Mortality After Ischemic Stroke in Patients with Alzheimer’s Disease Dementia and Other Dementia Disorders
Abstract
Background:
Stroke and dementia are interrelated diseases and risk for both increases with age. Even though stroke incidence and age-standardized death rates have decreased due to prevention of stroke risk factors, increased utilization of reperfusion therapies, and other changes in healthcare, the absolute numbers are increasing due to population growth and aging.
Objective:
To analyze predictors of death after stroke in patients with dementia and investigate possible time and treatment trends.
Methods:
A national longitudinal cohort study 2007–2017 using Swedish national registries. We compared 12,629 ischemic stroke events in patients with dementia with matched 57,954 stroke events in non-dementia controls in different aspects of patient care and mortality. Relationship between dementia status and dementia type (Alzheimer’s disease and mixed dementia, vascular dementia, other dementias) and death was analyzed using Cox regressions.
Results:
Differences in receiving intravenous thrombolysis between patients with and without dementia disappeared after the year 2015 (administered to 11.1% dementia versus 12.3% non-dementia patients, p = 0.117). One year after stroke, nearly 50% dementia and 30% non-dementia patients had died. After adjustment for demographics, mobility, nursing home placement, and comorbidity index, dementia was an independent predictor of death compared with non-dementia patients (HR 1.26 [1.23–1.29]).
Conclusion:
Dementia before ischemic stroke is an independent predictor of death. Over time, early and delayed mortality in patients with dementia remained increased, regardless of dementia type. Patients with≤80 years with prior Alzheimer’s disease or mixed dementia had higher mortality rates after stroke compared to patients with prior vascular dementia.
INTRODUCTION
In the past years, stroke incidence and age-standardized death rates have decreased [1] due to prevention of stroke risk factors, of stroke recurrence, complications after stroke, other changes in healthcare [2], and increased utilization of reperfusion therapies [3]. However, population growth and aging resulted in a greater absolute number of strokes, and improved stroke survival led to a greater overall stroke burden [1], since 50% of survivors still remain chronically disabled [4].
Dementia and stroke are strongly interrelated diseases, sharing risk factors and increasing the chances of one leading to the other. Dementia is common in older people and age by itself is a prognostic factor for a poor outcome after stroke [5].
It is estimated that there are 150,000 people living with dementia in Sweden [6] and every year around 20,000 Swedes have an ischemic stroke [7]. Previous studies have reported that dementia leads to an increased risk for death after stroke [8–12]; however, some studies did not differentiate between pre-stroke and post-stroke dementia [8, 10] or characterized pre-stroke dementia retrospectively [9]. Differences in stroke outcomes might be primarily driven by other conditions than by dementia itself [11]. In our prior study, performed in a similar population, pre-stroke dementia was associated with excess mortality risk 3 months after stroke; however, this was partially mediated by poorer pre-stroke mobility [13]. Another possible explanation for increased mortality could be the higher occurrence of cardioembolic strokes in patients with dementia due to underutilization of anticoagulation in patients with dementia and atrial fibrillation (AF), as these strokes are more severe [14–17]. Even though intravenous thrombolysis and mechanical thrombectomy have lower population benefits (lives saved from death or dependency) than, e.g., hospitalization in a stroke unit or pharmacological prevention [18], lower utilization of these in patients with dementia [19] could lead to worse outcomes and worse survival.
Patients with dementia have a shorter life expectancy [20]. Dementia was the third leading cause of death in the United States while Alzheimer’s disease was the sixth in 2017 [21]. In a previous study conducted on patients with dementia in Sweden (SveDem cohort), the most frequent cause of death was cardiovascular (37%), followed by dementia (30%) and respiratory causes (26%) [22]. After receiving a dementia diagnosis, factors associated with increased death risk were age, male sex, worse cognition, higher number of medications, and nursing home placement [23]. After non-fatal stroke, the most frequent causes of death are heart or cerebrovascular diseases with 2- to 8-times increased risk of death respectively [24]; however, causes of death after stroke in patients with pre-existing dementia are poorly characterized. Furthermore, most studies reported increased mortality rates for patients with vascular cognitive impairment compared to Alzheimer’s disease dementia (AD) [25–28], or no difference [29–31], but it remains unexplored if certain dementia types worsen survival after stroke.
The aim of our study was to analyze predictors of death after stroke in patients with dementia and investigate possible time trends.
METHODS
Quality registries and study population
A longitudinal cohort study of patients diagnosed with dementia who subsequently suffered an ischemic stroke was performed. Patients with dementia were identified either from 1) SveDem, the Swedish Dementia Registry [6], 2) having ICD-10 codes F00-F03, G30, and G31 according to the Swedish National Inpatient Register, covering all in-hospital and hospital-based outpatient visits [32], or 3) having been prescribed anti-dementia drugs (ATC codes N06D; including donepezil, rivastigmine, galantamine and memantine) according to the Swedish Prescribed Drug Register.
The occurrence of ischemic stroke was determined by Riksstroke, the Swedish national quality registry for stroke [7], which collects information on the entire chain of stroke care and contains both process and outcome variables.
Ischemic stroke events in patients with pre-stroke dementia were compared to ischemic stroke events in a population without dementia, matched by age (±3 years), sex, year of stroke, and geographic region. Non-dementia controls were excluded if they ever had a SveDem registration, were diagnosed with dementia or confusional syndrome (ICD-10 codes F00–F09 or G30–G32), or took anti-dementia drugs (ATC codes N06DX and N06DA; including donepezil, rivastigmine, galantamine, and memantine). Patients and controls were matched on date of first stroke.
