 267 | n = 198 | n = 141 | ||
| Sex | |||||
| Males/Females | 219/387 | 114/153 | 61/137 | 44/97 | 0.012 |
| Males (%) | 36.10 | 42.70 | 30.80 | 31.20 | |
| Age (y) | |||||
| Mean±SD | 70.57±4.71 | 70.73±4.73 | 70.63±4.61 | 70.89±4.83 | 0.567 |
| Range | 60.00 –85.00 | 61.00 –85.00 | 60.00 –83.00 | 60.00 –82.00 | |
| MMSE | |||||
| Mean±SD | 15.87±5.49 | 14.87±5.45 | 19.14±4.26 | 13.20±4.92 | < 0.0001 |
| Range | 0 –23.80 | 0 –23.80 | 0 –23.70 | 0 –22.70 | |
| FAB | |||||
| Mean±SD | 7.04±5.21 | 6.14±5.01 | 9.61±4.86 | 5.00±4.63 | < 0.0001 |
| Range | 0 –18.00 | 0 –17.00 | 0 –18.00 | 0 –16.00 | |
| CDT | |||||
| Mean±SD | 4.98±1.41 | 5.05±1.31 | 4.54±4.62 | 5.46±1.08 | < 0.0001 |
| Range | 1.00 –6.00 | 1.00 –6.00 | 1.00 –6.00 | 1.00 –6.00 | |
| ADL | |||||
| Mean±SD | 3.83±1.64 | 3.75±1.68 | 4.30±1.53 | 3.33±1.53 | < 0.0001 |
| Range | 1.00 –6.00 | 1.00 –6.00 | 1.00 –6.00 | 1.00 –6.00 | |
| IADL | |||||
| Mean±SD | 1.94±2.38 | 1.64±2.21 | 2.83±2.64 | 1.27±1.94 | < 0.0001 |
| Range | 0 –7.00 | 0 –7.00 | 0 –7.00 | 0 –7.00 | |
| NPI Total score | |||||
| Mean±SD | 31.05±20.96 | 30.41±21.18 | 28.25±20.32 | 36.14±20.67 | 0.002 |
| Range | 0 –90.00 | 0 –90.00 | 0 –87.00 | 0 –87.00 | |
| NPI Distress Total score | |||||
| Mean±SD | 11.42±6.73 | 11.27±6.80 | 10.52±6.63 | 12.95±6.51 | 0.004 |
| Range | 0 –33.00 | 0 –30.00 | 0 –32.00 | 0 –33.00 | |
| NPI-Delusion | |||||
| Mean±SD | 1.24±3.12 | 1.60±3.44 | 0.72±2.49 | 1.30±3.19 | 0.011 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Hallucination | |||||
| Mean±SD | 1.21±3.01 | 1.59±3.30 | 0.72±2.40 | 1.20±3.13 | 0.009 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Agitation/Aggression | |||||
| Mean±SD | 3.88±3.93 | 4.29±4.05 | 3.03±3.53 | 4.32±4.07 | 0.001 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Depression | |||||
| Mean±SD | 4.95±4.51 | 4.08±4.23 | 5.47±4.62 | 5.88±4.61 | < 0.0001 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Anxiety | |||||
| Mean±SD | 3.25±4.42 | 2.44±3.95 | 4.00±4.66 | 3.73±4.69 | <0.0001 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Euphoria | |||||
| Mean±SD | 0.04±0.47 | 0.03±0.35 | 0.07±0.71 | 0 | 0.373 |
| Range | 0 –9.00 | 0 –4.00 | 0 –9.00 | 0 | |
| NPI-Apathy/Indifference | |||||
| Mean±SD | 6.52±4.70 | 6.81±4.66 | 5.28±4.68 | 7.69±4.43 | < 0.0001 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Disinhibition | |||||
| Mean±SD | 0.07±0.67 | 0.15±0.97 | 0 | 0.03±0.34 | 0.041 |
| Range | 0 –9.00 | 0 –9.00 | 0 | 0 –4.00 | |
| NPI-Irritability/Lability | |||||
| Mean±SD | 3.60±4.13 | 3.72±4.27 | 3.04±3.83 | 4.16±4.19 | 0.041 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Aberrant Motor Behavior | |||||
| Mean±SD | 0.25±1.48 | 0.44±1.96 | 0.06±0.86 | 0.16±1.02 | 0.020 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –9.00 | |
| NPI-Sleep/night | |||||
| time behavior | |||||
| Mean±SD | 4.09±4.35 | 3.60±4.34 | 4.00±4.12 | 5.14±5.52 | 0.003 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| NPI-Appetite/eating change | |||||