Variables
Data on stroke event, demographics, functional status, living arrangements, medication therapy, comorbidities, and outcome variables were obtained from Riksstroke. From patients registered in SveDem, information on dementia type was used. Modified Rankin Scale (mRS) was used to compare functional premorbid states and stroke outcomes. As mRS score is not included in Riksstroke registry, it was estimated by translation of 5 self-reported Riksstroke functional outcome variables with high precision [33], with conversion of lowest scores limited to categories mRS 0–3. Independence in daily activities was defined as independence in dressing, toilet use, and moving around indoors and outdoors without the help of another person, while the use of assistive devices was permitted. Stroke alarm was defined as an activation of a protocol for management for those eligible for acute reperfusion therapies. The Charlson Comorbidity Index (CCI) was calculated using data on comorbidities obtained from the Swedish National Inpatient Register [34]. Date of death was obtained from the Swedish Cause of Death Register, with data available until October 2019.
Outcome measures
The primary outcome was all-cause mortality at a 1-month, 3- month, 1-year, and 3-year period.
Statistical analysis
Categorical variables are presented as number of cases and percentages and continuous variables as median (±interquartile range–IQR). To calculate intergroup differences, Mann-Whitney U-test and Chi-square or Fisher’s exact tests were used for continuous and categorical variables respectively. Tests were 2-tailed with p value < 0.05 considered significant.
Relationship between dementia status and time to death was analyzed using Cox regressions. The proportionality of hazards was checked with log minus log plot. We added the covariates in a stepwise manner to adjust for possible confounding. Model 1 of regression analyses is adjusted for age and sex. In model 2, we adjusted for age, sex, independent mobility, and nursing home placement prior to stroke. In model 3, we additionally adjusted for covariates of overall comorbidity using Charlson comorbidity index (CCI). In model 4, we used dementia subtypes instead of dementia diagnosis. Adjusted hazard ratios (HRs) with 95% CIs are presented and additionally dichotomized for patients≤80 years and > 80 years of age.
IBM Statistical Package for Social Sciences (IBM SPSS) for Windows, Sciences software version 22 (IBM Corporation, Armonk, NY, USA) was used.
Standard protocol approvals and patient consent
This study complies with the Declaration of Helsinki and was approved by the regional ethical review board in Stockholm, Sweden (dnr 2015/743–31/4; 2018/563–32). Patients and relatives were informed of inclusion in the registries at the time of diagnosis and could decline participation or withdraw consent. Data were de-identified before analysis.
RESULTS
Between January 2007 and December 2017, there were 45,798 stroke events in patients with dementia, which were matched to 66,784 stroke events in non-dementia controls. Hemorrhagic strokes, duplicated stroke entries, and stroke events in patients with post-stroke dementia were excluded (n = 41,999). For final analyses 12,629 ischemic stroke events in dementia and 57,954 ischemic stroke events in non-dementia patients were available. The patient selection process is illustrated in Fig. 1.
Fig. 1
The flowchart of patient selection.
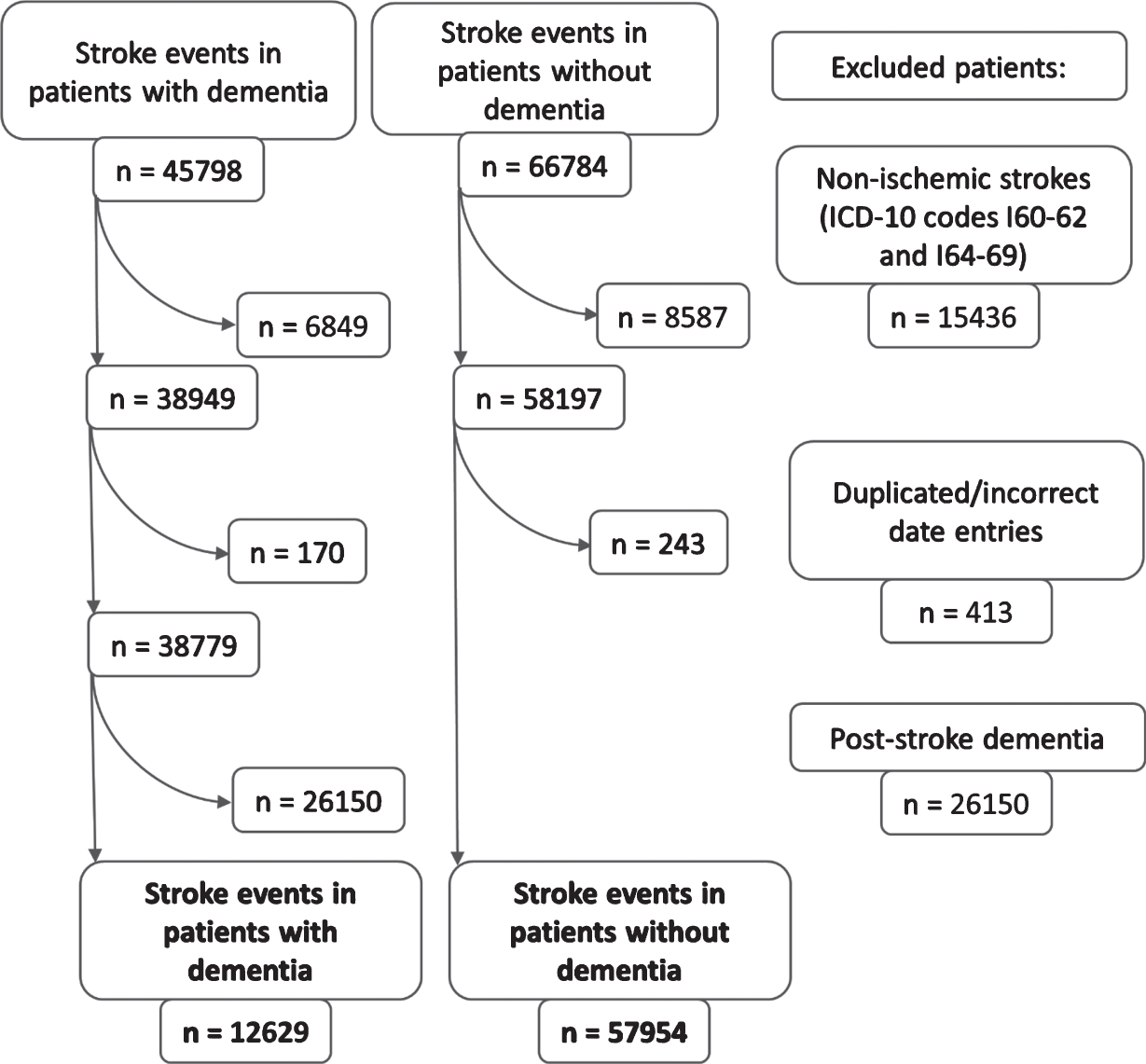
Information on dementia type was available only for 3,756 stroke events in patients registered in SveDem; there were 754 (20.1%) with AD, 809 (21.5%) with mixed, 1,100 (29.3%) with vascular dementia and 1,093 (29.1%) with other dementias. Out of 12,629 ischemic stroke events in dementia, 8,873 stroke events occurred in patients with dementia not registered in the Swedish national dementia registry, SveDem.
In Table 1 and Supplementary Table 1, characteristics of patients prior to stroke, activation of stroke alarm, use of acute reperfusion treatments, and stroke outcomes are presented for the following time intervals: 2007–2009, 2016–2017 (Table 1) and 2010–2012, 2013–2015 (Supplementary Table 1). Before stroke, dementia patients were more likely to be nursing home residents, to be dependent in everyday activities, and to have more comorbidities. They were less likely to be receiving stroke preventive medication except for antiplatelets. The difference in activation of stroke alarm disappeared after the year 2012 (activated in 27.7% dementia and 27.9% non-dementia patients, p = 0.772). After the year 2015, a similar proportion of patients with and without dementia received intravenous thrombolysis (11.1% dementia and 12.3% non-dementia patients, p = 0.117). Differences for acute reperfusion treatments (intravenous thrombolysis and endovascular thrombectomies) remained (years 2016-17, 11.8% in dementia and 13.7% in non-dementia patients, p = 0.010), inferring less patients with dementia receive endovascular thrombectomy.
Table 1
Characteristics, functional outcome, and mortality of patients prior and after ischemic stroke with and without dementia in years 2007–2009 and 2016-2017
| 2007–2009 | 2016-2017 | |||||
| Strokes events in dementia | Stroke events in non-dementia | p | Strokes events in dementia | Stroke events in non-dementia | p | |
| (n = 2,260) | (n = 12,778) | (n = 2,544) | (n = 10,805) | |||
| Age, median (IQR) | 83 (9) | 83 (9) | 0.538 | 84 (10) | 84 (10) | < 0.001 |
| Female sex | 1,273 (56.3) | 7,277 (56.9) | 0.582 | 1,459 (57.4) | 5,981 (55.4) | 0.068 |
| Nursing home | 838 (37.1) | 1,288 (9.6) | < 0.001 | 1,078 (42.4) | 845 (7.8) | < 0.001 |
| Independence in daily activities | 987 (45.5) | 10,406 (82.9) | < 0.001 | 864 (38.1) | 8,547 (82.3) | < 0.001 |
| mRS before stroke | ||||||
| 0–3 | 1,143 (53.1) | 10,926 (87.4) | < 0.001 | 1,208 (49.9) | 9,179 (87.0) | < 0.001 |
| 4 | 679 (31.5) | 1,152 (9.2) | < 0.001 | 851 (35.2) | 1,073 (10.2) | < 0.001 |
| 5 | 331 (15.4) | 425 (3.4) | < 0.001 | 362 (15.0) | 296 (2.8) | < 0.001 |
| CCI at stroke, median (IQR) | 2 (2) | 1 (2) | < 0.001 | 3 (3) | 2 (3) | < 0.001 |
| AF (old or new) | 821 (36.9) | 4,814 (38.1) | 0.250 | 988 (39.0) | 4,016 (37.2) | 0.106 |
| Previous stroke or TIA | 956 (42.9) | 4,298 (33.9) | < 0.001 | 962 (37.9) | 3,036 (28.2) | < 0.001 |
| Previous stroke | 846 (37.4) | 3,700 (29.0) | < 0.001 | 835 (32.8) | 2,388 (22.1) | < 0.001 |
| Previous TIA | 245 (11.5) | 1,212 (9.9) | 0.027 | 294 (11.6) | 1,122 (10.4) | 0.078 |
| Diabetes (old or new) | 498 (22.2) | 2,575 (20.3) | 0.038 | 658 (25.9) | 2,320 (21.5) | < 0.001 |
| Smoking | 113 (5.8) | 963 (8.6) | < 0.001 | 125 (6.0) | 739 (8.0) | 0.002 |
| Antihypertensives | 1,543 (69.5) | 9,430 (74.8) | < 0.001 | 1,799 (71.0) | 8,036 (74.6) | < 0.001 |
| Statins | 473 (21.3) | 3,068 (24.4) | 0.002 | 677 (26.7) | 3,431 (31.9) | < 0.001 |
| Antiplatelets | 1,361 (61.3) | 6,675 (52.9) | < 0.001 | 1,043 (41.1) | 3,960 (36.8) | < 0.001 |
| In AF | 574 (70.8) | ,2775 (58.4) | < 0.001 | 309 (31.3) | 1,061 (26.5) | 0.002 |
| In nonAF | 768 (55.6) | 3,826 (49.5) | < 0.001 | 731 (47.3) | 2,893 (42.8) | 0.001 |
| Anticoagulants in AF | 73 (9.0) | 847 (17.8) | < 0.001 | 313 (31.7) | 1,610 (40.1) | < 0.001 |
| Consciousness at arrival | ||||||
| Fully awake | 1,568 (70.4) | 10,210 (80.7) | < 0.001 | 1,864 (75.0) | 9,171 (86.0) | < 0.001 |
| Lethargic | 495 (22.2) | 1,763 (13.9) | 481 (19.4) | 1142 (10.7) | ||
| Unconscious | 164 (7.4) | 678 (5.4) | 139 (5.6) | 346 (3.2) | ||
| Stroke alarm | 179 (8.1) | 1,276 (10.2) | 0.002 | 869 (34.4) | 3,461 (32.4) | 0.057 |
| IVT | 34 (1.5) | 409 (3.2) | < 0.001 | 283 (11.1) | 1,323 (12.3) | 0.117 |
| IVT or EVT | 34 (1.5) | 418 (3.3) | < 0.001 | 299 (11.8) | 1,479 (13.7) | 0.010 |
| New nursing home placement | 352 (18.0) | 1,389 (11.9) | < 0.001 | 277 (13.6) | 808 (8.6) | < 0.001 |
| Dead after 1 month | 534 (23.6) | 2,108 (16.5) | < 0.001 | 658 (25.9) | 1,568 (14.5) | < 0.001 |
| mRS at 3 months | ||||||
| 0–3 | 420 (21.2) | 5,783 (50.2) | < 0.001 | 350 (16.9) | 5,063 (55.3) | < 0.001 |
| 4 | 382 (16.9) | 1,383 (10.8) | < 0.001 | 397 (15.6) | 1,087 (10.1) | < 0.001 |
| 5 | 408 (18.1) | 1257 (9.8) | < 0.001 | 363 (14.3) | 739 (6.8) | < 0.001 |
| (dead) 6 | 772 (39.0) | 3,105 (26.9) | < 0.001 | 958 (46.3) | 2,260 (24.7) | < 0.001 |