| Mean±SD | 1.93±3.36 | 1.66±3.06 | 1.86±3.33 | 2.52±3.84 | 0.048 |
| Range | 0 –12.00 | 0 –12.00 | 0 –12.00 | 0 –12.00 | |
| Homocysteine, μmol/L | |||||
| Mean±SD | 12.55±8.16 | 11.46±6.14 | 13.59±11.02 | 13.16±6.42 | 0.012 |
| Range | 3.70 –90.00 | 3.70 –49.90 | 4.80 –90.00 | 6.1 –47.90 | |
| Vitamin B12, ng/mL | |||||
| Mean±SD | 327.50±326.91 | 296.69±213.41 | 333.46±217.83 | 377.35±548.96 | 0.064 |
| Range | 4.00 –6000.00 | 4.00 –2000.00 | 4.00 –2000.00 | 59.00 –6000.00 | |
| Folate, pg/mL | |||||
| Mean±SD | 10.39±25.63 | 10.71±33.18 | 10.79±21.47 | 9.27±10.67 | 0.839 |
| Range | 2.00 –443.00 | 2.00 –443.00 | 2.00 –252.00 | 2.00 –100.00 | |
| Haemoglobin, g/dL | |||||
| Mean±SD | 13.44±1.63 | 13.67±1.60 | 13.30±1.53 | 13.19±1.75 | 0.007 |
| Range | 8.00 –19.00 | 8.00 –19.00 | 10.00 –17.00 | 8.00 –18.00 | |
| CRP, mg/dL | |||||
| Mean±SD | 0.07±0.37 | 0.04±0.15 | 0.12±0.60 | 0.06±0.18 | 0.090 |
| Range | 0 –6.90 | 0 –1.70 | 0 –6.90 | 0 –1.70 | |
| ESR, mm/min | |||||
| Mean±SD | 28.59±18.72 | 27.06±15.99 | 29.44±17.92 | 30.29±23.84 | 0.197 |
| Range | 2.00 –174.00 | 3.00 –85.00 | 2.00 –83.00 | 2.00 –174.00 | |
| Cholesterol, mg/dL | |||||
| Mean±SD | 185.28±41.72 | 190.42±42.78 | 182.05±41.03 | 180.11±39.79 | 0.026 |
| Range | 54.00 –338.00 | 54.00 –338.00 | 71.00 –305.00 | 82.00 –271.00 | |
| Triglycerides, mg/dL | |||||
| Mean±SD | 113.94±57.76 | 111.16±53.07 | 115.08±52.91 | 117.56±71.16 | 0.543 |
| Range | 33.00 –721.00 | 36.00 –519.00 | 33.00 –383.00 | 41.00 –721.00 | |
| Glycemia, mg/dL | |||||
| Mean±SD | 102.81±37.03 | 102.20±36.00 | 101.70±33.23 | 105.86±43.48 | 0.535 |
| Range | 51.00 –371.00 | 64.00 –298.00 | 59.00 –267.00 | 51.00 –371.00 | |
| Hypertension | |||||
| Yes –N (%) | 485 (80.0) | 214 (80.1) | 158 (79.8) | 113 (80.1) | 0.975 |
| No –N (%) | 121 (20.0) | 53 (19.9) | 40 (20.2) | 28 (19.9) | |
| Alcohol consumption | |||||
| Yes –N (%) | 27 (4.5) | 13 (4.9) | 8 (4.0) | 6 (4.3) | 0.905 |
| No –N (%) | 579 (95.5) | 254 (95.1) | 190 (96.0) | 135 (95.7) | |
| Tobacco use | |||||
| Yes –N (%) | 84 (13.9) | 37 (13.9) | 26 (13.1) | 21 (14.9) | 0.898 |
| No –N (%) | 522 (86.1) | 230 (86.1) | 172 (86.9) | 120 (85.1) |
MMSE, Mini-Mental State Examination; FAB, Frontal Assessment Battery; CDT, Clock Drawing Test; ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; NPI, Neuropsychiatric Inventory; CRP, C-Reactive Protein; ESR, Erythrocyte Sedimentation Rate.
Poss-AD patients were more cognitively impaired in MMSE (p < 0.001), FAB (p < 0.001), and CDT (p < 0.001), showed obviously a major impairment in ADL (p < 0.001) and IADL (p < 0.001), and a major neuropsychiatric implication in NPI Total score (p = 0.002), NPI Distress Total score (p = 0.004), NPI-Agitation/Aggression (p = 0.001), NPI-Depression (p < 0.001), NPI-Apathy/Indifference (p < 0.001), NPI-Irritability/Lability (p = 0.041), NPI-Sleep/night time behavior (p = 0.003), and NPI-Appetite/eating change (p = 0.048), than other groups of patients.