| Worse mRS at 3 months | 1,336 (70.7) | 6,530 (57.9) | < 0.001 | 1,409 (71.7) | 3,680 (41.3) | < 0.001 |
| Dead after 1 year | 1,048 (46.4) | 4,319 (33.8) | < 0.001 | 1,246 (49.0) | 3,040 (28.1) | < 0.001 |
| Dead after 3 years | 1,563 (69.2) | 6,591 (51.6) | < 0.001 | / | / | / |
Data are presented as number of cases (n) and percentage proportion (%) or median and interquartile range (IQR). In variables where n (%) are reported, p-values were obtained by chi-square, whereas in variables where median (IQR) are reported, p-values were obtained by Mann-Whitney test. AF, atrial fibrillation; CCI, Charlson comorbidity index; IQR, interquartile range; IVT, intravenous thrombolysis; EVT, endovascular thrombectomy; mRS, modified Rankin scale; TIA, transient ischemic attack.
Over the years, early and delayed mortality in patients with dementia remained increased. One month after stroke, approximately 25% dementia and 15% non-dementia patients died (in 2007–2009 23.6% dementia and 16.5% non-dementia patients and in 2016–2017 25.9% dementia and 14.5% non-dementia patients were dead). One year after stroke, nearly 50% dementia and 30% non-dementia patients had died (in 2007–2009 46.4% dementia and 33.8% non-dementia patients and in 2016–2017 49.0% dementia and 28.1% non-dementia patients died). Adjusted analyses with Cox hazard ratios (HRs) with 95% CIs for days until death for the whole study period are presented in Table 2. Dementia was an independent predictor of death in all of the models.
Table 2
Cox hazard regression models for time to death. Stroke events in patients without dementia are the reference category
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Dementia | 1.86 (1.82–1.90)*** | 1.39 (1.36–1.43)*** | 1.26 (1.23–1.29)*** | / |
| Age | 1.09 (1.08–1.09)*** | 1.08 (1.08–1.08)*** | 1.08 (1.08–1.08)*** | 1.08 (1.08–1.08)*** |
| Female sex | 0.92 (0.91–0.94)*** | 0.87 (0.85–0.89)*** | 0.92 (0.90–0.93)*** | 0.92 (0.90–0.93)*** |
| Dependent mobility | / | 1.80 (1.75–1.84)*** | 1.67 (1.63–1.71)*** | 1.67 (1.63–1.71)*** |
| Nursing home placement | / | 1.46 (1.42–1.50)*** | 1.44 (1.40–1.48)*** | 1.43 (1.40–1.47)*** |
| Year of stroke | / | 0.96 (0.96–0.96)*** | 0.95 (0.95–0.96)*** | 0.95 (0.95–0.96)*** |
| CCI | / | / | 1.14 (1.13–1.14)*** | 1.14 (1.13–1.14)*** |
| Vascular dementia | / | / | / | 1.16 (1.08–1.24)*** |
| AD and mixed dementia | / | / | / | 1.23 (1.16–1.31)*** |
| Other or unspecified dementias | / | / | / | 1.28 (1.24–1.31)*** |
| Only patients≤80 years of age | ||||
| Dementia | 2.56 (2.45–2.67)*** | 1.73 (1.65–1.82)*** | 1.47 (1.40–1.54)*** | / |
| Age | 1.08 (1.07–1.08)*** | 1.07 (1.07–1.08)*** | 1.07 (1.07–1.07)*** | 1.07 (1.07–1.07)*** |
| Female sex | 0.92 (0.89–0.95)*** | 0.87 (0.84–0.90)*** | 0.92 (0.88–0.95)*** | 0.91 (0.88–0.95)*** |
| Dependent mobility | / | 2.25 (2.13–2.38)*** | 1.94 (1.84–2.05)*** | 1.94 (1.84–2.05)*** |
| Nursing home placement | / | 1.64 (1.54–1.76)*** | 1.70 (1.59–1.82)*** | 1.69 (1.58–1.81)*** |
| Year of stroke | / | 0.96 (0.96–0.97)*** | 0.95 (0.94–0.95)*** | 0.95 (0.94–0.96)*** |
| CCI | / | / | 1.19 (1.18–1.19)*** | 1.19 (1.18–1.19)*** |
| Vascular dementia | / | / | / | 1.23 (1.09–1.40)*** |
| AD and mixed dementia | / | / | / | 1.46 (1.30–1.64)*** |
| Other or unspecified dementias | / | / | / | 1.50 (1.43–1.59)*** |
| Only patients > 80 years of age | ||||
| Dementia | 1.68 (1.64–1.73)*** | 1.27 (1.24–1.31)*** | 1.18 (1.14–1.21)*** | / |
| Age | 1.09 (1.08–1.09)*** | 1.08 (1.08–1.08)*** | 1.08 (1.08–1.09)*** | 1.08 (1.08–1.09)*** |
| Female sex | 0.92 (0.91–0.94)*** | 0.87 (0.85–0.89)*** | 0.91 (0.89–0.93)*** | 0.91 (0.89–0.93)*** |
| Dependent mobility | / | 1.70 (1.65–1.75)*** | 1.61 (1.56–1.65)*** | 1.61 (1.56–1.65)*** |
| Nursing home placement | / | 1.45 (1.41–1.50)*** | 1.42 (1.37–1.46)*** | 1.42 (1.37–1.46)*** |
| Year of stroke | / | 0.96 (0.96–0.97)*** | 0.95 (0.95–0.96)*** | 0.95 (0.95–0.96)*** |
| CCI | / | / | 1.12 (1.11–1.12)*** | 1.12 (1.11–1.13)*** |
| Vascular dementia | / | / | / | 1.10 (1.01–1.19)*** |
| AD and mixed dementia | / | / | / | 1.16 (1.08–1.25)*** |
| Other or unspecified dementias | / | / | / | 1.19 (1.15–1.22)*** |
Results are presented as hazards ratios (HRs) with 95% CI. *p < 0.05, **p < 0.01, ***p≤0.001 CCI, Charlson comorbidity index. Independent mobility, nursing home placement and CCI are reported prior to stroke.
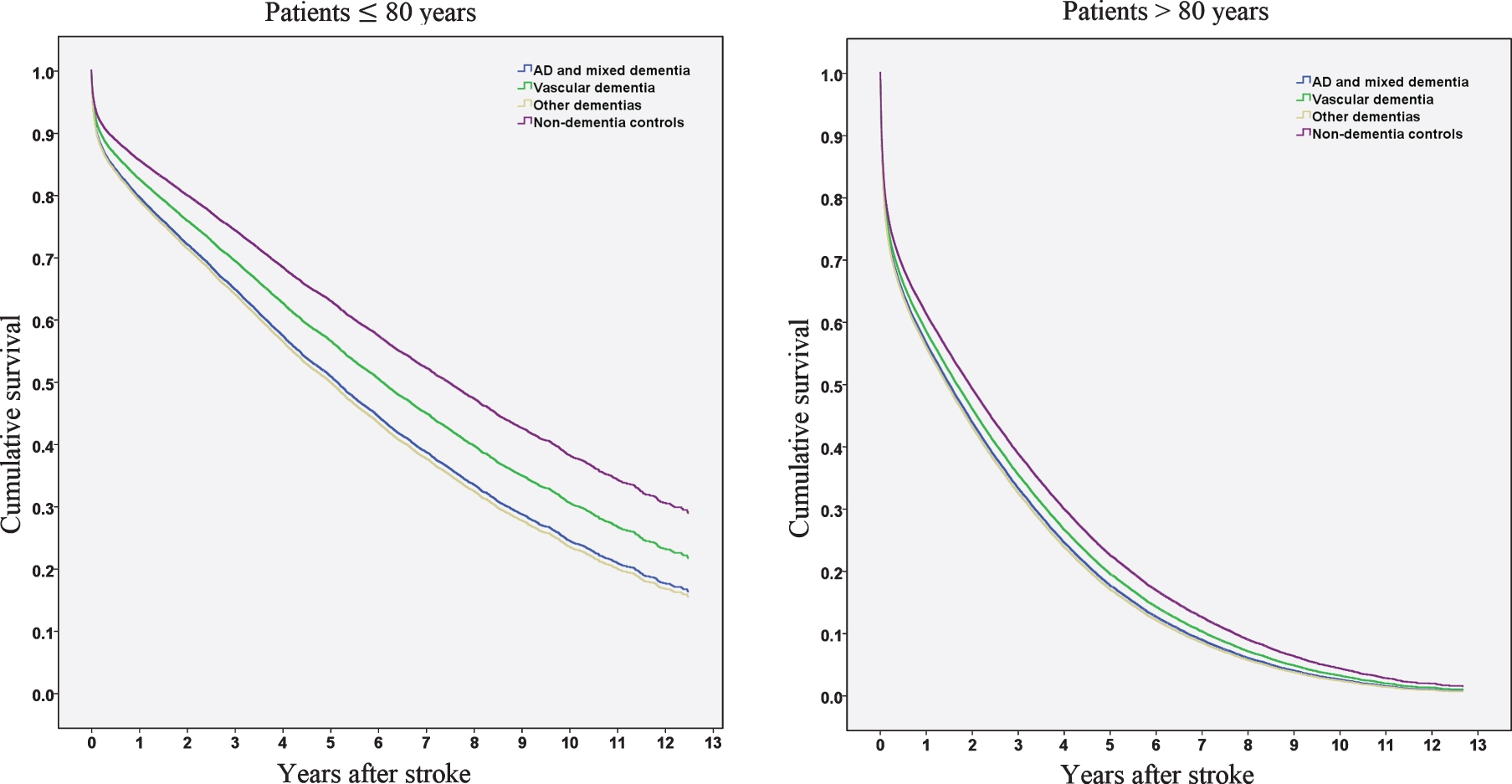
We performed Cox hazard analyses for dementia types (Alzheimer’s disease and mixed dementia, vascular dementia, and other dementias), stratified by age (Table 2, Fig. 2). All dementia subcategories presented significantly higher mortality in the hazard regression model compared to controls, regardless of dementia type. When patients with Alzheimer’s disease and mixed dementia were used as a reference category (results not presented in the table), patients with vascular dementia did not display significantly increased mortality rates (HR 0.94 [0.86–1.03]). However, when the analysis was stratified to patients≤80 years and > 80 years, patients with vascular dementia in the younger strata had significantly decreased mortality compared to patients with AD or mixed dementia (≤80 years, HR 0.84 [0.72–0.99]), but not in the older strata (> 80 years, HR 1.03 [0.91–1.16). Survival function patterns for patients≤80 years and > 80 years are presented in Fig. 2.