However, Prob-AD patients had a higher percentage of males (p = 0.012), and a major neuropsychiatric implication in NPI-Delusion (p = 0.011), NPI-sHallucination (p = 0.009), NPI-Disinhibition (p = 0.041), and NPI-Aberrant Motor Behavior (p = 0.020), than VaD and Poss-AD patients.
Moreover, VaD patients had a major neuropsychiatric implication in NPI-Anxiety (p = 0.020), than other patient groups.
The serum homocysteine concentration is higher in VaD patients (p = 0.012), than Prob-AD and Poss-AD patients.
Besides, Poss-AD patients had a lower level of hemoglobin (p = 0.007), than other patients. Finally, Prob-AD patients had a higher level of cholesterol (p = 0.026).
The groups did not differ in age (p = 0.567), NPI-Euphoria (p = 0.373), vitamin B12 (p = 0.064), folate (p = 0.839), CRP (p = 0.090), ESR (p = 0.197), triglycerides (p = 0.543), glycemia (p = 0.535), alcohol consumption (p = 0.905), and tobacco use (p = 0.898).
In Fig. 1, distribution of homocysteine levels according to the CogI presence/absence and severity, and dementia types was shown. CogI patients showed a higher frequency of homocysteine > 10 (OR = 1.782; 95% CI = 1.212–2.620; p = 0.003), than NoCogI group. According to cognitive impairment severity, patients with moderate and severe dementia had a higher frequency of homocysteine > 10 (OR = 2.003; 95% CI = 1.509–2.658; p < 0.0001), than MCI and mild dementia. Moreover, according to dementia types, Poss-AD and VaD had a higher frequency of homocysteine > 10 (OR = 1.628; 95% CI = 1.176–2.255; p = 0.003), than Prob-AD patients.
Fig. 1
Distribution of homocysteine levels according to the cognitive impairment presence/absence and severity, and dementia types. CogI, cognitive impairment; NoCogI, no cognitive impairment; MCI, mild cognitive impairment; Prob-AD, Probable Alzheimer’s disease; VaD, Vascular dementia; Poss-AD, Possible Alzheimer’s disease.
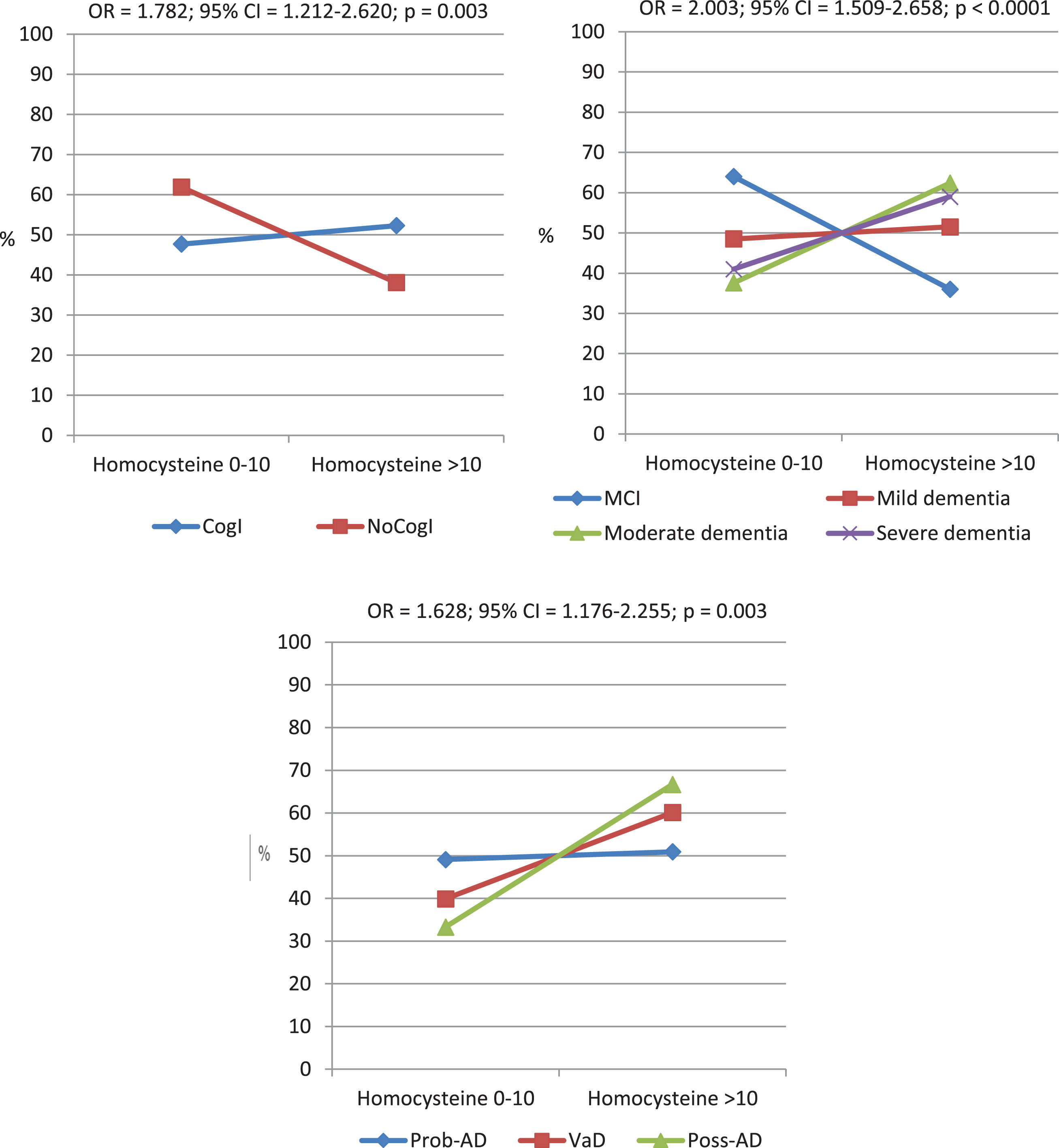
In Fig. 2, the homocysteine > 10 frequency increases with raising of neuropsychiatric symptom severity (OR = 2.043; 95% CI = 1.930–2.157; p < 0.0001). Likewise, in Fig. 3, homocysteine > 10 frequency is directly proportional to increased functional impairment severity, respectively for ADL (OR = 1.022; 95% CI = 0.930–1.113; p < 0.0001), and IADL (OR = 1.330; 95% CI = 1.212–1.448; p < 0.0001). In Table 3, considering homocysteine levels and MMSE, NPI, ADL, and IADL scores as continues variables, it emerged that hyperhomocysteine seems to be a risk factor for cognitive impairment (MMSE: OR = 21.378; 95% CI = 20.557–22.200; p < 0.0001), neuropsychiatric symptoms (NPI: OR = 31.673; 95% CI = 28.587–34.759; p < 0.0001), and functional impairment (respectively, ADL: OR = 4.972; 95% CI = 4.770–5.173; p < 0.0001, and IADL: OR = 5.018; 95% CI = 4.603–5.432; p < 0.0001).
Fig. 2
Distribution of homocysteine levels according to the neuropsychiatric symptom (NS) severity evaluated by Neuropsychiatric Inventory (NPI).
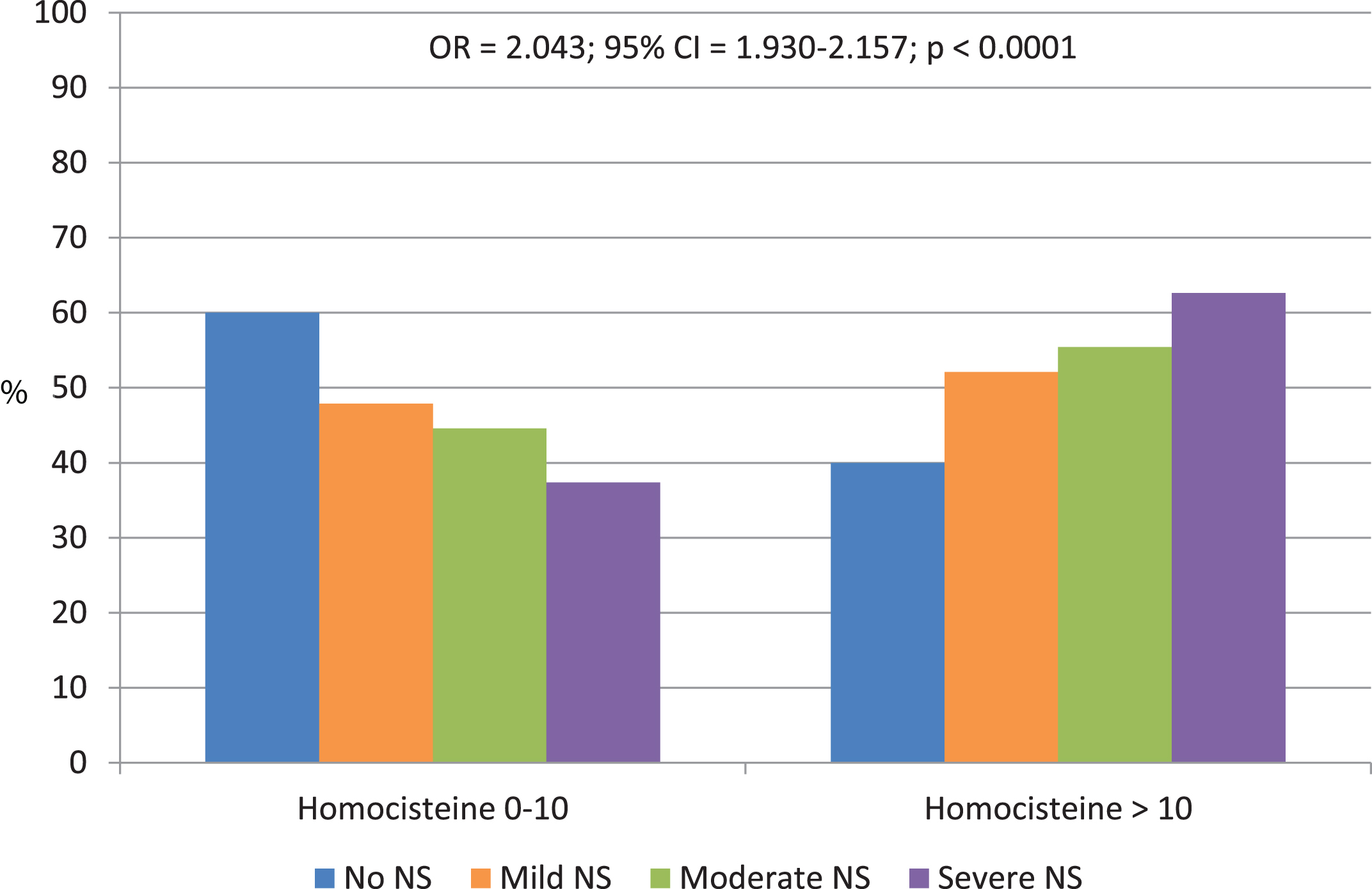
Fig. 3
Distribution of homocysteine levels according to the functional impairment (FI) severity evaluated by Activity of Daily Living (ADL) and Instrumental Activity of Daily Living (IADL).
DISCUSSION
Cognitive impairment is a chronic process that results from the interaction of multiple factors. All possible modifiable risk factors should be explored. There are numerous recent works attesting to a link between hyperhomocysteinemia and cognitive impairment [19, 52–58]. Among all the known damage mechanisms, we are convinced of the importance of homocysteine [28]. For this reason, we have dosed levels in our patient population. This study explores the relationship of plasma homocysteine levels with cognitive