Fig. 2
Cumulative survival in years after stroke in patients≤80 years and > 80 years.
DISCUSSION
The main findings of our study are 1) patients with dementia continued to have increased early and late mortality after stroke during the whole study period, 2) dementia is an independent predictor of mortality after stroke, and 3) patients with prior Alzheimer’s disease and mixed dementia may have worse survival after stroke when compared to patients with vascular dementia.
Comorbidities have a strong impact on outcome and mortality after stroke. Another observational Swedish Stroke Register (Riksstroke) study demonstrated high comorbidity burden in first-ever ischemic stroke patients, which more than doubled the proportion of poor outcome (the strongest being dementia, kidney, and heart failure) [35]. Since cardioembolic strokes in general are more severe and fatal, the increased risk of death in patients with dementia could be partly explained by a stroke mechanism. Even though a similar proportion of patients with and without dementia had AF (35% –40%), patients with dementia and AF were less likely to be receiving anticoagulation in the beginning of the study period (e.g., in 2010–2012, 9.6% of dementia and 20.9% of non-dementia patients received anticoagulants, p < 0.001), hence AF could partly account for the increased mortality among patients with dementia in our study.
Nonetheless, even though previous studies have demonstrated increased mortality in patients with dementia [8–12], pre-stroke dementia was not an independent predictor for death [9, 11]. In a study by Henon et al., 54.5% of patients with pre-stroke dementia and 24.9% non-dementia patients died in a 6-month period after stroke, but probably due to stroke characteristics rather than pre-stroke dementia [9]. In contrast, our study demonstrates increased early and delayed mortality in patients with dementia. This completes a previous study from our group showing that prestroke mobility was worse in patients with dementia, and together with dementia was associated with mortality at 1 and 3 months after stroke [13].
Patients with pre-existing dementia might be more severely affected by stroke because of reduced neuroplasticity and neuronal loss, which may explain worsening of dementia and poorer recovery after stroke [36]. We did not observe a difference in stroke mortality in a previous study on outcomes of intravenous thrombolysis in dementia; however, patients receiving acute reperfusion treatment might have had a better functional status at baseline [19]. Stroke detection may be delayed in patients with dementia, missing the window for acute reperfusion treatments. These treatments may be withheld in patients with reduced life expectancy [14]. Nevertheless, during the study time period, utilization of stroke alarm increased in both groups and the differences between its use in patients with and without dementia disappeared. Additionally, the gap between dementia and non-dementia patients for acute stroke treatment with intravenous thrombolysis also decreased, with the differences dissappearing in 2016–2017. The difference in endovascular thrombectomy persisted.
Even though global stroke incidence and age-standardized death rates have decreased [1], we observed this trend only in patients without dementia, where mortality continuously decreased over the study period (e.g., 1-year mortality decreased by approximately 5%, from 33.8% in 2007–2009 to 28.1% in 2016–2017). In patients with dementia, a similar trend was not observed, on the contrary, at most mortality increased by approximately 2%. In Sweden, there are no or small differences for most aspects of stroke care (direct access to stroke units, CT, swallowing assessments, etc.) in patients with dementia [37], and stroke care is improving over time. Riksstroke only covers hospital stroke events, and this could explain the increase in mortality in patients with dementia. With the development and greater accessibility of reperfusion treatments, more nursing home residents with dementia and other comorbidities, who previously died in their residences, could be referred to hospitals and hence increase hospital stroke mortality.
AD and vascular dementia were found to be important predictors of death [25]. While most other studies found poorer survival in patients with vascular dementia compared to AD [23, 25–28], or no differences [29–31], we found worse survival in AD and mixed dementia in ‘younger’ patients (≤ 80 years) compared to vascular dementia after stroke. Similar findings were reported in an older study, where patients with AD had worse survival compared to multi-infarct dementia [38]. On the other hand, a majority of these studies are older and survival in patients with vascular dementia might have improved over the years. Another Swedish study including Riksstroke and the Swedish Cause of Death Register found vascular dementia as the most common subtype in patients with dementia who died because of ischemic stroke while AD was the most common subtype in those who died from other causes [39].
Importantly, the patients received dementia diagnosis subtypes prior to the stroke event. Consequently, patients with AD or mixed pre-stroke dementia could then suffer from additional post-stroke vascular cognitive impairment, which might have had a greater impact on survival. The combination of pre-stroke AD and new ischemic stroke lesions might have a synergistic and more detrimental effect on outcome compared to ischemic stroke in a patient with a prior vascular cognitive decline. In older patients with dementia (> 80 years), dementia subtypes seem less important. The reasons behind this remain unknown; it could also be a reflection of substantial increases in mortality in all old patients (including non-dementia) or the fact that pure AD becomes uncommon with increasing age. Nonetheless, ‘younger’ patients with prior AD or mixed dementia and ischemic stroke having worse survival compared to vascular dementia and ischemic stroke is a novel finding of this study.