impairment and its psychobehavioral and functional complications. We have already said that development of cognitive impairment is a multifactorial condition, therefore the best approach to preventing dementia is to identify modifiable risk factors. Homocysteine is an important risk factor but, interestingly, manageable given that plasma levels can be controlled through adequate nutritional and lifestyle interventions. Our work was inspired by recent conclusions from a panel of experts on the evidence concerning the causal role of raised homocysteine in dementia. They conclude that raised homocysteine is a strong and modifiable risk factor for cognitive impairment [59]. It is necessary to define the threshold value of homocysteine beyond which the harmful effect on the vascular and neuronal tissue becomes important. Knowing this certainly helps us to take clinical action to reduce the risk. We studied the different proposals regarding the threshold value to be used in clinical prevention [60–62]. After analyzing our data, we propose 10μmol/L as the plasma homocysteine threshold value. In fact, our results show that beyond this value, the risk of cognitive impairment increases (p = 0.003). Patients were studied for degree of severity and type of diagnosis. Higher homocysteine values are associated with moderate and severe dementia (p < 0.0001), and Poss-AD and VaD (p = 0.003) than Prob-AD.
Table 3
Logistic Regression P-P normality plot Standardized residual of homocysteine levels as continue variable related to cognitive, neuropsychiatric and functional scores
| OR | 95% CI | p | |
| MMSE | 21.378 | 20.557–22.200 | < 0.0001 |
 | |||
| NPI | 31.673 | 28.587–34.759 | < 0.0001 |
 | |||
| ADL | 4.972 | 4.770–5.173 | < 0.0001 |
 | |||
| IADL | 5.018 | 4.603–5.432 | < 0.0001 |
 |
MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living.