Strengths and limitations
A major strength of this study is the use of national quality registries, SveDem and Riksstroke, which have high coverage. SveDem is connected to all specialist memory clinics in Sweden and to increasing number of primary care centers and nursing homes; however, registered cases may not be representative of the general dementia population. Riksstroke’s follow-up of functional outcomes have tolerable proportion of missing (approximately 15%). However, it is worthwhile mentioning there might be some residual confounding we did not adjust for and other contributing disease states could be involved, for example frailty, a risk factor for developing stroke, including factors such as sarcopenia, mobility, and cognitive status [40]. Frail people with dementia often reside in nursing homes and may be underrepresented in this study if they are not referred to hospital for treatment in ischemic stroke, since Riksstroke covers only hospital stroke events. Additionally, it was impossible to determine dementia severity at the time of stroke, while stroke severity, assessed by the National Institutes of Health Stroke Scale (NIHSS), had too many missing values to be useful for analyses. Nonetheless, Riksstroke contains information on living arrangements and independence in daily activities prior to stroke. We were unable to differentiate between mRS 0–3 pre-stroke and mRS 0–2 poststroke, since Riksstroke only allows an mRS estimation. Information on dementia subtype was available only for patients registered in SveDem, limiting reliability of dementia subtype diagnosis, since some patients in ‘other dementias’ group could have had vascular, mixed or AD dementia.
CONCLUSION
Over time we did not observe a decrease in early or delayed mortality in patients with dementia; however, some aspects of stroke care improved (e.g., stroke alarm, access to intravenous thrombolysis). Dementia before ischemic stroke is an independent predictor of death after stroke. Patients≤80 years with prior Alzheimer’s disease and mixed dementia might have higher mortality rates after stroke compared to patients with prior vascular dementia.
ACKNOWLEDGMENTS
The authors are grateful to SveDem, Riksstroke, all patients, caregivers, and staff. SveDem is supported by the Swedish Association of Local Authorities and Regions and Swedish Brain Power. This study has been financially supported by FORTE grant # 2017–01646, Swedish Society for Medical Research, Swedish Stroke Association, KI Research Foundations, KI Foundation for Diseases of Aging, Loo and Hans Osterman’s Foundation for Medical Research, Swedish Research Council (2018–02843) Swedish Order of St. John/Johanniterorden i Sverige and Margaretha af Ugglas foundation. The funding organizations did not influence study design or data interpretation.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1459r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-201459.
REFERENCES
[1] | GBD 2016 Stroke Collaborators ((2019) ) Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: , 439–458. |
[2] | Lackland DT , Roccella EJ , Deutsch AF , Fornage M , George MG , Howard G , Kissela BM , Kittner SJ , Lichtman JH , Lisabeth LD , Schwamm LH , Smith EE , Towfighi A; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; Council on Functional Genomics and Translational Biology ((2014) ) Factors influencing the decline in stroke mortality: A statement from the American Heart Association/American Stroke Association. Stroke 45: , 315–353. |
[3] | Man S , Schold JD , Uchino K ((2020) ) Case fatality decline from 2009 to 2013 among Medicare beneficiaries with ischemic stroke. J Stroke Cerebrovasc Dis 29: , 104559. |
[4] | Donkor ES ((2018) ) Stroke in the 21(st) century: A snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat 2018: , 3238165. |
[5] | Fonarow GC , Reeves MJ , Zhao X , Olson DM , Smith EE , Saver JL , Schwamm LH ((2010) ) Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation 121: , 879–891. |
[6] | SveDem, Swedish Dementia Registry, http://www.ucr.uu.se/svedem/in-english, Accessed May 25, 2020. |
[7] | Riksstroke, The Swedish Stroke Register, Riksstroke, Medicincentrum, Norrlands universitetssjukhus, http://www.riksstroke.org/eng/, Accessed May 25, 2020. |
[8] | Desmond DW , Moroney JT , Sano M , Stern Y ((2002) ) Mortality in patients with dementia after ischemic stroke. Neurology 59: , 537–543. |
[9] | Henon H , Durieu I , Lebert F , Pasquier F , Leys D ((2003) ) Influence of prestroke dementia on early and delayed mortality in stroke patients. J Neurol 250: , 10–16. |
[10] | Tatemichi TK , Paik M , Bagiella E , Desmond DW , Pirro M , Hanzawa LK ((1994) ) Dementia after stroke is a predictor of long-term survival. Stroke 25: , 1915–1919. |
[11] | Saposnik G , Kapral MK , Cote R , Rochon PA , Wang J , Raptis S , Mamdani M , Black SE ((2012) ) Is pre-existing dementia an independent predictor of outcome after stroke? A propensity score-matched analysis. J Neurol 259: , 2366–2375. |
[12] | Saposnik G , Cote R , Rochon PA , Mamdani M , Liu Y , Raptis S , Kapral MK , Black SE ((2011) ) Care and outcomes in patients with ischemic stroke with and without preexisting dementia. Neurology 77: , 1664–1673. |
[13] | Garcia-Ptacek S , Contreras Escamez B , Zupanic E , Religa D , von Koch L , Johnell K , von Euler M , Kåreholt I , Eriksdotter M ((2018) ) Prestroke mobility and dementia as predictors of stroke outcomes in patients over 65 years of age: A cohort study from the Swedish Dementia and Stroke Registries. J Am Med Dir Assoc 19: , 154–161. |
[14] | Zupanic E , Kramberger MG , von Euler M , Norrving B , Winblad B , Secnik J , Fastbom J , Eriksdotter M , Garcia-Ptacek S ((2020) ) Secondary stroke prevention after ischemic stroke in patients with Alzheimer’s disease and other dementia disorders. J Alzheimers Dis 73: , 1013–1021. |