This data is in line with current knowledge according to which vascular damage mechanisms also play a decisive role in the possible forms of AD [63].
Many patients in our population show psychobehavioral disorders often related to the severity of the cognitive deficit. These patients are characterized by generally higher levels of homocysteinemia and the same thing happens with patients with functional impairment regardless of severity of cognitive impairment. Accordingly, homocysteine > 10 frequency is appeared directly proportional to increased neuropsychiatric symptom severity (p < 0.0001), and functional impairment severity respectively for ADL (p < 0.0001) and IADL (p < 0.0001).
The association of hyperhomocysteinemia with neuropsychiatric disorders from an early age is known, and even in our population this data has been found.
In its entirety, the functional ability is the overall integrity outcome of the neuromuscular systems closely related to the muscular system integrity of which, as already mentioned, homocysteine is an endothelial damage marker. Functional ability is the result of the contemporary integrity of cognitive and neuromuscular functions. In the absence of one of the two functions, the patient will still experience a state of neurocognitive or neuromuscular disability.
Some limitations of the present study must be acknowledged. The follow-up on homocysteine levels and worsening of cognitive impairment, particularly in mild forms, is missing. Furthermore, the study population comprising only Caucasian patients recruited in a single center, so it could be possible that our results may not be applicable in other populations.
To conclude, based on information from numerous studies according to which having a low homocysteine level is protective against a risk of cognitive impairment or slows down the aggravation, we want to suggest that screening and treatment should be done much before the age of 65.
ACKNOWLEDGMENTS
This work was fully supported by “Ministero della Salute”, I.R.C.C.S. Research Program, Ricerca Corrente “Malattie complesse e terapie innovative” and by the “5 x 1000” voluntary contribution.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0166r1).
REFERENCES
[1] | World Health Organization. Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 11 December 2020. |
[2] | Kapasi A , DeCarli C , Schneider J ((2017) ) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 134: , 171–186. |
[3] | Eckerström C , Eckerström M , Göthlin M , Molinder A , Jonsson M , Kettunen P , Svensson J , Rolstad S , Wallin A ((2020) ) Characteristic biomarker and cognitive profile in incipient mixed dementia. J Alzheimers Dis 73: , 597–607. |
[4] | Hainsworth A , Yeo N , Weekman E , Wilcock D ((2016) ) Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta 1862: , 1008–1017. |
[5] | Roman G ((2001) ) Diagnosis of vascular dementia and Alzheimer’s disease. Int J Clin Practice 120: , 9–13. |
[6] | Erkinjuntti T ((2001) ) Clinical deficits of Alzheimer’s disease with cerebrovascular disease and probable VaD. Int J Clin Practice 120: , 14–23. |
[7] | de la Torre J , Mussivand T ((1993) ) Can disturbed brain microcirculation cause Alzheimer’s disease?. Neurol Res 15: , 146–153. |
[8] | de la Torre J ((2000) ) Critically attained threshold of cerebral hypoperfusion (CATCH): Can it cause Alzheimer’s disease?. Ann NY Acad Sci 903: , 424–436. |
[9] | Hadi H , Suwaidi J ((2007) ) Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3: , 853–876. |
[10] | Xu J , Zou M ((2009) ) Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 120: , 1266–1286. |
[11] | Yannoutsos A , Levy B , Safar M , Slama G , Blacher J ((2014) ) Pathophysiology of hypertension: Interactions between macro and microvascular alterations through endothelial dysfunction. J Hypertens 32: , 216–224. |
[12] | Grammas P , Martinez J , Miller B ((2011) ) Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med 13: , e19. |
[13] | Stabler S , Marcell P , Podell E , Allen R , Savage D , Lindenbaum J ((1988) ) Elevations of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J Clin Invest 81: , 466–474. |
[14] | Wald D , Law M , Morris J ((2002) ) Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 325: , 1202. |
[15] | Wald D , Wald N , Morris J , Law M ((2006) ) Folic acid, homocysteine, and cardiovascular disease: Judging causality in the face of inconclusive trial evidence. BMJ 333: , 1114–1117. |
[16] | Yi F , Li P ((2008) ) Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol 28: , 254–264. |
[17] | Bertsch T , Mielke O , Höly S , Zimmer W , Casarin W , Aufenanger J , Walter S , Muehlhauser F , Kuehl S , Ragoschke A , Fassbender K ((2001) ) Homocysteine in cerebrovascular disease: An independent risk factor for subcortical vascular encephalopathy. Clin Chem Lab Med 39: , 721–724. |
[18] | Papatheodorou L , Weiss N ((2007) ) Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal 9: , 1941–1958. |
[19] | Clarke R , Harrison G , Richards S , Group VT ((2003) ) Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. J Intern Med 254: , 67–75. |
[20] | Riggs K , Spiro AI , Tucker K , Rush D ((1996) ) Relations of vitamin B12, vitamin B6, folate and homocysteine to cognitive performance in the normative aging study. Am J Clin Nutr 63: , 306–314. |
[21] | Ravaglia G , Forti P , Maioli F , Zanardi V , Dalmonte E , Grossi G , Cucinotta D , Macini P , Caldarera M ((2000) ) Blood homocysteine and vitamin B levels are not associated with cognitive skills in healthy normally ageing subjects. J Nutr Health Aging 4: , 218–222. |
[22] | Budge M , de Jager C , Hogervorst E , Smith A ((2002) ) Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. J Am Geriatr Soc 50: , 2014–2018. |
[23] | Duthie S , Whalley L , Collins A , Leaper S , Berger K , Deary I ((2002) ) Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr 75: , 908–913. |
[24] | Prins ND , Den Heijer T , Hofman A , Koudstaal PJ , Jolles J , Clarke R , Breteler MM; Rotterdam Scan Study ((2002) ) Homocysteine and cognitive function in the elderly. The Rotterdam Scan Study. Neurology 59: , 1375–1380. |
[25] | Smith A , Refsum H ((2012) ) Do we need to reconsider the desirable blood level of vitamin B12?. J Intern Med 271: , 179–182. |
[26] | de Jager C , Oulhaj A , Jacoby R , Refsum H , Smith A ((2012) ) Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int J Geriatr Psychiatry 27: , 592–600. |
[27] | Blasko I , Hinterberger M , Kemmler G , Jungwirth S , Krampla W , Leitha T , Heinz Tragl K , Fischer P ((2013) ) Conversion from mild cognitive impairment to dementia: Influence of folic acid and vitamin B12 use in the vita cohort. J Nutr Health Aging 16: , 687–694. |
[28] | Kruman II , Culmsee C , Chan SL , Kruman Y , Guo Z , Penix L , Mattson MP ((2000) ) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hyper sensitivity to exitotoxicity. J Neurosci 20: , 6920–6926. |
[29] | Lipton SA , Kim WK , Choi YB , Kumar S , D’Emilia DM , Rayudu PV , Arnelle DR , Stamler JS ((1997) ) Neurotoxicity associated with dual actions of homocysteine at the N-methylD-aspartate receptor. Proc Natl Acad Sci U S A 94: , 5923–5928. |
[30] | Madsen S , Rajagopalan P , Joshi S , Toga A , Thompson P , Alzheimer’s Disease Neuroimaging Initiative (ADNI) ((2015) ) Higherhomocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer’s disease neuroimaging initiative. Neurobiol Aging 36: (Suppl 1), s203–s210. |
[31] | Welch G , Loscalzo J ((1998) ) Homocyteine and atherothrombosis. N Engl J Med 338: , 1042–1050. |
[32] | Herrmann W ((2001) ) The importance of hyperhomocysteinemia as a risk factor for disease: An overview. Clin Chem Lab Med 39: , 666–674. |
[33] | Boysen G , Brander T , Christensen H , Gideon R , Truelsen T ((2003) ) Homocysteine and risk of recurrent stroke. Stroke 34: , 1258–1261. |
[34] | Fassbender K , Mielke O , Bertsch T , Nafe B , Froschen S , Hennerici M ((1999) ) Homocysteine in cerebral macroangiopathy. Lancet 353: , 1586–1587. |
[35] | den Heijer T , Vermeer SE , Clarke R , Oudkerk M , Koudstaal PJ , Hofman A , Breteler MM ((2003) ) Homocysteine and brain atrophy on MRI of non-demented elderly. Brain 126: , 170–175. |
[36] | McCaddon A , Davies G , Hudson P , Tandy S , Cattell H ((1998) ) Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry 13: , 235–239. |
[37] | Clarke R , Smith A , Jobst K , Refsum H , Sutton L , Ueland P ((1998) ) Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 55: , 1449–1455. |
[38] | Hu Q , Teng W , Li J , Hao F , Wang N ((2016) ) Homocysteine and Alzheimer’s disease: Evidence for a causal link from Mendelian randomization. J Alzheimers Dis 52: , 747–756. |