[15] | Tanislav C , Milde S , Schwartzkopff S , Sieweke N , Kramer HH , Juenemann M , Misselwitz B , Kaps M ((2014) ) Secondary stroke prevention in atrial fibrillation: A challenge in the clinical practice. BMC Neurol 14: , 195. |
[16] | Shah R , Li S , Stamplecoski M , Kapral MK ((2016) ) Low use of oral anticoagulant prescribing for secondary stroke prevention: Results from the Ontario Stroke Registry. Med Care 54: , 907–912. |
[17] | Subic A , Cermakova P , Religa D , Han S , von Euler M , Kareholt I , Johnell K , Fastbom J , Bognandi L , Winblad B , Kramberger MG , Eriksdotter M , Garcia-Ptacek S ((2018) ) Treatment of atrial fibrillation in patients with dementia: A cohort study from the Swedish Dementia Registry. J Alzheimers Dis 61: , 1119–1128. |
[18] | Gilligan AK , Thrift AG , Sturm JW , Dewey HM , Macdonell RA , Donnan GA ((2005) ) Stroke units, tissue plasminogen activator, aspirin and neuroprotection: Which stroke intervention could provide the greatest community benefit? Cerebrovasc Dis 20: , 239–244. |
[19] | Zupanic E , von Euler M , Kåreholt I , Escamez BC , Fastbom J , Norrving B , Religa D , Kramberger MG , Winblad B , Johnell K ((2017) ) Thrombolysis in acute ischemic stroke in patients with dementia A Swedish registry study. Neurology 89: , 1860–1868. |
[20] | Kua EH , Ho E , Tan HH , Tsoi C , Thng C , Mahendran R ((2014) ) The natural history of dementia. Psychogeriatrics 14: , 196–201. |
[21] | Kramarow EA , Tejada-Vera B ((2019) ) Dementia mortality in the United States, 2000-2017. Natl Vital Stat Rep 68: , 1–29. doi: |
[22] | Garcia-Ptacek S , Kareholt I , Cermakova P , Rizzuto D , Religa D , Eriksdotter M ((2016) ) Causes of death according to death certificates in individuals with dementia: A cohort from the Swedish Dementia Registry. J Am Geriatr Soc 64: , e137–e142. |
[23] | Garcia-Ptacek S , Farahmand B , Kareholt I , Religa D , Cuadrado ML , Eriksdotter M ((2014) ) Mortality risk after dementia diagnosis by dementia type and underlying factors: A cohort of 15,209 patients based on the Swedish Dementia Registry. J Alzheimers Dis 41: , 467–477. |
[24] | Brønnum-Hansen H , Davidsen M , Thorvaldsen P ((2001) ) Long-term survival and causes of death after stroke. Stroke 32: , 2131–2136. |
[25] | Aevarsson Ó , Svanborg A , Skoog I ((1998) ) Seven-year survival rate after age 85 years: Relation to Alzheimer disease and vascular dementia. Arch Neurol 55: , 1226–1232. |
[26] | Barclay LL , Zemcov A , Blass JP , Sansone J ((1985) ) Survival in Alzheimer’s disease and vascular dementias. Neurology 35: , 834–840. |
[27] | Katzman R , Hill LR , Yu ES , Wang ZY , Booth A , Salmon DP , Liu WT , Qu GY , Zhang M ((1994) ) The malignancy of dementia. Predictors of mortality in clinically diagnosed dementia in a population survey of Shanghai, China. Arch Neurol 51: , 1220–1225. |
[28] | Garre-Olmo J , Ponjoan A , Inoriza JM , Blanch J , Sánchez-Pérez I , Cubí R , de Eugenio R , Turró-Garriga O , Vilalta-Franch J ((2019) ) Survival, effect measures, and impact numbers after dementia diagnosis: A matched cohort study. Clin Epidemiol 11: , 525–542. |
[29] | Rockwood K , Wentzel C , Hachinski V , Hogan DB , MacKnight C , McDowell I ((2000) ) Prevalence and outcomes of vascular cognitive impairment. Neurology 54: , 447–447. |
[30] | Rössler W , Hewer W , Fätkenheuer B , Löffler W ((2018) ) Excess mortality among elderly psychiatric in-patients with organic mental disorder. Br J Psychiatry 167: , 527–532. |
[31] | van Dijk PT , Dippel DW , Habbema JD ((1991) ) Survival of patients with dementia. J Am Geriatr Soc 39: , 603–610. |
[32] | Ludvigsson JF , Andersson E , Ekbom A , Feychting M , Kim JL , Reuterwall C , Heurgren M , Olausson PO ((2011) ) External review and validation of the Swedish national inpatient register. BMC Public Health 11: , 450. |
[33] | Eriksson M , Appelros P , Norrving B , Terént A , Stegmayr B ((2007) ) Assessment of functional outcome in a national quality register for acute stroke can simple self-reported items be transformed into the modified Rankin Scale? Stroke 38: , 1384–1386. |
[34] | Charlson ME , Pompei P , Ales KL , MacKenzie CR ((1987) ) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: , 373–383. |
[35] | Sennfält S , Pihlsgård M , Petersson J , Norrving B , Ullberg T ((2020) ) Long-term outcome after ischemic stroke in relation to comorbidity–An observational study from the Swedish Stroke Register (Riksstroke). Eur Stroke J 5: , 36–46. |
[36] | Altman KW , Richards A , Goldberg L , Frucht S , McCabe DJ ((2013) ) Dysphagia in stroke, neurodegenerative disease, and advanced dementia. Otolaryngol Clin North Am 46: , 1137–1149. |
[37] | Zupanic E , Kareholt I , Norrving B , Secnik J , von Euler M , Winblad B , Religa D , Kramberger MG , Johnell K , Eriksdotter M , Garcia-Ptacek S ((2018) ) Acute stroke care in dementia: A cohort study from the Swedish Dementia and Stroke Registries. J Alzheimers Dis 66: , 185–194. |
[38] | Belloni-Sonzogni A , Tissot A , Tettamanti M , Frattura L , Spagnoli A ((1989) ) Mortality of demented patients in a geriatric institution. Arch Gerontol Geriatr 9: , 193–197. |
[39] | Subic A , Zupanic E , von Euler M , Norrving B , Cermakova P , Religa D , Winblad B , Kramberger MG , Eriksdotter M , Garcia-Ptacek S ((2018) ) Stroke as a cause of death in death certificates of patients with dementia: A cohort study from the Swedish Dementia Registry. Curr Alzheimer Res 15: , 1322–1330. |
[40] | Lichtman JH , Krumholz HM , Wang Y , Radford MJ , Brass LM ((2002) ) Risk and predictors of stroke after myocardial infarction among the elderly. Circulation 105: , 1082–1087. |