[39] | Shen W , Gao C , Cueto R , Liu L , Fu H , Shao Y , Yang WY , Fang P , Choi ET , Wu Q , Yang X , Wang H ((2020) ) Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling. Redox Biol 28: , 101322. |
[40] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[41] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan E ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–944. |
[42] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[43] | Román GC , Tatemichi TK , Erkinjuntti T , Cummings JL , Masdeu JC , Garcia JH , Amaducci L , Orgogozo JM , Brun A , Hofman A , et al. ((1993) ) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: , 250–260. |
[44] | Folstein M , Folstein S , McHugh P ((1975) ) Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[45] | Dubois B , Litvan I ((2000) ) The FAB: A frontal assessment battery at bedside. Neurology 55: , 1621–1626. |
[46] | Rouleau I , Salmon D , Butters N , Kennedy C , McGuire K ((1992) ) Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn 18: , 70–87. |
[47] | Hachinski VC , Iliff LD , Zilhka E , Du Boulay GH , McAllister VL , Marshall J , Russell RW , Symon L ((1975) ) Cerebral blood flow in dementia. Arch Neurol 32: , 632–637. |
[48] | Cummings J , Mega M , Gray K , Rosenberg-Thompson S , Carusi D , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[49] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: (5 Suppl 6), S10–S16. |
[50] | Katz S , Downs T , Cash H , Grotz R ((1970) ) Progress in the development of an index of ADL. Gerontologist 10: , 20–30. |
[51] | Lawton M , Brody E ((1969) ) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: , 179–186. |
[52] | Miller J , Green R , Mungas D , Reed B , Jagust W ((2002) ) Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology 58: , 1471–1475. |
[53] | Stewart R , Asonganyi B , Sherwood R ((2002) ) Plasma homocysteine and cognitive impairment in an older British African-Caribbean population. J Am Geriatr Soc 50: , 1227–1232. |
[54] | Miller JW , Green R , Ramos MI , Allen LH , Mungas DM , Jagust WJ , Haan MN ((2003) ) Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 78: , 441–447. |
[55] | Ravaglia G , Forti P , Maioli F , Muscari A , Sacchetti L , Arnone G , Nativio V , Talerico T , Mariani E ((2003) ) Homocysteine and cognitive function in healthy elderly community dwellers in Italy. Am J Clin Nutr 77: , 668–673. |
[56] | Smith A , Refsum H ((2016) ) Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 36: , 211–239. |
[57] | Kim S , Choi B , Nam J , Kim M , Oh D , Yang Y ((2019) ) Cognitive impairment is associated with elevated serum homocysteine levels among older adults. Eur J Nutr 58: , 399–408. |
[58] | Ansari Z ((2016) ) Homocysteine and mild cognitive impairment: Are these the tools for early intervention in the dementia spectrum?. J Nutr Health Aging 20: , 155–160. |
[59] | Smith AD , Refsum H , Bottiglieri T , Fenech M , Hooshmand B , McCaddon A , Miller JW , Rosenberg IH , Obeid R ((2018) ) Homocysteine and dementia: An international consensus statement. J Alzheimers Dis 62: , 561–570. |
[60] | Whalley LJ , Duthie SJ , Collins AR , Starr JM , Deary IJ , Lemmon H , Duthie AC , Murray AD , Staff RT ((2014) ) Homocysteine, antioxidant micronutrients and late onset dementia. Eur J Nutr 53: , 277–285. |
[61] | Tucker K , Qiao N , Scott T , Rosenberg I , Spiro A 3rd ((2005) ) High homocysteine and low B vitamins predict cognitive decline in aging men: The Veterans Affairs Normative Aging Study. Am J Clin Nutr 82: , 627–635. |
[62] | Refsum H , Smith AD , Ueland PM , Nexo E , Clarke R , McPartlin J , Johnston C , Engbaek F , Schneede J , McPartlin C , Scott JM ((2004) ) Facts and recommendations about total homocysteine determinations: An expert opinion. Clin Chem 50: , 3–32. |
[63] | Hooshmand B , Polvikoski T , Kivipelto M , Tanskanen M , Myllykangas L , Erkinjuntti T , Mäkelä M , Oinas M , Paetau A , Scheltens P , van Straaten EC , Sulkava R , Solomon A ((2013) ) Plasma homocysteine, Alzheimer and cerebrovascular pathology: A population-based autopsy study. Brain 136: (Pt 9), 2707–2716. |
[64] | Golden E , Josephs K ((2014) ) Clinical aspects of dementia. In Neuropathology of Neurodegenerative Diseases: A Practical Guide, Kovacs G , ed. Cambridge University Press, Cambridge, pp. 8–28. |




